Abstract
Threat-related emotional function is supported by a neural circuit that includes the prefrontal cortex (PFC), hippocampus, and amygdala. The function of this neural circuit is altered by negative life experiences, which can potentially affect threat-related emotional processes. Notably, Black-American individuals disproportionately endure negative life experiences compared to White-American individuals. However, the relationships among negative life experiences, race, and the neural substrates that support threat-related emotional function remains unclear. Therefore, the current study investigated whether the brain function that supports threat-related emotional processes varies with racial differences in negative life experiences. In the present study, adolescent violence exposure, family income, and neighborhood disadvantage were measured prospectively (i.e., at 11–19 years of age) for Black-American and White-American volunteers. Participants then, as young adults (i.e., 18–23 years of age), completed a Pavlovian fear conditioning task during functional magnetic resonance imaging (fMRI). Cued and non-cued threats were presented during the conditioning task and behavioral (threat expectancy) and psychophysiological responses (skin conductance response; SCR) were recorded simultaneously with fMRI. Racial differences were observed in neural (e.g., fMRI activity), behavioral (e.g., threat expectancy), and psychophysiological (SCR) responses to threat. These threat-elicited responses also varied with negative life experiences (violence exposure, family income, and neighborhood disadvantage). Notably, racial differences in brain activity to threat were smaller after accounting for negative life experiences. The present findings suggest that racial differences in the neural and behavioral response to threat are due, in part, to exposure to negative life experiences and may provide new insight into the mechanisms underlying racial disparities in mental health.
Keywords: Fear, Brain imaging, Race differences, Social neuroscience, Health disparities, Stress
Introduction
Within the United States, there is a stark racial disparity in negative life experiences between Black-American and White-American individuals (DeNavas-Walt, Proctor, & Smith, 2015; Schuster et al., 2012; Slopen et al., 2016; Williams & Collins, 2001; Zimmerman & Messner, 2013). Specifically, Black-American individuals are more often exposed to violence (Zimmerman & Messner, 2013), have lower income (DeNavas-Walt et al., 2015), and live in disproportionally disadvantaged neighborhoods (Williams & Collins, 2001) compared to White-American individuals. Negative life experiences are associated with neurobiological changes that include alterations of prefrontal cortex (PFC), hippocampus, and amygdala activity (Kim et al., 2013; McCrory et al., 2011; Sripada et al., 2014). These brain regions support threat-related processes that have an important impact on emotional function (Goodman, Harnett, & Knight, 2018). Therefore, there may be racial differences in the neural activity that supports threat-related emotional function, which may stem from racial differences in exposure to negative life events during development. A better understanding of racial differences in the neural activity that supports threat-related emotional function may provide new insight into racial disparities in mental health (Alvarez et al., 2018; Kessler et al., 2005; McLaughlin et al., 2007; Vilsaint et al., 2019).
Threat-related emotional processes can be studied using Pavlovian fear conditioning procedures. During Pavlovian fear conditioning, an innocuous cue (conditioned stimulus; CS) is paired with a naturally aversive threat (unconditioned stimulus; UCS) that produces an innate threat-elicited emotional response (unconditioned response; UCR). As the innocuous cue and aversive threat are repeatedly paired, individuals learn that the cue predicts the threat. Thus, the cue becomes a warning signal (i.e., a CS), which is evidenced by expression of a conditioned response (CR) to the warning signal in anticipation of the aversive threat. Importantly, development of the association between the warning signal and the threat also produces changes in the threat-elicited emotional response (Baxter, 1966; Knight et al., 2011; Rust, 1976). Specifically, the threat-elicited response is diminished when the warning signal precedes the aversive threat (i.e., the threat is cued or predictable) compared to when the aversive threat is presented alone or follows a safety signal (i.e., the threat is not cued or unpredictable) (Knight et al., 2011; Wood, Ver Hoef, & Knight, 2012). Thus, diminution of the threat-elicited response provides a method for studying emotion regulation (Goodman et al., 2018a). Prior work has demonstrated that threat-related responses are supported by brain regions that include the PFC, inferior parietal lobule (IPL), insula, hippocampus, and amygdala (Dunsmoor et al., 2008; Fullana et al., 2016; Goodman et al., 2018b; Knight et al., 2010; Linnman et al., 2011; Patrick et al., 2019; Wheelock et al., 2014; Wood et al., 2014; Wood et al., 2013; Wood et al., 2012). In particular, the amygdala appears to be critical for expression of the peripheral emotional response (Cheng et al., 2006; Cheng et al., 2003; Ganella et al., 2018; Harnett et al., 2015; Knight et al., 2005; LeDoux et al., 1988; Maren, 2001; Marin et al., 2019). Further, components of the PFC (i.e., dorsolateral, dorsomedial, and ventromedial PFC) support detection and regulation processes that modulate the amygdala-mediated response to threat (Atlas et al., 2014; Delgado et al., 2008; Goodman et al., 2018b, Motzkin et al., 2015; Urry et al., 2006). Taken together, these brain regions are critical for the regulation and expression of threat-related emotional responses.
Notably, neural activity within brain regions that are associated with emotional processes appear to vary with negative life events such as violence exposure and poverty (Kim et al., 2013; McCrory et al., 2011; Sripada et al., 2014). For example, prior functional magnetic resonance imaging (fMRI) research has observed that exposure to violent media (e.g., movies) is associated with decreased pain empathy-related activity within the dorsomedial PFC and insula (Guo et al., 2013). Further, exposure to familial violence in childhood is associated with greater amygdala and insula activation to angry faces (McCrory et al., 2011). In addition, early life poverty is associated with decreased activity within dorsolateral and ventrolateral PFC during emotion-regulation (Kim et al., 2013). Thus, violence exposure and early life poverty appear to be linked to neural changes that enhance emotional expression and reduce emotion regulation. Importantly, exposure to violence and early life poverty are often observed in disadvantaged communities (Aisenberg & Herrenkohl, 2008). Prior research has found that neighborhood disadvantage is linked to both adolescent stress reactivity (Hackman et al., 2012) and childhood mental health (Xue et al., 2005). Therefore, neighborhood disadvantage may also have an impact on neural activity within brain regions that support threat-related emotional processes. Taken together, exposure to negative life experiences appears to modulate neural activity within brain regions that support threat-related emotional function.
Importantly, negative life experiences (e.g., violence exposure, poverty, and neighborhood disadvantage) are disproportionately experienced by Black-American individuals (DeNavas-Walt et al., 2015; Williams & Collins, 2001; Zimmerman & Messner, 2013). This exposure to negative life experiences may play an important role in racial health disparities that have been observed in prior work (Anderson & Mayes, 2010; McLaughlin et al., 2007; Wallander et al., 2012). Given the racial disparities in exposure to negative life events, Black-American and White-American individuals likely exhibit differences in brain activity within regions that mediate threat-related emotional function. However, limited research has investigated potential racial differences in the brain function that supports threat-related emotional processes. Thus, investigating race-related differences in neural reactivity to threat is important for our understanding of the neural consequences of negative life experiences.
The present study investigated racial differences in the neural substrates of threat-related emotional function. Specifically, we investigated racial differences in threat-elicited brain activity, behavior, and psychophysiology during Pavlovian fear conditioning. Given the racial disparities in negative life experiences, we hypothesized Black-American and White-American participants would show differences in the magnitude of threat-elicited behavioral and psychophysiological responses. In addition, we hypothesized that brain activity within regions that support threat-related emotional processes would differ between Black-American and White-American individuals. Specifically, we hypothesized neural activity within the dorsolateral PFC, dorsomedial PFC, ventromedial PFC, hippocampus, and amygdala would differ between Black-American and White-American participants. Further, we hypothesized that racial differences in threat-elicited brain and behavioral responses would also vary with exposure to negative life experiences during development, and that accounting for negative life experiences would diminish racial differences in threat-elicited brain and behavioral responses. The present study extends our understanding of the racial differences in threat-related brain and emotional processes and provides new insights that support our understanding of racial disparities in mental health.
