Abstract
Dynamic nuclear polarization (DNP) is a technique to polarize the nuclear spin population. As a result of the hyperpolarization, NMR sensitivity of the nuclei in molecules can be enhanced dramatically. Recent application of the hyperpolarization technique has led to advances in biochemical and molecular studies. A major problem is the short lifetime of the polarized nuclear spin state. Generally, in solution, the polarized nuclear spin state decays to a thermal spin equilibrium, resulting in loss of the enhanced NMR signal. This decay is correlated directly with the spin-lattice relaxation time T1. Here we report [13C,D14]tert-butylbenzene as a new scaffold structure for designing hyperpolarized 13C probes. Thanks to the minimized spin-lattice relaxation (T1) pathways, its water-soluble derivative showed a remarkably long 13C T1 value and long retention of the hyperpolarized spin state.
Keywords: dynamic nuclear polarization, hyperpolarization, nuclear magnetic resonance, spin-lattice relaxation time, chemical probe
Graphical Abstract
Here we report [13C,D14]tert-butylbenzene as a new scaffold structure for designing hyperpolarized 13C probes. Thanks to the minimized spin-lattice relaxation (T1) pathways, its water-soluble derivative showed a remarkably long 13C T1 value and long retention of the hyperpolarized spin state.
Dynamic nuclear polarization (DNP) is a technique to polarize the nuclear spin population.[1–3] As a result of the hyperpolarization, NMR sensitivity of the nuclei in molecules can be enhanced dramatically. Recent application of the hyperpolarization technique has led to advances in biochemical and molecular studies.[4] Hyperpolarized (HP) molecules have enabled highly sensitive and real-time monitoring of metabolic fluxes[4,5] and chemical status[6] in vivo. DNP has not only been applied to these biomedical studies, but also to molecular interaction analysis and chemical reaction monitoring.[7] Such extensive research has demonstrated the high potential of HP-NMR techniques.
A major problem is the short lifetime of the polarized nuclear spin state. Generally, in solution, the polarized nuclear spin state decays to a thermal spin equilibrium, resulting in loss of the enhanced NMR signal. This decay is correlated directly with the spin-lattice relaxation time T1. In solution, the T1 value of 13C in small molecules is typically from a few to tens of seconds.4 This short lifetime has hampered the flexible design of HP chemical probes.
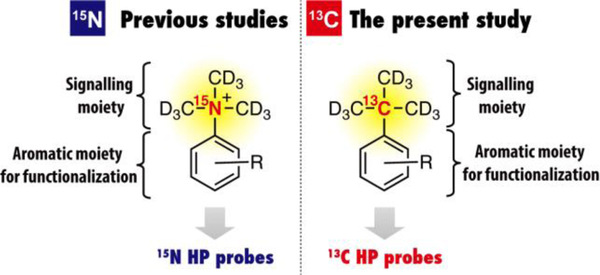
To overcome the decay problem and to generate a series of HP probes systematically, we have proposed a scaffold strategy.[8–11] A representative scaffold structure is [15N,D]trimethylphenylammonium (TMPA), which comprises a long-lived HP signalling moiety (–15N(CD3)3) and an aromatic ring for facile chemical modifications (Figure 1). We have successfully demonstrated that a series of HP probes could be developed from the [15N,D]TMPA by introducing a molecular sensing unit R.[9,10] However, in principle, the 15N NMR sensitivity is lower than that of 13C nuclei because of its smaller gyromagnetic ratio. Therefore, a 13C HP scaffold structure with a long T1 value has been highly sought after.[11]
Figure 1.
Schematic illustration of 15N and 13C scaffold structures reported in previous and this study, respectively.
Here, we report a 13C scaffold structure, [13C,D14]tert-butylbenzene, that can be used to design long-lived HP 13C probes.
There are two approaches to achieving a long-lived HP spin state. One is to design a molecule with long T1, because the HP spin state is directly correlated with T1.[4] The other is to use a nuclear singlet state with a magnetically equivalent spin −1/2 pair.[12] Both approaches are attractive and have advanced long-lived hyperpolarization.[8–10,13–18] In the present study, we adopt the more straightforward former approach.
