Abstract
Background
In 2011, England introduced the Public Health Responsibility Deal (RD), a public-private partnership (PPP) which gave greater freedom to the food industry to set and monitor targets for salt intakes. We estimated the impact of the RD on trends in salt intake and associated changes in cardiovascular disease (CVD) and gastric cancer (GCa) incidence, mortality and economic costs in England from 2011–2025.
Methods
We used interrupted time series models with 24 hours' urine sample data and the IMPACTNCD microsimulation model to estimate impacts of changes in salt consumption on CVD and GCa incidence, mortality and economic impacts, as well as equity impacts.
Results
Between 2003 and 2010 mean salt intake was falling annually by 0.20 grams/day among men and 0.12 g/d among women (P-value for trend both < 0.001). After RD implementation in 2011, annual declines in salt intake slowed statistically significantly to 0.11 g/d among men and 0.07 g/d among women (P-values for differences in trend both P < 0.001). We estimated that the RD has been responsible for approximately 9900 (interquartile quartile range (IQR): 6700 to 13,000) additional cases of CVD and 1500 (IQR: 510 to 2300) additional cases of GCa between 2011 and 2018. If the RD continues unchanged between 2019 and 2025, approximately 26 000 (IQR: 20 000 to 31,000) additional cases of CVD and 3800 (IQR: 2200 to 5300) cases of GCa may occur.
Interpretation
Public-private partnerships such as the RD which lack robust and independent target setting, monitoring and enforcement are unlikely to produce optimal health gains.
Keywords: cardiovascular disease, chd/coronorary heart, diet, policy
Introduction
Public-private partnerships (PPPs), involving public and private sector organisations establishing collective initiatives to improve health have been promoted as a key mechanism to address non-communicable diseases.1 They have been presented as a promising middle option between industry self-regulation, which is argued to lack sufficient oversight, and legislative and regulatory approaches, which can be powerful but politically contentious.2 PPPs have especially been promoted as a mechanism to meet nutritional targets to reduce population intakes of sugar, fat and salt set by national governments and the WHO.3 Despite their popularity, PPPs remain poorly evaluated and evidence on how best to engage the private sector to improve public health nutrition is lacking.2
The Public Health Responsibility Deal (RD), a PPP in operation in England from 2011–2017, aimed to engage government, the voluntary sector and the commercial sector to work in partnership to improve population health.4 Prior to the RD, from 2003 to 2010 the independent Food Standards Agency (FSA) undertook a multicomponent strategy to reduce salt intake, including the use of agreements with the food industry to reformulate processed foods, increase public awareness and introduce food labelling.5 While the FSA strategy was arguably a PPP, it differed in important ways from the RD (Box 1). Industry had almost no role in the formulation of policy and specific strategies to reduce population-level salt intake and targets were set by the FSA for reductions to be achieved within 4 years, with mid-point review at 2 years alongside independent monitoring.6 7 Additionally, while agreements with the food industry were technically 'voluntary', they were backed with the repeated ministerial threat of mandatory imposition in the event of poor compliance.8 9 While there have been evaluations of the process of setting targets and the mechanisms of the RD, a lack of available data has previously precluded a quantitative evaluation of the change from the FSA salt reduction strategy to the RD.5 Such evaluations could provide important lessons, both internationally and for England which is set to soon revise its salt reduction strategy. We have therefore estimated the impact of the RD on trends in population-level salt intake and associated changes in cardiovascular disease (CVD) and gastric cancer (GCa) incidence and mortality, and their economic costs in England from 2011 to 2025.
Box 1. Key differences between FSA and RD salt reduction strategies.
Food Standards Agency salt reduction strategy 2003–2010
Targets: The FSA strategy involved specific targets for 85 categories of food (of approximately 10–20% reductions), which were developed in 2005. In 2006 the FSA published industry salt targets to be achieved by 2010. Meeting these targets was subsequently delayed to 2012.
Activities: Reformulation of foods known to contain high levels of salt, and introduction of food labelling and public awareness campaigns (e.g. to reduce salt use when cooking).
Monitoring: Monitoring of progress was by the independent FSA, alongside the establishment of national monitoring of population-level salt intakes to monitor progress. Results of progress were publicly available.
Involvement: 'Voluntary' involvement and targets were underpinned by direct pressure from the FSA, non-governmental organisations (NGOs) and Government Ministers threatening further regulation.
Reduction strategy 2011–2017
Targets: There was an original commitment to the 2012 FSA targets for salt, with targets after this set by industry partners themselves.
Activities: There were a range of food pledges which could be signed up to for catering and food production sectors. These included chef training in using less salt; providing salt content on menus; and reformulation, all within 2 years of signing RD pledges.
