Abstract
The safety of a novel microbial muramidase (Muramidase 007) as a feed additive for swine was evaluated in a target animal safety study (Experiment 1). Forty weanling pigs were allotted to 4 dietary treatments: T1 control group, and 3 groups receiving Muramidase 007 in increasing doses: T2 65,000 (1X), T3 325,000 (5X) and T4 650,000 (10X) LSU(F)/kg feed. The efficacy of Muramidase 007 on growth performance was evaluated in a feeding experiment (Experiment 2). A total of 288 piglets were allotted to two groups: T1 control group and T2 receiving Muramidase 007 at 50,000 (LSU(F)/kg feed. In Experiment 1, no growth depression of pigs was observed. No adverse effects of Muramidase 007 were observed for any of the hematology and serum chemistry parameters measured or on pig health status. Post-mortem evaluation showed no adverse effects due to Muramidase 007 supplementation in the gross pathology or in the histological examination. In Experiment 2, Muramidase 007 significantly increased overall (d 0–42) average daily gain (ADG) and tended to improve overall average daily feed intake (ADFI) and day 42 body weight of nursery pigs and had no effect on feed conversion ratio (FCR). Overall, results of these studies show that there were no adverse effects of Muramidase 007 compared to the control group.
Keywords: Agriculture, Microbiology, Toxicology, Animal nutrition, Agricultural policy, Microorganism, Microbial biotechnology, Enzymology, Safety, Tolerance, Feed, Muramidase, NOAEL, SWINE, Regulatory
Agriculture; Microbiology; Toxicology; Animal nutrition; Agricultural policy; Microorganism; Microbial biotechnology; Enzymology; Safety; Tolerance; Feed; Muramidase; NOAEL; SWINE; Regulatory
1. Introduction
Effective gastrointestinal functionality is a very important topic in animal nutrition and health, as it plays a key role in profitability and sustainable production (Celi et al., 2017). The addition of exogenous enzymes to animal feed is a common practice to enhance the digestibility of nutrients (Menezes-Blackburn and Greiner, 2015) and to support the digestion for the benefit of the animal and its microbiota (Bedford and Cowieson, 2012). Recently, a novel enzyme was shown to support the gastrointestinal functionality and to improve growth performance in broiler chickens (Lichtenberg et al., 2017; Goodarzi Boroojeni et al., 2019). This novel enzyme is a muramidase (EC 3.2.1.17), referred to in this paper as Muramidase 007 which belongs to the class of N-acetylmuramidases, also known as lysozymes. Muramidases are ubiquitous in nature and present in many organisms such as bacteria, viruses, plants, invertebrates, and animals (Callewaert and Michiels, 2010). Muramidase cleaves the β-1, 4 glycosidic linkages between N-acetylmuramic acid and N-acetyl glucosamine in the carbohydrate backbone of peptidoglycan, the major structural polymer uniquely found in nature in bacterial cell walls (Vollmer et al., 2008).
Catalysis of the depolymerization of peptidoglycans via dietary muramidase has been found to improve feed efficiency (May et al., 2012; Oliver and Wells, 2013; Oliver et al., 2014; Ma et al., 2017; Vanrolleghem et al., 2019). Muramidase 007 is proposed to be used in the feed market as a digestive aid to support gastrointestinal functionality in swine. Its safety for application in poultry feed has been recently established (Lichtenberg et al., 2017). The objectives of the studies detailed in this paper were to assess the safety of Muramidase 007 in growing pigs and determine if supplementation of the enzyme can also improve growth performance.
2. Material and methods
2.1. Description of the muramidase
The Muramidase 007 was supplied by Novozymes A/S (Bagsvaerd, Denmark) and it was produced as described by Lichtenberg et al. (2017). The enzyme activity is expressed in muramidase units, coded LSU(F), and reflects the ability of the enzyme to lyse peptidoglycans. One LSU(F) unit is defined as the amount of enzyme that is needed to increase the fluorescence of a 12.5 μg/ml fluorescein-labelled peptidoglycan suspension by a value that corresponds to the fluorescence of 0.077 mM fluorescein isothiocyanate (FITC), per minute at pH 7.5 and 30 °C.
2.2. Experiment 1 (safety study)
The target animal safety study with pigs was conducted at Charles River Laboratories Edinburgh Ltd. facilities in East Lothian, UK. This study was performed in accordance with the OECD Principles of Good Laboratory Practice as incorporated into the United Kingdom Statutory Instrument for GLP. The animal study protocol was approved by the Animal Ethics Committee of Charles River.
2.2.1. Animals and treatments
A total of forty weaned piglets (Landrace/Large White), 20 uncastrated males and 20 females, were sourced from the local commercial supplier and used in a 42-day study. All piglets were acclimated in two separate group pens, one for males and one for females, for 14 days followed by a further 14 days of acclimation in individual pens. Pigs were weaned at 28 days and were 56 days old at the start of the study with an average body weight (BW ± SD) of 18.7 ± 2.3 kg. Pigs were randomized into four treatment groups, each group containing ten animals, five uncastrated males and five females. The experiment consisted of four dietary treatments as follows:
-
•
T1: control non-supplemented basal diet;
-
•
T2: basal diet supplemented with Muramidase 007 at 65,000 LSU(F)/kg feed (1X);
-
•
T3: basal diet supplemented with Muramidase 007 at 325,000 LSU(F)/kg feed (5X):
-
•
T4: basal diet supplemented with Muramidase 007 at 650,000 LSU(F)/kg feed (10X).
The Muramidase 007 enzyme used in this study had an analyzed muramidase activity of 87,850 LSU(F)/g. Muramidase 007 was included in the diets in dry form. Pigs were fed treatment diets from a period of 42 days.
2.2.2. Housing, management, diet and feeding
Throughout the study pigs were individually housed in a 4 m2 pen. Housing room temperatures and humidity were recorded daily and ranged from 12 to 23 °C and 14 to 83%, respectively. Animals were fed individually with both feed and water available ad libitum. Individual feed and water intake were recorded daily throughout the study. During the acclimation period, animals were offered a commercially available wheat-based diet in mash form without Muramidase 007. The diet was formulated to comply with National Research Council recommendations (NRC, 2012). Pigs were weighed on day 0, 14, 28 and 42.
2.2.3. Measurements and examinations
2.2.3.1. Feed sampling and composition
Feed sampling was performed at each of the two feed manufacturing occasions for the two batches of feed prepared. On each occasion, 10 samples of 500 g each were collected. For quality control, samples of the experimental diets were analyzed for chemical proximate composition (energy – MJ/kg, moisture content, ash, crude protein, sugar (as sucrose), fiber (neutral detergent), starch, calcium, sodium, phosphorus and potassium and oil) at Sciantec Analytical Services Ltd. (Cawood, North Yorkshire, UK) before approval was granted for feeding the diets to the animals. The in feed muramidase activity was determined at Charles River laboratories (Edinburg, UK) to confirm the proper supplementation of Muramidase 007 in the experimental diets. The ingredient and nutrient composition of the basal diets are described in Table 1.
Table 1.
Composition and nutritive value of the experimental diets used in the safety study (experiment 1).
