Abstract
Naltrexone (NTX) has been widely studied for the treatment of alcohol use disorder with overall support for its efficacy. The mechanisms of action of naltrexone are thought to involve attenuation of the hedonic effects of alcohol and potentiation of its aversive effects. In order to provide a quantitative estimate of the effects of naltrexone on subjective response to alcohol, the aims of this meta-analytic review are to examine the effects of naltrexone across four domains of subjective response. Meta-analyses of naltrexone effects on alcohol craving (k=16, N=686), stimulation (k=15, N=675), sedation (k=18, N=777), and negative mood (k=9, N=281) suggested that under laboratory conditions and compared to placebo, naltrexone reduces craving (Hedge’s g= −0.252, SE=0.054, 95% CI [−0.375, −0.130], p<0.01), reduces stimulation (g= −0.223, SE=0.067, 95% CI [−0.372, −0.074], p<0.01), increases sedation (g=0.251, SE=0.064, 95% CI [0.112, 0.389], p<0.01), and increases negative mood (g=0.227, SE=0.047, 95% CI [0.100, 0.354], p<0.01). Results were robust when drinks per month and alcohol dose were added to the models as covariates. The effects of naltrexone varied by severity of alcohol use with medication effects on craving and stimulation being observed in sample of both heavy drinkers and AUD individuals. These results are consistent with the hypothesized mechanisms of action of NTX, although the effects are of small magnitude. This meta-analysis aggregates across multiple human laboratory studies of NTX’s effects on subjective response to alcohol, providing a comprehensive summary of a key mechanism of NTX efficacy, namely alteration of the subjective experience of alcohol.
Keywords: naltrexone, meta-analysis, subjective response, craving, human laboratory, effect size
INTRODUCTION
Alcohol Use Disorders (AUD) are among the most common and costly psychiatric disorders with relatively few established treatment options (Grant et al., 2015; Litten et al., 2016b; Miller et al., 2011; Rehm et al., 2009). The opioid receptor antagonist naltrexone has garnered considerable empirical support for the treatment of both alcohol (Anton et al., 2006; Donoghue et al., 2015; O’Malley et al., 1992; Rosner et al., 2010) and opioid (Johansson et al., 2006) use disorders.
Though naltrexone appears to promote alcohol abstinence, greater effect sizes are typically observed for reductions in heavy drinking on naltrexone compared to placebo. Specifically, meta-analytic results of randomized controlled trials observed a risk ratio (RR) for the outcome “return to any drinking” of 0.96, 95% CI [0.92, 1.00], whereas the RR for “return to heavy drinking” was 0.83, 95% CI [0.76, 0.90] (Rosner et al., 2010). This same pattern of results was then replicated in a later meta-analysis (Jonas et al., 2014) with greater effect sizes observed for heavy drinking outcomes as compared to abstinence outcomes. Furthermore, secondary analysis of the large, multisite COMBINE trial (Anton et al., 2006) suggested that individuals who drank more regularly during the trial showed greater benefits of naltrexone (Ray et al., 2010c). These results suggest that naltrexone’s clinical efficacy is partially a result of its interaction with the pharmacodynamics effects of alcohol.
Alcohol’s pharmacodynamic interactions are complex, affecting a host of neurotransmitter systems including GABA, glutamate, dopamine, and endogenous opioids (Vengeliene et al., 2008). The reinforcing effects of alcohol are in part a consequence of β-endorphin release in mesolimbic reward systems (Gianoulakis, 2004, 2009). Further, animal studies have demonstrated that naltrexone reduces ethanol self-administration by interfering with the dopamine-mediated effects of ethanol in the nucleus accumbens (Gonzales and Weiss, 1998). Multiple candidate gene studies of the μ-opioid receptor gene (OPRM1) have shown genetic variation in this opioidergic receptor to affect level of alcohol reward and reinforcement in terms of subjective responses to alcohol (Ray et al., 2013; Ray and Hutchison, 2004; Ray et al., 2010d), alcohol self-administration (Hendershot et al., 2016), and striatal activity in the PET environment (Ramchandani et al., 2011). As a competitive opioid antagonist with primary affinity for μ-opioid receptors, one proposed mechanism of action for naltrexone is the suppression of alcohol’s rewarding subjective effects (Heilig et al., 2010; Ray et al., 2010a). In support, a PET study found that naltrexone at the 50 mg dose produced near complete inhibition of the mu-opioid receptor in a sample of individuals with AUD in early abstinence (Weerts et al., 2008).
The human behavioral pharmacology laboratory is ideal for testing this biobehavioral mechanism of action via controlled alcohol administration paradigms (Bujarski and Ray, 2016; Ray et al., 2010b; Zimmermann et al., 2013). A systematic review of the alcoholism medication development literature identified 15 different pharmacological compounds that have been tested for their effects on laboratory outcomes (Yardley and Ray, 2017) and naltrexone is by far the most widely studied medication in the human laboratory (e.g., Anton et al., 2012; de Wit et al., 1999; Drobes et al., 2004; King et al., 1997; O’Malley et al., 2002; Ray and Hutchison, 2007). Furthermore, while these studies appear to provide a consistent picture wherein naltrexone reduces the rewarding effects of alcohol and alcohol craving, only one systematic review and meta-analysis has been published addressing a subset of human laboratory outcomes, namely alcohol craving and self-administration (Hendershot et al., 2017). Hendershot et al (2017) analyzed data from 20 placebo-controlled studies on the effects of naltrexone on craving in response to alcohol administration and/or cue presentation, as well as the effects of naltrexone on alcohol self-administration. Hendershot and colleagues observed significant, though relatively small effect sizes for naltrexone on craving (Hedge’s g = −0.29, 95% CI [−0.42, −0.16]) and self-administration (Hedge’s g = −0.28 95% CI [−0.42, −0.13]) in the human laboratory.
While Hendershot et al. (2017) is the first study to quantify the magnitude of naltrexone effects on measures of alcohol reinforcement (i.e., craving and self-administration), no study has examined the putative mechanism of action of blunting hedonic responses to alcohol and/or potentiating aversive responses (King et al., 2014; Rueger et al., 2009). The importance of a quantitative review, as compared to a qualitative review, is underscored by the common practice in human laboratory research of collecting many outcomes within a given study (Bujarski and Ray, 2016). As there are no established guidelines on the assessment of subjective alcohol responses, there is strong potential for systematic bias in reporting of statistical results and potentially inflating the apparent reliability and effect sizes of naltrexone (Simmons et al., 2011). Furthermore, most behavioral pharmacology studies involve small samples (~20–40 subjects), which may increase these risks (Gelman and Carlin, 2014). Therefore, the aims of this meta-analytic review are to examine the effects of naltrexone on subjective response to alcohol across the four domains of (a) craving, (b) stimulation, (c) sedation, and (d) negative affect. These four domains of subjective response to alcohol have been identified in previous factor-analytic work as capturing the full spectrum of SR in the human laboratory (Bujarski et al., 2015; Ray et al., 2009). Specifically, by synthesizing data across a wide range of human laboratory studies of naltrexone, this meta-analysis examines whether naltrexone reduces alcohol-induced craving, and stimulation while increasing alcohol-induced sedation and negative affect. These behavioral pharmacology endpoints, in turn, represent some of the strongest putative mechanisms of action of naltrexone, based on single studies and qualitative reviews of the literature (Ray et al., 2010a).
