Abstract
Cullin-RING ligases (CRLs) comprise a large group of modular eukaryotic E3 ubiquitin ligases. Within this family, the CRL4 ligase (consisting of the Cullin4 [CUL4] scaffold protein, the Rbx1 RING finger domain protein, the DNA damage-binding protein 1 [DDB1], and one of many DDB1-associated substrate receptor proteins) has been intensively studied in recent years due to its involvement in regulating various cellular processes, its role in cancer development and progression, and its subversion by viral accessory proteins. Initially discovered as a target for hijacking by the human immunodeficiency virus accessory protein r, the normal targets and function of the CRL4 substrate receptor protein DDB1–Cul4-associated factor 1 (DCAF1; also known as VprBP) had remained elusive, but newer studies have begun to shed light on these questions. Here, we review recent progress in understanding the diverse physiological roles of this DCAF1 in supporting various general and cell type-specific cellular processes in its context with the CRL4 E3 ligase, as well as another HECT-type E3 ligase with which DCAF1 also associates, called EDD/UBR5. We also discuss emerging questions and areas of future study to uncover the dynamic roles of DCAF1 in normal physiology.
Keywords: V(D)J recombination, Merlin, Dicer, p53, EDD/DYRK2, TET, Hippo
Introduction
Protein ubiquitylation is a widespread and evolutionarily conserved form of post-translational modification that regulates most, if not all, cellular processes at some level. The covalent attachment of ubiquitin to substrate proteins is a multistep process involving three classes of enzymes, termed ubiquitin-activating (E1), ubiquitin-conjugating (E2), and ubiquitin ligases (E3). E1s, of which only two are present in mammals, catalyze the attachment of ubiquitin to one of ~40 E2 proteins via a thioester bond. The E2 then interacts with an E3 ubiquitin ligase, which, depending on the ligase type, transfers ubiquitin to a substrate lysine residue either directly from the E2 enzyme (really interesting new gene [RING] E3 ligases) or via a ubiquitin-charged E3 intermediate (homologous to E6AP carboxyl terminus [HECT] and RING-between-RING [RBR] E3 ligases). Additional ubiquitin molecules may be appended to the first ubiquitin monomer to form polyubiquitin chains. The outcome of substrate ubiquitination depends on the number of ubiquitin molecules conjugated to the target protein and the topology of polyubiquitin chain linkages. Polyubiquitin chains linked via K11 or K48 trigger proteasomal degradation, whereas attachment of single ubiquitin molecules (monoubiquitylation) or polyubiquitin chains using alternative linkages can modify protein function and localization (Rape, 2018).
The Cullin RING ligases (CRLs) comprise the largest family of E3 ubiquitin ligases. CRLs are modular, multi-subunit complexes in which E2-binding and substrate recruitment activities are delegated to separate subunits. The common CRL architecture includes one of seven Cullin scaffold proteins (CUL1, CUL2, CUL3, CUL4A, CUL4B, CUL5, and CUL7) assembled with a RING domain-containing protein (either Rbx1 or Rbx2) and one of many substrate recognition subunits. This organizational scheme allows for assembly of different substrate binding modules with a common catalytic core, creating flexibility in CRL specificity and function. In the case of CRL4, which can contain either Cul4A or Cul4B, the substrate recognition subunit is bridged to the Cul4 protein by DNA damage-binding protein 1 (DDB1). Thus, substrate receptors for CRL4 have been termed DDB1 and CUL4-associated factors (DCAFs) (Angers et al., 2006; Jin et al., 2006). The number of identified and putative DCAFs is in excess of 50 members (Lee and Zhou, 2007) and is likely to continue growing. Moreover, orthologs of human DCAF1 have been identified other non-vertebrate organisms including Drosophila melanogaster (Tamori et al., 2010) and Arabidopsis thaliana (Zhang et al., 2008).
DCAF1 was originally discovered as the cellular target of the HIV-1 accessory protein viral protein r (Vpr) and hence was originally named Vpr binding protein (VprBP) (Zhang et al., 2001). Since the discovery of its association with CRL4, DCAF1 has attracted the interest of investigators working in many subfields of molecular and cellular biology. Furthermore, the broad expression pattern of DCAF1 has prompted the study of DCAF1 function in many tissues and cell types. Germline deletion of Dcaf1 was determined to be embryonically lethal in mice (McCall et al., 2008), a finding which has prompted the use of knockdown and conditional knockout approaches to study DCAF1 function in specific cell types. As many of the studies described in this review indicate, DCAF1 deficiency is often associated with defects in cell cycle, cell growth, cell division, and cell survival.
Interestingly, DCAF1 can direct the ubiquitylation of substrate proteins via both CRL4 and a separate HECT-type E3 ligase in which the DCAF1–DDB1 module forms a complex with dual-specificity tyrosine (Y)-phosphorylation regulated kinase (DYRK2) and the E3 ligase identified by differential display (EDD, also called UBR5) (Nakagawa et al., 2013). Several DCAF1-directed substrates of this so-called ‘EDVP’ complex have been identified, none of which is shared with CRL4, making DCAF1 unique among DCAFs in its ability to service two distinct E3 ubiquitin ligases (Nakagawa et al., 2013).
Here, we review recent advances in the knowledge of DCAF1 structure and function. We begin by highlighting recent studies of the structural basis of DCAF1–DDB1 interaction. We discuss the role of DCAF1 in regulating cell cycle, cell growth, cell division, and the contribution of DCAF1 to the development of certain cancers. We discuss several recently recognized roles for DCAF1 in diverse cellular processes, including oocyte development, microRNA biogenesis, and the development and function of B and T lymphocytes. Finally, we address outstanding questions and yet-unresolved issues in the current understanding of DCAF1 function. Given the breadth of these topics, in this review we will not consider the roles of other DCAFs, DCAF1-independent functions of CRL4, or the subversion of CRL4DCAF1 by primate lentiviruses, which has recently been reviewed elsewhere (Romani and Cohen, 2012; Strebel, 2013; Blondot et al., 2014; Guenzel et al., 2014; Rice and Kimata, 2015).
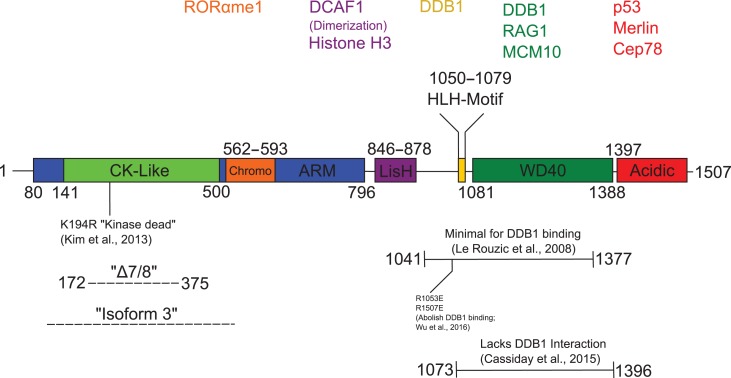
Domain organization of DCAF1 and structural characterization of DCAF1-containing complexes
DCAFs were initially identified by proteomic screens of co-purifying proteins in tandem affinity preparations of DDB1 and CUL4A (Angers et al., 2006; He et al., 2006; Higa et al., 2006; Jin et al., 2006). Most DCAFs identified in these studies contain sequences resembling WD40 repeats, a 40–60 amino acid motif terminating in a Trp-Asp dipeptide (Xu and Min, 2011). Structurally, each WD40 repeat consists of four anti-parallel β-sheets, which constitute one ‘blade’ of a seven-bladed β-propeller (Xu and Min, 2011). WD40 domains adopt a planar, solvent-exposed structure which facilitates protein–protein interactions. They are found in a wide variety of proteins with diverse functions and are conserved among all eukaryotes (Xu and Min, 2011). In DCAF WD40 domains, including the one found in DCAF1, the Trp-Asp dipeptide is frequently followed by a single non-conserved amino acid and a conserved arginine residue, referred to as a ‘WDXR’ motif. Mutating the terminal arginine of the WDXR motif impairs or abolishes DCAF binding to DDB1, suggesting that DCAF WD40 domains are crucial for interaction with DDB1 (Angers et al., 2006; Jin et al., 2006).