Monitoring: A plenary group of senior representatives from the business community, NGOs, public health organisations and local government oversaw the RD, with monitoring by the Department of Health. Partners were asked to report on their progress by the end of April each year. For some pledges, partners were asked to report using pre-defined quantitative measures, while for others they were asked for a narrative update.
Involvement: Voluntary
Methods
We used interrupted time series (ITS) analysis to estimate the impact of the RD on trends in population-level salt intake and microsimulation modelling to estimate associated changes in CVD and GCa incidence and mortality, and their economic costs within a synthetic English population.
Data sources
Data for population salt intake come from 24 hours' urine samples collected in the National Diet and Nutrition Survey (NDNS) 2000/01, and national sodium surveys conducted in 2006, 2008, 2011 and 2014. All sodium surveys were of adults aged 19–64 years and designed to produce nationally representative estimates of salt intake. The NDNS is a dietary survey of children and adults in the UK, and includes an interview, diary sample and urine collection (only among adults), and selects participants using a multistage random probability design. The response rate for the survey overall was 61% and of these 66% consented to 24 hours' urine collection.10 The 2006 sodium survey was drawn from the nationally representative Health Survey for England (HSE) 2005, while the subsequent sodium surveys used random samples of postcodes and random digit dialling of telephone numbers within these.11 Response rates ranged from 43% in 2006 to 61% in 2014 and sample sizes for all surveys are provided in table 1 and elsewhere.11
Table 1.
Surveys with 24 hours' urinary salt data included
| Dates of 24 hours' urine collection | N included in analyses | |
| National Diet and Nutrition Survey 2000/1 | July 2000 to June 2001 | 1029 |
| England 2006 sodium survey | October 2005 to July 2006 | 445 |
| UK 2008 sodium survey | January to May 2008 | 571 |
| England 2011 sodium survey | July to December 2011 | 499 |
| England 2014 sodium survey | May to September 2014 | 622 |
| National Diet and Nutrition Survey Rolling Programme (sensitivity analyses only) | ||
| 2008 | January to December 2008 | 75 |
| 2009 | January to December 2009 | 96 |
| 2010 | January to December 2010 | 101 |
| 2011 | January to December 2011 | 154 |
| 2012 | January to December 2012 | 153 |
| 2013 | January to June 2013 | 88 |
The NDNS and four sodium surveys all adhered to a standardised protocol with participants asked to collect all urine during a 24-hour period. With the exception of the NDNS 2000/1, all surveys used para-amino benzoic acid (PABA) tablets to assess completion of urine collection and we only included data where PABA excretion ≥70%.12 In line with official reports, we used data from NDNS and the four sodium surveys data which were adjusted for changes in the instruments to estimate salt intakes over time.
Interrupted time series modelling
We used an ITS analysis which is a quasi-experimental design and is appropriate for natural experiments when an intervention occurs at a well-defined moment in time.13 For this analysis, we identified 2010 as the last year of the FSA strategy followed by the introduction of the RD in 2011. Our ITS was a Generalised Linear Model and a Gamma distributed-dependent variable to fit the positively skewed salt intake data. The model included two time-based explanatory variables, an annual time trend between 2000/1 and 2010 and a post-RD trend from 2011 to 2014. The model was also adjusted for age group (19–34, 35–49, and 50–64 years) and stratified by sex. We used this model to estimate the pre-RD trend, and the post-RD trend and report P-values for whether the trend post-RD is different to that pre-RD. We did not include a term for an immediate step change in intakes as we did not expect any changes from the transition to the RD to be immediate.
In a sensitivity analysis, we also used 24 hours' urinary salt data collected in the NDNS Rolling Programme (2008/9–2013/4). Here salt intake was assessed from participants with PABA excretion ≥70%, consistent with the main analysis. While this data did provide estimates for additional years, sample sizes were small, ranging from six to 42 participants in each age and sex group, and were therefore not included in the main analyses.
IMPACTNCD
We estimated the effect of changes in salt intake on CVD and GCa outcomes using the IMPACTNCD model, which has been previously used to quantify the effect of salt policies in England.14 IMPACTNCD is a microsimulation model which generates synthetic individuals to simulate the impacts of changing risk factors on disease outcomes and uses probabilistic sensitivity analysis to estimate uncertainty of outcomes. Population information by age, sex and socioeconomic status comes from the Office for National Statistics (ONS) and additional data on risk factors for CVD and GCa from the Health Survey for England (HSE). We used effect sizes of the association between risk factors and CVD and GCa from published meta-analyses and longitudinal studies (online supplementary appendix table 7). The impact of salt on CVD was mediated through blood pressure with a 5-year median lag time (range 1 to 10 years) and effects on GCa were modelled directly with an 8-year median lag time (range 1 to 10 years).14 We informed the model with the socioeconomic gradient from HSE spot-urine salt data to overcome the lack of consistent socioeconomic information in the 24-hours' sodium surveys. As the 24-hours' urine data did not include adults aged over 64 years we also extrapolated salt intakes for older adults based on the HSE data. A more detailed description of IMPACTNCD is provided in the online appendix supplementary material.
