Abstract
This article investigates the influence of family back-ground and neighborhood conditions during childhood on health later in life, with a focus on hypertension. To document the proportion of current adult racial health disparities rooted in early-life factors, I use nationally representative longitudinal data from the PSID spanning four decades. The results indicate that racial differences in early life neighborhood conditions and family background characteristics play a substantial role in explaining racial disparities in hypertension through at least age 50. Contemporaneous socioeconomic factors account for relatively little of the racial disparities in this health condition in adulthood. Second, I match the Panel Study of Income Dynamics data to county-level data on Medicaid expenditures during these cohorts’ childhoods, and provide new causal evidence on the long-run returns to childhood Medicaid spending: Medicaid-induced increases in access to public health insurance led to significant reductions in the likelihood of low birth weight, increased educational attainment and adult income, and reduced adult mortality and the annual incidence of health problems.
Keywords: health disparities, racial inequality, neighborhood quality, effects of child health insurance
African Americans experience high rates of premature aging. In the United States, blacks can expect to live six fewer years than whites, and can expect to live more years with chronic health problems. Most of the black-white difference in life expectancy stems from racial differences in mortality rates prior to age 65. Blacks’ higher prevalence of cardiovascular disease-related risk factors account for more than half of the racial disparity (Barghaus et al. 2008), with hypertension the leading culprit. Hypertension is a major risk factor for heart disease and stroke, the leading causes of death in the United States. The prevalence of hypertension and diabetes is two to three times higher among blacks relative to whites.
Every seven minutes a black person dies prematurely in the United States; more than 200 black people die daily who would not have if the health of blacks and whites were equal (Williams 2005). At age 25, there is a five-year life expectancy gap between blacks and whites; and the gap by education for both blacks and whites is even larger than the racial gap (Braveman et al. 2010). But adult socioeconomic status (SES) is only part of the story underlying racial health disparities, as we see white high school graduates who did not attend college live longer than blacks with a college degree or more education. So why does race matter?
Racial health disparities are not only large but appear to widen over the life cycle, suggestive of an important role of childhood conditions. Thus, understanding sources of racial health disparities requires the investigation of exposures to socioeconomic conditions and risk factors earlier in the life cycle.
While these health inequities have been well documented (Williams and Collins 1995; Johnson 2011; Meara, Richards, and Cutler 2008; Chetty et al. 2016), they are not immutable—not the product of our genes but the consequences of our policies and history. The greatest black-white convergence in various dimensions of health over the life course occurred for cohorts born between 1965 and 1980. This period coincided with the rollout of Medicaid, both hospital and school desegregation, and Head Start, which each substantially improved early life health and educational investments that significantly narrowed opportunity gaps for minority and poor children. Too often the various strands of the inequality literature are ahistorical—e.g., research panning for contemporary period-specific factors to account for current health disparities. The search for clues to the root causes of contemporary racial health disparities in adulthood, and how best to promote population health, may lie in first extracting the important lessons from some of our most significant expansions of public investments in childhood health and education.
This article provides new evidence on the long-run effects of policies designed to increase children’s early, and equal, healthcare access. Medicaid, first established in 1965, was part of President Lyndon Johnson’s “War on Poverty” to create a public health care system to improve the health of the poor. Medicaid’s founding architects aimed to play a foundational role in restructuring American health care inequalities by poverty and race. I examine the extent to which those aims have been realized, and highlight goals yet unattained—the unfinished work of health equity. Currently, Medicaid and the Children’s Health Insurance Program (CHIP) provide low-cost health care coverage to nearly 44 million children, covering one-half of all low-income children (Council of Economic Advisers 2016).
Emerging research has sought to identify whether, and how, early life differences in access to health care and exposures to stressful life conditions “get under the skin.” Recent findings in neuroscience highlight prolonged exposure to stress hormones (e.g., cortisol) can suppress the body’s immune response and cause greater vulnerability to chronic health conditions. Early life experiences of toxic stress, even in the womb, may have profound implications for later-life health (Johnson and Schoeni 2011). Identifying sources of race differences in risk factors that stem from childhood conditions and lead to elevated levels of adult morbidity and mortality may shed light on extant inequalities in life chances.
The aim of this article is two-fold. First, I document the proportion of current adult racial health disparities rooted in early life factors. For this investigation, I use nationally representative longitudinal data from the United States, spanning four decades to estimate hazard models of onset of hypertension, cardiovascular disease, adult mortality, and general health status through mid-life for cohorts born between 1950 and 1970. The national descriptive portrait of the evolution of health over the life course is presented by race/ethnicity, childhood SES, and childhood neighborhood poverty. This is among the first such studies of the full U.S. population. The dataset, the Panel Study of Income Dynamics (PSID, 1968–2015), with information collected on both age of onset of specific health conditions and early childhood experiences, has the additional unique feature of allowing analyses of siblings and child neighbors throughout much of the sample’s life course. I use the resemblance between neighboring children’s subsequent likelihood of hypertension in adulthood in comparison to the similarity between siblings to bound the proportion of inequality in each health condition that can be attributed to disparities in neighborhood and family background. I estimate four-level hierarchical random effects hazard models of the onset of hypertension, which provide a better understanding of the relative importance of family and neighborhood backgrounds. Hypertension is a focal point here because it is the leading risk factor of cardiovascular-related disease.
Second, I seek to identify which childhood family and neighborhood factors matter most, with a focus on those that are amenable to policy reforms. In particular, I provide new causal evidence on the long-run effects of childhood public health insurance access on educational attainment, adult earnings and poverty status, and adult health status and mortality. For this second aim, I match the PSID data to administrative data on county Medicaid expenditures that prevailed when these cohorts were children. The research design uses the staggered introduction of Medicaid across states (1966–1982) and the federal mandate that states cover all cash welfare recipients to isolate the effects of child health insurance on the adult outcomes of poor children. This work builds on recent research by Goodman-Bacon (2018), who found that Medicaid’s introduction significantly reduced black infant mortality, and reduced childhood mortality among black children by 20 percent in the 1960s and 1970s. This extends that work to show the long-run returns to childhood Medicaid spending, using a difference-in-difference and instrumental variables approach.
My results indicate that racial differences in early life neighborhood conditions and family background characteristics play a significant role in explaining racial disparities in hypertension through at least age 50, while contemporaneous socioeconomic factors account for relatively little of the racial disparities in this health condition in adulthood.
For child cohorts after Medicaid implementation, childhood health care utilization increased—and more rapidly so among children from high-Medicaid- eligibility states. I find these increases in childhood public health insurance access led to significant reductions in the likelihood of low birth weight, increased educational attainment and the likelihood of graduating from high school, increased adult earnings, reduced the annual incidence of poverty in adulthood, and significantly reduced adult mortality and the annual incidence of health problems.
I begin this article with a discussion of how childhood neighborhood and family background may affect an individual’s health trajectory in adulthood, and risk of hypertension and cardiovascular disease in particular. I briefly summarize relevant previous research and highlight the relevant theoretical issues that motivate the empirical analyses to follow. The following section lays out the methodological challenges in estimating the relative importance of childhood family and neighborhood factors, and the causal effects of child health insurance access. The data are then described, followed by a discussion of the econometric model and estimation methods. I then present the results and wrap up with concluding statements.
Related Research
There is a strong, well-developed theoretical foundation that motivates this empirical investigation. The early-life origins of adult disease may begin in the womb. When a fetus receives limited nutrition, its metabolic and physiological makeup fundamentally changes. While the consequences may not be evident at birth, or even in early childhood, they can appear much later in life. This phenomenon is known as the “fetal origins hypothesis,” developed by epidemiologist David Barker (1998). A voluminous empirical literature supports Barker’s hypothesis, mostly drawn from the UK (see Barker 1998 for a review), and I have previously documented the long-run impacts of poor infant health in the United States using the PSID (Johnson and Schoeni 2011). Recent evidence in the developmental origins of adult disease and neuroscience literatures emphasizes the critical period of development from conception to age five as one that is extremely sensitive to stressful environmental conditions, as the speed of growth is more rapid than any other stage of the life course and the nutritional and health care needs are greatest (e.g., see Lynch and Smith 2005 for a review; Heckman 2007, and references therein).
Recent findings in neuroscience also indicate that developmental health trajectories can be altered more readily during sensitive periods of rapid developmental change than during other periods. Heckman (2007) emphasizes that, “common developmental processes are at work where some cognitive and noncognitive skills and health capabilities at one stage in childhood cross-fertilize the productivity of investment at later stages” (p. 13254). Research evidence from this field increasingly supports the notion that the greatest opportunities to invest in health occur during the first 20 years of life. This suggests a need to shift some of the emphasis on treatment in later stages of disease toward the promotion of earlier, more effective prevention, and an investment-oriented approach to health spending aimed at its most productive uses.
Chronic health conditions, like hypertension, typically grow out of socioeconomic conditions over a lengthy life-cycle period rather than from circumstances at a single point in time. The life-course perspective taken here emphasizes that health problems early in life could affect health later in life because the problem is chronic, because the health shock damaged health stock making it more susceptible to deterioration later in life, and because the health problem affects socioeconomic outcomes such as education, which in turn influences health later in life (Kuh and Wadsworth 1993). Poor black children are less likely to escape poverty than poor white children (Bhattacharya and Mazumder 2011). Johnson (2016) shows that blacks are trapped in high poverty neighborhoods for a significant share of the life course to a far greater extent than whites.1 Stressful neighborhood conditions, due to high poverty rates, crime, violence, and weaker sources of social support, may lead to increased risk of high blood pressure.
The hypothesis that the differential burden of lifetime stress contributes to racial and socioeconomic disparities in health builds on a solid scientific foundation. Prolonged exposure to stress produces elevated risks of a condition known as allostatic load, which refers to the physiological costs of chronic overactivity or underactivity of systems within the body (e.g., the hypothalamic-pituitary-adrenal axis or the autonomic nervous system) that fluctuate to meet demands of chronic exposure to environmental stressors (McEwen 1998). Persistent exposures to disadvantaged neighborhood and family conditions may have a cumulative toll in the form of “weathering” (Geronimus 1996). Recent findings in neuroscience indicate that early life risk factors compound over the life cycle—often-cited examples of the adaptive cost of stress-induced wear and tear (“weathering”) include pushing the endocrine system toward diabetes or the cardiovascular system toward coronary artery disease and hypertension (Halfon and Hochstein 2002). How people are affected and adapt to stressful family circumstances and neighborhood environments may depend, in part, on their access to informal sources of social support. Blacks appear to face more stress than comparable whites (Geronimus et al. 2001), as evidenced in studies that document higher cortisol levels—a hormone that is a marker for stress—among blacks even after accounting for family income (DeSantis et al. 2007).
A prior study (Fang, Madhavan, and Alderman 1996) showed that the health status of blacks living in New York City as adults was associated with their place of birth: the northern United States, the southern United States, or the Caribbean. The health status of those from the Caribbean was similar to New York City whites, and better than those from the northern United States; in turn, those from the northern United States had better health status than those from the southern United States. These findings point to the enduring influence of childhood circumstances, while not identifying what aspects of upbringing matter most.
Recent research has highlighted the importance of examining potential complementarities between early life health and educational investments, or synergies between early childhood investments and public K-12 spending (Johnson and Jackson 2017). Interconnections over the life cycle and feedback between improvements in early life health, cognitive ability, access to quality schools, and formation of noncognitive skills lead to gains in educational and economic attainments in adulthood. Dynamic complementarities may exist between early and later human capital investments (Cunha and Heckman 2007) that magnified the interactive impacts of desegregation, Head Start, improved access to school resources, and the roll-out of various antipoverty programs in ways that far exceed what the sum of each one of these initiatives could have achieved in isolation.
The fact that health investments in different life stages may be interactive has important implications for the most effective design of public policies to promote healthy development and child well-being. Maximizing the combined, interactive effects of child health and human capital policies and antipoverty programs can break the generational persistence of low economic and health status for the 42 percent of American low-income children who spend their entire life in the bottom income quintile (Jantti et al. 2006). Poor infant health has been shown to be an important transmission mechanism underlying this relative immobility (wherein only 8 percent of U.S. men at the bottom rise to the top quintile) (Johnson and Schoeni 2011).2 For example, there were substantial improvements in the intergenerational mobility of successive cohorts of black children born from the early 1960s to early 1970s, because they were differently exposed to desegregation in both schools and hospitals, and they had better access to quality schools (Johnson 2016). Without these ripple-like interactions across human capital investments, the impacts would have likely been smaller.