T1 relaxation is affected by several factors, such as dipole–dipole (DD) interactions, chemical shift anisotropy (CSA), spin–rotation, scalar coupling.[19,20] For 13C small organic molecules, especially in high magnetic field, DD and CSA relaxations are dominant factors.[4,6]
To minimize the CSA relaxation, sp3 carbon with symmetrical environment is preferable. The tert-butyl carbon has an sp3 hybridization. This carbon centre is also attractive for HP applications because it experiences only a small DD-induced T1 relaxation because of the absence of neighbouring 1H. Indeed, tert-butanol and 1,1-cyclopropane dimethanol have been shown to have long T1 values and have been proposed as potential HPMRI probes.[21,22]
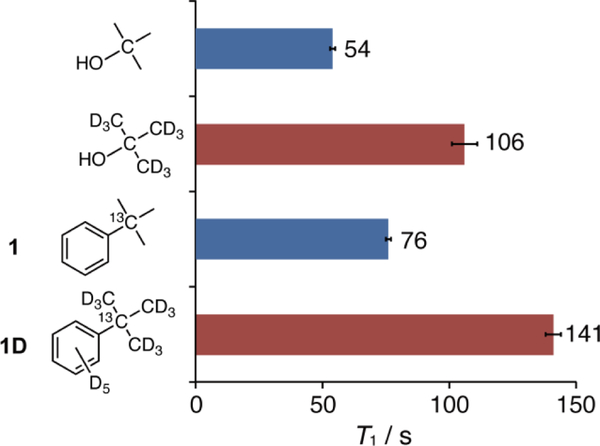
We then designed [13C]tert-butylbenzene (1) as a potential 13C scaffold structure. Molecule 1 is composed of a 13C tert-butyl group attached to an aromatic ring for feasible chemical modification to incorporate functional groups such as sensing moieties. 1 was synthesized from [13C]benzoic acid in 2 steps. The 13C T1 value of 1 was 76 ± 1 s (CD3OD, 9.4 T, 25 °C). The 13C T1 of [13C]benzoic acid, which was another candidate for a 13C scaffold utilizing a 13C carboxyl group as a HP unit, was 33.9 ± 0.3 s (CD3OD, 9.4 T, 25 °C), suggesting an advantage in utilizing sp3 carbon as a long-lived HP unit.
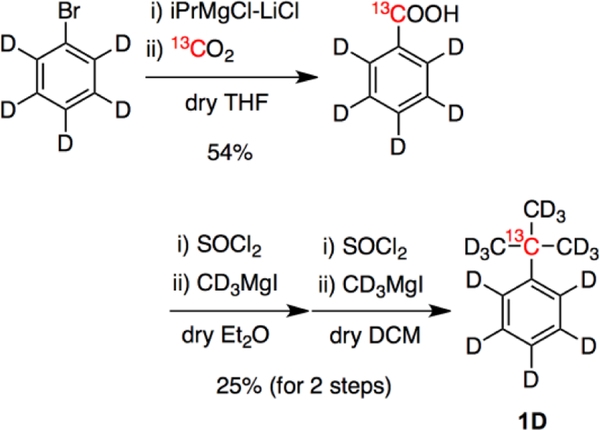
To extend the 13C T1 of 1 further, we then prepared [13C,D14]tert-butylbenzene (1D, Figure 2), wherein all 1H were replaced with 2H (D) to minimize 13C-1H DD relaxation. This 1D was synthesized from [D5]bromobenzene in 3 steps (Scheme 1). The 13C T1 of 1D reached over 100 s (141 ± 3 s, CD3OD, 9.4 T, 25 °C), which was 1.3 times longer than that of deuterated tert-butanol (106 s).
Figure 2.
T1 of tert-butanol and tert-butylbenzene (1 and 1D). 13C T1 were determined by using inversion recovery method (9.4 T, 25 °C, CD3OD, tert-butanol = 1.0 M, [D9]tert-butanol = 1.5 M, 1 = 0.05 M, 1D = 0.05 M)
Scheme 1.
Synthesis of [13C,D14]tert-butylbenzene (1D).
