Abstract
This reconnaissance study was undertaken in 2012 to examine the occurrence of common perfluoroalkyl substances (PFAS), including perfluoroalkyl sulphonic acids and perfluoroalkyl carboxylic acids in rivers and estuaries in Port Philip Bay, Victoria, Australia. In total, 19 PFAS were screened in grab samples of water using a combination of solid phase extraction and liquid chromatography - mass spectrometry measurement techniques. Eighteen of the PFAS screened were observed in samples. The highest level of PFOS observed at a freshwater site was 0.045 μg/L; this concentration is approximately half the draft Australian 95% species protection level for total PFOS. The highest level of PFOA in the study (0.014 μg/L) was some four orders of magnitude lower than the draft Australian trigger value for PFOA (220 μg/L). However, none of the PFAS observed at the freshwater sites had research quotient (RQ) or toxicity unit (TU) values above 1 or -3, respectively. The highest concentration of PFOS observed at an estuarine site was 0.075 μg/L; the highest level of PFOA, 0.09 μg/L). There are no Australian marine water quality trigger values for PFAS, so potential risk was assessed using the European environment quality standards (EQS) adopted in EU Directive 2013/39/EU, RQ and TU methods. In that context, none of the PFAS observed at estuary sites had concentrations higher than the EU standards, or RQ above 1 or Log10TU above -3. Together these assessments suggest none of the PFAS screened would have posed an acute risk to organisms in the fresh or estuary waters studied at the time of sampling on an individual or collective basis. However, the detection of these PFAS in Victorian estuaries highlights that the issue is not just an issue for more densely populated countries in the northern hemisphere, but also potentially of concern in Australia. And, in that context, more sampling campaigns in Port Philip Bay are of paramount importance to assess the potential risk pose by these compounds to aquatic ecosystems.
Keywords: Environmental science, Environmental analysis, Environmental assessment, Environmental chemistry, Environmental pollution, Water quality, Perfluoroalkyl sulphonic acids, Estuary, Port Philip Bay, Perfluoroalkyl carboxylic acids
1. Introduction
Perfluoroalkyl substances (PFAS), such as PFOS (perfluorooctanesulfonic acid) and PFOA (perfluorooctanoic acid) and related shorter and longer chain analogues, are ubiquitous contaminants of soils, water and biota (Wang et al., 2017). Such chemicals were, and are still, designed to be chemically and biologically stable in the products into which they were/are applied and so they are persistent in the environment. They have been frequently found in terrestrial and marine wildlife and also human blood. Acute toxicity data for many of these compounds are limited compared to that available for other persistent organic chemicals (Wang et al., 2017), with mortality not observed until organisms are exposed to moderate to high (mg/L) concentrations (Giesy et al., 2010). Laboratory animal experiments have, however, suggested that potential developmental, reproductive, and other systematic effects are observed at high μg/L concentrations, and that long chain PFAS are bio-accumulative and potentially carcinogenic (as reviewed in DeWitt et al., 2015; Wang et al., 2017).
In Australia much of the recent concern around PFAS has been driven by the recognition that the PFAS in aqueous film forming foams (AFFFs) used for emergency response and regular training exercises at military and civilian airports and training bases has resulted in contamination of local soils and groundwaters (Baduel et al., 2017), with some recognition of the potential for contaminated landfill leachate to contaminate aquatic ecosystems (Gallen et al., 2017). There have been reports of groundwater contamination in all Australian states and territories, with perhaps the most publicised cases contamination of drinking water bores in Katherine, 270 km southeast of Darwin in the Northern Territory, and around the RAAF Williamtown airbase, 170 km north east of Sydney in New South Wales. In Victoria, the Environment Protection Authority has notified the public of elevated PFAS levels near Department of Defence locations in East Sale, Wodonga and Hastings, and fire training facilities in Fiskville, Penshurst, Bangholme, Wangaratta, Huntly, Fulham and Longerenong, all as a result of chemicals used in firefighting foam (Australian Water Association, 2017).
While there is now a reasonable understanding of the ubiquitous nature of PFAS contamination of water and biota internationally, at the time of this study (2012) there were no published data on PFAS in natural, surface waters in Victoria, Australia. This reconnaissance survey, therefore, sought to obtain a snapshot of the occurrence and concentrations of common PFAS in surface water samples collected from seven creeks and estuaries in Port Philip Bay catchment, Victoria, and to assess the risk to aquatic organisms therein by comparing observed concentrations with regulatory guideline values.
2. Materials and methods
2.1. Study sites
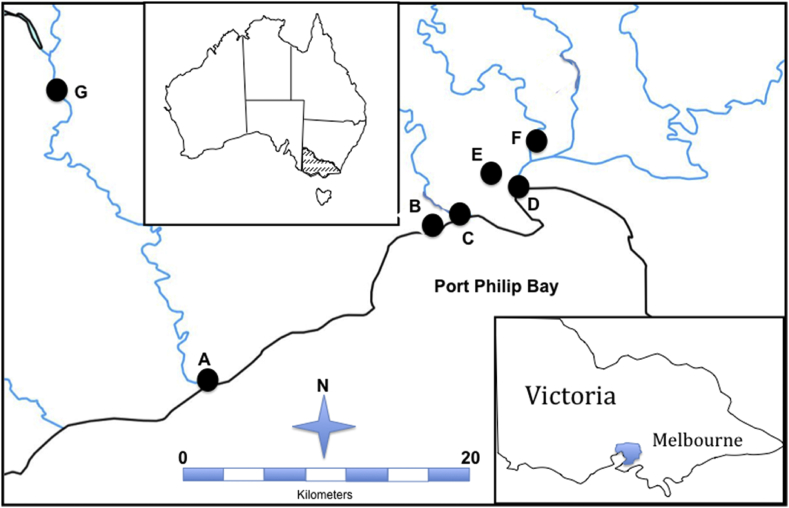
Seven sites in and around Melbourne were chosen for this study (Fig. 1; A-G). The sites were selected because they were part of other on-going ecological investigations of river health. A single ‘grab’ or spot sample of water (1 L) was collected from each of the seven sites on a single occasion during September 2012.
Fig. 1.
Approximate location of sampling sites in and around the city of Melbourne (M) in Victoria, Australia.
Sites A (estuary) and G (freshwater) were on the Werribee River. Approximately 25% of the Werribee River catchment is forested, with ∼66% of the total catchment area used for grazing. The remaining 10% of the catchment is urbanised. At the time of this study there were two small sewage treatment plants (STPs) in the upper Werribee River catchment licensed to discharge to the river (<∼5 000 m3 annual discharge). Site B (estuarine) was on Laverton Creek a few hundred metres from its discharge into PPB. The entire length of this creek is within the western suburbs of Melbourne, including through heavily industrialized areas. Site C (estuary) was on Kororoit Creek. This long stream is ∼50 km long flows almost entirely through the suburbs of north western Melbourne, including several industrialised areas before emptying into Altona Bay; there are no known STPs discharging into either Lavefrton or Kororoit creek upstream of sampling sites. Site D (estuary) was in the Yarra River estuary. This river is ∼240 km long, and originates in the Yarra Ranges east of Melbourne and several small STPs discharged treated effluent into the upper Yarra River (<∼5000 m3 total annual flow) in 2012. Site E (freshwater) was on Stony Creek in the western suburbs of Melbourne downstream of an industrialised area. As far as the Authors know, there are no STP discharges into this creek. Site F (estuary) was at the end of the Maribyrnong River prior to its confluence with the Yarra River. Most (∼80%) of the Maribyrnong River is used for agriculture, principally grazing and horticulture, with only ∼10% of the catchment urbanised. In 2012 there were two small STPs in the upper catchment discharging to the river (<∼3000 m3 annual discharge).