Methods
Participants
One-hundred and ninety-eight right-handed young adults (Age: 20.73 ± 1.35 SD) from the Birmingham-Metropolitan area participated in the present study (143 Black-American, 55 White-American; 98 Male, 100 Female). All participants previously participated, as part of a larger Birmingham-Metropolitan area cohort (n = 1,594), in the Healthy Passages study, a multisite longitudinal study of adolescent health (Schuster et al., 2012; Windle et al., 2004). Participants were initially recruited from 5th grade classrooms in local public schools and interviewed along with their primary caregivers at three time points (T1 Age: 11.17 ± 0.46, T2 Age: 13.03 ± 0.46, T3 Age: 16.20 ± 0.47, Mean ± SD). An additional T4 interview was conducted with the youth only (T4 Age: 19.59 ± 1.13 years) as part of the present study. Neuroimaging was completed shortly after T4 on ~12% of the initial Healthy Passages cohort. Given only a fraction of the initial cohort completed neuroimaging, we assessed demographic differences between participant groups who did and did not complete the neuroimaging session. There was no difference in the percentage of male participants compared to female participants who did (49% Male) versus did not complete (50% Male) neuroimaging [χ2(1) = 0.03, p = 0.86]. A greater percentage of Black-American than White-American participants completed (72% Black-American) neuroimaging than did not (58% Black-American) [χ2(1) = 14.88, p < 0.001]. Violence exposure (see below for more information) at T1 did not differ between the participant group that did (M = 1.06, SD = 1.03) versus the participant group that did not (M = 0.98, SD = 0.99) complete the neuroimaging session [t(1483) = 1.08, p = 0.28]. Family income (see below for more information) at T1 differed between the participant group that did (M = 225.40, SD = 254.30) compared to participant group that did not (M = 286.61, SD = 300.71) complete the neuroimaging session [t(270.20) = −3.03, p = 0.003, adjusted for unequal variance]. Neighborhood disadvantage (see below for more information) at T1 differed between the participant group that did (M = 0.17, SD = 0.93) compared to the participant group that did not (M = −0.02, SD = 1.00) complete the neuroimaging session [t(267.26) = 2.71, p = 0.007, adjusted for unequal variance]. All participants in the present study provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Stimuli
Warning and safety signals (i.e., CS) (10 s duration) consisted of two pure tones (700 Hz and 1300 Hz). The warning signal (CS+) co-terminated with the threat (i.e., UCS) (100-dB white noise, 0.5 s duration; 100% pairing rate), while the safety signal (CS−) was presented without the threat (i.e., 0% pairing rate). The tones that served as the warning and safety signal were counter-balanced across participants. In addition, the threat was presented alone (UCS alone) on some trials. A total of 72 trials (18 s inter-trial interval) were presented across two fMRI scans (36 trials per scan; 12 CS+, 12 CS−, 12 UCS alone trials). Stimuli were presented in a pseudorandom order such that no more than two trials of any stimulus (CS+, CS−, and UCS alone) were presented consecutively. The current study investigated racial differences in the emotional response to threat. Thus, all analyses focused on responses to cued threat (i.e., UCS paired with the CS+; CS+UCS) and non-cued threat (i.e., UCS presented alone; UCS alone). Responses to the warning signal (CS+) and safety signal (CS−) were not the focus of the present study and will be reported separately.
Threat expectancy
Threat expectancy was measured continuously (40 Hz sampling rate) throughout the conditioning session using methods described in prior work (Knight & Wood, 2011). A rating scale ranging from 0 to 100 was displayed on an IFIS-SA LCD monitor (InVivo Corp.; Gainesville, FL) using Presentation software (NeuroBehavioral Systems, Inc.; Albany, CA). Participants viewed the screen through a mirror attached to the head coil. Participants rated their expectancy of the threat using a magnetic resonance imaging (MRI) compatible joystick (Current Designs; Philadelphia, PA) on a scale of 0 (certain the threat would not be presented) to 100 (certain the threat would be presented). A rating of 50 indicated that the participant was unsure whether or not the threat would be presented. Threat expectancy was calculated as the average expectancy rating during the 1 s prior to threat (i.e., UCS) presentation.
Skin conductance response
Skin conductance response (SCR) data were collected using an MRI compatible Biopac MP150 data acquisition system (Biopac Systems; Goleta, CA). SCR data were sampled (10 kHz) from the thenar and hypothenar prominence of the left hand. Analyses were performed offline using Biopac AcqKnowledge 4.1 software as described previously (Knight & Wood, 2011). In short, a 1 Hz low pass filter was applied, and data were resampled at 250 Hz. An additional 0.5 Hz low pass filter was applied prior to resampling when data were collected on the Siemens Prisma system, due to differences in the radio frequency artefacts produced by the Siemens Allegra and Prisma scanners (see Functional MRI section for additional neuroimaging details). Threat-elicited (i.e., unconditioned) SCRs (during the 10 s after UCS presentation) were calculated as the difference in the participants’ skin conductance level at response onset (i.e., the onset of response itself and not the UCS presentation) from the peak skin conductance during the response. The onset of the response is often delayed by about 2 seconds after stimulus presentation (i.e., stimulus onset) (Boucsein et al., 2012). Response onset reflects the initial increase from baseline skin conductance level. During trials wherein there was a) no response, b) a response smaller than 0.05 uS, or c) a decrease in skin conductance, a value of 0 was assigned for that trial, as is typical in the analysis of SCR data (Boucsein et al., 2012). On average, about 18.5 trials were scored as zeros. A Shapiro-Wilks’ test for normality revealed SCR data were not normally distributed (WCS+UCS = 0.51, p < 0.001; WUCS alone = 0.48, p < 0.001). Thus, SCR data were square-root transformed prior to statistical analyses. Although the square-root transformation did not result in a normal distribution, the distribution was marginally improved (Wcs+ucs = 0.76, p < 0.001; Wucs alone = 0.77, p < 0.001) and transformed data were used for statistical analyses.
Violence Exposure
Violence exposure was assessed for each participant at four time points (T1–4; described above). At T1-T3, participants provided the information along with their primary caregiver. At T4, participants provided the information alone. At each time point, participants were asked how frequently within the past twelve months they had witnessed 1) threat of violence, 2) physical violence, and 3) threat or physical violence involving a weapon. Participants were also asked how frequently they had been victimized within the past twelve months by 1) threat of violence, 2) physical violence, 3) threat or violence involving a weapon, and 4) violent injury requiring medical treatment. Participants rated items for both victimization and witnessing on a four-point frequency scale (0, “Never”; 1, “Once”; 2, “A few times”; and 3, “Many times”). The responses were averaged separately for witnessing and victimization, and the summed total score was used as a measure of violence exposure at each of the four time points. The average score of all four time points was used as an index of violence exposure in the present study.
Family Income
Family income was reported at T1-T3 by primary caregivers. Family income was then transformed into a percentage of the federal poverty line (FPL) for the caregiver-reported household size. Income as a percentage of the FPL was averaged across T1-T3 to provide an overall index of family income during childhood and adolescence. Family income was entirely unavailable for one participant. Thus, this participant was excluded from analyses involving family income.
Neighborhood disadvantage
Neighborhood disadvantage scores were computed for each participant using geocoded addresses from the T1 assessment and block-level (~600–3000 residents) data from the 2000 United States census. Specifically, a factor score was derived from a principal component analysis of the following neighborhood variables loading on a single dimension: percentage of households below the FPL, percentage of single-parent families, percentage of adult residents without a high-school diploma, percentage of unemployed adults, percentage of non-Hispanic Black-American residents, as well as median household income and percentage of owner-occupied housing units, which had negative loadings (absolute loading values ranged from 0.71 to 0.93).
Functional MRI
MRI scans were completed on both a 3T Siemens Allegra (n = 145) scanner and a 3T Siemens Prisma (n = 53) scanner. High resolution anatomical scans (MPRAGE) were collected in the sagittal plane [Allegra: (TR = 2300 ms, TE = 3.90 ms, flip angle = 12°, FOV = 25.6 cm, matrix = 256×256, slice thickness = 1 mm, 0.5 mm gap); Prisma: (TR = 2300 ms, TE = 2.98 ms, flip angle = 9°, FOV = 25.6 cm, matrix = 256×256, slice thickness = 1 mm, 0.5 mm gap)]. The blood-oxygen-level-dependent (BOLD) fMRI signal was measured with a gradient-echo echoplanar pulse sequence in an oblique axial orientation [Allegra: (TR = 2000 ms, TE = 30 ms, flip angle = 70°, FOV = 24 cm, matrix = 64×64, voxel size = 3.75 × 3.75 × 4 mm, no gap); Prisma: (TR = 2000 ms, TE = 30 ms, flip angle = 70°, FOV = 24 cm, matrix = 64×64, voxel size = 3.75 × 3.75 × 4 mm, no gap)]. Data analyses were performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Echoplanar image time-series data were corrected for slice-timing offset, motion corrected, spatially smoothed with a 4 mm full-width-at-half-maximum Gaussian filter, concatenated, and coregistered with the structural image.
Functional maps were generated at the individual participant level using a multiple linear regression. Volumes in which there was a high amount of motion (i.e., any volumes wherein greater than 3% of voxels were considered outliers using AFNI’s 3dToutcount) were censored. On average, 97% of volumes were retained per participant. The fMRI signal was modeled with a gamma variate hemodynamic response function with reference waveforms for all stimuli (i.e., CS+, CS−, CS+UCS, UCS alone) and nuisance regressors to account for participant head motion and joystick movement. Joystick motion was modeled by identifying TRs wherein the location of the threat expectancy rating bar moved more than 10 points (51 pixels) on the threat expectancy scale between the current and previous TR. The resulting vector was transformed to a stick function convolved with a gamma variate hemodynamic response function. Regressors of interest for the fMRI analysis modeled the UCRs to the CS+UCS and the UCS alone. Percent signal change was used as an index of the amplitude of the fMRI signal response to stimulus presentation. Data were then normalized to the Talairach and Tournoux stereotaxic coordinate system (Talairach & Tournoux, 1988) and resampled to 1 mm3 resolution.