The finding that more-educated individuals have higher general health status, life expectancy, lower morbidity, and experience lower incidence and risks of onset of specific health conditions is one of the most strongly established facts in the health disparities literature. Educational attainment is one of the strongest and robust determinants of health, has been documented throughout the life course, and in all countries as far back as data exist, and even intergenerationally (i.e., parental education influences child health and well-being). Beyond the veil of these large gaps in morbidity and mortality between more- and less-educated individuals, the nature and sources of this relationship between education and health are less well understood. For policy purposes, descriptive evidence of the strong links between education and health are currently insufficient to provide precise guidance on how best to improve overall population health and narrow racial and SES-related health disparities. Research is needed that uncovers the relationships between childhood conditions and children’s short- and long-run health outcomes, and identifies the causal chain that links measures of segregation, childhood neighborhood quality, childhood health insurance access, and adult health.
Studies using quasi-experimental methods find that legislative expansions of Medicaid eligibility since 1980 led to large reductions in mortality for infants, children, and adults (Currie and Gruber 1996a, 1996b; Sommers, Baicker, and Epstein 2012; Wherry and Meyer 2015). As Card and Shore-Sheppard (2004) show, the corresponding increases in any insurance coverage are relatively small during this later period, however, leaving considerable uncertainty about the mechanism for these effects. The Oregon Health Insurance Experiment (Finkelstein et al. 2012), while providing the strongest research design to tease out health insurance coverage effects, is limited to only short-run health outcomes. Recent studies find these Medicaid expansions (since 1980) led to improvements in poor children’s academic outcomes (Cohodes et al. 2016; Levine and Schanzenbach 2009).
Hospital desegregation
Parallel, compelling evidence of the long-run effects of early childhood access to health care comes from analyses of hospital desegregation. Hospital desegregation was a monumental policy shift that, alongside the rollout of school desegregation, impacted the overall long-term well-being of minority children of the post-Brown era. The desegregation of hospitals in the South can be initially dated from 1964, when federally mandated policies began to be enforced. In particular, developments in all three branches of government—judicial, executive, legislative—were influential. First, the Fourth Federal Circuit Court ruled that the “separate but equal” clause in the Hill-Burton Act was unconstitutional, rendering hospital segregation unlawful in certain hospitals that had received federal funding in a handful of southern states under the Fourth Circuit’s purview. Second, Title VI of the Civil Rights Act of 1964 broadened the evisceration of the “separate but equal” doctrine beyond select hospitals and past Virginia, North Carolina, and other Fourth Circuit states, making it illegal for any private institution that received government funds to withhold service on the basis of race. Third, with the introduction of Medicaid and Medicare in 1965, a hospital had to be racially desegregated to be eligible to receive Medicaid/Medicare funding (Johnson, forthcoming). The staggered timing of hospital desegregation in the South led to differences in the timing of improved access to hospital care for minorities, and resulted in timing differences in the implementation of Medicare in parts of the South that had not desegregated their hospitals prior to 1965.
Using the American Hospital Association’s Annual Survey of Hospitals along with the Centers for Medicare Provider of Service data files to identify the precise date in which a Medicare-certified hospital was established in each county of the United States (an accurate marker for hospital desegregation compliance), I find that one-quarter of counties in the South—and 75 percent of counties in the Mississippi Delta—lacked a Medicare-certified hospital by the end of 1966. Almond, Chay, and Greenstone (2006), using this variation in the timing of hospital desegregation in Mississippi, document substantial declines in blacks’ postneonatal infant mortality from diarrhea and pneumonia in counties that had desegregated by February 1969 relative to counties whose hospitals remained segregated through the late 1960s. These are early life health conditions that require immediate access to adequate hospital care to prevent mortality. They show a 40 percent reduction in black infant mortality between 1964 and 1972. In the early 1960s, black infant mortality rates were significantly higher in the twenty-one states, plus the District of Columbia, with de jure segregation than in non-Jim Crow, northern states. By the late 1960s, these differences in black infant mortality rates in the South vs non-South had disappeared (Krieger et al. 2013). Furthermore, Chay, Guryan, and Mazumder (2009) provide evidence that racial convergence in early life health and hospital access during ages zero to five years subsequently led to a significant narrowing of the black-white test score gap for cohorts born during the mid-to-late 1960s.
My prior research findings demonstrate that both school desegregation and hospital desegregation independently contributed to the improvement in adult attainment of black cohorts that transitioned from the era of segregated to desegregated and integrated hospital and school contexts (Johnson, forthcoming). In this prior work, I looked at the timing of both school desegregation and hospital desegregation in the same model, and linked that with nationally representative data. The analysis used age at which hospital desegregation occurred and distance to the nearest hospital as an index of segregation and access during childhood (where the location of black hospitals was included as well as whether the nearest public hospital was open to blacks). I explored how improved access to school quality and health care services interacted with blacks’ subsequent life trajectories, adult SES, and health outcomes. The findings show healthier children are better learners. I find significant impacts of both hospital desegregation and school desegregation that led to significant narrowing of health disparities in adulthood for blacks, which once again underscored the importance of considering the interrelationship between early childhood investments in health and public school spending (Johnson and Jackson 2017). When these two types of investments occur together, the combined effect can be substantial, and larger than the sum of the two investments in isolation.
Methodological Challenges in Estimating Causal Effects
Neighborhood effects
Childhood residential segregation influences access to high-quality schools and medical care, exposure to crime and violence, housing and neighborhood conditions, and future employment opportunities. Black children are substantially more likely than white children to both grow up in concentrated poverty neighborhoods and attend high-poverty schools.3
The primary methodological challenge in estimating the causal effects of neighborhoods on health status is that unobserved factors that affect health may also be correlated with neighborhood factors, leading to biased estimates of neighborhood effects. This can arise from the endogeneity of residential location. That is, individuals and families choose where they live based on the characteristics they value (Tiebout 1956), although constraints such as racial discrimination and exclusionary zoning may be placed on that decision. In this context, families and individuals who care more about their health will be less likely to choose to live in an area with high crime, pollution, or a poor health care system. Furthermore, the set of complex and nuanced characteristics that influence neighborhood choices are not likely to be well measured and accounted for appropriately in econometric models.
The most powerful way to address selection is through a randomized trial. But an experimental design where neighborhoods are randomly assigned is rare. A significant exception is the evaluation of the Move to Opportunity (MTO) program, where an experimental design is used to estimate the effects of offering housing assistance that allows individuals to move out of low-income, poor neighborhoods. Evidence from MTO demonstrates that moves to better neighborhoods had beneficial effects on the health of children and adults (Katz, Kling, Liebman 2001; Leventhal and Brooks-Gunn 2003). This evidence is consistent with the claim that neighborhood factors do in fact influence health status, at least in the short-run, among poor families.4
The question of whether and how neighborhood socioeconomic features influence long-run health trajectories is particularly ill-suited for the typical methods by which microeconometricians solve endogeneity problems (e.g., instrumental variables and fixed effect approaches) for several reasons. First, most health outcomes are a product of cumulative exposures to advantaged/disadvantaged environments spanning decades, or exhibit long latent periods before problems manifest. Therefore, the connection between current neighborhood and current health may say little about the overall influence of neighborhood factors over the life cycle. As well, it may be important to conceptualize neighborhood effects as cumulative and variable over the life course as opposed to isolated and unchanging. Because most methods for overcoming endogenous neighborhood choice are based on small short-run changes in the neighborhood environment, these approaches might be limited to uncovering effects only for rapidly responding intermediate outcomes such as health behaviors (e.g., smoking/drinking, exercise/diet). An additional issue is that neighborhood variables of the underlying neighborhood feature of interest are notoriously measured with a great deal of noise. The neighborhood attributes of interest change slowly over time, so most year-to-year variations in the characteristic measured are noise.
The first part of this article takes a different approach that largely side-steps these challenges by exploiting a unique feature of the PSID. Specifically, the initial PSID sample in 1968 was highly clustered, allowing one to compare the similarity in onset of hypertension between siblings who grew up together, versus unrelated individuals who grew up in the same narrowly defined neighborhood.5 Instead of performing another typical regression analysis focused on particular neighborhood characteristics, the first goal of the analysis is focused on an overall assessment of the relative contributions of individual, childhood family, and neighborhood effects on the onset of hypertension. I use the resemblance between neighboring children’s subsequent likelihood of hypertension in adulthood to bound the proportion of inequality in this health condition that can be attributed to disparities in neighborhood background.
The resemblance in the risk of hypertension onset in adulthood among childhood neighbors reflects the lasting and composite influence of factors shared by individuals from the same neighborhood—measured and unmeasured. This approach avoids the difficulty of defining neighborhood quality at the outset and, instead, compares the relative magnitudes of the child neighborhood and family components, placing an upper bound on the neighborhood influence. The magnitude of the overall neighborhood component (from the unconditional random effects hazard model of onset of hypertension) represents an omnibus measure of the overall scope of child neighborhood effects, but is an upper bound because some of the influence may emanate from child neighbors having similar family background characteristics. A central aim of this work is to disentangle true causal influences of neighborhoods from influences stemming from familial selection into neighborhoods, and to examine whether (and how) child neighborhood context influences subsequent hypertension risk in a way that cannot be reduced to the characteristics of the individuals themselves.
Data
The PSID began interviewing a national probability sample of families in 1968, and it has re-interviewed the members of those families every year since, with bi-annual interviewing beginning in 1997. Most importantly, when children of the 1968 PSID families became adults and left their parents’ homes, these children themselves were interviewed in each year.
The PSID used a “cluster sample” when it started in 1968 to economize on interviewing costs. This design effect is typically a liability in statistical analyses because one has to account for nonindependence across individuals within the same cluster. But for our purposes the clustering provides the unique opportunity to examine health outcomes for adults who were childhood neighbors in 1968. Moreover, because all 1968 children within a given family are followed throughout their lives, we can examine the similarity in health outcomes over the life-course of siblings and childhood neighbors.
In the analyses, I define the neighborhood of upbringing as the census block where the child lived in 1968.6 Thus, I am able to use a narrower, compact definition of neighborhood than the vast majority of previous studies of neighborhood effects. Typical studies use census tracts to define neighborhoods, and census tracts, which consist of roughly 5,000 families, are much larger than the neighborhood construct we employ. Although the neighboring families in the PSID sample may or may not have been social neighbors in the sense of interacting closely with each other, they did live in close geographic proximity to each other, and this neighborhood construct should capture important environmental influences. In urban areas, neighboring 1968 families in the PSID may have been a city block or even just part of a block. In rural areas, the families were spread farther apart, but still were among each other’s closest neighbors (Solon, Page, and Duncan 2000).
Most prior studies of the connection between early life health and economic status and adult health have relied on health surveys that have very limited economic data. The PSID is among the best equipped data sources to investigate the linkages between health and economic status in the initial stages of life and the subsequent onset of specific health conditions. Spanning from 1968 through 2015, it is the longest-running nationally representative U.S. sample of households, is one of the premier data sources of income in the world, and it also contains significant detail on health. The data include extensive socioeconomic measures in childhood and adulthood and do not have to rely on retrospective reports of early life economic conditions.
All persons in PSID families in 1968 have the PSID “gene,” which means that they are followed in subsequent waves. In addition, anyone born to or adopted by PSID sample members acquires the PSID “gene” themselves and therefore is followed. When children with the “gene” become adults and leave their parents’ homes, they become their own PSID “family unit” and are interviewed in each wave. This sample of “split offs” has been found to be representative (Fitzgerald, Gottschalk, and Moffitt 1998). Moreover, the genealogical design implies that the PSID sample today includes numerous adult sibling groupings and parent-child groupings who have been members of PSID-interviewed families for nearly four decades.
The sample chosen for the focus of the study consists of PSID sample members who were children when the study began and who have been followed into adulthood. Specifically, I choose PSID sample members born between 1951 and 1968, which consists of children 0–17 years old in the first wave of interviewing in 1968. I then obtain all available information on these individuals for each wave, 1968 to 2005. Therefore, by 2005 the oldest person in the sample is 57 and the youngest is 37. The ages of these respondents by the end of the sample period correspond to the years of life (fifties) in which both socioeconomic differences in adult health status begin to approach peak levels, and rates of deterioration in health begin to accelerate for disadvantaged persons (House et al. 1994; Johnson and Schoeni 2011).