| Ingredients (g/kg) | Starter diet | Grower diet |
|---|---|---|
| Micro Oats | 200.00 | 0 |
| Micro Wheat | 110.15 | 192.26 |
| Whey powder | 159.38 | 100.00 |
| Soybean meal | 100.00 | 100.00 |
| Full Fat Soya | 146.55 | 150.00 |
| Soya Protein Concentrate | 55.00 | 81.10 |
| Wheat | 66.66 | 199.99 |
| Milk powder | 55.00 | 0 |
| Barley | 0 | 70.00 |
| Soya oil | 40.76 | 40.00 |
| Dextrose | 25.00 | 25.00 |
| Limestone | 4.19 | 0 |
| Mono-Calcium Phosphate | 8.81 | 12.48 |
| NaCl | 1.83 | 3.31 |
| Pig Premix1 | 15.00 | 15.00 |
| L-Lysine HCl | 5.20 | 5.00 |
| DL- Methionine | 2.90 | 2.87 |
| L-Threonine | 2.49 | 2.17 |
| L-Tryptophan | 0.80 | 0.57 |
| Vanilla | 0.25 | 0.25 |
| Calculated composition (%) | ||
| Pig DE (MJ/kg)2 | 15.5 | 14.8 |
| Crude protein | 21.4 | 20.2 |
| Calcium | 0.79 | 0.78 |
| Phosphorus | 0.69 | 0.71 |
| Starch | 26.9 | 31.3 |
| Sugars | 13.6 | 10.3 |
| Analyzed composition (%) | ||
| Crude protein | 21.1 | 21.1 |
| Crude fat | 9.2 | 7.6 |
| Calcium | 0.93 | 0.80 |
| Phosphorus | 0.58 | 0.61 |
| Starch | 25.4 | 30.5 |
| Sugars | 10.8 | 9.5 |
Premix supplied per kilogram of diet: vitamin A, 10,000 IU; vitamin D3, 2,000 IU; vitamin E, 75 mg; vitamin K3, 2.0 mg; vitamin B1, 1.0 mg; vitamin B2, 3.0 mg; vitamin B6, 2.0 mg; vitamin B12, 10 μg; D-calcium pantothenate, 15 mg; folic acid, 1.0 mg; niacin, 20 mg; Cu (copper sulphate), 160 mg; I (calcium iodate), 1.5 mg; Fe (ferrous sulfate), 200 mg; Mn (manganese oxide), 404.5 mg; Zn (zinc oxide), 100 mg; Se (sodium selenite), 0.35 mg; choline (choline chloride), 200 mg; and Ca (calcium carbonate) 0.6 g.
Pig DE = Digestible Energy DEdm/MJ/Kg dry matter = 17.47 + (0.079 x CRPdm) + (0.158 x OAHdm) - (0.331 x Ashdm) - (0.140 x NDFdm).
2.2.3.2. General health observations and fecal scoring
Each animal was observed daily for general health and behavior from the beginning of acclimation to the end of the study by qualified personnel. All animals were inspected by a veterinarian on arrival and were subject to a physical examination on day -3, 2, 16, 30 and 42. These examinations were performed by a masked veterinarian and included the following: general appearance and behavior, integumentary system, musculoskeletal system, cardiovascular system, respiratory system, gastrointestinal system, urinary system, reproductive system, lymphatic system, nervous system, ocular system. The following vital signs were also measured, rectal body temperature (°C), heart rate (beats/min), respiration rate (breaths/min). The feces of each animal were checked visually on days -9, 2 and 42 to assess the consistency according to the following score guide: 1 = normal; 2 = semi-solid and 3 = watery diarrhea, and the presence/absence of mucous and/or blood.
2.2.3.3. Urine collection and analysis
Urine samples were collected from each animal for urinalysis on days -3, 2, 21 and 42. Urine was collected by placing each animal in an individual crate containing a tray suitable for the collection of urine. Once produced, urine (≥1 mL) was collected from the tray and transferred into a labelled 50 mL plastic tube and stored at 4 °C until analysis. Urine samples were analyzed on the day of collection using the Clinitek Advantus Urine Chemistry Analyzer (Siemens) for color, protein, pH, ketones, bilirubin, urobilinogen. Microscopic examination of sediment was performed for crystals, casts, red blood cells and white blood cells. Specific gravity was measured on a refractometer.
2.2.3.4. Clinical pathology
Blood samples were collected from the jugular vein on day 42. Blood specimens were collected for each animal into the following tubes; potassium EDTA (hematology), lithium heparin tubes with no anticoagulant (serum biochemistry) and tri-sodium citrate (coagulation). Blood tubes were immediately placed on ice (except for the heparin tubes which were kept at room temperature) pending processing. Blood in the EDTA tubes was not processed as it was used for hematological analysis. The heparin and tri-sodium citrate tubes were centrifuged at 2500 × g for 15 min at room temperature. Hematological examinations were performed using a Siemens Advia 2120 Hematology System (Beckman Coulter, Inc) and included hemoglobin, hematocrits, total white blood cell count, differential white blood cell count (neutrophils, lymphocytes, monocytes, eosinophils, basophils, large unclassified cells), red blood cell count, platelet count, reticulocyte count, mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration. Coagulation parameters were measured using a Sysmex CA 1500 Coagulation Instrument (Siemens Healthcare Diagnostics, Inc.) and included prothrombin time, activated partial thromboplastin time and fibrinogen. Clinical chemistry analysis was performed using a Roche/Hitachi P Modular 800 clinical chemistry analyzer (Roche Diagnostics) and included alanine aminotransferase, albumin, albumin-globulin ratio, alkaline phosphatase, amylase, aspartate aminotransferase, calcium, chloride, creatinine, creatinine phosphokinase, gamma glutamyl transferase, globulin, glucose, glutamate dehydrogenase, haptoglobin, lactate dehydrogenase, magnesium, phosphate, phospholipids, potassium, triglycerides, sodium, sorbitol dehydrogenase, total cholesterol, total protein, urea, total bilirubin, direct bilirubin and indirect bilirubin.
2.2.3.5. Pathology
On day 42, all pigs were sacrificed by captive bolt, pithing and exsanguination. Each animal was subject to a detailed necropsy and tissues collection for histopathological evaluation. The necropsy consisted of an external and internal examination of all major organs. All gross lesions were recorded in descriptive terms, including location(s), approximate size (in mm), shape, color, consistency and number. Organ weights, representative of the major organ systems, were obtained for the heart, brain, spleen, liver, gonads (testes or ovaries) lungs, kidneys and brain. Tissue collection for histopathological evaluation included: artery (aorta), bone marrow, bone, brain, caecum, cervix, colon, duodenum, epididymis, eye, gall bladder, gland (adrenal, mammary, pituitary, thyroid, parathyroid), heart, ileum, jejunum, kidney, larynx, liver, lymph node, lung, muscle (skeletal), nasal cavity, esophagus, ovary, pancreas, pharynx, prostate, rectum and spinal cord. All specimens were preserved in 10% neutral buffered formalin which was refreshed every 24 h. Tissues slides from all treatment groups were prepared for histopathological evaluation as follows. Tissue sections were cut ca 4–6 μm thick, processed and stained with haematoxylin and eosin. The marrow smear was stained with May-Grunwald's Giemsa stain. Stained sections of tissues were evaluated by a pathologist for all pigs in the study.
2.2.4. Statistical analyses
Data obtained on each quantitative study parameter measured more than one time during the treatment period (with the exception of body weight gain) was statistically evaluated via repeated-measured analysis of covariance with treatment (T1, T2, T3, T4), sex (male, female) and time (health observations days 2, 16, 30, 42; urine days 2, 21, 42) as the main effects, along with all the interactions of the main effects. Pretreatment value was used as a covariate in the model and was included as a random effect. Endpoint parameters (blood hematology, clinical chemistry, coagulation, organ weights) were analyzed as one-way analysis of variance (ANOVA) with treatment (T1, T2, T3, T4) and sex (male, female) as the main effects, along with their interaction. Growth performance data were analyzed as one-way analysis of variance (ANOVA) with treatment (T1, T2, T3, T4) as a fixed effect. All statistical analysis was conducted using Fit Model platform of JMP 13.0 (SAS Inst. Inc., Cary, NC). For all response criteria, pen was the experimental unit. Variability in the data was expressed as pooled SEM, and statistical significance was determined at p < 0.05. Means separation was determined using Tukey's honest significant difference test.
2.3. Experiment 2 (efficacy study)
The efficacy study was conducted at the Nursery Pig House of the DSM Animal Nutrition Research Center Co., Ltd. (Bazhou, P. R. China). The animal study protocol was approved by the Animal Welfare Committee of DSM (China) Animal Nutrition Research Center.
2.3.1. Animals and treatments
A total of 288, 21-day old commercial cross breed (PIC L1050 x L337) barrows and gilts with an average initial body weight of 6.2 ± 0.7 kg were used in a 42-day study. Piglets were randomly assigned by initial body weight into 24 replicate blocks in an environmentally controlled experimental room and each block consisted of two pens with six piglets each (three males and three females). Each pen measured 3.0 × 1.8 m, resulting in a stocking density of 0.9 m2 per pig and it was equipped with fully-slatted flooring, two nipple drinkers and one trough. Room temperature and ventilation were computer-controlled to maintaining an optimal environment according to the age of the pigs. The environmental control system comprised a heating system, a negative pressure ventilation system, and a water-curtain-enabled air-cooling system (Big Dutchman, Vechta, Germany). Temperature was set at 30 °C at the initiation of the trial and then decreased by 1 °C per week until reaching 24 °C. Relative humidity varied from 55 to 75%. Water and feed in mash form were supplied ad libitum. The lighting was adjusted to bright during the day and to dim during the night. Throughout a 42-day observation period, piglets were fed experimental diets in two phases consisting of a pre-starter diet from d 0–14 and a starter diet from d 15–42 (Table 3). Piglets were fed one of two dietary treatments: (T1) a non-supplemented basal diet or (T2) basal diet supplemented with Muramidase 007 at 50,000 LSU(F)/kg diet (Table 2). Seven pigs were removed from the study due to diarrhea or emaciation (five from control treatment and two from the Muramidase 007 treatment).