Statistical Analyses
Statistical analyses were completed using AFNI and IBM SPSS Statistics Version 24 (IBM Corp., Armonk, NY). Demographic data for Black-American and White-American participants were compared using independent samples t-tests and chi-square tests. Analyses of covariance (ANCOVA) were conducted in SPSS to assess main effects and interactions for threat type (i.e., cued versus non-cued) and racial group, including a covariate for scanner type, on threat expectancy and SCR data. Multiple linear regressions were completed to test the relationship that violence exposure, family income, and neighborhood disadvantage had with threat expectancy and SCR. These regression analyses did not include race as a covariate to 1) avoid multicollinearity issues, given that negative life experiences differed between Black-American and White-American participants and 2) allow for the assessment of race differences before and after accounting for negative life experiences.
Functional maps representing the percent BOLD signal change elicited by the cued (i.e., CS+UCS trials) and non-cued (i.e., UCS alone trials) threat were entered into a linear mixed-effects model (3dLME) in AFNI with factors for threat type (i.e., cued and non-cued) and race (Black-American and White-American individuals), with a covariate for scanner type (Chen et al., 2013). The analysis was restricted using a cortical gray matter mask that also included the hippocampus and amygdala. The functional maps were set to a corrected false-discovery-rate threshold of q < 0.05 and cluster volume of 200 mm. Group-level fMRI analyses were visualized using a combination of AFNI (Cox, 1996) and Surf Ice (https://www.nitrc.org/projects/surfice/). For follow-up analyses of the fMRI data, the average fMRI signal response within a 3 mm radius of the peak voxel was extracted from a priori regions of interest. Specifically, we investigated the fMRI signal responses within the dorsolateral PFC, dorsomedial PFC, ventromedial PFC, hippocampus, and amygdala. The extracted fMRI signal responses were included as dependent variables in multiple linear regressions that included violence exposure, family income, and neighborhood disadvantage as independent variables. For all regression analyses, a nominal significance threshold of p ≤ 0.05 was used. Bonferroni corrections were applied to the follow-up regression analyses on the extracted fMRI signal responses to maintain a family-wise error of α = 0.05 across the multiple regression models (nine models in total, p = 0.05/9 = ).
Results
Participant demographics
A series of chi-square and independent samples t-tests were completed to assess differences in demographic variables between Black-American and White-American participants (Table 1). On average, Black-American participants (M = 20.87, SD = 1.30) were older by 0.49 years [t(196) = 2.28, p = 0.023] than the White-American participants (M = 20.38, SD = 1.43) at the neuroimaging assessment. The Black-American and White-American groups did not differ in gender composition [χ2 = 3.36, p = 0.067], and an equivalent proportion of Black-American and White-American participants were scanned on both the Allegra and Prisma systems [χ2 = 0.07, p = 0.796]. The Black-American and White-American groups also differed in all three types of negative life experiences: Black-American participants had higher (~2.5 times higher) levels of violence exposure [t(149.31) = 8.90, p < 0.001, adjusted for unequal variance], lower (~4 times lower) family income levels [t(58.60) = −8.55, p < 0.001, adjusted for unequal variance], and greater (~1.5 times greater) neighborhood disadvantage [t(121.06) = 13.71, p < 0.001, adjusted for unequal variance] than the White-American participants (Table 1).
Table 1.
Participant Demographics
| White-American (n = 55) | Black-American (n = 143) | p-value | |
|---|---|---|---|
| Age | 20.38 (1.43) | 20.87 (1.30) | 0.023 |
| Female | 22 | 78 | 0.067 |
| Violence Exposure | 1.88 (1.78) | 4.86 (2.77) | < 0.001 |
| Family Income (% FPL) | 519.48 (326.75) | 135.19 (107.63) | < 0.001 |
| Neighborhood Disadvantage | −0.83 (0.59) | 0.55 (0.73) | < 0.001 |
Note: Neighborhood Disadvantage scores are a principle component score. FPL = Federal Poverty Line. Values are presented as: Mean (Standard Deviation).
Threat expectancy
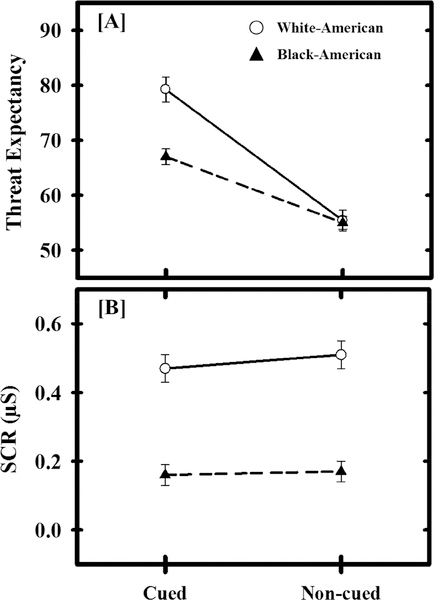
An ANCOVA was completed that included a within-subjects factor of threat type (i.e., cued and non-cued), a between-subjects factor of race (i.e., Black-American and White-American), and a covariate for scanner type (i.e., Allegra or Prisma). No significant effects of scanner type were observed (all p > 0.05). We observed a significant main effect of threat type [F(1,195) = 109.96, p < 0.001]. Specifically, threat expectancy was greater during cued (M = 73.11, SEM = 1.35) than non-cued trials (M = 55.19, SEM = 1.10). The ANOVA also revealed a main effect of race [F(1,195) = 10.14, p = 0.002], such that Black-American participants’ threat expectancies (M = 60.99, SEM = 1.05) were lower than White-American participants’ threat expectancies (M = 67.31, SEM = 1.69). A significant interaction was also observed such that the effect of threat type on expectancy was dependent upon participant race [F(1,195) = 16.35, p < 0.001] (Figure 1). Post-hoc contrasts revealed a significant difference between Black-American and White-American participants’ threat expectancy during cued threat trials [F(1,195) = 20.57, p < 0.001], but not non-cued threat trials [F(1,195) = 0.04, p = 0.849]. Specifically, Black-American participants showed lower expectancy of cued threats (M = 67.00, SEM = 1.42) than White-American participants (M = 79.22, SEM = 2.29), but Black-American participants (M = 54.98, SEM = 1.16) and White-American participants (M = 55.40, SEM = 1.88) did not differ in their expectancy of non-cued threats. Thus, there was a significant difference in expectation of threat during cued trials between Black-American and White-American participants.
Figure 1.
Racial differences in behavioral and psychophysiological responses. White-American (White Circles) participants had greater threat expectancy to cued, but not non-cued, threat than Black-American (Black Triangles) participants (a). Further, White-American participants produced larger threat-elicited skin conductance responses (SCR; b) than Black-American participants. Circles (White-Americans) and triangles (Black-Americans) represent mean threat expectancy and SCR for each trial-type and group. Error bars reflect standard error of the mean. SCR data were square-root transformed.
Our ANCOVA results suggested there was a racial difference in the expectation of threat during the Pavlovian fear conditioning task. Therefore, a multiple linear regression analysis was completed to determine whether threat expectancy varied with violence exposure, family income, and neighborhood disadvantage. The regression model predicted threat expectancy during the cued threat [F(3,193) = 4.39, p = 0.005, R = 0.25]. Thus, expectancy of cued threat varied with negative life experiences as a whole, however, expectancy of cued threat did not vary with violence exposure, family income, or neighborhood disadvantage individually (p > 0.05). An identical regression comparing the variables of interest (e.g., violence exposure, family income, and neighborhood disadvantage) and expectancy of non-cued threat did not reveal a significant relationship (all p > 0.05).
Our analyses of threat expectancy demonstrated that Black-American participants had lower expectancies of cued threat than White-American participants. Further, these analyses revealed that expectations of cued threat also varied with exposure to negative life experiences. We next sought to determine whether accounting for negative life experiences attenuated racial differences in expectancy of cued threat. Estimates of effect size (Cohen’s d) were calculated both on differences in the raw expectancy to cued threat and on the differences in the residual expectancy to cued threat (i.e., the residuals from the prior multiple linear regression of negative life experiences on expectancy to cued threat). Cohen’s d for the raw difference between Black-American and White-American participants’ expectancy of cued threat was high (d = 0.78). However, a small effect of race was observed after accounting for negative life experiences (d = 0.28). These results suggest that racial disparities in negative life experiences partially mediate racial differences in expectation of cued threat.
Skin Conductance Response
SCR data from eight volunteers (five Black-American and three White-American) were discarded due to equipment malfunction. Therefore, 190 participants were included in SCR analyses. An ANCOVA was completed that included a within-subjects factor of threat type (i.e., cued and non-cued), a between-subjects factor of race (i.e., Black-American and White-American), and a covariate for scanner type (i.e., Allegra or Prisma). No significant effects of scanner type were observed (all p > 0.05). We observed a significant main effect of threat type [F(1,187) = 4.89, p = 0.028]. Specifically, threat-elicited SCRs were smaller during cued (M = 0.31, SEM = 0.02) than non-cued threat (M = 0.34, SEM = 0.03). The ANCOVA also revealed a main effect of race [F(1,187) = 44.59, p < 0.001], such that threat-elicited SCRs were smaller for Black-American participants (M = 0.17, SEM = 0.03) than White-American participants (M = 0.49, SEM = 0.04) (Figure 1). The interaction between threat type and race was not significant [F(1,187) = 2.97, p = 0.086]. These results demonstrate that threat-elicited SCRs vary independently with both threat type and participant race.