jech-2018-211749supp001.pdf (14.9MB, pdf)
We modelled two scenarios which were informed by the findings of the ITS. First, in our counterfactual scenario we assumed that the approximately linear decline in salt intake that was observed before 2011 continued, as if the RD has never been implemented. Second, we modelled a RD scenario which assumed that the post-RD trend estimated from our ITS continued until 2025. In a separate, one-way sensitivity analysis we assumed a logarithmic decline calibrated to reach a population mean salt intake of 7.0 g/day (vs 6.5 g/day in the main analysis) by 2020, and 6.6 g/day (vs 5.8 g/day) by 2025, for the baseline scenario. This assumption produces more conservative salt exposure estimates in comparison to our main data-driven scenarios.
We estimated the additional number of CVD and GCa cases and deaths under the RD scenario between 2011 and 2025 among English adults aged 30–84 years by age group, sex and quintile group of Index of Multiple Deprivation (IMD). Taking into account time lags between exposure and disease, IMPACTNCD accumulates cases and deaths up to 2035. To maximise policy relevance we report our results up to 2025 and based on the time of exposure, rather than when the event occurred. To separate estimates into those which have already occurred and those which may occur if the policy is not changed, we present estimates for 2011–2018 and 2019–2025 separately. We summarise the output distributions by reporting medians and interquartile ranges (IQRs) in the form of first and third quartiles. We also report the computed probability (Ps) that the RD is superior to the counterfactual scenario.
We estimated healthcare costs and workplace productivity losses from CVD and GCa based on published estimates from the UK and Ireland. CVD tariff costs varied by first year since diagnosis, subsequent years and year of death. Stroke costs included rehabilitation but not ongoing social care costs. We weighted healthcare costs by deprivation as there is good evidence that costs for the same disease show a social gradient.15 We inflated all costs to 2018 using UK Treasury GDP inflator tables from April 2018 and used an annual discount rate of 3.5%.
Results
In 2000/1, mean salt intake was 10.5 grams/day (95% CI: 10.1 to 11.0) in men and 8.0 grams/day (95% CI: 7.7 to 8.3) in women (figure 1). Between 2003 and 2010, mean salt intake was reducing annually by 0.20 (95% CI: 0.12 to 0.29, P for trend <0.001) g/d among men and by 0.12 (95% CI: 0.06 to 0.18, P for trend <0.001) g/d among women (table 2). From 2011 to 2014, after the RD was implemented, annual reductions in salt intake were reduced: 0.11 (95% CI: 0.06 to 0.15, P for difference to pre-RD trend <0.001) g/d among men and 0.07 (95% CI: 0.04 to 0.11, P for difference to pre-RD trend <0.001) g/d among women.
Figure 1.
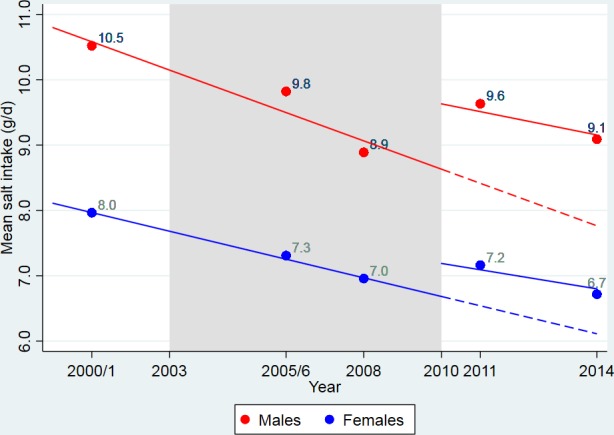
Pre- and post-Responsibility Deal trends of salt intake in England 2000/01 to 2014.
Table 2.