Multiple studies have shown that DCAF1 fragments overlapping the WD40 motif can associate with DDB1 (Le Rouzic et al., 2008; McCall et al., 2008; Cassiday et al., 2015). However, a minimal fragment containing only the residues comprising the seven-bladed β-propeller motif of DCAF1 fails to bind DDB1, suggesting another interface helps stabilize the interaction with DDB1 (Cassiday et al., 2015). DDB1 itself is comprised of three WD40 β-propeller domains (designated BPA, BPB, and BPC) which adopt a planar, three-bladed conformation. Previous studies of other DCAF family members bound to DDB1 identified a helix-loop-helix motif, called an H-box, that inserts into the BPA–BPC cleft of DDB1, and this motif was proposed to represent a common mode of DCAF–DDB1 interaction (Angers et al., 2006; Li et al., 2010a; Fischer et al., 2011). Indeed, structural predictions identified a putative H-box motif in DCAF1 (Gerard et al., 2014). Recently, crystallographic analysis of the DCAF1–DDB1 complex confirmed the presence of a helix-loop-helix motif spanning aa1050–1079 of DCAF1 amino-terminal to the WD40 domain that inserts into the BPA–BPC cleft of DDB1 (Wu et al., 2016) (see Figure 1). Importantly, Wu et al. (2016) showed that mutation of several residues within the DCAF1 H-box motif abolished DDB1 interaction.
Figure 1.
Domain organization of DCAF1. The 1507-amino acid human DCAF1 isoform encoded by transcript variant ENST00000563997 (Ensembl database) is shown with structural features indicated. Interacting proteins are listed above and color-coded according to the site of interaction with DCAF1. Below, dashed lines indicate endogenous or recombinant deletions and solid lines indicate recombinant truncation mutants from the listed reference.
In addition to the WD40 domain, DCAF1 also contains a large N-terminal domain characterized by Armadillo (ARM)-like folds, which provides an interface for protein–protein interactions (Figure 1) (Peifer et al., 1994). Interspersed within the ARM folds is a recently characterized casein kinase (CK)-like domain (Kim et al., 2013) and a chromo-like domain that resembles those found in chromatin-modifying proteins (Messmer et al., 1992). The H box–WD40 module is flanked on the N-terminal side by a Lis1-homology (LisH) domain which is required for DCAF1 dimerization (Ahn et al., 2011), and on the C-terminal side by an acidic domain which provides interactions with additional proteins (Huang and Chen, 2008; Wang et al., 2016) (Figure 1).
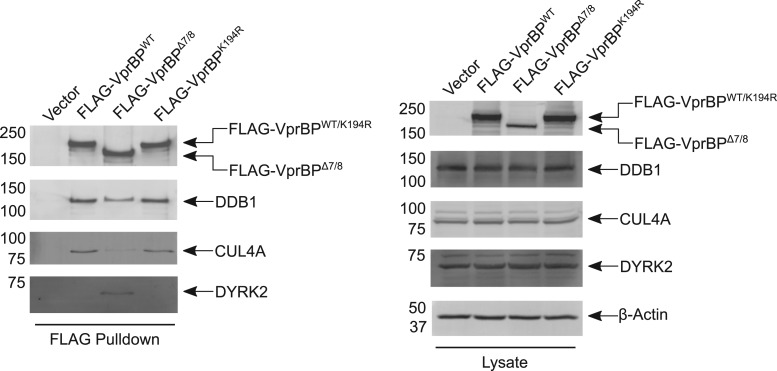
Though the H-box motif and WD40 domain are key structural features of DCAF1 that mediate its interaction with DDB1 in the CRL4 complex, there is evidence that other portions of DCAF1 may contribute to this interaction. First, two reports demonstrate that truncated forms of DCAF1 lacking both the H-box and WD40 domain can interact with DDB1, albeit more poorly than full-length DCAF1 (McCall et al., 2008; Kassmeier et al., 2012). Second, a naturally occurring isoform of DCAF1, harboring an internal truncation spanning the casein kinase (CK)-like domain, the chromo-like domain, and a portion of the ARM domain (‘isoform 3’, Uniprot ID Q9Y4B6-3), shows impaired DDB1 interaction relative to full-length DCAF1 (Cassiday et al., 2015). Third, we show here that a DCAF1 mutant lacking residues encoded by exons 7 and 8 of murine Dcaf1 (‘DCAF1Δ7/8’, aa172–375) exhibits reduced association with DDB1 upon affinity purification of FLAG-tagged DCAF1Δ7/8 from HEK293T cells compared to wild-type DCAF1 (Figure 2). Interestingly, DCAF1Δ7/8 also shows increased association with DYRK2 under these conditions (Figure 2). This observation suggests that this region may control the differential association of DCAF1 with the CRL4 and EDD-DYRK2 E3 ubiquitin ligases. Although DCAF1 has established roles in recruiting substrates to both E3 ligase complexes, no mechanism regulating the association of DCAF1 with one or the other has been identified (Nakagawa et al., 2013). Such a mechanism is unlikely to involve DCAF1’s kinase activity (see below), because a putative active-site mutant of DCAF1 (DCAF1K194R) shows levels of DDB1 association comparable to DCAF1WT (Figure 2). Further work will be needed to determine how aa172–375 promotes association with DDB1, as well as its possible significance for regulating DCAF1-mediated protein ubiquitylation via CRL4 versus EDD-DYRK2.
Figure 2.
DCAF1Δ7/8 exhibits impaired association with CRL4. FLAG epitope-tagged murine DCAF1WT, DCAF1Δ7/8, and DCAF1K194R were expressed in HEK 293T cells and purified using anti-FLAG agarose resin (clone M-2, Biolegend) as described previously (Kassmeier et al., 2012). Proteins were analyzed by SDS-PAGE and western blotting to detect FLAG (Clone M2, Sigma-Aldrich), DDB1 (Clone D4C8, Cell Signaling Technology), CUL4A (Rabbit polyclonal #2699, Cell Signaling Technology), DYRK2 (Rabbit polyclonal #37912, Abcam), and β-actin (Clone AC-15, Sigma-Aldrich).
Function of DCAF1 in normal physiology and cancer
Regulation of p53-mediated transcription
Recent work has highlighted the importance of DCAF1 in regulating cell-cycle progression and apoptosis through both p53-dependent and -independent mechanisms. Hrecka et al. (2007) provided the first evidence that DCAF1 negatively regulates p53. This study showed that DCAF1 depletion in U2OS cells increases expression of p53 and several of its target genes, including those encoding the cyclin-dependent kinase inhibitors p21 and p27. Consistent with this finding, conditional inactivation of Dcaf1 in T lymphocytes was found to stabilize p53 levels in DCAF1-deficient T cells and increase levels of p21 and the pro-apoptotic protein Bax (both products of p53 target genes) (Guo et al., 2016). p53 is known to be polyubiquitylated and degraded by the E3 ligase MDM2 (Haupt et al., 1997; Kubbutat et al., 1997). However, regulation of p53 ubiquitylation remains incompletely understood and may involve other E3 ligases (Brooks and Gu, 2006). In keeping with this, Guo et al. (2016) found that MDM2 overexpression increased p53 polyubiquitylation in U2OS cells, and that this effect was reversed by concomitant loss of DCAF1. However, the exact mechanism by which DCAF1 promotes ubiquitylation and degradation of p53 via MDM2 remains to be determined.