Table 3.
Composition and nutritive value of the experimental diets used in the efficacy study (experiment 2).
| Ingredient, g/kg | Pre-starter | Starter |
|---|---|---|
| Corn | 382.50 | 475.50 |
| Corn, extruded | 130.00 | 180.00 |
| Soybean meal, 47% CP | 70.00 | 100.00 |
| Soy protein concentrate | 70.00 | 35.00 |
| Full-fat soy bean, extruded | 100.00 | 70.00 |
| Whey permeate | 100.00 | 60.00 |
| Fish meal | 60.00 | 50.00 |
| Lactose | 50.00 | 0 |
| Soybean oil | 10.00 | 5.00 |
| Salt | 2.50 | 3.00 |
| Limestone | 7.505 | 7.405 |
| Monocalcium phosphate | 2.00 | 1.50 |
| Phytase1 | 0.075 | 0.075 |
| ZnO | 2.50 | 0 |
| L-Lysine·HCl | 4.50 | 4.50 |
| DL-Methionine | 0.80 | 0.60 |
| L-Threonine | 1.50 | 1.50 |
| L-Tryptophan | 0.40 | 0.20 |
| Muramidase 007 or SiO2 | 0.72 | 0.72 |
| DNP premix 42052, 0.5% | 5.00 | 5.00 |
| Calculated nutrients & energy3 | ||
| Crude protein, % | 20.6 | 19.0 |
| ME, kcal/kg | 3,420 | 3,373 |
| Total Ca, % | 0.81 | 0.73 |
| Total P, % | 0.71 | 0.65 |
| SID, % | ||
| Lys | 1.33 | 1.20 |
| Met | 0.39 | 0.35 |
| Thr | 0.78 | 0.72 |
| Trp | 0.23 | 0.19 |
| Val | 0.80 | 0.73 |
| Analyzed components, % | ||
| Moisture | 9.5 | 10.1 |
| Crude protein | 20.2 | 18.8 |
| Starch | 37.4 | 42.9 |
| Crude fat | 5.7 | 4.9 |
| Crude fiber | 1.1 | 1.7 |
RONOZYME® NP at the supplementation level of 75 g/metric ton of feed is equal to 1.27 kg total P and 1.06 kg total Ca/metric ton of feed.
DSM 4205 premix supplied per kilogram of diet: vitamin A, 9,750 IU; vitamin D3, 3,000 IU; vitamin E, 63 mg; vitamin K3, 3.0 mg; vitamin B1, 3.0 mg; vitamin B2, 9.6 mg; vitamin B6, 4.5 mg; vitamin B12, 36 μg; D-biotin, 240 μg; D-calcium pantothenate, 30 mg; folic acid, 1.8 mg; niacin, 36 mg; Cu (tribasic copper chloride), 190 mg; I (potassium iodate), 0.6 mg; Fe (ferrous sulfate), 120 mg; Mn (manganese sulfate), 60 mg; Zn (zinc sulfate), 120 mg; Se (sodium selenite), 450 μg; choline (choline chloride), 300 mg; and Ca (calcium carbonate) 0.6 g.
Nutrients and energy levels were calculated according to values in NRC (2012).
Table 2.
Analyzed Muramidase activity in experimental diets used in the safety (experiment 1) and efficacy (experiment 2) studies.
| Treatment group | Muramidase (LSU(F)/kg∗ |
|
|---|---|---|
| Inclusion level | Analyzed activity | |
| Experiment 1 | ||
| Treatment 1 (control) | 0 | LOQ∗∗ |
| Treatment 2 (1X) | 65,000 | 59,177 |
| Treatment 3 (5X) | 325,000 | 302,691 |
| Treatment 4 (10X) | 650,000 | 635,282 |
| Experiment 2 | ||
| Treatment 1 (control) | ||
| Pre-Starter | 0 | < LOQ∗∗ |
| Starter | 0 | < LOQ∗∗ |
| Treatment 2 | ||
| Pre-Starter | 50,000 | 44,180 |
| Starter | 50,000 | 43,860 |
One unit of muramidase (LSU(F)) is the amount of enzyme that increases the fluorescence of a 12.5 μg/ml fluorescein-labelled peptidoglycan suspension by a value that corresponds to the fluorescence of 0.077 mM fluorescein isothiocyanate (FITC), per minute at pH 7.5 and 30 °C.
LOQ: Limit of quantification.
2.3.2. Feed sampling and measurements
Two representative feed samples (approx. 500 g) of each treatment at each study phase were collected by pooling samples from 20-kg feed bags at the time the feed was dispensed in bags for storage and transportation. For quality control, samples of the experimental diets were scanned using a FOSS NIRS DS2500 (FOSS NIRSystems Inc., Laurel, MD, USA) before approval was granted for feeding the diets to the animals. The muramidase activity of the dietary samples was analyzed by Biopract GmbH (Berlin, Germany) to confirm the correct supplementation of Muramidase 007 to the experimental diets. The ingredient and nutrient composition of the basal diets of pre-starter and starter pigs are shown in Table 3. The body weight of the pigs was recorded on day 0, 14, and 42 of the study. Feed allowance and leftover were recorded every 14 days. The number of pig-days per period and pen was also recorded. Data were pen-aggregated (averaged by pen) prior to statistical analysis. Average daily gain was calculated by adding the individual weight gains of pigs per pen and divided by the number of pig-days of that specific pen. Data of culled pigs were included in the analysis with the body weight on their last day in trial. Pen-aggregated average daily body weight gain in the relevant period was then calculated using these data and the number of pig days per pen. Feed intake was measured by pen only, and the average daily feed intake (ADFI) was calculated by dividing the feed intake of that specific pen by the number of pig-days of that pen. Feed conversion ratio (FCR) was calculated by dividing ADFI by the average daily gain (ADG). Fecal score was recorded on a pen basis on day 7, 14, 21, 28, 35 and 42 according to a 4-points score system with 0 for normal feces, 1 for soft feces, 2 for mild diarrhea, and 3 for severe diarrhea.
2.3.3. Statistical analysis
Data were analyzed with a two-sided two-sample t-test procedure of SAS version 9.3 (SAS Inc., Cary, NC) with pen-aggregated data. The t value and the degree of freedom (n-1) were used to generate the corresponding p value to indicate whether the difference between the control and Muramidase 007 treatment reached a statistically significant level. Normality of the data points for each treatment were checked graphically (Q-Q plot) and formally by applying Shapiro-Wilk’s test.
3. Results
3.1. Experiment 1: safety study
3.1.1. Feed analyses
The nutrient analyses of the starter and grower diets fed to pigs during the target animal safety study (Table 1) showed a good agreement with expected values. The analyzed muramidase activity of the diets used in the safety study are shown in Table 2. The supplementation of Muramidase 007 in feed resulted in an average muramidase activity of 59,177 LSU(F)/kg feed for the T2, 302,691 LSU(F)/kg feed for T3 and 635,282 LSU(F)/kg feed for T4 which accounted for 91%, 93% and 98% of the intended muramidase activity of 65,000, 325,000 and 650,000 LSU(F)/kg feed for T2, T3, and T4, respectively. The analyzed value of muramidase activity in the control treatment (T1) was below the limit of quantification of the assay.
