Key Points
Question
Can RNA genetic testing (RGT) improve the diagnostic outcome of DNA genetic testing (DGT) in patients with suspected hereditary cancer predisposition?
Findings
In this diagnostic study, performing RGT clarified 88% of inconclusive DGT results evaluated, and in 47% of cases, the results were consistent with a molecular diagnosis of hereditary cancer predisposition, compared with 41%, in which inconclusive results were clarified as negative. Of 307 812 patients who underwent DGT for hereditary cancer, 7265 had putative splicing variants that were identified as likely pathogenic or of uncertain significance, indicating that they could benefit from RGT.
Meaning
In this diagnostic study, RGT as a supplement to DGT was associated with an improved outcome of hereditary cancer predisposition genetic testing and could affect medical management in at least 1 in 43 patients if performed concurrently.
This diagnostic study evaluates whether RNA genetic testing (RGT) is associated with an improvement in the diagnostic outcome of DNA genetic testing (DGT) and in the delivery of personalized cancer risk management for patients with hereditary cancer predisposition.
Abstract
Importance
Performing DNA genetic testing (DGT) for hereditary cancer genes is now a well-accepted clinical practice; however, the interpretation of DNA variation remains a challenge for laboratories and clinicians. Adding RNA genetic testing (RGT) enhances DGT by clarifying the clinical actionability of hereditary cancer gene variants, thus improving clinicians’ ability to accurately apply strategies for cancer risk reduction and treatment.
Objective
To evaluate whether RGT is associated with improvement in the diagnostic outcome of DGT and in the delivery of personalized cancer risk management for patients with hereditary cancer predisposition.
Design, Setting, and Participants
Diagnostic study in which patients and/or families with inconclusive variants detected by DGT in genes associated with hereditary breast and ovarian cancer, Lynch syndrome, and hereditary diffuse gastric cancer sent blood samples for RGT from March 2016 to April 2018. Clinicians who ordered genetic testing and received a reclassification report for these variants were surveyed to assess whether RGT-related variant reclassifications changed clinical management of these patients. To quantify the potential number of tested individuals who could benefit from RGT, a cohort of 307 812 patients who underwent DGT for hereditary cancer were separately queried to identify variants predicted to affect splicing. Data analysis was conducted from March 2016 and September 2018.
Main Outcomes and Measures
Variant reclassification outcomes following RGT, clinical management changes associated with RGT-related variant reclassifications, and the proportion of patients who would likely be affected by a concurrent DGT and RGT multigene panel testing approach.
Results
In total, 93 if 909 eligible families (10.2%) submitted samples for RGT. Evidence from RGT clarified the interpretation of 49 of 56 inconclusive cases (88%) studied; 26 (47%) were reclassified as clinically actionable and 23 (41%) were clarified as benign. Variant reclassifications based on RGT results changed clinical management recommendations for 8 of 18 patients (44%) and 14 of 18 families (78%), based on responses from 18 of 45 clinicians (40%) surveyed. A total of 7265 of 307 812 patients who underwent DGT had likely pathogenic variants or variants of uncertain significance potentially affecting splicing, indicating that approximately 1 in 43 individuals could benefit from RGT.
Conclusions and Relevance
In this diagnostic study, conducting RNA testing resolved a substantial proportion of variants of uncertain significance in a cohort of individuals previously tested for cancer predisposition by DGT. Performing RGT might change the diagnostic outcome of at least 1 in 43 patients if performed in all individuals undergoing genetic evaluation for hereditary cancer.
Introduction
Germline DNA genetic testing (DGT) identifies individuals with cancer predisposition syndromes, enabling the implementation of personalized strategies for cancer prevention, early detection, and/or targeted therapy. This approach has been associated with decreases in cancer-associated morbidity and mortality.1,2,3 Although advances in nucleic acid sequencing technology have facilitated the implementation of DGT in clinical practice, interpretation of the data remains a challenge. Anywhere from 2% to 44% of variants identified by DGT are classified as variants of uncertain significance (VUS), depending on the genes tested,4,5 which poses significant challenges to laboratories, the medical community, and patients.6 Patients with VUS in the BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185) genes are also more likely to obtain a second opinion from medical oncologists for cancer treatment and are at greater risk for anxiety and medical mismanagement of hereditary risks.7,8 The presence of a VUS may also preclude patient access to targeted therapies, such as poly(adenosine diphosphate-ribose) polymerase inhibitors, which were recently approved by the US Food and Drug Administration to treat certain types of BRCA1/2-mutated ovarian and breast cancers.9,10,11 Inconclusive DGT results, or VUS, also limit the identification of family members facing increased cancer risks and their options for risk reduction. The ability to reach family members and translate the results of an individual’s genetic testing into a family management strategy is a critical component that supports the cost-effectiveness models for genetic testing.12 Considering the clinical implications of DGT on hereditary cancer prevention and treatment, clarification of a variant’s significance as either benign or pathogenic is of utmost importance.
