Abstract
Loss of muscle mass and function, or sarcopenia, is a common feature of cirrhosis and contributes significantly to morbidity and mortality in this population. Sarcopenia is a main indicator of adverse outcomes in this population, including poor quality of life, hepatic decompensation, mortality in patients with cirrhosis evaluated for LT, longer hospital and intensive care unit stay, higher incidence of infection following LT, and higher overall health care cost. While it is clear that muscle mass is an important predictor of LT outcomes, many questions remain, including the best modality for assessing muscle mass, the optimal cut-off values for sarcopenia, the ideal timing and frequency of muscle mass assessment, and how to best incorporate the concept of sarcopenia into clinical decision making. For that reason, we assembled a group of experts to form the North American Working Group on Sarcopenia in Liver Transplantation to use evidence from the medical literature to address these outstanding questions regarding sarcopenia in LT. We believe sarcopenia assessment should be considered in all patients with cirrhosis evaluated for liver transplantation. Skeletal muscle index (SMI) assessed by computed tomography constitutes the best studied technique for assessing sarcopenia in patients with cirrhosis. Cut-off values for sarcopenia, defined as SMI <50 cm2/m2 in male and <39 cm2/m2 in female patients constitute the validated definition for sarcopenia in patients with cirrhosis. The management of sarcopenia requires a multi-pronged approach including nutrition, exercise and additional pharmacological therapy as deemed necessary.
Future studies should evaluate whether recovery of sarcopenia with nutritional management in combination with an exercise program is sustainable, and how improvement in muscle mass might be associated with improvement in clinical outcomes.
Introduction
Sarcopenia, the disproportionate loss of muscle mass, is common in patients with cirrhosis awaiting liver transplantation (LT). Sarcopenia has been shown to be a significant risk factor for waitlist mortality, postoperative complications, and post-LT death (1–5). The new international statistical classification of diseases and related health problems, 10th revision (ICD-10-CM) (M62.84) code for sarcopenia represents a significant recognition of sarcopenia as a disease (6). While it is clear that muscle mass is an important predictor of LT outcomes, many questions remain, including the best modality for measuring muscle mass, the optimal cut-off values for sarcopenia, the ideal timing and frequency of muscle mass assessment, and how to best incorporate the concept of sarcopenia into clinical decision making.
Therefore, in 2018, we convened a group of experts to form the North American Working Group on Sarcopenia in Liver Transplantation to use evidence from the medical literature to address these outstanding questions regarding sarcopenia in LT. This document represents the product of our efforts to develop a statement by experts in the field on the current state of knowledge and key gaps for future high impact research on sarcopenia in the LT setting.
The importance of sarcopenia as a construct
Sarcopenia is a term derived from the Greek sarco (flesh) and penia (deficiency). Sarcopenia was initially defined as age-related loss of skeletal muscle (7), but has since been expanded to reflect low muscle mass leading to negative effects on physical performance and clinical outcomes across a broad range of disease states outside of geriatric populations.
Conceptually, sarcopenia is only one – but likely the dominant – component of the larger construct of global physical dysfunction that is prevalent in patients with cirrhosis that has most recently been described with the term “frailty”. While frailty is the manifesting symptom of impaired global physical functioning, loss of muscle mass is an obvious sign that frailty may be present. Sarcopenia is the metric of clinically-relevant skeletal muscle depletion that can be objectively measured in clinical practice and is least likely to be affected by acute illness or alterations in cognitive function. For this reason, our working group chose to define sarcopenia using only muscle mass, although we acknowledge that muscle function (e.g., grip strength) is incorporated into definitions of sarcopenia by other groups including the European Working Group on Sarcopenia in Older People and Asian Working Group for Sarcopenia (8, 9).
Although multiple definitions of sarcopenia for patients with cirrhosis exist in the literature, low muscle mass, regardless of how it is measured, is a powerful predictor of clinically relevant adverse outcomes, including poor quality of life (10), hepatic decompensation (11), mortality in patients with cirrhosis on the LT waitlist (3, 11–14), longer hospital and intensive care unit stay (2, 15), higher incidence of infection following LT (2, 16), higher overall health care cost (17), and post-LT mortality (3, 18).
Aside from being an important marker of pre and post-LT mortality, sarcopenia is associated with additional important clinical parameters independent of scoring systems for severity of liver dysfunction. Skeletal muscle plays an integral role in ammonia detoxification, and sarcopenia has been identified as an independent risk factor for hepatic encephalopathy (HE) in patients with cirrhosis (19). Other complications such as ascites and infections are more common in sarcopenic patients compared to non-sarcopenic patients with cirrhosis (2).
Modalities to Evaluate Muscle Mass in the Liver Transplant Candidate
There currently exists significant heterogeneity in the metrics used to define sarcopenia in the published domain. The great challenge in identifying a single standard is that many modalities exist for muscle mass quantification, including anthropometry, bioelectrical impedance analysis (BIA), dual-energy X-ray absorptiometry (DEXA), ultrasound (US), magnetic resonance imaging (MRI) and computed tomography (CT). The advantages and limitations of each modality as they relate to measurement of muscle mass in patients with end-stage liver disease (ESLD) are summarized in Table 1.
Table 1.