3.1.2. Body weight, feed and water intake, fecal score and general health of pigs
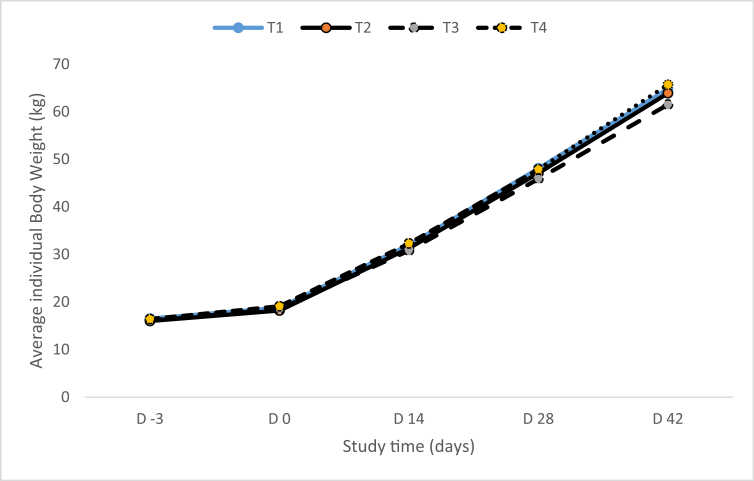
The pattern of BW change during the study is reported in Fig. 1 and the overall (d 0–42) average daily feed intake and water consumption are detailed in Table 5. There was no significant ‘treatment × sex’ interaction for BW, overall average daily feed intake and water consumption. Likewise, there was no effect of treatment on BW recorded on days 0, 14, 28 or 42 of the study and all pigs gained weight equally. Overall (d 0–42) average daily feed intake and water consumption were not affected by treatment also. The incidence of diarrhea was sporadic during the study and not affected by treatment (data not shown). No treatment-related effects on animal health were observed during clinical examinations (day 2, 16, 30 and 42) of the pigs. Descriptive statistics for rectal temperature (RT), heart rate (HR) and respiratory rate (RR) are presented in Table 4. There was no significant ‘treatment x time’ interactions for RT, HR or RR. Likewise, there was no main effect of treatment on RT, HR or RR recorded on day 2, 16, 30 and 42.
Fig. 1.
Effects of dietary supplementation with increasing levels of Muramidase 007 (T1 = 0 LSU(F)/kg feed, T2 = 65,000 LSU(F)/kg feed, T3 = 325,000 LSU(F)/kg feed, T4 = 650,000 LSU(F)/kg feed) on pig body weight on day -3, 0, 14, 28 and 42 of the study (kg).
Table 5.
Effect of dietary supplementation with increasing levels of Muramidase 007 (T1 = 0 LSU(F)/kg feed, T2 = 65,000 LSU(F)/kg feed, T3 = 325,000 LSU(F)/kg feed, T4 = 650,000 LSU(F)/kg feed) on average daily feed and water consumption (day 0–42) and hematology profile for all pigs on day 42 of the study. Different letters indicate significant differences between the groups (p < 0.05).
| T1 | T2 | T3 | T4 | SE | |
|---|---|---|---|---|---|
| Average daily water consumption d 0–42 (L/d) | 3.64 | 3.32 | 3.34 | 3.86 | 0.221 |
| Average daily feed intake d 0–42 (kg/d) | 1.86 | 1.74 | 1.73 | 1.81 | 0.061 |
| HgB (g/L) | 133.5 | 139.1 | 137.3 | 137.7 | 2.63 |
| HCT (L/L) | 0.41 | 0.43 | 0.42 | 0.43 | 0.01 |
| WBC (109/L) | 14.8 | 15.6 | 17.2 | 17.4 | 1.03 |
| RBC (1012/L) | 7.2 | 7.6 | 7.4 | 7.6 | 0.15 |
| MCV (fL) | 57.7 | 56.6 | 57.1 | 56.9 | 0.71 |
| MCH (pg) | 18.7 | 18.3 | 18.7 | 18.1 | 0.26 |
| MCHC (g/dL) | 32.3ab | 32.3ab | 32.7a | 31.8b | 0.18 |
| Platelet (PLT, 109/L) | 338.1 | 347.3 | 373.7 | 345.9 | 30.19 |
| Neutrophils (NEU, 109/L) | 4.17 | 4.06 | 4.63 | 4.85 | 0.299 |
| NEU percent of WBC (%) | 27.8 | 26.2 | 27.8 | 28.2 | 2.32 |
| Lymphocytes (LYM, 109/L) | 9.65 | 10.29 | 11.28 | 11.10 | 0.973 |
| LYM percent of WBC (%) | 64.9 | 65.8 | 64.7 | 636.6 | 2.56 |
| Monocytes (MON, 109/L) | 0.58 | 0.72 | 0.69 | 0.72 | 0.062 |
| MON percent of WBC (%) | 3.93 | 4.55 | 4.13 | 4.15 | 0.40 |
| Eosinophils (EOS, 109/L) | 0.35 | 0.35 | 0.34 | 0.48 | 0.069 |
| EOS percent of WBS (%) | 2.38 | 2.28 | 2.03 | 2.86 | 0.450 |
| Basophils (BAS, 109/L) | 0.07 | 0.10 | 0.15 | 0.13 | 0.031 |
| BAS as percent of WBC (%) | 0.46 | 0.65 | 0.78 | 0.70 | 0.122 |
| Large unstained cells (LUC, 109/L) | 0.059 | 0.069 | 0.076 | 0.089 | 0.011 |
| LUC as percent of WBC (%) | 0.412 | 0.447 | 0.450 | 0.520 | 0.066 |
| PT (sec) | 10.9 | 11.2 | 10.8 | 10.9 | 0.17 |
| APTT (sec) | 9.9a | 10.5a | 8.7b | 9.6ab | 0.35 |
| Fibrinogen (mg/dL) | 6.1 | 5.8 | 6.2 | 5.8 | 0.22 |
HgB, Hemoglobin; HCT, Hematocrit; WBC, White Blood Count; PLT, platelet; RBC, Red Blood Count; MCV, Mean Corpuscular Volume; MCH, Mean Corpuscular Hemoglobin; MCHC, Mean Corpuscular Haemoglobin Concentration; PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time.
Table 4.
Effects of dietary supplementation with increasing levels of Muramidase 007 (T1 = 0 LSU(F)/kg feed, T2 = 65,000 LSU(F)/kg feed, T3 = 325,000 LSU(F)/kg feed, T4 = 650,000 LSU(F)/kg feed) on rectal temperature, heart rate and respiration rate of all pigs (days 2, 16, 30 & 42).
| Treatment |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | SEM | TRT | Day | Trt × Day | |
| Heart rate, beats/min | 160.53 | 161.43 | 161.39 | 162.57 | 3.06 | 0.973 | 0.001 | 0.706 |
| Respiratory rate, breaths/min | 44.66 | 43.77 | 45.01 | 46.96 | 1.43 | 0.456 | 0.011 | 0.611 |
| Rectal Temperature, °C | 39.27 | 39.25 | 39.25 | 39.24 | 0.04 | 0.890 | 0.001 | 0.112 |
3.1.3. Clinical pathology
3.1.3.1. Hematology and serum chemistry
Descriptive statistics for the day 42 hematology and serum chemistry are summarized in Tables 5 and 6, respectively. There was a significant ‘treatment × sex’ interaction for large unstained cells (LUC; p = 0.04) and LUC as a percent of white blood cells (p = 0.04). Females receiving T3 and males receiving T1 (LUC; 0.05 × 103/μL and 0.047 × 103/μL, respectively) had significantly lower LUC's and LUC's as a percent of white blood cells (p = 0.04) than females receiving T4 and males receiving T3 (LUC; 0.10 × 103/μL and 0.103 × 103/μL, respectively), but were not different to all other treatments (data not shown). There was a significant effect of treatment on mean corpuscular hemoglobin concentration (MCHC; p = 0.004) and activated partial thromoboplast in time (APTT; p = 0.006). Pigs fed T3 had significantly higher MCHC values than pigs fed T4 (32.7 vs 31.8 g/dL; p = 0.004) but were not different to the control group T1 and group T2. The APTT value of pigs fed T3 was significantly lower than pigs fed T1 or T2 (8.7 vs 9.9 or 10.5 s; p = 0.006), however none of the treatment were different to group T4. There was no ‘treatment × sex’ interactions or main effect of treatment identified for any other hematology parameter measured. There was a main effect of sex on hemoglobin (139.9 vs 133.9 g/L; p = 0.01) and hematocrit (0.434 vs 0.414 L/L; p = 0.008) where by values for both were higher in females than in males (data not shown).
Table 6.