2.2. Sample preparation and chemical analysis
In total, 19 PFAS were screened including the sulphonates PFHxS and PFOS, and the carboxylates PFHxA and PFOA (Table 1). Detailed analytical procedures are reported in Taniyasu et al. (2008) but, in short, samples were extracted with Oasis® WAX cartridges (150 mg, 6 mL, 30 μm, Waters) and eluted with 0.1% NH4OH in methanol. The concentrations of PFAS in the extracts were determined using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). In short, an HP1100 HPLC-system (Agilent Technologies, Palo Alto, CA) was equipped either with a Betasil C18 column (2.1 mm i.d. 50 mm in length, 5 μm; Thermo Hypersil-Keystone, Bellefonte, PA), with a XDB-C8 (12.5 mm 2.1 mm, 5 um; Agilent Technologies, Foster City, CA) as a guard column (for perfluorosulphonates analysis), or a RSpak JJ-50 2D column (2.0 mm i.d. 150 mm in length, 5 μm; Shodex, Showa Denko K.K., Kawasaki, Japan) with an OPTI-GUARD 1mm DVB guard column (Optimize Technologies, OR, USA) (for perfluorocarboxylates analysis). A triple-quadrupole mass spectrometer, supplied by Micromass (Quattro Ultima Pt, Beverly, MA), used an electrospray ionization (ESI) interface in negative ionization mode. The flow rate was set to 300 μL/min and 10 μL of the sample was injected. Limits of Quantitation (LOQ) varied depending on the chemical and column/instrument consitions used, but ranged from 0.02 to 0.50 ng L−1. For full details of the instrumental conditions and quantification for PFAS analysis, readers are directed to Taniyasu et al. (2008).
Table 1.
Summary of PFAS detected in water samples.
| Chemical | Site |
||||||
|---|---|---|---|---|---|---|---|
| Estuarine sites |
Freshwater sites |
||||||
| A | B | C | D | F | E | G | |
| Sulfonates | (ng/L) | (ng/L) | |||||
| PFBS | 0.8 | 7.0 | 6.7 | 0.4 | 0.8 | 2.6 | 0.5 |
| PFHxS | 4.2 | 42.3 | 29.6 | 3.0 | 7.0 | 15.4 | 2.9 |
| PFOS | 3.9 | 74.9 | 74.1 | 5.1 | 13.9 | 45.2 | 6.5 |
| PFDS | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | |
| FOSA | 0.0 | 0.1 | 0.3 | 0.0 | 0.1 | 1.0 | 0.0 |
| N-EtFOSA | |||||||
| N-EtFOSAA | 0.2 | 0.3 | 0.8 | 0.2 | 1.5 | 1.9 | 0.1 |
| Carboxylates | |||||||
| PFBA | 2.9 | 10.2 | 11.1 | 1.7 | 2.8 | 8.8 | 2.7 |
| PFPeA | 3.1 | 9.7 | 6.4 | 1.2 | 2.1 | 9.7 | 3.5 |
| PFHxA | 2.1 | 22.6 | 10.9 | 1.5 | 2.1 | 11.4 | 2.4 |
| PFHpA | 1.8 | 8.9 | 6.9 | 1.2 | 1.5 | 6.0 | 1.8 |
| PFOA | 2.2 | 9.2 | 8.5 | 1.7 | 2.2 | 14.5 | 1.2 |
| PFNA | 0.2 | 1.2 | 6.4 | 0.4 | 3.3 | 2.2 | 0.1 |
| PFDA | 0.2 | 0.5 | 1.7 | 0.4 | 1.2 | 4.2 | 0.7 |
| PFUnDA | 0.1 | 0.2 | 1.1 | 0.2 | 0.7 | 0.5 | 0.2 |
| PFDoDA | 0.0 | 0.1 | 0.3 | 0.2 | 1.1 | 0.9 | 0.1 |
| PFTeDA | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | ||
| PFHxDA | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | ||
| PFOcDA | 0.0 | 0.0 | |||||
No value, <LOR.
2.3. Data analysis and risk methodology
Data has been truncated to report only positive detects relevant to exceedances of regulatory guidelines or ecotoxicological benchmarks. Observed concentrations were first compared with the ANZECC and ARMCANZ (2000) water quality guidelines trigger values for water discharged to receiving freshwater and marine aquatic environments. Where no local guideline values were available, concentrations were compared to European environment quality standards (EQS) adopted in EU Directive 2013/39/EU. Thereafter two additional methods were used to evaluate the potential risk to aquatic organisms and ecosystems: the risk quotient (RQ) and toxicity unit (TU) methods. For the RQ we followed the process outlined by Thomatou et al. (2013) to generate general case (RQmedian) and the worst case (RQmax) scenarios. Long-term ecotoxicological effects data were extracted from the U.S. Environmental Protection Agency (2019). In the first instance, data for the rainbow trout (Onchorynchus mykis) was used for the calculation of the PNECs; where this data was not available any alternative data for a fish was used. Similar processes were used for aquatic invertebrates, plants and algae. Short term EC50 (lethal/effect) data were obtained from the database when long-term data were not available. The PNEC values were calculated by dividing the lowest effect concentration of the most sensitive species by 10 where chronic data was available from all four trophic levels, 50 where long-term data was available from three trophic levels or at least one effect level used was an acute value, and 100 for all other cases. We calculated the toxic unit (Log10TU) for each PFAS according to the method of Liess and Von Der Ohe (2005) by dividing the observed water concentration by a measure of the toxicity (as used for measuring RQ). The toxic units for the PFCAs and PFSAs screened were considered individually and on a group basis for both freshwater and estuarine sites.
3. Results and discussion
3.1. PFAS concentrations at freshwater sites E and G
The main aim of this reconnaissance survey was to obtain a snapshot of the occurrence of 19 common PFAS in five urban/peri-urban estuaries, one urban freshwater stream and one rural river. In that context, water samples collected from the urban freshwater site E were more highly contaminated than the rural freshwater site G (site E, ΣPFAS 125 ng/L cf. site G, 23 ng/L). Moreover, site E was more contaminated with PFOS than the rural freshwater site G (site E, 45 ng/L cf. site G, 6.5 ng/L, respectively; Table 1)). There were, and still are, no published studies of perfluoroalkylsulphonic acid (PFSA) concentrations in Victorian freshwaters with which to compare our data. However, PFSA concentrations observed at site G in this study were consistent with levels in Queensland (Gallen et al., 2014). Specifically, the levels of PFOS at site G (6.5 ng/L) are consistent with levels reported in the freshwater sections of the Brisbane River (up to 4.9, respectively; Gallen et al., 2014)). The PFSA concentrations at site E were, however, an order of magnitude higher than reported in the Brisbane River (Gallen et al., 2014), except for PFBS, which was higher in a tributary. Overall, our freshwater site data is consistent with reports of PFSA concentrations in rivers in China (e.g. Zhao et al., 2016), Germany (e.g. Möller et al., 2010), Japan (Ye et al., 2014), Korea (Lam et al., 2016), Norway (Kwok et al., 2013), and the USA (Newsted et al., 2017) (see Table 2a).
Table 2a.