DCAF1 has also been implicated in controlling the transcriptional activity of p53 independently of p53 degradation. Kim et al. (2012) demonstrated that DCAF1 interacts with p53 and is recruited to the promoters of p53 target genes, functioning to block transcriptional activation via acetylation of histone-H3 tails. The LisH domain of DCAF1 was shown to preferentially interact with the unmodified tail of H3, enabling recruitment of histone deacetylase 1 (HDAC1) to p53-dependent promoters, as well as blocking the histone acetyltransferase activity of CREB-binding protein (CBP, also known as p300) (Kim et al., 2012). Interestingly, the repression of p53-mediated transcription by DCAF1 was recently shown to be controlled by acetylation of p53 itself. Acetylation of six lysine residues in the C-terminal domain (CTD) of p53 was shown to block p53 interaction with DCAF1, as well as the oncoprotein SET, another p53 repressor (Vonlindern et al., 1992; Wang et al., 2016). The authors determined that interaction with the Lys-rich CTD of p53 is mediated by the C-terminal acidic patch of DCAF1, which is enriched in Asp and Glu residues (Wang et al., 2016) (Figure 1). Acetylation of Lys residues in the CTD of p53 is thought to neutralize their positive charge and thus reduce electrostatic attraction to the DCAF1 acidic domain. In support of this possibility, acetylation of p53 abrogated DCAF1 binding in vitro, and K→Q replacement mutations in the p53 CTD blocked DCAF1 interaction in vivo (Wang et al., 2016). Taken together, these studies describe a central role for DCAF1 in regulating p53 and its downstream targets, both by mediating MDM2-dependent p53 degradation, and by suppressing p53-mediated transcription.
p53-independent regulation of cell cycle, cell growth, division, and survival
Discovery and characterization of DCAF1 serine/threonine kinase activity
Besides p53, DCAF1 is known to regulate a variety of other factors and processes related to cell cycle and cell survival, several of which have been linked to cancer. Interestingly, Kim et al. (2013) identified a CK-like domain spanning residues 141–500 of DCAF1 (Figure 1). The authors determined that DCAF1 can phosphorylate histone H2A at T120 and identified a putative active site mutant lacking this activity, DCAF1K194R (Figure 1). Increased DCAF1 expression was associated with elevated H2AT120p levels in prostate, bladder, and breast cancer tissue sections (Kim et al., 2013). RNA microarray analysis of DCAF1-depleted DU145 cells identified a set of tumor-suppressor genes whose expression was elevated, suggesting that the kinase activity of DCAF1 promotes tumor growth. The authors also reported a small-molecule inhibitor of DCAF1 kinase activity, called B32B3, which displayed tumoristatic activity when administered to DU145 tumor-engrafted mice (Kim et al., 2013). Thus, this study identified DCAF1 as a potential cancer therapeutic target and provided additional evidence for DCAF1’s involvement in regulating gene expression through control of histone modification.
Functional significance of DCAF1 in Merlin-deficient tumors
Recent work has identified CRL4DCAF1 as an inhibitory target of the tumor-suppressor protein Merlin, which is encoded by the neurofibromatosis type II (NF2) gene. Mutations in NF2 are linked to several glial cancers including schwannomas, mesotheliomas, and meningiomas (Ammoun and Hanemann, 2011). A tandem affinity purification-mass spectrometry (TAP-MS) screen identified DCAF1 and DDB1 as Merlin-associated proteins (Li et al., 2010b). However, rather than being a substrate of CRL4DCAF1, Merlin was found to inhibit CRL4DCAF1-mediated ubiquitylation, with the interaction between Merlin and DCAF1 being essential to Merlin’s tumor-suppressive function (Li et al., 2010b). Recent reports suggesting that Merlin exerts its tumor-suppressive function by activating the anti-proliferative Hippo kinase pathway (Zhao et al., 2007; Zhang et al., 2010) raised the possibility that DCAF1 normally functions to inactivate Hippo kinase signaling (Li et al., 2014). Using the Merlin-deficient mesothelioma cell line Meso-33, Li et al. (2014) determined that depleting DCAF1 increased inhibitory phosphorylation of the oncoprotein and Hippo regulatory target YAP and suppressed the expression of target genes of the transcription factor TEAD, which is activated by YAP. Interestingly, DCAF1 was found to interact with Lats1 and Lats2 (collectively called Warts), the kinases that directly phosphorylate YAP in the Hippo pathway (Li et al., 2014). DCAF1 was shown to direct the polyubiquitylation and proteasomal degradation of Lats1 by CRL4, while Lats2 was found to be oligoubiquitylated and inactivated by CRL4DCAF1. Ubiquitylation of both Lats1 and Lats2 was inhibited by Merlin overexpression.
Finally, Li et al. (2014) established the role of DCAF1-mediated Lats1/2 inactivation in Merlin-deficient tumorigenesis. While siRNA-mediated DCAF1 depletion impaired the growth of Merlin-deficient FC-1801 schwannoma cells in mouse xenograft experiments, simultaneous depletion of Lats1/2 rescued this effect. Furthermore, using gene expression profiling experiments in Meso-33 cells, the authors demonstrated that depleting DCAF1 alone, or simultaneously depleting YAP and TAZ (which together activate TEAD), repressed YAP/TAZ-dependent genes. Of the genes identified in this experiment, expression of those dependent on both YAP/TAZ and DCAF1 were found to be more highly enriched in a panel of human mesotheliomas than those dependent only on DCAF1 or YAP/TAZ (Li et al., 2014). Thus, this study established DCAF1 as an important regulator of the Hippo pathway and revealed the molecular mechanism by which Merlin inhibits DCAF1-mediated tumorigenesis.
Mori et al. (2014) solved the crystal structure of the Merlin 4.1/Ezrin/Radixin/Moesin (FERM) domain complexed with the acidic C-terminal domain (CTD) of DCAF1 (aa1417–1506), which was previously shown to mediate Merlin interaction (Li et al., 2010b). The authors identified two regions of the Merlin FERM domain which synergistically facilitate DCAF1 interaction. Based on structural similarity between the Merlin–DCAF1 CTD complex and complexes containing other FERM-domain proteins and cytoplasmic domains of transmembrane signaling molecules, including CD43 and CD44, the authors speculated that the CD44 intracellular domain (ICD) competes with DCAF1 for Merlin binding. The ICD of CD44 and other adhesion molecules can be liberated by proteolytic cleavage and subsequently participate in downstream signaling and transcription (Okamoto et al., 2001; Murakami et al., 2003). In keeping with this, the presence of DCAF1 abolished Merlin interaction with the ICD of CD44 in an in vitro binding assay (Mori et al., 2014). The authors propose a mechanism in which cleavage of the CD44 ICD enables it to block the Merlin–DCAF1 interaction, releasing CRL4DCAF1 from Merlin-mediated inhibition and promoting tumorigenesis (Mori et al., 2014). Though intriguing, further investigation will be needed to confirm the in vivo relevance of this mechanism.
Though significant progress toward understanding the pathways controlled by Merlin-mediated inhibition of CRL4DCAF1 has been made in recent years, several questions remain to be addressed. First, whether degradation of all or only a subset of DCAF1 substrates is blocked by Merlin remains unclear. It would be interesting to determine whether other pathways controlled by CRL4DCAF1, including those that have not been linked to tumorigenesis, are also subject to regulation by Merlin. Second, the structural basis for how Merlin interferes with the ubiquitylation of CRL4DCAF1 substrates remains to be determined. Of note, Merlin appears to block Lats1 ubiquitylation by CRL4 without disrupting the interaction between DCAF1 and Lats1 (Li et al., 2014). This suggests that Merlin does not directly compete with CRL4DCAF1 substrates for DCAF1 binding. Whether Merlin interferes with Rbx1–E2 interaction, ubiquitin transfer between the E2 and the substrate, or another step in the ubiquitylation process remains to be established. Third, one report purports to show that Merlin itself is targeted for proteasomal degradation through polyubiquitylation by CRL4DCAF1 (Huang and Chen, 2008), a finding which has been challenged by Li et al. (2010b). Whether Merlin degradation by CRL4DCAF1 is a physiologically relevant phenomenon, and under what conditions it enables release of CRL4DCAF1 inhibition remains to be determined. Finally, whether Merlin can inhibit other tumor promoting-activities of DCAF1, including those that appear to be independent of CRL4 E3 ubiquitin ligase activity (i.e. kinase activity or p53 inhibition/destabilization, described above) is also unclear.
Additional cell-cycle regulatory functions of DCAF1
Multiple lines of evidence implicate DCAF1 in the regulation of DNA replication, cell cycle progression, and cell division. McCall et al. (2008) provided early evidence for DCAF1 involvement in S-phase progression and DNA replication. Specifically, depletion of DCAF1 in U2OS cells increased the proportion of cells in the S and G2 cell cycle phases and was associated with increased ‘firing’ of new replication forks and increased apoptosis. Interestingly, subcellular fractionation experiments showed association of both DCAF1 and CUL4A with chromatin during S and G2 (McCall et al., 2008). Moreover, DCAF1 was shown to direct the degradation of the licensing factor MCM10 in a stress-dependent manner (Kaur et al., 2012). Whether these observations are mechanistically linked remains to be determined.
