Abstract
Background:
We systematically evaluated the impact of the location and burden of extranodal testicular germ cell tumor (TGCT) metastases on survival using a large, nationally representative population-based cancer registry.
Methods:
Men with stage III TGCT captured by the Surveillance, Epidemiology, and End Results registry from 2010–2015 with distant extranodal metastases were identified. Clinicopathologic information were collected, and patients were subdivided based on specific organ site(s) of metastatic involvement (lung, liver, bone, and/or brain). Kaplan-Meier analysis and multivariable Cox regression were used to evaluate cancer-specific survival (CSS), and model performance was assessed using Harrell’s C-statistic.
Results:
969 patients with stage III TGCT were included, with predominantly nonseminomatous histology (84%). Most patients (91%) had pulmonary metastases, while 20%, 10%, and 10% had liver, bone, and brain metastases, respectively. Over a median follow-up of 21 months, 19% of men died of TGCT. When grouped by primary site of metastasis, patients with more than one extrapulmonary metastasis exhibited the worst CSS (HR 4.27 (95% CI 2.60–7.00), vs. isolated pulmonary involvement, p<0.01). Among patients with isolated extrapulmonary involvement, those with brain metastases had the poorest survival (HR 3.24 (95% CI 1.98–5.28), p<0.01), followed by liver (HR 2.29 (95% CI 1.56–3.35), p<0.01) and bone (HR 1.97 (95% CI 1.11–3.50), p=0.02). Multivariable Harrell’s C-statistic was 0.71.
Conclusions:
Site of metastatic involvement impacts survival outcomes in patients with TGCT, which may reflect both the aggressive biology and challenging treatment of these tumors. Further incorporation of organotropism into current prognostic models for metastatic TGCT warrants attention.
Keywords: testicular cancer, metastasis, organotropism, outcomes, epidemiology
Precis:
Site of metastatic involvement impacts survival outcomes in patients with testicular cancer, which may reflect both the aggressive biology and challenging treatment of these tumors. Further incorporation of organotropism into current prognostic models for metastatic testicular cancer warrants attention.
INTRODUCTION
Testicular germ cell tumors (TGCT) are the most common malignancy in young men, and approximately 9,560 new cases are expected to be diagnosed in the U.S. in 2019.1 Given the multimodal strategies available to manage these tumors, patients with TGCT tend to exhibit favorable clinical outcomes, with >80% 5-year overall survival and fewer than 500 cancer-specific deaths annually in the U.S.1,2 Poor outcomes in TGCT are driven primarily by extranodal metastatic involvement (clinical stage III disease), and even among patients with metastatic disease, predictors of survival are multifactorial.3
The current staging system for testis cancer attempts to differentiate among sites of metastatic involvement by categorizing non-pulmonary metastatic disease (M1b, stage IIIC) into a higher stage compared to pulmonary or non-regional lymph node involvement (M1a, predominantly stage IIIA-B).4 This classification is based on earlier studies suggesting relatively worse outcomes in patients that have non-pulmonary metastatic sites involved, and accordingly, patients with M1b disease are categorized into a worse risk stratification according to the International Germ-Cell Cancer Cooperative Group (IGCCCG).2 Aside from the lung, the most common extranodal sites of TGCT involvement include the liver, bone, and brain; involvement of these sites—particularly the bone and brain—has been associated with worse outcomes.5–9 However, the relative impact of involved organ sites on survival among patients with M1b disease has not been systematically assessed.
Herein, we systematically evaluate the impact of the location and burden of extranodal TGCT metastases on survival using a large, nationally representative, population-based cancer registry. Elucidating the specific impact of organotropism on survival outcomes may help refine current prognostic models for metastatic TGCT and provide insight into the heterogeneous behavior of these tumors.
METHODS
Patient Population, Variables, and Outcomes
A cohort of men with stage III TGCT from 2010–2015 captured by the Surveillance, Epidemiology, and End Results (SEER) Program were identified. Men with sites of known distant extranodal metastasis (lung, bone, liver, or brain) were included (M1a or M1b). Extragonadal GCT, patients under 16 years of age, and those without distant metastasis were excluded. Demographic and clinical data included age, race, year of diagnosis, histology (seminoma or nonseminoma (NSGCT)), laterality, and staging (AJCC, 7th Edition) classified as IIIA, IIIB, IIIC, or III not otherwise specified (NOS). The independent variable of interest was site of distant metastasis. The primary outcome of interest was cancer-specific survival (CSS; death due to testicular cancer).