Summary of selected (most commonly reported) PFSA concentrations reported in rivers in Australia and internationally (ng/L).
| Country | PFBS | PFHxS | PFOS | PFDS | FOSA | Reference |
|---|---|---|---|---|---|---|
| Australia | 0.5–2.6 | 2. 9–15 | 6.5–45 | 0.0–0.1 | 0.0–1.0 | This study |
| Australia | n.d. - 2.5 | n.d. - 4.9 | Gallen et al. (2014) | |||
| Canada | n.d. - 14 | n.d. - 180 | n.d. - 190 | D'Agostino and Mabury (2017) | ||
| China | n.d. - 6.7 | 2.7–41 | Zhao et al. (2016) | |||
| China | 0.0–14 | n.d. - 1.4 | n.d. - 9.7 | n.d. - 2.5 | Lu et al. (2015) | |
| China | n.d. - 31 | 0.3–290 | n.d. - 0.2 | Pan et al. (2014a) | ||
| China | n.d. - 42 | n.d. - 4.5 | n.d. - 0.2 | n.d. - 0.6 | Pan et al. (2014b) | |
| China | n.d. - 8.4 | Yao et al. (2014) | ||||
| China | 0.16. -1.01 | 0.01–0.43 | 0.01–1.57 | Loi et al. (2013) | ||
| China | n.d. - 2.25 | Ju et al. (2008) | ||||
| France | n.d. - 3.1 | 0.3–7.8 | 0.3–16 | n.d. - 0.1 | n.d. - 0.2 | Munoz et al. (2018) |
| France | Bach et al. (2017) | |||||
| France | n.d. - 6 | Boiteux et al. (2017) | ||||
| France | n.d. - 29 | n.d. - 217 | n.d. - 173 | n.d. - 0.21 | n.d. - 0.73 | Munoz et al. (2015) |
| Germany | n.d. - 5.6 | n.d. - 4.6 | Llorca et al. (2012) | |||
| Germany | 0.22–118 | n.d. - 14.5 | 0.89–10.1 | Möller et al. (2010) | ||
| Germany | n.d. - 3.7 | n.d - 0.5 | 0.2–2.9 | Ahrens et al. (2009) | ||
| India | n.d. - 10 | n.d. - 0.3 | n.d. - 1.7 | n.d. - 0.1 | n.d. - 0.1 | Sharma et al. (2016) |
| Italy | n.d. - 5 | n.d. 43 | Castiglioni et al. (2015) | |||
| Italy | n.d. - 1670 | n.d. - 36 | n.d. - 150 | Valsecchi et al. (2015) | ||
| Italy | n.d. - 25 | Loos et al. (2008) | ||||
| Japan | 0.1–2.0 | n.d. - 6.8 | 0.5–8.0 | n.d. - 0.1 | Ye et al. (2014) | |
| Japan | n.d. - 22 | n.d. - 99 | n.d. - 4.0 | Zushi et al. (2011) | ||
| Japan | n.d. – 4.6 | Takagi et al. (2008) | ||||
| Korea | n.d. - 4.0 | n.d. - 15 | Lam et al. (2014) | |||
| Korea | n.d. - 2.3 | n.d. - 6.5 | n.d. - 33 | n.d. - 0.3 | Lam et al. (2016) | |
| Malta | n.d. - 2.5 | n.d. - 8.6 | Sammut et al. (2017) | |||
| Norway | n.d. - 0.4 | n.d. - 0.7 | Kwok et al. (2013) | |||
| Singapore | 1.4–55 | 0.4–16 | 1.5–24 | n.d. - 6.1 | Chen et al. (2017) | |
| Singapore | 1.2–68 | 1.3–156 | n.d. - 3.15 | Nguyen et al. (2011) | ||
| South Africa | n.d. - 25 | n.d. - 7.6 | 0.4–36 | Groffen et al. (2018) | ||
| Spain | 12–37 | 0.01–128 | Campo et al. (2016) | |||
| Spain | 0.4–4.1 | 14–33 | 0.0–271000 | Campo et al. (2015) | ||
| Spain | 20–348 | Flores et al. (2013) | ||||
| Spain | n.d. - 37 | n.d. - 2700 | Llorca et al. (2012) | |||
| Sweden | 0.0–19 | 0.1–18 | 0.0–6.9 | 0.0–0.5 | Nguyen et al. (2017) | |
| Switzerland | 0.4. - 20 | 5.8–20 | 29–82 | Huset et al. (2008) | ||
| USA | n.d. - 24 | Newsted et al. (2017) | ||||
| USA | n.d. - 84 | n.d. - 371 | n.d. - 296 | Nakayama et al. (2010) | ||
| USA | n.d. - 4.05 | n.d. 9.3 | n.d. - 0.34 | n.d. - 0.47 | Kim and Kannan (2007) | |
| USA | 16.8–144 | Hansen et al. (2002) | ||||
| Vietnam | n.d. - 5.3 | n.d. - 6.6 | Duong et al. (2015) | |||
| Vietnam | n.d. - 8.3 | n.d. - 6.0 | n.d. - 40 | Lam et al. (2017) |
Perfluoroalkylcarboxylic acid (PFCA) chemical concentrations were also higher at site E than at site G (e.g. PFOA 14.5 ng/L cf. 1.2 ng/L; Table 1). Again, there were, and still are, no published studies of PFCA concentrations in Victorian freshwaters with which to compare our data, although PFCA concentrations observed at site G in this study were consistent with levels in Queensland (Gallen et al., 2014). For example, the levels of PFOA levels at site G (1.2 ng/L) are consistent with levels reported in the freshwater sections of the Brisbane River (up to 1.4 ng/L; Gallen et al., 2014)). The PFCA concentrations at site E were, however, an order of magnitude higher than reported in the Brisbane River (Gallen et al., 2014), again with the exception of a higher PFBS level observed in a tributary. Site E is downstream of an industrial park, perhaps suggesting a point source for PFAS in that catchment. Overall, however, our freshwater site data is consistent with reports of PFCAs concentrations in rivers in China (e.g. Pan et al., 2014a,b), France (e.g. Munoz et al., 2018), Korea (Lam et al., 2014), Switzerland (Huset et al., 2008), and Vietnam (Duong et al., 2015) (see Table 2b).
Table 2b.
Summary of selected (most commonly reported) PFCA concentrations reported in rivers in Australia and internationally (ng/L).
| Country | PFBA | PFPeA | PFHxA | PFHpA | PFOA | PFNA | PFDA | Reference |
|---|---|---|---|---|---|---|---|---|
| Australia | 2.6–8.8 | 3.5–9.7 | 2.4–11 | 1.8–6.0 | 1.1–14 | 0.1–2.2 | 0.7–4.2 | This study |
| Australia | 0.12–1.1 | n.d. - 0.5 | 0.3–1.4 | n.d. - 0.1 | Gallen et al. (2014) | |||
| Canada | n.d. - 190 | n.d. - 120 | n.d. - 60 | n.d. - 59 | D'Agostino and Mabury (2017) | |||
| China | 6.4–54 | 8.0–47 | n.d. - 3.8 | 2.0–42 | 0.8–25 | 0.2–101 | Zhao et al. (2016) | |
| China | n.d. - 5 | n.d. - 3.5 | 0.1–41 | n.d. - 4.3 | 0.3–200 | n.d. - 2.9 | n.d. - 2.6 | Lu et al. (2015) |