Effect of dietary supplementation with increasing levels of Muramidase 007 (T1 = 0 LSU(F)/kg feed, T2 = 65,000 LSU(F)/kg feed, T3 = 325,000 LSU(F)/kg feed, T4 = 650,000 LSU(F)/kg feed) on serum chemistry profile for all pigs on day 42 of the study. Different letters indicate significant differences between the groups (p < 0.05).
| T1 | T2 | T3 | T4 | SE | |
|---|---|---|---|---|---|
| Urea (mml/L) | 4.0 | 3.9 | 4.1 | 4.3 | 0.249 |
| Creatinine (μmol/L) | 79.5 | 83.2 | 71.7 | 81.3 | 3.82 |
| Glucose (mmol/L) | 6.6 | 6.9 | 6.1 | 6.4 | 0.26 |
| Sodium (mmol/L) | 146.8 | 146.1 | 147.7 | 149.2 | 1.03 |
| Potassium (mmol/L) | 5.14 | 5.37 | 5.35 | 5.55 | 0.233 |
| Chloride (mmol/L) | 102.4 | 102.6 | 102.7 | 103.0 | 0.78 |
| ALT (U/L) | 34.2 | 34.2 | 35.6 | 35.9 | 2.44 |
| ALP (U/L) | 189.1 | 190.4 | 182.0 | 215.1 | 11.35 |
| AST (U/L) | 33.7b | 39.0ab | 53.0a | 37.9ab | 4.77 |
| CPK (U/L) | 2184.3 | 1961.7 | 2547.5 | 2320.4 | 522.1 |
| GGT (U/L) | 35.8 | 36.5 | 36.6 | 38.2 | 2.14 |
| GDH (U/L) | 1.2 | 1.2 | 2.7 | 1.3 | 1.02 |
| LDH (U/L) | 562.9 | 566.3 | 602.5 | 618.5 | 30.42 |
| SDH (U/L) | 33.2ab | 29.3b | 30.6ab | 41.4a | 3.97 |
| Total Protein (g/L) | 56.9 | 56.7 | 56.5 | 57.4 | 1.07 |
| Albumin (g/L) | 47.3 | 47.4 | 47.1 | 47.7 | 0.64 |
| Globulin (g/L) | 9.8 | 9.2 | 9.4 | 9.9 | 1.00 |
| A/G (ratio) | 5.16 | 5.43 | 5.36 | 5.55 | 0.66 |
| Calcium (mmol/L) | 2.94 | 2.93 | 2.94 | 2.97 | 0.027 |
| Phosphate (mmol/L) | 3.10 | 3.16 | 3.20 | 3.19 | 0.087 |
| Magnesium (mmol/L) | 0.731 | 0.768 | 0.767 | 0.767 | 0.021 |
| Total Cholesterol (mmol/L) | 2.28 | 2.29 | 2.31 | 2.23 | 0.107 |
| Triglycerides (mmol/L) | 0.253 | 0.298 | 0.250 | 0.299 | 0.036 |
| Phospholipids (mmol/L) | 1.32 | 1.35 | 1.31 | 1.31 | 0.061 |
| Amylase (U/L) | 2474.7 | 2382.7 | 2782.7 | 2287.7 | 178.8 |
| Total Bilirubin (μmol/L) | <1.3 | <1.3 | <1.3 | <1.3 | 0 |
| Direct Bilirubin (μmol/L) | <0.8 | <0.8 | <0.8 | <0.8 | 0 |
| Haptoglobin (g/L) | 1.07 | 0.84 | 0.94 | 1.01 | 0.148 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; GGT, gamma glutamyl transferase; GDH, glutamate dehydrogenase; LDH, lactic dehydrogenase; SDH, sorbitol dehydrogenase.
There was no significant ‘treatment × sex’ interactions for any of the serum chemistry parameters measured on day 42 (Table 6). There was a main effect of treatment for asparate aminotransferase (AST; p = 0.03) and sorbitol dehydrogenase (SDH; p = 0.04). Pigs fed T3 had significantly higher AST values than control pigs fed T1 (53.0 vs 33.7 U/L; p = 0.03) but were not different to all other treatments. For SDH, pigs fed T4 had significantly higher values than pigs fed T2 (41.4 vs 29.3 U/L; p = 0.04) but neither were different to the control pigs (T1) or pigs fed T3. A significant effect of sex was identified for urea (4.32 vs 3.84 mml/L; p = 0.04), lactic dehydrogenase (LDH; 617.9 vs 557.5 U/L; p = 0.03), magnesium (Mg; 0.782 vs 0.734 mmol/L; p = 0.02), cholesterol (2.47 vs 2.08 mmol/L; p = 0.001) and phospholipids (1.406 vs 1.240 mmol/L; p = 0.002) whereby the values for females were higher than the values for males (data not shown). There was no main effect of treatment or sex identified for any other serum chemistry parameter measured on day 42.
3.1.4. Urine analyses
Descriptive statistics for all urinalysis are presented in Table 7. No interactions involving treatment group was statistically significant for any of the two quantitative urinalysis parameters (pH and specific gravity). Likewise, there was no main effect of treatment on pH or specific gravity. All other non-quantitative parameters (bilirubin, urobilirubin, ketone bodies, protein and color) were either negative or within the range found pretrial in the control animals.
Table 7.
Effects of dietary supplementation with increasing levels of Muramidase 007 (T1 = 0 LSU(F)/kg feed, T2 = 65,000 LSU(F)/kg feed, T3 = 325,000 LSU(F)/kg feed, T4 = 650,000 LSU(F)/kg feed) on urine characteristics of all pigs (day 2, 21, 42). Different letters indicate significant differences between the groups (p < 0.05).
| T1 | T2 | T3 | T4 | SE | |
|---|---|---|---|---|---|
| pH | 7.69 | 7.78 | 7.80 | 7.97 | 0.160 |
| Specific gravity | 1.019 | 1.021 | 1.021 | 1.019 | 0.001 |
| Bilirubin | NEG | NEG | NEG | NEG | |
| Urobilirubin | NEG | NEG | NEG | NEG | |
| Ketone bodies | NEG | NEG | NEG | NEG | |
| Protein | NEG | NEG | NEG | NEG | |
| Color | Pale yellow | Pale yellow | Pale yellow | Pale yellow |
3.1.5. Pathological findings
3.1.5.1. Necropsy and organs weight
Descriptive statistics for weights – both absolute (g) and as a percent of final BW – for all organs examined at necropsy (brain, epididymis, adrenal gland, pituitary gland, thyroid gland, heart, kidneys, liver, lung, ovary, prostate, spleen, uterus, testes, and thymus) are presented in Table 8. There was no significant ‘treatment × sex’ interaction for any of the organ weights measured. There was a main effect of treatment on the mean adrenal gland weight as a percent of final BW (ADR%) with pigs fed T3 having significantly heavier ADR% compared to pigs fed T1 and T2 (0.006 vs 0.005 and 0.005%, respectively; p = 0.032) with T4 being intermediate to all other treatments. There was no main effect of treatment on any other organ measured. There was a main effect of sex on the weight of the brain (77.2 vs 87.0 g; p = 0.006), thyroid gland (5.74 vs 7.04 g; p = 0.002), kidneys (296.6 vs 335.9 g; p = 0.005), liver (1405.2 vs 1689.7 g; p = 0.001), thymus (226.7 vs 296.1; p = 0.0006) and the mean weight of the thyroid and thymus as a percent of final BW (0.009 vs 0.010%; p = 0.045 and 0.368 vs 0.437%; p = 0.008, respectively) whereby the organs in males were significantly heavier than in females (data not shown). Following histopathological examination of all organs all gross necropsy findings were not treatment related.
Table 8.