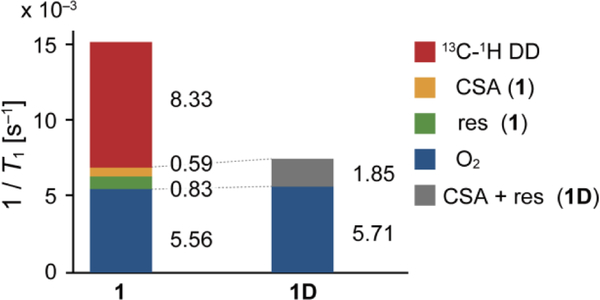
T1 relaxation of 1D was investigated further by decomposing it to each relaxation mechanism: 13C–1H DD (T1 CH-DD), CSA (T1 CSA), dissolved oxygen (T1 O2), and residual factors (T1 res). Total T1 can be described using these decomposed T1 values as equation 1. These T1 contributions were determined according to previous reports (experimental details, see: supporting information).[19,20,23–28]
| (eq 1) |
The estimated T1 contributions in 1 and 1D are summarized in Figure 3. As shown in equation 1, a larger 1/T1 X indicates the larger contribution of the relaxation mechanism X to shorten the apparent T1. These data include three important implications. First, 13C–1H DD relaxation of 1D is almost completely suppressed by full deuteration, while 1 suffers from non-negligible 13C–1H DD relaxation (red bar). Second, the sp3 carbon of 1 and 1D experience low CSA relaxation, as designed. Third, as a result of sufficient minimization of 13C–1H DD and CSA relaxation mechanisms, the relaxation from dissolved oxygen (T1 O2) becomes the major contributor for T1 relaxation (blue bar). In the case of 1D, T1 O2 governs the majority of the apparent T1 relaxation, resulting in the considerably longer T1 value of 1D in degassed condition (541 ± 18 s, toluene-d8, 9.4 T, 25 °C). These data support the validity of our design and the potential of 1D as a long-lived HP scaffold.
Figure 3.
Stacked bar graph of 1/T1 X of 1 and 1D (50 mM, toluene-d8, 25 °C, 9.4 T). 1/T1 X values were determined experimentally (see, supporting information).
Furthermore, we tried to achieve a T1 value longer than 1D based on the understanding of T1 contributions of 1D shown in Figure 3. Considering that the dissolved oxygen is a dominant relaxation factor of 1D, it was considered that a longer T1 could be achieved by changing the solvent to water in which the oxygen concentration is lower than that in organic solvents.[29] We then attempted to add the water solubility into 1D.
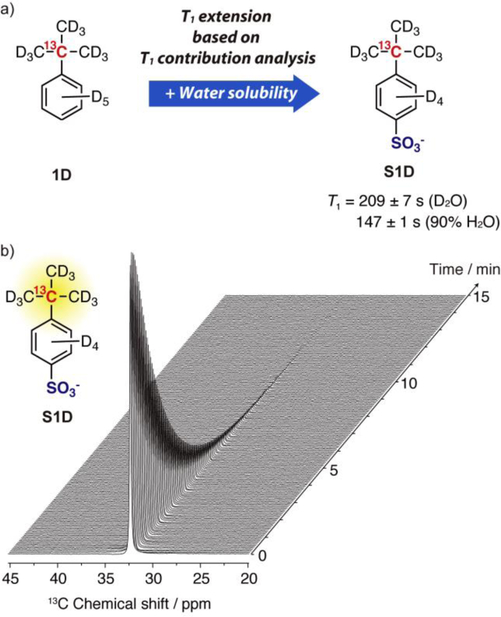
In order to increase the water solubility of 1D, sulfonated 1D (S1D, Figure 4a) was designed. Thanks to the rich chemical functionality of conjugated aromatic systems, a variety of substitutions can be introduced to the benzene ring of 1D. In this case, 1D was diversified by aromatic sulfonation with fuming sulphuric acid to produce S1D (Scheme S1) as an HP 13C probe with excellent water solubility (> 0.4 mol/L, r.t.).
Figure 4.
