Abstract
BACKGROUND:
Black women have worse breast cancer survival after controlling for clinical factors, suggesting that treatment differences may contribute to poorer outcomes. Delays in initiating and completing treatment are one proposed mechanism.
METHODS:
The Carolina Breast Cancer Study Phase III is a large population-based cohort of women with incident breast cancer. Study participants comprised black (n=1328) and white (n=1331) women with stage I-III disease whose treatment included surgery with or without adjuvant therapies. We employed a novel treatment pathway grouping to benchmark treatment duration (surgery only; surgery + chemotherapy; surgery + radiation; or all three). Models controlled for treatment pathway, age, and tumor characteristics, and demographic factors related to healthcare access. We performed exploratory analyses of the association between delays and cancer recurrence.
RESULTS:
In fully adjusted analyses, blacks had 1.73 times higher odds of treatment initiation >60 days after diagnoses compared to whites (OR: 1.73, 95% CI: 1.04–2.90). Race was also associated with longer treatment duration. Blacks were also more likely to be in the highest quartile of treatment duration (OR: 1.69, 95% CI: 1.41, 2.02), even after adjustment for demographic and tumor characteristics (OR: 1.31, 95% CI: 1.04–1.64). A non-significant trend toward higher recurrence risk was observed for patients with delayed initiation (HR 1.44, 95% CI 0.89–2.33) or longest duration (HR 1.17, 95% CI 0.87–1.59).
CONCLUSIONS:
Black women more often had delayed treatment initiation and longer duration than whites receiving similar treatment. Interventions that target access barriers may be needed to improve timely delivery of care.
Keywords: breast cancer, health care disparities, minority health
Precis
Black women more often have delays across the breast cancer treatment continuum than whites receiving similar treatment. Interventions that target access barriers may be needed to improve timely delivery of care.
Background
Despite comparable or lower incidence of breast cancer than White women in the United States, Black women have higher breast cancer mortality, a survival gap that has persisted for more than four decades.1–3 Survival differences between Black and White women are attributable in part to the greater proportion of hormone receptor- and HER2 receptor-negative or “triple negative” cancers among young black women,1,4 more advanced stage3 and higher grade at diagnosis,5 and other adverse tumor features in Black women.5–7 However, the survival decrement that remains after accounting for tumor stage and subtype suggests that post-diagnosis factors also contribute to racial disparities in US breast cancer outcomes.8
Disparities in diagnostic and treatment patterns, including treatment delays, have been described for Black women, and may contribute to racial differences in breast cancer survival. Black women experience longer intervals than Whites between first symptom, screening, or consult and biopsy-proven diagnosis.9,10 Black women are also more likely to experience delays in surgical treatment after a new diagnosis than Whites.11,9,12,13,14,15 Radiation is also delayed in Black women, and these delays appear to be explained in part by differences in geographic access to radiation facilities.16,17 Black patients experience delays in initiating and completing chemotherapy,18–21 and experience longer time to initiation of appropriate endocrine therapy.22 Although differential insurance coverage may explain some of these differences, Black women are still more likely to experience delays9,11,12,16 and to fare worse12,18,19,23 after controlling for insurance type. Unfortunately, delays are particularly impactful on clinical outcomes of triple negative breast cancer, a subtype more common among Black women.18,23
Significant gaps exist in understanding the drivers of treatment delay across the continuum of cancer care. Although race, geography, insurance access, and other socioeconomic factors all have been individually implicated in treatment delays, the literature describing these patterns has several gaps. First, large studies of racial and geographic factors associated with treatment delay typically have relied on neighborhood-level characteristics rather than individual-level data on education and income, limiting the ability to account for the impact of socioeconomic status. Second, studies with more detailed information on individual characteristics, such as chart reviews, generally focus on patients within a restricted geographic location or institution, where access may be relatively homogenous. Third, most studies assess only one fragmentary measure of treatment delay, such as time from diagnosis to surgery or time from surgery to radiation, rather than trying to understand more precisely how delays may be compounded across the care continuum. Finally, most studies focus on delays in initiation of treatment, rather than a broader consideration of the timeliness of completion of the entire treatment plan. A more complete understanding of how delays occur and in which patients, whether they accumulate for the same patients across the spectrum of cancer care, and how race and geographic factors interact in treatment delays, is needed.