Effect of dietary supplementation with increasing levels of Muramidase 007 (T1 = 0 LSU(F)/kg feed, T2 = 65,000 LSU(F)/kg feed, T3 = 325,000 LSU(F)/kg feed, T4 = 650,000 LSU(F)/kg feed) on the absolute organ weights (g) and as a percentage (%) of final body weight of all pigs on day 42 of the study. Different letters indicate significant differences between the groups (p < 0.05).
| T1 | T2 | T3 | T4 | SE | |
|---|---|---|---|---|---|
| Brain (BRA) | 81.3 | 84.7 | 80.9 | 81.5 | 3.58 |
| BRA % | 0.124 | 0.131 | 0.143 | 0.124 | 0.008 |
| Epididymis (EPI) | 41.7 | 43.3 | 47.4 | 41.9 | 3.23 |
| EPI % | 0.061 | 0.065 | 0.075 | 0.060 | 0.005 |
| Adrenal gland (ADR) | 3.9 | 3.2 | 3.6 | 3.8 | 0.22 |
| ADR % | 0.005a | 0.005a | 0.006b | 0.0056ab | 0.0002 |
| Pituitary gland (PIT) | 0.17 | 0.15 | 0.21 | 0.19 | 0.035 |
| PIT % | 0.0002 | 0.0002 | 0.0003 | 0.0003 | 0.00005 |
| Thyroid gland (THY) | 6.8 | 6.3 | 5.7 | 6.7 | 0.439 |
| THY % | 0.010 | 0.009 | 0.009 | 0.010 | 0.0004 |
| Heart (HEA) | 327.5 | 299.4 | 277.9 | 305.4 | 14.66 |
| HEA% | 0.495 | 0.463 | 0.472 | 0.461 | 0.016 |
| Kidney (KID) | 322.9 | 312.6 | 301.6 | 328.1 | 14.30 |
| KID % | 0.488 | 0.483 | 0.513 | 0.494 | 0.0145 |
| Liver (LIV) | 1600.8 | 1539.9 | 1448.8 | 1600.3 | 76.21 |
| LIV % | 2.43 | 2.44 | 2.45 | 2.40 | 0.077 |
| Lung (LUN) | 703.9 | 621.8 | 701.5 | 711.3 | 52.33 |
| LUN % | 1.066 | 0.966 | 1.185 | 1.081 | 0.072 |
| Ovary (OVA) | 7.3 | 5.4 | 4.2 | 3.0 | 1.04 |
| OVA % | 0.012 | 0.008 | 0.007 | 0.005 | 0.0016 |
| Prostate (PRO) | 4.1 | 4.6 | 5.1 | 4.1 | 0.44 |
| PRO % | 0.006 | 0.007 | 0.008 | 0.006 | 0.0007 |
| Spleen (SPL) | 116.8 | 133.9 | 116.7 | 131.3 | 6.91 |
| SPL % | 0.177 | 0.208 | 0.199 | 0.197 | 0.0096 |
| Uterus (UTE) | 99.2 | 71.2 | 69.3 | 61.9 | 17.71 |
| UTE % | 0.158 | 0.115 | 0.119 | 0.100 | 0.0295 |
| Testes (TES) | 83.9 | 93.8 | 86.4 | 94.7 | 10.71 |
| TES % | 0.122 | 0.141 | 0.137 | 0.132 | 0.0151 |
| Thymus (TYM) | 273.8 | 238.6 | 237.8 | 295.4 | 21.02 |
| TYM % | 0.411 | 0.369 | 0.387 | 0.443 | 0.0244 |
3.2. Experiment 2: efficacy study
3.2.1. Feed analyses
The analyzed muramidase activity of the diets used in the efficacy study are shown in Table 2. The Muramidase 007 used in the current study had an analyzed muramidase activity of 57,950 LSU(F)/g product. The supplementation of Muramidase 007 in feed resulted in an average muramidase activity of 44,180 LSU(F)/kg feed for the pre-starter phase and 43,860 LSU(F)/kg feed for the starter phase, which accounted for 88.4% and 87.7% of the intended muramidase activity of 50,000 LSU(F)/kg feed, respectively. The analytical results of the experimental diets by NIRS (Table 3) showed a good agreement with expected values in dietary parameters for the NC and Muramidase 007 treatments.
3.2.2. Health status and mortality
In general, the piglets remained healthy throughout the study and the growth performance was judged as acceptable by historical comparison with previous trials in this facility. The fecal scores revealed only few cases of diarrhea at the beginning of the study, which were considered sporadic and not related to the dietary treatment, indicating a high level of healthy status of the experimental animals (data not shown). The mortality and morbidity rate (pigs identified as being ill and not thriving were removed from the study) were both 2.5% and was not treatment related.
3.2.3. Growth performance
The pig growth performance of pigs by phase (pre-starter; d 0–14, starter; d 14–42) and overall (d 0–42) are shown in Table 9. There was no significant effect of treatment on BW at day 0 or 14 of the study. During the pre-starter phase (d 0–14), there was no significant effect of Muramidase 007 supplementation on average daily gain (ADG) and average daily feed intake (ADFI), however there was a significant reduction in feed conversion ratio (FCR) with Muramidase 007 supplementation reducing FCR by 10 points compared to the control treatment (1.44 vs 1.54; p = 0.02). During the starter phase (d 14–42) there was a significant effect of treatment on both ADG (p = 0.04) and ADFI (p = 0.04) associated to Muramidase 007 supplementation. Overall (d 0–42d) ADG was significantly improved (p = 0.04), and ADFI showed a positive tendency (p = 0.06), resulting in an improvement in ADG (starter; 6.5%, and overall 7.1%) and ADFI (starter; 6.4% and overall 6.3%), respectively. There was no effect of treatment on FCR during the starter phase (d 14–42) or overall (d 0–42). There was also a positive tendency for pigs supplemented with Muramidase 007 which were on average 1.1 kg heavier on day 42 of the study compared to the unsupplemented pigs (p = 0.10).
Table 9.
Effect of dietary supplementation with Muramidase 007 (50,000 LSU(F)/kg feed) compared to an unsupplemented control on the growth performance of nursery pigs over a 42-day period.
| Muramidase activity LSU(F)/kg | 0 |
50,000 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD1 | n | Mean | SD1 | n | T value | df | P value | |
| Body weight, kg | |||||||||
| d 0 | 6.2 | 0.7 | 24 | 6.2 | 0.7 | 24 | -0.02 | 46 | 0.98 |
| d 14 | 8.5 | 1.0 | 24 | 8.8 | 1.0 | 24 | -0.88 | 46 | 0.38 |
| d 42 | 22.9 | 2.4 | 24 | 24.0 | 2.1 | 24 | -1.69 | 46 | 0.10 |
| Pre-Starter (d 0 to 14) | |||||||||
| ADG, g/d | 169 | 39 | 24 | 187 | 37 | 24 | -1.73 | 46 | 0.09 |
| ADFI, g/d | 254 | 43 | 24 | 268 | 47 | 24 | -1.06 | 46 | 0.29 |
| FCR, g/g | 1.54 | 0.19 | 24 | 1.44 | 0.10 | 24 | 2.41 | 46 | 0.02 |
| Starter (d 14 to 42) | |||||||||
| ADG, g/d | 508 | 64 | 24 | 541 | 46 | 24 | -2.03 | 46 | 0.04 |
| ADFI, g/d | 844 | 100 | 24 | 898 | 79 | 24 | -2.07 | 46 | 0.04 |
| FCR, g/g | 1.67 | 0.07 | 24 | 1.65 | 0.05 | 24 | 0.87 | 46 | 0.39 |
| Overall (d 0 to 42) | |||||||||
| ADG, g/d | 395 | 53 | 24 | 423 | 39 | 24 | -2.11 | 46 | 0.04 |
| ADFI, g/d | 647 | 78 | 24 | 688 | 65 | 24 | -1.95 | 46 | 0.06 |
| FCR, g/g | 1.64 | 0.05 | 24 | 1.62 | 0.04 | 24 | 1.25 | 46 | 0.22 |
SD: Standard Deviation.
4. Discussion
4.1. Safety study
In the present target animal safety study, no significant differences in general health parameters or effects in the vast majority of the clinical pathology parameters evaluated were found. There was an effect of treatment on hematological parameters MCHC and APTT. For MCHC, while feeding 650,000 LSU(F)/kg of Muramidase 007 increased MCHC compared to feeding 325,000 LSU(F)/kg of Muramidase 007, neither of these treatments were different to the control.