Quantitative Tools Evaluating Sarcopenia in Cirrhosis
| Modalities | Experience | Advantages/Limitations |
|---|---|---|
| Dual-energy X-ray absorptiometry (DEXA) |
Advantages:
|
|
| Bioelectrical impedance analysis (BIA) |
|
Advantages:
|
| Ultrasound (thigh muscle thickness) |
|
Advantage:
|
| Mid-arm muscle circumference (MAMC) |
Advantage:
|
|
| CT/ MRI |
Advantage:
|
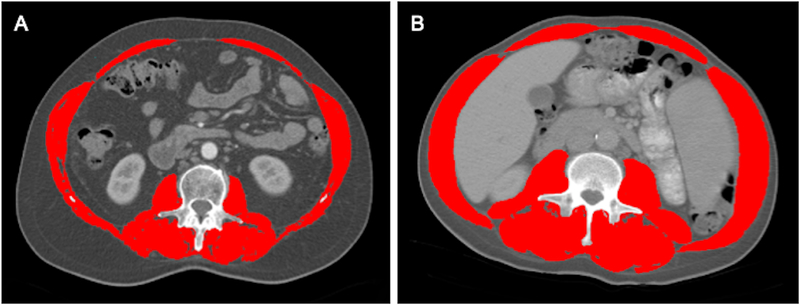
We advocate for the use of CT-based skeletal muscle area, measured at the third lumbar vertebra on an abdominal CT scan (either non-contrast or contrast-enhanced scans are acceptable), in patients with cirrhosis for the following reasons (Please see example in Figure 1). First, cross-sectional imaging is commonly used in transplant centers to monitor for hepatocellular carcinoma (HCC) and to evaluate the vascular and biliary anatomy for surgical planning. While preliminary reports suggest that MRI-based imaging yields equivalent results to CT scans (20), CT scans tend to be more widely available, of lower cost, and more rapidly performed in clinical practice. Second, the majority of published reports on sarcopenia in LT have used standard-of-care CT imaging, summarized in Table 2. Several key questions remain regarding the use of CT-based estimation of skeletal muscle mass, including the use of values below specific percentiles (i.e. 5th percentile) of age and sex-matched population or optimal cut-points for mortality discrimination, sensitivity of changes over time as well as the validity of measurement of psoas alone versus the total muscle area (21).
Figure 1.
Total Muscle Area Quantification at the Level of 3rd.Lumbar Vertebra using Abdominal CT Images from Two Male Patients with Cirrhosis. Figure 1 A and B, respectively, present a patient who had low SMI (46 cm2/m2) and high SMI (60 cm2/m2) as indicated by the red shading.
Table 2.
Summary of studies investigating the CT-determined low muscle mass in patients with ESLD
| Author/ Year |
Study Population/ Center (Single, Multi) |
Sarcopenia Definition |
Muscle Included | Frequency of Sarcopenia |
|---|---|---|---|---|
| Englesbe et al., 2010 (1) | 163 LT recipients/ Single |
Lowest TPA quartile: TPA< 1420 mm2 at the level of the fourth lumbar vertebra (L4) |
Total psoas areas | 25% |
| Montano-Loza et al., 2012 (59) | 112 patients with cirrhosis evaluated for LT/ Single |
Mortality associated SMI cut-offs in cancer* (60) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 50% Males 18% Females |
| Tandon et al., 2012 (61) | 142 patients listed for LT/ Single |
Mortality associated SMI cut-offs in cancer* (60) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 54% Males 21% Females |
| Meza-Junco et al., 2013(62) | 116 patients with HCC evaluate for LT/ Single |
Mortality associated SMI cut-offs in 1475 patients with solid tumors¥ (63) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 31% Males 28% Females |
| Krell et al., 2013 (16) | 207 LT recipients/Single | Lowest TPA Tertile: TPA <1499 mm2 for men and <954 mm2 for women |
Total psoas areas | 33% |
| DiMartini et al., 2013 (14) | 338 LT recipients/Single | Mortality associated SMI cut-offs in cancer* (60) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 76% Males 51% Females |
| Masuda et al., 2014 (15) | 204 patients undergoing LT/Single | PMA below the 5th percentile for each sex: PMA <800 cm2 for men and <380 cm2 for women |
Sum of the areas of the 2 psoas | 58% Males 36% Females |
| Durand et al., 2014 (12) | 562 patients listed for LT/Single | Optimal cut-offs of TPMT/height to discriminate waiting list mortality Transversal psoas muscle thickness [TPMT/height (mm/m)] at the level of umbilicus≤ 16.8 mm/m |
Right psoas muscle | NA |
| Yadav et al., 2015 (4) | 213 patients listed for LT/Single | Mortality associated SMI cut-offs in cancer* (60) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 28% Males 13% Females |
| Carey et al., 2017 (22) | 396 patients listed for LT/Multi | Optimal cut-offs of SMI to discriminate waiting list mortality: SMI <39 cm2/m2 for women and <50 cm2/m2 for men |
Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 50% Males 33% Females |
| Tandon et al., 2016 (57) | 159 patients evaluated for LT/Single | Mortality associated SMI cut-offs in cancer* (60) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 57% Males 25% Females |
| Van Vugt et al., 2017 (64) | 585 patients listed for LT/Multi | Mortality associated SMI cut-offs in 1475 patients with solid tumors¥ (63) | Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 42% Males 47% Females |
| Van Vugt et al., 2018 (17) | 224 patients listed for LT/Single | Lowest sex-specific quartile of SMI: L3 SMI <44.1 for men and <37.9 for women |
Total cross-sectional areas of psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis (Normalized to height) | 25% Males 24% Females |
Abbreviations: PMA, Psoas Muscle Area, PMI, Psoas Muscle Index, SMI, Skeletal Muscle Index; TPA, Total Psoas Area; TPMT, Transversal Psoas Muscle Thickness
L3 SMI ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men
Defined as L3 SMI <=41 cm2/m2 for women and <=53 cm2/m2 for men with body mass index (BMI) >=25 and <=43 cm2/m2 in patients with BMI<25.