The Carolina Breast Cancer Study offers is a population-based, minority-enriched study representing 44 urban and rural counties in North Carolina and includes detailed treatment data. The study’s Phase III population (CBS3) comprises 2,998 racially diverse women with newly diagnosed breast cancer in North Carolina, a state with a spectrum of urban and rural geographies and demographic heterogeneity. The study includes a wealth of patient-reported individual data on socioeconomic status, place of residence and locations of treatment, and detailed medical record abstraction of breast cancer treatments and dates. In this study, we examine the relationship between delays throughout the cancer care continuum, while considering stage, race, socioeconomic status, and geography as potential determinants, and using a novel benchmarking approach to assess treatment timeliness.
Methods
Data source and study population
Women eligible for CBCS3 were identified through the Rapid Case Ascertainment of the North Carolina Central Cancer Registry (NCCCR), and selected by randomized recruitment within four strata defined by race (African American (AA vs. non-AA) and age (<50 vs. 50+ years of age at diagnosis). Participants underwent extensive structured in-home baseline interviews with a trained nurse, during which anthropomorphic data and a germline DNA sample were also gathered. Women also consented to medical record review, from which detailed cancer treatment data were abstracted.
This analysis was restricted to women who i) were diagnosed with stage I-III cancers, ii) self-reported African American/Black or White race, and iii) underwent surgery for breast cancer within 18 months of diagnosis (n=2,761). To avoid confusion between primary treatment and treatment of early relapses, we restricted the analysis to patients without documented relapse within 18 months of diagnosis.
Covariate assessment
Putative predictors of treatment delay were ascertained from home-based structured interviews with a nurse. These covariates included age at diagnosis (<40, 40–49, 50–59, 60+ years), self-reported race (Black, White) educational attainment (<high school education, high school graduate-some college, college graduate or higher), annual family income (<$20,000, $20-<50,000, $50,000+), insurance type (uninsured, any Medicaid, Medicare (no Medicaid), private/other), marital status and county of residence. Since many counties had small numbers of cases, county of residence was grouped into nine Area Health Education Center (AHEC) regions. Tumor characteristics including stage at diagnosis, hormone receptor (HR) positivity, and human epidermal growth factor receptor 2 (HER2) positivity, were ascertained from pathology reports.
Outcome assessment
The primary outcomes of interest, delayed treatment initiation and prolonged treatment duration, were defined based on dates observed in the medical record. Treatment initiation was defined as the first treatment-directed surgery, radiation, or chemotherapy event observed after diagnosis. Time from diagnosis date to treatment initiation was measured in days and those exceeding 60 days were categorized as having ‘delayed initiation’. The 60 day threshold was chosen based on clinical judgement and distribution of time to first treatment to represent a clinically significant and unusual delay in care, and based on prior research associating pre-operative intervals of greater than 60 days with survival decrements.24
To assess timeliness of treatment completion, we characterized treatment duration as the interval between treatment initiation and the last day of the latest treatment observed in the medical record prior to a cancer recurrence. Given the chronic nature of maintenance biologic therapy for HER2+ disease (1 year duration) and adjuvant endocrine therapy (5–10 year duration), these targeted therapies were not included in our assessment. Patients were classified into four treatment patterns: surgery only, surgery and radiation, surgery and chemotherapy, or all three modalities. Within each treatment group, a patient’s duration of treatment was benchmarked against other patients who received the same therapies. We calculated quartiles of treatment duration within each group and classified women who were above the 75th percentile of treatment duration compared to others in their group as having ‘prolonged treatment duration’. Time to first recurrence was ascertained from medical records abstraction.
Statistical analysis
For descriptive analyses, chi-square tests utilizing CBCS3 randomized recruitment sampling weights were used to test whether covariate distributions differed by treatment timing. Log-binomial and logistic regression models then were used to evaluate the relationship between race and delayed initiation and prolonged treatment duration. Three sets of models were performed: (1) minimally-adjusted models controlling only for age in 10-year age categories, and (2) models adjusted for age and demographic characteristics (marital status, income, education, insurance type, and AHEC region), and (3) models adjusted for age and demographic and tumor characteristics (stage, HR, HER2).