The variations in MCHC are therefore not dose dependent. Secondly, these observations are not corroborated by the variations in MCH, nor MCV. Furthermore, all treatments groups had MCHC levels within the normal biological range for pigs (Thorn, 2000). Therefore, the finding on MCHC appear to be of minor relevance for the safety determination. For APTT, pigs fed 325,000 LSU(F)/kg of Muramidase 007 had lower values than the control treatment and pigs fed 65,000 LSU(F)/kg of Muramidase 007. The values of APTT observed in this study (8.7–10.5 s) were below the normal physiological mean value for pigs which has been reported as 34.5 s (Drescher et al., 2002). It is not thought that these low levels are treatment related as they are not different to values recorded in these pigs pre-trial. From a clinical perspective extended APTT is more of a concern and shortened APTT can often be attributed to preanalytical problems such as difficulty in blood collection (Lippi et al., 2010). No trend in APTT decrease has been reported by Lichtenberg et al., 2017 in their hematology investigations upon oral administration of the same Muramidase 007. Given these elements, the finding on APTT appear to be of minor relevance for the safety determination. There was a significant ‘treatment x sex’ interaction for LUC and LUC as a percentage of WBC. Female pigs fed 65,000 LSU(F)/kg of Muramidase 007 and males fed the control diet had significantly lower counts of LUC and LUC as a percentage of WBC compared to females fed 650,000 LSU(F)/kg of Muramidase 007 and females fed 325,000 LSU(F)/kg of Muramidase 007. However, the LUC counts for all treatments were within the normal biological range for pigs (0.1–1.4 × 109/L; Klem et al., 2010) therefore, any differences are not thought to be biologically relevant. Pigs fed 325,000 LSU(F)/kg of Muramidase 007 had higher serum concentration of AST compared to the control group however, the highest dose (650,000 LSU(F)/kg feed) was not different to the control. All treatment values for AST were within the normal biological range for pigs (Cooper et al., 2014) and there was a lack of a clear dose correlation suggesting that these differences could be considered within the expected biological variability for the parameter and species. The safety evaluation of Muramidase 007 in broilers found no effects of this enzyme on AST concentrations in serum (Lichtenberg et al., 2017). Similarly, serum SDH was higher in T4 than T2 but neither were different to the control and all levels were within the normal biological range for pigs (Cooper et al., 2014) therefore, this response was not considered to be biological relevant. Feeding Muramidase 007 was found to increase the relative weight of adrenal glands when fed at 65,000 LSU(F)/kg feed and 325,000 LSU(F)/kg feed but not at 650,000 LSU(F)/kg feed. There were no effects of dietary supplementation of Muramidase 007 on the absolute weight of adrenal gland and both gross and microscopic histopathological examination revealed no abnormalities related to treatment. Therefore, these differences were not thought to be of clinical relevance.
In summary, statistically significant changes in hematology, coagulation and clinical chemistry parameters were not considered clinically relevant or adverse for one or more of the following reasons: a) Their magnitude was low, b) Most values were within the overall reference ranges, c) There was a lack of clear dose correlation, d) There was a lack of in life or histological correlations, and/or e) The findings were transient in nature and were considered within the expected biological variability for the parameter and species.
The absence of observed adverse effects due to dietary Muramidase 007 supplementation in this safety study conducted in weaned pigs indicates that this enzyme is safe for piglets. This establishes a margin of safety of at least a factor of 10 (650,000 LSU(F)/kg feed) the same as was found in a safety study with broiler chickens (Lichtenberg et al., 2017).
Both, the current safety study and the one performed in broiler chickens (Lichtenberg et al., 2017) can also be read as experimental confirmation of the interspecies in silico conservative approach proposed by EFSA (2017), suggesting the consideration of a x100 safety factor applied to the no-observed-adverse-effect-level (NOAEL) for establishing a safe feed level for each target species (Table 10). Indeed, Lichtenberg et al. (2017) has experimentally established the NOAEL for the Muramidase 007 at 384 616 LSU/F)/kg body weight (highest dose tested). Applying a x100 safety factor to this exposure and considering standard feed intakes and body weights (specified in Table 10), along the proposal of EFSA (2017), the safe feed level of Muramidase 007 for broiler chickens and piglets would be 42,843 and 76,923 LSU(F)/kg feed, respectively. These levels are well below (by almost a factor 10) the 450,000 and 650,000 LSU(F)/kg feed evidenced as safe by Lichtenberg et al. (2017) and in the current safety study, thereof confirming, on two distinct animal categories, that the NOAEL derived approach proposed by EFSA (2017) is indeed conservative and thus suitable for safety determination in given target species, at least when applied to Muramidase 007. Beyond the intra-species consideration, allowing extending the safety conclusions evidenced in piglets (the most sensitive model) to other types of pigs (pigs for fattening, sows), the NOAEL/100 approach further documents the absence of safety concern for other poultry categories than the ones documented in Lichtenberg et al. (2017), namely the layers and the turkeys (specified in Table 10).
Table 10.
Established safe feed levels for Muramidase 007 in various animal categories.
| Experimentally proven safe levels of LSU(F)/kg feed | ||
| Growing pigs1 | 650 000 | |
| Broiler chickens2 | 450 000 | |
| Experimentally proven safe levels of LSU(F)/kg body weight/day (NOAEL) | ||
| Rats2 | 384 616 | |
| Maximum safe feed levels extrapolated from NOAEL/100, in LSU(F)/kg feed3 | ||
| Piglets | 76 923 | |
| Fattening pigs | 91 476 | |
| Lactating sows | 112 821 | |
| Broiler chickens | 42 843 | |
| Layers | 63 861 | |
| Turkey | 57 366 | |
| Proposed intended levels in feed, in LSU(F)/kg feed | ||
| Poultry | Up to 45 000 | |
| Pig | Up to 65 000 | |
This study.
Maximum safe concentration in feed = ((NOAEL/100)/Feed intake) x 1 000 × 0.88 with a feed intake (expressed in g dry matter intake per day and per kg body weight) set at 44, 37, 30, 79 for piglets, growing pigs, lactating sows and broiler chickens, respectively (EFSA 2017).
Thus, in conclusion, the consideration of both experimental safety studies and the in silico conservative approach, allow concluding, in confidence, that feeding piglets, pigs for fattening or sows with a feed delivering a Muramidase 007 enzymatic activity between 40,000 and 65,000 LSU(F)/kg feed is of no safety concern for swine.
4.2. Efficacy study
All muramidases (or lysozymes, EC 3.2.1.17) are characterized by catalyzing the same reaction: splitting the β-(1,4)-glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine of the peptidoglycan in bacterial cell walls. Conventional type (c-type) muramidases, such as the well-studied from hen egg white and human milk, are the main muramidases produced by most vertebrates with a notable contribution to antibacterial defense (Callewaert and Michiels, 2010). It was traditionally believed that the muramidase activity of lysozymes was mainly responsible for its antimicrobial action. However, it was demonstrated by Ibrahim et al. (2001) that its antimicrobial action is operationally independent of its muramidase activity and could be due to structural factors. In an in-vivo model, Nash et al. (2006) proved that the muramidase activity of this enzyme is not required for bactericidal activity. This function of fighting harmful bacteria by muramidase appeared to have been largely abandoned in animals with foregut fermentation (e.g. cow) and such muramidases have evolved to function as digestive enzymes (Jollès et al., 1989). The stomach muramidases in cows make the bacteria entering the stomach from the foregut available for hydrolysis by conventional digestive enzymes (Dobson et al., 1984). The digestive muramidase in cows is conformationally more rigid and thus more resistant to pepsin than the one found in hen egg white (Nonaka et al., 2009). The increase in gene number in the muramidase in cows is also considered as a result of evolutionary selection for efficient digestion of rumen bacteria (Mackie, 2002).
In the current study, weaned pigs fed Muramidase 007 demonstrated an increase in ADG and FI but no difference in FCR after 42 days supplementation. Studies supplementing hen egg white lysozyme (HEWL) to the diets of young piglets have been shown to improve performance (May et al., 2012; Oliver and Wells, 2013; Oliver et al., 2014; Ma et al., 2017). However, Nyachoti et al. (2012) found that weaned pigs supplemented with HEWL via the drinking water during an ETEC challenged did not perform better than the controls. Studies with transgenic goat milk containing human lysozyme reported no improvements in piglet growth compared with milk without lysozyme (Maga et al., 2006; Brundige et al., 2008). The variability in piglet growth response may be attributable to the differences in enzyme dose, application or the origin of the lysozymes. Given the diversity in origin between different lysozymes evaluated in-vivo, it might be speculated that the mode of action could be also different. In the current study, Muramidase 007 was encoded by the muramidase gene from the fungus Acremonium alcalophilum and was confirmed not to possess any antibacterial activity at intended doses (EFSA, 2018). To our knowledge, no previous studies have used this novel enzyme on piglets. However, Lichtenberg et al. (2017) showed an increase on the feed efficiency without main differences on microbial composition in the caeca of Muramidase 007 supplemented broiler chickens. In addition, Goodarzi Boroojeni et al. (2019), recently demonstrated that adding Muramidase 007 to broiler diets increased the feed efficiency and the apparent ileal digestibility of crude protein, fat and phosphorus, compared to unsupplemented birds. In contrast, in pig studies using HEWL did not increase the apparent digestibility of gross energy and crude protein (Ma et al., 2017). Goodarzi Boroojeni et al. (2019) suggested that the accumulation of bacterial cell wall fragments on the surface of the gut, rich in peptidoglycans, could impair nutrient digestion and absorption and the microbial muramidase might have counteracted this impact. Nevertheless, exploring the mode of action of this novel Muramidase 007 and its role in improving gastrointestinal functionality, further research is warranted.