METHODS
Literature Review and Study Coding
Inclusion criteria were (1) randomized placebo-controlled administration of naltrexone in individuals who consume alcohol for the purposes of testing AUD outcomes (e.g. studies aiming to test smoking outcomes were not included), (2) alcohol administration in the laboratory to a target BrAC via alcohol challenge or priming for self-administration paradigms1, (3) subjective response outcomes measured via self-report questionnaires, (4) reported in the English language, or translated to English, and (5) publication in a peer reviewed and PubMed indexed journal.
Literature searching consisted of multiple stages. First, published reviews of alcohol use disorder (AUD) psychopharmacology were reviewed to identify studies of naltrexone on subjective responses to alcohol (Bujarski and Ray, 2016; Litten et al., 2012; Litten et al., 2016a; Yardley and Ray, 2017). Second, PubMed searches were conducted with the following phrases: “naltrexone”, “alcohol challenge,” “alcohol response,” “response* to alcohol,” “alcohol response,” “alcohol priming,” “alcohol intoxication,” “ethanol intoxication,” “response* to ethanol,” “ethanol response.” These PubMed searches yielded a total of 88 citations which were assessed for relevance in the present paper via abstract review.
Based on abstract reviews, from these 88 initial studies, 28 were deemed relevant for full text review. The 60 citations that were excluded from abstract review were excluded because they were either (1) review papers, (2) animal studies, (3) clinical trials, or (4) observational studies, From the 28 studies reviewed in full, eight studies were excluded based on full text review (four for lack of controlled alcohol administration, two for lack of SR outcomes, and two for reporting previously published results which were already included in this analysis, see Figure 1). This resulted in a final sample of 20 studies that were included in this analysis comprising 21 independent samples with 822 total subjects. All studies were coded by at least two raters (SB, DJOR, or RG). Where coding discrepancies arose, all raters met in person to reach a consensus. Furthermore, when sufficient data to generate effect size estimates were not reported in the published paper, corresponding authors were contacted via email in an attempt to obtain the necessary information. The DigitizeIt software (Bormann, 2012) was also utilized to extract data from published figures (Rakap et al., 2016) where necessary. Assigning of outcome variables to SR domains was also determined through consensus discussion among all study coders referencing the prior factor analytic work (Bujarski et al., 2015; Ray et al., 2009), other published articles, and/or through referencing the specific items. The full list of variables included in each domain can be found in the supplemental materials. Studies were also coded on the following design and sample characteristics in order to control for them when estimating naltrexone effects on the laboratory outcomes of interest: (a) final/target BrAC, (b) mean drinks per month for the study sample, and (c) target naltrexone dose.
Figure 1.
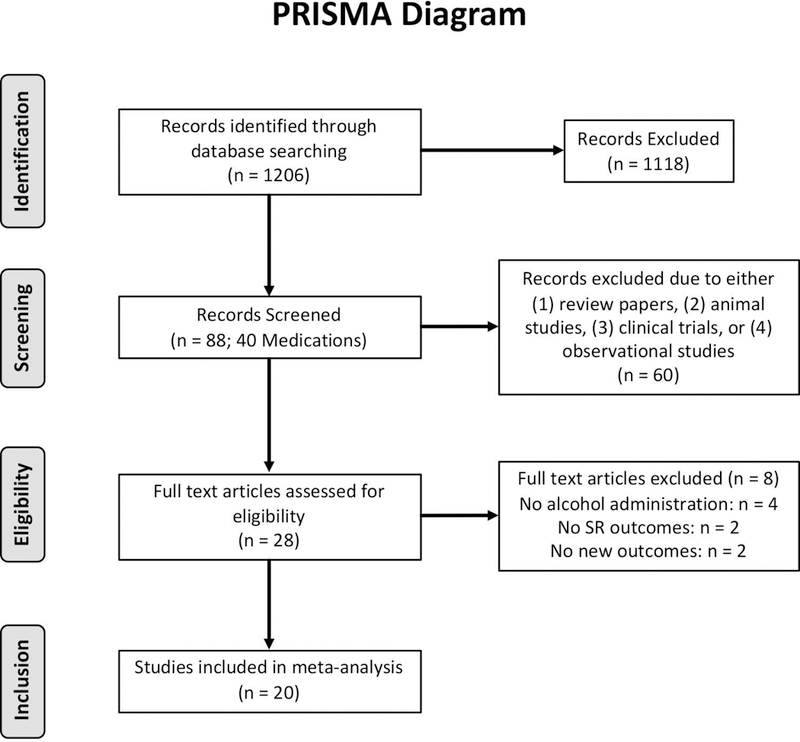
PRISMA diagram outlining the record identification, screening, eligibility, and inclusion.
Effect Size Computation
Hedge’s g effect sizes, which are unbiased estimators of standardized mean differences (Hedges, 1981), were computed for naltrexone main effects based on the reported results, extraction of data from published figures, or through contact with the study authors. Effect sizes from within-subjects studies were converted to a “raw score” metric which represents expected effect size and error variance as if the estimate was coming from a between-subjects study design thus permitting the comparison with other between-subject studies (Morris and DeShon, 2002). Where results were described as “non-significant” or omitted from the study results and data was not made available from the authors, two approaches were used. First, a moderate approach was employed wherein missing results were imputed with an effect size associated with a p-value of 0.50. Second, a conservative approach was employed wherein a Hedge’s g estimate of 0 was imputed. These measures were taken to address effect size inflation resulting from publication bias. Importantly, this was a common occurrence (47 of the 171 total study-level outcomes) suggesting that there is a high risk of publication bias and associated effect size inflation. Our approach to measure selection was to include all available measures. Notably however, while these methods will address the issue of publication bias from individual studies selectively reporting significant outcomes, it does not affect the related problem of whole studies going unreported.