The analysis focuses on the prevalence and age of onset of hypertension. Respondents in the 1999–2015 PSID survey interviews were asked whether a doctor ever told them they have a particular disease, and if so, at what age the initial diagnosis occurred. It is important to bear in mind that this self-report measure may be affected by differential access and interaction with the health care system. In particular, poor families’ and African Americans’ lower levels of health care use relative to affluent and white families, with the exception of emergency room care, is expected to bias the socioeconomic gradient and racial health gaps toward equality, making our estimates of these differences in hypertension conservative.
I found no evidence that estimates from the adult sample suffer significant bias from health-related attrition due to selective mortality; any potential bias suggests that early mortality will tend to reduce the estimated effect of neighborhood disadvantage during childhood on later outcomes.
To increase the sample size as well as the proportion of poor and black families in the sample, I include both the Survey Research Center (SRC) component and the Survey of Economic Opportunity (SEO) component, commonly known as the “poverty sample,” of the PSID sample. I appropriately apply multi-level sample weights at the neighborhood and family levels to produce nationally representative estimates.7 The results are robust to the exclusion of the SEO sample, as estimates that exclude the SEO sample exhibit the same patterns as those reported in the article.8
The ability to conduct analyses within families and between neighboring families is a unique feature of this research. Because this study is among the first to report evidence of sibling similarity in the onset of hypertension, I include all neighborhoods to increase the effective sample size for the sibling comparison estimates. Results on the subsample that is restricted to neighborhoods containing children from at least two different families yields very similar magnitudes of sibling and child neighbor resemblance in hypertension risk.
The analysis sample of PSID original sample children consists of 125,164 person-year observations from 2,942 individuals from 1,410 families from 991 original neighborhoods in 95 different counties. The mean age at the most recent survey interview is about 48, with age ranging from 34 to 64. The sample is about 41 percent black due to the oversampling of low-income families.
Income is the total for the family in which the child lives, and it is measured from the five-year average for the years 1967–1972. All dollar values are expressed in 1997 dollars using the Consumer Price Index for All urban Consumers (CPI-U). The parental income measure is specified as the income-to- needs ratio, and we explore nonlinearities in effects at the bottom of the income distribution (child poverty).
Child health insurance coverage is measured through information collected in the first five waves of the PSID (1968–1972) on whether the parent (head of household) had access to private health insurance coverage and, if so, whether the entire family was covered. I define three categories of child health insurance coverage: continuously covered by private health insurance in childhood years during 1968–1972; intermittent coverage during those years; and lacking private health insurance coverage in all of these years. Lack of private health insurance may discourage preventive medical care use. For those who lacked private coverage for their children, the data suggest that public health insurance coverage was used to some extent, but there were not enough individuals in our sample who persistently lacked public and private insurance during these childhood years to define “no public or private insurance during childhood” as a reference category.
The parental health status measures are the proportion of years spent when the parent was in their fifties in which they were in problematic health (based on selfreports of work-limiting conditions and/or general health status). These ages correspond to years in life when rates of health deterioration typically begin to accelerate.
Descriptive analyses
Using the PSID original sample children born between 1951 and 1968, I begin by producing nationally representative estimates of the cumulative risks through middle-age of hypertension, stroke/heart attack/heart disease, and mortality by race/ethnicity, child poverty, child health insurance coverage status, and childhood neighborhood poverty. The results for the annual mortality hazard and cumulative mortality hazard are presented in Figure 1A and 1B, respectively, separately by race. The results for hypertension are summarized in Table 1 and in Figure 2. Sampling weights are used to produce nationally representative estimates. I report Nelson-Aalen estimates of the cumulative risk of hypertension onset by various ages over the life course.
figure 1.
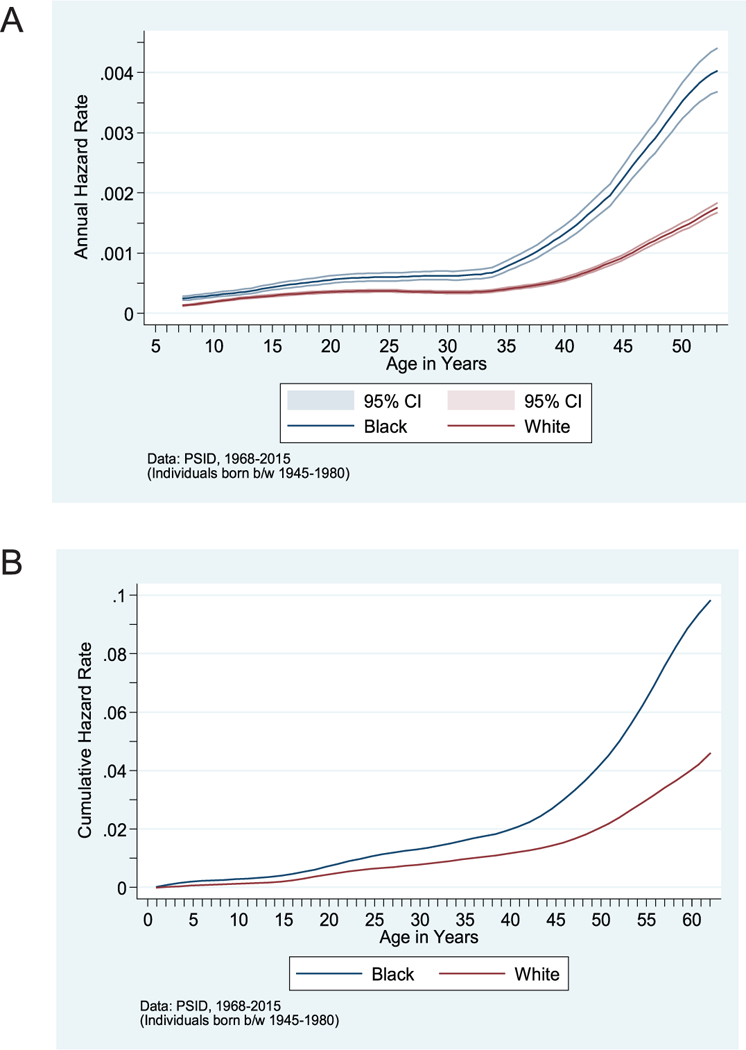
(A) Mortality Hazard, by Race. (B) Cumulative Mortality Hazard, by Race
SOURCE: PSID data, 1968–2015.
NOTE: Individuals born between 1945 and 1980. NOTE: Individuals born between 1950 and 1975.
TABLE 1.
Proportion with Hypertension, by Race and Child Socioeconomic Status
| Proportion ever diagnosed with hypertension by age: | ||||
|---|---|---|---|---|
| 25 | 35 | 45 | 50 | |
| By Pace | ||||
| Black, non-Hispanic | 0.0380 | 0.1568 | 0.4705 | 0.8078 |
| White, non-Hispanic | 0.0223 | 0.0893 | 0.2386 | 0.3908 |
| By Child Neighborhood Poverty Status | ||||
| High Poverty Neighborhood | 0.0529 | 0.1601 | 0.4335 | 0.7813 |
| Medium Poverty Neighborhood | 0.0201 | 0.1425 | 0.4062 | 0.6002 |
| Low Poverty Neighborhood | 0.0225 | 0.0850 | 0.2339 | 0.3893 |
| By Child Poverty Status | ||||
| child Poverty | 0.0259 | 0.1520 | 0.4566 | 0.6835 |
| Non-poor | 0.0201 | 0.0841 | 0.2262 | 0.3906 |
| By Child Health Insurance Status | ||||
| No childhood Health Insurance | 0.0290 | 0.1380 | 0.4169 | 0.6283 |
| Health Insurance | 0.0219 | 0.0755 | 0.2198 | 0.3651 |
Data: PSID, 1968–2015 (Individuals born between 1950 and 1968).
NOTES: Sampling weights are used to provide nationally-representative estimates.
FIGURE 2.
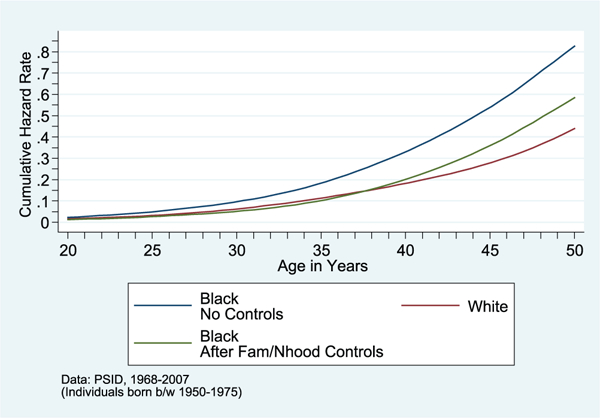
Cumulative Hazard of Onset of Hypertension, by Race, before and after Controls for Childhood Family/Neighborhood
SOURCE: PSID data, 1968–2007.
NOTE: Individuals born between 1950 and 1975.
Linking childhood neighborhood poverty to hypertension
The set of results summarized in Table 1 (and Figure 2) displays striking differences in the age of onset of hypertension, depending on whether the individual grew up in a low-poverty neighborhood, medium poverty neighborhood, or high-poverty neighborhood; depending on low birth weight status and whether average childhood income was below, close to, or well above the poverty line. I tested for the equality of the survivor functions along these lines and the reported differences are statistically significant in the vast majority of cases.
As shown in Table 1, the differences in risks of onset of hypertension by neighborhood poverty rate, low birth weight status, child poverty, health insurance coverage, and race/ethnicity are all stark. I find that by age 45, 42 percent of low- birth weight individuals had hypertension (compared with one-quarter of normal birth weight individuals); 45.7 percent of individuals raised in poverty had hypertension (compared with 22.6 percent of individuals with parental incomes during childhood that were at least two times the poverty line); 41.7 percent of individuals who lacked access to private health insurance coverage during childhood had hypertension (compared with 22 percent among those with access); and 47 percent of African Americans had hypertension (compared with less than one- quarter of whites).
It is important to note that the magnitudes of these differences in the risks of hypertension tend to become more pronounced as individuals age, a result that mirrors findings in Johnson and Schoeni (2011). The strong associations between neighborhood poverty rate, childhood socioeconomic factors, and the likelihood of onset of hypertension shown in Table 1 do not prove that neighborhood poverty itself is the cause of these differences. Of course, families who exhibit different patterns of disease onset are different from one another in a multitude of ways that may also contribute to the raw differences in their children’s adult health status outcomes that we observed. Family economic conditions in adulthood may be what really matters. Or perhaps some third factor, such as inadequate parental education or parental health status, is the cause of poor childhood neighborhood conditions and family poverty as well as their children’s subsequent poorer health in adulthood. We would expect these other factors to affect risks of hypertension, independent of early life neighborhood and child socioeconomic characteristics. A portion of the regression analysis in the subsequent section is devoted to attempting to identify whether it is childhood neighborhood poverty itself and its attendant stressors that leads to the higher disease risks, or these other differences in family characteristics (e.g., child health insurance) that are the main causal factors.
Overview of empirical strategy for aim #1
A primary goal of the analysis is focused on an overall assessment of the relative contributions of individual, family, and neighborhood effects on the onset of hypertension. I analyze the relative contribution of a parsimonious set of measured individual, household, and neighborhood covariates to the total variation from each component, and test specific hypotheses about the effects of specific characteristics of households and neighborhoods.
The strategy for assessing the importance of contextual effects involves estimating the fraction of variation in the risk of hypertension risk that lies between families and neighborhoods, to provide an upper bound on the possible effect of these contexts. The intuition motivating the use of this strategy is that, if family background and residential community are important determinants of the onset of hypertension, there will be a strong resemblance between siblings in disease onset, as compared with two arbitrarily chosen individuals. And if the neighborhood where the child grew up is important, it will show up as a strong similarity between neighboring children’s subsequent risks of hypertension in adulthood. This is the first study to simultaneously analyze individual, family, and child neighborhood-level variation in the onset of hypertension in a nationally representative sample that covers diverse neighborhood environments. By decomposing disparities into within- and between-area components, it advances our understanding of the possible contribution of residential segregation experienced in childhood to racial and socioeconomic disparities in hypertension in adulthood.
The similarity in health outcomes between siblings captures the effects of all measured and unmeasured factors shared by siblings that may have an impact on these outcomes, such as the SES of parents, genetic traits shared by siblings, family structure, as well as neighborhood effects stemming from the quality of neighborhood conditions (e.g., the school quality and availability of social services, housing and environmental conditions, peer group and role model influences, perceived/actual availability of economic opportunities), and sorting of similar family types within neighborhoods. Documenting the resemblance in health between siblings alone cannot identify the separate effects of family and neighborhood origins.