(a) Molecular design of S1D. T1s in D2O and 90% H2O were determined by inversion recovery method at 50 mM S1D, 37 °C, 9.4 T. (b) 13C NMR spectra of the hyperpolarized probe S1D (2 mM in H2O), stacked from 0 to 15 min (every 2 s, pulse angle 5°).
The probe S1D showed a remarkably long T1 in water. The 13C T1 exceeded 200 s in D2O (209 ± 7 s, 9.4 T, 37 °C), which was much longer than that of the original (1D) in organic solvent despite the increased molecular weight. It was shortened in 90% H2O, but still maintained a long T1 (147 ± 1 s, 9.4 T, 37 °C). These values were longer than those for [D9]tert-butanol (140 ± 7 in D2O, 77± 1 s in H2O, 9.4 T, 37 °C).
Finally, S1D was applied for a DNP-NMR study in water. S1D was hyperpolarized efficiently by dissolution DNP. The liquid state 13C NMR signal of the probe dissolved in water was enhanced by approximately 29,000-fold (%P13C = 23.5%, T = 298 K, B0 = 9.4 T) compared with the thermally polarized signal, allowing detection of 13C NMR signal by 1 scan. Figure 4b shows the time course stack of 13C NMR spectra of HP S1D. Because of the long T1 value, S1D could retain a hyperpolarized spin state for a relatively long time, allowing observation of the HP 13C NMR signal for over 10 min under our experimental conditions. These results strongly indicate that this approach toward T1 extension based on an analysis of the T1 contribution is a useful approach to the design of HP probes.
In summary, we developed a new HP 13C scaffold [13C,D14]tert-butylbenzene (1D). The significance of this scaffold can be summarized as follows. First, it satisfies the requirement of a long T1. The 1D scaffold was designed by minimizing each relaxation mechanism to achieve longer T1 value. Notably, S1D, developed from 1D, afforded a remarkably long 13C T1 (209 s in D2O and 147 s in 90%H2O, non-degassed, 9.4 T, 37 °C). To our knowledge, this is the longest among the water-soluble 13C small organic molecules used for HP-NMR research. Second, it satisfies a facile functionalization of HP probes by feasible chemical modification. As we reported in the present and the previous papers, thanks to the rich organic chemistry of aromatic compounds, a variety of HP probes may potentially be developed using this 13C HP scaffold. Development of water soluble S1D from 1D scaffold is a good example of this versatility. Third is higher sensitivity. The present 13C scaffold is more sensitive than the previous 15N scaffold. Although further optimization may be necessary for practical applications, these advantages indicate the high potential of 1D as a new scaffold structure for designing a series of HP 13C MR probes.
Supplementary Material
Acknowledgements
The authors thank Ms. Kaori Inoue of Kyushu University for her help in DNP-NMR measurements. This work was supported by CREST (JPMJCR13L4), Japan Science and Technology Agency (JST). The use of HyperSense was in part supported by the funding program ‘Creation of Innovation Centers for Advanced Interdisciplinary Research Areas’ from JST. We also thank the US National Institutes of Health (NIH 2P41-EB015908) and Department of Defense (W81XWH-12-1-0134) for financial support.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Conflicts of interest
There are no conflicts to declare.