The previous ANCOVA analysis demonstrated racial differences in the threat-elicited SCR (i.e., average SCR during cued and non-cued presentations). Therefore, a subsequent analysis investigated whether threat-elicited SCRs varied with violence exposure, family income, and neighborhood disadvantage. A multiple linear regression revealed these independent variables together predicted threat-elicited SCRs [F(3,185) = 10.15, p < 0.001, R = 0.38]. Further, a unique relationship between family income and threat-elicited SCRs was observed, such that greater income predicted larger threat-elicited SCRs [t(186) = 3.36, β = 0.28 p = 0.001].
Our analyses of threat-elicited SCRs demonstrated that White-American participants produced larger threat-elicited SCRs than Black-American participants. Further, the amplitude of threat-elicited SCRs also varied with exposure to negative life experiences during development. We next sought to determine whether accounting for negative life experiences attenuated racial differences in threat-elicited SCRs. Estimates of effect size (Cohen’s d) were calculated both on differences in the raw threat-elicited SCRs and on the differences in the residual threat-elicited SCRs (i.e., the residuals from the prior multiple linear regression of negative life experiences on threat-elicited SCRs). Cohen’s d for the raw difference between Black-American and White-American participants’ threat-elicited SCRs was high (d = 0.94). However, a moderate effect of race was observed after accounting for negative life experiences (d = 0.34). These results suggest racial disparities in negative life experiences partially mediate racial differences in threat-elicited SCRs.
Functional Imaging
Threat type
Voxel-wise analysis of the fMRI data was completed using AFNI’s 3dLME. The linear mixed-effects model revealed a significant main effect of threat type (i.e., cued versus non-cued) consistent with prior work (Dunsmoor et al., 2008; Goodman et al., 2018b; Harnett et al., 2015; Knight et al., 2010; Wood et al., 2013; Wood et al., 2012). Specifically, the fMRI signal response was greater during non-cued than cued threats within the dorsolateral PFC, ventromedial PFC, IPL, insula, and precuneus. In contrast, the fMRI signal response was greater during cued than non-cued threats within the cuneus, lingual gyrus, superior temporal gyrus, precentral gyrus, and postcentral gyrus (Table 2).
Table 2.
Regional differences in the fMRI signal response to cued and non-cued threat
| Structure | Direction | Hemisphere | F-statistic | Volume (mm3) | Talairach Coordinates | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Dorsolateral PFC | NC > C | Left | 16.75 | 1155 | −45 | 13 | 49 |
| NC > C | Right | 16.25 | 1170 | 40 | 0 | 57 | |
| SFG | NC > C | Left | 22.06 | 363 | −19 | 50 | 38 |
| NC > C | Left | 11.92 | 318 | −24 | 43 | 18 | |
| NC > C | Right | 40.82 | 31994 | 9 | 22 | 60 | |
| Ventromedial PFC | NC > C | Right | 15.03 | 240 | 11 | 46 | −19 |
| Precentral Gyrus | C > NC | Right | 15.37 | 1082 | 34 | −23 | 63 |
| Postcentral Gyrus | C > NC | Left | 17.13 | 691 | −21 | −49 | 67 |
| Mid. Cingulate | NC > C | Bilateral | 12.17 | 748 | 2 | −29 | 32 |
| IPL | NC > C | Left | 24.31 | 4328 | −56 | −55 | 37 |
| NC > C | Right | 26.76 | 10850 | 56 | −47 | 25 | |
| STG | C > NC | Left | 34.27 | 9282 | −54 | −27 | 14 |
| C > NC | Right | 39.89 | 9225 | 58 | −13 | 7 | |
| Temporal Pole | NC > C | Left | 11.40 | 237 | −31 | 21 | −29 |
| Insula | NC > C | Left | 39.55 | 7252 | −27 | 21 | −4 |
| NC > C | Right | 31.08 | 9222 | 32 | 21 | −4 | |
| Precuneus | NC > C | Left | 21.22 | 6340 | −12 | −72 | 50 |
| Cuneus | C > NC | Bilateral | 13.94 | 221 | −2 | −89 | 31 |
| C > NC | Right | 18.74 | 588 | 6 | −88 | 32 | |
| Lingual Gyrus | C > NC | Right | 11.92 | 904 | 12 | −57 | −9 |
Location, F-statistic, volume, and Talairach and Tournoux (1988) coordinates of the peak voxel for significant (q = 0.05; k = 200) areas of activation. NC = non-cued threat, C = cued threat, PFC = Prefrontal Cortex, SFG = Superior Frontal Gyrus, IPL = Inferior Parietal Lobule, STG = Superior Temporal Gyrus. Original voxel dimensions = 3.75 × 3.75 × 4 mm.
Racial differences in neural activity
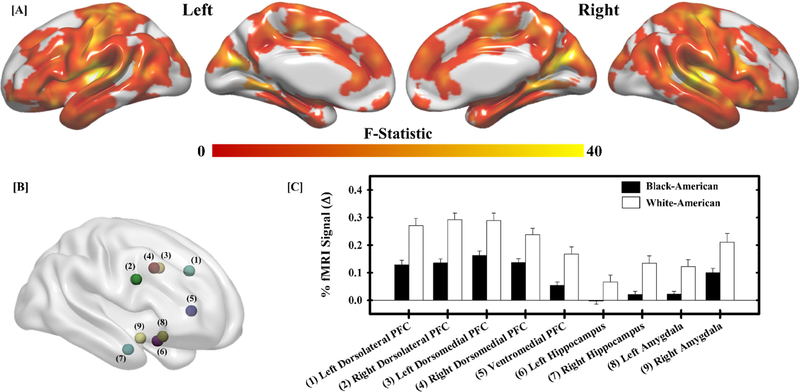
We also observed a significant main effect of race, with peak activations within the superior frontal gyrus, superior temporal gyrus, fusiform gyrus, cuneus, and lingual gyrus (Table 3; Figure 2a). The clusters of activation spanned multiple brain regions, including those in which we had strong a priori hypotheses (i.e., dorsolateral PFC, dorsomedial PFC, ventromedial PFC, hippocampus, and amygdala). Therefore, follow-up analyses focused on fMRI signal responses within the dorsolateral PFC, dorsomedial PFC, ventromedial PFC, hippocampus, and amygdala as prior work has demonstrated that these regions are important for the threat-related emotional processes that were under investigation (Dunsmoor et al., 2008; Goodman et al., 2018a; Harnett et al., 2015; Knight et al., 2010; Wood et al., 2012). The average fMRI signal response within a 3 mm radius of the peak activation within these regions was extracted for subsequent follow-up analyses (Figure 2b). Black-American participants showed lower fMRI signal responses to threat within the PFC, hippocampus, and amygdala than White-American participants (Figure 2c). These results demonstrate racial differences in the neural response to threat.
Table 3.
Regions showing race-related differences in the fMRI signal response to threat
| Structure | Hemisphere | F-statistic | Volume (mm3) | Talairach Coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| SFG | Right | 6.98 | 213 | 37 | 56 | 9 |
| STG | Left | 71.62 | 323176 | −60 | −21 | 15 |
| Fusiform Gyrus | Left | 10.28 | 953 | −50 | −59 | −11 |
| Cuneus | Right | 11.92 | 627 | 21 | −96 | 3 |
| Lingual Gyrus | Right | 7.50 | 216 | 8 | −82 | −16 |
Location, F-statistic, volume, and Talairach and Tournoux (1988) coordinates of the peak voxel for significant (q = 0.05; k = 200) areas of activation. SFG = Superior Frontal Gyrus, STG = Superior Temporal Gyrus. Original voxel dimensions = 3.75 × 3.75 × 4 mm.
Figure 2.
Racial differences in the neural response to threat. A significant main effect of race (White-American versus Black-American) was observed across the brain (a). Follow-up analyses were completed on significant peaks of activation in regions important for threat-related emotional function (b). Within these regions, White-American participants (white bars) exhibited a greater fMRI signal response (% change) to threat than Black-American participants (black bars) (c). Bars reflect the mean fMRI signal response for each group and error bars reflect the standard error of the mean. Numbers next to region labels in (c) correspond to numbered regions in (b).