| China | 0.2–2.5 | 0.2–4.6 | n.d. - 4.6 | n.d. - 6.2 | 0.2–22 | 0.2–2.0 | 0.2–1.4 | Pan et al. (2014a) |
| China | 0.4–8.4 | n.d. - 2.6 | 0.1–23 | n.d. - 2.6 | 0.5–18 | n.d. - 0.9 | n.d - 0.3 | Pan et al. (2014b) |
| China | 6.4–2.6 | Yao et al. (2014) | ||||||
| China | 0.15–1.18 | 0.31–2.48 | 0.04–0.56 | 0.01–0.39 | Loi et al. (2013) | |||
| China | 0.17–36 | Ju et al. (2008) | ||||||
| France | 0.1–6.4 | 0.2–7.7 | 0.1–2.8 | 0.2–5.9 | n.d. - 2.0 | n.d. - 1.5 | Munoz et al. (2018) | |
| France | n.d. - 188 | Bach et al. (2017) | ||||||
| France | n.d. - 4 | n.d. - 4 | n.d. - 8 | n.d. - 7 | n.d. - 13 | Boiteux et al. (2017) | ||
| France | n.d. - 11 | n.d. - 35 | n.d. - 86 | n.d. - 16 | n.d. - 36 | n.d. - 30 | n.d. - 10 | Munoz et al. (2015) |
| Germany | n.d. - 23 | n.d. - 9.4 | n.d. - 13 | n.d. - 24 | n.d. - 6.5 | Llorca et al. (2012) | ||
| Germany | n.d. - 188 | n.d. - 27.6 | n.d. - 13.8 | n.d - 1.31 | 0.61–17.9 | Möller et al. (2010) | ||
| Germany | 0.7–2.8 | 1.6–5.0 | 0.8–2.4 | 2.6–9.7 | 0.2–0.9 | n.d. -0.7 | Ahrens et al. (2009) | |
| India | n.d. - 1.0 | n.d. - 3.0 | 0.4–4.7 | 0.3–3.3 | 0.1–1.2 | n.d. - 0.2 | n.d. - 0.2 | Sharma et al. (2016) |
| Italy | n.d. - 52 | n.d. - 41 | n.d. - 62 | n.d. - 93 | 3–303 | n.d. - 174 | n.d. - 99 | Castiglioni et al. (2015) |
| Italy | n.d. - 401 | n.d. - 505 | n.d. - 346 | n.d. - 946 | n.d - 6480 | n.d. - 70 | n.d. -51 | Valsecchi et al. (2015) |
| Italy | n.d. - 18 | 1–1270 | n.d. - 13 | Loos et al. (2008) | ||||
| Japan | n.d. - 92 | Takagi et al. (2008) | ||||||
| Japan | n.d. - 1.4 | 0.5–2.4 | 0.3–5.0 | 0.2–2.2 | 0.6–5.7 | 0.3–5.0 | n.d. 0.2 | Ye et al. (2014) |
| Japan | n.d. - 423 | n.d. - 460 | n.d - 3703 | n.d. - 1120 | n.d. - 122 | Zushi et al. (2011) | ||
| Korea | n.d. - 7.9 | n.d. - 3.4 | n.d. - 8.3 | n.d. - 4.4 | n.d. - 4.8 | Lam et al. (2014) | ||
| Korea | n.d. - 9.0 | n.d. - 7.2 | n.d. - 42 | n.d. - 7.6 | n.d. - 7.0 | Lam et al. (2016) | ||
| Malta | n.d. - 17 | n.d. - 11 | n.d. - 16 | n.d. - 2.5 | n.d. - 4.4 | Sammut et al. (2017) | ||
| Norway | 0.5–1.8 | n.d. - 2.4 | 0.2–0.3 | 0.1–0.2 | 0.1–0.4 | <0.1 | <0.1 | Kwok et al. (2013) |
| Singapore | 1.0–22 | 0.5–19 | 0.4–7.1 | 1.1–14 | 2.0–21 | 1.2–530 | 1.2–8.6 | Chen et al. (2017) |
| Singapore | n.d - 14.4 | 5.4–38 | 0.9–78 | 0.7–28 | Nguyen et al. (2011) | |||
| South Africa | 5.7–45 | n.d. - 20 | n.d - 2.5 | 0.6–4.6 | n.d. - 1.8 | Groffen et al. (2018) | ||
| Spain | 5.2–644 | 0.1–2.8 | 1.4–18 | 0.6–20 | 0.1–52 | 0.9–20 | 0.1–213 | Campo et al. (2016) |
| Spain | 0.1–111 | 0.1–2.5 | 0.6–25 | 0.6–31 | 0.1–146 | 0.8–52 | 0.1–4.3 | Campo et al. (2015) |
| Spain | 4.9–44 | Flores et al. (2013) | ||||||
| Spain | n.d. - 125 | n.d. - 13 | n.d. - 31 | n.d. - 27 | n.d. - 68 | n.d. - 52 | n.d. - 213 | Llorca et al. (2012) |
| Sweden | 0.5–3.7 | 0.5–4.2 | 0.4–1.7 | 0.2–4.2 | 0.1–5.8 | 0.0–4.4 | Nguyen et al. (2017) | |
| Switzerland | n.d. - 8.7 | 3.6. - 11 | Huset et al. (2008) | |||||
| USA | n.d. - 19 | n.d. - 6.1 | Newsted et al. (2017) | |||||
| USA | n.d. - 458 | n.d. - 45 | n.d. -140 | n.d. - 90 | n.d. - 125 | n.d. - 73 | n.d. - 42 | Nakayama et al. (2010) |
| USA | 1.15–12.7 | 3.27–15.8 | n.d. -3.51 | 0.25–3.58 | Kim and Kannan (2007) | |||
| USA | n.d. - 598 | Hansen et al. (2002) | ||||||
| Vietnam | n.d. - 5.8 | n.d. - 5.6 | n.d. - 9.2 | n.d. - 18 | n.d. - 0.9 | n.d. - 1.3 | Duong et al. (2015) | |
| Vietnam | n.d. - 4.3 | n.d. - 7.8 | n.d. - 54 | n.d. - 4.8 | n.d. - 1.4 | Lam et al. (2017) |
3.2. PFAS concentrations at estuary sites A, B, C, D and F
Water samples collected from estuary sites B and C (ΣPFAS 187 and 165 ng/L, respectively) were much more highly contaminated than sites A, D and F (ΣPFAS 22, 17 and 42 ng/L, respectively). And, in that context, estuaries B and C were also the most highly contaminated with PFOS (75 ng/L and 74 ng/L, respectively; Table 1) and PFHxS (42 ng/L and 30 ng/L, respectively). These two sites are downstream of some of the most heavily industrialised areas of Melbourne and both also had significant levels of PFOA (site B, 9 ng/L; site C, 9 ng/L), PFHxA (site B, 23 ng/L; site C, 11 ng/L), and PFBA (site B, 10 ng/L; site C, 11 ng/L). There are fewer studies of PFAS in estuaries with which to compare our data than those reported for freshwaters, although the PFAS concentrations observed in our estuaries are consistent with levels reported elsewhere in Australia. For example, the levels of PFOS and PFOA concentrations (up to 74 and 9.1 ng/L) are consistent with levels reported in the estuarine sections of the Brisbane River (up to 34 and 11 ng/L, respectively; Gallen et al., 2014) and Sydney harbour (up to 21 and 6.4 ng/L, respectively; Thompson et al., 2011). Overall, our estuary data is consistent with reports of PFAS concentrations in estuaries in China (e.g. Shao et al., 2016; Wang et al., 2011), Japan (Ahrens et al., 2010), Korea (Lam et al., 2016; Hong et al., 2013), and the USA (Konwick et al., 2008) (see Table 3).
Table 3.
Summary of selected (most commonly reported) PFAS concentrations reported in estuaries and embayments.