In exploratory analyses, we examined whether delayed initiation and treatment duration were associated with recurrence-free survival. Cox proportional hazards models were used with adjustment for HR and HER2 receptor status, grade, nodal status, size, and 10-year age group. Survival time was assessed beginning at 18 months because the full cohort was required to be recurrence-free at 18 months. For analysis of treatment duration, we additionally excluded individuals who had continuous active treatment for more than 18 months after diagnosis (n=18), because of the concern for undocumented recurrent disease driving the treatment plan, or who experienced a documented recurrence (n=108) and/or were lost to follow up (n=81) prior to 18 months from diagnosis.
Results
The analysis included 2,659 CBCS3 participants. The median time to treatment initiation was 24 days (interquartile range: 13–36). A total of 117 (4.4%) women initiated treatment more than 60 days after diagnosis. In bivariate analyses, women with delayed treatment initiation were significantly more likely to be black, unmarried, have lower income, be uninsured or insured through Medicaid, and to have later stages at diagnosis (Table 1). They also were more likely to live in the Charlotte AHEC region, and less likely to live in the Wake AHEC region; these regions correspond to the two largest metropolitan regions in the study catchment area. Those who had delayed treatment initiation were more likely to receive surgery only, and were less likely to receive all modalities.
Table 1.
Cohort demographics by time to treatment initiation and treatment duration quartile.
| Treatment initiation | Treatment duration | |||||
|---|---|---|---|---|---|---|
| ≤60 days (N=2542) |
>60 days (N=117) |
X2
p-value* |
Q1–Q3 (N=2001) |
Q4 (N=658) |
X2
p-value* |
|
| Race | N (%) | N(%) | <0.0001 | N (%) | N (%) | <0.0001 |
| White | 1297 (51.0) | 34 (29.1) | 1066 (53.3) | 265 (40.3) | ||
| Black | 1245 (49.0) | 83 (70.9) | 935 (46.7) | 393 (59.7) | ||
| Age | 0.4222 | 0.0180 | ||||
| <40 | 294 (11.6) | 13 (11.1) | 230 (11.5) | 77 (11.7) | ||
| 40–49 | 949 (37.3) | 43 (36.8) | 715 (35.7) | 277 (42.1) | ||
| 50–59 | 568 (22.3) | 33 (28.2) | 457 (22.8) | 144 (21.9) | ||
| 60–74 | 731 (28.8) | 28 (23.9) | 599 (29.9) | 160 (24.3) | ||
| Marital status | 0.0043 | 0.0197 | ||||
| Unmarried | 1087 (42.8) | 69 (59.0) | 840 (42.0) | 316 (48.0) | ||
| Married | 1455 (57.2) | 48 (41.0) | 1161 (58.0) | 342 (52.0) | ||
| Income | 0.0039 | <0.0001 | ||||
| <$20,000 | 524 (24.7) | 41 (43.6) | 363 (21.8) | 202 (36.7) | ||
| $20–50,000 | 459 (21.7) | 20 (21.3) | 351 (21.1) | 128 (23.3) | ||
| >$50,000 | 1137 (53.6) | 33 (35.1) | 950 (57.1) | 220 (40.0) | ||