Relationship between the fMRI signal response to threat and negative life experiences
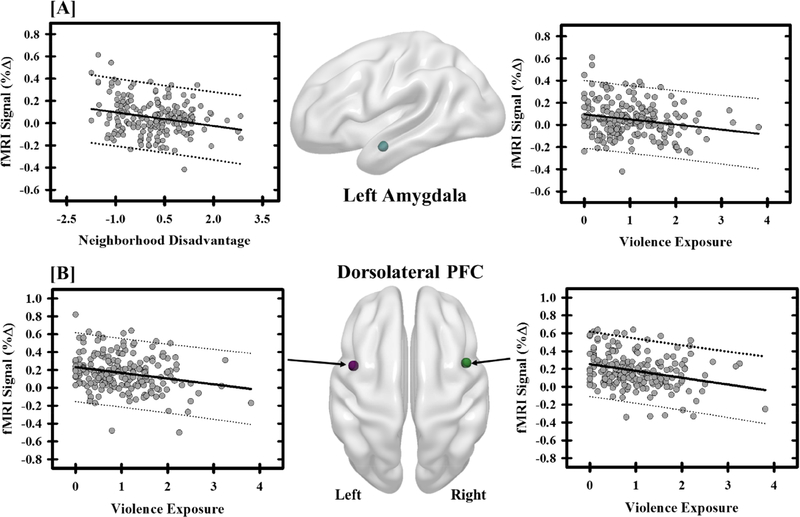
The linear-mixed effects model revealed a strong main effect of race on threat-related reactivity across many brain regions. Importantly, Black-American and White-American participants also showed significant differences in negative life experiences (i.e., violence exposure, family income, and neighborhood disadvantage). Therefore, we sought to determine whether threat-related neural activity related to racial differences varied with negative life experiences. Multiple regression models were completed on the percent fMRI signal response (one model per brain region) and included violence exposure, family income, and neighborhood disadvantage as independent variables. Following a Bonferroni correction, the regression models showed a significant relationship between negative life experiences and the fMRI signal response within the left and right dorsolateral PFC, ventromedial PFC, right hippocampus, and left amygdala (Figure 3; Table 4). These results demonstrate neural activity varies with negative life experiences.
Figure 3.
Negative life experiences influence threat-elicited brain activity. The fMRI signal response (% change) within the amygdala showed negative relationships with neighborhood disadvantage and violence exposure (a). Specifically, participants with more neighborhood disadvantage and violence exposure showed diminished amygdala activity. Negative relationships were also observed between violence exposure and the fMRI signal response to threat within the dorsolateral prefrontal cortex (PFC) (b). Graphs depict the mean fMRI signal response (% change) and negative life experience (i.e., neighborhood disadvantage and violence exposure) for each participant (grey dots). The solid black line reflects the line of best fit, and the dashed lines represent the prediction bands.
Table 4.
Regressions with negative life experience measures and functional ROIs
| Structure | Full Model (F-Statistic) | R-Statistic | Violence Exposurea β (t) | Family Incomea β (t) | Neighborhood Disadvantagea β (t) | Talairach Coordinates | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Left Dorsolateral PFC┼ | 6.08*** | 0.29 | −0.18 (−2.44)* | −0.00 (−0.05) | −0.20 (−2.37)* | −43 | 8 | 33 |
| Right Dorsolateral PFC┼ | 8.92*** | 0.35 | −0.22 (−3.04)** | 0.16 (1.91) | −0.07 (−0.85) | 44 | 9 | 27 |
| Left Dorsomedial PFC | 4.06** | 0.24 | −0.15 (−2.04)* | 0.00 (0.09) | −0.15 (−1.80) | −3 | 4 | 35 |
| Right Dorsomedial PFC | 3.29* | 0.22 | −0.15 (−2.04)* | 0.02 (0.24) | −0.11 (−1.30) | 5 | 4 | 35 |
| Ventromedial PFC┼ | 5.43** | 0.28 | −0.16 (−2.16)* | 0.07 (0.78) | −0.14 (−1.68) | 1 | 31 | 5 |
| Left Hippocampus | 2.83* | 0.21 | −0.09 (−1.18) | −0.05 (−0.56) | −0.19 (−2.27)* | −21 | −6 | −16 |
| Right Hippocampus┼ | 4.78** | 0.26 | −0.15 (−2.05)* | −0.11 (−1.29) | −0.24 (−2.85)** | 20 | −9 | −22 |
| Left Amygdala┼ | 5.89*** | 0.29 | −0.16 (−2.24)* | −0.04 (−0.41) | −0.22 (−2.70)** | −21 | −2 | −13 |
| Right Amygdala | 3.30* | 0.22 | −0.11 (−1.47) | 0.00 (0.01) | −0.17 (−1.98)* | 27 | 4 | −14 |
Talairach and Tournoux (1988) coordinates of the location of the center of mass of each sphere used for the %fMRI signal extractions are presented.
Symbol indicates the full model survived a Bonferroni Correction (0.05/9 = )
Values are presented as β-coefficients (t-statistic).
= p ≤ 0.05
= p ≤ 0.01
= p ≤ 0.001
Accounting for negative life experiences attenuates race-related differences in neural activity
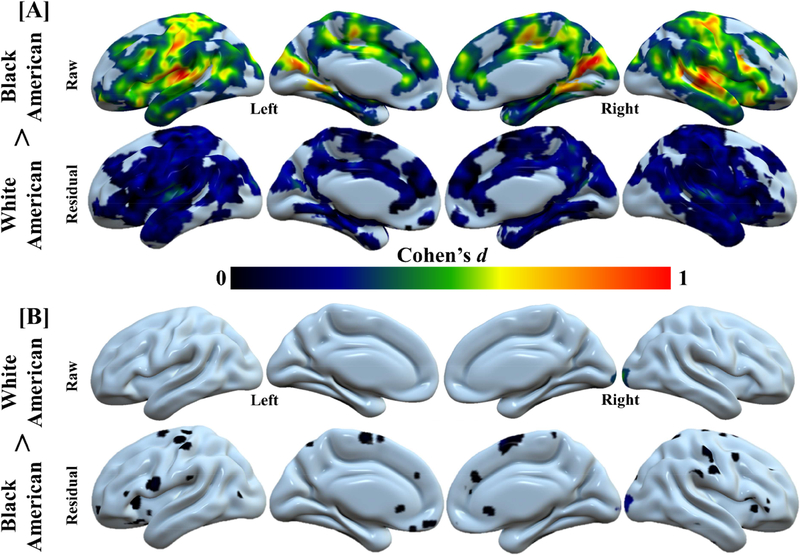
Our voxel-wise analyses demonstrated racial differences in neural reactivity to threat. Further, negative life experiences also varied with neural reactivity to threat. Therefore, we next sought to determine whether negative life experiences accounted for racial differences in the neural response to threat. Estimates of effect size (Cohen’s d) were calculated both on differences in the raw fMRI signal response and on the differences in the residual fMRI signal response (after accounting for negative life experiences) to threat. Voxel-wise residuals were obtained using AFNFs 3dttest++ by specifying a one-sample t-test of the average threat-elicited fMRI signal response (i.e., neural activity collapsed over threat type) with covariates for scanner type, violence exposure, family income, and neighborhood disadvantage. Estimates of effect size for both the raw and residual fMRI signals were masked by the regions that showed a significant main effect of race from the prior voxel-wise linear mixed-effect model. In general, a moderate to large effect size for race was observed across the brain before accounting for differences in negative life experiences. However, after accounting for negative life experiences, racial differences were diminished to small and moderate magnitudes (Figure 4). We further calculated 95% confidence intervals for the observed effect sizes (Lee, 2016) to determine whether the effect sizes for the raw and residual fMRI signal responses confidently excluded zero. Before accounting for differences in negative life experiences, the 95% confidence interval excluded zero across the majority of brain regions, suggesting neural reactivity to threat was different between Black-American and White-American participants. After accounting for differences in negative life experiences, the 95% confidence interval included zero across the majority of brain regions suggesting the Black-American and White-American groups were not confidently different from one another (Figure S1). These data suggest negative life experiences partially explain racial differences in the neural response to threat.
Figure 4.
Race-related differences in brain activity are accounted for by negative life experience. Negative life experience diminishes the effect of race (i.e., White-American and Black-American) on the neural response to threat. Cohen’s d values from “raw” (i.e., the % fMRI signal responses obtained from the first-level analyses) and “residual” (i.e., the residuals of the model that assessed the relationship between negative life experiences and % fMRI signal responses) neural activity for White-American > Black-American (a) and Black-American > White-American (b) analyses. Large effects of race were observed in the threat-elicited fMRI signal response (i.e., White-American > Black-American: raw). However, these racial differences were reduced (i.e., White-American > Black-American: residual) after accounting for negative life experiences (i.e., violence exposure, family income, and neighborhood disadvantage). A comparison of the confidence intervals for the raw and residual effect sizes is presented in Figure S1.
Threat type by race interaction
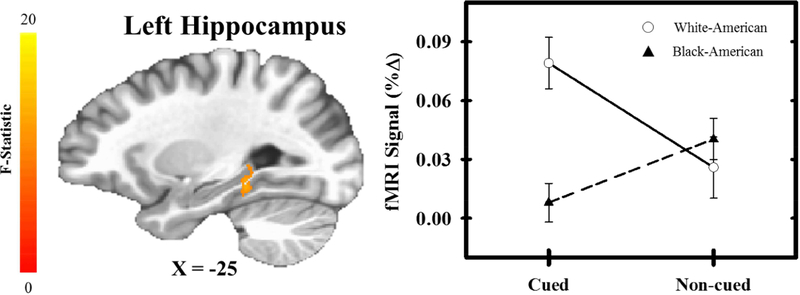
The voxel-wise analysis also revealed a significant threat type (cued versus non-cued) by race interaction within the superior temporal gyrus, cuneus, postcentral gyrus, IPL, and hippocampus (Table 5; Figure 5). Given the role of the hippocampus in declarative memory and threat expectancy processes (Knight et al., 2009), we performed post-hoc analyses to understand the interaction within this brain region. Black-American participants showed greater diminution of fMRI signal responses during cued threat than White-American participants [t(196) = 3.98, p < 0.001], but no group difference was observed for non-cued threat [t(196) = 0.46, p = 0.459]. Further, White-American participants showed significantly greater fMRI signal responses during cued threat than non-cued threat [t(54) = −3.61, p < 0.001], while Black-American participants showed the reverse pattern [t(142) = 2.44, p = 0.016] within the hippocampus. These results suggest cued threat, but not non-cued threat, differentially activated the hippocampus for Black-American and White-American participants.