Defining sarcopenia
A large, multi-center study of 396 patients with cirrhosis from five North American liver transplant centers established standardized cut-off values of skeletal muscle index (SMI) at <50 cm2/m2 and <39 cm2/m2 to define sarcopenia in men and women with cirrhosis, respectively (22). These sex-specific SMI cut-points were strongly associated with pre-LT mortality independent of age and MELD score (15).
Although muscle mass has been shown in multiple studies to be associated with post-LT mortality (3, 18, 23), data reporting pre-LT muscle indexes associated with post-LT adverse outcome is limited. Recently, SMI<48 cm2/m2 in acutely ill men undergoing urgent evaluation and LT, was associated with higher post-LT mortality (24).
When assessing sarcopenia by SMI on an abdominal CT scan, there does not appear to be a large difference between measurements at L3 versus L4 vertebra (25). There is also excellent agreement between the various software programs (i.e. SliceOmatic, ImageJ, etc.) with respect to measurements of abdominal skeletal muscle area (26).
SMI seems to be a more complete and robust measurement than individual measurement of the psoas muscle or the psoas muscle index (PMI), especially in men with cirrhosis. In addition, low PMI identifies an incomplete subset of patients at increased risk of mortality indicated by low SMI (21).
While sarcopenia has classically been associated with increased mortality in both men and women with cirrhosis, emerging evidence suggests that sarcopenia is associated with disproportionately higher rates of mortality in men as compared to women (27). This emphasizes the importance of survival analysis stratification by sex rather than simply adjusting multivariable models for sex. Furthermore, the role of ethnicity in baseline muscle mass and muscle loss has not yet been determined in patients with cirrhosis (28). While more data are needed, the prevalence of sarcopenia within each BMI category is another consideration when building a definition to incorporate into clinical practice. Lastly, divergent study outcomes such as overall mortality in evaluated patients, waitlist mortality in listed patients, post-LT mortality in patients undergoing LT, and short term vs. long-term outcomes, confound the comparison between published studies and development of generalized definitions.
Key points
Standardized tools and validation between techniques are important considerations for the evaluation of sarcopenia in patients with end-stage liver disease awaiting LT.
CT constitutes the best-studied technique for measuring sarcopenia in patients with cirrhosis.
FLEXIT (Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium) cut-off values (SMI <50 cm2/m2 in men and <39 cm2/m2 in women) constitute the most robust definition for sarcopenia in patients with end-stage liver disease awaiting LT.
Recommendations
We recommend the use of SMI as a marker of sarcopenia for outcome prediction.
We recommend the use of FLEXIT cut-off values to define sarcopenia in cirrhosis in order to characterize cohorts of patients for prospective clinical trials.
Future studies to define sarcopenia should be established for use in clinical practice with consideration of sex, age and ethnicity.
Incorporating Sarcopenia into Clinical Practice
Although severe sarcopenia may be easy to identify visually (i.e., the “eyeball test”), objective and validated measures are needed to detect early stages of muscle loss, when interventions to slow the progression may be more effective. Ascites, obesity, and body fat distribution may render sarcopenia less apparent, and early stages of muscle loss are often not visually obvious. Furthermore, quantification of muscle mass provides objective data, which is especially critical in the setting of LT.
One of the advantages of determination of muscle mass is that it can be done on cross-sectional imaging (e.g., abdominal CT or MRI), which is often performed in LT patients as standard of care. Radiologists can provide total abdominal skeletal muscle area at the L3 vertebral level from these routine scans, from which SMI can easily be calculated. We anticipate that this will enable more widespread incorporation of the information provided from muscle mass measurement into clinical practice.
Objective assessment of sarcopenia in the LT candidate is important to two major reasons: 1) clinical decision-making and 2) intervention. We expand upon both of these areas here.
Incorporating sarcopenia into clinical decision-making
It is important to note that the North American Working Group on Sarcopenia in Liver Transplantation recommends that sarcopenia should not be the sole criterion for declining or de-listing candidates for LT. A significant limitation of utilizing sarcopenia for risk stratification is the lack of a threshold value of muscle loss that predicts health outcomes prohibitive for a particular intervention such as LT. Furthermore, the threshold of “futility” is not universally defined but, instead, varies by center, clinician, and patient values. Rather, we advocate that an objective metric of sarcopenia be taken into consideration within the full context of the other medical, physical, functional and psychosocial factors of each individual patient with respect to their transplant candidacy as well as their own values of what a successful transplant looks like.
That being said, sarcopenia has important implications for a patient awaiting LT. At the minimum, in the outpatient setting, a patient with sarcopenia should be counseled that his or her higher risk of waitlist mortality exceeds that predicted by the MELDNa score and that they have a higher risk of complication after LT. This information may help to motivate the patient to seek a faster path to transplant, including living donor LT or to accept higher-risk donor livers. In addition, this information may motivate patients and providers to engage in interventions that might mitigate muscle loss (see next section).