| Missing | 422 | 23 | 337 | 108 | ||
| Primary insurance | 0.0269 | <0.0001 | ||||
| Uninsured | 136 (5.4) | 12 (10.3) | 98 (4.9) | 50 (7.6) | ||
| Medicaid | 365 (14.4) | 32 (27.6) | 234 (11.7) | 163 (24.8) | ||
| Medicare | 485 (19.1) | 18 (15.5) | 393 (19.7) | 110 (16.7) | ||
| Private/other | 1555 (61.2) | 54 (46.6) | 1274 (63.7) | 335 (50.9) | ||
| Missing | 1 | 1 | 2 | 0 | ||
| Education | 0.4211 | 0.0018 | ||||
| <HS | 1338 (52.6) | 56 (47.9) | 1027 (51.3) | 367 (55.8) | ||
| HS-Some college | 1010 (39.7) | 48 (41.0) | 837 (41.8) | 221 (33.6) | ||
| College+ | 194 (7.6) | 13 (11.1) | 137 (6.9) | 70 (10.6) | ||
| AHEC region | 0.0472 | <0.0001 | ||||
| UNC-Chapel Hill | 169 (6.7) | 10 (8.6) | 151 (7.6) | 28 (4.3) | ||
| Area L | 101 (4.0) | 3 (2.6) | 70 (3.5) | 34 (5.2) | ||
| Charlotte | 484 (19.0) | 35 (29.9) | 409 (20.4) | 110 (16.7) | ||
| Eastern | 255 (10.0) | 11 (9.4) | 170 (8.5) | 96 (14.6) | ||
| Greensboro | 332 (13.1) | 21 (18.0) | 255 (12.7) | 98 (14.9) | ||
| Northwest | 308 (12.1) | 17 (14.5) | 249 (12.4) | 76 (11.6) | ||
| South East | 137 (5.4) | 6 (5.1) | 113 (5.7) | 30 (4.6) | ||
| Southern | 206 (8.1) | 3 (2.6) | 147 (7.4) | 62 (9.4) | ||
| Wake | 550 (21.6) | 11 (9.4) | 437 (21.8) | 124 (18.8) | ||
| Stage | 0.0470 | <0.0001 | ||||
| I | 1140 (44.9) | 41 (35.0) | 941 (47.0) | 240 (36.5) | ||
| II | 1059 (41.7) | 60 (51.3) | 825 (41.2) | 294 (44.7) | ||
| III | 343 (13.5) | 16 (13.7) | 235 (11.7) | 124 (18.8) | ||
| HR status | 0.4726 | 0.4982 | ||||
| Negative | 602 (23.9) | 20 (17.5) | 467 (23.6) | 155 (23.9) | ||
| Positive | 1913 (76.1) | 94 (82.5) | 1514 (76.4) | 493 (76.1) | ||
| Missing | 27 | 3 | 20 | 10 | ||
| HER2 status | 0.7684 | 0.0121 | ||||
| Negative | 2095 (83.4) | 98 (85.2) | 1685 (85.1) | 508 (78.4) | ||
| Positive | 418 (16.6) | 17 (14.8) | 295 (14.9) | 140 (21.6) | ||
| Missing | 29 | 2 | 21 | 10 | ||
| Patient group | 0.1885 | -- | ||||
| Surg. only | 326 (12.8) | 26 (22.2) | -- | -- | ||
| Surg. + rad. | 604 (23.8) | 28 (23.9) | -- | -- | ||
| Surg. + chemo. | 343 (13.5) | 21 (18.0) | -- | -- | ||
| All | 1269 (49.9) | 42 (35.9) | -- | -- | ||
| Delayed initiation | -- | 0.2915 | ||||
| No | -- | -- | 1917 (95.8) | 625 (95.0) | ||
| Yes | -- | -- | 84 (4.2) | 33 (5.0) | ||
Weighted for CBCS3 sampling probabilities.
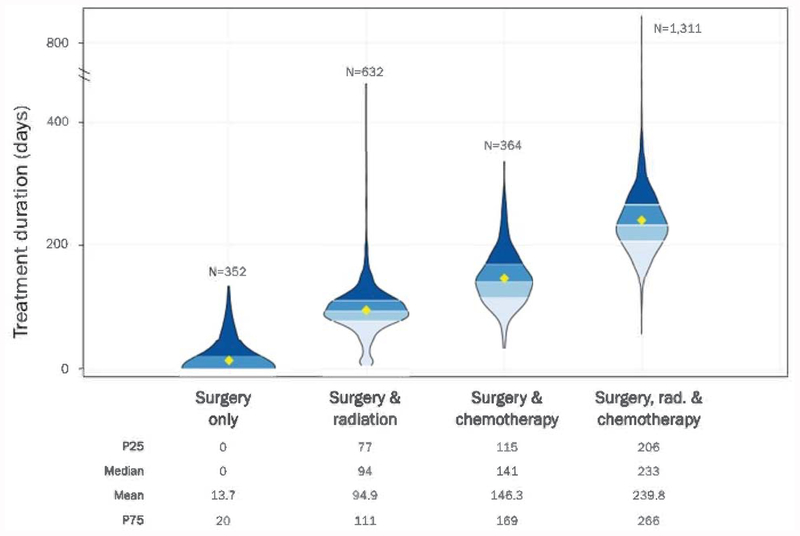
The distribution of treatment durations, measured from the first day of first treatment to the last day of final treatment, is presented by treatment group (Figure 1). There was substantial variation in treatment duration within each treatment group. As expected, increasing number of treatment modalities was associated with longer treatment duration.