Interrupted time series results of salt intake (grams/day) 2000/01–2014 pre- and post-Responsibility Deal implementation
| Men | Coefficient | 95% CI | P-value | |
| Intercept | 11.07 | 10.43 | 11.7 | <0.001 |
| Change in salt intake per year (annual time effect) | −0.20 | −0.29 | −0.12 | <0.001 |
| Slope change after the Responsibility Deal (time*intervention interaction) | 0.09 | 0.03 | 0.17 | 0.004 |
| Post-Responsibility Deal annual trend | −0.11 | −0.15 | −0.06 | <0.001 |
| Women | ||||
| Intercept | 8.75 | 8.30 | 9.19 | <0.001 |
| Change in salt intake per year (annual time effect) | −0.12 | −0.18 | −0.06 | <0.001 |
| Slope change after the Responsibility Deal (time*intervention interaction) | 0.05 | 0.01 | 0.09 | 0.031 |
| Post-Responsibility Deal annual trend | −0.07 | −0.10 | −0.04 | <0.001 |
| Sensitivity analysis | ||||
| Men | Coefficient | 95% CI | P - value | |
| Intercept | 10.98 | 10.4 | 11.57 | <0.001 |
| Change in salt intake per year (annual time effect) | −0.16 | −0.24 | −0.09 | <0.001 |
| Slope change after the Responsibility Deal (time*intervention interaction) | 0.06 | 0.01 | 0.12 | 0.032 |
| Post-Responsibility Deal annual trend | −0.10 | −0.15 | −0.06 | <0.001 |
| Women | ||||
| Intercept | 8.63 | 8.22 | 9.04 | <0.001 |
| Change in salt intake per year (annual time effect) | −0.11 | −0.17 | −0.06 | <0.001 |
| Slope change after the Responsibility Deal (time*intervention interaction) | 0.04 | 0.00 | 0.08 | 0.036 |
| Post-Responsibility Deal annual trend | −0.07 | −0.10 | −0.04 | <0.001 |
CI, confidence Interval.
Including data from the NDNS Rolling Programme in a sensitivity analysis did not substantially change these results.
Health impacts
IMPACTNCD estimated that the slower fall in salt consumption may have generated approximately 9900 (IQR: 6700 to 13,000, Ps=2%) additional cases of CVD and approximately 710 (IQR: −510 to 2300) CVD deaths, between 2011 and 2018 in England (table 3). Likewise, approximately 1500 (IQR: 510 to 2300, Ps=16%) additional cases of GCa and 610 (IQR: −310 to 1500) additional GCa deaths between 2011 and 2018.
Table 3.
IMPACTNCD estimates of additional cardiovascular disease (CVD) and gastric cancer cases linked to Responsibility Deal 2011–2025. Negative values in the lower bound of the interquartile ranges (IQR) are a consequence of stochastic noise in the model
| Disease | Period of exposure | Absolute number of additional cases (IQR) | Absolute number of additional deaths (IQR) | Probability of superiority * |
| CVD | ||||
| 2011–2018 | 9900 (6700 to 13,000) | 710 (-510 to 2300) | 2.0% | |
| 2019–2025 | 26 000 (20 000 to 31,000) | 5500 (2800 to 8500) | 0.2% | |
| 2011–2025 | 35 000 (29 000 to 42,000) | 6400 (3200 to 9400) | <0.1% | |
| GCa | ||||
| 2011–2018 | 1500 (510 to 2300) | 610 (-310 to 1500) | 16.0% | |
| 2019–2025 | 3800 (2200 to 5300) | 1900 (790 to 3100) | 5.3% | |
| 2011–2025 | 5300 (3400 to 7200) | 2500 (920 to 3900) | 5.8% |
Numbers are rounded to the second significant digit.
*Probability of superiority represents the probability that the Responsibility Deal scenario had fewer cases than the counterfactual scenario.
GCa, gastric cancer.
If the RD continues unchanged from 2019 to 2025, the model estimated approximately 26 000 (IQR: 20 000 to 31,000, Ps=0.2%) additional cases of CVD and some 5500 (IQR: 2800 to 8500) CVD deaths, plus approximately 3800 (IQR: 2200 to 5300, Ps=5%) additional cases of GCa.
Equity impacts
Estimated cases linked to the RD were greater in the more deprived areas than more affluent areas (table 4). For the first period (2011–2018), approximately 1600 (IQR: −200 to 3600) additional CVD cases may have occurred in the most affluent areas (QIMD 1) compared with approximately 2000 (IQR: 200 to 4000) additional cases in the most deprived areas (QIMD 5). The model estimated approximately 1200 (IQR: −150 to 2700) new CVD cases in QIMD 1 vs 1500 (IQR: 150 to 2800) in QIMD 5 per 100,000 CVD cases.
Table 4.