In conclusion, results from the safety study found the absence of any adverse effects on health parameters measured when Muramidase 007 was supplemented in feed compared to the control group. In the case of the efficacy study, Muramidase 007 was even found to improve growth performance of weanling pigs.
Declarations
Author contribution statement
Wolfgang Schliffka, Heng-Xiao Zhai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Estefania Pérez Calvo, Sabine van Cauwenberghe, Maria C. Walsh, Rual Lopez-Ulibarri: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
The authors received no funding from an external source.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Bedford M.R., Cowieson A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012;173(1-2):76–85. [Google Scholar]
- Brundige D.R., Maga E.A., Klasing K.C., Murphy J.D. Lysozyme transgenic goats’ milk influences gastrointestinal morphology in young pigs. J. Nutr. 2008;138:921–926. doi: 10.1093/jn/138.5.921. [DOI] [PubMed] [Google Scholar]
- Callewaert L., Michiels C.W. Lysozymes in the animal kingdom. J. Biosci. 2010;35(1):127–160. doi: 10.1007/s12038-010-0015-5. [DOI] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.-M., Verlhac V. Animal Feed Science and Technology; 2017. Gastrointestinal Functionality in Animal Nutrition and Health: New Opportunities for Sustainable Animal Production. [Google Scholar]
- Cooper C.A., Moraes L.E., Murray J.D., Owens S.D. Hematologic and biochemical reference intervals for specific pathogen free 6-week-old Hampshire-Yorkshire crossbred pigs. J. Anim. Sci. Biotechnol. 2014;5(1):5. doi: 10.1186/2049-1891-5-5. Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson D.E., Prager E.M., Wilson A.C. Stomach lysozymes of ruminants. I. Distribution and catalytic properties. J. Biol. Chem. 1984;259:11607–11616. [PubMed] [Google Scholar]
- Drescher W., Li H., Jensen S.D., Ingerslev J., Hansen E.S., Hauge E.M., Bunger C. The effect of long-term methylprednisolone treatment on the femoral head in growing pigs. J. Orthop. Res. 2002;20:662–668. doi: 10.1016/S0736-0266(01)00183-8. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Guidance on the assessment of the safety of feed additives for the target species. EFSA J. 2017;15(10):5021. doi: 10.2903/j.efsa.2017.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Safety and efficacy of muramidase from Trichoderma reesei DSM 32338 as a feed additive for chickens for fattening and minor poultry species. EFSA J. 2018;16(7):5342. doi: 10.2903/j.efsa.2018.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi Boroojeni F., Männer K., Rieger J., Pérez Calvo E., Zentek J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019;98(5):2080–2086. doi: 10.3382/ps/pey556. [DOI] [PubMed] [Google Scholar]
- Ibrahim H.R., Matsuzaki T., Aoki T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001;506:27–32. doi: 10.1016/s0014-5793(01)02872-1. [DOI] [PubMed] [Google Scholar]
- Jollès J., Jollès P., Bowman B.H., Prager E.M., Stewart C.-B., Wilson A.C. Episodic evolution in the stomach lysozymes of ruminants. J. Mol. Evol. 1989;28:528–535. doi: 10.1007/BF02602933. [DOI] [PubMed] [Google Scholar]
- Klem T.B., Bleken E., Morberg H., Thoresen S.I., Framstad T. Hematologic and biochemical reference intervals for Norwegian crossbreed grower pigs. Vet. Clin. Pathol. 2010;39(2):221–226. doi: 10.1111/j.1939-165X.2009.00199.x. Jun. [DOI] [PubMed] [Google Scholar]
- Lichtenberg J., Perez Calvo E., Madsen K., Østergaard Lund T., Kramer Birkved F., van Cauwenberghe S., Mourier M., Wulf-Andersen L., Jansman A.J.M., Lopez-Ulibarri R. Safety evaluation of a novel muramidase for feed application. Regul. Toxicol. Pharmacol. 2017;89:57–69. doi: 10.1016/j.yrtph.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Lippi G., Salvagno G.L., Ippolito L., Franchini M., Favaloro E. Shortened activated partial thromboplastin time: causes and management. 2010;21(5):459–463. doi: 10.1097/mbc.0b013e328338dbe8. [DOI] [PubMed] [Google Scholar]
- Ma X., Zhang S., Pan L., Piao X. Effects of lysozyme on the growth performance, nutrient digestibility, intestinal barrier and microbiota of weaned pigs fed diets containing spray-dried whole egg or albumen powder. Can. J. Anim. Sci. 2017;97:466–475. [Google Scholar]
- Mackie R.I. Mutualistic fermentative digestion in the gastrointestinal tract: diversity and evolution. Integr. Comp. Biol. 2002;42:319–326. doi: 10.1093/icb/42.2.319. [DOI] [PubMed] [Google Scholar]
- Maga E.A., Walker R.L., Anderson G.B., Murray J.D. Consumption of milk from transgenic goats expressing human lysozyme in the mammary gland results in the modulation of intestinal microflora. Transgenic Res. 2006;15:515–519. doi: 10.1007/s11248-006-0014-3. [DOI] [PubMed] [Google Scholar]
- May K.D., Wells J.E., Maxwell C.V., Oliver W.T. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J. Anim. Sci. 2012;90(4):1118–1125. doi: 10.2527/jas.2011-4297. [DOI] [PubMed] [Google Scholar]
- Menezes-Blackburn D., Greiner R. Enzymes used in animal feed: leading technologies and forthcoming developments. In: Cirillo G., Gianfranco Spizzirri U., Iemma F., editors. vol. 2. 2015. pp. 47–73. (Functional Polymers in Food Science: from Technology to Biology). [Google Scholar]
- Nash J.A., Ballard T.N.S., Weaver T.E., Akinbi H.T. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J. Immunol. 2006;177:519–526. doi: 10.4049/jimmunol.177.1.519. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 11th revised edition. Acad. Press; Washington DC: 2012. Nutrients Requirements of Swine. [Google Scholar]
- Nonaka Y., Akieda D., Aizawa T., Watanabe N., Kamiya M., Kumaki Y., Mizuguchi M., Kikukawa T., Demura M., Kawano K. X-ray crystallography and structural stability of digestive lysozyme from cow stomach. FEBS J. 2009;276:2192–2200. doi: 10.1111/j.1742-4658.2009.06948.x. [DOI] [PubMed] [Google Scholar]
- Nyachoti C.M., Kiarie E., Bhandari S.K., Zhang G., Krause D.O. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 2012;90(1):252–260. doi: 10.2527/jas.2010-3596. [DOI] [PubMed] [Google Scholar]
- Oliver W.T., Wells J.E. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J. Anim. Sci. 2013;91(7):3129–3136. doi: 10.2527/jas.2012-5782. [DOI] [PubMed] [Google Scholar]
- Oliver W.T., Wells J.E., Maxwell C.V. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J. Anim. Sci. 2014;92(11):4927–4934. doi: 10.2527/jas.2014-8033. [DOI] [PubMed] [Google Scholar]
- Thorn C.E., editor. Normal Hematology of the Pig. Schalm’s Veterinary Hematology. 5 ed. 2000. pp. 1089–1095. [Google Scholar]
- Vollmer W., Blanot D., De Pedro M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008;32(2):149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- Vanrolleghem W., Tanghe S., Verstringe S., Bruggeman G., Papadopoulos D., Trevisi P., Zentek J., Sarrazin S., Dewulf J. Potential dietary feed additives with antibacterial effects and their impact on performance of weaned piglets: a meta-analysis. Vet. J. 2019;249:24–32. doi: 10.1016/j.tvjl.2019.04.017. [DOI] [PubMed] [Google Scholar]