Sir,
The recent article by Ladislau et al. on the pathogenicity of type I interferon (IFN) and the effect of type I IFN pathway blockade in vitro was received with great interest. Increasing evidence suggests a role for types I and II IFN in juvenile and adult dermatomyositis (JDM and DM, respectively), including elevated IFN-response gene signatures in the muscle, skin and blood (Ladislau et al., 2018; Reed et al., 2019). Ladislau et al. demonstrated in primary human skeletal muscle cells from a healthy donor that type I IFN pathway activation impaired muscle repair, decreased myotube differentiation, upregulated myotube atrophy-associated genes, and induced endothelial dysfunction in a dermal microvascular endothelial cell line that mimicked some pathogenic findings observed in DM/JDM muscle and skin. These findings were abrogated in vitro by treatment with ruxolitinib, a Janus kinase (JAK) inhibitor, which targets the IFN pathway. Ladislau also reported four adults with refractory DM whose skin disease activity and IFN score improved following ruxolitinib therapy. (Ladislau et al., 2018) The letter by Papadopoulou et al. (2019) reported a patient with refractory JDM who responded well to treatment with baricitinib, a JAK1/2 inhibitor (Papadopoulou et al., 2019), with corresponding downregulation in selected IFN-response genes. Additional reports of responses to JAK inhibition in DM patients have focused on refractory cutaneous manifestations (Kurtzman et al., 2016; Moghadam-Kia et al., 2019), and recently, on improvement of myositis-associated interstitial lung disease (ILD) (Kurasawa et al., 2018; Kato et al., 2019), although these cases have been limited to adults. Here we describe two JDM patients with anti-MDA5 autoantibodies, elevated blood IFN-response gene signatures, as well as refractory muscle, skin, and ILD, with well-documented responses to therapy with the JAK inhibitor tofacitinib, and with corresponding improvement in IFN-response gene signatures and STAT1 phosphorylation. Further details on the patients and methods are provided in the Supplementary material.
Patient 1
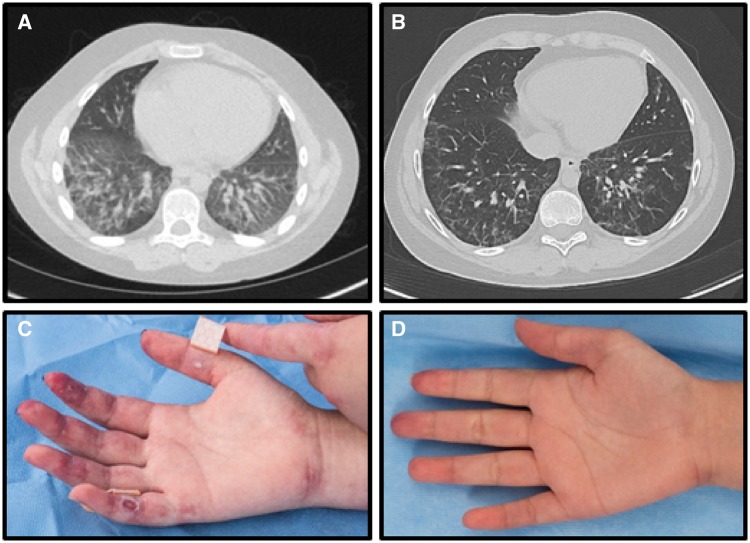
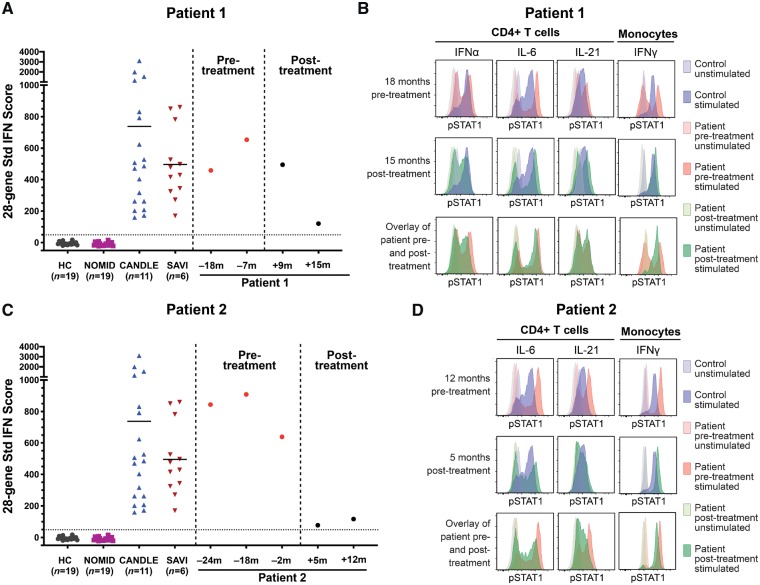
A 12-year-old Caucasian male with anti-MDA5 autoantibody-positive JDM presented with malar and heliotrope rashes, Gottron’s papules, polyarthritis, weight loss, dyspnoea on exertion, and severe proximal weakness, with diffuse myofascial oedema on short-tau inversion recovery (STIR) MRI. He initially improved with pulse methylprednisolone, intravenous immunoglobulin (IVIg), and methotrexate but developed several disease flares. His JDM remained poorly controlled following abatacept therapy, with a Physician Global Activity (PGA) of 3.8 (normal 0; range 0–10), Manual Muscle Testing 8 score (MMT8) of 130 (normal 150; range 0–150), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) score of 21 (range 0–100), and Disease Activity Score (DAS) of 12.5 (range 0–20), indicative of moderate myositis disease activity (Rider et al., 2017). Pulmonary function tests showed diffusing capacity of the lungs for carbon monoxide (DLCO) of 55% predicted value and high-resolution CT of the chest showed bilateral lower lobe reticular nodular infiltrates and juxta-pleural scarring in the lingula (Fig. 2A). Lung biopsy revealed a non-specific interstitial pneumonitis with areas of fibrosis. He also had an elevated 28-gene IFN score, ranging from 458 to 654 (upper limit of normal <49) (Fig. 1A) (Kim et al., 2018). Moreover, in comparison with cells from a healthy control, the patient’s CD4+ T cells had an increased STAT1 phosphorylation after stimulation with IFNα, IL-6, and IL-21, and the patient’s monocytes had a significant upregulation of STAT1 phosphorylation upon stimulation with IFNγ (Fig. 1B). He had multiple disease flares requiring hospitalization, despite addition of mycophenolate mofetil (MMF) and rituximab. Eighteen months after diagnosis he started tofacitinib 5 mg twice a day, which was increased to 10 mg twice a day. Within 