IMPACTNCD estimates of additional cardiovascular disease (CVD) and gastric cancer cases linked to Responsibility Deal 2011–2025 by deprivation group. Negative values in the lower bound of the interquartile ranges (IQR) are a consequence of stochastic noise in the model
| Disease | QIMD (5=most deprived) | Absolute number of additional cases (IQR) | Rate per 100 000 person-years (IQR) | Rate per 100 000 new cases |
| 2011–2018 * | ||||
| CVD | 1 | 1600 (-200 to 3600) | 3.0 (-0.38 to 6.7) | 1200 (-150 to 2700) |
| 2 | 1900 (200 to 4100) | 3.6 (0.38 to 7.5) | 1300 (130 to 2700) | |
| 3 | 1900 (100 to 4100) | 3.6 (0.19 to 7.5) | 1300 (65 to 2800) | |
| 4 | 2000 (2800 to 4100) | 3.9 (0.52 to 7.7) | 1500 (200 to 2900) | |
| 5 | 2000 (200 to 4000) | 4.1 (0.4 to 7.8) | 1500 (150 to 2800) | |
| GCa | 1 | 200 (-310 to 820) | 0.37 (-0.75 to 1.5) | 910 (-4,400 to 5400) |
| 2 | 310 (-310 to 920) | 0.56 (-0.56 to 1.7) | 1000 (-4,000 to 6100) | |
| 3 | 310 (-310 to 820) | 0.57 (-0.56 to 1.5) | 420 (-3,500 to 5900) | |
| 4 | 410 (-200 to 940) | 0.76 (-0.39 to 1.8) | 1300 (-4,100 to 6800) | |
| 5 | 310 (-200 to 920) | 0.59 (-0.39 to 1.7) | 1200 (-4,200 to 7100) | |
| 2019–2025 * | ||||
| CVD | 1 | 4300 (1400 to 7100) | 9.1 (2.9 to 15) | 3600 (1100 to 6100) |
| 2 | 5100 (2000 to 8300) | 11 (4.3 to 17) | 4100 (1600 to 6600) | |
| 3 | 5300 (1900 to 8400) | 11 (4.1 to 18) | 4200 (1500 to 6600) | |
| 4 | 5400 (2200 to 8700) | 11 (4.7 to 18) | 4500 (1800 to 7100) | |
| 5 | 5800 (2300 to 9100) | 12 (5.1 to 19) | 4500 (1900 to 7100) | |
| GCa | 1 | 710 (0 to 1300) | 1.5 (0 to 2.8) | 7100 (-1,600 to 17,000) |
| 2 | 820 (0 to 1500) | 1.7 (0 to 3.3) | 7800 (-930 to 20,000) | |
| 3 | 820 (0 to 1500) | 1.7 (0.42 to 3.3) | 9000 (-1,200 to 20,000) | |
| 4 | 820 (0 to 1500) | 1.7 (0 to 3.2) | 10 000 (-1,300 to 21,000) | |
| 5 | 820 (0 to 1500) | 1.7 (0 to 3.2) | 9200 (-1,500 to 22,000) |
Numbers are rounded to the second significant digit.
*Years refer to the years in which the relevant policies were in operation, although outcomes can be accumulated up to 2035.
GCa, gastric cancer; QIMD, Quantile group of Index of Multiple Deprivation.
Economic impacts
We estimated the incremental economic impact of the RD to date (2011–2018) as approximately £160 million (IQR: £88 to £230 million, Ps=6.6%) (table 5). This includes approximately £110 million (IQR: £61 to £160 million) in additional healthcare costs and some £47 million (IQR: £12 to £80 million) in workplace productivity losses through people of working age living with CVD and people dying of CVD or GCa. For 2019–2025 the model estimated the additional incremental economic impact of continuing the RD to be approximately £970 million (IQR: £760 to £1200 million, Ps=0.1%).
Table 5.
Incremental healthcare and workplace productivity costs in the Responsibility Deal scenario compared with the counterfactual scenario. costs in 2018 GBP
| Disease | Healthcare costs in million (IQR) | Workplace productivity costs in million (IQR) | Total |
| 2011–2018 * | |||
| CVD | £83 (£50 to £120) | £37 (£13 to £61) | |
| Gastric cancer | £30 (-£6.4 to £66) | £8.4 (-£16 to £34) | |
| Total 2011–2018 | £110 (£61 to £160) | £47 (£12 to £80) | £160 (£88 to 230) |
| 2019–2025 * | |||
| CVD | £500 (£380 to £620) | £290 (£170 to £400) | |
| Gastric cancer | £150 (£68 to £220) | £27 (-£8.0 to £61) | |
| Total 2019–2025 | £650 (£450 to £840) | £320 (£162 to 460) | £970 (£760 to £1,200) |
| Combined costs 2011–2025 * | |||
| CVD | £583 (£430 to £740) | £327 (£183 to £461) | |
| Gastric cancer | £180 (£61 to £286) | £35.4 (-£24 to £95) |
Negative values in the lower bound of the interquartile ranges are a consequence of stochastic noise in the model.
Numbers are rounded to the second significant digit.
*Years refer to the years in which the relevant policies were in operation, although outcomes can be accumulated up to 2035.
CVD, cardiovascular disease; IQR, interquartile range.