Table 5.
Regions showing an interaction between threat type and race
| Structure | Hemisphere | F-statistic | Volume (mm3) | Talairach Coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| STG | Left | 24.36 | 7412 | −62 | −19 | 12 |
| Right | 30.68 | 6024 | 56 | −6 | 8 | |
| Postcentral Gyrus | Left | 23.89 | 3095 | −25 | −39 | 65 |
| Left | 17.81 | 735 | −54 | −17 | 49 | |
| Right | 14.52 | 245 | 21 | −51 | 64 | |
| Right | 19.62 | 2848 | 33 | −33 | 64 | |
| IPL | Right | 27.66 | 1261 | 59 | −28 | 44 |
| Right | 18.98 | 412 | 65 | −24 | 22 | |
| Hippocampus/PHG | Left | 13.89 | 332 | −27 | −34 | −9 |
| Fusiform Gyrus | Left | 19.93 | 963 | −42 | −33 | −18 |
| Right | 20.16 | 333 | 37 | −32 | −17 | |
| Cuneus | Right | 18.30 | 3162 | 8 | −88 | 31 |
| Lingual Gyrus | Left | 14.89 | 286 | −12 | −59 | −10 |
| Right | 17.05 | 995 | 6 | −64 | −7 | |
Location, F-statistic, volume, and Talairach and Tournoux (1988) coordinates of the peak voxel for significant (q = 0.05; k = 200) areas of activation. STG = Superior Temporal Gyrus, IPL = Inferior Parietal Lobule, PHG = Parahippocampal Gyrus. Original voxel dimensions = 3.75 × 3.75 × 4 mm.
Figure 5.
Interaction between race and threat type. A significant interaction effect was observed within the hippocampus such that White-American (White Circles) participants exhibited a greater fMRI signal response (% change) to cued compared to non-cued threat. In contrast, Black-American (Black Triangles) participants exhibited greater fMRI signal responses to non-cued compared to cued threat. Circles (White-Americans) and triangles (Black-Americans) represent mean fMRI signal response for each trial-type and group. Error bars reflect standard error of the mean.
Relationship between hippocampal reactivity and negative life experiences
The hippocampus showed a significant threat type by race interaction. Specifically, Black-American participants showed lower hippocampal activation during cued threat than White-American participants. Therefore, a subsequent analysis investigated whether threat-related hippocampal reactivity varied with violence exposure, family income, and neighborhood disadvantage. The regression model predicted hippocampal reactivity during the cued threat [F(3,193) = 3.19, p = 0.025, R = 0.22]. Thus, negative life experiences as a whole varied with hippocampal reactivity to cued threat. However, hippocampal reactivity to cued threat did not vary with violence exposure, family income, or neighborhood disadvantage individually (p > 0.05). An identical regression model comparing the variables of interest (e.g., violence exposure, family income, and neighborhood disadvantage) and hippocampal reactivity to non-cued threat was not significant [F(3,193) = 2.47, p = 0.063, R = 0.19]. However, within the model, family income was uniquely related to hippocampal reactivity to non-cued threat [t(193) = −2.61, β = −0.23, p = 0.01]. These results demonstrate hippocampal reactivity to threat varies with negative life experiences.
The voxel-wise analysis demonstrated that hippocampal reactivity to cued threat was greater in White-American participants than Black-American participants. Further, the hippocampal regression analysis demonstrated hippocampal reactivity to cued threat varied with negative life experiences. We next sought to determine whether accounting for negative life experiences attenuated racial differences in hippocampal reactivity to cued threat. Estimates of effect size (Cohen’s d) were calculated both on differences in the raw hippocampal reactivity and on the differences in the residual hippocampal reactivity (i.e., the residuals from the prior multiple linear regression of negative life experiences on hippocampal reactivity to cued threat. Cohen’s d for the raw difference between Black-American and White-American participants’ hippocampal reactivity to cued threat was high (d = 0.66, 95% CI [0.34, 0.98]). However, a small effect of race was observed after accounting for negative life experiences (d = 0.26, 95% CI [−0.05, 0.57]). Before accounting for differences in negative life experiences, the 95% confidence interval excluded zero, suggesting hippocampal reactivity to cued threat was different between Black-American and White-American participants. After accounting for differences in negative life experiences, the 95% confidence interval included zero suggesting the Black-American and White-American groups were not confidently different from one another. These results suggest racial disparities in negative life experiences contribute to racial differences in hippocampal reactivity to cued threat.
Discussion
Negative life experiences can have formative effects on an individual’s development and may subsequently affect threat-related emotional processes (Pechtel & Pizzagalli, 2011). In general, Black-American individuals tend to endure more negative life experiences than White-American individuals (DeNavas-Walt et al., 2015; Schuster et al., 2012; Slopen et al., 2016; Williams & Collins, 2001; Zimmerman & Messner, 2013). As a result, there may be racial differences in the neural, behavioral, and psychophysiological processes that mediate threat-related emotional function. Thus, the present study investigated the relationship between race, negative life experiences, and brain function that supports threat-related emotional processes. We observed differences in threat expectancy, psychophysiological responses, and neural reactivity to threat in Black-American and White-American participants. Importantly, threat-elicited brain, behavioral, and psychophysiological responses also varied with participants’ history of exposure to negative life events (e.g., violence exposure, poverty, and neighborhood disadvantage). In fact, accounting for negative life experiences dramatically reduced racial differences in threat-elicited brain reactivity (Figure 4). The results of the present study suggest that negative life experiences contribute to and explain racial differences in the neural function that supports threat-related emotional processes.
The present study suggests that racial differences in threat-related emotional expression are partially due to disparities in negative experiences during adolescence. In the present study, White-American participants demonstrated heightened autonomic responses (i.e., SCRs) to threat compared to Black-American participants. However, these differences were attenuated once disparities in exposure to negative life experiences were considered in the analysis. Prior psychophysiological research has also found reduced SCRs in Black-American compared to White-American individuals (Johnson & Landon, 1965; Kredlow et al., 2017). However, prior work has provided limited insight into psychosocial factors that may influence these racial differences in the psychophysiological response. In the present study, we found that threat-elicited SCRs varied with exposure to negative life experiences. Reduced autonomic reactivity is often observed in adolescents exposed to negative life experiences (Busso et al., 2017; Ruttle et al., 2011). In the present study, Black-American participants had greater violence exposure, lower family income, and greater neighborhood disadvantage during adolescence compared to White-American participants. By accounting for differences in these negative life experiences, the magnitude of racial differences in threat-elicited SCRs was reduced. Taken together, the current findings suggest that racial differences in emotional reactivity are due, in part, to disparities in negative experiences during adolescence.
Negative life experiences may also affect processes related to the prediction of aversive threats. In the present study, Black-American participants showed lower expectancy of cued threat than White-American participants (Figure 1). However, Black-American and White-American participants did not differ in their expectancy of non-cued threat. The same pattern was observed within the hippocampus, where Black-American participants showed less hippocampal reactivity to cued threat than White-American participants, but no group difference was observed to non-cued threat (Figure 5). Further, hippocampal responses to cued threat varied with negative life experiences. Importantly, the magnitude of racial differences in hippocampal reactivity to cued threat was reduced after accounting for differences in negative life experiences. The hippocampus is a critical component of the neural circuit responsible for conscious expectations of impending threat (Haritha et al., 2013; Knight et al., 2009). Thus, differences in threat-elicited hippocampal activity may partially underlie the threat expectancy differences observed between Black-American and White-American participants in the present study. Further, racial differences in responses to cued threat may be due, in part, to differences in negative life experiences.
Differences in exposure to negative life experiences also appear to underlie racial differences in the brain activity that supports threat-related emotional function. We observed differences in threat-elicited fMRI signal responses within the dorsolateral PFC, dorsomedial PFC, ventromedial PFC, hippocampus, and amygdala between Black-American and White-American participants (Figure 2). However, functional brain activity within these regions also varied with negative life experiences. Specifically, the fMRI signal response within the dorsolateral PFC (Figure 3), ventromedial PFC, hippocampus, and amygdala (Figure 3) varied with individual differences in negative life experiences (i.e., violence exposure, family income, and neighborhood disadvantage). Further, the magnitude of racial differences in brain activity was attenuated by accounting for these negative life experiences (Figure 4). Together, these findings illustrate the pronounced effect that exposure to negative life experiences has on the neural activity that supports threat-related emotional function. The racial differences in brain activity were observed within brain regions that are part of a neural circuit that supports important threat-related emotional processes. The amygdala is critical for expression of the peripheral emotional response (Cheng et al., 2008; Cheng et al., 2006; Harnett et al., 2015; Knight et al., 2005; Orem et al., 2019). Further, the dorsolateral, dorsomedial, and ventromedial PFC play important roles in the modulation of amygdala-mediated autonomic responses (Delgado et al., 2008; Goodman, Harnett, Wheelock, et al., 2018; Knight et al., 2010; Motzkin et al., 2015; Wheelock et al., 2016; Wood et al., 2012). Taken together, the results of the present study suggest that differences in negative life experiences may lead to measurable differences in threat-related brain and behavior responses.