It is estimated that approximately 15% to 25% of DNA variants in genes associated with hereditary cancer disrupt the respective RNA splicing of the gene,13,14 yet these alterations are most often classified as VUS because of a lack of functional RNA evidence. As such, developing high-throughput functional assays to clarify DGT results is an important step toward addressing the urgent need for improved genetic testing outcomes.15,16,17,18 In 2016, we implemented an RNA genetic testing (RGT) program to facilitate classification of putative splicing variants identified by DGT in hereditary cancer predisposition genes. In the present study, we investigated the variant reclassification outcomes following RGT and explored clinical management changes associated with variant reclassifications resulting from RGT. In addition, we estimated the proportion of patients who would likely be affected by a concurrent DGT and RGT approach.
Methods
RNA Genetic Testing
Study participants submitted samples to Ambry Genetics’ Translational Genomics Laboratory for complementary RNA studies between March 2016 and April 2018. Patients were eligible to receive RGT if a germline variant was predicted to impact splicing by at least 1 in silico tool (ie, Human Splicing Finder,19 MaxEnt,20 Berkeley Drosophila Genome Project,21 ESEfinder,22 or SpliceScanII23) or if a germline variant occurring at the native donor or acceptor splice site, regardless of in silico prediction, was identified on DGT in any hereditary cancer gene with known RNA expression in blood and with loss of function as the mechanism of disease. In addition, the individual had to be available to submit a blood sample for RNA extraction. Patients were offered participation by direct contact between study staff and clinicians during the entirety of the study or through informational materials attached to clinical test reports beginning in October 2017. The clinicians who ordered genetic testing from Ambry Genetics as part of patient workup were not directly involved in this study, with the exception of clinicians at 4 participating institutions (ie, Dana-Farber Cancer Institute, Cedars-Sinai Medical Center, Rutgers Cancer Institute of New Jersey, and University of Kansas Cancer Center). When available, family members were also tested. Peripheral blood was collected in PAXgene Blood RNA Tubes (PreAnalytiX) from patients according to manufacturer instructions, and RNA sequencing analysis was performed by CloneSeq as previously described.24 Patients were included in the present study if RGT-eligible germline variants were identified in the following genes associated with hereditary breast and ovarian cancer (HBOC), ie, ATM (OMIM 607585), BRCA1, BRCA2, BRIP1 (OMIM 605882), PALB2 (OMIM 610355), RAD50 (OMIM 604040), RAD51C (OMIM 602774), RAD51D (OMIM 602954); Lynch syndrome (LS), ie, MLH1 (OMIM 120436), MSH2 (OMIM 609309), MSH6 (OMIM 600678), PMS2 (OMIM 600259); or hereditary diffuse gastric cancer (HDGC), ie, CDH1 (OMIM 192090). The use of data produced from this supplemental RNA testing was approved and carried out in accordance with the recommendations of the Western Institutional Review Board (Puyallup, Washington). All patients described were evaluated by genetic counselors and provided informed consent for testing. This study followed the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guideline.
Interpretation of Genetic Test Results
Classification of germline variants identified by DGT was performed following the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) guidelines.25,26,27 Multiple lines of evidence were combined to reach 1 of the 5 following classifications: (1) pathogenic variant; (2) variant, likely pathogenic (VLP); (3) VUS; (4) variant, likely benign (VLB); or (5) benign variant.27 Guidelines from the ACMG/AMP place greater weight on experimental evidence of abnormal splicing than on evidence from in silico predictors.25,26,27 In accordance with ACMG/AMP guideline recommendations, RGT results were considered strong evidence of pathogenicity or strong evidence of benignity when applicable. Results from RGT were then combined with existing evidence to reassess variant classification toward pathogenic or benign classification.25,27 Amended reports were issued to clinicians for all patients who carried reclassified variants, including those who had been previously identified through clinical testing at Ambry Genetics but were not participants in this study. In addition, updated variant classifications were made publicly available at ClinVar.28
Clinical Management Survey
To evaluate whether RNA-related variant reclassifications altered clinical management, we conducted a survey of clinicians who received amended test results for patients who underwent RGT. The survey was designed to assess whether the updated variant interpretation had resulted in changes to clinical management for the tested patient and/or their family members (eMethods in the Supplement). Clinicians were sent an invitation email for each amended test result received, which contained a link to complete an anonymous survey hosted by Qualtrics, a secure online survey service. Individual survey responses could not be linked to the survey respondent or associated case; however, there were 4 selected cases for which 5 of us (H.Q.R., S.C., J.H., D.T., D.C.) provided detailed case descriptions, representing at least 1 case from each disease area studied. The survey protocol was deemed exempt from informed consent because it was already covered, approved, and carried out in accordance with the recommendations of the Western Institutional Review Board.