| Country | Chemical |
Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFBS |
PFHxS |
PFOS |
PFBA |
PFPeA |
PFHxA |
PFHpA |
PFOA |
PFNA |
PFDA |
PFUnDA |
||
| (ng/L) | ||||||||||||
| Australia | 0.4–7.0 | 3.0–30 | 3.9–74 | 1.7–11 | 1.1–9.7 | 1.5–22 | 1.2–8.9 | 2.1–9.1 | 0.2–6.4 | 0.2–1.7 | 0.0–1.1 | This study |
| 0.6–2.6 | 0.1–17 | 0.3–34 | 0.4–6.2 | 0.2–3.7 | 0.1–11 | 0.2–1.3 | 0.1–1.2 | Gallen et al. (2014) | ||||
| 152 +/- 24 | 323 +/- 25 | 231 +/- 17 | 253 +/- 40 | 225 +/- 33 | 303 +/- 36 | 334 +/- 53 | Kaserzon et al. (2012) | |||||
| 1.2–1.5 | 2.7–4.3 | 7.5–21 | 2.8–3.2 | 1.4–2.0 | 4.2–6.4 | 0.6–2.0 | 0.8–1.6 | 0.2–0.3 | Thompson et al. (2011) | |||
| China | 2.3–9.6 | n.d. - 0.6 | n.d. - 0.8 | 25–105 | 20–79 | 4–11 | 0.8–2.3 | 9–16 | 0.4–0.8 | n.d. - 0.3 | Shao et al. (2016) | |
| 2.38 | 107 | 142 | 289 | 211 | 13666 | 1.75 | Heydebreck et al. (2015) | |||||
| n.d. - 30.9 | 2.58–81.7 | Wang et al. (2011) | ||||||||||
| n.d. - 2.25 | 0.17–37 | Ju et al. (2008) | ||||||||||
| n.d. - 99 | n.d. - 260 | So et al. (2007) | ||||||||||
| Germany | 0.37–1.65 | n.d. - 1.62 | n.d. - 3.11 | 0.81–3.83 | 1.31–4.38 | n.d. - 7.74 | 1.57–11.56 | 0.12–2.25 | n.d. - 2.12 | n.d. - 0.34 | Heydebreck et al. (2015) | |
| Japan | 0.5–1.0 | 0.9–2.2 | 8–20 | 2–4 | 0.5–1.5 | 0.4–1.5 | 2.5–9.0 | 2–5 | Ahrens et al. (2010)∗ | |||
| 0.78–17 | 2.7–63 | Sakurai et al. (2010) | ||||||||||
| 0.02–56 | 0.34–57 | 1.8–192 | 0.16–71 | Yamashita et al. (2005) | ||||||||
| 3.3–5.6 | 12.7–25.4 | 154–192 | Yamashita et al. (2004) | |||||||||
| 0.5–25.2 | Saito et al. (2003) | |||||||||||
| Korea | n.d. - 0.4 | n.d. - 1.1 | n.d. - 4.2 | n.d. - 2.0 | n.d. - 8.7 | n.d. - 16 | n.d. - 1.5 | n.d. - 1.0 | Lam et al. (2016) | |||
| 1.0–15 | 0.83–14 | 0.57–26 | 0.78–9.5 | 0.41–5.1 | 0.81–10 | 2.4–34 | 1.3–23 | 0.45–7.7 | 0.17–4.9 | 0.32–5.2 | Hong et al. (2013) | |
| n.d. - 16 | n.d. - 8.7 | 0.35–47 | n.d. - 110 | 0.54–31 | 0.20–5.9 | 0.20–9.3 | 0.22–1.3 | Naile et al. (2013) | ||||
| 2.24. -651 | 0.9–62 | Rostkowski et al. (2006) | ||||||||||
| Norway | n.d. - 0.1 | 0.0–0.2 | 0.0–0.1 | 0.3–0.4 | 0.0–0.1 | 0.0–0.1 | Kwok et al. (2013) | |||||
| USA | 2.5–2.8 | 2.6–3.7 | Konwick et al. (2008) | |||||||||
Estimated from Author figures.
Simcik and Dorweiler (2005) proposed that a PFHpA/PFOA ratio greater than one is indicative of atmospheric PFAS sources, but of non-atmospheric sources associated with urban areas when lower than one. In that context, only Site G had a ratio >1 (1.55), suggesting long distance atmospheric transport as the source of much of the PFAS entering the upper reaches of the Werribee River. All other sites had a PFHpA/PFOA ratio less than one, with Site E ratio 0.4, further suggesting a point source of PFAS on Stony Creek above that sampling point.
3.3. PFAS loads entering Port Philip Bay
To estimate the amount of PFAS entering Port Philip Bay the PFAS concentration at the Yarra and Werribee River outlet sites was multiplied by river flow in 2012–13 (no flow data could be obtained for Laverton Creek or Kororoit Creek for the period in question; the Maribyrnong River enters the Yarra River upstream of the Yarra River site and so contributes to the flux observed at Site D). The estimated yearly PFAS input to PPB is, however, subject to uncertainties due to the possible variations in PFAS concentrations in the river throughout the year, which was not covered in our sampling, resulting from both changes in inputs and the inherent variability of flows in south eastern Australian rivers. During 2012–13, for instance, rainfall across the Melbourne region was generally below-average, which translated into a reduction of runoff into most of Melbourne's surface waters, including the Werribee River, but not the Yarra River, which had higher than average flow during the sampling period (BOM, 2019). Assuming the data obtained in this pilot study was representative of PFAS concentrations in the sampled waters in 2012–13, then at least 11 kg ΣPFAS entered PPB via the Yarra River and 1 kg ΣPFAS from the Werribee River. The PFAS flux estimated in this study is of the same order of magnitude estimated by Ahrens et al. (2015) in Sweden, but at least a thousand times lower than the amount of PFAS estimated to enter German rivers by Möller et al. (2010).
3.4. Ecological risk of observed PFAS concentrations
The current ANZECC & ARMCANZ 2000 do not provide trigger values for any PFAS. The Guidelines are currently being revised, with the development of draft standards for PFOS and PFOA being fast-tracked (EPA Victoria, 2017). zThe default value used in Australia is the 95% species protection value, which is designed to allow for 95% species protection in “slightly-moderately disturbed ecosystems.” This description would apply to Site G. The level of PFOS observed at this site (0.0065 μg/L) is two orders of magnitude lower than the draft Australian guideline level for total PFOS (0.13 μg/L). The highest level of PFOS observed at a freshwater was 0.045 μg/L at site E (Table 1); this concentration is approximately half the draft 95% species protection level for total PFOS. Site E also returned the highest level of PFOA in the study (0.014 μg/L), some four orders of magnitude lower than the draft Australian trigger value for total PFOA (220 μg/L). The risk quotients (RQmed and RQmax) did not exceed 1 for any residue at either site. When the toxic unit (log10TU; Liess and Von Der Ohe, 2005) was calculated no chemical had a log10TUf > -3; further suggesting that observed PFAS would not have posed a short-term risk to organisms in the freshwaters studied at the time of sampling.
No Australian marine water quality trigger values have as yet been proposed. The European environment quality standards (EQS) adopted in EU Directive 2013/39/EU (EU, 2013) provides for a maximum allowable concentration of PFOS in ‘other surface waters,’ which would seem to include estuaries and coastal waters of the type sampled in this pilot program. None of the estuarine samples would have exceeded the EU ‘other waters’ guideline level (7.2 μg/L). Moreover, none of the PFAS observed at estuary sites had RQ above 1 or Log10TU above -3.
The toxic effects of mixtures can occur at much lower concentrations than observed for individual chemicals (Baas et al., 2009). In that context, six PFSAs (PFBS, PFHxS, PFO, PFDA, FOSA, and N-EtFOSAA) were observed in one or more water sample; these PFSAs will have similar modes of action, yet when the sum of toxic units (Σlog10TU) was calculated, no sites had log10TUf or log10Tualg above -3 (the level suggested by Liess and Von Der Ohe (2005) as being problematic). When this process was completed for the PFCAs, no water sample had log10TU above -3, further suggesting little or no short-term risk to organisms in the estuary waters studied at the time of sampling.
4. Conclusions
The main aim of this reconnaissance was to examine the occurrence of common PFAS in water samples collected from seven creeks and estuaries in the Port Philip Bay catchment, Victoria, and to assess the risk to aquatic organisms therein by comparing observed concentrations with regulatory guideline values. Eighteen of the 19 screened PFAS were observed on one or more occasion in one or more samples. The detection of common perfluoroalkyl sulphonates such as PFBS (up to 7.0 ng/L), PFHxS (up to 42 ng/L), and PFOS (up to 75 ng/L), as well as perfluoroalkyl carboxylates such as PFBA (up to 11 ng/L, respectively), PFOA (up to 8.5 ng/L) and PFNA (up to 6 ng/L) in these Victorian estuaries proves that the existence of these PFAS in the Port Philip environment is potentially of concern in Australia. The lack of exceedances of the draft Australian guideline values for PFOS and PFOA in the freshwater water samples would in and of themselves likely not ‘trigger’ a management response, however further investigation of the potential aquatic ecosystem impact of PFAS in metropolitan Melbourne's urban waters is still warranted.
Declarations
Author contribution statement
Mayumi Allinson: Conceived and designed the experiments; Performed the experiments.