With respect to clinical decision-making, sarcopenia may hold a unique place within the inpatient setting where performance-based assessments of frailty may be misleading due to acute changes in functional performance that may not accurately reflect their underlying “steady state” physiologic reserve. An objective assessment of muscle mass may indicate that the patient has good underlying physiologic reserve that will support a full peri-operative recovery. For a patient without multiple risk factors for poor post-LT outcomes, sarcopenia alone is not sufficient to deny LT but may guide the decision about the quality of liver to accept in attempt to minimize liver-related complications and optimize overall patient recovery (29). For a patient with multiple co-morbidities that may also negatively impact post-LT outcomes, identification of sarcopenia - in combination with these other medical risk factors - may be added objective evidence to not proceed with LT.
Incorporating sarcopenia into management and treatment of the LT candidate
We recommend that measurement of muscle mass best be integrated into clinical practice to identify patients for “prehabilitation” programs focused on optimizing nutrition and physical activity (30). The management of sarcopenia requires a multi-pronged approach including nutrition, exercise and additional pharmacological therapy as deemed necessary. The incorporation of behavioral change strategies while delivering nutrition (31) and exercise prescriptions (32) encourages exploration of the patient’s individual personal and social factors to motivate and increase the likelihood of patient engagement (33). We recommend the following strategies based on the current available evidence:
- A nutrition prescription. At minimum, this consists of three major components:
- A target caloric intake recommendation. For non-obese individuals (BMI <30 kg/m2), the latest European Association for the Study of the Liver (EASL) Clinical Practice Guidelines on nutrition in chronic liver disease recommend an optimal daily energy intake of at least 35 kcal/kg of actual body weight corrected for fluid retention. In obese patients, a tailored moderately hypocaloric diet (with a reduction of 500 to 800 kcal/day) has been suggested (34). The International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) Consensus (35) provides additional BMI-stratified target caloric recommendations based on an ideal body weight (also corrected for fluid retention), including BMI ranges of 20–30, 30–40 and >40 kg/m2. Supplemental enteral nutrition should be considered in hospitalized patients who are unable to meet calorie intake targets with oral intake alone (34, 36).
- A target protein intake recommendation. Protein restriction is not necessary in patients with hepatic encephalopathy (37). Guidelines recommend a daily protein intake of 1.2–1.5 grams/kg protein (34, 35) with further study required as to whether dairy/vegetable protein may have a benefit over meat protein in the setting of hepatic encephalopathy (34) and the variable impact of protein across the spectrum of liver disease severity.
- A late-evening snack recommendation. In an attempt to shorten the overnight fasting period, patients are advised to take a snack shortly before bedtime or during nighttime hours and to eat breakfast (34, 38). We would support the recommendation of a late-night snack across Child Pugh classes. The optimal composition of the late-evening snack is not clearly defined, with recommendations varying from branched chain amino acids (BCAA) containing supplements (39) to snacks containing ~50 grams of carbohydrates and 10–20 grams of protein (31).
Exercise. Although an evidence-based exercise prescription is still not available for patients with cirrhosis, extrapolating current knowledge from the 11 published studies on exercise in patients with cirrhosis (please refer to Table 4) into practice, it is recommended for patients to perform moderate intensity exercise for no less than 30 minutes per day, starting with a brief warm-up (5–10 minutes) and ending with a stretching / cool-down phase (5–10 minutes), 3–5 times per week, aiming for a total of 150–200 minutes of exercise per week (32). Exercise bouts should have a duration of no less than 5–10 minutes, depending on disease status and tolerance, with patients commonly having to undergo multiple exercise bouts per day in order to accomplish these goals. In general, it is recommended that aerobic and resistance training be combined in a 3:2 ratio, although for the purpose of improving sarcopenia this ratio should favor resistance training (40).
- Pharmacotherapy (Table 3)
- Vitamin D3. Vitamin D deficiency is highly prevalent in cirrhosis and is a well-defined contributor to sarcopenia. Detection and repletion with cholecalciferol is standard practice in transplant hepatology (41).
- Hormonal therapy. Standard hormonal therapy to sustain a euthyroid and euglycemic state defends muscle mass and function. In addition, the majority of men with advanced cirrhosis have hypogonadism as measured by decreased total and free testosterone levels. A randomized trial showed that intramuscular androgen therapy of hypogonadal cirrhotic men improved muscle mass, bone mass and hemoglobin A1C (45); transdermal therapy also appears effective with fewer potential adverse events.
- L-Carnitine. L-carnitine is a quaternary amine (3-hydroxy-4-N-trimethylaminobutyrate) needed for fatty acid oxidation. Binding of L-carnitine to acetyl groups enables movement of acetylated fatty acids into mitochondria and their oxidation to generate energy in the form of ATP. Carnitine supplementation was recently reported to suppress muscle loss in an initial study of cirrhotic patients (46).
Table 4.