Statistical Analysis
Baseline characteristics were tabulated for included patients. Univariable and multivariable Cox proportional hazards regression models were constructed to evaluate the impact of each variable on CSS. The relative impact of metastasis to the lung, liver, bone, and brain was assessed. Patients were further categorized into five groups of interest by their primary site of metastasis including the following: lung metastasis only, bone metastasis (with or without lung metastasis), liver metastasis (with or without lung metastasis), brain (with or without lung metastasis), and multiple non-pulmonary sites of metastasis. Model performance was assessed by computing Harrell’s C-statistic. Kaplan-Meier curves were created to visualize the five groups of interest, and survival probabilities were tabulated at 2 years and 3 years for CSS. Statistical analyses were performed using STATA v.15.0 (STATA Corp, College Station, TX, 2017) with two-sided alpha set to 0.05.
RESULTS
Cohort
A total of 969 patients diagnosed with stage III TGCT with distant metastasis were included. The median age was 29 years (IQR 23–38). The majority of patients were white (55.2%) or Hispanic (34.9%), and the predominant histology was NSGCT (84.2%) (Table 1). In this cohort with high metastatic burden, most patients had distant metastasis to the lung (90.5%), while approximately 20% of patients had metastasis to the liver and 10% each had bone and brain metastases.
Table 1.
Demographics and sites of distant metastases for included patients with stage III primary testicular germ cell tumors. Surveillance, Epidemiology, and End Results Program 2010–2015.
| Overall | |||
|---|---|---|---|
| Value | (% or IQR) | ||
| N | 969 | - | |
| Age, years (median) | 29 | 23–38 | |
| Race | White | 535 | 55.2% |
| Black | 38 | 3.9% | |
| Hispanic | 338 | 34.9% | |
| Asian | 39 | 4.0% | |
| Native1 | 18 | 1.9% | |
| Unknown | 1 | 0.1% | |
| Year of Diagnosis | 2010 | 146 | 15.1% |
| 2011 | 167 | 17.2% | |
| 2012 | 152 | 15.7% | |
| 2013 | 192 | 19.8% | |
| 2014 | 164 | 16.9% | |
| 2015 | 148 | 15.3% | |
| Laterality | Right | 480 | 49.5% |
| Left | 433 | 44.7% | |
| Unknown | 56 | 5.8% | |
| Histology | Seminoma | 153 | 15.8% |
| NSGCT | 816 | 84.2% | |
| Metastasis | Lung | 877 | 90.5% |
| Liver | 203 | 20.9% | |
| Bone | 94 | 9.7% | |
| Brain | 95 | 9.8% | |
| Stage | III NOS | 232 | 23.9% |
| IIIA | 196 | 20.2% | |
| IIIB | 94 | 9.7% | |
| IIIC | 447 | 46.1% | |
Native American or Alaskan
NOS = not otherwise specified; SD = standard deviation
Impact of Individual Metastatic Sites on CSS
Over a median follow-up of 21 months (IQR 8–43), 185 (19.1%) men were confirmed to have died due to testicular cancer, while 225 (23.2%) died of any cause. In total, 23 (2.4%) were confirmed as deaths due to non-testis cancer causes while 17 (1.8%) were not definitively confirmed as deaths either related or unrelated to testis cancer (Supplemental Table 1). Increasing age was a statistically significant predictor for worse CSS, while histology, year of diagnosis, and lymphovascular invasion were not associated with CSS (Table 2). Stage IIIC disease was strongly associated with CSS on multivariable analysis (HR 5.17 (95% CI 2.83–9.42), p<0.01). On assessment of individual sites of metastasis, metastasis to the brain appeared to have the greatest association with poor CSS among included sites (HR 2.49 (95% CI 1.69–3.66), p<0.01) followed by liver and bone. Harrell’s C-statistic for multivariable models ranged between 0.71 and 0.73.
Table 2.
Survival analysis evaluating impact of stage and metastatic sites on cancer-specific survival among included stage III primary testicular germ cell tumor patients. Model 1 Harrell’s C 0.723. Model 2 Harrell’s C 0.714. Surveillance, Epidemiology, and End Results Program 2010–2015.