Recently, DCAF1 has been implicated in the transcriptional control of cell cycle progression. Using a high-throughput screening method to identify CRL substrates, Emanuele et al. (2011) and Wang et al. (2017) discovered that the stability of the transcription factor FoxM1 is regulated by CRL4DCAF1. FoxM1 is a member of the Forkhead-box family of transcription factors and regulates genes involved in cell proliferation; notably, dysregulation of FoxM1 target genes was identified in human high-grade serous ovarian cancer (HGSOC) (Ye et al., 1999; Kandoth et al., 2013). Wang et al. (2017) demonstrated that FoxM1 interacts with DCAF1 and is degraded by CRL4. Interestingly, DCAF1 is reported to transcriptionally co-activate FoxM1. Knockdown of DCAF1 impaired FoxM1 target gene expression, as well as progression from G2 to M phase (Wang et al., 2017). Chromatin immunoprecipitation (ChIP) experiments revealed that DCAF1 association with promotors of FoxM1 target genes in G2/M-synchronized cells was accompanied by decreased association of DCAF1 with other CRL4 components (Wang et al., 2017). Based on these observations, the authors proposed a model in which, during S-phase, DCAF1 directs FoxM1 polyubiquitylation and degradation via CRL4, whereas during G2/M, DCAF1 disengages from CRL4 and instead promotes FoxM1-dependent transcription. Finally, DCAF1 was found to be upregulated in HGSOC tumor samples relative to normal ovarian tissue, which the authors speculate explains the increased expression of FoxM1 target genes that is frequently found in HGSOC (Wang et al., 2017).
The above report represents one of several lines of evidence that implicate DCAF1 in the control of mitotic entry and cell division. Maddika and Chen (2009) identified the microtubule-severing enzyme Katanin as a ubiquitylation and degradation target of the EDVP complex. Katanin is required for the proper segregation of sister chromatids during mitosis (Roll-Mecak and Mcnally, 2010). Overexpression of Katanin or depletion of EDVP components in HeLa cells causes accumulation of cells in G2/M, an effect which is rescued in the latter case by concurrent depletion of Katanin (Maddika and Chen, 2009). This finding indicates that DCAF1 helps control cytoskeletal and genome reorganization to facilitate mitosis. In keeping with this, a recent report implicated DCAF1 in regulating centrosome structure and function (Hossain et al., 2017). The centrosome, composed of a pair of centrioles, is the center of microtubule organization in the eukaryotic cell and plays an important role in organelle positioning and cell division (Bornens, 2012). Each centriole is a barrel-like structure composed of hundreds of proteins organized by a scaffold of tubulin rods. Centrosomes are duplicated during S and G2 phases, and during M phase, organize the formation of the mitotic spindle and enable proper segregation of daughter chromatids prior to cytokinesis (Bornens, 2012; Fu et al., 2015). The protein composition of the centrosome and the functions of centrosomal proteins are subjects of ongoing inquiry. Hossain et al. (2017) identified an interaction between the DCAF1 CTD and centrosomal protein of 78 kDa (Cep78) and showed that Cep78 co-immunoprecipitates with components of the EDVP complex, but not those of CRL4. Rather than being a substrate of EDVP, Cep78 was found to block ubiquitylation of known EDVP targets, but had no effect on CRL4DCAF1 substrates (Hossain et al., 2017). The authors found that CP110, another centrosomal protein previously shown to interact with Cep78, was ubiquitylated by EDVP and subsequently degraded. Depletion of Cep78 or overexpression of DCAF1 was associated with the formation of elongated centrioles, as well as premature formation of cilia in proliferating cells (Hossain et al., 2017). Interestingly, as was the case for EDVP-mediated degradation of Katanin (Maddika and Chen, 2009), phosphorylation of CP110 by DYRK2 was shown to be required for its ubiquitylation (Hossain et al., 2017). Also, as was true for Merlin-mediated inhibition of Lats1 ubiquitylation by CRL4DCAF1 (Li et al., 2014), Cep78 did not block CP110 ubiquitylation by disrupting the CP110–DCAF1 interaction. An interesting mechanistic question raised by these studies is how phosphorylation of EDVP substrates by DYRK2 triggers their ubiquitylation. The observation by Hossain et al. (2017) that a form of CP110 harboring mutations in putative DYRK2 phosphorylation sites lost its ability to interact with DCAF1 suggests that phosphorylation by DYRK2 is involved in recruiting substrates to the EDVP complex.
Role of DCAF1 in other cellular processes
Functions in gametogenesis and reproduction
Recent work by two groups has highlighted a role for DCAF1 in oocyte and zygotic development. In the ovaries of adult female mice and humans, oocyte-containing primordial follicles are maintained for the length of the reproductive lifespan in a dormant state (McGee and Hsueh, 2000; Adhikari and Liu, 2009). To produce oocytes capable of being fertilized, primordial follicles continuously give rise to primary, second, and antral follicles (characterized by the formation of an antral cavity). At the antral stage, most follicles degenerate through an apoptotic process called atresia, whereas a subset is rescued by follicle-stimulating hormone (FSH) and become preovulatory follicles, which in turn give rise to mature oocytes during ovulation (McGee and Hsueh, 2000).
To investigate potential functions of CRL4DCAF1 in oocytes, Yu et al. (2013) generated a series of mouse strains in which Dcaf1 or Ddb1 is conditionally inactivated at specific stages of oocyte maturation. Pan-oocyte deletion of Dcaf1 or Ddb1 (in Dcaf1fl/fl; Gdf9-cre or Ddb1fl/fl;Gdf9-cre mice) led to a complete absence of follicles in the ovaries of 12 week-old mice, indicating that CRL4DCAF1 is required for maintaining primordial follicles (Yu et al., 2013). In a subsequent study, this group determined that deleting Dcaf1 in activated oocytes (using Dcaf1fl/fl;Zp3-cre mice) increased the frequency of atretic follicles compared to control mice (Yu et al., 2015b). This was followed by complete loss of activated follicles by 6 weeks, though primordial follicles were intact as late as 20 weeks, indicating that CRL4DCAF1 is required for both primordial follicle maintenance and oocyte activation (Yu et al., 2015b).
Interestingly, although oocyte numbers were comparable with wild-type mice at postnatal day 1, DDB1-deficient oocytes displayed reduced expression of several genes required for oocyte survival (Yu et al., 2013). Loss of DDB1 or DCAF1 was associated with increased formation of 5-methyl cytosine (5mC) at the promoters of these genes, suggesting that CRL4DCAF1 regulates epigenetic control of oocyte maturation (Yu et al., 2013). 5mC levels are regulated by the ten eleven translocation (TET) family of dioxygenases, which catalyze the conversion of 5mC to 5-hydroxymethyl cytosine (5hmC), the first reaction in a multistep process that reverses cytosine methylation (Ito et al., 2010). Loss of CRL4DCAF1 activity resulted in a global decrease in 5hmC levels in oocytes (Yu et al., 2013). Importantly, this study also demonstrated that TET1 interacts with DCAF1 via residues N-terminal to the WD40 domain, and that inhibition of CRL activity by MLN4924 abolishes DNA binding by TET1, TET2, and TET3 (Yu et al., 2013). However, the exact mechanism of TET activation by CRL4DCAF1 remained to be determined.
In agreement with these observations, Yue Xiong’s group demonstrated that DCAF1 interacts with all three TET family members (Nakagawa et al., 2015). As was the case for Ddb1fl/fl;Zp3-cre mice (Yu et al., 2013), the male pronuclei of zygotes harvested from mated Dcaf1fl/fl;Zp3-cre females exhibited reduced 5hmC and increased 5mC levels as compared to Dcaf1fl/fl counterparts, and failed to initiate embryogenesis (Nakagawa et al., 2015). Interestingly, the authors determined that CRL4DCAF1 monoubiquitylates TET1, TET2, and TET3 at evolutionarily conserved lysine residues, including K1299 of TET2, which is recurrently mutated in patients with myeloid cancers (Delhommeau et al., 2009; Kosmider et al., 2009). Whereas recombinant WT TET2 bound unmethylated oligonucleotides in vitro, TET2 K1299E and K1299N mutants exhibited no detectable binding to these targets (Nakagawa et al., 2015). ChIP-qPCR analysis revealed that both mutants exhibited reduced binding to TET-target promoters compared to WT when overexpressed in U2OS cells (Nakagawa et al., 2015). Finally, the authors determined that overexpressing a ubiquitylation site mutant of murine TET2 blocked 5hmC formation in U2OS cells. Notably, this mutant retained its ability to interact with DCAF1, as well as its catalytic activity in an in vitro assay (Nakagawa et al., 2015). Taken together, these two studies identify DCAF1 as a key epigenetic regulator in both oocyte maturation and zygotic development. However, aside from the TET proteins, there may be additional factors whose expression or activity is regulated by CRL4DCAF1 in oocyte development. Of importance, Nakagawa et al. (2015) note that ablation of TET3 does not recapitulate the developmental defect caused by loss of DCAF1 in murine zygotes, although loss of 5hmC in the male pronucleus occurred in both cases (Gu et al., 2011; Wossidlo et al., 2011).