Meta-Analytic Approach
Because these laboratory studies typically reported several outcomes within each SR domain, robust variance estimation (RVE) meta-analysis methods were implemented via the robumeta package in R (Fisher and Tipton, 2015). RVE techniques were utilized as opposed to more traditional analytic methods for the following reasons: (1) RVE methods allow for the estimation of an overall effect size accounting for the dependence of multiple related outcomes reported in a given study (Fisher and Tipton, 2015; Hedges et al., 2010; Tipton, 2015), and (2) RVE methods are able to correct for small sample sizes which are common in behavioral pharmacology studies (Tipton, 2015). Given the significant methodological variation between studies, we expected a substantial degree of heterogeneity. I2 was used to test for inter-study heterogeneity (Deeks et al., 2008; Higgins, 2008; Higgins et al., 2003) and τ2 was used to provide an estimate of between study variance regardless of sample size (Rucker et al., 2008). Funnel plots were examined to detect signs of publication and other reporting biases (Egger et al., 1997; Sterne et al., 2011).
Because study population (i.e. AUD, heavy drinking, or light drinking sample) is a potentially integral component to the transition of laboratory studies to clinical trials, further analysis was also conducted examining naltrexone’s effect on subjective responses to alcohol in these different population groups separately. Due to the strong, nearly tautological relationship between study population and mean drinks per month, intercept only models are reported for these analyses.
Random RVE intercept models were first conducted to estimate the average effect size of naltrexone on a given SR domain. Following the fitting of intercept-only models, meta-regression techniques were employed to determine whether between study factors impact naltrexone effect sizes and test whether naltrexone effects were robust to controlling for study differences. Specifically, the following continuous and objective metrics were entered into RVE meta-regression models: final target BrAC (centered at 0.06 g/dl) and mean drinks per month for the study sample (log centered at 100 drinks per month). Study population was not entered as a covariate because of (1) the limited number of categories as compared to the continuous variable of mean drinks per month, and (2) potential inconsistency in terminology which was not an issue with the objective measures included.
RESULTS
Study Characteristics
In total, 20 studies met the inclusion criteria for this analysis with 822 total subjects. On average, studies included 35.74 ± 21.39 subjects (range: 10 – 85). A majority of these studies (k = 14/20) utilized a within-subjects design for the primary medication condition (e.g. Naltrexone versus matched placebo). Studies typically employed controlled alcohol challenge paradigms (80%), with a smaller subset utilizing a priming dose of alcohol (20%). Overall the average target BrAC of these alcohol administration studies was .054 ± 0.022 g/dl. However, as expected there were sizeable differences in the target BrAC between alcohol challenge and priming paradigms (priming mean = 0.024 ± 0.009; challenge mean = 0.062 ± 0.017; t (8.84) = 5.94, p < 0.001). Only a minority of studies recruited AUD samples (25%), with most recruiting either light or heavy drinkers (40% and 35%, respectively). In terms of the average alcohol consumed by the study samples, there was a wide range of drinking behavior (mean drinks per month = 84.37 ± 81.15); however as expected drinking magnitude was differentiated by the target population being enrolled in the study (light drinking mean = 33.7 ± 17; heavy drinking mean = 59.5 ± 33.8; AUD mean = 200 ± 78; F (2, 7) = 23.53, p < 0.001). Nearly every study tested a 50mg/day dose of NTX with one study testing a 25mg/day dose and one study testing a100mg/day dose. Several studies included multiple (repeated) within-subjects observations of a given outcome. In those cases where multiple time points were available, a single effect size was derived by computing the effect size from the repeated measures analyses which encompass all time points, as opposed to the deriving an effect size for each time point in analysis. Table 1 provides a description of each study that was included in this meta-analysis. Forest Plots describing the effect size for study included in this meta-analysis are provided as Supplementary Materials for each of the four domains of subjective response examined.
Table 1:
List of studies included in the meta-analysis and study characteristics. (Anton et al., 2004) (Anton et al., 2012) (Bujarski et al., 2012) (Anton et al., 2004) (King et al., 1997) (McCaul et al., 2000) (Na and Lee, 2002) (O’Malley et al., 2002) (Peterson et al., 2006) (Plebani et al., 2011) (Ray et al., 2012b) (Ray et al., 2014) (Ray and Hutchison, 2007; Ray et al., 2008) (Ray et al., 2007) (Setiawan et al., 2011) (Spagnolo et al., 2014) (Swift et al., 1994) (Doty et al., 1997) (de Wit et al., 1999)
| Study | Max Naltrexone Dose | Within-Subjects Design | N | Alcohol Administration
Paradigm |
Study Population | Average Drinks per Month | Target Alcohol Administration Dose (g/dl) |
Outcomes Measured |
|---|---|---|---|---|---|---|---|---|
| Anton et al.2004 | 50 | Between-Subjects | 40 | Priming | Alcohol Use Disorder | 160 | 0.03 | Craving: AUQ Stimulation: BAES Stimulation Sedation: BAES Sedation, SHAS |
| Anton et al.2012 | 50 | Between-Subjects | 83 | Priming | Alcohol Use Disorder | 174.9 | 0.025 | Craving: AUQ Stimulation: BAES Stimulation Sedation: BAES Sedation, SHAS |
| Bujarski et al.2012 | 50 | Within-Subjects | 35 | Challenge | Heavy Drinkers | 48.1 | 0.06 | Craving: Intensity of Demand, Omax, Pmax, Breakpoint |
| Drobes et al.2004 | 50 | Between-Subjects | 85 | Challenge | Alcohol Use Disorder & Light Drinkers | AUD Sample: 186.75 Light Drinking Sample: 17.82 |
0.055 | Craving: ACQ Craving Stimulation: BAES Stimulation Sedation: BAES Sedation |
| King et al.1997 | 50 | Within-Subjects | 27 | Challenge | Light Drinkers | 41.7 | 0.06 | Stimulation: BAES Stimulation, POMS
Vigor Sedation: BAES Sedation, POMS Fatigue, POMS Confusion Negative Mood: POMS Anxiety, POMS Depression, POMS Tension |
| McCaul et al.2000 | 100 | Within-Subjects | 23 | Challenge | Heavy Drinkers | 55.2 | 0.088 | Craving: Desire to Drink,