Analyzing the extent to which hypertension risk in adulthood is correlated among siblings versus childhood neighbors enables us to bound the relative importance of family and neighborhood factors. The sibling resemblance can be decomposed into a part arising from shared neighborhood origins and a part related to family background characteristics. I assess the extent to which hypertension risk is correlated among neighboring children above and beyond the correlation that exists because of similar family backgrounds.
Results
Sibling and child neighbor correlations in hypertension
I first present the unadjusted family, neighborhood, and county components in the onset of hypertension, and examine how much of these effects can be explained by the fact that families in a neighborhood tend to be similar. I begin by estimating an unconditional four-level (hierarchical) random effects discretetime hazard model of onset of hypertension, which includes random components at the county, neighborhood, and family levels (see appendix for further details). About one-third of individuals in the sample became hypertensive at some point during early-to-mid adulthood. The results are presented in Table 2. Estimates of the random effects of the neighborhood (σn) and family (σf) components indicate that neighborhood and family background have large and significant effects on the likelihood of hypertension onset.9 For example, the estimated σn of .502 implies the odds of onset of hypertension in adulthood for children who grow up in neighborhoods that are one standard deviation below average neighborhood quality are 1.7 times [exp(.502)] the corresponding odds of individuals who grow up in neighborhoods of average quality. Similarly, the estimated σf of .652 implies the odds of onset of adult hypertension for children who grow up in families that are one standard deviation below average family background are 92 percent higher than for individuals who grow up in families of average background. The county-level random components are much smaller in magnitude but are significant as well. The results indicate that children who grow up in counties that are one standard deviation below average have 27 percent higher odds of onset of hypertension relative to individuals who grow up in counties of average quality. The four-level models were estimated as a robustness check to ensure that the childhood neighborhood random effects components were not primarily driven by effects operating at higher geographic levels of aggregation. The three-level hierarchical models yielded fairly similar patterns as the more computationally intensive four-level models; so, for computational simplicity, I report only the results from the three-level models here.
TABLE 2.
Importance of Child Neighborhood and Family Background on Onset of Hypertension (Discrete-Time Hazard Model of Onset of Hypertension)
| Hierarchical Logit Model | 3-Level (1) |
4-Level (2) |
|---|---|---|
| Age – 40 | 0.1114*** | 0.1103*** |
| (0.0071) | (0.0071) | |
| (Age – 40)2 | −0.0016** | −0.0010 |
| (0.0006) | (0.0009) | |
| (Age – 40)3 | −0.0000 | −0.0000 |
| (0.0000) | (0.0000) | |
| Year born – 1960 | 0.0398*** | 0.0344*** |
| (0.0105) | (0.0126) | |
| Female | −0.2086** | −0.1950 + |
| (0.0924) | (0.1457) | |
| Constant | −4.0253*** | −4.0859*** |
| (0.0914) | (0.1151) | |
| Random Effects, Unmeasured (Std Dev) | ||
| childhood county component | 0.2415*** | |
| (0.0525) | ||
| childhood Neighborhood component | 0.5925*** | 0.5020*** |
| (0.0952) | (0.1468) | |
| childhood Family component | 0.5267*** | 0.6519*** |
| (0.1542) | (0.1681) | |
| Log-likelihood | −66753.161 | −2939400.6 |
| Number of counties | 95 | 95 |
| Number of neighborhoods | 991 | 991 |
| Number of families | 1,410 | 1,410 |
| Number of individuals | 2,942 | 2,942 |
| Number of person-year observations | 125,164 | 125,164 |
p < 0.01,
p < 0.05,
p < 0.10
NOTE: Robust standard errors in parentheses and all standard errors are Huber-corrected, clustered on county.
I next investigate how much of the magnitude of the estimated neighborhood random effects component may arise from childhood neighbors having similar observed family background characteristics as opposed to emanating from neighborhood effects per se. I find that observable family sorting (controlling for a broad array of family background characteristics) explains a significant share, but not all, of the resemblance in adulthood hypertension risk among unrelated individuals who grew up in the same neighborhood. I estimated the resultant adjusted neighborhood component after appropriately extracting the part arising from childhood neighbors having similar observed family background character- istics.10 The adjusted between-neighborhood variance (random effects) component is roughly 50 percent lower than the unadjusted between-neighborhood variance estimate. The adjusted neighborhood random effects component estimates imply that the odds of onset of hypertension in adulthood for children who grow up in neighborhoods that are one standard deviation below average neighborhood quality are 1.5 times [exp(.40)] the corresponding odds of individuals who grow up in neighborhoods of average quality.
For aim 1, the remainder of the analysis and presentation of results proceeds in three stages. First, I estimate the raw black-white gap in the risk of onset of hypertension in adulthood (controlling for age, year of birth, and gender). I then attempt to explain the racial disparity in this specific health condition by adding only childhood family and individual-level factors to the baseline model, including parental education, parental income-to-needs ratio, child health insurance coverage, low birth weight, parental smoking and alcohol expenditures, whether born into two-parent family, and birth order. Second, I examine how adjusting for neighborhood context changes estimates of family- and individual-level disparities in the risk of hypertension onset.11 In the third set of models, I add an extensive set of observable neighborhood-level variables into the hierarchical model, including childhood neighborhood poverty rate, school district per-pupil expenditures, measures of residential segregation, parental connectedness to informal sources of help, parental expectations of children’s educational attainment, and parental self-reports of neighborhood and housing quality collected in the PSID.12
The results are presented in Table 3. As shown in column (2), blacks have twice [exp(.708)] the odds of onset of hypertension in adulthood than whites experience. The racial gap is cut in half after inclusion of childhood family- and individual-level factors. The most prominent childhood family background factors on hypertension risk include parental education, low birth weight, and child health insurance coverage (parental income was not statistically significant after inclusion of these factors). For example, children with parents who did not graduate from high school have 1.4 times [exp(.361)] the odds of hypertension onset in adulthood as those with parents who were high school graduates (without college attendance); and college-educated parents had children whose odds of hypertension in adulthood was roughly 30 percent lower than that of individuals whose parents were high school graduates (without college attendance). Low birth weight children have, on average, nearly 75 percent higher odds of hypertension onset in adulthood (relative to normal birth weight individuals); and individuals who had access to health insurance coverage during childhood had about 20 percent lower odds of hypertension onset in adulthood (relative to those who lacked continuous coverage during childhood). These latter findings confirm those reported in Johnson and Schoeni (2011). The patterns of results for the childhood family factors remain after neighborhood adjustments (column 3) and inclusion of the set of childhood neighborhood factors (column 4).
TABLE 3.
Race Differences in Onset of Hypertension: Importance of Child Neighborhood and Family Background (Discrete-Time Hazard Model of Onset of Hypertension)
| 3-Level Hierarchical Logit Model | |||||||
|---|---|---|---|---|---|---|---|
| Childhood factors | Unconditional model |
Raw race gap |
No adjustment for neighborhood |
Neighborhood adjustmenta |
Controls for Child Nhood + Fam bckgrd |
Only Adult Nhood + SES |
Child bckgrd + Adult SES |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
| Black | 0.7081*** | 0.3553*** | 0.3559 | 0.2334 + | 0.4592*** | 0.1894+ | |
| (0.1082) | (0.1314) | (0.5518) | (0.1450) | (0.1199) | (0.1467) | ||
| Family income-to-needs ratio (avg during 1967–1972) | −0.0184 | −0.0599 | −0.0081 | 0.0177 | |||
| (0.0352) | (0.0484) | (0.0369) | (0.0369) | ||||
| High school dropout | 0.3612*** | 0.2285 + | 0.3029** | 0.2890** | |||
| High school graduate (reference categoiy) | (0.1236) | (0.1724) | (0.1240) | (0.1263) | |||
| College-educated | −0.3427** | −0.3914* | −0.3528** | −0.2855* | |||
| (0.1519) | (0.2082) | (0.1533) | (0.1568) | ||||
| Health insurance coverage in all years 1968 to 1972 | −0.2012* | −0.1941 | −0.1780+ | −0.1507+ | |||
| (0.1187) | (0.1713) | (0.1150) | (0.1150) | ||||
| Low birth weight | 0.5487*** | 0.5896*** | 0.5400*** | 0.5179*** | |||
| (0.1764) | (0.1746) | (0.1762) | (0.1794) | ||||
| Birth order | −0.0246 | 0.0114 | −0.0208 | −0.0292 | |||
| (0.0266) | (0.0278) | (0.0259) | (0.0257) | ||||
| Mother unmarried at child’s birth | 0.1435 | 0.2612** | 0.1556 + | 0.1428 | |||
| (0.1155) | (0.1170) | (0.1171) | (0.1177) | ||||
| Smoked cigarettes at some point, 1968–1972 | −0.0764 | −0.1081 | −0.0785 | −0.1233 | |||
| (0.1190) | (0.1519) | (0.1155) | (0.1149) | ||||
| Annual cigarette expenditures (in $100’s), | 0.0036 | 0.0077 | 0.0042 | 0.0056 | |||
| 5-year average 1968–1972 | (0.0099) | (0.0135) | (0.0099) | (0.0101) | |||
| Child Neighbo rhood factors | |||||||
| Residential segregation dissimilarity index, 1970 (MSA) | 0.2446 | −0.0262 | |||||
| (0.7622) | (0.7535) | ||||||
| Proportion of childhood years lived in low poverty neighborhood | −0.3020* | −0.2312 + | |||||
| (0.1703) | (0.1716) | ||||||
| Neighborhood incarceration rate, 1970 | 0.1169” | 0.1275** | |||||
| (0.0527) | (0.0524) | ||||||
| County school expenditures per pupil, 1962 | −0.2536** | −0.2022** | |||||
| (0.1008) | (0.1016) | ||||||
| Parental high expectations for child achievement | −0.1194 | −0.0838 | |||||
| (0.1232) | (0.1247) | ||||||
| Adulthood SES | |||||||
| Proportion of adulthood years lived in low poverty neighborhood | −0.4583*** | −0.3547** | |||||
| (0.1445) | (0.1520) | ||||||
| Years of completed education | −0.0916*** | −0.0530** | |||||
| (0.0242) | (0.0268) | ||||||
| Ln(annual labor earnings) | −0.0775* | −0.0649 + | |||||
| (0.0431) | (0.0443) | ||||||
| Random Effects, Unmeasured (Std Dev) | |||||||
| Childhood Neighborhood component | 0.5925*** | 0.5079*** | 0.4446*** | 0.5969*** | 0.4172*** | 0.4320*** | 0.3747*** |
| (0.0952) | (0.1138) | (0.1217) | (0.0983) | (0.1261) | (0.1091) | (0.1281) | |
| Childhood F amily component | 0.5267*** | 0.5107*** | 0.4763*** | 0.4719*** | 0.4492*** | 0.4489*** | 0.4564*** |
| (0.1542) | (0.1541) | (0.1440) | (0.1515) | (0.1472) | (0.1557) | (0.1413) | |
| Log-likelihood | −66753.161 | −66509.944 | −65933.001 | −66397.549 | −65738.665 | −6.5890.274 | −6.5359.924 |
| Number of counties | 95 | 95 | 95 | 95 | 95 | 95 | 95 |
| Number of neighborhoods | 991 | 991 | 991 | 991 | 991 | 991 | 991 |
| Number of families | 1,410 | 1,410 | 1,410 | 1,410 | 1,410 | 1,410 | 1,410 |
| Number of individuals | 2,942 | 2,942 | 2,942 | 2,942 | 2,942 | 2,942 | 2,942 |
| Number of person-year observations | 125,164 | 125,164 | 125,164 | 125,164 | 125,164 | 125,164 | 125,164 |
p < 0.01,
p < 0.05,
p < 0.10,
p < 0.10 (one-tailed test)
NOTE: All models include a constant and controls for age, age squared, age cubed, year of birth, gender, and columns (3)—(5) and (7) includes indices intended to capture parental connectedness to informal sources of help, parental aspirations/motivation and long-term planning (coefficients supressed to conserve space).
Neighborhood adjustment refers to centering all covariates around their neighborhood means, which is the analogous random effects formulation of a neighborhood fixed effects model.
The black-white gap is substantially reduced after inclusion of both the childhood neighborhood and family background characteristics—indeed, blacks have just 25 percent higher odds of hypertension onset in adulthood after accounting for these childhood factors (and the race coefficient ceases to be statistically significant at the 10-percent level).