Exercise clinical trials investigating the effect on muscle mass or function
| Author/ Year |
Design | Main Exercise Intervention | Dietary Intervention |
Skeletal Muscle Assessment | N of subjects | Primary aim(s) | Skeletal Muscle Mass Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Active | Control | |||||||
| Konishi et al, 2011(67) | ONCT | Walking (6 months) | Optimal kCal | BIA lean mass (muscle weight) | 3 | NA | HOMA-IR change | 22.5 kg (before) vs. 22.8 kg (after) |
| Patullo et al, 2013 (68) | ONCT | Walking (24 weeks) | Optimal kCal/protein | Handgrip strength | 7 | NA | HOMA-IR change | 45 lbs (before) vs. 44 lbs (after) |
| Roman et al, 2014 (69) | Open RCT | Cycle ergometry (12 weeks) | Leucine | Arm and thigh circumferences | 10 | 10 | 6MWT, 2MST, muscle mass, and HRQoL | MAMC ↓ 0.5 cm in active & ↑ 0.6 cm in control; TC ↑ 5 cm in active¶ & ↓ 1 cm in control |
| Zenith et al, 2014 (70) | Open RCT | Cycle ergometry (8 weeks) | Optimal kCal/protein | Thigh US & circumference | 9 | 10 | Peak VO2, muscle mass and HRQoL | Thigh US* ↑ 0.05 cm/m2 in active¶ & no change in control; TC ↑ 1.2 cm in active¶ & 0.2 cm in control |
| Debette-Gratien et al, 2015 (71) | ONCT | Cycle ergometry (12 weeks) | NA | Quadriceps Strength | 13 | NA | Acceptability of exercise program | 30 kg (before) to 37 kg (after)¶ |
| Macias-Rodriguez et al, 2016 (72) | Open RCT | Cycle ergometry (14 weeks) | Optimal kCal/protein | BIA phase angle | 14 | 15 | HRQoL and HVPG | Phase angle ↑ 0.3 in active¶ & no change in control |
| Roman et al, 2016 (73) | Open RCT | Cycle ergometry (12 weeks) | NA | DXA, Thigh circumference | 15 | 10 | Peak VO2, fat/lean body mass, and risk of falls | Lean mass ↑ 1.05 kg in active & ↓ 0.05 kg in control |
| Berzigotti et al, 2017 (74) | ONCT | Aerobic & resistance (16 weeks) | kCal reduction | BIA lean mass | 60 | NA | HVPG and body weight | 56.7 kg (before) vs. 55.6 kg (after) |
| Nishida et al, 2017(75) | ONCT | Bench step-ups (12 months) | BCAA | Intramuscular adipose content | 9 | NA | Anaerobic threshold, fat in liver and muscle, and glycemic control | −0.47 (before) vs. −0.42 (after) |
| Hiraoka et al, 2017(76) | ONCT | Walking (3 months) | BCAA (as late night snack) | BIA-SMI, handgrip and leg strength | 33 | NA | Muscle volume and function | ↑ 13% in muscle volume¶, ↑ 5% in leg strength¶, and ↑ 10% in handgrip¶ |
| Kruger et al, 2018 (77) | Open RCT | Cycle ergometry (8 weeks) | Optimal kcal/protein | Thigh US & circumference | 20 | 20 | Peak VO2 | Thigh US** ↑ 0.06 cm/m2 in active¶ & 0.06 in control; TC ↑ 1.8 cm in active¶ & 0.6 cm in control |
Denotes comparison was statistically significant
Average feather index showed was significant in active group, whereas average compression index was not.
Average feather index showed a p=0.05 in active group, whereas average compression index was not significant.
6MWT, six-minute walk test; 2MST, 2-min step tests; BCAA, branched-chain amino acids; BIA, bioelectrical impedance analysis; DXA, dual energy X-ray absorptiometry; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance, HRQoL, health-related quality of life; HVPG, hepatic vein pressure gradient; kCal, kilocalories (nutritional requirement); MAMC, mid-arm muscle circumference; NA, not available; ONCT, open non-controlled clinical trial; RCT, randomized clinical trial; SMI, skeletal muscle index; TC, lower thigh circumference; US, ultrasound
Table 3.
Pharmacotherapy for Cirrhotic Sarcopenia
| Author/ Year |
Intervention | Typical Dosing | Comment |
|---|---|---|---|
| Corey al., 2014 (41) | Cholecalciferol | 2000 IU/d | Deficiency common in cirrhosis |
| Davuluri et al., 2016 (43) | Leucine | 7.5 g/d typically in divided doses with additional amino acids | Included in many nutritional supplements |
| Tsien et al., 2015 (44) | |||
| Holecek et al., 2017 (65) | 2-hydroxymethyl butyrate (HMB) | 1 g TID | Metabolite of leucine Nutritional supplement with anti-catabolic action |
| Sinclair et al., 2016 (45) | Testosterone in androgen deficient men | Testosterone undecanoate 1000 mg IM, schedule per RCT; or transdermal gel 50 mg/d (66) | Gel preferred for sustained physiologic levels, concerns for thrombosis and prostate cancer |
| Ohara et al., 2018 (46) | L-Carnitine | 1000 mg/d or BID | Essential nutrient for fatty acid metabolism One fourth is synthesized in the kidney and liver |
Key Points
We believe sarcopenia should not be a sole criterion on which to determine candidacy for LT.
Attaining nutritional goals appropriate for patients with cirrhosis is currently the predominant treatment for sarcopenia.
Recommendations
We recommend that the presence or absence of sarcopenia be considered as part of the multidisciplinary assessment in cirrhosis.
Until more evidence is available, we recommend for patients with cirrhosis and sarcopenia to exercise 150–200 minutes per week, including both aerobic and resistance training (ratio favoring the latter), along with a nutritional intervention with an adiposity-tailored caloric intake that favors protein with key amino acids and aims to prevent starvation.
Male patients with cirrhosis and sarcopenia are potentially eligible for pharmacologic treatment with testosterone replacement.
Sarcopenia in Children
Children with irreversible liver disease offer a complex challenge to clinical care teams that is distinct from adults since they have a time-limited opportunity for growth and development and may suffer lifelong consequences if not expeditiously transplanted.
The PELD (Pediatric End-Stage Liver Disease) score does not adequately capture waitlist and peri-operative risk nor the extent to which ESLD impairs functional development. Clinical (weight, height, anthropometrics) and biochemical (serum total protein and albumin) data fail to fully characterize malnutrition in chronically ill children/those with end-stage organ/liver failure and are often confounded. Objective nutritional biomarkers are lacking for children with ESLD.