Liking Sedation: Worst, I've Felt, Clumsy, Confused, Slurred Speech, Sleepy, Nauseated, Trouble Concentrating, Dizze, ARCI PCAG, Drunk |
| Na and Lee2002 | 50 | Within-Subjects | 15 | Challenge | Light Drinkers | 19 | 0.074 | Craving: Urge Stimulation: ASS Elated, ASS Happy, ASS Talkative Sedation: ASS Dizziness, ASS Headache, ASS Nausea, ASS Feel Sleepy Negative Mood: ASS Anxious, ASS Depressed ASS Relaxed (reverse scored) |
| O'Malley et al.2002 | 50 | Between-Subjects | 16 | Priming | Alcohol Use Disorder | 142.7 | 0.03 | Craving: AUQ Sedation: Nausea |
| Peterson et al.2006 | 50 | Within-Subjects | 20 | Challenge | Light Drinkers | Not Reported | 0.07 | Stimulation: POMS Confident Unsure (reverse
scored), POMS Energetic-Tired (reverse scored) Sedation: SHAS, POMS Clearheaded-Confused Negative Mood: POMS Composed-Anxious, POMS Elated-Depressed |
| Plebani et al.2011 | 50 | Within-Subjects | 43 | Challenge | Light Drinkers | 14.14 | 0.0565 | Stimulation: BAES Stimulation, POMS
Vigor Sedation: BAES Sedation, SHAS, POMS Fatigue |
| Ray et al.2012 | 50 | Within-Subjects | 32 | Challenge | Heavy Drinkers | 48.4 | 0.06 | Craving: AUQ Stimulation: BAES Stimulation, POMS Pos. Mood, POMS Vigor Sedation: BAES Sedation, SHAS Negative Mood: POMS Negative Mood, POMS Tension |
| Ray et al.2014 | 25 | Between-Subjects | 60 | Challenge | Heavy Drinkers | 135.4 | 0.067 | Craving: AUQ Stimulation: BAES Stimulation, POMS Pos. Mood, POMS Vigor Sedation: BAES Sedation Negative Mood: POMS Negative Mood, POMS Tension |
| Ray et al. 2007 | 50 | Within-Subjects | 40 | Challenge | Heavy Drinkers | 44 | 0.06 | Craving: AUQ, ARS
Liking Stimulation: BAES Stimulation, POMS Pos. Mood, POMS Vigor Sedation: BAES Sedation, SHAS Negative Mood: POMS Negative Mood, POMS Tension |
| Ray et al.2008 | 50 | Within-Subjects | 38 | Challenge | Heavy Drinkers | 41.7 | 0.033 | Craving: AUQ Stimulation: BAES Stimulation, POMS Pos. Mood, POMS Vigor Sedation: BAES Sedation, SHAS Negative Mood: POMS Negative Mood, POMS Tension |
| Ray et al.2007 | 50 | Within-Subjects | 10 | Challenge | Heavy Drinkers | 43.7 | 0.06 | Craving: Urge |
| Setiawan et al.2011 | 50 | Within-Subjects | 40 | Priming | Light Drinkers | 66.4 | 0.01 | Craving: VAS Desire a Drink, VAS Want a
Drink, VAS Like the Drink Stimulation: VAS Euphoria, VAS Mind Racing, VAS Alert, VAS Energetic, VAS Excited, VAS Rush Sedation: SHAS Nauseated, SHAS Trouble Concentrating, SHAS Clumsy, SHAS Sleepy, SHAS Drunk, SHAS Muddled/Confused, SHAS Dizzy, SHAS Slurred Speech, VAS Sedated, VAS Intoxicated |
| Spagnolo et al.2014 | 50 | Between-Subjects | 63 | Challenge | Alcohol Use Disorder | 336.6 | 0.08 | Craving: DEQ Want More, DEQ Like
Drug Sedation: DEQ Intoxicated |
| Swift et al.1994 | 50 | Within-Subjects | 19 | Challenge | Light Drinkers | 17.9 | 0.05 | Stimulation: BAES
Stimulation Sedation: BAES Sedation, ASS Anesthesia, ASS Impaired Functioning, ASS Nausea, ASS Central Stimulant |
| Doty et al.1997 | 50 | Within-Subjects | 25 | Challenge | Light Drinkers | 39 | 0.025 | Craving: VAS Want More, VAS
Like Stimulation: VAS Elated, VAS Good Natured, VAS Full of Pep, ARCI BG, ARCI MBG Sedation: VAS Confused, VAS Intoxicated, VAS Drowsy, VAS Lightheaded, ARCI LSD, ARCI PCAG, ARCI Central Stimulation Negative Mood: VAS Relaxed (reverse scored) |
| de Wit et al.1999 | 50 | Within-Subjects | 24 | Challenge | Light Drinkers | 34.3 | 0.09 | Craving: DEQ More, DEQ
Like Stimulation: BAES Stimulation, POMS Elation, POMS Friendliness, POMS Vigor, ARCI MBG, ARCI Amphetamine, ARCI BG, VAS Stimulated Sedation: BAES Sedation, POMS Confusion, POMS Fatigue, ARCI PCAG, ARCI LSD, VAS Sedated, VAS Nauseous Negative Mood: VAS Down, VAS Anxious, POMS Depression, POMS Anxiety |
In terms of individual outcomes, obtained from each study a total of 171 outcomes (i.e., study-level effects) were coded across these 20 studies. Sedation/motor intoxication outcomes were the most common with 77 outcomes, followed by stimulation/hedonic reward (44 outcomes), then craving (29 outcomes) and lastly negative affect (21 outcomes). As previously stated, 27.5% of all the outcomes reported in the methods section of these manuscripts did not have detailed statistical results suggesting a clear risk of publication bias and effect size inflation.
Naltrexone effects on Alcohol Craving
A total of 16 studies reported one or more craving outcomes with a total sample size of 686 subjects and 29 total outcomes (i.e., study-level effects), 7 (24.14%) of which had no statistics reported. Using a moderate imputation of missing outcomes, naltrexone was found to have a significant, albeit small effect in reducing craving in the context of alcohol administration (Hedge’s g = −0.252, 95% CI [−0.375, −0.130], SE = 0.054, t (8.99) = −4.65, p < 0.01). This effect was slightly diminished when controlling for mean drinks per month and target alcohol dose (Hedge’s g = −0.202, 95% CI [−0.326, −0.077]); although none of these covariates significantly predicted craving effect sizes (p ≥ 0.133). Effect size heterogeneity was substantial (I2 = 26.81%, τ2 = 0.014, Figure 2A) and thus accurately modeled through a random effects approach.
Figure 2.

Funnel plots for NTX effect sizes on the SR domains of (A) craving, (B) stimulation, (C) sedation, and (D) negative affect. Each point represents an individual outcome, and thus several points might be from the same study, however the RVE meta-analysis approach that was used is able to account for this nested structure when computing an overall effect. The solid black vertical line represents the point estimate, or average effect size from all the studies reported in the literature (without any imputation for missing data) analyzed using a random effects RVE meta-analysis model without inclusion of study covariates. The sloping lines comprising the triangle represent the 95% CI range of variability based on study standard error (which is strongly related to study sample size), and thus observations that fall in the grey region are those that are outside the 95% CI of the mean. Asymmetry in these plots can be interpreted as representing reporting bias. these funnel plots are relatively symmetric and thus are not indicative of bias which would produce effect size inflation. The sign of the effect size is representative of the effect naltrexone had on that outcome (i.e., a positive sign means that naltrexone increased this subjective response, and a negative sign means naltrexone blunted the response).