Figure 1:
Distribution of Treatment Duration by Treatment Group
In bivariate analyses, women with the longest treatment durations (highest quartile) compared to others in their own treatment pathway were more likely to be black, younger, lower income, uninsured or have Medicaid, less educated, higher stage at diagnosis, and have HER2 positive disease (Table 1). The association with HER2-positive was observed despite the fact that maintenance HER2-directed therapy was not considered as part of the treatment duration. Delayed initiation did not appear to be strongly associated with prolonged duration in bivariate analysis (X2 p-value 0.29).
Multivariable-adjusted associations between race and treatment delay (60+ days after diagnosis) and treatment duration (highest quartile) are summarized in Table 2. Black women more frequently experienced delayed treatment (age-adjusted Odds ratio (OR): 2.50, 95% confidence interval (CI): 1.66, 3.77). Adjustment for demographic characteristics and geographic region of residence attenuated this difference, but significantly higher frequency of delay among black women persisted. Adjustment for tumor characteristics only slightly attenuated the association; in the fully adjusted model black women had almost twice the frequency of delayed initiation compared to white women (OR: 1.73, 95% CI: 1.04–2.90).
Table 2.
Odds ratios (and 95% confidence intervals) for the association between race and treatment initiation and duration.
| Age-adjusted | Adjusted for demographics* | Fully adjusted* | |
|---|---|---|---|
| Initiation >60 days | 2.50 (1.66, 3.77) | 1.80 (1.09–2.97) | 1.73 (1.04–2.90) |
| Q4 duration | 1.69 (1.41, 2.02) | 1.33 (1.07–1.66) | 1.31 (1.04–1.64) |
Additionally adjusted for income, education, AHEC region, marital status and insurance status
Additionally adjusted for stage, and HER2 and HR status
Race was also associated with treatment duration. In age-adjusted models, black women were significantly more likely to be in the highest quartile of treatment duration compared to white women (OR: 1.69, 95% CI: 1.41, 2.02). Adjustment for demographic characteristics and geographic region of residence slightly attenuated this association, as did additional adjustment for tumor characteristics. However, after adjustment for both demographic and tumor characteristics, Black women remained more likely to experience the longest treatment durations (OR: 1.31, 95% CI: 1.04–1.64).
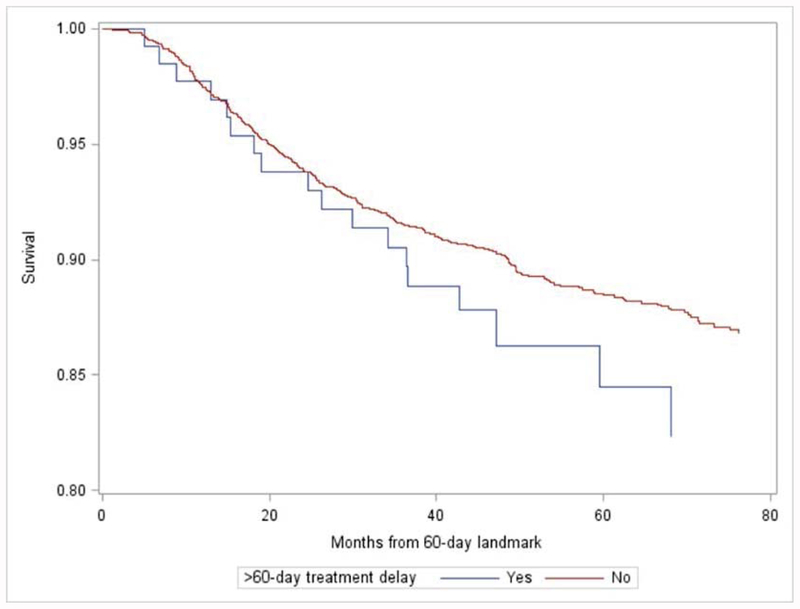
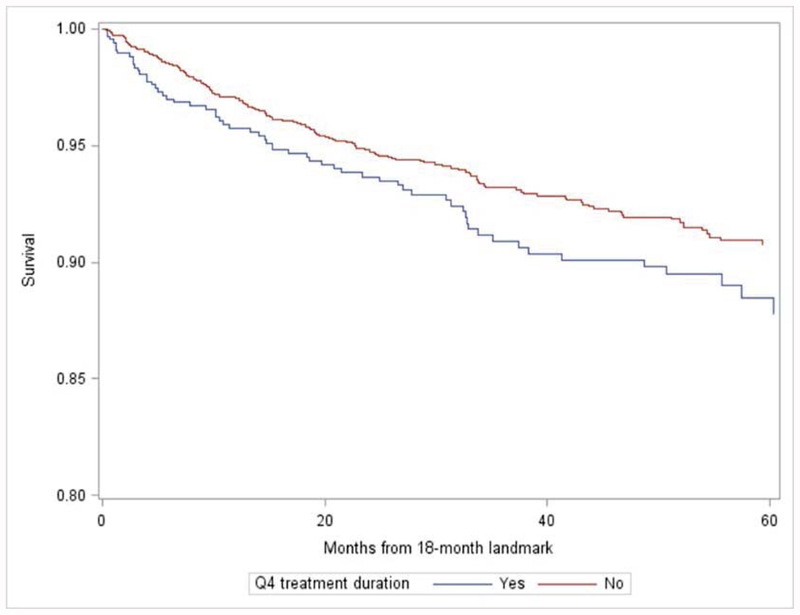
To assess the impact of delayed initiation and treatment prolongation, we performed exploratory analyses of the association between delayed initiation, prolonged duration and recurrence-free survival (Figure 2). After adjustment for age, receptor status, grade, and tumor size, a non-significant trend association with recurrence was suggested for patients with delayed initiation (HR: 1.44, 95% CI: 0.89, 2.33) or longest quartile of duration (HR 1.17, 95% CI 0.87–1.59) compared to those receiving timely care.