Nobuyoshi Yamashita: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Sachi Taniyasu, Eriko Yamazaki: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Graeme Allinson: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Department of Primary Industries, Victoria (Project #06889).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
At the time of the study, CAPIM was funded by The Victorian Science Agenda Investment Fund managed by the Department of Business and Innovation, Victoria (DBI) (www.innovation.vic.gov.au) with partner funding contributed from Melbourne Water, Department of Primary Industries (Victoria), and Environment Protection Authority (Victoria).
References
- Ahrens L., Felizeter S., Sturm R., Xie Z., Ebinghaus R. Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the River Elbe, Germany. Mar. Pollut. Bull. 2009;58(9):1326–1333. doi: 10.1016/j.marpolbul.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Ahrens L., Taniyasu S., Yeung L.W.Y., Yamashita N., Lam P.K.S., Ebinghaus R. Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan. Chemosphere. 2010;79:266–272. doi: 10.1016/j.chemosphere.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Ahrens L., Norström K., Viktor T., Cousins A.P., Josefsson S. Stockholm Arlanda Airport as a source of per- and polyfluoroalkyl substances to water, sediment and fish. Chemosphere. 2015;129:33–38. doi: 10.1016/j.chemosphere.2014.03.136. [DOI] [PubMed] [Google Scholar]
- ANZECC and ARMCANZ . Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand; Canberra, Australia: 2000. Australian and New Zealand Guidelines for Fresh and marine Water Quality. [Google Scholar]
- Australian Water Association EPA warns of PFAS contamination in multiple Victorian water sources. 2017. http://www.awa.asn.au/AWA_MBRR/Publications/Latest_News/EPA_warns_of_PFAS_contamination_in_multiple_Victorian_water_sources.aspx Online, last accessed 22 December 2017.
- Baas J., Jager T., Kooijman S.A.L.M. A model to analyze effects of complex mixtures on survival. Ecotoxicol. Environ. Saf. 2009;72:669–676. doi: 10.1016/j.ecoenv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Baduel C., Mueller J.F., Rotander A., Corfield J., Gomez-Ramos M.-J. Discovery of novel per- and polyfluoroalkyl substances (PFASs) at a fire fighting training ground and preliminary investigation of their fate and mobility. Chemosphere. 2017;185:1030–1038. doi: 10.1016/j.chemosphere.2017.06.096. [DOI] [PubMed] [Google Scholar]
- Bach C., Dauchy X., Boiteux V., Colin A., Hemard J., Sagres V., Rosin C., Munoz J.-F. The impact of two fluoropolymer manufacturing facilities on downstream contamination of a river and drinking water resources with per- and polyfluoroalkyl substances. Environ. Sci. Pollut. Res. 2017;24:4916–4925. doi: 10.1007/s11356-016-8243-3. [DOI] [PubMed] [Google Scholar]
- Boiteux V., Dauchy X., Bach C., Colin A., Hemard J., Sagres V., Rosin C., Munoz J.-F. Concentrations and patterns of perfluoroalkyl and polyfluoroalkyl substances in a river and three drinking water treatment plants near and far from a major production source. Sci. Total Environ. 2017;583:393–400. doi: 10.1016/j.scitotenv.2017.01.079. [DOI] [PubMed] [Google Scholar]
- BOM . 2019. National Water Account 2013. Australian Melbourne: Water Overview. Australian Government Bureau of Meteorology.http://www.bom.gov.au/water/nwa/2013/melbourne/contextual/wateroverview.shtml Available online. Last accessed 2 February 2019. [Google Scholar]
- Campo J., Lorenzo M., Pérez F., Picó Y., Farré M., Barceló D. Analysis of the presence of perfluoroalkylsubstances in water, sediment and biota of the Jucar River (E Spain). Sources, partitioning and relationships with water physical characteristics. Environ. Res. 2016;147:503–512. doi: 10.1016/j.envres.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Campo J., Pérez F., Masiá A., Picó Y., Farré M., Barceló D. Perfluoroalkyl substance contamination of the Llobregat River ecosystem (Mediterranean area, NE Spain) Sci. Total Environ. 2015;503–504:48–57. doi: 10.1016/j.scitotenv.2014.05.094. [DOI] [PubMed] [Google Scholar]
- Castiglioni S., Valsecchi S., Polesello S., Rusconi M., Melis M., Palmiotto M., Manenti A., Davoli E Zuccato E. Sources and fate of perfluorinated compounds in the aqueous environment and in drinking water of a highly urbanized and industrialized area in Italy. J. Hazard Mater. 2015;282:51–60. doi: 10.1016/j.jhazmat.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Chen H., Reinhard M., Nguyen T.V., You L., He Y., Gin K.Y.-H. Characterization of occurrence, sources and sinks of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a tropical urban catchment. Environ. Pollut. 2017;227:397–405. doi: 10.1016/j.envpol.2017.04.091. [DOI] [PubMed] [Google Scholar]
- D’Agostino L.A., Mabury S.A. Certain perfluoroalkyl and polyfluoroalkyl substances associated with aqueous film forming foam are widespread in Canadian surface waters. Environ. Sci. Technol. 2017;51:13603–13613. doi: 10.1021/acs.est.7b03994. [DOI] [PubMed] [Google Scholar]
- DeWitt J.C. In: Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. DeWitt J.C., editor. Humana Press; Cham: 2015. [Google Scholar]
- Duong H.T., Kadokami K., Shirasaka H., Hidaka R., Chau H.T.C., Kong L., Nguyen T.Q., Nguyen T.T. Occurrence of perfluoroalkyl acids in environmental waters in Vietnam. Chemosphere. 2015;122:115–124. doi: 10.1016/j.chemosphere.2014.11.023. [DOI] [PubMed] [Google Scholar]
- EPA Victoria . 2017. Incoming Water Standards for Aquatic Ecosystem protection: PFOS and PFOA Publication 1633.2, August 2017.www.epa.vic.gov.au/~/media/Publications/1633%202.pdf Available online: Last accessed 25 December 2017. [Google Scholar]
- EU Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union. 2013;L226:1–17. [Google Scholar]
- Flores C., Ventura F., Martin-Alonso J., Caixach J. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in N.E. Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Sci. Total Environ. 2013;461–462:618–626. doi: 10.1016/j.scitotenv.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Gallen C., Baduel C., Lai F.Y., Thompson K., Thompson J., Warne M., Mueller J.F. Spatio-temporal assessment of perfluorinated compounds in the Brisbane River system, Australia: impact of a major flood event. Mar. Pollut. Bull. 2014;85:597–605. doi: 10.1016/j.marpolbul.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Gallen C., Drage D., Eaglesham G., Grant S., Bowman M., Mueller J.F. Australia-wide assessment of perfluoroalkyl substances (PFASs) in landfill leachates. J. Hazard Mater. 2017;331:132–141. doi: 10.1016/j.jhazmat.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Giesy J.P., Naile J.E., Khim J.S., Jones P.D., Newsted J.L. Rev Environ Contam Toxicol 202. Springer; New York, NY: 2010. Aquatic toxicology of perfluorinated chemicals; pp. 1–55. [DOI] [PubMed] [Google Scholar]
- Groffen T., Wepener V., Malherbe W., Bervoets L. Distribution of perfluorinated compounds (PFASs) in the aquatic environment of the industrially polluted Vaal River, South Africa. Sci. Total Environ. 2018;627:1334–1344. doi: 10.1016/j.scitotenv.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Hansen K.J., Johnson H.O., Eldridge J.S., Butenhoff J.L., Dick L.A. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee river. Environ. Sci. Technol. 2002;36(8):1681–1685. doi: 10.1021/es010780r. [DOI] [PubMed] [Google Scholar]