Naltrexone’s effect size in reducing craving did not differ substantially between study populations. Among the 5 studies recruiting AUD samples, the estimated effect size was −0.247, 95% CI [−0.366, −0.127]. For heavy drinking samples (7 studies) the effect size was −0.273, 95% CI [−0.452, −0.094]. For studies that recruited light drinking samples, the point estimate of the effect size nearly identical at −0.270, however the variability was greater and thus this effect was not statistically significant (95% CI, [−0.707, 0.168]). Thus, while the mean estimate across these three populations was consistent, these findings suggest that light drinking samples are more variable and thus less reliable in determining efficacy.
As expected, when employing a conservative approach to missing data, the effect of NTX was reduced, but remained statistically significant. (Hedge’s g = −0.221, 95% CI [−0.333, −0.108], SE = 0.052, t (11.9) = −4.28, p < 0.01). Again, no covariates predicted alcohol craving effect sizes (p ≥ 0.112), but their inclusion in the meta-regression model reduced the effect size estimate further (Hedge’s g = −0.167, 95% CI [−0.272, −0.063]). Estimates of heterogeneity were relatively unchanged (I2 = 29.04%, τ2 = 0.015). The estimated effect of naltrexone on craving obtained without imputation was higher; Hedge’s g = −0.287, 95% CI [−0.429, −0.145].
Naltrexone Effects on Alcohol Stimulation
Stimulation outcomes were measured in 15 studies, including 675 total subjects and 44 outcomes (i.e., study-level effects), 9 (20.45%) of which did not have reported statistics. With a moderate imputation approach, naltrexone significantly reduced alcohol stimulation, though again the effect size was small (Hedge’s g = −0.223, 95% CI [−0.372, −0.074], SE = 0.067, t (10.3) = −3.31, p < 0.01). This effect size was virtually identical when accounting for study covariates (Hedge’s g = −0.228, 95% CI [−0.440, −0.016]); although, as with craving, none of the covariates significantly predicted the stimulation outcome (p ≥ 0.891). Effect size heterogeneity was also observed (I2 = 31.25%, τ2 = 0.017, Figure 2B).
Only three studies recruited an AUD sample and measured stimulation outcomes; However, based on these three studies the estimated effect size was −0.215, 95% CI [−0.287, −0.143]. Among the 4 studies that recruited heavy drinkers, the point estimate of naltrexone on stimulation was slightly larger, but with significantly more variability (Hedge’s g = −0.287 95% [−0.711, 0.138]). Lastly, Naltrexone’s effect in blunting stimulation was considerably smaller for light drinking samples (9 studies, Hedge’s g = −0.151, 95% CI [−0.367, 0.065]. Thus, similar to craving, studies that recruited light drinkers demonstrated smaller and less reliable effect sizes.
Using a conservative imputation approach, naltrexone reduced alcohol stimulation, and this effect remained statistically significant, although the effect size was diminished (Hedge’s g = −0.186, 95% CI [−0.316, −0.056], SE = 0.060, t (12.1) = −3.11, p < 0.01). No covariates were predictive of stimulation effect sizes (p ≥ 0.881), and the inclusion of covariates had minimal effect on the effect size of NTX (Hedge’s g = −0.191, 95% CI [−0.371, −0.011]). Estimates of heterogeneity were greater using a conservative approach (I2 = 41.61%, τ2 = 0.023). The estimated effect of naltrexone on stimulation obtained without imputation was higher; Hedge’s g = −0.232, 95% CI [−0.384, −0.079].
Naltrexone Effects on Alcohol Sedation
Alcohol sedation was the most common outcome reported, which was measured in 18 studies including 777 subjects, and 77 total outcomes (i.e., study-level effects), 22 (28.57%) of which were missing statistical outcomes. Naltrexone was found to modestly increase alcohol sedation (Hedge’s g = 0.251, 95% CI [0.112, 0.389], SE = 0.064, t (13.2) = 3.91, p < 0.01). Controlling for study covariates increased the estimate of naltrexone effects, though still within the small range (Hedge’s g = 0.321, 95% CI [0.146, 0.495]). Alcohol dose was found to increase the effect sizes of naltrexone on alcohol sedation (B = 5.24, t (4.78) = 3.25, p < 0.05). Average drinks per month was not a significant covariate (p = 0.323). Substantial heterogeneity was observed (I2 = 24.57%, τ2 = 0.016, Figure 2C).
Among AUD samples, Naltrexone did not significantly increase subjective sedation (5 studies, Hedge’s g = 0.170, 95% CI [−0.308, 0.648]. The effect size was marginally larger among heavy drinking samples, though still not statistically significant (5 studies, Hedge’s g = 0.297, 95% CI [−0.121, 0.715]. Studies that recruited light drinkers were the only subgroup where naltrexone produced a significant increase in sedation (9 studies, Hedge’s g = 0.211, 95% CI [0.023, 0.400]). In sum, sedation was increased to a significant degree only among light drinkers which was a different pattern of results than other domains.
The effect size of naltrexone on alcohol-induced sedation was still evident using a conservative approach (Hedge’s g = 0.214, 95% CI [0.095, 0.333], SE = 0.056, t (15) = 3.83, p < <0.01). This effect was slightly increased with the inclusion of study covariates (Hedge’s g = 0.291, 95% CI [0.145, 0.437]), and alcohol dose significantly predicted sedation effect sizes (B = 5.60, t (5.88) = 3.80, p < 0.01). Heterogeneity was substantially increased with a conservative imputation approach (I2 = 51.32%, τ2 = 0.038). The estimated effect of naltrexone on sedation obtained without imputation was higher; Hedge’s g = 0.306, 95% CI [0.149, 0.464].
NTX Effects on Negative Affect
Negative affect was substantially less likely to be included as a measured outcome in these studies. Specifically, it was measured in only 9 studies, including 281 subjects, and only 21 total outcomes (i.e., study-level effects), 9 (42.86%) of which were unreported in the results. Naltrexone modestly increased negative affect in the context of alcohol administration (Hedge’s g = 0.227, 95% CI [0.100, .354], SE = 0.047, t (4.23) = 4.85, p < 0.01). Though the point estimate did not change much, when covarying for study characteristics, naltrexone no longer significantly impacted negative affect (Hedge’s g = 0.282, 95% CI [−0.797, 1.360]), though none of the covariates significantly predicted naltrexone’s effect sizes (p ≥ 0.741). Due to the small number of studies, heterogeneity was not able to be estimated in this model (Figure 2D).