Figure 2:
Unadjusted recurrence-free survival by timeliness of initiation (A) and treatment duration (B), beginning from 60-day (A) or 18 month (B) landmarks.
Discussion
In this large population-based cohort of racially diverse patients with newly diagnosed non-metastatic breast cancer, we found that compared to White women, Black women with similar treatment plans were more likely to experience delays in treatment initiation and longer treatment duration. Interestingly, the effect of urban geography was variable by the particular metropolitan area, suggesting that the effect of urban geography may vary depending on other characteristics of local health services. Interesting, delayed initiation was not strongly associated with prolonged treatment duration, suggesting that different patients may be at risk for delays at different points on the care continuum.
Our findings add to recent work from the National Comprehensive Cancer Network, the National Cancer Database, and medical chart reviews that report longer times from symptom onset to diagnosis,9 diagnosis to surgery25 and from diagnosis to adjuvant chemotherapy26 for Black patients, and provide a novel approach to benchmarking treatment timeliness against other patients with similar treatment plans. Our data also suggest a trend toward increased recurrence associated with both delayed initiation and prolonged duration. These findings supplement earlier research that delays in initiation of surgical treatment beyond 60 days24, as well as delays in time between surgery and initiation of chemotherapy18,23 are associated with decrements in breast cancer survival.
It has been noted that therapeutically appropriate interventions, including breast reconstruction and gene expression profile testing, can drive some treatment delays.26; however, given that reconstruction27–29 and gene expression profile testing are used at similar rates30 or lower rates31–34 among Black women, and that complications of breast reconstruction are similar across race35,36, such clinically appropriate delays are unlikely to explain the racial disparities we observed. Some previous literature has suggested that racial disparities in breast cancer treatment quality and timeliness may partially be explained by the characteristics of the institutions at which minority patients seek care,37,38, their insurance status, and/or the distribution of minority patients in geographic areas with lower access or longer distance to care.16,39 However, our study suggests that after controlling for these aspects using high-quality individual-level data, race remains an independent predictor of delay.