Expanding on their previous work, Yu et al. (2015b) observed increased apoptosis among granulosa cells in the ovaries of Dcaf1fl/fl;Gdf9-cre and Ddb1fl/fl;Gdf9-cre mice. Granulosa cells are somatic cells which initially form a single layer surrounding the oocyte, subsequently expanding as the follicle becomes activated and advances to the antral stage (McGee and Hsueh, 2000). Granulosa cells provide steroid hormones and growth factors to the developing oocyte, but also themselves receive stimulatory signals from the oocyte (McGee and Hsueh, 2000; Pangas and Matzuk, 2004). Because Gdf9 is not expressed in granulosa cells and DDB1 expression was unaltered in these cells in Gdf9-cre transgenic mice, this finding suggests that CRL4DCAF1 functions in oocytes to control granulosa cell expansion and survival in a non-autonomous manner (Yu et al., 2015b).
In addition to promoting of granulosa cell survival, this group identified a second TET-independent function for CRL4DCAF1 in oocytes. The surge in levels of follicle-stimulating and luteinizing hormones that precedes ovulation stimulates meiotic reentry of dormant oocytes in preovulatory follicles, an event termed germinal vesicle breakdown (GVBD) (Mattheij et al., 1994; McGee and Hsueh, 2000). Histological analysis of the ovaries of Dcaf1fl/fl;Gdf9-cre and Ddb1fl/fl;Gdf9-cre mice revealed significantly reduced levels of GVBD in response to hCG injection in both knockout strains as compared to WT mice (Yu et al., 2015a). Fluorescent microscopy analysis of oocytes collected from superovulated mice revealed that this was accompanied by defects in both knockout strains in chromosome alignment at prometaphase I and impaired chromosome segregation during anaphase (Yu et al., 2015b). In Ddb1fl/fl;Gdf9-cre oocytes, structural maintenance of chromosomes 3 (SMC3), a component of the cohesin complex that holds homologous chromosomes together during metaphase, was retained on chromosome arms, whereas it was relocalized to the centromere in WT oocytes (Yu et al., 2015a). DDB1-deficient oocytes also displayed increased ectopic localization of protein phosphatase 2A (PP2A) on chromosome arms, whereas it normally co-localizes with cohesin complexes during the metaphase-anaphase transition and protects cohesin against removal from the chromosome (Liu et al., 2013; Yu et al., 2015a). The authors determined that CRL4DCAF1 targets the scaffold subunit of PP2A, PP2A-A, for proteasomal degradation, whereas it does not appear to regulate the stability of the regulatory or catalytic subunits (PP2A-B and PP2A-C, respectively (Yu et al., 2015a)). The authors present a model in which CRL4DCAF1 limits PP2A-A expression in order to ensure proper chromatid alignment and segregation during meiotic reentry of activated oocytes (Yu et al., 2015a). Taken together, these studies illuminate previously unknown functions for CRL4DCAF1 female fertility and oogenesis.
Lipid metabolism
A pair of recent studies suggest that DCAF1 regulates lipid metabolism (Yoshizawa et al., 2014; Karim et al., 2017). The authors were originally interested in determining the function of Sirtuin-7 (SIRT7) in lipid uptake in the liver (Yoshizawa et al., 2014). The seven SIRT proteins found in mammals are nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases that have been implicated in a wide variety of cellular processes (Houtkooper et al., 2012). Deletion of SIRT1, SIRT3, or SIRT6 was shown to increase hepatic lipid uptake, prompting Yoshizawa et al. (2014) to investigate a role for SIRT7 in lipid metabolism. In contrast to SIRT1, SIRT3, and SIRT6, the authors found that both conventional and liver-specific deletion of SIRT7 resulted in decreased hepatic lipid uptake in mice fed with a high-fat diet, as well as resistance to obesity. These effects were accompanied by a transcription-independent decrease in the expression level of the nuclear orphan receptor TR4 in hepatocytes of SIRT7-deficient mice (Yoshizawa et al., 2014). Importantly, the authors note that SIRT7 deficiency recapitulates several of the effects of TR4 deficiency on hepatic lipid metabolism (Kang et al., 2011).
In a proteomic screen of mouse hepatocyte nuclear extracts for TR4-interacting proteins, the authors identified DCAF1 and DDB1, suggesting that TR4 interacts with CRL4DCAF1 (Yoshizawa et al., 2014). This finding, along with the observations that SIRT7 knockdown shortens TR4 half-life, and that overexpressing SIRT7 inhibits TR4 polyubiquitination in COS-7 cells, led the authors to hypothesize that SIRT7 blocks the ubiquitylation and degradation of TR4 by CRL4DCAF1 (Yoshizawa et al., 2014). In support of this possibility, loss of TR4 in SIRT7-depleted Hepa1-6 cells was partially reversed by simultaneous depletion of CUL4B (Yoshizawa et al., 2014). However, the authors were unable to detect an interaction between DCAF1 and SIRT7, leaving the precise mechanism of inhibition unclear.
In a subsequent study, this group found that, rather than interacting with DCAF1 or CUL4B, SIRT7 interacts directly with DDB1 (Karim et al., 2017). Given that DDB1 was identified as a substrate of the lysine acetyltransferase p300/CBP associated factor (PCAF) (Li et al., 2012), Karim et al. (2017) investigated the role of SIRT7 in regulating DDB1 function. The authors demonstrated that SIRT7 deacetylates DDB1, resulting in impaired DDB1–DCAF1 interaction and stabilization of TR4 (Karim et al., 2017). Taken together, these two studies indicate a novel role for DCAF1 in regulating lipid metabolism, as well as a role for lysine acetylation in regulating CRL4DCAF1 assembly.
Skeletal muscle cell differentiation
A recent study identified a previously unrecognized role for DCAF1 in regulating skeletal muscle cell differentiation (Jung et al., 2015). In both embryo and adult, myocytes differentiate from mesenchymal stem cells by first proceeding through a myoblast stage that is characterized by rapid proliferation. Terminally differentiated myotubes arise when myoblasts exit cell cycle and fuse with one another, forming elongated, multinucleate cells. The transcription factor MyoD has been identified as a key regulator of stem cell commitment to the myocyte lineage (Rudnicki and Jaenisch, 1995). MyoD expression and activity is in turn regulated post-translationally through modulation of MyoD stability by the lysine methyltransferase G9a, though the mechanistic details remained obscure (Ling et al., 2012a, b; Jung et al., 2015).