In the 4 studies that recruited heavy drinkers, naltrexone was found to significantly increase negative mood (Hedge’s g = 0.220, 95% CI [0.001, 0.440]. In the 5 studies on light drinkers, Naltrexone was also associated with a significant increase in negative mood (Hedge’s g = 0.272, 95% CI [0.232, 0.312]. No studies that recruited alcohol use disorder patients included measures of negative affect, therefore no estimate of effect size is possible. In sum NTX was associated with an increase in negative mood for both heavy and light drinkers, but no information was available for AUD samples.
Using the conservative imputation of missing statistics, the effect size of naltrexone was smaller (Hedge’s g = 0.141, 95% CI [0.037, .245], SE = 0.044, t (7.15) = 3.20, p < 0.05). This effect was not robust to controlling for study covariates (Hedge’s g = 0.261, 95% CI −0.830, 1.350], though no covariates were significant predictors (p ≥ 0.597). Heterogeneity was estimated to be a bit smaller than other outcome domains (I2 = 15.42 %, τ2 = 0.005). The estimated effect of naltrexone on sedation obtained without imputation was higher; Hedge’s g = 0.249, 95% CI [0.101, 0.398].
DISCUSSION
Naltrexone is by far the most widely studied pharmacotherapy for AUD. Studies of naltrexone encompass clinical trials as well as behavioral pharmacology trials combining alcohol administration with acute naltrexone dosing (typically ranging from 3 to 7 days of medication/placebo). The overall consensus regarding the clinical efficacy of naltrexone is that its effects are small in magnitude and stronger for outcomes involving reduced drinking, compared to abstinence outcomes (Maisel et al., 2013). Interestingly, recent analyses have suggested that the clinical effects of naltrexone may be “declining over time of publication,” such that year of publication predicts trial outcomes with more recent trials having smaller effect sizes (Del Re et al., 2013). This may be due to increased quality of clinical trials over time or with the broader issue of replicability. On the other hand, recent studies have reported larger effect sizes for naltrexone when accounting for key variables such as smoking status (Anton et al., 2018; Schacht et al., 2017) and reward drinking (Mann et al., 2018), thus suggesting that it possible to identify naltrexone responders. Insofar as the effects of naltrexone are more robust for outcomes involving alcohol intake, the effects of naltrexone in altering the pharmacodynamic effects of alcohol and the associated subjective experience of alcohol have long been postulated (Volpicelli et al., 1995).
Given the mature status of the literature on naltrexone, including its effects during controlled alcohol administration, this quantitative review of the literature sought to quantify the effects of naltrexone across four domains of subjective response to alcohol, namely craving, stimulation, sedation, and negative mood. Results revealed a significant effect of naltrexone, versus placebo, in attenuating craving and alcohol-induced stimulation, while exacerbating sedation and negative affect in the human laboratory (Hedge’s g’ of −0.252, −0.223, 0.251, and 0.227, respectively). Notably, the observed estimates were all in the small effect size range. The results presented using a moderate (i.e., “middle of the road”) imputation approach were generally consistent with those obtained with a more conservative imputation approach as well as with analyses that did not include an imputation of missing data. A clear pattern is observed by which the effect size estimate using the conservative imputation approach is smaller, followed by the effect size resulting from the moderate imputation approach next, and with the no-imputation method resulting in larger effect sizes. Nonetheless, as a whole, the complete set of results provided herein coalesce within the small effect size range and should be interpreted as such.
The effect sizes for naltrexone-induced increases in the sedative and aversive effects of alcohol were by and large similar to effect sizes for decreases in the stimulant and rewarding effects of alcohol. In the behavioral pharmacology literature, more studies tend to focus on naltrexone’s attenuation of rewarding effects (King et al., 1997; Volpicelli et al., 1995), as compared to increases in the sedative and aversive effects of alcohol (McCaul et al., 2000; Ray et al., 2008). Nevertheless, the similar increase in aversive effects highlights how these biobehavioral mechanisms of action for naltrexone may be working in comparable magnitude, and possibly synergistically, towards the clinical efficacy of naltrexone for AUD. Importantly, analyses of naltrexone effects at each study population found that naltrexone potentiated the sedative effects of alcohol among light drinkers, but this effect was not significant in heavy drinking or AUD samples. It is plausible to hypothesize that perhaps light drinkers experience more of the sedative effects of alcohol and that naltrexone, in turn, may potentiate these effects more strongly in this subset of drinkers.
The similarity in effect size across study populations, particularly for craving, stimulation and negative affect, suggests that naltrexone exerts a comparable effect on subjective responses to alcohol spanning a range of drinking levels. However, it should be noted that high levels of alcoholism severity are generally not well-represented in human laboratory studies. This is critical given that the higher severity and associated “dark side” of addiction is thought represent a discrete phenotype characterized by a chronic and relapsing pattern of alcohol misuse with significant dysregulation of mood and stress systems (Koob, 2009; Koob and Le Moal, 2005). Although it should be noted that efforts to characterize the allostatic model in humans, including our own (Bujarski et al., 2018; Bujarski and Ray, 2014), have not fully supported the model, particularly with regard to the “dark side” of addiction (King et al., 2016).
Regarding naltrexone’s effects on craving in the human laboratory, the results obtained in this study are largely consistent with those recently reported by Hendershot and colleagues (2017), which also found significant medication effects that were small in magnitude. When contrasted to the clinical trials literature, however, the effects of naltrexone have been somewhat smaller, with Hedge’s g of 0.116 and 0.189 for abstinence and heavy drinking, respectively (Maisel et al., 2013). Maisel et al. (2013) also reported a somewhat smaller effect size for subjective craving with a Hedge’s g of 0.144. A possible explanation for the slight discrepancy in naltrexone effect size between laboratory studies and clinical trials is differences in sample characteristics. Recent studies from our group (Ray et al., 2017) and others (Rohn et al., 2017) suggested that non-treatment seekers, which comprise the vast majority of participants in human laboratory studies of naltrexone, vary widely from treatment-seeking individuals with AUD. Further, a recent meta-analysis by Klemperer and colleagues (2018) found that study characteristics accounted for 48% of the variance among naltrexone clinical trials for AUDs after controlling for medication characteristics (Klemperer et al., 2018). In the context of laboratory studies, the covariates examined in the present analyses (mean drinks per month, target alcohol dose, and target NTX dose) did not alter the significance of our effects, except for negative affect. Whether study characteristics are more influential in the context of clinical trials than laboratory studies, remains to be determined.