A number of additional factors, outside the scope of this analysis, may vary by race and impact treatment timeliness, including difficulty with transportation or sick leave, affordability of shared costs, distrust of medical providers, or toxicity experiences. We have recently reported greater financial toxicity of breast cancer among Black women.40 Other sources suggest that toxicities of radiation and chemotherapy, which could potentially prolong therapy, are more common among Black women.41–44 Thus, there are several barriers not measured in this study that warrant future investigation.
This study has a number of strengths, including a racially diverse cohort with detailed information regarding timing of treatments and sociodemographic data, and an analytic approach that copes with clinically appropriate differences in treatment trajectories. We also recognize some limitations of our analysis. AHEC region was used as a gross measure of health care system factors within a geographic region, but does not control for all characteristics of oncology care in a given area. We did not adjudicate the clinical appropriateness of delays. Finally, while we did examine the relationship between delays and recurrence in exploratory analyses, CBCS3 has a limited follow up (median 5.5 years at this analysis), and longer follow-up is needed to evaluate long term impact of delays particularly among HR+ cases, where risk of distant recurrence persists for at least two decades after diagnosis.45
In summary, we found that after accounting for geographic, insurance, demographic, and disease characteristics, as well as the complexity of the treatment plan, Black women remained more likely than Whites to experience delays in initiation and completion of primary breast cancer treatment. Our findings of treatment timeliness disparities are particularly troubling given that Black women are more likely to be diagnosed at advanced stages and are disproportionately affected by biologically aggressive breast cancer subtypes4,7 associated with survival decrements after even brief delays (>30 days) in adjuvant therapy.18 The combination of delayed diagnosis, biologically aggressive disease, and treatment delays across the care continuum underscore the complexity of racial disparities in breast cancer and suggest multifactorial strategies are required to reduce breast cancer disparities. Interventions such as targeted patient navigation aimed at overcoming barriers to treatment may be worthy of investigation as means to forestall treatment delays.
Funding
Dr. Reeder-Hayes was supported in part during this research by a Komen Career Catalyst Award (CCR15333140), a Conquer Cancer Foundation Career Development Award, and an Alliance Scholar Award. Dr. Troester was supported by the University of North Carolina Breast Cancer Specialized Programs of Research Excellence (SPORE) grant CA058223 and a National Institutes of Health UO1 grant (CA179715).
Footnotes
Conflicts of Interest
The authors have no conflicts to report.
REFERENCES
- 1.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Journal of the National Cancer Institute.106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: a cancer journal for clinicians. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 3.Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2013. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. [DOI] [PubMed] [Google Scholar]
- 5.Chen VW, Correa P, Kurman RJ, et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1994;3(2):127–135. [PubMed] [Google Scholar]
- 6.Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–2542. [DOI] [PubMed] [Google Scholar]
- 7.Troester MA, Sun X, Allott EH, et al. Racial Differences in PAM50 Subtypes in the Carolina Breast Cancer Study. Journal of the National Cancer Institute. 2018;110(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112(1):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. Journal of women’s health (2002). 2015;24(3):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–2252. [DOI] [PubMed] [Google Scholar]
- 11.Halpern MT, Schrag D. Effects of state-level medicaid policies and patient characteristics on time to breast cancer surgery among medicaid beneficiaries. Breast Cancer Res Treat. 2016;158(3):573–581. [DOI] [PubMed] [Google Scholar]
- 12.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to Treatment: Measuring Quality Breast Cancer Care. Ann Surg Oncol. 2016;23(10):3392–3402. [DOI] [PubMed] [Google Scholar]
- 13.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516–523. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard VB, Oppong BA, Hampton R, et al. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015;22(9):2902–2911. [DOI] [PubMed] [Google Scholar]