References
- [1].Lee JH, Okuno Y, Cavagnero S, J. Magn. Reson 2014, 241, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nikolaou P, Goodson BM, Chekmenev EY, Chem. Eur. J 2015, 21, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ardenkjær Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K, Proc. Natl. Acad. Sci. U.S.A 2003, 100, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Keshari KR, Wilson DM, Chem. Soc. Rev 2014, 43, 1627–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, Deberardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR, Neoplasia 2011, 13, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meier S, Jensen PR, Karlsson M, Lerche MH, Sensors 2014, 14, 1576–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Keshari KR, Kurhanewicz J, Macdonald JM, Wilson DM, Analyst 2012, 137, 3427–3429.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee Y, Heo GS, Zeng H, Wooley KL, Hilty C, J. Am. Chem. Soc 2013, 135, 4636–4639. [DOI] [PubMed] [Google Scholar]
- [8].Doura T, Hata R, Nonaka H, Ichikawa K, Sando S, Angew. Chem. Int. Ed 2012, 51, 10114–10117. [DOI] [PubMed] [Google Scholar]
- [9].Nonaka H, Hata R, Doura T, Nishihara T, Kumagai K, Akakabe M, Tsuda M, Ichikawa K, Sando S, Nat. Commun 2013, 4, 2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nonaka H, Hirano M, Imakura Y, Takakusagi Y, Ichikawa K, Sando S, Sci. Rep 2017, 7, 40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nishihara T, Kameyama Y, Nonaka H, Takakusagi Y, Hyodo F, Ichikawa K, Sando S, Chem. Asian. J 2017, 12, 949–953. [DOI] [PubMed] [Google Scholar]
- [12].Pileio G, Hill-Cousins JT, Mitchell S, Kuprov I, Brown LJ, Brown RCD, Levitt MH, J. Am. Chem. Soc 2012, 134, 17494–17497. [DOI] [PubMed] [Google Scholar]
- [13].Allouche-Arnon H, Lerche MH, Karlsson M, Lenkinski RE, Katz-Brull R, Contrast Media Mol. Imaging 2011, 6, 499–506. [DOI] [PubMed] [Google Scholar]
- [14].Vuichoud B, Milani J, Bornet A, Melzi R, Jannin S, Bodenhausen G, J. Phys. Chem. B 2014, 118, 1411–1415. [DOI] [PubMed] [Google Scholar]
- [15].Rodrigues TB, Serrao EM, Kennedy BWC, Hu D, Kettunen MI, Brindle KM, Nat. Med 2014, 20, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tayler MCD, Marco-Rius I, Kettunen MI, Brindle KM, Levitt MH, Pileio G, J. Am. Chem. Soc 2012, 134, 7668–7671. [DOI] [PubMed] [Google Scholar]
- [17].Stevanato G, Hill-Cousins JT, Håkansson P, Roy SS, Brown LJ, Brown RCD, Pileio G, Levitt MH, Angew. Chem. Int. Ed 2015, 54, 3740–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Theis T, Ortiz GX Jr., Logan AWJ, Claytor KE, Feng Y, Huhn WP, Blum V, Malcolmson SJ, Chekmenev EY, Wang Q, Warren WS, Sci. Adv 2016, 2, e1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levy GC, Acc. Chem. Res 1973, 6, 161–169. [Google Scholar]
- [20].Breitmaier E, Spohn KH, Berger S, Angew. Chem. Int. Ed 1975, 14, 144–159. [Google Scholar]
- [21].Cheng T, Mishkovsky M, Junk MJN, Münnemann K, Comment A, Macromol. Rapid Commun 2016, 37, 1074–1078. [DOI] [PubMed] [Google Scholar]
- [22].von Morze C, Larson PE, Hu S, Yoshihara HA, Bok RA, Goga A, Ardenkjaer-Larsen JH, Vigneron DB, Magn. Reson. Imaging 2014, 72, 1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kuhlmann KF, Grant DM, J. Am. Chem. Soc 1968, 90, 7355–7357. [Google Scholar]
- [24].Levy GC, Cargioli JD, Anet FAL, J. Am. Chem. Soc 1973, 95, 1527–1535. [Google Scholar]
- [25].Giannini DD, Armitage IM, Pearson H, Grant DM, Roberts JD, J. Am. Chem. Soc 1975, 97, 3416–3419. [Google Scholar]
- [26].Gust D, Pearson H, Armitage IM, Roberts JD, J. Am. Chem. Soc 1976, 98, 2723–2726. [Google Scholar]
- [27].Alger TD, Hamill WD Jr., Pugmire RJ, Grant DM, Silcox GD, Solum M, J. Phys. Chem 1980, 84, 632–636. [Google Scholar]
- [28].Wong TC, Ang TT, Guziec FS, Moustakis CA, J. Magn. Reson 1984, 57, 463–470. [Google Scholar]
- [29].a) Quaranta M, Murkovic M, Klimant I, Analyst 2013, 138, 6243–6245; [DOI] [PubMed] [Google Scholar]; b) Sato T, Hamada Y, Sumikawa M, Araki S, Yamamoto H, Ind. Eng. Chem. Res 2014, 53, 19331–19337. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.