A number of limitations of the present study should be considered. These limitations include the fact that while recent studies have suggested a host of potential moderators of the clinical effects of naltrexone (Anton et al., 2018; Garbutt et al., 2014; Garbutt et al., 2016; Mann et al., 2018; Savulich et al., 2017; Schacht et al., 2017), these were not assessed and/or reported frequently enough in human laboratory trials to allow for a systematic examination of predictors of medication response. Notably, genetic factors, including variants at OPRM1 loci, were not accounted for in this meta-analysis and may clearly play a role in subjective responses to NTX in the laboratory (Ray et al., 2012a; Ray and Hutchison, 2007). Additionally, the present study relied heavily on U.S.-based studies and recent work has suggested discrepancies in AUD pharmacotherapy trials between U.S. and European countries (Donoghue et al., 2015); although the results for naltrexone were consistent across countries. This study does not directly address the degree to which naltrexone effects in the laboratory in fact predict clinical trial outcomes. Such comparisons are ultimately needed, yet they would require within-subject collection of both human laboratory outcomes and clinical outcomes. Lastly, as described in detail above, our approach to measure selection was to include all available measures. While these methods address the issue of publication bias from individual studies selectively reporting significant outcomes, it does not affect the related problem of whole studies going unreported. However, given the relative difficulty and costs associated with pharmacotherapy studies, we believe unreported outcomes will be a significantly larger problem than unreported studies. Nevertheless, the issue of selective reporting remains a problem and recent calls for transparency in science, across a range of disciplines (Miguel et al., 2014), underscore this important issue. This meta-analysis suggested that selective reporting was an in fact issue in the literature on the effects of naltrexone on subjective responses to alcohol. Requirements to pre-register trials and a-priori outcomes as well as to share data, represent a few of the steps towards addressing this broader issue of scientific integrity and accountability (Alberts et al., 2015).
In conclusion, the current meta-analysis aggregates across multiple human laboratory studies of naltrexone effects on subjective responses to alcohol, providing a comprehensive summary of key putative mechanisms of naltrexone efficacy, namely alteration of the subjective experience of alcohol intake. While the effect sizes in the laboratory are marginally larger than those obtained in clinical trials, both are uniformly small in magnitude. Insofar as these putative mechanisms of action, in this case subjective response to alcohol, are closer to the underlying biological effects of naltrexone, larger effect sizes might be expected. This expectation is also consistent with an endophenotype approach to psychiatric phenotypes, AUD included (Gottesman and Gould, 2003; Gottesman and Shields, 1972; Hines et al., 2005). Thus, a broader conclusion from the meta-analytic findings on naltrexone, both in the clinic and in the human laboratory, is that the small effect size estimates are prevalent across levels of analysis and that until reliable predictors of treatment-efficacy are detected, the clinical utility of naltrexone for the treatment of alcohol use disorders remains limited. Whether the prediction of naltrexone response will be driven by human laboratory constructs, by self-report measures (e.g., Mann et al., 2018), or by emerging research on brain imaging (e.g., Schacht et al., 2017), the overarching goal is to optimize the use of this pharmacotherapy for AUD.
Supplementary Material
Figure 3.


Domain assignment table describing the items used to capture each of the four domains of subjective response captured in this meta-analysis.
Acknowledgements
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to LAR (R01AA021744). LAR has received study medication from Pfizer Medicinova and consulted for GSK and Mitsubishi Tanabe.
Footnotes
None of the authors have conflicts of interest to disclose.
Studies that only reported subjective response data in the context of a self-administration paradigm were excluded due to the potential for large confounding effects of BrAC differences between medication groups.
REFERENCES
- Alberts B, Cicerone RJ, Fienberg SE, Kamb A, McNutt M, Nerem RM, Schekman R, Shiffrin R, Stodden V, Suresh S, Zuber MT, Pope BK, Jamieson KH (2015) SCIENTIFIC INTEGRITY. Self-correction in science at work. Science 348:1420–1422. [DOI] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D (2004) Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 173:32–40. [DOI] [PubMed] [Google Scholar]
- Anton RF, Latham PK, Voronin KE, Randall PK, Book SW, Hoffman M, Schacht JP (2018) Nicotine-Use/Smoking Is Associated with the Efficacy of Naltrexone in the Treatment of Alcohol Dependence. Alcohol Clin Exp Res 42:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A (2012) Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin Exp Res 36:2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann I (2012) DigitizeIt.
- Bujarski S, Hutchison KE, Roche DJ, Ray LA (2015) Factor Structure of Subjective Responses to Alcohol in Light and Heavy Drinkers. Alcohol Clin Exp Res 39:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Jentsch JD, Roche DJO, Ramchandani VA, Miotto K, Ray LA (2018) Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, MacKillop J, Ray LA (2012) Understanding naltrexone mechanism of action and pharmacogenetics in Asian Americans via behavioral economics: a preliminary study. Exp Clin Psychopharmacol 20:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA (2014) Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend 140:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA (2016) Experimental psychopathology paradigms for alcohol use disorders: Applications for translational research. Behav Res Ther 86:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Svenson J, York A (1999) Non-specific effect of naltrexone on ethanol consumption in social drinkers. Psychopharmacology (Berl) 146:33–41. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG (2008) Analysing data and undertaking meta‐analyses Cochrane handbook for systematic reviews of interventions: Cochrane book series:243–296. [Google Scholar]
- Del Re AC, Maisel N, Blodgett J, Finney J (2013) The declining efficacy of naltrexone pharmacotherapy for alcohol use disorders over time: a multivariate meta-analysis. Alcohol Clin Exp Res 37:1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C (2015) The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction 110:920–930. [DOI] [PubMed] [Google Scholar]
- Doty P, Kirk JM, Cramblett MJ, de Wit H (1997) Behavioral responses to ethanol in light and moderate social drinkers following naltrexone pretreatment. Drug Alcohol Depend 47:109–116. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K (2004) Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res 28:1362–1370. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Z, Tipton E (2015) Robumeta: An R-package for robust variance estimation in meta-analysis. arXiv preprint arXiv:150302220. [Google Scholar]
- Garbutt JC, Greenblatt AM, West SL, Morgan LC, Kampov-Polevoy A, Jordan HS, Bobashev GV (2014) Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence. Addiction 109:1274–1284. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy AB, Kalka-Juhl LS, Gallop RJ (2016) Association of the Sweet-Liking Phenotype and Craving for Alcohol With the Response to Naltrexone Treatment in Alcohol Dependence: A Randomized Clinical Trial. JAMA Psychiatry 73:1056–1063. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin J (2014) Beyond Power Calculations: Assessing Type S (Sign) and Type M (Magnitude) Errors. Perspect Psychol Sci 9:641–651. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C (2004) Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 4:39–50. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C (2009) Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 9:999–1015. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18:10663–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160:636–645. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Shields J (1972) Schizophrenia and Genetics: A Twin Study Vantage Point. Academic: London. [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (1981) Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat 6:107–128. [Google Scholar]