The results indicate that children who grew up in low-poverty neighborhoods had about 25 percent lower odds of hypertension onset in adulthood relative to children who spent their childhood living in medium- and high-poverty neighborhoods. Additionally, growing up in high-crime neighborhoods (as proxied by childhood neighborhood incarceration rate) is linked with increased risk of hypertension in adulthood—in particular, a one standard deviation increase in the neighborhood incarceration rate (or roughly a 1-percentage point increase) corresponds with roughly a 12 percent increase in the odds of hypertension onset in adulthood.
It is striking that the lion’s share of the enormous racial disparities in hypertension risk can be explained by differences in these childhood factors, particularly neighborhood poverty rate, birth weight, parental education, and child health insurance coverage. In the case of hypertension, the black-white gap remains but its magnitude is a small fraction of the unconditional raw differences after inclusion of these early life factors. Taken together, the cumulative set of childhood neighborhood and family background factors account for one-half of the neighborhood-level variance in hypertension risk during adulthood (implied quasi-R2 at the neighborhood level); the fact that these measures account for less of the family-level variance may be the result of the fact that family-level influences include hereditary risk factors.
A substantial literature has investigated whether contemporaneous economic factors can account for the racial disparities in adult health. In column (6) of Table 3, I use our models to re-examine this issue and find that 65 percent of the black-white gap in hypertension risk remains after accounting for adult socioeconomic factors. The final model includes both the full set of childhood neighborhood and family background factors along with contemporaneous adult SES measures (adult neighborhood poverty rate, educational attainment, adult earnings). As shown in column (7) of Table 3, the results reveal that racial differences in onset of hypertension in adulthood can be accounted for by childhood family and neighborhood factors, while contemporaneous economic factors account for relatively little of this gap (see also Figures 2 and 3).13 These findings parallel results of Johnson and Schoeni (2011), in which they find that early life factors can account for the entire black-white gap in general health status, while contemporaneous economic factors account for relatively little of this gap.
figure 3.
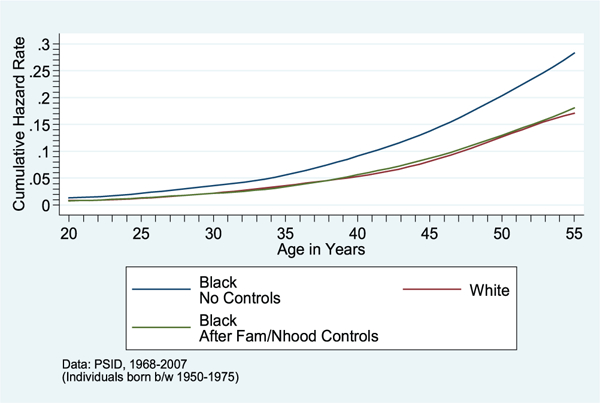
Cumulative Hazard of Onset of Stroke/Heart Attack/Heart Disease, by Race, before and after Controls for Childhood Family/Neighborhood
SOURCE: PSID data, 1968–2007.
NOTE: Individuals born between 1950 and 1975.
To probe the robustness of a causal inference, I use an empirical approach, first proposed by Altonji, Elder, and Taber (2005), to gauge how sensitive estimates of the effects of childhood neighborhood poverty are to selection on unobserved variables. I find that even a large amount of selection on unobservable factors does not completely eliminate the significant effect of child neighborhood poverty on hypertension later in life. The ratio of selection on unobservables to selection on observables would have to exceed 80 percent for one to attribute the entire effect of neighborhood poverty to selection bias.14
Long-run impacts of access to child health insurance (aim #2)
As aforementioned, child health insurance access is among the important childhood factors that is significantly correlated with subsequent health trajectories and racial health disparities—and one that is amenable to policy reforms. For the second aim, I sought to determine if that childhood link has causal roots. A key theme of my research agenda, and one emphasized here, is that human capital policy is health policy (and vice-versa). Thus, when we examine the long-term impacts of childhood health investments, we should not only consider impacts on health-related outcomes, but adult socioeconomic ones, too.
In this vein, I provide new causal evidence on the long-run effects of childhood public health insurance access on educational attainment, adult earnings and poverty status, and adult health status and mortality. For this second aim, I use data on all PSID respondents born between 1945 and 1975 followed into adulthood, geocoded identifiers for the county in which they grew up, and match the PSID data to administrative data on county Medicaid expenditures that prevailed when these cohorts were children.15 These cohorts straddle the period of the rollout of Medicaid during their childhood years. The sample includes 13,381 individuals from 1,070 childhood counties across all 50 states—this includes 5,623 individuals whose childhood families were low income (and potentially eligible for Medicaid depending on state and year of birth), providing sufficient power to detect effects of Medicaid spending. We examine all person-year observations in adulthood (ages 20–55) using PSID data through 2015.
The identification strategy uses the staggered introduction of Medicaid across states (1966–1982) and the federal mandate that states cover all cash welfare recipients to isolate the effects of child health insurance on the adult outcomes of poor children. As documented by Goodman-Bacon (2018), federally mandated Medicaid coverage of all cash welfare recipients induced substantial cross-state variation in the share of children immediately eligible for the program. Furthermore, black children were six times as likely to be eligible for Medicaid under the categorical welfare eligibility provision as white children (18 percent versus 3 percent) (Goodman-Bacon 2018). The timing of Medicaid implementation is presented in the maps shown in Figure 4; and the cross-state variation in Medicaid eligibility for all children is shown in Figure 5A, and separately for black and white children in Figures 5B and 5C, respectively. This timing and cross-state variation in Medicaid eligibility is leveraged to isolate the causal effects of childhood health insurance access and the long-run returns to childhood Medicaid spending, using a difference-in-difference and instrumental variables approach.
FIGURE 4.
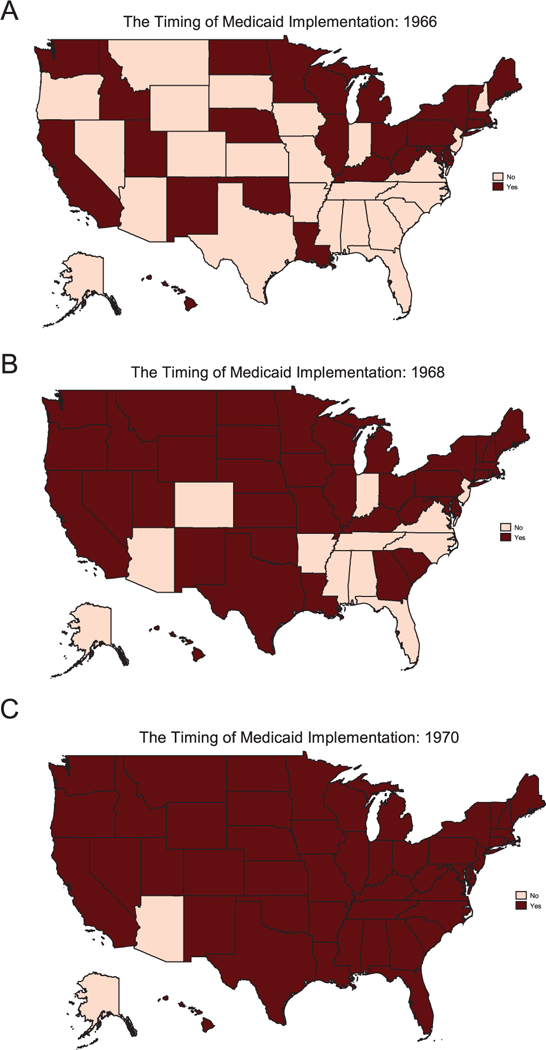
The Timing of Medicaid Implementation
FIGURE 5.
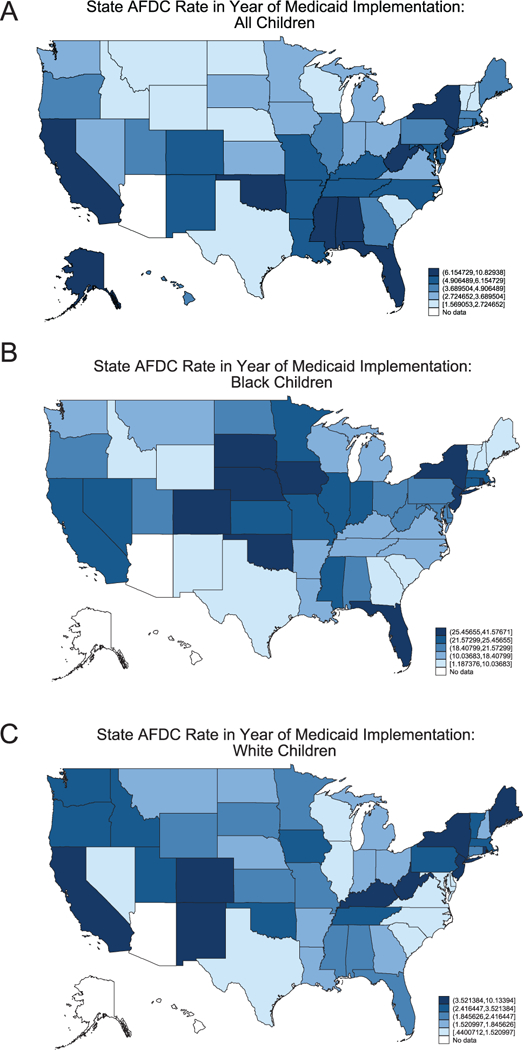
State AFDC Rate in Year of Medicaid Implementation: (A) All Children, (B) Black Children, (C) White Children
The research design compares children in areas where Medicaid was not yet available with children from those same areas after Medicaid became available. The changing availability of public health insurance coverage is beyond the control of parents and does not affect children independently of the programs themselves, which helps to insulate us from potential bias arising from omitted child family factors. In particular, I compare the adult outcomes of children before and after Medicaid implementation (first difference) between higher- and lower-eligibility states (second difference). In this way, the empirical strategy compares otherwise-similar children exposed to differing number of childhood years potentially eligible for Medicaid due to state differences in eligibility levels, and identifies a “dose- response” relationship. I analyze their life trajectories through adulthood.16
Because local areas with high versus low levels of per-capita Medicaid spending may differ in ways that could confound our comparisons, my identification strategy relies only on the variation in Medicaid spending during one’s childhood years induced by the precise timing of Medicaid implementation in the state of upbringing and the pre-Medicaid state AFDC (welfare) rates that determined eligibility during the program’s rollout, which in turn determined the generosity and availability of public health insurance for children. To do this, I examine per- capita Medicaid spending in county c (), and construct instrumental variables for it with an indicator variable of whether Medicaid had been implemented in one’s birth year in his/her childhood county and the pre-Medicaid state aFdC (welfare) rates (dosec).Formally, to identify effects of Medicaid spending on the probability of low birth weight we estimate the following system of equations by two-stage-least-squares (2SLS)
| 1 |
| 2 |
In similar fashion, to identify effects of Medicaid spending on the adult outcomes of children I use the analogous instrumented average per-capita Medicaid spending during ages 0–17, where represents the number of years of one’s childhood in which Medicaid existed (potential childhood exposure)—i.e., I estimate the following system of equations by 2SLS
| 3 |
| 4 |
where and are fitted values from first-stage regressions.
To further reduce the possibility of confounding effects, vector Cicb includes a variety of individual, childhood family, and childhood county controls. These include parental SES, mother’s marital status at birth, and gender; and the adult outcomes include flexible controls for age (cubic). I include birth-year fixed effects Cicb also includes race-by-region-specific birth cohort trends, and birth-cohort linear trends interacted with various 1960 characteristics of the childhood county (poverty rate, percent black, average education, percent urban, and population size). Standard errors are clustered at the state level.
Results.
We first show that children from states with higher and lower welfare-based eligibility had similar trends in children’s subsequent adult outcomes before Medicaid implementation (i.e., this evidence supports the parallel trends assumption that is central to the difference-in-difference approach). For child cohorts after Medicaid implementation, childhood health care utilization increased—and more rapidly so among children from high-Medicaid-eligibility states (as shown in Figure 6, A and B).
figure 6.