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||||||
| Univariable | Multivariable Model 11 | Multivariable Model 21 | |||||||||||
| Age | 1.03 | 1.02 | 1.04 | <0.01 | 1.03 | 1.01 | 1.04 | <0.01 | 1.03 | 1.01 | 1.04 | <0.01 | |
| Histology | NSGCT | REF | - | - | - | REF | - | - | - | REF | - | - | - |
| Seminoma | 0.92 | 0.61 | 1.38 | 0.68 | 0.59 | 0.13 | 0.38 | 0.92 | 0.73 | 0.47 | 1.14 | 0.16 | |
| Metastasis2 | Lung | 0.85 | 0.53 | 1.35 | 0.49 | - | - | - | - | 1.38 | 0.83 | 2.30 | 0.21 |
| Liver | 2.29 | 1.69 | 3.10 | <0.01 | - | - | - | - | 2.04 | 1.46 | 2.84 | <0.01 | |
| Bone | 1.94 | 1.29 | 2.92 | <0.01 | - | - | - | - | 1.62 | 1.04 | 2.53 | 0.03 | |
| Brain | 2.79 | 1.95 | 4.01 | <0.01 | - | - | - | - | 2.49 | 1.69 | 3.66 | <0.01 | |
| Stage | IIIA | REF | - | - | - | REF | - | - | - | - | - | - | - |
| IIIB | 2.74 | 1.28 | 5.86 | 0.01 | 3.10 | 1.44 | 6.66 | <0.01 | - | - | - | - | |
| IIIC | 5.63 | 3.12 | 10.17 | <0.01 | 5.17 | 2.83 | 9.42 | <0.01 | - | - | - | - | |
| III NOS | 1.82 | 0.92 | 3.60 | 0.08 | 1.64 | 0.82 | 3.27 | 0.16 | - | - | - | - | |
Multivariable Cox-proportional hazards models also adjusted for race, diagnosis year, laterality, and presence of lymphovascular invasion.
HR = hazard ratio, 95% CI = 95% confidence interval
Reference group for each organ site corresponds to the lack of involvement of the corresponding organ
Comparison of CSS by Primary Site of Metastasis
When grouped by primary site of metastasis, patients with primary brain metastasis were confirmed to have the worst prognosis (HR 3.24 (95% CI 1.98–5.28), p<0.01), while patients with liver and bone metastasis appeared to have similar CSS (Table 3). Harrell’s C-statistic was 0.71 for the multivariable model. Brain metastasis was associated with presence of choriocarcinoma as the primary histology (41.1% of patients with brain metastasis vs. 11.1% without brain metastasis, p<0.01). On a sensitivity analysis of histology, both brain metastasis (HR 2.98 (95%CI 1.79–4.95), p<0.01) and presence of choriocarcinoma (HR 1.86 (95% CI 1.09–3.19, p=0.02) remained statistically significant predictors of CSS in a multivariable model (Supplemental Table 2).
Table 3.
Survival analysis comparing groups by primary site of distant metastasis for cancer-specific survival among included stage III primary testicular germ cell tumor patients. Harrell’s C 0.713. Surveillance, Epidemiology, and End Results Program 2010–2015.
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||||
| Univariable | Multivariable1 | ||||||||
| Age | 1.03 | 1.02 | 1.04 | <0.01 | 1.03 | 1.01 | 1.04 | <0.01 | |
| Histology | NSGCT | REF | - | - | - | REF | - | - | - |
| Seminoma | 0.92 | 0.61 | 1.38 | 0.68 | 0.65 | 0.42 | 1.02 | 0.06 | |
| Metastasis | Lung Only | REF | - | - | - | REF | - | - | - |
| Liver (+/− Lung) | 2.40 | 1.65 | 3.48 | 0.01 | 2.29 | 1.56 | 3.35 | <0.01 | |
| Bone (+/− Lung) | 2.10 | 1.21 | 3.64 | <0.01 | 1.97 | 1.11 | 3.50 | 0.02 | |
| Brain (+/− Lung) | 3.21 | 1.99 | 5.18 | <0.01 | 3.24 | 1.98 | 5.28 | <0.01 | |
| Multiple Nonpulmonary Sites | 4.94 | 3.13 | 7.79 | <0.01 | 4.27 | 2.60 | 7.00 | <0.01 | |
Multivariable Cox-proportional hazards models also adjusted for race, diagnosis year, laterality, and presence of lymphovascular invasion.