Frequency of Splicing Alterations in a Multigene Panel Testing Cohort
To estimate the proportion of patients who would likely be affected by a concurrent DGT and RGT approach, we assessed the frequency of DNA splicing variants in a multigene panel testing cohort. Results from all patients undergoing multigene DGT for hereditary cancer susceptibility at Ambry Genetics between March 2012 and September 2018 (n = 307 812) were queried for VLP and VUS predicted to generate abnormal messenger RNA (mRNA) transcripts across a broader range of cancer predisposition genes with known RNA expression in blood, ie, APC (OMIM 611731), ATM, BRCA1, BRCA2, BRIP1, CHEK2 (OMIM 604373), CDH1, MLH1, MSH2, MSH6, PMS2, MUTYH (OMIM 604933), NF1 (OMIM 613113), PTEN (OMIM 601728), PALB2, RAD51C, RAD51D, and TP53 (OMIM 191170). The following 4 alteration types were included: (1) variants located in the native splice sites, (2) missense and synonymous alterations predicted deleterious by at least 1 splicing in silico model, (3) structural variations such as exonic intragenic duplications, and (4) alterations in 5′ and 3′ untranslated regions. Query results were used to calculate the relative proportion of VLP and VUS attributable to splicing alterations as well as the proportion of patients with VLP and VUS predicted to impact splicing.
Statistical Analysis
Statistical analysis was conducted with Python version 3.7.4 (Python Software Foundation). Statistical significance was set at P < .05. All tests were 2-tailed.
Results
Classification of Germline Splicing Alterations Following RGT and Associated Diagnostic Outcomes
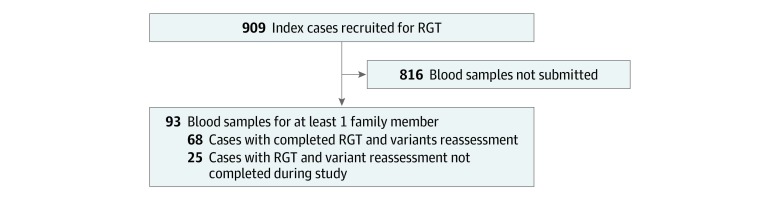
In total, 93 of 909 eligible families (10.2%) submitted samples for RGT. At the time of data analysis, variant assessment and reclassification had been completed for 64 variants from 68 families for whom RGT had been completed (Figure 1; eTable in the Supplement). Of the 64 unique alterations studied, 56 (88%) had been classified as VUS and 8 (13%) as VLP prior to RGT. Approximately half of the alterations studied were in HBOC-associated genes (30 [47%]), and alterations in genes associated with LS and HDGC accounted for 14 (22%) and 20 (31%) alterations tested, respectively (eTable in the Supplement). Overall, RGT provided sufficient evidence to reclassify 55 variants (86%) analyzed in this study (Figure 2). A summary of the detailed evidence used to reclassify these alterations, including corresponding ACMG/AMP classification criteria, is provided in the eTable in the Supplement.
Figure 1. Ascertainment of Cases Studied by RNA Genetic Testing (RGT).