The present findings complement a growing literature that details the impact early exposure to negative life experiences has on brain function and emotional processes (De Brito et al., 2013; Herringa et al., 2013; McLaughlin et al., 2014; Sheridan et al., 2012). Contemporary theories suggest negative life experiences broadly reflect dimensions of threat and deprivation that alter neural development (McLaughlin et al., 2014). Threat (e.g., violence exposure) is conceptualized as events that pose actual or perceived harm directly to an individual, while deprivation (e.g., poverty or neighborhood disadvantage) is conceptualized as the absence of sensory and cognitive stimuli in the environment (McLaughlin et al., 2014). The threat and deprivation dimensions of negative life experiences during development may have dissociable relationships with neural and behavioral function (Lambert et al., 2017; Miller et al., 2018). For example, threat may lead to emotional overreactivity, mediated by hypoactivity of the ventromedial PFC and hyperactivity of the amygdala. This pattern of activation is commonly observed in posttraumatic stress disorder patients (Hayes et al., 2012; Liberzon & Sripada, 2007; Milad et al., 2009; Rougemont-Bucking et al., 2011). Further, prior work that has assessed the independent effects of threat and deprivation has found that threat alters automatic emotional regulation processes, while deprivation alters cognitive control processes (Lambert et al., 2017). Therefore, one might have expected the different negative life experiences indexed in the present study to show dissociable relationships with neural and behavioral responses to threat. Specifically, one might have predicted that violence exposure would show one pattern with the response to threat, while family income and neighborhood disadvantage would show a different pattern. Importantly, negative life experiences assessed in the present study largely showed similar, negative relationships with brain and behavioral responses. Specifically, violence exposure, family income, and neighborhood disadvantage varied inversely with threat expectancy, threat-elicited SCRs, and threat-elicited fMRI signal responses. Therefore, we did not observe a dissociation between the effects of threat and deprivation dimensions on emotional brain and behavioral responses in the present study. Instead, greater exposure to negative life experiences was related to a blunting of emotional function as evidenced in both the brain and behavioral relationships. However, qualitative differences in the strength of the relationships between the different types of negative life experiences and regional brain activity was observed. In general, dorsolateral PFC activity was more strongly tied to violence exposure than to neighborhood disadvantage. In contrast, amygdala and hippocampal activity were more strongly associated with neighborhood disadvantage than with violence exposure. Therefore, brain reactivity to threat may show some differentiation that is dependent on the type of negative life experience, although in the opposite direction from what might be theorized based on prior work (Lambert et al., 2017; McLaughlin et al., 2014; Miller et al., 2018). Specifically, the present neural data may suggest violence exposure has stronger effects on PFC-mediated cognitive processes, whereas neighborhood disadvantage has stronger effects on amygdala/hippocampus-mediated affective processes. Taken together, the present findings highlight the potential influence negative life experiences during adolescence have on threat-related processes in adulthood.
Several limitations should be considered when drawing inferences from the results of the present study. First, Black-American and White-American participants were not matched for negative life experiences in the present study. It is difficult to match Black-American and White-American participants on certain demographic variables due to persistent socioeconomic inequalities within the United States. However, matching participants would allow researchers to better disentangle environmental effects that may influence racial differences in neural and behavioral responses to threat. A second consideration in the present study is that although participants were exposed to cued and non-cued threats, the observed racial differences in SCR may not be specific to threat-related stimuli. More specifically, we did not compare racial differences in the SCRs elicited by threat and non-threat conditions. Prior research has observed differences in basal skin conductance levels between Black-American and White-American individuals, suggesting that non-threat-specific mechanisms may underlie racial differences in skin conductance (Johnson & Landon, 1965). Therefore, without a non-threat comparison, we cannot unequivocally claim the racial differences in SCRs are threat-specific. A third limitation to consider in the present study is that participants only completed a single neuroimaging session after the T4 assessment. Given that the reports of negative life experiences were collected in the years prior to neuroimaging, it appears that the developmental environment strongly affected neural function later in life. However, without longitudinal neuroimaging data, it is difficult to determine the timeline and mechanisms by which the developmental environment may have led to the observed differences in emotional brain function. Specifically, differences in neural activity could pre-exist or interact with negative life experiences over time in ways that the current study could not assess. Therefore, future neuroimaging work should investigate brain function longitudinally with concurrent assessments of the developmental environment.
In conclusion, the present study investigated racial differences in neural, behavioral, and psychophysiological responses to threat. We observed racial differences in threat expectancy to cued threats, threat-elicited SCRs, and fMRI signal responses to threat during a Pavlovian fear conditioning task. However, we also observed neural, behavioral, and psychophysiological responses that varied with negative life experiences, including violence exposure, low family income, and neighborhood disadvantage. Further, racial differences in the fMRI signal response to threat were reduced after accounting for differences in these negative life experiences. The current findings suggest that racial differences in threat-related emotional processes are due, at least in part, to differences in negative life experiences. Further, our findings provide new insight into factors that may contribute to racial disparities in mental health.
Supplementary Material
Figure S1. Confidence intervals for racial differences in neural reactivity to threat. For each voxel, the 95% confidence interval for the Cohen’s d values from “raw” (i.e., the % fMRI signal responses obtained from the first-level analyses) and “residual” (i.e., the residuals of the model that assessed the relationship between negative life experiences and % fMRI signal responses) were calculated. Prior to accounting for negative life experiences, Cohen’s d values across several regions were confidently different from zero. After accounting for negative life experiences, Cohen’s d values across most of these regions were no longer confidently different from zero.
Acknowledgments
The authors would like to thank Danielle Hurst, Benjamin Pody, Kristina Bell, Juliann Purcell, Heather Dark, and Jordan Ladnier for assistance with this manuscript. The original Healthy Passages Study was funded by the Centers for Disease Control and Prevention through cooperative agreements (CCU409679, CCU609653, CCU915773, U48DP000046, U48DP000057, U48DP000056, U19DP002663, U19DP002664, and U19DP002665). This research was funded by the National Institute of Mental Health R01MH098348 (S. M. & D. C. K.), the University of Alabama at Birmingham Office for Equity and Diversity (N. G. H.), and the Ford Foundation (N. G. H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisenberg E, & Herrenkohl T (2008). Community violence in context: Risk and resilience in children and families. Journal of interpersonal violence, 23(3), 296–315. [DOI] [PubMed] [Google Scholar]
- Alvarez K, Fillbrunn M, Green JG, Jackson JS, Kessler RC, McLaughlin KA,… Alegria M (2018). Race/ethnicity, nativity, and lifetime risk of mental disorders in US adults. Social psychiatry and psychiatric epidemiology, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, & Mayes LC (2010). Race/ethnicity and internalizing disorders in youth: a review. Clin Psychol Rev, 30(3), 338–348. doi: 10.1016/j.cpr.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Atlas LY, Lindquist MA, Bolger N, & Wager TD (2014). Brain mediators of the effects of noxious heat on pain. Pain, 155(8), 1632–1648. doi: 10.1016/j.pain.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R (1966). Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. J Exp Psychol, 71(3), 447–451. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2008). Neural mechanisms underlying selective attention to threat. Ann N Y Acad Sci, 1129(1), 141–152. doi: 10.1196/annals.1417.016 [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2009). Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci, 12(1), 92–98. doi: 10.1038/nn.2242 [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT,… & Filion DL (2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49(8), 1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, & Sheridan MA (2017). Dimensions of Adversity, Physiological Reactivity, and Externalizing Psychopathology in Adolescence: Deprivation and Threat. Psychosom Med, 79(2), 162–171. doi: 10.1097/PSY.0000000000000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, & Cox RW (2013). Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage, 73, 176–190. doi: 10.1016/j.neuroimage.2013.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, & Desmond JE (2008). Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci U S A, 105(23), 8108–8113. doi: 10.1073/pnas.0800374105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, & Helmstetter FJ (2006). Human amygdala activity during the expression of fear responses. Behavioral neuroscience, 120(6), 1187–1195. doi: 10.1037/0735-7044.120.5.1187 [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, & Helmstetter FJ (2003). Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behavioral neuroscience, 117(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, & McCrory EJ (2013). Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of child psychology and psychiatry, 54(1), 105–112. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, & Phelps EA (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829–838. doi: 10.1016/j.neuron.2008.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD, & Smith JC (2015). Income and poverty in the United States: 2014: United States Census Bureau. [Google Scholar]