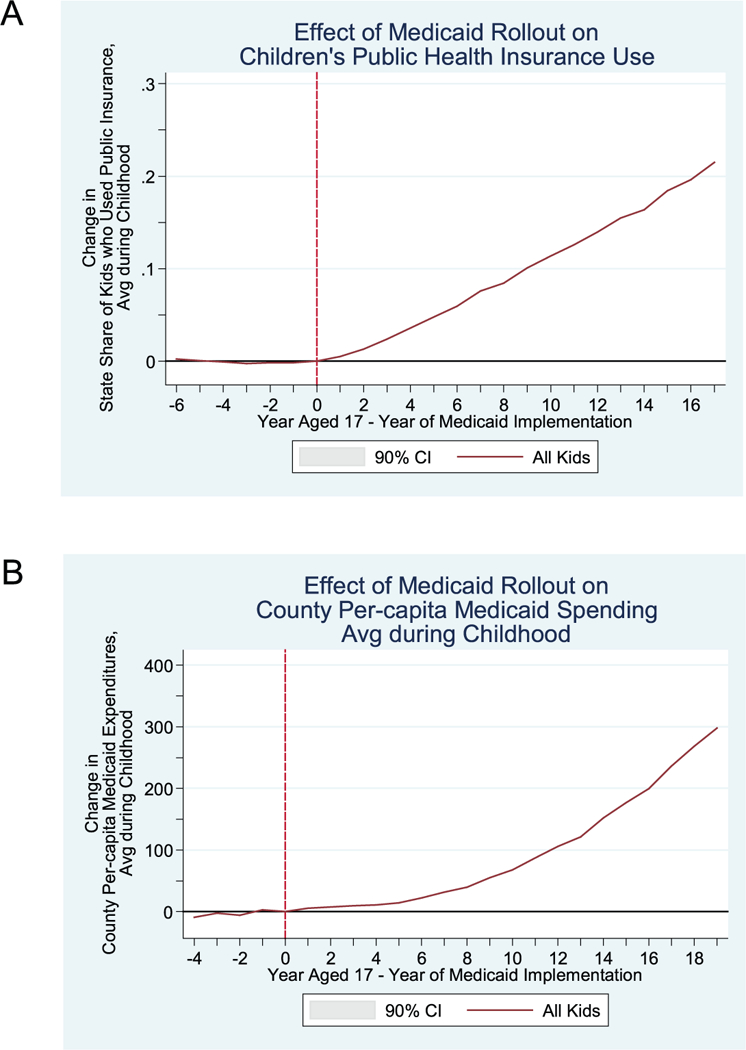
Effect of Medicaid Rollout on (A) Children’s Public Health Insurance use and (B) County Per-Capita Medicaid Spending Average during Childhood
To provide visual evidence of Medicaid spending effects, Figure 7 plots the estimated changes in years of educational attainment for cohorts before and after Medicaid implementation for children from high-Medicaid-eligibility states (i.e., with high predicted spending increases in dosec) and those from low-Medicaid- eligibility states (i.e., low predicted increases). Note that this is the identifying variation used in the 2SLS models.
FIGURE 7.
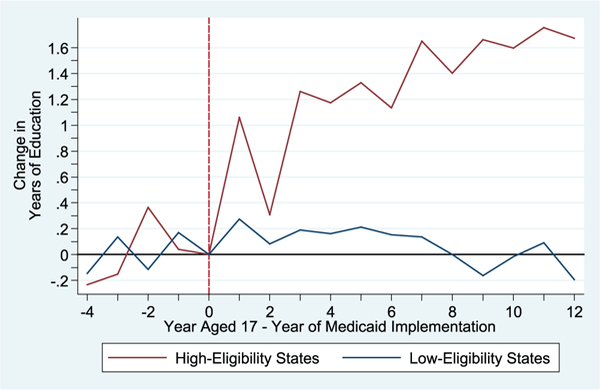
Effect of Medicaid Rollout on Educational Attainment
I find these increases in childhood public health insurance access led to significant reductions in the likelihood of low birth weight, increased educational attainment and the likelihood of graduating from high school, increased adult earnings, reduced the annual incidence of poverty in adulthood, and significantly educed adult mortality and the annual incidence of health problems (e.g., Figure 8). All these changes were much more pronounced among children from high-Medicaid-eligibility states—particularly poor, minority children—and, I see larger improvements in adult outcomes among cohorts for whom the increases in county Medicaid spending and health care access occurred at younger ages (i.e., relative to “unexposed” cohorts who reached age 18 before Medicaid implementation).
FIGURE 8.
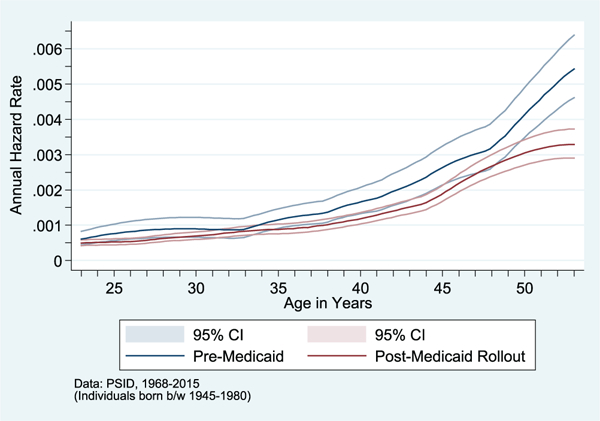
Mortality Hazard Pre- and Post-Medicaid Rollout
As shown in Table 4, for low-income children, I find a standard deviation increase in county per-capita Medicaid spending in utero is associated with a 5.9 percentage-point reduction in the likelihood of low birth weight. The results indicate that a standard deviation increase in average county per-capita Medicaid spending during childhood (ages 0–17) is associated with a 0.28 increase in completed years of education, 0.35 increase in adult family income-to-needs ratio, and a 2.5 percentage-point reduction in the annual incidence of problematic health in adulthood.
TABLE 4.
2SLS-IV Estimates of Effects of Childhood Medicaid Spending on Educational Attainment, and Adult economic and Health Status Outcomes of Children: using Timing of Medicaid Implementation and Pre-Medicaid State AFDC Rates as Instruments
| PSID children born b/w 1945-1975 | ||||
|---|---|---|---|---|
| Dependent variable: | ||||
| (1) | (2) | (3) | (4) | |
| Key Explanatory Variables: | Prob- (Low Birth Weight) |
Years of Education |
Annual Family Income-to- Needs Ratio, ages 20–50 |
Annual Incidence of Problematic Health, ages 20–50 |
| Instrumented County per-capita | −0.0589* | |||
| Medicaid Spending(age 0) | (0.0331) | |||
| Instrumented county per-capita | 0.2823** | 0.3452** | −0.0250* | |
| Medicaid Spending(age 0–17) | (0.1392) | (0.1621) | (0.0132) | |
NOTE: County per-capita Medicaid spending is expressed in standard deviation units to facilitate ease of interpretation of magnitudes of 2SLS regression coefficient estimates.
Conclusion
Summary
This study provides among the first evidence of the influence of childhood neighborhood conditions on the onset of hypertension through middle-age for a nationally representative sample of the U.S. population, and new causal evidence of the long-run impacts of childhood Medicaid spending. I focused on hypertension, as it is the leading modifiable risk factor of cardiovascular-related disease, an important contributor to the burden of disease, disability, and death in the united States, with a significant socioeconomic gradient. I find childhood neighborhood poverty and its attendant stressors play an influential role in shaping risks of onset of hypertension in middle-age. The results indicate that racial differences in these early life neighborhood conditions and family background characteristics play a significant role in explaining racial disparities in hypertension through at least age 50. The results from this study point to the potential for targeting neighborhood conditions as a means of improving population health and confronting health inequality.
Racial differences in mortality and morbidity in adulthood are large in the United States. I find that a few early life factors—neighborhood poverty, birth weight, child health insurance access, parental SES—can account for the lion’s share of the gaps in the prevalence of hypertension in adulthood. While contemporaneous socioeconomic factors have been the focus of the literature on racial disparities in health, the results suggest that adult health condition disparities may be better explained by early life factors. Taken together, these findings suggest that early life interventions have great promise as cost-efficient approaches to promote human capital development and the quantity of years lived without health problems.
Given the known lengthy latency periods before health effects like hypertension manifest, it was important to examine whether later-life racial health disparities are rooted in early life childhood circumstances. Racial differences in rates of hypertension are in part the result of a long-term cumulative process of socioeconomic environmental exposures over the life cycle. This article sought to identify causal influences of the life-cycle trajectory of inequality in this major cardiovascular disease risk factor. In contrast to cross-sectional evidence linking neighborhoods and health in a static framework, this article considered the life-course residential neighborhood quality histories and their implications for later-life health. This analysis examined whether differences across neighborhoods in the prevalence of hypertension, the leading risk factor of cardiovascular-related disease, do in fact reflect causal processes operating over the life course.
I estimated four-level hierarchical random effects hazard models of the onset of hypertension, which provide a better understanding of the relative importance of childhood family and neighborhood backgrounds. I used maximum “marginal” likelihood estimation procedures to allow for the multilevel components at the county, neighborhood, and family levels including measured and unmeasured components at each of these levels. Upon uncovering a significant overall scope of the influence of both childhood neighborhood conditions and family background on the onset of hypertension over the life course, I empirically tested the hypotheses that childhood exposure to one specific neighborhood factor and one family factor in particular—childhood neighborhood poverty and access to health insurance, respectively—influence the disease process, risk of hypertension later in life, and adult socioeconomic attainments.
The results indicated that neighborhood poverty during childhood significantly increases risks of hypertension through mid-life. I find that even a large amount of selection on unobservable factors does not completely eliminate the significant effect of child neighborhood poverty on hypertension later in life.
The results also revealed significant beneficial long-run impacts of Medicaid spending in childhood on educational attainment, income, and health status in adulthood. The research design exploited the staggered introduction of Medicaid across states (1966–1982) and cross-state variation in Medicaid eligibility (initial AFDC rates) to isolate causal effects of childhood health insurance access and long-run returns to Medicaid spending. A number of robustness checks were performed that support a causal interpretation to the findings on the effects of access to childhood health insurance. These public health investments led to a narrowing of racial health disparities in adulthood.
There is a need to unpack the specific exposures and pathways through which disadvantaged childhood conditions lead to poor health outcomes. The innovative research design and unique measures collected on aspects of childhood family SES, infant health, access to medical care, and neighborhood environments helped to illuminate what lies along the “chain of causation” from poverty to health outcomes over the life course. This clarity is imperative for policy prescriptions that address health inequities.
The value of the PSID
Longitudinal data that include information on initial health conditions and later-life health, adjoined with geocoded data of childhood residential location at the census block level are rare, which has limited efforts to estimate long-term effects of conditions in utero and during childhood. Few studies follow individuals from birth through middle age. The PSID, which is the longest running nationally representative household survey in the world, enables researchers to make contributions to the literature along these lines. The oversampling of low- income and black families enables issues of poverty and race to be examined.17 The PSID maintains wave-to-wave response rates of 95 to 98 percent, which is central to its ability to maintain its national representativeness over time. Specific reasons for attrition enabled us to examine health-related attrition that is central to analyze policy impacts on mortality, and analyses of disease onset, which also take into account the competing risk of mortality. The array of childhood measures of socioeconomic background can more fully account for the broad set of early life family and neighborhood socioeconomic factors that may lead to onset of disease in adulthood; and this augmented with significant retrospective health information collected on specific childhood health conditions.18,19 Future data efforts in collection of biomarkers may prove valuable to further aging research, and its early antecedents, in the field going forward.
This article focused on black-white differences primarily because data limitations in the PSID reduce the sample sizes of other racial or ethnic groups that have been followed from birth to adulthood (particularly among original sample children). Given the increasing racial/ethnic diversity of the U.S. population, it is imperative that future work consider the health of other groups, particularly Hispanics. The “Hispanic paradox, which refers to the finding that Hispanic Americans have relatively good health outcomes, especially lower mortality risk, is a main exception to the general finding that racial/ethnic minorities experience worse health outcomes than whites.20
My current ongoing work explores potential interactive effects between improved access to school quality and healthcare services on children’s subsequent life trajectories and adult SES and health outcomes. The findings may further inform how the long-term evolution of racial health disparities is shaped at different points in time by racial differences in access to health care and school quality.
Current U.S. health policy debates fixate on rising costs of health care and how to curb them, without as much attention on the long-term value and productivity of human capital investments, particularly when they are strategically timed (e.g., early childhood access to quality care and quality schools pre-K-12) that shape future health trajectories and augment productive capacities.