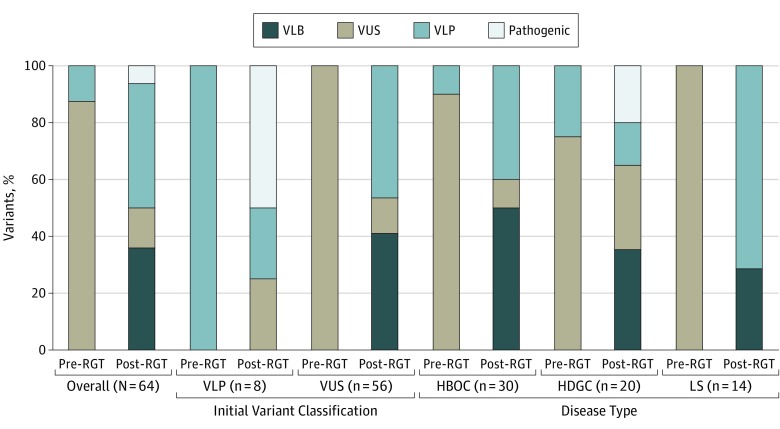
Figure 2. Variant Classification Before and After RNA Genetic Testing (RGT).
Variant classifications before and after RGT for all variants tested, stratified by initial classification and disease type. HBOC indicates hereditary breast and ovarian cancer; HDGC, hereditary diffuse gastric cancer; LS, Lynch syndrome; VLB, variant, likely benign; VLP, variant, likely pathogenic; VUS, variant of uncertain significance.
The addition of RGT decreased the number of VUS classifications, with 49 VUS (88%) upgraded to VLP (26 [47%]) or downgraded to VLB (23 [41%]) (Figure 2). The remaining 7 VUS (13%) were not reclassified because of insufficient evidence (eg, clinical history not informative) or because RGT identified in-frame transcript(s) with uncertain significance (eTable in the Supplement). Of all examined VLP, 4 (50%) were upgraded to pathogenic, 2 (25%) were downgraded to VUS, and 2 (25%) remained VLP (Figure 2). All VLPs upgraded to pathogenic were CDH1 alterations with additionally supportive clinical, population-based, and computational evidence (eTable in the Supplement). For the two VLPs downgraded to VUS, ie, BRCA1 c.670+1G>T and CDH1 c.387+1G>A, RGT identified in-frame transcripts of uncertain functional significance. In addition, both families lacked supporting phenotypes.
As per clinical laboratory protocol, amended reports were sent to clinicians of all patients who had been reported previously as carriers of these alterations. A total of 440 previously tested patients received reclassification reports as a result of performing RGT for these 64 variants. This included 88 patients (20.0%) who received reports amended to positive and 322 patients (73.2%) with inconclusive results now clarified as negative.
Of the 64 tested variants, 53 (83%) were predicted to have a deleterious effect on splicing by at least 1 of 5 in silico predictors queried, and 48 (75%) were predicted to impact splicing by at least 3 models. Most variants (52 of 64 [82%]) affected a native splice site; the remainders were coding variants predicted by in silico models to create a novel splice site or strengthen an alternate, so-called cryptic splice site. Based on our RGT results, in silico models incorrectly predicted a splicing impact for 16% to 25% of the 64 variants tested (ie, false-positives) and failed to predict a splicing impact for 3% to 9% of the variants (ie, false-negatives) (eTable in the Supplement), which demonstrated the risk of relying solely on splicing in silico models to predict the pathogenicity of DNA variants identified by clinical genetic tests.
Clinical Management Changes Associated With RGT-Related Variant Reclassifications
To assess the clinical management changes associated with RGT-related variant classifications, 4 clinicians (9%) who received amended results for patients in the RGT program were asked to provide detailed case descriptions, including post-RGT management recommendations, and 41 (91%) were offered a survey to evaluate clinical management decisions following RGT. Genetic counselors represented most clinicians invited to participate in the survey (26 [63%]), with physicians (11 [27%]) and advanced practice nurses or physician assistants (4 [10%]) accounting for the rest. Of the 41 cases eligible for the survey, 14 (34%) completed the survey. Additionally, detailed case descriptions, including clinical management recommendations, were obtained for 4 cases (Figure 3).
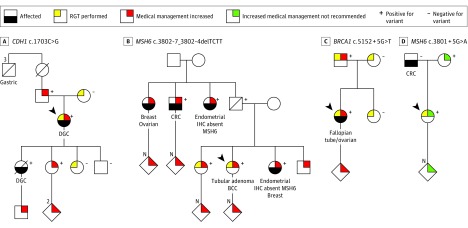
Figure 3. Pedigrees Demonstrating Clinical Management Changes From Selected Cases Undergoing RNA Genetic Testing.