Jung et al. (2015) determined that methylation of MyoD by G9a stimulates MyoD polyubiquitylation and proteasomal degradation in mouse C2C12 myoblast cells. This activity was inhibited both by overexpression of Jmjd2C, a member of the JmjC domain demethylase family, and by treatment of C2C12 cells with a specific inhibitor of G9a (Jung et al., 2015). Because CRL4DCAF1 was previously shown to mediate the degradation of methylated proteins (Lee et al., 2012), the authors sought to investigate CRL4DCAF1 involvement in MyoD turnover. The authors found that DCAF1 overexpression stimulates polyubiquitylation of MyoD in C2C12 cells. Furthermore, in C2C12 cells, overexpression of either of two truncated forms of DCAF1, one containing only the WD40 domain and one containing only the chromo-like domain, which specifically binds methylated proteins (Lee et al., 2012), had a dominant-negative effect on polyubiquitylation of MyoD (Jung et al., 2015). Thus, these data indicate that DCAF1 controls myogenesis by promoting the turnover of MyoD.
microRNA biogenesis
microRNAs (miRNAs) are small (~22 nt) non-coding RNAs that mediate post-transcriptional gene silencing (Bushati and Cohen, 2007). miRNAs are generated in a multistep process that begins with transcription of a primary miRNA, which forms a double-stranded stem-loop structure through self-complementarity. The primary miRNA, which can be 100S of nucleotides in length, is then trimmed to ~70 bp by the enzyme Drosha, before undergoing nuclear export. In the cytoplasm, the now-precursor miRNA is further processed by the endoribonuclease Dicer, which in a second cleavage step removes the loop, generating a miRNA duplex of 21–23 nt (Jaskiewicz and Filipowicz, 2008). One strand of the duplex is then incorporated into an RNA-induced silencing complex (RISC), which is targeted to specific mRNAs through miRNA-mRNA complementarity. In most cases, the RISC mediates destabilization of the target mRNA through deadenylation, but can also repress mRNA translation independently of stability (Filipowicz et al., 2008).
Ren et al. (2016) reported that stimulation of HCT116 colon cancer cells with lipopolysaccharide or interleukin-6 (IL-6) induces polyubiquitylation and proteasomal degradation of Dicer via CRL4DCAF1. Dicer degradation was correlated with increased activation of signal transducer and activator of transcription 3 (STAT3), a downstream target of the IL-6 receptor (Ren et al., 2016). Interestingly, ChIP-PCR revealed binding of STAT3 to the promoters of both CUL4A and DCAF1, suggesting that IL-6 stimulates transcriptional upregulation of the CRL4DCAF1 components, resulting in increased ubiquitylation and degradation of Dicer (Ren et al., 2016).
In surprising contrast to this report, we recently showed that conditional deletion of Dcaf1 in mouse B cell progenitors results in a profound, transcription-independent loss of Dicer in these cells (Schabla et al., 2018). Although the mechanism underlying this observation remains to be determined, it raises the possibility that DCAF1 has tissue-specific roles in regulating Dicer levels. Further study will be required to investigate cell type-specific regulation of Dicer by DCAF1, and the signaling pathways that orchestrate this activity.
Role in lymphocyte development and function
Recently, our laboratory and others have investigated the function of DCAF1 in lymphocytes. Guo et al. (2016) found that stimulation of T cell receptor (TCR) signaling in naïve CD4+ T cells elicits a transcription-independent increase in DCAF1 protein levels that is correlated with cell growth and proliferation, suggesting a role for DCAF1 in activated T cells. T cell-intrinsic inactivation of Dcaf1 in vivo at the double-positive stage of T cell development by Cd4Cre reduced numbers of both CD4+ and CD8+ T cells in spleen and peripheral lymph node, though T cell development in the thymus was not significantly perturbed (Guo et al., 2016). CD4+ T cells isolated from Dcaf1fl/fl;Cd4Cre mice failed to enter cell cycle and increase in size upon stimulation of TCR signaling. The former defect was rescued by ablating p53 in DCAF1-deficient T cells, whereas as the defect in cell growth was not (Guo et al., 2016). The authors also demonstrated that loss of DCAF1 impaired T cell-mediated immunity in a model of lymphocytic choriomeningitis virus infection. Thus, in addition to identifying a role for DCAF1 in regulating the stability of p53 (as discussed above), this study also highlights the importance of DCAF1 function in T cell activation and proliferation.
Our laboratory recently discovered an interaction between DCAF1 and the recombination activating gene 1 (RAG1) protein (Kassmeier et al., 2012). RAG1 and its cofactor RAG2 together form a site-specific endonuclease that catalyzes DNA cleavage during the assembly of immunoglobulin (Ig) and TCR genes from variable, diverse, and joining gene segments, a process termed V(D)J recombination (Schatz and Swanson, 2011). B cell-intrinsic deletion of DCAF1 caused a profound block in B cell development in vivo, but this defect could be partially rescued through expression of an Eμ-Bcl2 transgene on the DCAF1-deficient background (Palmer et al., 2015). Interestingly, most peripheral B cells developing on this genetic background express the Igλ light chain, rather than the Igκ light chain, which normally predominates. This reflects a ~10-fold loss in the total number of Igκ+ B cells, and is accompanied by increased levels of ‘Igκ-deletion’, a type of secondary V(D)J rearrangement in which an Igκ allele undergoes inactivation through RAG-mediated excision of the κ-constant exon and transcriptional enhancer elements from the chromosome.
We found that RAG1 levels were increased in B cell progenitors of these mice, in a manner that was independent of levels of Rag1 transcript and RAG2 protein (Schabla et al., 2018). Cycloheximide pulse-chase experiments revealed that loss of DCAF1 stabilizes RAG1 (Schabla et al., 2018), which is normally has a short half-life of ~15–30 min (Sadofsky et al., 1993; Grawunder et al., 1996), prompting the hypothesis that DCAF1 directs the degradation of RAG1 via CRL4. Consistent with this hypothesis, treatment of B cell progenitors with MLN4924 or the proteasome inhibitor Bortezomib mimics the effect of DCAF1 loss on RAG1 stability (Schabla et al., 2018). Thus, we have identified DCAF1 as a key regulator of RAG1 turnover, and by extension, V(D)J recombination and B cell repertoire development.
Concluding remarks
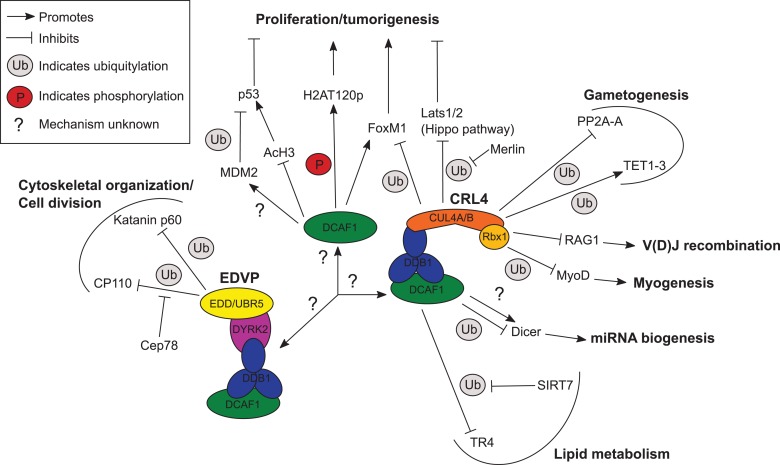
Since the discovery of DCAF1 nearly 20 years ago (Zhang et al., 2001), much progress has been made in understanding its function. However, it has only been more recently that interest in DCAF1’s physiological functions, as opposed to its subversion by lentiviruses, has prompted an accelerated growth in the body of literature on this subject, much of which is described here. The picture currently emerging is of DCAF1 as a multi-functional protein that regulates a wide variety of cellular processes through the CRL4DCAF1 and EDVP complexes. These include some cell type-specific processes, such as V(D)J recombination (Kassmeier et al., 2012; Palmer et al., 2015; Schabla et al., 2018) and others common to all tissues, such as p53 activity (Hrecka et al., 2007; Kim et al., 2012; Guo et al., 2016) and cell division (Hrecka et al., 2007; Maddika and Chen, 2009; Hossain et al., 2017). A graphical summary of current knowledge of the physiological regulatory targets of DCAF1 and their associated biological processes is shown in Figure 3.
Figure 3.
Illustration of pathways controlled by DCAF1. The DCAF1 regulatory targets reported in the studies cited here are shown, with bold text indicating the overarching biological processes with which they are associated. Targets are grouped according to regulation by CRL4DCAF1 (right), EDVP (left), or E3 ligase-independent (or unconfirmed) DCAF1 (center). Mechanisms are represented according to the legend at the top left.