HR = hazard ratio, 95% CI = 95% confidence interval
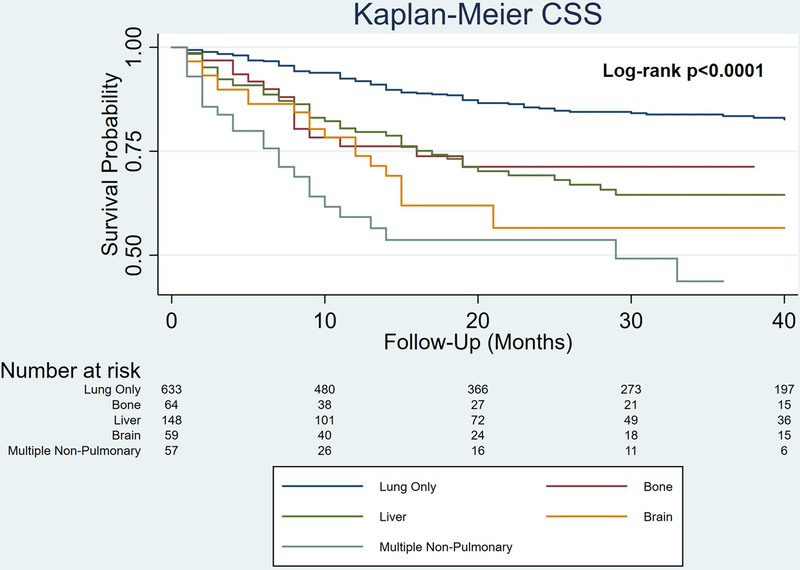
Kaplan-Meier curves estimated 3-year CSS of 83.5% for lung only, 71.3% for bone, 64.5% for liver, 56.6% for brain, and 43.7% for multiple non-pulmonary sites (log-rank p<0.0001; Figure 1). The latter group included 52 patients with 2 non-pulmonary sites and 5 patients with all 3 non-pulmonary sites.
Figure 1.
Kaplan-Meier survival curves of cancer-specific survival stratified by site of distant metastases for men with stage III primary testicular germ cell tumors (log-rank p<0.0001). Surveillance, Epidemiology, and End Results Program 2010–2015.
DISCUSSION
The distribution of metastatic involvement has been shown to carry prognostic value in several malignancies.10–17 The tendency of a tumor to metastasize to a particular organ site may reflect an interplay between the underlying biology of the tumor cells and a permissive host organ microenvironment.18–21 In the setting of TGCT, involvement of extranodal non-pulmonary sites is traditionally associated with poor outcomes.2 Here, we have systematically evaluated a large, nationally representative, population-based cancer registry to evaluate the impact of the specific location and extent of metastases on survival in patients with TGCT using multivariable regression modeling. We found that among stage III patients with only one non-pulmonary site involved, those harboring brain metastases exhibited the worst survival, followed by those with liver or bone involvement, which appeared to have a more similar impact on CSS. Consistent with current staging schema,4 patients with isolated pulmonary involvement tended to exhibit the best survival outcomes. Furthermore, patients with involvement of more than one non-pulmonary site had worse outcomes than those with only one non-pulmonary metastasis, including the brain.
While metastasizing tumor clones from testicular cancer tend to follow a predictable pattern of lymphatic drainage to the retroperitoneum,22 hematogenous routes enabling spread beyond the retroperitoneum, or even occasionally skipping the retroperitoneum altogether,23 facilitate colonization of solid organ sites. For reasons that are not understood, when the lung is the only organ involved, outcomes appear to be superior to cases involving either isolated or concomitant metastasis to other organ sites. The tropic pattern of spread may conceivably reflect the aggressiveness of the tumor biology, though importantly, the lack of a clear consensus on the optimal management strategy for extranodal metastases and the frequently resistant nature of these tumor clones to conventional therapies may contribute to the decreased survival observed.5–9,24,25
In a recent analysis of 1,594 patients with GCT who experienced treatment failure with cisplatin-based frontline chemotherapy, the International Prognostic Factors Study Group attempted to construct a prognostic model to guide salvage strategies.5 Notably, 50%, 17%, 7%, and 13% of their cohort exhibited lung, liver, bone, and brain metastases, respectively. On combining liver, bone, and brain metastases into a composite variable (LBB), they identified LBB as an independently poor prognostic feature on multivariable analysis for patients with NSGCT and found it to be the only significant predictor in their seminoma cohort. However, the influence of each involved organ site on outcome was not independently assessed.