One-way sensitivity analyses
In sensitivity analyses for the period 2011–2018, IMPACTNCD estimated that the RD may have generated 6100 (IQR: 2700 to 9500, Ps=9.8%) additional CVD cases and 1000 (IQR: 100 to 1800, Ps=24%) additional GCa cases. IMPACTNCD estimated the total incremental cost for this period may have been approximately £100 million (IQR: £28 to £170 million, Ps=17%). For 2019–2025, the model estimated that RD may cause 14 000 (IQR: 8600 to 19,000, Ps=3.0%) additional CVD cases and 2100 (IQR: 920 to 3200, Ps=12%) additional GCa cases. The socioeconomic gradient remained although was reduced in comparison with our main analysis.
Discussion
Key findings
Previous reductions in population-level salt intake in England slowed significantly after implementation of the Public Health Responsibility Deal in 2011. We suggest that this slowing was associated with approximately 10 000 additional cases of CVD and 1500 cases of GCa to date (2011–2018), with an additional 26 000 cases of CVD and 3800 cases of GCa projected if this policy is continued until 2025. Modelled health impacts were larger among more deprived populations, thus potentially widening inequalities and the associated healthcare and productivity costs exceeded £1 billion.
Strengths and limitations
This is the first study to estimate the impact of the RD on population-level salt intake, health and economic outcomes, building on the foundations laid by MacGregor et al.6 Estimates of salt intake are derived from 24 hours' urine excretion verified using para-aminobenzoic acid (PABA) which is the 'gold standard' for population-level monitoring.16 Our findings are informed by an ITS which is in line with best practice in answering questions about population- level interventions and effects.13
There are nonetheless limitations, including a lack of longitudinal data collections on salt intakes in the same people, meaning that we cannot ascribe causality to the RD. The cost and burden of undertaking 24 hours' urine collections means that data come from a small number of participants in some years and the relatively low number of data points included only two post-RD in our main analyses . However, sensitivity analyses with additional data points from the NDNS Rolling Programme produced consistent findings. Our modelling assumed that persons≥64 years would have similar trends in salt intake to those found in spot urine data from the HSE.17 The 24-hour sodium surveys had limited information on the socioeconomic position of participants and so we used spot urine data from the HSE to estimate socioeconomic gradient in salt intake (see online supplementary appendix p9-12 for additional details). This approach assumed that the transition from the FSA strategy to the RD did not alter the equity impacts of reformulation activities, although this assumption is nonetheless consistent with other empirical evidence.18
Our counterfactual scenario assumed that the ongoing linear decline in salt intake observed during the period of the FSA scheme continues linearly beyond 2010. To address uncertainty around this assumption we undertook a one-way sensitivity analysis that assumed logarithmic decline of mean salt intake and these findings concur with our main analyses. It is possible that we may have underestimated the attenuation of the decline in salt intake from the RD as it is likely that reformulation activities planned as part of the FSA strategy would not have stopped immediately.Finally, the estimates of disease costs presented here were based on workplace productivity costs, and not other costs including the economic value of quality adjusted life years, so this study likely represents a conservative estimate of the true economic impact of the RD. Nonetheless, if we examined total societal costs then it is possible the net monetary benefits may be negative if many of the life years gained are in older age groups, when many people have moved from being net producers to net consumers.
Comparison with other research
Salt reduction strategies are generally implemented nationally and few countries routinely collect representative 24 hours' urine data with sufficient frequency to assess policy impacts. Emerging data from country case studies, including from South Africa and Brazil, suggest that interventions of a structural nature, such as reformulation or food procurement policies, are most effective at reducing population-level salt intake.19 20 This is confirmed by findings from a recent systematic review which indicated that whole-population interventions have the potential to substantially reduce salt intake, particularly if they are multi-component.21 However, there remains very little evaluation of different models of industry engagement and whether regulatory approaches are more effective than voluntary targets, which is an important evidence gap as that the majority of countries with targets for salt reduction have voluntary rather than mandatory targets.22
Proponents of the RD argued that the increased role of industry would deliver more effective action to reduce salt intakes at lower cost than the FSA strategy,4 but our findings suggest this not to be the case. The design and implementation of the RD has been criticised for being underpinned by pledges made by the food industry, not following evidence of effectiveness to improve diets.5 While 46% of food industry salt reduction pledges did include reformulation of their products by 2013, independent evaluation concluded that none of these measures were prompted by the RD.23 24 Our findings that population levels of salt intake were decreasing until 2010 but then attenuated are consistent with official analyses and predictions by MacGregor et al.6 12 25 26 Our findings suggest that the RD may have had a particularly negative impact among more deprived populations, consistent with other research on the hierarchy of effectiveness in nutritional interventions.18 21 27 28
Policy implications
There is renewed policy interest in identifying and implementing optimal strategies to reduce population-level salt intake, and England is due to soon publish a new salt reduction strategy.29 Food industry engagement in such strategies is vitally important to optimise health and economic benefits, especially in high- income countries where the majority of dietary salt comes from processed and ultra-processed foods.30 Our findings suggest that if PPPs are used they require independent setting of targets, robust monitoring, and the use of incentives and tough sanctions to ensure compliance as highlighted by recent studies.2 Furthermore, the standard UK government approach to regulation is to try voluntary measures and legislate only if these fail.31 The shift from a semi-regulated voluntary agreement to a looser agreement (from the FSA to RD) could thus be viewed as a retrograde political move. Public Health England plans to review the future of the salt reduction programme very soon and we hope that this will consider the earlier FSA successes and evidence favouring a robust policy approach.