- Domjan M (2005). Pavlovian conditioning: a functional perspective. Annu Rev Psychol, 56, 179–206. doi: 10.1146/annurev.psych.55.090902.141409 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, & Knight DC (2008). Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage, 40(2), 811–817. doi: 10.1016/j.neuroimage.2007.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. doi: 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, & Radua J (2016). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21(4), 500–508. doi: 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- Ganella DE, Drummond KD, Ganella EP, Whittle S, & Kim JH (2018). Extinction of conditioned fear in adolescents and adults: a human fMRI study. Frontiers in human neuroscience, 11, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag C, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage, 34(3), 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Harnett NG, & Knight DC (2018). Pavlovian conditioned diminution of the neurobehavioral response to threat. Neuroscience & BiobehavioralReviews, 84, 218–224. doi: 10.1016/j.neubiorev.2017.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Harnett NG, Wheelock MD, Hurst DR, Orem TR, Gossett EW,… Knight DC (2018). Anticipatory prefrontal cortex activity underlies stress-induced changes in Pavlovian fear conditioning. Neuroimage, 174, 237–247. doi: 10.1016/j.neuroimage.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zheng L, Wang H, Zhu L, Li J, Wang Q,… Yang Z (2013). Exposure to violence reduces empathetic responses to other’s pain. Brain Cogn, 82(2), 187–191. doi: 10.1016/j.bandc.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, & Farah MJ (2012). Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci, 6, 277. doi: 10.3389/fnhum.2012.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, & Weinberger DR (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry, 53(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Haritha AT, Wood KH, Ver Hoef LW, & Knight DC (2013). Human trace fear conditioning: right-lateralized cortical activity supports trace-interval processes. Cogn Affect Behav Neurosci, 13(2), 225–237. doi: 10.3758/s13415-012-0142-6 [DOI] [PubMed] [Google Scholar]
- Harnett NG, Wheelock MD, Wood KH, Ladnier JC, Mrug S, & Knight DC (2015). Affective state and locus of control modulate the neural response to threat. Neuroimage, 121, 217–226. doi: 10.1016/j.neuroimage.2015.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Wood KH, Wheelock MD, Knight AJ, & Knight DC (2017). Anticipation and the Neural Response to Threat Anticipation and Medicine (pp. 219–228): Springer. [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord, 2(1), 9. doi: 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, & Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences, 110(47), 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LC, & Landon MM (1965). Eccrine sweat gland activity and racial differences in resting skin conductance. Psychophysiology, 1(4), 322–329. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6), 593–602. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE,… Phan KL (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci U S A, 110(46), 18442–18447. doi: 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Lewis EP, & Wood KH (2011). Conditioned diminution of the unconditioned skin conductance response. Behav Neurosci, 125(4), 626–631. doi: 10.1037/a0024324 [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, & Bandettini PA (2005). The role of the human amygdala in the production of conditioned fear responses. Neuroimage, 26(4), 1193–1200. doi: 10.1016/j.neuroimage.2005.03.020 [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, & Bandettini PA (2009). Neural substrates of explicit and implicit fear memory. Neuroimage, 45(1), 208–214. doi: 10.1016/j.neuroimage.2008.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, & Bandettini PA (2010). Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage, 49(1), 843–848. doi: 10.1016/j.neuroimage.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, & Wood KH (2011). Investigating the neural mechanisms of aware and unaware fear memory with FMRI. Journal of Visualized Experiments: JoVE(56). doi: 10.3791/3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredlow MA, Pineles SL, Inslicht SS, Marin MF, Milad MR, Otto MW, & Orr SP (2017). Assessment of skin conductance in African American and Non-African American participants in studies of conditioned fear. Psychophysiology, 54(11), 1741–1754. doi: 10.1111/psyp.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, & Phelps EA (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron, 20(5), 937–945. [DOI] [PubMed] [Google Scholar]
- Lambert HK, King KM, Monahan KC, & McLaughlin KA (2017). Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Development and psychopathology, 29(3), 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, & Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci, 8(7), 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK (2016). Alternatives to P value: confidence interval and effect size. Korean journal of anesthesiology, 69(6), 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, & Sripada CS (2007). The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research, 167, 151–169. [DOI] [PubMed] [Google Scholar]
- Linnman C, Zeffiro TA, Pitman RK, & Milad MR (2011). An fMRI study of unconditioned responses in post-traumatic stress disorder. Biology of mood & anxiety disorders, 1(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001). Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci, 24(1), 897–931. doi: 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- Marin MF, Barbey F, Rosenbaum BL, Hammoud MZ, Orr SP, & Milad MR (2019). Absence of conditioned responding in humans: A bad measure or individual differences? Psychophysiology, e13350. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E (2011). Heightened neural reactivity to threat in child victims of family violence. CurrBiol, 21(23), R947–948. doi: 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Hilt LM, & Nolen-Hoeksema S (2007). Racial/ethnic differences in internalizing and externalizing symptoms in adolescents. J Abnorm Child Psychol, 35(5), 801–816. doi: 10.1007/s10802-007-9128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological psychiatry, 76(8), 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB,… Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry, 66(12), 1075–1082. doi: 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, Sheridan MA, Hanson JL, McLaughlin KA, Bates JE, Lansford JE,… Dodge KA (2018). Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. Journal of abnormal psychology, 127(2), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological psychiatry, 77(3), 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem TR, Wheelock MD, Goodman AM, Harnett NG, Wood KH, Gossett EW,… Knight DC (2019). Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress. Behavioral neuroscience, 133(2), 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick F, Kempton MJ, Marwood L, Williams SC, Young AH, & Perkins A (2019). Brain activation during human defensive behaviour: A systematic review and preliminary meta-analysis. Neuroscience & Biobehavioral Reviews. [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J,… Milad MR (2011). Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther, 17(4), 227–236. doi: 10.1111/j.1755-5949.2010.00152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust J (1976). Unconditioned response diminution in the skin resistance response. J Gen Psychol, 95(1st Half), 77–84. doi: 10.1080/00221309.1976.9710867 [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB, Stack DM, & Schwartzman AE (2011). Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm Behav, 59(1), 123–132. doi: 10.1016/j.yhbeh.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster MA, Elliott MN, Kanouse DE, Wallander JL, Tortolero SR, Ratner JA,… Banspach SW (2012). Racial and ethnic health disparities among fifth-graders in three cities. New England Journal of Medicine, 367(8), 735–745. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences, 109(32), 12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, & Williams DR (2016). Racial disparities in child adversity in the US: Interactions with family immigration history and income. American journal of preventive medicine, 50(1), 47–56. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, & Liberzon I (2014). Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology, 39(9), 2244–2251. doi: 10.1038/npp.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. New York: Thieme. [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS,… Alexander AL (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26(16), 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsaint CL, NeMoyer A, Fillbrunn M, Sadikova E, Kessler RC, Sampson NA,… Chen R (2019). Racial/ethnic differences in 12-month prevalence and persistence of mood, anxiety, and substance use disorders: Variation by nativity and socioeconomic status. Comprehensive psychiatry, 89, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander JL, Fradkin C, Chien AT, Mrug S, Banspach SW, Davies S,… Schuster MA (2012). Racial/ethnic disparities in health-related quality of life and health in children are largely mediated by family contextual differences. Acad Pediatr, 12(6), 532–538. doi: 10.1016/j.acap.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Wheelock MD, Harnett NG, Wood KH, Orem TR, Granger DA, Mrug S, & Knight DC (2016). Prefrontal Cortex Activity Is Associated with Biobehavioral Components of the Stress Response. Frontiers in human neuroscience, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Sreenivasan KR, Wood KH, Ver Hoef LW, Deshpande G, & Knight DC (2014). Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage, 102 Pt 2, 904–912. doi: 10.1016/j.neuroimage.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Collins C (2001). Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep, 116(5), 404–416. doi: 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Grunbaum JA, Elliott M, Tortolero SR, Berry S, Gilliland J,… Schuster M (2004). Healthy passages. A multilevel, multimethod longitudinal study of adolescent health. Am JPrevMed, 27(2), 164–172. doi: 10.1016/j.amepre.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, & Knight DC (2014). The amygdala mediates the emotional modulation of threat-elicited skin conductance response. Emotion, 14(4), 693–700. doi: 10.1037/a0036636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Kuykendall D, Ver Hoef LW, & Knight DC (2013). Neural substrates underlying learning-related changes of the unconditioned fear response. Open Neuroimag J, 7, 41–52. doi: 10.2174/1874440001307010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, & Knight DC (2012). Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage, 60(1), 787–799. doi: 10.1016/j.neuroimage.2011.12.048 [DOI] [PubMed] [Google Scholar]
- Xue Y, Leventhal T, Brooks-Gunn J, & Earls FJ (2005). Neighborhood residence and mental health problems of 5- to 11-year-olds. Arch Gen Psychiatry, 62(5), 554–563. doi: 10.1001/archpsyc.62.5.554 [DOI] [PubMed] [Google Scholar]
- Zimmerman GM, & Messner SF (2013). Individual, family background, and contextual explanations of racial and ethnic disparities in youths’ exposure to violence. Am J Public Health, 103(3), 435–442. doi: 10.2105/AJPH.2012.300931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Confidence intervals for racial differences in neural reactivity to threat. For each voxel, the 95% confidence interval for the Cohen’s d values from “raw” (i.e., the % fMRI signal responses obtained from the first-level analyses) and “residual” (i.e., the residuals of the model that assessed the relationship between negative life experiences and % fMRI signal responses) were calculated. Prior to accounting for negative life experiences, Cohen’s d values across several regions were confidently different from zero. After accounting for negative life experiences, Cohen’s d values across most of these regions were no longer confidently different from zero.