- Hedges LV, Tipton E, Johnson MC (2010) Robust variance estimation in meta‐regression with dependent effect size estimates. Research synthesis methods 1:39–65. [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS (2010) Translating the neuroscience of alcoholism into clinical treatments: From blocking the buzz to curing the blues. Neurosci Biobehav Rev 35:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA (2016) Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol 21:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Samokhvalov AV, Rehm J (2017) Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict Biol 22:1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP (2008) Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 37:1158–1160. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LM, Ray L, Hutchison K, Tabakoff B (2005) Alcoholism: the dissection for endophenotypes. Dialogues Clin Neurosci 7:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BA, Berglund M, Lindgren A (2006) Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction 101:491–503. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama 311:1889–1900. [DOI] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D (2016) A Prospective 5-Year Re-examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder. Biol Psychiatry 79:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry 75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP (1997) Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 129:15–22. [DOI] [PubMed] [Google Scholar]
- Klemperer EM, Hughes JR, Naud S (2018) Study characteristics influence the efficacy of substance abuse treatments: A meta-analysis of medications for alcohol use disorder. Drug Alcohol Depend 190:229–234. [DOI] [PubMed] [Google Scholar]
- Koob GF (2009) Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 Suppl 1:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8:1442–1444. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A (2012) Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17:513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB (2016a) Discovery, Development, and Adoption of Medications to Treat Alcohol Use Disorder: Goals for the Phases of Medications Development. Alcohol Clin Exp Res 40:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Wilford BB, Falk DE, Ryan ML, Fertig JB (2016b) Potential medications for the treatment of alcohol use disorder: An evaluation of clinical efficacy and safety. Subst Abus 37:286–298. [DOI] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW (2013) Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108:275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Roos CR, Hoffmann S, Nakovics H, Lemenager T, Heinz A, Witkiewitz K (2018) Precision Medicine in Alcohol Dependence: A Controlled Trial Testing Pharmacotherapy Response Among Reward and Relief Drinking Phenotypes. Neuropsychopharmacology 43:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ (2000) Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 22:480–492. [DOI] [PubMed] [Google Scholar]
- Miguel E, Camerer C, Casey K, Cohen J, Esterling KM, Gerber A, Glennerster R, Green DP, Humphreys M, Imbens G, Laitin D, Madon T, Nelson L, Nosek BA, Petersen M, Sedlmayr R, Simmons JP, Simonsohn U, Van der Laan M (2014) Social science. Promoting transparency in social science research. Science 343:30–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Book SW, Stewart SH (2011) Medical treatment of alcohol dependence: a systematic review. Int J Psychiatry Med 42:227–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SB, DeShon RP (2002) Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods 7:105–125. [DOI] [PubMed] [Google Scholar]
- Na C, Lee YS (2002) Alcohol urge and plasma beta-endorphin change after alcohol challenge with naltrexone pretreatment in social drinkers. Prog Neuropsychopharmacol Biol Psychiatry 26:663–670. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B (1992) Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49:881–887. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 160:19–29. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Conrod P, Vassileva J, Gianoulakis C, Pihl RO (2006) Differential effects of naltrexone on cardiac, subjective and behavioural reactions to acute ethanol intoxication. J Psychiatry Neurosci 31:386–393. [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Oslin DW, Lynch KG (2011) Examining naltrexone and alcohol effects in a minority population: results from an initial human laboratory study. Am J Addict 20:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakap S, Rakap S, Evran D, Cig O (2016) Comparative evaluation of the reliability and validity of three data extraction programs: UnGraph, GraphClick, and DigitizeIt. Computers in Human Behavior 55:159–166. [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M (2011) A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry 16:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF (2012a) The role of the Asn40Asp polymorphism of the mu opioid receptor gene (OPRM1) on alcoholism etiology and treatment: a critical review. Alcohol Clin Exp Res 36:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K (2012b) Pharmacogenetics of naltrexone in asian americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacology 37:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, MacKillop J, Courtney KE, Monti PM, Miotto K (2013) Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res 37 Suppl 1:E116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE (2017) Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse 43:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K (2010a) Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets 9:13–22. [DOI] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED (2014) Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology (Berl) 231:3843–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2004) A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res 28:1789–1795. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2007) Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry 64:1069–1077. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, MacKillop J, Miranda R Jr., Audette A, Swift R, Monti PM (2008) Effects of naltrexone during the descending limb of the blood alcohol curve. Am J Addict 17:257–264. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M (2010b) Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des 16:2149–2158. [DOI] [PubMed] [Google Scholar]
- Ray LA, Krull JL, Leggio L (2010c) The Effects of Naltrexone Among Alcohol Non-Abstainers: Results from the COMBINE Study. Front Psychiatry 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE (2009) Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res 33:2154–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R Jr., Kahler CW, Leventhal AM, Monti PM, Swift R, Hutchison KE (2007) Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology (Berl) 193:449–456. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R Jr., Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM (2010d) Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol 119:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373:2223–2233. [DOI] [PubMed] [Google Scholar]
- Rohn MC, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L (2017) Differences Between Treatment-Seeking and Nontreatment-Seeking Alcohol-Dependent Research Participants: An Exploratory Analysis. Alcohol Clin Exp Res 41:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M (2010) Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev:CD001867. [DOI] [PubMed] [Google Scholar]
- Rucker G, Schwarzer G, Carpenter JR, Schumacher M (2008) Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ, King AC (2009) Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES). Alcohol Clin Exp Res 33:916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savulich G, Riccelli R, Passamonti L, Correia M, Deakin JF, Elliott R, Flechais RS, Lingford-Hughes AR, McGonigle J, Murphy A, Nutt DJ, Orban C, Paterson LM, Reed LJ, Smith DG, Suckling J, Tait R, Taylor EM, Sahakian BJ, Robbins TW, Ersche KD (2017) Effects of naltrexone are influenced by childhood adversity during negative emotional processing in addiction recovery. Transl Psychiatry 7:e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF (2017) Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology 42:2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M (2011) The effect of naltrexone on alcohol’s stimulant properties and self-administration behavior in social drinkers: influence of gender and genotype. Alcohol Clin Exp Res 35:1134–1141. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U (2011) False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci 22:1359–1366. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, Diamond KA, Phillips MJ, George DT, Momenan R, Heilig M (2014) Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcohol Clin Exp Res 38:3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002. [DOI] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H (1994) Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry 151:1463–1467. [DOI] [PubMed] [Google Scholar]
- Tipton E (2015) Small sample adjustments for robust variance estimation with meta-regression. Psychological Methods 20:375. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R (2008) Neuropharmacology of alcohol addiction. Br J Pharmacol 154:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP (1995) Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry 152:613–615. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME (2008) Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology 33:653–665. [DOI] [PubMed] [Google Scholar]
- Yardley MM, Ray LA (2017) Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol 22:581–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