A, The variant CDH1 c.1703C>G was identified by DNA genetic testing in an index patient meeting diagnostic criteria for hereditary diffuse gastric cancer (DGC; ie, 2 gastric cancer cases in the family, regardless of age, at least 1 confirmed DGC, and 1 case of DGC diagnosed at age <40 years). The variant was initially classified as a variant of uncertain significance, and subsequent to the reclassification to a likely pathogenic variant, the index patient was referred to the high-risk breast clinic for management of increased lobular breast cancer risk and consideration of risk-reducing bilateral mastectomy. The reclassification also impacted the clinical management of family members, as they became eligible for cascade genetic testing. B, Index patient was diagnosed with colorectal tubular adenoma and was found to carry the variant MSH6 c.3802-7_3802-4delTCTT on a 67-gene panel test. The patient’s sister, who had been previously diagnosed with endometrial adenocarcinoma showing loss of MSH6 (OMIM 600678) protein expression on immunohistochemistry (IHC), also carried this variant. Both sisters opted to participate in this study, and reclassification of the variant from a variant of uncertain significance to a likely pathogenic variant confirmed a diagnosis of Lynch syndrome. Both sisters elected risk-reducing total abdominal hysterectomy with bilateral salpingo-oophorectomy and were recommended to continue additional screening per National Comprehensive Cancer Network guidelines. Genetic testing for their adult children and other at-risk relatives was also recommended. C, A 24-gene panel identified the variant BRCA1 c.5152 + 5G>T in a woman diagnosed with papillary serous carcinoma of the right fallopian tube and both ovaries. The patient and her family opted to participate in this study, which resulted in the variant being reclassified from a variant of uncertain significance to a likely pathogenic variant. Subsequently, the patient was offered high-risk breast screening as defined by the National Comprehensive Cancer Network. The patient’s father was identified to carry the likely pathogenic variant and was recommended increased screening for prostate and breast cancers. D, A 34-gene panel identified the variant MSH6 c.3801 + 5G>A in an index patient with a family history of cancer. Colorectal cancer (CRC) screening recommendations for the patient included colonoscopy every 5 years based on family history. No abnormal transcripts were identified by RGT, resulting in reclassification from a variant of uncertain significance to a likely benign variant and no recommendation of increased medical management. No further familial testing was recommended for this variant. Diagonal lines indicate that the individual is deceased; arrows indicate index patients. BCC indicates basal cell carcinoma.
Overall, clinical management recommendations for patients changed in 8 of 18 cases (44%) surveyed, while recommendations for family members changed in 14 of 18 cases (78%). In 8 of 10 cases (80%) for which the variant was upgraded from VUS to clinically actionable variants (ie, VLP or pathogenic variant), clinicians recommended increased screening and/or risk-reducing interventions for the patient. Examples included an annual colonoscopy for a patient with LS and referrals to discuss risk-reducing bilateral salpingo-oophorectomy and mastectomy for HBOC. In 9 of 10 cases (90%), increased screening and/or genetic testing for family members was also recommended. The remaining responses related to cases for which a VUS was downgraded to benign or VLB. In accordance with published recommendations,29 patient management did not change as a result of the benign reclassification in any of these cases; however, family management strategy changed in most cases (5 of 8 [63%]). For example, clinicians recommended against family genetic testing and decreased the number of follow-up visits in several cases. In addition, multiple clinicians commented on the reassurance that these downgrades offered the families.
On a case-by-case level, the specific medical management recommendations varied based on the gene and clinical history (Figure 3). Upgrades from VUS to clinically actionable variants resulted in increased surveillance and/or recommendations to perform risk-reducing surgeries for all individuals heterozygous for CDH1 c.1703C>G (Figure 3A), MSH6 c.3802-7_3802-4delTCTT (Figure 3B), or BRCA1 c.5152 + 5G>T (Figure 3C). The reclassifications also resulted in recommendations for cascade testing of relatives with unknown carrier status (Figure 3A-C). In the case of MSH6 c.3801 + 5G>A (Figure 3D), no abnormal splicing was identified by RGT, supporting reclassification of the variant to VLB. For this patient, recommendations for screening did not change; however, consideration for risk-reducing surgery was now not advised. Additionally, no further familial testing was recommended for this variant.