- 15.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(7):1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler SB, Carpenter WR, Peppercorn J, Schenck AP, Weinberger M, Biddle AK. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133(1):333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold HT, Thwin SS, Buist DS, et al. Delayed radiotherapy for breast cancer patients in integrated delivery systems. The American journal of managed care. 2009;15(11):785–789. [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA oncology. 2016;2(3):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Medical oncology (Northwood, London, England). 2013;30(1):419. [DOI] [PubMed] [Google Scholar]
- 20.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–6646. [DOI] [PubMed] [Google Scholar]
- 21.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28(27):4135–4141. [DOI] [PubMed] [Google Scholar]
- 22.Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, Wheeler SB. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145(3):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the united states. JAMA oncology. 2016;2(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liederbach E, Sisco M, Wang C, et al. Wait times for breast surgical operations, 2003–2011: a report from the National Cancer Data Base. Ann Surg Oncol. 2015;22(3):899–907. [DOI] [PubMed] [Google Scholar]
- 26.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. Journal of the National Cancer Institute. 2013;105(2):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler PD, Familusi O, Serletti JM, Fox JP. Influence of race, insurance status, and geographic access to plastic surgeons on immediate breast reconstruction rates. American journal of surgery. 2017. [DOI] [PubMed] [Google Scholar]
- 28.Butler PD, Nelson JA, Fischer JP, et al. Racial and age disparities persist in immediate breast reconstruction: an updated analysis of 48,564 patients from the 2005 to 2011 American College of Surgeons National Surgery Quality Improvement Program data sets. American journal of surgery. 2016;212(1):96–101. [DOI] [PubMed] [Google Scholar]
- 29.Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2014;24(3):e261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts MC, Weinberger M, Dusetzina SB, et al. Racial Variation in the Uptake of Oncotype DX Testing for Early-Stage Breast Cancer. J Clin Oncol. 2016;34(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeder-Hayes KE, Wheeler SB, Baggett CD, et al. Influence of provider factors and race on uptake of breast cancer gene expression profiling. Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 33.Davis BA, Aminawung JA, Abu-Khalaf MM, et al. Racial and Ethnic Disparities in Oncotype DX Test Receipt in a Statewide Population-Based Study. J Natl Compr Canc Netw. 2017;15(3):346–354. [DOI] [PubMed] [Google Scholar]
- 34.Press DJ, Ibraheem A, Dolan ME, Goss KH, Conzen S, Huo D. Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat. 2017. [DOI] [PubMed] [Google Scholar]
- 35.Berlin NL, Momoh AO, Qi J, et al. Racial and ethnic variations in one-year clinical and patient-reported outcomes following breast reconstruction. American journal of surgery. 2017;214(2):312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler PD, Nelson JA, Fischer JP, et al. African-American women have equivalent outcomes following autologous free flap breast reconstruction despite greater preoperative risk factors. American journal of surgery. 2015;209(4):589–596. [DOI] [PubMed] [Google Scholar]
- 37.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47(7):765–773. [DOI] [PubMed] [Google Scholar]
- 38.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142–150. [DOI] [PubMed] [Google Scholar]
- 39.Markossian TW, Hines RB. Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women & health. 2012;52(4):317–335. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE. Financial Impact of Breast Cancer in Black Versus White Women. Journal of Clinical Oncology. 2018;36(17):1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hertz DL, Roy S, Motsinger-Reif AA, et al. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol. 2013;24(6):1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. Journal of the National Cancer Institute. 2003;95(20):1545–1548. [DOI] [PubMed] [Google Scholar]
- 43.Wright JL, Takita C, Reis IM, Zhao W, Lee E, Hu JJ. Racial variations in radiation-induced skin toxicity severity: data from a prospective cohort receiving postmastectomy radiation. International journal of radiation oncology, biology, physics. 2014;90(2):335–343. [DOI] [PubMed] [Google Scholar]
- 44.Check DK, Reeder-Hayes KE, Basch EM, Zullig LL, Weinberger M, Dusetzina SB. Investigating racial disparities in use of NK1 receptor antagonists to prevent chemotherapy-induced nausea and vomiting among women with breast cancer. Breast Cancer Res Treat. 2016;156(2):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colleoni M, Sun Z, Price KN, et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016;34(9):927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]