Studies evaluating unique cohorts of GCT patients exhibiting brain metastases have reported on the attempted use of multimodal therapies that include, often, some combination of chemotherapy, radiotherapy, and surgery,6,24 yet the benefit of utilizing a multimodal approach was more evident among patients with brain metastases at relapse rather than at initial diagnosis.6 As in our cohort, brain metastases usually coexist with pulmonary metastases,6,24 and the presence of multiple brain metastases and concomitant involvement of liver or bone metastases were found to be independent adverse prognostic features.6 While we did not have data on the number of brain metastases present in our SEER cohort, we similarly noted that a higher burden of non-pulmonary metastases conferred worse outcomes. Attesting to the poor outcomes of patients with brain metastases, Feldman et al. reported that over 50% of patients with brain metastases experience disease progression and death within one year of identifying intracranial involvement.6 They note that these poor outcomes are likely driven, in large part, by chemoresistance, given that mortality in these patients is frequently due to systemic progression rather than uncontrolled brain metastases.
The optimal management of bone and liver metastases from TGCT also remains challenging without a clear consensus.7–9,25 The role for surgery in managing hepatic metastases has been debated,9,25 and the advantage of multimodal treatment for bone metastases has not been clearly demonstrated.7 In fact, Oing et al. reported that the outcomes of NSGCT patients with bone metastases were even worse than expected for a typical IGCCCG poor risk cohort.7 Furthermore, they noted that while the presence of concomitant liver or brain metastases was significant for poor progression-free survival on univariable analysis, significance was lost on multivariable analysis, and seminomatous histology was found to be the strongest independent predictor for favorable outcomes, which was not reflected in our analysis. Their cohort was notably limited by a small sample size and included patients with primary mediastinal and retroperitoneal GCT in addition to TGCT.
Limitations inherent to the use of the SEER registry, including its retrospective nature, selection bias, and missing data for certain variables of interest, such as serum tumor markers and the number and size of metastatic lesions, must be considered in the context of our findings. Patients with stage III disease driven by elevated serum tumor markers only (cM0S2–3) were also not included for analysis. While a prior study noted TNM staging errors for GCT in SEER, this was largely driven by the S and N categories, which are avoided in the present analysis;26 the present cohort includes only M1a and M1b patients with identified sites of distant metastasis and excludes node-only disease. More recent studies have also relied on extent of disease variables to recreate TNM staging for early-stage GCT.27,28 Most cases of extrapulmonary metastases were confounded by concomitant pulmonary involvement in the majority of patients. Involvement of other organ sites beyond the lung, liver, bone, and brain were also not captured in SEER, though for TGCT, these are typically the most common sites involved. Treatment strategies for patients with metastatic TGCT, including chemotherapy agents which are not captured by SEER, vary considerably among practitioners and would conceivably influence outcomes in our cohort; however, this heterogeneity reflects the challenges and lack of guidelines in managing these patients. Nonetheless, our study is strengthened by its large population-based cohort, representative of national practice patterns across multiple centers.
CONCLUSION
We have systematically evaluated the impact of the location and extent of extranodal metastases on survival in patients with TGCT using a large, nationally representative, population-based cancer registry. Patients with more than one extrapulmonary site involved exhibit the worst outcomes, and among patients with isolated extrapulmonary involvement, those with brain metastases tend to have the poorest survival. These outcomes may reflect both the aggressive biology of these tumors and the challenging nature of treating these patients. Further incorporation of organotropism into current prognostic models for metastatic TGCT warrants attention.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support and sponsorship: This work was supported, in part, by the Ruth L. Kirschstein National Research Service Award T32 CA136515–09 (N.S.), the University of Texas Southwestern Medical Center Physician Scientist Training Program (N.S.), and Dedman Family Scholarship in Clinical Care (A.B).