Children with ESLD suffer from malnutrition, muscle wasting, and deconditioning that result from the insidious effects of underlying hepatic synthetic dysfunction and systemic inflammation. Broader non-laboratory assessment metrics are needed to more fully capture the extent of ill health status associated with chronic or end-stage liver disease in children. Pilot studies have demonstrated that children with ESLD have smaller psoas muscle areas than healthy controls, and that the psoas muscle area does not correlate with weight z-scores nor PELD score (47, 48).
Significant gaps exist, including the (Table 5) absence of unifying definition of sarcopenia in growing children, the paucity of evidence to date of HOW to measure sarcopenia in children, with different modalities utilized, the evolving validated reference data for total psoas muscle area (tPMA) (via CT abdominal images) in children, the near term impact of interventions such as nutritional support, exercise programs etc. limited by challenges of longitudinal/serial evaluation of sarcopenia due to cumbersome (DEXA) or radiation heavy (CT) assessment methods, the lack of full understanding of how the presence of sarcopenia pre-LT affect outcomes post LT, and whether sarcopenia be considered in clinical decision-making.
Table 5.
Summary of Published Literature on Sarcopenia in Pediatric Liver Disease
| Article | Design | Study Population | Definition of Sarcopenia | Key Findings/Clinical Outcomes |
|---|---|---|---|---|
| Mager et al, 2018 (78) | Retrospective | N=41 children post-LT (age 0.5 – 17 years) | DEXA – to measure appendicular skeletal muscle mass (SMM) z-score <−2 |
|
| Lurz et al, 2018 (79) | Retrospective | N=23 children with ESLD with clinically indicated CT Control: 2:1 age and sex matched healthy controls ( trauma victims with CT) |
CT - PMSA at L3/4 and L4/5 |
|
| Mangus et al. 2017 (48) | Retrospective | N=81 subjects with organ failure – ESLD (35), ESRD (20), IF (26) Control (39) |
CT - PMSA at L2/3 | Reduction in muscle mass (ESLD 23%, ESRD 19%, IF 24%) vs. healthy controls Serum total protein and albumin, and BMI, fail to fully characterize malnutrition in chronically ill children |
CT, computed tomography; DEXA, dual energy X-ray absorptiometry; ESLD, end stage liver disease; ESRD, end stage renal disease; IF, intestine failure; LOS, length of stay; LT, liver transplantation; PICU, Pediatric Intensive Care Unit; PMSA, psoas muscle surface area
Key Points
The majority of children are too young to undergo functional or performance-based (frailty) testing before time of liver transplantation, underscoring the special role and importance of muscle mass assessment in this vulnerable patient population.
Pilot studies demonstrate that total psoas muscle area (tPMA) increases over time until late adolescence and is smaller in pediatric ESLD than healthy controls.
Recommendations
Sarcopenia assessment should be considered in children with ESLD. Amongst those pediatric patients requiring clinically indicated CT imaging, the availability of recent pediatric specific novel tools providing age- and sex-specific reference growth curves will facilitate targeted interventions and their near-term impacts.
Targeting non-invasive assessment strategies for children is a high needs research area.
Sarcopenia in Special Patient Populations
Sarcopenic obesity can be present in 20–40% of cirrhotic LT candidates (49, 50). Patients with sarcopenia are more likely to be obese and those with sarcopenic obesity are more likely to have non-alcoholic fatty liver disease as the etiology of liver disease (50). Of particular concern is the fact that sarcopenia can be difficult to recognize in the presence of obesity. Future research should include measures of obesity beyond BMI such as CT measures of adipose tissue.
Chronic kidney disease (CKD) alone can affect muscle mass. Sarcopenia occurs in roughly 10% of patients with CKD alone and is associated with increased mortality (51). In renal transplant recipients, sarcopenia may persist for years after transplantation, with age and duration of dialysis being important predictors (52). Little is known about the relationship between sarcopenia and CKD in LT candidates. Normal renal function in patients with cirrhosis has been correlated with higher measures of muscle mass (53). Data on measures such as duration of renal insufficiency and renal replacement therapy in LT candidates with sarcopenia are not available.
Future directions for research
Sarcopenia has become a topic of prolific exploration in patients with ESLD over the last few years. Currently, the evaluation of sarcopenia in patients with cirrhosis appears in over 1,500 publications. Important research questions that merit exploration for sarcopenia assessment in patients with ESLD include 1) the availability of reliable, accessible and practical tools to be used in clinical practice, 2) the optimal frequency of measurement over time, 3) how to assess the clinical meaning of changes over time, whether they have prognostic value independent of other measurements, and 4) how they can best be used to engage patients in self-motivation.
One of the most important knowledge gaps in cirrhosis care is our limited understanding of the time frame associated with the efficacy of interventions. We lack information on whether recovery of sarcopenia with nutritional management in combination with an exercise program is sustainable, and how an improvement in muscle mass might be associated with improvement in clinical outcomes.
Financial Support:
Jennifer C. Lai received NIH K23AG048337 and NIH R01AG059183.
Srinivasan Dasarathy received NIH R21 AA022742, RO1 DK 113196, RO1 GM119174, P50 AA024333, UO1 AA021890, UO1 AA026975, UO1 DK061732, and the Mikati Foundation Grant support.
Aldo J. Montano-Loza received Canadian National Transplant Research Program (CNTRP) 2018 ATIF Innovation Grant Award.