Conclusions
Our findings suggest that declines in salt intake slowed after implementation of the Public Health Responsibility Deal in England, resulting in excess CVD and cancer burdens, plus additional healthcare and societal costs. Public-private partnerships such as the RD which lack robust and independent target setting, monitoring and enforcement, are unlikely to produce optimal health gains.
What is already known on this subject.
Public-private partnerships (PPPs) are often promoted as a key mechanism to improve population health, including addressing dietary risk factors for non-communicable disease
Salt intake is a leading risk factor for both cardiovascular disease and gastric cancer
Between 2003 and 2010, the United Kingdom had an internationally recognised scheme to reduce population-level salt intake, led by the Food Standards Agency (FSA) and backed by governmental threat of mandatory imposition in the event of poor industry compliance. This was replaced by a different PPP known as the Public Health Responsibility Deal (RD) in England in 2011.
What this study adds.
Using individual-level data from 24 hours' urine samples we estimated that reductions in salt intake during the FSA scheme slowed significantly after introduction of the RD.
Using a microsimulation model, we estimate that transition from the FSA scheme to the RD may have generated approximately 9900 cases of CVD and 1500 cases of GCa between 2011 and 2018. If the RD continues unchanged through to 2025 we estimate an additional 26 000 cases of CVD and 3800 cases of GCa. These impacts were proportionally greater among more disadvantaged groups which may widen health inequalities. Associated excess healthcare and societal costs exceed £1 billion during the period 2011–2025.
Without independent targets and monitoring, PPPs are unlikely to deliver the improvements in population health claimed by their proponents.
Acknowledgments
CM, AAL and PS are funded by the National Institute for Health Research (NIHR Research Professorship 2014-04-032) and the Public Health Policy Evaluation Unit at Imperial College London is grateful for the support of the NIHR School of Public Health Research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Lead authors can take responsibility for the integrity of the data and manuscript is an honest, accurate, and transparent account of the study being reported.
Footnotes
Contributors: Study design was by all authors with analyses by CK and PS. AL produced the first draft and all authors revised the manuscript for important intellectual content and approved the final version. AL and CK contributed equally to this manuscript as did MoF and CM
Funding: UK Prevention Research Partnership. UKPRP Consortium Development Grant. UKPRP_CO1_105. QUEST: QUantifying Equitable Solutions To prevent Non-Communicable Diseases.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Bryden A, Petticrew M, Mays N, et al. Voluntary agreements between government and business – a scoping review of the literature with specific reference to the Public Health Responsibility Deal. Health Policy 2013;110:186–97. 10.1016/j.healthpol.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 2. Moodie R, Stuckler D, Monteiro C, et al. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. The Lancet 2013;381:670–9. 10.1016/S0140-6736(12)62089-3 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization Global action Plan for the prevention and control of non-communicable diseases (2013–2020). Geneva: World Health Organization, 2013. [Google Scholar]
- 4. Jebb SA. The Public Health Responsibility Deal Food Network. Nutr Bull 2012;37:355–8. 10.1111/j.1467-3010.2012.01992.x [DOI] [Google Scholar]
- 5. Knai C, Petticrew M, Durand MA, et al. Has a public–private partnership resulted in action on healthier diets in England? An analysis of the Public Health Responsibility Deal food pledges. Food Policy 2015;54:1–10. 10.1016/j.foodpol.2015.04.002 [DOI] [Google Scholar]
- 6. MacGregor GA, He FJ, Pombo-Rodrigues S. Food and the responsibility deal: how the salt reduction strategy was derailed. BMJ 2015;350 10.1136/bmj.h1936 [DOI] [PubMed] [Google Scholar]
- 7. Food Standards Agency Impact assessment of the revised salt reduction targets, 2009. Available: https://www.legislation.gov.uk/ukia/2009/86/pdfs/ukia_20090086_en.pdf
- 8. The UK Food Standards Agency’s programme on salt reduction Dr Corinne Vaughan Deputy head – nutrition, 2009. Available: http://www.nationalacademies.org/hmd/~/media/E927FD0F64414A119A3097CD8786BFAB.ashx
- 9. Wyness LA, Butriss JL, Stanner SA. Reducing the population's sodium intake: the UK food standards agency's salt reduction programme. Public Health Nutr 2012;15:254–61. 10.1017/S1368980011000966 [DOI] [PubMed] [Google Scholar]
- 10. Food Standards Agency The National Diet & Nutrition Survey: adults aged 19 to 64 years. Vitamin and mineral intake and urinary analytes, 2003. Available: http://webarchive.nationalarchives.gov.uk/20100409185714/http://www.food.gov.uk/multimedia/pdfs/ndnsv3.pdf