Frequency of Variants Predicted to Affect Splicing in a Large Cohort of Patients Undergoing DGT
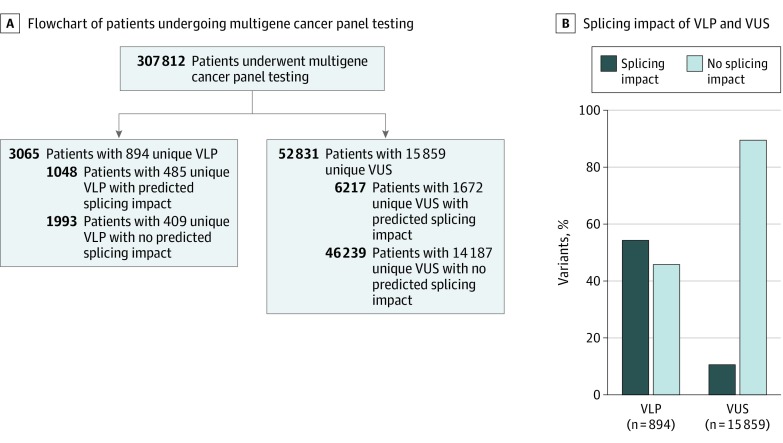
To estimate the potential impact of RGT if performed concurrently with DGT for selected hereditary cancer genes, we assessed the frequency of variants with a predicted splicing impact in a cohort of 307 812 patients undergoing DGT (Figure 4A). In total, 3065 individuals were identified with 894 unique VLP, and 52 831 individuals were identified with 15 859 unique VUS (Figure 4A). Variants predicted to affect splicing accounted for 1672 of 15 859 (10.5%) reported VUS and 485 of 894 (54.2%) reported VLP (Figure 4B). Collectively, 1048 and 6217 individuals were carriers for VLP and VUS predicted to affect splicing, respectively, encompassing at total of 7265 individuals (2.4%) in the tested cohort, indicating that approximately 1 in 43 individuals (7265 of 307 812) could benefit from RGT.
Figure 4. Distribution of Variants Likely Pathogenic (VLP) and Variants of Uncertain Significance (VUS) in a Multigene Panel Testing Cohort.
A, In a cohort of 307 812 patients receiving multigene panel testing, 3065 individuals were identified with 894 unique VLPs, and 52 831 individuals were identified with 15 859 unique VUS in the following genes: APC, ATM, BRCA1, BRCA2, BRIP1, CHEK2, CDH1, MLH1, MSH2, MSH6, PMS2, MUTYH, NF1, PTEN, PALB2, RAD51C, RAD51D, TP53. A total of 7265 individuals (2.4%) were carriers for variants predicted to affect splicing. B, Distribution of VLP and VUS in the studied cohort. Variants predicted to affect splicing accounted for 485 VLPs (54%) and 1672 VUS (11%).
Discussion
Clinicians in oncology routinely use DGT to inform clinical decisions surrounding cancer treatment and surveillance. For example, identification of a germline pathogenic variant in BRCA1 in a female patient with breast cancer could be the determining factor in her decision to pursue bilateral mastectomy vs lumpectomy or her eligibility for poly(adenosine diphosphate-ribose) polymerase inhibitor therapy. The benefits of genetic test results extend beyond the index patient, as cascade testing of at-risk family members identifies those who may benefit from increased surveillance and risk-reducing surgical procedures. Negative genetic testing results are also helpful, as they distinguish patients and family members who are not as likely to benefit from such interventions.
The clinical utility of germline DGT relies on the ability to detect and characterize disease-causing variants, yet multigene panel testing currently yields inconclusive results (ie, VUS) in 5% to 40% of cases. As such, methods to reduce the VUS burden are of high interest to those reporting and receiving genetic testing results. A substantial proportion of inconclusive results arise from the detection of variants that are predicted to generate abnormal mRNA transcripts but lack functional evidence. We applied a high-throughput RGT assay to generate functional evidence for such variants, with the goal of reducing VUS burden. Of 56 VUS studied, the addition of RGT resulted in reclassification of 88% as either clinically actionable or benign. Data collected on clinical management demonstrated the medical relevance of RGT-based variant reclassification. Recommendations for screening and risk-reducing surgery increased for probands and their family members when VUS were upgraded to pathogenic or VLP; downgrading VUS to benign or VLB also resulted in changes in management, most often in the direction of less testing and fewer follow-up visits for family members. In 2 cases, RGT led to a VLP being downgraded to VUS, exemplifying the need for functional evidence at the time of initial variant classification even when DNA variants are expected to be pathogenic based on their location at the canonical splice site. Furthermore, these cases serve as key examples of the limitations of in silico splicing prediction models, as both were predicted deleterious. In silico models yielded a 25% false-positive rate among the 64 variants studied. Classification of canonical splice site variants as likely pathogenic based on prediction alone risks the overdiagnosis of hereditary cancer predisposition, as in these 2 cases.