- Heydebreck F., Tang J., Xie Z., Ebinghaus R. Alternative and legacy perfluoroalkyl substances: differences between European and Chinese river/estuary systems. Environ. Sci. Technol. 2015;49:8386–8395. doi: 10.1021/acs.est.5b01648. [DOI] [PubMed] [Google Scholar]
- Hong S., Khim J.S., Park J., Kim M., Kim W.-K., Jung J., Hyun S., Kim J.-G., Lee H., Choi H.J., Codling G., Giesy J.P. In situ fate and partitioning of waterborne perfluoroalkyl acids (PFAAs) in the Youngsan and Nakdong River Estuaries of South Korea. Sci. Total Environ. 2013;445–446:136–145. doi: 10.1016/j.scitotenv.2012.12.040. [DOI] [PubMed] [Google Scholar]
- Huset C.A., Chiaia A.C., Barofsky D.F., Jonkers N., Kohler H.-P.E., Ort C., Giger W., Field J.A. Occurrence and mass flows of fluorochemicals in the Glatt valley watershed, Switzerland. Environ. Sci. Technol. 2008;42(17):6369–6377. doi: 10.1021/es703062f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X.D., Jin Y.H., Sasaki K., Saito N. Perfluorinated surfactants in surface, subsurface water and microlayer from Dalian Coastal waters in China. Environ. Sci. Technol. 2008;42:3538–3542. doi: 10.1021/es703006d. [DOI] [PubMed] [Google Scholar]
- Kaserzon S.L., Kennedy K., Hawker D.W., Thompson J., Carter S., Roach A.C., Booij K., Mueller J.F. Development and calibration of a passive sampler for perfluorinated alkyl carboxylates and sulfonates in water. Environ. Sci. Technol. 2012;46:4985–4993. doi: 10.1021/es300593a. [DOI] [PubMed] [Google Scholar]
- Kim S.-K., Kannan K. Perfluorinated acids in air, rain, snow, surface runoff, and lakes: relative importance of pathways to contamination of urban lakes. Environ. Sci. Technol. 2007;41(24):8328–8334. doi: 10.1021/es072107t. [DOI] [PubMed] [Google Scholar]
- Konwick B.J., Tomy G.T., Ismail N., Peterson J.T., Fauver R.J., Higginbotham D., Fisk A.T. Concentrations and patterns of perfluoroalkyl acids in Georgia, USA surface waters near and distant to a major use source. Environ. Toxicol. Chem. 2008;27(1):2011–2018. doi: 10.1897/07-659.1. [DOI] [PubMed] [Google Scholar]
- Kwok K.Y., Yamazaki E., Yamashita N., Taniyasu S., Murphy M.B., Horii Y., Petrick G., Kallerborn R., Kannan K., Murano K., Lam P.K.S. Transport of perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: implications for sources. Sci. Total Environ. 2013;447:46–55. doi: 10.1016/j.scitotenv.2012.10.091. [DOI] [PubMed] [Google Scholar]
- Lam N.H., Cho C.-R., Kannan K., Choa H.-S. A nationwide survey of perfluorinated alkyl substances in waters, sediment and biota collected from aquatic environment in Vietnam: distributions and bioconcentration profiles. J. Hazard Mater. 2017;323:116–127. doi: 10.1016/j.jhazmat.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Lam N.H., Min B.-K., Cho C.-R., Park K.-H., Ryu J.-S., Kim P.-J., Choi K.-H., Morita M., Cho H.-S. Distribution of perfluoroalkyl substances in water from industrialized bays, rivers and agricultural areas in Korea. Toxicol. Environ. Health Sci. 2016;8(1):43–55. [Google Scholar]
- Lam N.-H., Cho C.-R., Lee J.-S., Soha H.-Y.S., Lee B.-C., Lee J.-A., Tatarozako N., Sasaki K., Saito N., Iwabuchi K., Kannan K., Cho H.-S. Perfluorinated alkyl substances in water, sediment, plankton and fish from Korean rivers and lakes: a nationwide survey. Sci. Total Environ. 2014;491–492:154–162. doi: 10.1016/j.scitotenv.2014.01.045. [DOI] [PubMed] [Google Scholar]
- Liess M., Von Der Ohe P.C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005;24(4):954–965. doi: 10.1897/03-652.1. [DOI] [PubMed] [Google Scholar]
- Llorca M., Farré M., Picó Y., Müller J., Knepper T.P., Barceló D. Analysis of perfluoroalkyl substances in waters from Germany and Spain. Sci. Total Environ. 2012;431:139–150. doi: 10.1016/j.scitotenv.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Loi E.I.H., Yeung L.W.Y., Mabury S.A., Lam P.K.S. Detections of commercial fluorosurfactants in Hong Kong marine environment and human blood: a pilot study. Environ. Sci. Technol. 2013;47(9):4677–4685. doi: 10.1021/es303805k. [DOI] [PubMed] [Google Scholar]
- Loos R., Locoro G., Huber T., Wollgast J., Christoph E.H., de Jager A., Gawlik B.M., Hanke G., Umlauf G., Zaldívar J.-M. Analysis of perfluorooctanoate (PFOA) and other perfluorinated compounds (PFCs) in the River Po watershed in N-Italy. Chemosphere. 2008;71(2):306–331. doi: 10.1016/j.chemosphere.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Lu Z., Song L., Zhao Z., Ma Y., Wang J., Yang H., Mad H., Cai M., Codling G., Ebinghaus R., Xie Z., Giesy J.P. Occurrence and trends in concentrations of perfluoroalkyl substances (PFASs) in surface waters of eastern China. Chemosphere. 2015;119:820–827. doi: 10.1016/j.chemosphere.2014.08.045. [DOI] [PubMed] [Google Scholar]
- Möller A., Ahrens L., Surm R., Westerveld J., Van Der Wielen F., Ebinghaus R., De Voogt P. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environ. Pollut. 2010;158(10):3243–3250. doi: 10.1016/j.envpol.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Munoz G., Fechner L.C., Geneste E., Pardon P., Budzinski H., Labadie P. Spatio-temporal dynamics of per and polyfluoroalkyl substances (PFASs) and transfer to periphytic biofilm in an urban river: case-study on the River Seine. Environ. Sci. Pollut. Res. 2018;25(24):23574–23582. doi: 10.1007/s11356-016-8051-9. [DOI] [PubMed] [Google Scholar]
- Munoz G., Giraudel J.-L., Botta F., Lestremau F., Dévier M.-H., Budzinski H., Labadie P. Spatial distribution and partitioning behavior of selected poly- and perfluoroalkyl substances in freshwater ecosystems: A French nationwide survey. Sci. Total. Environ. 2015;517:48–56. doi: 10.1016/j.scitotenv.2015.02.043. [DOI] [PubMed] [Google Scholar]
- Naile J.S., Khim J.S., Hong S., Park J., Kwon B.-O., Ryu J.S., Hwang J.H., Jones P.D., Giesy J.P. Distributions and bioconcentration characteristics of perfluorinated compounds in environmental samples collected from the west coast of Korea. Chemosphere. 2013;90:387–394. doi: 10.1016/j.chemosphere.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Nakayama S.F., Strynar M.J., Reiner J.L., Delinsky A.D., Lindstrom A. Determination of perfluorinated compounds in the Upper Mississippi River basin. Environ. Sci. Technol. 2010;44:4103–4109. doi: 10.1021/es100382z. [DOI] [PubMed] [Google Scholar]
- Newsted J.L., Holem R., Hohenstein G., Lange C., Ellefson M., Reagen W., Wolf S. Spatial and temporal trends of poly- and perfluoroalkyl substances in fish fillets and water collected from pool 2 of the upper Mississippi River. Environ. Toxicol. Chem. 2017;36(11):3138–3147. doi: 10.1002/etc.3891. [DOI] [PubMed] [Google Scholar]
- Nguyen V.T., Reinhard M., Karina G.Y.-H. Occurrence and source characterization of perfluorochemicals in an urban watershed. Chemosphere. 2011;82(9):1277–1285. doi: 10.1016/j.chemosphere.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Nguyen M.A., Wiberg K., Ribeli E., Josefsson S., Futter M., Gustavsson J., Ahrens L. Spatial distribution and source tracing of per- and polyfluoroalkyl substances (PFASs) in surface water in Northern Europe. Environ. Pollut. 2017;220:1438–1446. doi: 10.1016/j.envpol.2016.10.089. [DOI] [PubMed] [Google Scholar]