The studies discussed in this review also raise many new questions that remain to be addressed. One outstanding issue is how association of DCAF1 with CRL4 versus EDVP is controlled. Though experimental evidence is lacking, an obvious possibility is post-translational modification of DCAF1, DDB1, or both. If this turns out to be the case, it would be interesting to determine which signaling pathways control exchange of DCAF1 between CRL4 and EDVP, and how this impacts the timing of substrate ubiquitylation with respect to cell cycle or differentiation. A second issue pertains to newly discovered, ubiquitin-independent functions of DCAF1. For example, H2A is to our knowledge the only recognized substrate of DCAF1’s Ser/Thr kinase activity (Kim et al., 2013). If other phosphorylation substrates of DCAF1 exist, their discovery would be of interest. In addition, whether DCAF1 can regulate the activity of transcription factors in addition to FoxM1 (Wang et al., 2017) and p53 (Kim et al., 2012; Wang et al., 2016) remains to be determined. A third issue centers on the significance of the DCAF1 CTD as a ‘reader’ of Lys-rich motifs in p53 and other proteins (Wang et al., 2016), and the circumstances under which such proteins are subjected to DCAF1 regulation.
The progress made over the last several years to understand DCAF1’s normal physiological activity is cause for optimism that these and other questions will be answered. However, we believe that the pleiotropic roles of DCAF1 as revealed by the studies discussed in this review provide reason for some caution in interpreting the phenotypic effects of deletion or knockdown of DCAF1. For example, biological processes that normally involve cellular proliferation may be expected to show deficits upon DCAF1 deletion. Our studies show that enforced Bcl2 expression can rescue some of these general deficits to enable analysis of B cell-intrinsic DCAF1 function, which may be a useful approach to study cell type-specific roles for DCAF1 in other systems.
Acknowledgements
The authors thank Yue Xiong for the murine DCAF1WT and DCAF1Δ7/8 expression constructs used in Figure 2.
Funding
P.C.S. gratefully acknowledges support from the National Institutes of Health (NIH) (R01GM102487) and revenue from Nebraska’s excise tax on cigarettes awarded to Creighton University through the Nebraska Department of Health & Human Services (DHHS). This publication’s contents represent the views of the authors and do not necessarily represent the official views of the State of Nebraska, DHHS, or the NIH.
Conflict of interest
none declared.
References
- Adhikari D., and Liu K. (2009). Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 30, 438–464. [DOI] [PubMed] [Google Scholar]
- Ahn J., Novince Z., Concel J., et al. (2011). The Cullin-RING E3 ubiquitin ligase CRL4-DCAF1 complex dimerizes via a short helical region in DCAF1. Biochemistry 50, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S., and Hanemann C.O. (2011). Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nat. Rev. Neurol. 7, 392–399. [DOI] [PubMed] [Google Scholar]
- Angers S., Li T., Yi X., et al. (2006). Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443, 590–593. [DOI] [PubMed] [Google Scholar]
- Blondot M.L., Dragin L., Lahouassa H., et al. (2014). How SLX4 cuts through the mystery of HIV-1 Vpr-mediated cell cycle arrest. Retrovirology 11, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M. (2012). The centrosome in cells and organisms. Science 335, 422–426. [DOI] [PubMed] [Google Scholar]
- Brooks C.L., and Gu W. (2006). p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N., and Cohen S.M. (2007). MicroRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175–205. [DOI] [PubMed] [Google Scholar]
- Cassiday P.A., DePaula-Silva A.B., Chumley J., et al. (2015). Understanding the molecular manipulation of DCAF1 by the lentiviral accessory proteins Vpr and Vpx. Virology 476, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F., Dupont S., Della Valle V., et al. (2009). Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 360, 2289–2301. [DOI] [PubMed] [Google Scholar]
- Emanuele M.J., Elia A.E.H., Xu Q.K., et al. (2011). Global identification of modular cullin-RING ligase substrates. Cell 147, 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Bhattacharyya S.N., and Sonenberg N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114. [DOI] [PubMed] [Google Scholar]
- Fischer E.S., Scrima A., Bohm K., et al. (2011). The molecular basis of CRL4(DDB2/CSA) ubiquitin ligase architecture, targeting, and activation. Cell 147, 1024–1039. [DOI] [PubMed] [Google Scholar]
- Fu J.Y., Hagan I.M., and Glover D.M. (2015). The centrosome and its duplication cycle. Cold Spring Harb. Perspect. Biol. 7, a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard F.C.A., Yang R.F., Romani B., et al. (2014). Defining the interactions and role of DCAF1/VPRBP in the DDB1-cullin4A E3 ubiquitin ligase complex engaged by HIV-1 Vpr to induce a G(2) cell cycle arrest. PLoS One 9, e89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U., Schatz D.G., Leu T.M.J., et al. (1996). The half-life of RAG-1 protein in precursor B cells is increased in the absence of RAG-2 expression. J. Exp. Med. 183, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T.P., Guo F., Yang H., et al. (2011). The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477, 606–610. [DOI] [PubMed] [Google Scholar]
- Guenzel C.A., Herate C., and Benichou S. (2014). HIV-1 Vpr-a still ‘enigmatic multitasker’. Front. Microbiol. 5, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.L., Kong Q., Liu C., et al. (2016). DCAF1 controls T-cell function via p53-dependent and -independent mechanisms. Nat. Commun. 7, 10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt Y., Maya R., Kazaz A., et al. (1997). Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299. [DOI] [PubMed] [Google Scholar]
- He Y.J., McCall C.M., Hu J., et al. (2006). DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev. 20, 2949–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa L.A., Wu M., Ye T., et al. (2006). CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat. Cell Biol. 8, 1277–1283. [DOI] [PubMed] [Google Scholar]
- Hossain D., Esfehani Y.J., Das A., et al. (2017). Cep78 controls centrosome homeostasis by inhibiting EDD-DYRK2-DDB1(VprBP). EMBO Rep. 18, 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper R.H., Pirinen E., and Auwerx J. (2012). Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrecka K., Gierszewska M., Srivastava S., et al. (2007). Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc. Natl Acad. Sci. USA 104, 11778–11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., and Chen J. (2008). VprBP targets Merlin to the Roc1-Cul4A-DDB1 E3 ligase complex for degradation. Oncogene 27, 4056–4064. [DOI] [PubMed] [Google Scholar]
- Ito S., D’Alessio A.C., Taranova O.V., et al. (2010). Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz L., and Filipowicz W. (2008). Role of Dicer in posttranscriptional RNA silencing. Curr. Top. Microbiol. Immunol. 320, 77–97. [DOI] [PubMed] [Google Scholar]
- Jin J., Arias E.E., Chen J., et al. (2006). A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell 23, 709–721. [DOI] [PubMed] [Google Scholar]
- Jung E.S., Sim Y.J., Jeong H.S., et al. (2015). Jmjd2C increases MyoD transcriptional activity through inhibiting G9a-dependent MyoD degradation. Biochim. Biophys. Acta 1849, 1081–1094. [DOI] [PubMed] [Google Scholar]
- Kandoth C., McLellan M.D., Vandin F., et al. (2013). Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.S., Okamoto K., Kim Y.S., et al. (2011). Nuclear orphan receptor TAK1/TR4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance. Diabetes 60, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M.F., Yoshizawa T., Sobuz S.U., et al. (2017). Sirtuin 7-dependent deacetylation of DDB1 regulates the expression of nuclear receptor TR4. Biochem. Biophys. Res. Commun. 490, 423–428. [DOI] [PubMed] [Google Scholar]
- Kassmeier M.D., Mondal K., Palmer V.L., et al. (2012). VprBP binds full-length RAG1 and is required for B-cell development and V(D)J recombination fidelity. EMBO J. 31, 945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M., Khan M.M., Kar A., et al. (2012). CRL4-DDB1-VPRBP ubiquitin ligase mediates the stress triggered proteolysis of Mcm10. Nucleic Acids Res. 40, 7332–7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Heo K., Choi J., et al. (2012). Vpr-binding protein antagonizes p53-mediated transcription via direct interaction with H3 tail. Mol. Cell. Biol. 32, 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim J.M., Kim J.S., et al. (2013). VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol. Cell 52, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider F., Delhommeau F., Dupont S., et al. (2009). Identification of Tet2 as a gene frequently mutated in myeloid disorders. Haematol. Hematol. J. 94, 216. [Google Scholar]
- Kubbutat M.H.G., Jones S.N., and Vousden K.H. (1997). Regulation of p53 stability by Mdm2. Nature 387, 299–303. [DOI] [PubMed] [Google Scholar]