Limitations
Results from this study are subject to limitations. First, the selection of variants in this study was limited by patient availability to submit an additional blood sample for RGT. Thus, studies designed to include RGT concurrently with DNA analysis are needed to fully assess the impact of RGT if available to all patients. Medical management data were also limited because of survey participation; however, based on current practice guidelines, we would expect results to remain consistent across the remaining cases. Results from this study may have also underestimated the clinical impact of variant reclassification, which extend well beyond the index family. More than 400 patients received amended reports as a result of reclassifications occurring in this study alone. Because these variants will continue to be seen in future patients tested, these reclassifications have a downstream impact as well. Further, as part of routine data sharing with ClinVar, these results would be expected to also affect patients with the same variants identified through other clinical laboratories, also not quantified in this study.
It is clear that RGT contributes to a decrease in VUS rates; however, there are substantial limitations to performing RGT after DGT has been conducted. The process of recontacting patients retrospectively places a significant time burden on clinicians and laboratory staff. In addition, RGT requires an additional blood sample, which can be logistically challenging to obtain after the fact. As shown in this study, samples were received for only 10% of index cases recruited for RGT. Performing RGT after DGT also imposes an inherent delay in the reclassification of VUS, which limits the clinical utility of the DGT results. Based on the frequency of splicing alterations in our multigene DGT cohort, we expect that RGT results could potentially lead to variant reclassifications for at least 1 of 43 patients undergoing genetic testing. As a comparator, gross deletion and/or duplication analysis, which is routinely included as a component of DGT, identifies pathogenic variants in 1 of 142 patients tested.30 Thus, if RGT were routinely performed alongside DGT, the potential consequences would exceed that of gross deletion and/or duplication analysis. This estimation also underestimates the overall number of individuals, since RGT may also detect intronic splicing variants outside the analytical range of clinical DGT that are currently not accounted for.31,32,33,34,35 It will be important to evaluate the use of concurrent DGT and RGT in a prospective cohort to assess the ability of RNA analysis to decrease VUS rates and increase the identification of clinically actionable variants in an unbiased fashion.
Conclusions
In this study, conducting RGT as a supplement to DGT improved the outcome of hereditary cancer predisposition genetic testing, clarifying 88% of inconclusive results. The ability to accurately define the significance of an identified variant relieves uncertainty and results in improved management for patients and their families. It can potentially ameliorate the collective strain that VUS place on the health care system by reducing the need for second opinions and focusing return visits on optimizing management,7 thereby allowing cancer screening and treatment resources to be allocated more appropriately. Along with the data presented in this study, these attributes support the implementation of RGT to the diagnostic workflow of clinical laboratories performing genetic testing for hereditary cancer genes.
eMethods. RNA Clinical Management Survey
eTable. Summary of Variants Analyzed by RGT
References
- 1.Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):-. doi: 10.1056/NEJMsr1501341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23-32. doi: 10.1001/jamaoncol.2014.168 [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JH, Enns R, Heidelbaugh J, Barkun A; Clinical Guidelines Committee . American Gastroenterological Association Institute guideline on the diagnosis and management of Lynch syndrome. Gastroenterology. 2015;149(3):777-782. [DOI] [PubMed] [Google Scholar]
- 4.LaDuca H, Stuenkel AJ, Dolinsky JS, et al. Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genet Med. 2014;16(11):830-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesaran T, Karam R, Huether R, et al. Beyond DNA: an integrated and functional approach for classifying germline variants in breast cancer genes. Int J Breast Cancer. 2016;2016:2469523. doi: 10.1155/2016/2469523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia C, Lyon L, Littell RD, Powell CB. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. Genet Med. 2014;16(12):896-902. [DOI] [PubMed] [Google Scholar]