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15(2):594–603. [DOI] [PubMed] [Google Scholar]
- 3.Woldu SL, Matulay JT, Clinton TN, et al. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urol Oncol. 2018;36(1):14 e17–14 e15. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Stage for TNM Edition 7. American Joint Committee for Cancer Staging. 2010. [Google Scholar]
- 5.International Prognostic Factors Study G, Lorch A, Beyer J, et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol. 2010;28(33):4906–4911. [DOI] [PubMed] [Google Scholar]
- 6.Feldman DR, Lorch A, Kramar A, et al. Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol. 2016;34(4):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oing C, Oechsle K, Necchi A, et al. Impact of primary metastatic bone disease in germ cell tumors: results of an International Global Germ Cell Tumor Collaborative Group G3 Registry Study. Ann Oncol. 2017;28(3):576–582. [DOI] [PubMed] [Google Scholar]
- 8.Jamal-Hanjani M, Karpathakis A, Kwan A, et al. Bone metastases in germ cell tumours: lessons learnt from a large retrospective study. BJU Int. 2013;112(2):176–181. [DOI] [PubMed] [Google Scholar]
- 9.Copson E, McKendrick J, Hennessey N, Tung K, Mead GZ. Liver metastases in germ cell cancer: defining a role for surgery after chemotherapy. BJU Int. 2004;94(4):552–558. [DOI] [PubMed] [Google Scholar]
- 10.Bowman IA, Bent A, Le T, et al. Improved Survival Outcomes for Kidney Cancer Patients With Brain Metastases. Clin Genitourin Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol. 2015;68(2):325–334. [DOI] [PubMed] [Google Scholar]
- 12.Gerratana L, Fanotto V, Bonotto M, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32(2):125–133. [DOI] [PubMed] [Google Scholar]
- 13.Guijarro A, Hernandez V, de la Morena JM, et al. Influence of the location and number of metastases in the survival of metastatic prostatic cancer patients. Actas Urol Esp. 2017;41(4):226–233. [DOI] [PubMed] [Google Scholar]
- 14.Kalra S, Atkinson BJ, Matrana MR, et al. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 2016;117(5):761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgenstern DA, London WB, Stephens D, et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database. Eur J Cancer. 2016;65:1–10. [DOI] [PubMed] [Google Scholar]
- 16.Ording AG, Heide-Jorgensen U, Christiansen CF, Norgaard M, Acquavella J, Sorensen HT. Site of metastasis and breast cancer mortality: a Danish nationwide registry-based cohort study. Clin Exp Metastasis. 2017;34(1):93–101. [DOI] [PubMed] [Google Scholar]
- 17.Singla N, Choi J, Onabolu O et al. Comprehensive molecular and genomic characterization of pancreatic tropism in metastatic renal cell carcinoma. J Clin Oncol. 2019;37(7_suppl):633. [Google Scholar]
- 18.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. [DOI] [PubMed] [Google Scholar]
- 19.Obenauf AC, Massague J. Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer. 2015;1(1):76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paget S The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 21.Smith HA, Kang Y. Determinants of Organotropic Metastasis. Annual Review of Cancer Biology. 2017;1(1):403–423. [Google Scholar]
- 22.Donohue JP, Zachary JM, Maynard BR. Distribution of nodal metastases in nonseminomatous testis cancer. J Urol. 1982;128(2):315–320. [DOI] [PubMed] [Google Scholar]
- 23.Weems WL. Hematogenous metastasis of nonseminomatous germ cell testicular cancer. Urology. 1985;26(6):583–584. [DOI] [PubMed] [Google Scholar]
- 24.Oechsle K, Kollmannsberger C, Honecker F, Boehlke I, Bokemeyer C. Cerebral metastases in non-seminomatous germ cell tumour patients undergoing primary high-dose chemotherapy. Eur J Cancer. 2008;44(12):1663–1669. [DOI] [PubMed] [Google Scholar]
- 25.Pietzak EJ, Assel M, Becerra MF, et al. Histologic and Oncologic Outcomes Following Liver Mass Resection With Retroperitoneal Lymph Node Dissection in Patients With Nonseminomatous Germ Cell Tumor. Urology. 2018;118:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faber KD, Carlos MC, Cortessis VK, Daneshmand S. Validation of Surveillance, Epidemiology, and End Results TNM staging for testicular germ cell tumor. Urol Oncol. 2014;32(8):1341–1346. [DOI] [PubMed] [Google Scholar]
- 27.Patel HD, Joice GA, Schwen ZR, et al. Retroperitoneal lymph node dissection for testicular seminomas: population-based practice and survival outcomes. World J Urol. 2018;36(1):73–78. [DOI] [PubMed] [Google Scholar]
- 28.Patel HD, Srivastava A, Alam R, et al. Radiotherapy for stage I and II testicular seminomas: Secondary malignancies and survival. Urol Oncol. 2017;35(10):606 e601–606 e607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.