- Le Rouzic E., Morel M., Ayinde D., et al. (2008). Assembly with the Cul4A-DDB1(DCAF1) ubiquitin ligase protects HIV-1 vpr from proteasomal degradation. J. Biol. Chem. 283, 21686–21692. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Lee J.S., Kim H., et al. (2012). EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Mol. Cell 48, 572–586. [DOI] [PubMed] [Google Scholar]
- Lee J., and Zhou P. (2007). DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol. Cell 26, 775–780. [DOI] [PubMed] [Google Scholar]
- Li W., Cooper J., Zhou L., et al. (2014). Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DcAF1)-mediated inhibition of the hippo pathway kinases latsl and 2 in the nucleus. Cancer Cell 26, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.T., Du Y.P., Wang L.K., et al. (2012). Characterization and prediction of lysine (K)-acetyl-transferase specific acetylation sites. Mol. Cell. Proteomics 11, M111.011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Robert E.I., van Breugel P.C., et al. (2010. a). A promiscuous α-helical motif anchors viral hijackers and substrate receptors to the CUL4-DDB1 ubiquitin ligase machinery. Nat. Struct. Mol. Biol. 17, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., You L., Cooper J., et al. (2010. b). Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 140, 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B.M.T., Bharathy N., Chung T.K., et al. (2012. a). Lysine methyltransferase G9a methylates the transcription factor MyoD and regulates skeletal muscle differentiation. Proc. Natl Acad. Sci. USA 109, 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B.M.T., Gopinadhan S., Kok W.K., et al. (2012. b). G9a mediates Sharp-1-dependent inhibition of skeletal muscle differentiation. Mol. Biol. Cell 23, 4778–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Rankin S., and Yu H.T. (2013). Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol. 15, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S., and Chen J. (2009). Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nat. Cell Biol. 11, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheij J.A.M., Swarts J.J.M., Hurks H.M.H., et al. (1994). Advancement of meiotic resumption in Graafian-follicles by lh in relation to preovulatory aging of rat oocytes. J. Reprod. Fertil. 100, 65–70. [DOI] [PubMed] [Google Scholar]
- McCall C.M., Miliani de Marval P.L., Chastain P.D. 2nd, et al. (2008). Human immunodeficiency virus type 1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol. Cell. Biol. 28, 5621–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee E.A., and Hsueh A.J.W. (2000). Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 21, 200–214. [DOI] [PubMed] [Google Scholar]
- Messmer S., Franke A., and Paro R. (1992). Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes Dev. 6, 1241–1254. [DOI] [PubMed] [Google Scholar]
- Mori T., Gotoh S., Shirakawa M., et al. (2014). Structural basis of DDB1-and-Cullin 4-associated Factor 1 (DCAF1) recognition by merlin/NF2 and its implication in tumorigenesis by CD44-mediated inhibition of merlin suppression of DCAF1 function. Genes Cells 19, 603–619. [DOI] [PubMed] [Google Scholar]
- Murakami D., Okamoto I., Nagano O., et al. (2003). Presenilin-dependent γ-secretase activity mediates the intramembranous cleavage of CD44. Oncogene 22, 1511–1516. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Lv L., Nakagawa M., et al. (2015). CRL4(VprBP) E3 ligase promotes monoubiquitylation and chromatin binding of TET dioxygenases. Mol. Cell 57, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Mondal K., and Swanson P.C. (2013). VprBP (DCAF1): a promiscuous substrate recognition subunit that incorporates into both RING-family CRL4 and HECT-family EDD/UBR5 E3 ubiquitin ligases. BMC Mol. Biol. 14, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto I., Kawano Y., Murakami D., et al. (2001). Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J. Cell Biol. 155, 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer V.L., Aziz-Seible R., Kassmeier M.D., et al. (2015). VprBP is required for efficient editing and selection of Igκ+ B cells, but is dispensable for Igλ+ and marginal zone B cell maturation and selection. J. Immunol. 195, 1524–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas M.S., and Matzuk M.M. (2004). Genetic models for transforming growth factor beta superfamily signaling in ovarian follicle development. Mol. Cell. Endocrinol 225, 83–91. [DOI] [PubMed] [Google Scholar]
- Peifer M., Berg S., and Reynolds A.B. (1994). A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76, 789–791. [DOI] [PubMed] [Google Scholar]
- Rape M. (2018). Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 19, 59–70. [DOI] [PubMed] [Google Scholar]
- Ren W.G., Shen S.R., Sun Z.Q., et al. (2016). Jak-STAT3 pathway triggers DICER1 for proteasomal degradation by ubiquitin ligase complex of CUL4A(DCAF1) to promote colon cancer development. Cancer Lett. 375, 209–220. [DOI] [PubMed] [Google Scholar]
- Rice A.P., and Kimata J.T. (2015). Subversion of cell cycle regulatory mechanisms by HIV. Cell Host Microbe 17, 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A., and Mcnally F.J. (2010). Microtubule-severing enzymes. Curr. Opin. Cell Biol. 22, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani B., and Cohen E.A. (2012). Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase. Curr. Opin. Virol. 2, 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M.A., and Jaenisch R. (1995). The myod family of transcription factors and skeletal myogenesis. Bioessays 17, 203–209. [DOI] [PubMed] [Google Scholar]
- Sadofsky M.J., Hesse J.E., McBlane J.F., et al. (1993). Expression and V(D)J recombination activity of mutated RAG-1 proteins. Nucleic Acids Res. 21, 5644–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabla N.M., Perry G.A., Palmer V.L., et al. (2018). VprBP (DCAF1) regulates RAG1 expression independently of dicer by mediating RAG1 degradation. J. Immunol. 201, 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D.G., and Swanson P.C. (2011). V(D)J recombination: mechanisms of initiation. Annu. Rev. Genet. Genetics 45, 167–202. [DOI] [PubMed] [Google Scholar]
- Strebel K. (2013). HIV accessory proteins versus host restriction factors. Curr. Opin. Virol. 3, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y., Bialucha C.U., Tian A.G., et al. (2010). Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 8, e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlindern M., Vanbaal S., Wiegant J., et al. (1992). Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3’ 1/2 to different genes—characterization of the set gene. Mol. Cell. Biol. 12, 3346–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.X., Arceci A., Bird K., et al. (2017). VprBP/DCAF1 regulates the degradation and nonproteolytic activation of the cell cycle transcription factor FoxM1. Mol. Cell. Biol. 37, e00609–e00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.L., Kon N., Lasso G., et al. (2016). Acetylation-regulated interaction between p53 and SET reveals a widespread regulatory mode. Nature 538, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wossidlo M., Nakamura T., Lepikhov K., et al. (2011). 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun. 2, 241. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou X.H., Barnes C.O., et al. (2016). The DDB1-DCAF1-Vpr-UNG2 crystal structure reveals how HIV-1 Vpr steers human UNG2 toward destruction. Nat. Struct. Mol. Biol. 23, 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., and Min J.R. (2011). Structure and function of WD40 domain proteins. Protein Cell 2, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H.G., Holterman A.X., Yoo K.W., et al. (1999). Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol. Cell. Biol. 19, 8570–8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T., Karim M.F., Sato Y., et al. (2014). SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway. Cell Metab. 19, 712–721. [DOI] [PubMed] [Google Scholar]
- Yu C., Ji S.Y., Sha Q.Q., et al. (2015. a). CRL4-DCAF1 ubiquitin E3 ligase directs protein phosphatase 2A degradation to control oocyte meiotic maturation. Nat. Commun. 6, 8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Xu Y.W., Sha Q.Q., et al. (2015. b). CRL4(DCAF1) is required in activated oocytes for follicle maintenance and ovulation. Mol. Hum. Reprod. 21, 195–205. [DOI] [PubMed] [Google Scholar]
- Yu C., Zhang Y.L., Pan W.W., et al. (2013). CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins. Science 342, 1518–1521. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng S., Chen F., et al. (2008). Arabidopsis DDB1-CUL4 ASSOCIATED FACTOR1 forms a nuclear E3 ubiquitin ligase with DDB1 and CUL4 that is involved in multiple plant developmental processes. Plant Cell 20, 1437–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Feng Y., Narayan O., et al. (2001). Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene 263, 131–140. [DOI] [PubMed] [Google Scholar]
- Zhang J.M., Ji J.Y., Yi M., et al. (2010). YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Cancer Res. 70, 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Wei X., Li W., et al. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]