- 7.Kurian AW, Friese CR, Bondarenko I, et al. Second opinions from medical oncologists for early-stage breast cancer: prevalence, correlates, and consequences. JAMA Oncol. 2017;3(3):391-397. doi: 10.1001/jamaoncol.2016.5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232-2239. doi: 10.1200/JCO.2016.71.6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies H, Glodzik D, Morganella S, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med. 2017;23(4):517-525. doi: 10.1038/nm.4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi: 10.1056/NEJMoa1506859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer. 2018;119(2):141-152. doi: 10.1038/s41416-018-0127-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampel H. Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer. 2016;15(3):423-427. doi: 10.1007/s10689-016-9893-5 [DOI] [PubMed] [Google Scholar]
- 13.Nielsen FC, van Overeem Hansen T, Sørensen CS. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer. 2016;16(9):599-612. doi: 10.1038/nrc.2016.72 [DOI] [PubMed] [Google Scholar]
- 14.Rhine CL, Cygan KJ, Soemedi R, et al. Hereditary cancer genes are highly susceptible to splicing mutations. PLoS Genet. 2018;14(3):e1007231. doi: 10.1371/journal.pgen.1007231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davy G, Rousselin A, Goardon N, et al. Detecting splicing patterns in genes involved in hereditary breast and ovarian cancer. Eur J Hum Genet. 2017;25(10):1147-1154. doi: 10.1038/ejhg.2017.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Findlay GM, Daza RM, Martin B, et al. Accurate classification of BRCA1 variants with saturation genome editing. Nature. 2018;562(7726):217-222. doi: 10.1038/s41586-018-0461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matreyek KA, Starita LM, Stephany JJ, et al. Multiplex assessment of protein variant abundance by massively parallel sequencing. Nat Genet. 2018;50(6):874-882. doi: 10.1038/s41588-018-0122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starita LM, Islam MM, Banerjee T, et al. A multiplex homology-directed DNA repair assay reveals the impact of more than 1,000 BRCA1 missense substitution variants on protein function. Am J Hum Genet. 2018;103(4):498-508. doi: 10.1016/j.ajhg.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67. doi: 10.1093/nar/gkp215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11(2-3):377-394. doi: 10.1089/1066527041410418 [DOI] [PubMed] [Google Scholar]
- 21.Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J Comput Biol. 1997;4(3):311-323. doi: 10.1089/cmb.1997.4.311 [DOI] [PubMed] [Google Scholar]
- 22.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31(13):3568-3571. doi: 10.1093/nar/gkg616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churbanov A, Vorechovský I, Hicks C. A method of predicting changes in human gene splicing induced by genetic variants in context of cis-acting elements. BMC Bioinformatics. 2010;11:22. doi: 10.1186/1471-2105-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farber-Katz S, Hsuan V, Wu S, et al. Quantitative analysis of BRCA1 and BRCA2 germline splicing variants using a novel RNA-massively parallel sequencing assay. Front Oncol. 2018;8:286. doi: 10.3389/fonc.2018.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou Tayoun AN, Pesaran T, DiStefano MT, et al. ; ClinGen Sequence Variant Interpretation Working Group (ClinGen SVI) . Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39(11):1517-1524. doi: 10.1002/humu.23626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K, Krempely K, Roberts ME, et al. Specifications of the ACMG/AMP variant curation guidelines for the analysis of germline CDH1 sequence variants. Hum Mutat. 2018;39(11):1553-1568. doi: 10.1002/humu.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Center for Biotechnology Information . ClinVar. https://www.ncbi.nlm.nih.gov/clinvar/. Accessed September 11, 2019.
- 29.Plon SE, Eccles DM, Easton D, et al. ; IARC Unclassified Genetic Variants Working Group . Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29(11):1282-1291. doi: 10.1002/humu.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2019. doi: 10.1038/s41436-019-0633-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings BB, Marshall JL, Tukiainen T, et al. ; Genotype-Tissue Expression Consortium . Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med. 2017;9(386):eaal5209. doi: 10.1126/scitranslmed.aal5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frésard L, Smail C, Ferraro NM, et al. ; Undiagnosed Diseases Network; Care4Rare Canada Consortium . Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts. Nat Med. 2019;25(6):911-919. doi: 10.1038/s41591-019-0457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamanaka K, Miyatake S, Koshimizu E, et al. RNA sequencing solved the most common but unrecognized NEB pathogenic variant in Japanese nemaline myopathy. Genet Med. 2019;21(7):1629-1638. [DOI] [PubMed] [Google Scholar]
- 34.Kernohan KD, Frésard L, Zappala Z, et al. Whole-transcriptome sequencing in blood provides a diagnosis of spinal muscular atrophy with progressive myoclonic epilepsy. Hum Mutat. 2017;38(6):611-614. doi: 10.1002/humu.23211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremer LS, Bader DM, Mertes C, et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun. 2017;8:15824. doi: 10.1038/ncomms15824 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. RNA Clinical Management Survey
eTable. Summary of Variants Analyzed by RGT