These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations:
- PMA
Psoas Muscle Area
- PMI
Psoas Muscle Index
- SMI
Skeletal Muscle Index
- TPA
Total Psoas Area
- TPMT
Transversal Psoas Muscle Thickness
Footnotes
Conflict of interest:
None.
References
- 1.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20:640–648. [DOI] [PubMed] [Google Scholar]
- 3.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, JN IJ. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant 2016;16:2277–2292. [DOI] [PubMed] [Google Scholar]
- 4.Yadav A, Chang YH, Carpenter S, Silva AC, Rakela J, Aqel BA, et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transplant 2015;29:134–141. [DOI] [PubMed] [Google Scholar]
- 5.Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One 2017;12:e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 10.Norman K, Kirchner H, Lochs H, Pirlich M. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol 2006;12:3380–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005;21:113–117. [DOI] [PubMed] [Google Scholar]
- 12.Durand F, Buyse S, Francoz C, Laouenan C, Bruno O, Belghiti J, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014;60:1151–1157. [DOI] [PubMed] [Google Scholar]
- 13.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol 2015;6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMartini A, Cruz RJ Jr., Dew MA, Myaskovsky L, Goodpaster B, Fox K, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl 2013;19:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014;20:401–407. [DOI] [PubMed] [Google Scholar]
- 16.Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 2013;19:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Vugt JLA, Buettner S, Alferink LJM, Bossche N, de Bruin RWF, Darwish Murad S, et al. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation-a retrospective study. Transpl Int 2018;31:165–174. [DOI] [PubMed] [Google Scholar]
- 18.Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013;13:1549–1556. [DOI] [PubMed] [Google Scholar]
- 19.Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int 2018;12:377–386. [DOI] [PubMed] [Google Scholar]
- 20.Tandon P, Mourtzakis M, Low G, Zenith L, Ney M, Carbonneau M, et al. Comparing the Variability Between Measurements for Sarcopenia Using Magnetic Resonance Imaging and Computed Tomography Imaging. Am J Transplant 2016;16:2766–2767. [DOI] [PubMed] [Google Scholar]
- 21.Ebadi M, Wang CW, Lai JC, Dasarathy S, Kappus MR, Dunn MA, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle 2017;8:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo SZ, Ahmad M, Dunn MA, Montano-Loza AJ, Carey E, Lin S, et al. Sarcopenia Predicts Post-Transplant Mortality in Acutely Ill Men Undergoing Urgent Evaluation and Liver Transplantation. Transplantation 2019. doi: 10.1097/TP.0000000000002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 26.van Vugt JL, Levolger S, Gharbharan A, Koek M, Niessen WJ, Burger JW, et al. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 2017;8:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC, et al. Low Subcutaneous Adiposity Associates with Higher Mortality in Female Patients with Cirrhosis. J Hepatol 2018;69:608–616. [DOI] [PubMed] [Google Scholar]
- 28.Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol 2010;22:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai JC. Transplant for the very sick: No limitations in donor quality? Liver Transpl 2017;23:S40–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waits SA, Englesbe MJ. Making Progress Toward Frailty Remediation in End-Stage Liver Disease. Transplantation 2016;100:2526. [DOI] [PubMed] [Google Scholar]
- 31.Lai JC, Tandon P. How I Approach It: Improving Nutritional Status in Patients With Cirrhosis. Am J Gastroenterol 2018;113:1574–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, et al. Exercise in cirrhosis: Translating evidence and experience to practice. J Hepatol 2018;69:1164–1177. [DOI] [PubMed] [Google Scholar]
- 33.Pollak KI, Alexander SC, Coffman CJ, Tulsky JA, Lyna P, Dolor RJ, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med 2010;39:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70(1):172–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013;58:325–336. [DOI] [PubMed] [Google Scholar]
- 36.Plauth M, Cabre E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285–294. [DOI] [PubMed] [Google Scholar]
- 37.Cordoba J, Lopez-Hellin J, Planas M, Sabin P, Sanpedro F, Castro F, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol 2004;41:38–43. [DOI] [PubMed] [Google Scholar]
- 38.Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology 2008;48:557–566. [DOI] [PubMed] [Google Scholar]
- 39.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 2012;27:430–441. [DOI] [PubMed] [Google Scholar]
- 40.Duarte-Rojo A, Ruiz-Margain A, Montano-Loza AJ, Macias-Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122–139. [DOI] [PubMed] [Google Scholar]
- 41.Corey RL, Whitaker MD, Crowell MD, Keddis MT, Aqel B, Balan V, et al. Vitamin D deficiency, parathyroid hormone levels, and bone disease among patients with end-stage liver disease and normal serum creatinine awaiting liver transplantation. Clin Transplant 2014;28:579–584. [DOI] [PubMed] [Google Scholar]
- 42.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, et al. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol 2016;65:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015;61:2018–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther 2016;43:765–777. [DOI] [PubMed] [Google Scholar]