Contemporary policy should consider the long-term educational, health, and economic benefits of improved infant and child health resultant from Medicaid and hospital desegregation. While completely different eras and policies, there are ties between the impacts of hospital desegregation and impacts of access to childhood health insurance on child development that may be relevant for contemporary Affordable Care Act implementation and the present contentious debates surrounding its potential repeal and replacement.21
Future research must make further strides to uncover the relationships between childhood conditions and children’s short-and long-run health outcomes to inform policy on how best to improve overall population health and narrow racial and SES-related health disparities. As research identifies the causal chain that links measures of segregation, childhood neighborhood quality, childhood health insurance access, and adult health, policy can design more effective instruments to alleviate health and economic disparities and narrow racial inequality. The ability to more precisely identify the mechanisms may enable more effective policy interventions to be implemented to ameliorate the burden of disease and the economic burden to the health care system. The economic drain may be reduced by greater investment in early life interventions. This work can assist in shifting the goal from secondary symptom amelioration to primary disease prevention. My findings suggest that the seeds of vulnerability to chronic health conditions are planted early in life.
Acknowledgments
NOTE: I wish to thank the Panel Study of Income Dynamics (PSID) staff for access to the confidential restricted-use PSID geocode data. I am grateful to seminar and conference participants at National Institutes of Health (NIH), Stanford University, and the University of Michigan for helpful comments; and thank Andrew Goodman-Bacon for sharing data on the timing of Medicaid implementation and pre-Medicaid state health insurance and welfare eligibility rates. This research was supported by the NIH and the Carnegie Corporation.
Appendix.
More Details on Estimation Methods and Econometric Model
To decompose the total variation in hypertension risk into the fraction that lies among neighborhoods, families, and individuals, I estimate a four-level hierarchical random effects discrete-time hazard model to analyze the age onset of hypertension through mid-life. I also test a subset of models that includes county-, neighborhood-, and family-level random effects. The data are hierarchical because we have data on individuals who are nested within families, which are nested within neighborhoods and counties. Multilevel modeling techniques can accommodate the hierarchical and unbalanced structure of our data, nonindependence of the (sometimes overlapping) pairs of siblings and neighbors, as well as the nonnormality of hypertension onset (Raudenbush and Bryk 2002).
The hazard function, hnfst, is the probability that individual s from family f in neighborhood n becomes hypertensive in year t, given the individual has never had hypertension in any previous year. The hazard is specified in a logit form, where in the baseline model, the explanatory variables include only a gender dummy indicator, a quadratic specification of age, a set of birth cohort dummy indicators, and a neighborhood-level random effect ( ηn ) and family-level random effect ():
In this model, we are implicitly assuming proportional odds—in particular, we assume the baseline logit hazard curves in the J neighborhoods are parallel to one another, and the baseline logit hazard curves in the K families in these neighborhoods are parallel to one another.22
In this formulation the random effects, which play the role of additional error terms, are assumed to be normally distributed with mean 0, and Here is an individual error term associated with individual s from family f in neighborhood n and is assumed to have a standard logistic distribution with mean 0 and variance
In this model, individuals from the same neighborhood, but not in the same family (i.e., neighbors), are correlated because they share the random effect and siblings are correlated because they share the random effects and Because we did not want our baseline random effects components to reflect the influence of age and gender, we adjusted for them in our baseline model.
I then estimate adjusted neighbor correlations, which are net of the resemblance/similarity arising from childhood neighbors having similar family background characteristics (due to sorting influences). To extract the impact of similar family backgrounds out of the neighbor correlation, I first estimate the following regression; for ease of exposition, here I omit the random effects terms that are included in the estimated model:
where is a vector of childhood family background characteristics including: average annual family income-to-needs ratio (based on the five-year average as reported in 1967–1972), parental education, parental family structure, race, child health insurance coverage (as reported in 1967–1972), parental annual expenditures on cigarette and alcohol consumption (based on the five-year average in 1967–1972), indicator for birth weight, parental connectedness to informal sources of help, parental expectations for child achievement, and housing plumbing; and insulation problems. is a vector of the 1968 neighborhood-level means of the same above variables.
Inclusion of family-level and neighborhood-level variables measuring the same concepts enables the vector α2 of coefficient estimates to capture the within-neighborhood effects of these family background characteristics. Using the within-neighborhood estimates of the family background effects of parental income, education, race, family structure, child health insurance coverage, parental health behaviors, birth weight, parental expectations for child achievement, parental connectedness to informal sources of help and housing quality on health in adulthood will ensure the coefficients (α2) will not be biased by omitted neighborhood variables. This follows from the fact that the neighborhood-level unmeasured factors can only be correlated with the neighborhood-level mean of the covariates. In combination, the resulting estimates of the effects of these family background characteristics can be taken as a conservative estimate of in equation (5).
I then estimate the inter-neighbor variance in by estimating a hierarchical random effects model of a on neighborhood-level, family-level, and individual- level random effects. I then subtract the estimate of the inter-neighbor variance in a from the estimate of the overall inter-neighbor variance in Dividing the resulting quantity by yields a tighter upper bound on the proportion of that can be attributed to child neighborhood effects.
Parental income and neighborhood poverty are dimensions of family and neighborhood background that I give particular emphasis to in the regression analysis. Growing up in a neighborhood with concentrated poverty may have grave consequences above and beyond those of growing up in a poor family because of the absence of positive role models, social isolation, weakened social institutions, unrelenting stress, inferior health care accessibility, and other factors. I control for parental education, birth order, whether child was low birth weight, born into a two-parent family, and year of birth. I also make use of a unique set of measures of parental expectations of children’s educational attainment, county school expenditures per pupil, residential segregation, neighborhood crime (proxied by neighborhood incarceration rate), parental connectedness to informal sources of help, parental aspirations/motivation and long-term planning, parental personality, habits and skills that were collected in the early years of the PSID. These factors may themselves be the product of growing up in a high poverty neighborhood and may represent important pathways through which exposure to depressed neighborhood environments during childhood affect health trajectories later in life. However, controlling for this myriad of ways in which children who grow up in high poverty neighborhoods may differ from children who grow up in affluent neighborhood environments allows us to generate a more conservative estimate of the effect of neighborhood poverty itself, as well as shed light on the factors that affect hypertension risk.
Sensitivity analysis
I conduct a sensitivity analysis to test the robustness of the estimated effects of childhood neighborhood poverty to selection bias due to an omitted variable. The goal is to assess how the point estimate and confidence interval of the effect of neighborhood poverty change under the presence of selection bias of varying strengths. I use a novel empirical approach, recently proposed by Altonji, Elder, and Taber (2005) and Krauth (2006), to perform the sensitivity analysis. This analysis allows us to determine the threshold of selection on unobservables, if any, at which neighborhood poverty during childhood no longer has a significant effect on hypertension risk. The approach uses the statistical relationship between observed explanatory variables in a regression as a guide to generate plausible estimates about the relationship between observed and unobserved variables. The sensitivity parameter, θ, can be defined as
where θ indexes the magnitude of the correlation between observables and unobservables relative to the analogous correlation among observables themselves. In other words, the correlation between the neighborhood poverty rate and the (effect-weighted) unobservables is proportional to the correlation between the neighborhood poverty rate and the effect-weighted observables. The standard exogeneity assumption is the special case of θ=0. This approach provides a way to construct bounds on the effect of neighborhood poverty during childhood on adult health based on the bounds one is willing to place on the sensitivity parameter θ (i.e., the relative correlation).
Altonji, Elder, and Taber (2005) argue that if the observable determinants of an outcome are truly just a random subset of the complete determinants, selection on observable characteristics must be equal to selection on unobservable characteristics. Because the PSID was conducted specifically to study family background factors that affect well-being, we would expect selection on observable factors to be greater than selection on unobservable factors; in other words, the extensive measures of family and neighborhood background captured in the PSID are likely to be the most important determinants of adult health. Thus, estimates obtained under the assumption of equal selection will be biased downward.
Sensitivity to selection bias
The estimates of the significant effects of neighborhood poverty during childhood on adult health reported in Table 3 in this article are based on models in which exogeneity is assumed. I next evaluate the robustness of these results to deviations from exogeneity. Figure 6 in this article displays the range of estimated coefficients and confidence intervals on neighborhood poverty as a function of the ratio of selection on unobservables to selection on observables. As the figure shows, the effect of child neighborhood poverty on the risk of onset of hypertension in adulthood remains large and significant even with a reasonably large amount of selection on unobservable factors. The correlation between neighborhood poverty and unobserved outcome-relevant factors would need to exceed 80 percent of the correlation between neighborhood poverty and observed-relevant factors to eliminate the estimated effect. In other words, the ratio of selection on unobservables to selection on observables would have to exceed 80 percent for one to attribute the entire effect of neighborhood poverty to selection bias.
Footnotes
Rucker Johnson is an economist and an associate professor at UC-Berkeley’s Goldman School of Public Policy. He was the recipient of the Andrew Carnegie Fellowship for his scholarship on the effects of desegregation, school finance reform, and Head Start. His research agenda broadly considers how poverty and inequality affect life chances.
Johnson: University of California, Berkeley and NBER. I wish to thank the PSID staff for access to the confidential restricted-use PSID geocode data.
In particular, my previous work shows that among cohorts born between 1951 and 1970, the average black child spent about one-quarter of their childhood years in high poverty neighborhoods (i.e., neighborhood poverty rates in excess of 30 percent), and about one-third of their early-to-mid adulthood years (ages 30–50) in high poverty neighborhoods, while only roughly 15 percent of these adulthood years were lived in low-poverty neighborhoods (i.e., less than 10 percent of households in poverty). In contrast, the comparable estimates for the average white child is only 3 percent of childhood and adulthood years spent in high poverty neighborhoods, while spending 80 percent of childhood years in low-poverty neighborhoods and more than half of early-to-mid adulthood years in low-poverty neighborhoods. Furthermore, black-white differences in adulthood exposure to neighborhood poverty are largely accounted for by differences in the likelihood of being born into a poor neighborhood, and to a lesser extent by differences in rates of upward and downward socioeconomic mobility over the life course.
The results also reveal substantial persistence in health status across generations that are linked in part to low intergenerational economic mobility.
For example, 45 percent of black students attend schools in which more than three-fourths of students are poor, while only 8 percent of white students attend such schools. Likewise, 30 percent of white students attend low-poverty schools (<25 percent poor), while only 8 percent of black students attend such schools.
However, neither of two randomized treatment groups that received housing vouchers to move between 1994 and 1997 experienced lower prevalence of hypertension at follow-up in 2002 compared with the control group that received no vouchers (Kling, Liebman, and Katz 2007). It is important to bear in mind that the treatment effects represent not only changes in neighborhoods, but also the process of moving, which could mask any beneficial effects of leaving a stressful neighborhood.
This approach was first used by Solon, Page, and Duncan (2000) to study determinants of educational attainment, and then I subsequently adapted the approach to examine the role of contextual factors on general health status (Johnson 2011).
The 1968 addresses were geocoded to census block identifiers using GDT geographic mapping technologies. Census blocks are the smallest level of geographic precision reported by the Census Bureau and represent a narrow definition of neighborhood. Census block identifiers are defined for the entire United States in 2000.
To be eligible for the SEO sample, households had to have income that was below two times the poverty line, which in theory could be problematic for our purposes because two neighboring families could enter that component of the PSID only if they had sufficiently low income. However, due to the significant degree of residential segregation by income, I find evidence that the typical neighbor of a low-income family was also low income; thus, in practice this does not present any significant within-neighbor-hood sample selection bias problems. In particular, in the 1968 SRC component of the PSID, the average family with income less than two times the poverty line (in that year) lived in neighborhoods in which neighbors’ average income was also among the bottom third of the income distribution. Similarly, using larger national samples geocoded to the census block, Hardman and Ioannides (2004) find that among the poorest 30 percent of households, roughly 75 percent live in neighborhoods in which neighbors’ median income is also among the poorest 30 percent of households.
Results available upon request.
Likelihood ratio tests are used to test the statistical significance of the family and neighborhood random effects.
These estimates are based in part on within-neighborhood estimates of effects of child family background on hypertension onset.
To accomplish this, I estimate a random effects formulation that is equivalent to a neighborhood fixed effect model by centering all family- and individual-level factors by their neighborhood means. This neighborhood-specific centering generates within-neighborhood estimates of these family- and individual- level factors.