- 11. NatCen Social Research, MRC Elsie Widdowson Laboratory National Diet and Nutrition Survey: Assessment of Dietary Sodium in Adults, 2006/09 and 2011/15. [data collection]. 2nd Edn UK Data Service, 2018. [Google Scholar]
- 12. Public Health England National Diet and Nutrition Survey: assessment of dietary sodium adults (19 to 64 years) in England, 2014, 2014. [Google Scholar]
- 13. Kontopantelis E, Doran T, Springate DA, et al. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350 10.1136/bmj.h2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kypridemos C, Guzman-Castillo M, Hyseni L, et al. Estimated reductions in cardiovascular and gastric cancer disease burden through salt policies in England: an IMPACTNCD microsimulation study. BMJ Open 2017;7 10.1136/bmjopen-2016-013791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charlton J, Rudisill C, Bhattarai N, et al. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J Health Serv Res Policy 2013;18:215–23. 10.1177/1355819613493772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization Reducing salt intake in populations: report of a WHO forum and technical meeting, 2006. Available: http://www.who.int/dietphysicalactivity/Salt_Report_VC_april07.pdf
- 17. NatCen Social Research, University College London Department of Epidemiology and Public Health. Health Survey for England, 2012, 2014. Available: http:// [Accessed 1 May 2014].
- 18. Millett C, Laverty AA, Stylianou N, et al. Impacts of a national strategy to reduce population salt intake in England: serial cross sectional study. PLoS ONE 2012;7 10.1371/journal.pone.0029836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nilson E, Spaniol A, Gonçalves V, et al. Sodium reduction in processed foods in Brazil: analysis of food categories and voluntary targets from 2011 to 2017. Nutrients 2017;9 10.3390/nu9070742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters S, Dunford E, Ware L, et al. The sodium content of processed foods in South Africa during the introduction of mandatory sodium limits. Nutrients 2017;9 10.3390/nu9040404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyseni L, Atkinson M, Bromley H, et al. The effects of policy actions to improve population dietary patterns and prevent diet-related non-communicable diseases: scoping review. Eur J Clin Nutr 2017;71:694–711. 10.1038/ejcn.2016.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trieu K, Neal B, Hawkes C, et al. Salt reduction initiatives around the world – a systematic review of progress towards the global target. PLoS One 2015;10:e0130247 10.1371/journal.pone.0130247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knai C, Petticrew M, Durand MA, et al. The Public Health Responsibility Deal: has a public-private partnership brought about action on alcohol reduction? Addiction 2015;110:1217–25. 10.1111/add.12892 [DOI] [PubMed] [Google Scholar]
- 24. Durand MA, Petticrew M, Goulding L, et al. An evaluation of the Public Health Responsibility Deal: informants’ experiences and views of the development, implementation and achievements of a pledge-based, public–private partnership to improve population health in England. Health Policy 2015;119:1506–14. 10.1016/j.healthpol.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 25. Sadler K, Nicholson S, Steer T, et al. National Diet and Nutrition Survey – assessment of dietary sodium in adults (aged 19 to 64 years) in England 2011;2011. [Google Scholar]
- 26. Public Health England Salt targets 2017: Progress report. A report on the food industry’s progress towards meeting the 2017 salt targets, 2018. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/765571/Salt_targ
- 27. Ji C, Cappuccio FP. Socioeconomic inequality in salt intake in Britain 10 years after a national salt reduction programme. BMJ Open 2014;4 10.1136/bmjopen-2014-005683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hyseni L, Elliot-Green A, Lloyd-Williams F, et al. Systematic review of dietary salt reduction policies: evidence for an effectiveness hierarchy? Plos One 2017;12:e0177535 10.1371/journal.pone.0177535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saltsmart Consortium consensus statement, 2015. Available: https://www.paho.org/hq/dmdocuments/2015/salt-smart-Consensus-statement-with-targets-FINAL.pdf
- 30. Barberio AM, Sumar N, Trieu K, et al. Population-level interventions in government jurisdictions for dietary sodium reduction: a Cochrane Review. Int J Epidemiol 2017;46:1551–1563. 10.1093/ije/dyw361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cairney P. A ‘Multiple Lenses’ approach to policy change: the case of tobacco policy in the UK. Br Polit 2007;2:45–68. 10.1057/palgrave.bp.4200039 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jech-2018-211749supp001.pdf (14.9MB, pdf)