- Pan C.-G., Zhao J.-L., Liu Y.-S., Zhang Q.-Q., Chen Z.-F., Lai H.-L., Peng F.-J., Liu S.-S., Ying G.-G. Bioaccumulation and risk assessment of per- and polyfluoroalkyl substances in wild freshwater fish from rivers in the Pearl River Delta region, South China. Ecotoxicol. Environ. Saf. 2014;107:192–199. doi: 10.1016/j.ecoenv.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Pan C.-G., Ying G.-G., Zhao J.-L., Liu Y.-S., Jiang Y.-X., Zhang Q.-Q. Spatiotemporal distribution and mass loadings of perfluoroalkyl substances in the Yangtze River of China. Sci. Total Environ. 2014;493:580–587. doi: 10.1016/j.scitotenv.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Rostkowski P., Yamashita N., So I.M.K., Taniyasu S., Lam P.K.S., Falandysz J., Lee K.T., Kim S.K., Khim J.S., Im S.H., Newsted J.L., Jones P.D., Kannan K., Giesy J.P. Perfluorinated compounds in streams of the Shihwa industrial zone and Lake Shihwa, South Korea. Environ. Toxicol. Chem. 2006;25:2374–2380. doi: 10.1897/05-627r.1. [DOI] [PubMed] [Google Scholar]
- Saito N., Sasaki K., Nakatome K., Harada K., Yoshinaga T., Koizumi A. Perfluorooctane sulfonate concentrations in surface water in Japan. Arch. Environ. Contam. Toxicol. 2003;45:149–158. doi: 10.1007/s00244-003-0163-9. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Serizawa S., Isobe T., Kobayashi J., Kodama K., Kume G., Lee J.H., Maki H., Imaizumi Y., Suzuki N., Horiguchi T., Morita M., Shiraishi H. Spatial, phase, and temporal distributions of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) in Tokyo Bay, Japan. Environ. Sci. Technol. 2010;44:4110–4115. doi: 10.1021/es1007609. [DOI] [PubMed] [Google Scholar]
- Sammut G., Sinagra E., Helmus R., de Voogt P. Perfluoroalkyl substances in the Maltese environment – (I) surface water and rain water. Sci. Total Environ. 2017;589:182–190. doi: 10.1016/j.scitotenv.2017.02.128. [DOI] [PubMed] [Google Scholar]
- Sharma B.M., Bharat G.K., Tayal S., Larssen T., Bečanová J., Karásková P., Whitehead P.G., Futter M.N., Butterfield D., Nizzetto L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: emissions and implications for human exposure. Environ. Pollut. 2016;208:704–713. doi: 10.1016/j.envpol.2015.10.050. [DOI] [PubMed] [Google Scholar]
- Shao M., Ding G., Zhang J., Wei L., Xue H., Zhang N., Li Y., Chen G., Sun Y. Occurrence and distribution of perfluoroalkyl substances (PFASs) in surface water and bottom water of the Shuangtaizi Estuary, China. Environ. Pollut. 2016;216:675–681. doi: 10.1016/j.envpol.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Simcik M.F., Dorweiler K.J. Ratio of perfluorochemical concentrations as a tracer of atmospheric deposition to surface waters. Environ. Sci. Technol. 2005;39(22):8678–8683. doi: 10.1021/es0511218. [DOI] [PubMed] [Google Scholar]
- So M.K., Miyake Y., Yeung W.Y., Ho Y.M., Taniyasu S., Rostkowski P., Yamashita N., Zhou B.S., Shi X.J., Wang J.X., Giesy J.P., Yu H., Lam P.K.S. Perfluorinated compounds in the Pearl River and Yangtze river of China. Chemosphere. 2007;68:2085–2095. doi: 10.1016/j.chemosphere.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Takagi S., Adachi F., Miyano K., Koizumi Y., Tanaka H., Mimura M., Watanabe I., Tanabe S., Kannan K. Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan. Chemosphere. 2008;72:1409–1412. doi: 10.1016/j.chemosphere.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Taniyasu S., Kannan K., Yeung L.W.Y., Kwok K.Y., Lam P.K.S., Yamashita N. Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (C2– C4) in precipitation by liquid chromatography–tandem mass spectrometry: comparison to patterns of long-chain perfluorinated acids (C5–C18) Anal. Chim. Acta. 2008;619:221–230. doi: 10.1016/j.aca.2008.04.064. [DOI] [PubMed] [Google Scholar]
- Thomatou A.-A., Zacharias I., Hela D., Konstantinou I. Determination and risk assessment of pesticide residues in Lake Amvrakia (W. Greece) after agricultural land use changes in the lake's drainage basin. Int. J. Environ. Anal. Chem. 2013;93(7):780–799. [Google Scholar]
- Thompson J., Roach A., Eaglesham G., Bartkow M.E., Edge K., Mueller J.F. Perfluorinated alkyl acids in water, sediment and wildlife from Sydney Harbour and surroundings. Mar. Pollut. Bull. 2011;62:2869–2875. doi: 10.1016/j.marpolbul.2011.09.002. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . 2019. ECOTOX User Guide: ECOTOXicology Knowledgebase System. Version 4.0.https://www.epa.gov/ecotox/ Available. [Google Scholar]
- Valsecchi S., Rusconi M., Mazzoni M., Viviano G., Pagnotta R., Zaghi C., Serrini G., Polesello S. Occurrence and sources of perfluoroalkyl acids in Italian river basins. Chemosphere. 2015;129(0):126–134. doi: 10.1016/j.chemosphere.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Wang T., Lu Y., Chen C., Naile J.S., Khim J.S., Park J., Luo W., Jiao W., Hua W., Giesy J.P. Perfluorinated compounds in estuarine and coastal areas of north Bohai Sea, China. Mar. Pollut. Bull. 2011;62:1905–1914. doi: 10.1016/j.marpolbul.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Wang Z., DeWitt J.C., Higgins C.P., Cousins I.T. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017;51:2508–2518. doi: 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Kannan K., Taniyasu S., Horii Y., Okazawa T., Petrick G., Gamo T. Analysis of perfluorinated acids at parts-per-quadrillion levels in seawater using liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol. 2004;38:5522–5528. doi: 10.1021/es0492541. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Kannan K., Taniyasu S., Horii Y., Petrick G., Gamo T. A global survey of perfluorinated acids in oceans. Mar. Pollut. Bull. 2005;51:658–668. doi: 10.1016/j.marpolbul.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Yao Y., Zhu H., Li B., Hua H., Zhang T., Yamazaki E., Taniyasu S., Yamashita N., Sun H. Distribution and primary source analysis of per-and poly-fluoroalkyl substances with different chain lengths in surface and groundwater in two cities, North China. Ecotoxicol. Environ. Saf. 2014;108:318–328. doi: 10.1016/j.ecoenv.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Ye F., Tokumura M., Islam M.S., Zushi Y., Oh J., Masunaga S. Spatial distribution and importance of potential perfluoroalkyl acid precursors in urban rivers and sewage treatment plant effluent - case study of Tama River, Japan. Water Res. 2014;67:77–85. doi: 10.1016/j.watres.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Zhao P., Xia X., Dong J., Xia N., Jiang X., Li Y., Zhu Y. Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river. Sci. Total Environ. 2016;568:57–65. doi: 10.1016/j.scitotenv.2016.05.221. [DOI] [PubMed] [Google Scholar]
- Zushi Y., Ye F., Motegi M., Nojiri K., Hosono S., Suzuki T., Kosugi Y., Yaguchi K., Masunaga S. Spatially detailed survey on pollution by multiple perfluorinated compounds in the Tokyo Bay basin of Japan. Environ. Sci. Technol. 2011;45:2887–2893. doi: 10.1021/es103917r. [DOI] [PubMed] [Google Scholar]