These measures include self-reports of whether it is a poor neighborhood for children, whether plumbing problems exist, housing structural problems, security problems, cockroach or rat problems, insulation problems, neighborhood cleanliness problems, overcrowding, noise, traffic problems, burglary, robbery, assault, drug use, or problems related to too few police in the neighborhood in which they live.
The coefficient estimates on the childhood neighborhood and family background factors are reduced to some extent with the inclusion of the adult SES measures, but the childhood factors remain large and significant.
The appendix provides further details on the estimation procedures.
I match respondents to their earliest available residential address in childhood (in most cases, 1968), so their childhood place of upbringing is measured prereform (or near or before the timing of Medicaid implementation), in most cases, to minimize potential bias from endogenous residential mobility.
I also estimated models separately for those from low-income families (who are disproportionately black), since nonpoor children are not eligible for Medicaid and therefore would not be expected to be significantly affected.
Sample weights account for oversampling and make the weighted sample nationally representative, and these weights are used in the analyses.
To determine childhood general health status as retrospectively reported in adulthood on the basis of self-rated general health status, participants were asked the following: “Consider your health while you were growing up, before you were 17 years old. Would you say that your health during that time was excellent, very good, good, fair, or poor?” The accuracy of retrospective reports of childhood health have been examined and found to be reliable (e.g., Smith 2009).
Disease onset was based on self-reported measures, and a patient’s knowledge of disease presence could have been affected by differential access to and interaction with the health care system. People in poor families have lower levels of health care utilization than does the general population and are less likely to have a disease diagnosed. The disparities in self-reported disease prevalence observed in our study are therefore probably smaller than disparities in true disease prevalence, making our estimates of these disparities conservative.
For a recent review, see Markides and Eschbach (2011).
ACAs broad Medicaid expansion to poor families was effectively turned into a state opt-in, and seven of the ten states with the highest black populations chose not to expand Medicaid. Consequently, more than half of the people without affordable health care coverage are minorities; 30 percent of them are black.
Maximum-likelihood estimates based on a numerical integration procedure were computed using the gllamm6 macro in Stata (Rabe-Hesketh 2001).
References
- Almond Douglas, Chay Kenneth, and Greenstone Michael. 2006. Civil rights, the war on poverty, and black-white convergence in infant mortality in the rural South and Mississippi. MIT Department of Economics Working Paper 07–04. [Google Scholar]
- Altonji Joseph, Todd Elder, and Christopher Taber. 2005. Selection on observed and unobserved variables: Assessing the effectiveness of Catholic schools. Journal of Political Economy 113 (1): 151–84. [Google Scholar]
- Barghaus Katherine, Cutler David M., Fryer Roland G., and Glaeser Edward L.. 2008. An empirical examination of racial differences in health. NBER Working Paper. Available from http://isites.harvard.edu/fs/docs/icb.topic709943.files/salt%20final.pdf.
- Barker David JP 1998. Mothers, babies and health in later life Edinburgh: Churchill Livingstone. [Google Scholar]
- Bhattacharya D, and Mazumder B. 2011. A nonparametric analysis of black-white differences in intergenerational income mobility in the United States. Quantitative Economics 2 (3): 335–79. [Google Scholar]
- Braveman Paula A., Cubbin Catherine, Egerter Susan, Williams David, and Pamuk Elsie. 2010. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health 100 (S1): S186–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card David, and Shore-Sheppard Lara D.. 2004. Using discontinuous eligibility rules to identify the effects of the federal Medicaid expansions on low-income children. The Review of Economics and Statistics 86:752–66. [Google Scholar]
- Chay Kenneth Y., Guryan Jonathan, and Mazumder Bhashkar. 2009. Birth cohort and the black- white achievement gap: The roles of access and health soon after birth NBER Working Paper Series, no. 15078, Cambridge, MA. [Google Scholar]
- Raj Chetty, Stepner Michael, Abraham Sarah, Lin Shelby, Scuderi Benjamin, Turner Nicholas, Bergeron Augustin, and Cutler David. 2016. The association between income and life expectancy in the United States, 2001– 2014. JAMA 315 (16): 1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Flavio, and Heckman James. 2007. The technology of skill formation NBER Working Paper No. 12840, Cambridge, MA. [Google Scholar]
- Cohodes Sarah, Kleiner Samuel, Lovenheim Michael F., and Grossman Daniel. 2016. The effect of child health insurance access on schooling: Evidence from public insurance expansions. Journal of Human Resources 51 (3): 727–59. [Google Scholar]
- Council of Economic Advisers. 2016. Economic report of the President. Washington, DC: Council of Economic Advisers. [Google Scholar]
- Currie Janet, and Gruber Jonathan. 1996a. Health insurance eligibility, utilization of medical care, and child health. The Quarterly Journal of Economics 111:431–66. [Google Scholar]
- Currie Janet, and Gruber Jonathan. 1996b. Saving babies: The efficacy and cost of recent changes in the Medicaid eligibility of pregnant women. Journal of Political Economy 104 (6): 1263–1296. [Google Scholar]
- Desantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, and Craske MG. 2007. Racial/ ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health 41 (1): 3–13. [DOI] [PubMed] [Google Scholar]
- Fang Jing, Madhavan Shantha, and Alderman Michael. 1996. The association between birthplace and mortality from cardiovascular causes among black and white residents of New York City. New England Journal of Medicine 335:1545–1551. [DOI] [PubMed] [Google Scholar]
- Finkelstein Amy, Taubman Sarah, Wright Bill, Bernstein Mira, Gruber Jonathan, Newhouse Joseph P., Allen Heidi, et al. 2012. The Oregon Health Insurance Experiment: Evidence from the first year. The Quarterly Journal of Economics 127:1057–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald John, Gottschalk Peter, and Moffitt Robert. 1998. The impact of attrition in the Panel Study of Income Dynamics on intergenerational analysis. Journal of Human Resources 33 (2): 300–44. [Google Scholar]
- Geronimus Arline T. 1996. Black/white differences in the relationship of maternal age to birthweight: A population-based test of the weathering hypothesis. Social Science Medicine 42 (4): 589–97. [DOI] [PubMed] [Google Scholar]
- Geronimus Arline T., Bound John, Waidmann Timothy A., Colen Cynthia G., and Steffick Dianne. 2001. Inequality in life expectancy, functional status, and active life expectancy across selected black and white populations in the United States. Demography 38 (2): 227–51. [DOI] [PubMed] [Google Scholar]
- Goodman-Bacon Andrew. 2018. Public insurance and mortality: Evidence from Medicaid implementation. The Journal of Political Economy 126:216–62. [Google Scholar]
- Halfon Neal, and Hochstein Miles. 2002. Life course health development: an integrated framework for developing health, policy, research. The Milbank Quarterly 80 (3): 433–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman Anna, and Ioannides Yannis. 2004. Neighbors’ income distribution: Economic segregation and mixing in US urban neighborhoods. Journal of Housing Economics 13 (4): 368–82. [Google Scholar]
- Heckman James. 2007. The economics, technology, and neuroscience of human capability formation. Proceedings of the National Academy of Sciences 104 (3333): 13250–13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House James S., Lepkowski James M., Kinney Ann M., Mero Richard P., Kessler Ronald C., and Regula Herzog A. 1994. The social stratification of aging and health. Journal of Health and Social Behavior 35:213–34. [PubMed] [Google Scholar]
- Jäntti Markus, Bratsberg Bernt, Knut Røed Oddbjørn Raaum, Naylor Robin, Österbacka Eva, Bjorklund Anders, Eriksson Tor. 2006. American exceptionalism in a new light: A comparison of intergenerational earnings mobility in the Nordic countries, the UK and the US IZA Discussion Papers, no. 1938, Bonn, Germany. [Google Scholar]
- Johnson Rucker C. 2011. Health dynamics and the evolution of health inequality over the life course: The importance of neighborhood and family background. B.E. Journal of Economic Analysis & Policy: Advances 11 (3) Article 6. [Google Scholar]
- Johnson Rucker C. 2016. Can schools level the intergenerational playing field? Lessons from equal educational opportunity policies. Washington, DC: Federal Reserve Board. [Google Scholar]
- Johnson Rucker C. Forthcoming. Children of the dream: Why school integration works. New York, NY: Basic Books and Russell Sage Foundation Press. [Google Scholar]
- Johnson Rucker C., and Kirabo Jackson C. 2017. Reducing inequality through dynamic complementarity: Evidence from Head Start and public school spending NBER Working Paper Series #23489, Cambridge, MA. [Google Scholar]
- Johnson Rucker C., and Schoeni Robert. 2011. The influence of early-life events on human capital, health status, and labor market outcomes over the life course. B.E. Journal of Economic Analysis & Policy: Advances 3 (3): pii: 2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz Lawrence, Kling Jeffrey, and Liebman Jeffrey. 2001. Moving to opportunity in Boston: Early results of a randomized mobility experiment. Quarterly Journal of Economics 116 (2): 607–54. [Google Scholar]
- Kling Jeffrey, Liebman Jeffrey, and Katz Lawrence. 2007. Experimental analysis of neighborhood effects. Econometrica 75 (1): 83–119. [Google Scholar]
- Krauth Brian. 2006. Simulation-based estimation of peer effects. Journal of Econometrics 133 (1): 243–71. [Google Scholar]
- Krieger Nancy, Jarvis T Chen Brent Coull, Waterman Pamela D., and Beckfield Jason. 2013The unique impact of abolition of Jim Crow laws on reducing inequities in infant death rates and implications for choice of comparison groups in analyzing societal determinants of health. American Journal of Public Hedth 103 (12): 2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh Diana J., and Wadsworth Michael E.. 1993. Physical health status at 36 years in a British national birth cohort. Social Science and Medicine 37 (7): 905–16. [DOI] [PubMed] [Google Scholar]
- Leventhal Tama, and Brooks-Gunn Jeanne. 2003. New York City site findings: the early impacts of Moving to Opportunity on children and youth In Choosing a better life? Evaluating the Moving to Opportunity Social Experiment, eds. Goering John and Feins Judith D., 213–45. Washington, DC: Urban Institute Press. [Google Scholar]
- Levine Philip B, and Schanzenbach Diane. 2009. The impact of children’s public health insurance expansions on educational outcomes. Forum for Health Economics & Policy 12 (1): 1–26 [Google Scholar]
- Lynch John, and Smith George Davey. 2005. A Life Course Approach to Chronic Disease Epidemiology. Annual Review of Public Health 26:1–35. [DOI] [PubMed] [Google Scholar]
- Markides Kyriakos S., and Eschbach Karl. 2011. Hispanic paradox in adult mortality in the United States In International handbook of adult mortality, eds. Rogers Richard G. and Crimmins Eileen M., 227–40. The Netherlands: Springer. [Google Scholar]
- McEwen Bruce S. 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences 840:33–44. [DOI] [PubMed] [Google Scholar]
- Meara Ellen, Richards Seth, and Cutler David. 2008. The gap gets bigger: Changes in mortality and life expectancy by education, 1981–2000. Health Affairs (Project Hope) 27 (2): 350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh Sophia. 2001. GLLAMM Manual Technical Report 2001/01. Unpublished manuscript. Department of Biostatistics and Computing, Institute of Psychiatry, University of London. [Google Scholar]
- Raudenbush Stephen, and Bryk Anthony S.. 2002. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Smith James P. 2009. Reconstructing childhood health histories. Demography 46 (2): 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon Gary, Page Marianne, and Duncan Greg. 2000. Correlations between neighboring children in their subsequent educational attainment. The Pieview of Economics and Statistics 82 (3): 383–92. [Google Scholar]
- Sommers Benjamin D., Baicker Katherine, and Epstein Arnold M.. 2012, Mortality and access to care among adults after state Medicaid expansions. New England Journal of Medicine 367:1025–1034. [DOI] [PubMed] [Google Scholar]
- Tiebout Charles M. 1956. A pure theory of local expenditures. Journal of Political Economy 64:416–24. [Google Scholar]
- Wherry Laura R., and Meyer Bruce D.. 2015. Saving teens: Using a policy discontinuity to estimate the effects of Medicaid eligibility. Journal of Human Resources 51 (3): 556–88. [Google Scholar]
- Williams David. 2005. Social sources of racial disparities in health. Health Affairs 24 (2): 325–34. [DOI] [PubMed] [Google Scholar]
- Williams David, and Collins Chiquita. 1995. U.S. socioeconomic and racial differences in health: Patterns and explanations. Annual Review of Sociology 21:349–86. [Google Scholar]