- 46.Ohara M, Ogawa K, Suda G, Kimura M, Maehara O, Shimazaki T, et al. L-Carnitine Suppresses Loss of Skeletal Muscle Mass in Patients With Liver Cirrhosis. Hepatol Commun 2018;2:906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lurz E, Quammie C, Englesbe M, Alonso EM, Lin HC, Hsu EK, Furuya KN, et al. Frailty in Children with Liver Disease: A Prospective Multicenter Study. J Pediatr 2018;194:109–115. [DOI] [PubMed] [Google Scholar]
- 48.Mangus RS, Bush WJ, Miller C, Kubal CA. Severe Sarcopenia and Increased Fat Stores in Pediatric Patients With Liver, Kidney, or Intestine Failure. J Pediatr Gastroenterol Nutr 2017;65:579–583. [DOI] [PubMed] [Google Scholar]
- 49.Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carias S, Castellanos AL, Vilchez V, Nair R, Dela Cruz AC, Watkins J, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kakiya R, Shoji T, Tsujimoto Y, Tatsumi N, Hatsuda S, Shinohara K, et al. Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int 2006;70:549–556. [DOI] [PubMed] [Google Scholar]
- 52.Yanishi M, Kimura Y, Tsukaguchi H, Koito Y, Taniguchi H, Mishima T, et al. Factors Associated With the Development of Sarcopenia in Kidney Transplant Recipients. Transplant Proc 2017;49:288–292. [DOI] [PubMed] [Google Scholar]
- 53.Pirlich M, Selberg O, Boker K, Schwarze M, Muller MJ. The creatinine approach to estimate skeletal muscle mass in patients with cirrhosis. Hepatology 1996;24:1422–1427. [DOI] [PubMed] [Google Scholar]
- 54.Belarmino G, Gonzalez MC, Sala P, Torrinhas RS, Andraus W, D’Albuquerque LA, et al. Diagnosing Sarcopenia in Male Patients With Cirrhosis by Dual-Energy X-Ray Absorptiometry Estimates of Appendicular Skeletal Muscle Mass. JPEN J Parenter Enteral Nutr 2018;42:24–36. [DOI] [PubMed] [Google Scholar]
- 55.Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, et al. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 2015;27:328–334. [DOI] [PubMed] [Google Scholar]
- 56.Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, et al. Sarcopenia and Sarcopenic Obesity Are Prognostic Factors for Overall Survival in Patients with Cirrhosis. Intern Med 2016;55:863–870. [DOI] [PubMed] [Google Scholar]
- 57.Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, et al. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2016;14:1473–1480. [DOI] [PubMed] [Google Scholar]
- 58.Praktiknjo M, Book M, Luetkens J, Pohlmann A, Meyer C, Thomas D, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology 2018;67:1014–1026. [DOI] [PubMed] [Google Scholar]
- 59.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–173. [DOI] [PubMed] [Google Scholar]
- 60.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 61.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–1216. [DOI] [PubMed] [Google Scholar]
- 62.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–870. [DOI] [PubMed] [Google Scholar]
- 63.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 64.van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2018;68:707–714. [DOI] [PubMed] [Google Scholar]
- 65.Holecek M Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle 2017;8:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yurci A, Yucesoy M, Unluhizarci K, Torun E, Gursoy S, Baskol M, et al. Effects of testosterone gel treatment in hypogonadal men with liver cirrhosis. Clin Res Hepatol Gastroenterol 2011;35:845–854. [DOI] [PubMed] [Google Scholar]
- 67.Konishi I, Hiasa Y, Tokumoto Y, Abe M, Furukawa S, Toshimitsu K, et al. Aerobic exercise improves insulin resistance and decreases body fat and serum levels of leptin in patients with hepatitis C virus. Hepatol Res 2011;41:928–935. [DOI] [PubMed] [Google Scholar]
- 68.Pattullo V, Duarte-Rojo A, Soliman W, Vargas-Vorackova F, Sockalingam S, Fantus IG, et al. A 24-week dietary and physical activity lifestyle intervention reduces hepatic insulin resistance in the obese with chronic hepatitis C. Liver Int 2013;33:410–419. [DOI] [PubMed] [Google Scholar]
- 69.Roman E, Torrades MT, Nadal MJ, Cardenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966–1975. [DOI] [PubMed] [Google Scholar]
- 70.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920–1926. [DOI] [PubMed] [Google Scholar]
- 71.Debette-Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, et al. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation 2015;99:145–150. [DOI] [PubMed] [Google Scholar]
- 72.Macias-Rodriguez RU, Ilarraza-Lomeli H, Ruiz-Margain A, Ponce-de-Leon-Rosales S, Vargas-Vorackova F, Garcia-Flores O, et al. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin Transl Gastroenterol 2016;7:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roman E, Garcia-Galceran C, Torrades T, Herrera S, Marin A, Donate M, et al. Effects of an Exercise Programme on Functional Capacity, Body Composition and Risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One 2016;11:e0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017;65:1293–1305. [DOI] [PubMed] [Google Scholar]
- 75.Nishida Y, Ide Y, Okada M, Otsuka T, Eguchi Y, Ozaki I, et al. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res 2017;47:E193–E200. [DOI] [PubMed] [Google Scholar]
- 76.Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2017;29:1416–1423. [DOI] [PubMed] [Google Scholar]
- 77.Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home Exercise Training Improves Exercise Capacity in Cirrhosis Patients: Role of Exercise Adherence. Sci Rep 2018;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mager DR, Hager A, Ooi PH, Siminoski K, Gilmour SM, Yap JYK. Persistence of Sarcopenia After Pediatric Liver Transplantation Is Associated With Poorer Growth and Recurrent Hospital Admissions. JPEN J Parenter Enteral Nutr 2019;43:271–280. [DOI] [PubMed] [Google Scholar]
- 79.Lurz E, Patel H, Frimpong RG, Ricciuto A, Kehar M, Wales PW, et al. Sarcopenia in Children With End-Stage Liver Disease. J Pediatr Gastroenterol Nutr 2018;66:222–226. [DOI] [PubMed] [Google Scholar]