6 months, he had significant clinical improvement, with decrease of PGA from 3.8 to 2.1, MMT8 increased from 130 to 148, CDASI decreased from 21 to 12, and DAS decreased from 12.5 to 8.5. Extramuscular activity improved from 4.2 to 2.5 (range 0–10), Childhood Health Assessment Questionnaire (CHAQ) from 0.625 to 0.25 (range 0–3), and serum aldolase from 9.6 to 7 U/l (upper limit of normal <8). He achieved moderate improvement in disease activity by the ACR/EULAR Response Criteria for JDM (Rider et al., 2017), with a Total Improvement Score of 52.5 (moderate improvement range 45–69). Repeat high-resolution CT showed near resolution of infiltrates with markedly improved consolidation and subpleural reticular opacities (Fig. 2B). DLCO on PFT improved to 96% predicted. Repeat MRI showed complete resolution of myofascial oedema. He was successfully weaned off prednisone, pulse methylprednisolone, and MMF. Repeat 28-gene IFN score decreased from 654 (maximum) to 121 15 months after treatment initiation (Fig. 1A). After tofacitinib therapy, the upregulation of STAT1 phosphorylation in patient CD4+ T cells stimulated with IFNα, IL-6, and IL-21 and monocytes stimulated with IFNγ reverted to the same or similar levels as the healthy control (Fig. 1B). The only notable adverse event during tofacitinib treatment was herpes simplex meningitis, with a prior history of recurrent mucositis, and he recovered following treatment with acyclovir.
Figure 2.
Lung high-resolution CT in Patient 1 and cutaneous findings in Patient 2, pre- and post-treatment with tofacitinib. (A) Lung view HRCT of Patient 1 showing bilateral lower lobe reticular nodular infiltrates and ground glass opacities 17 months prior to starting tofacitinib therapy. (B) Lung view HRCT of Patient 1 showing near resolution of infiltrates and ground glass opacities 16 months after tofacitinib therapy. (C) Palmar surface of right hand with erythema and ulcerations prior to tofacitinib therapy in Patient 2. (D) Palmar surface of right hand with complete resolution of erythema and ulceration 12 months after tofacitinib therapy in Patient 2.
Figure 1.
Twenty-eight gene IFN scores in peripheral blood and phosho-STAT signal in PBMCs pre- and post-treatment with tofacitinib. (A and C) Twenty-eight-gene interferon (IFN) scores in Patient 1 (A) and Patient 2 (C) in comparison with healthy and disease controls. (A) Patient 1 at 18 months and 7 months pre-treatment (red dots), and 9 months and 15 months post-treatment (black dots). (C) Patient 2 at 24 months, 18 months and 2 months pre-treatment (red dots), and 5 months and 12 months post-treatment (black dots). Time in months in relation to tofacitinib start date is shown as plus or minus number of months. (B and D) Overlays of flow cytometry histograms of phospho-STATs (pSTAT1) signal in unstimulated (tinted) and indicated cytokines-stimulated (solid) peripheral blood mononuclear cells (PBMCs) from a healthy control and from Patient 1 (B) and Patient 2 (D) pre- and post-treatment samples. Gating was on CD4+ T cells or monocytes by forward scatter (FSC) versus side scatter (SSC).
Patient 2
A 15-year-old Vietnamese female with anti-MDA5 autoantibody-positive JDM presented with malar rash, digital erythema and ulcers (Fig. 2C), hair loss, arthritis, weight loss, Raynaud phenomenon, and weakness, with moderate muscle and myofascial oedema on MRI of the pelvis and thighs. Her PGA was 5, Childhood Myositis Assessment Scale (CMAS) score was 35 (normal 52; range 0–52), MMT8 was 142, CDASI was 21, and DAS was 15. Other disease activity measures included extra-muscular activity of 5.3 and CHAQ of 1.6. Initial pulmonary function tests showed DLCO 60% predicted value and high-resolution CT had extensive right basilar ground-glass consolidation, bronchiectasis, and findings of microcystic ILD. A 28-gene IFN score was elevated at 844 (Fig. 1C). Compared to a healthy control, patient CD4+ T cells had an increased STAT1 phosphorylation upon stimulation with IL-6 and IL-21 and patient monocytes upregulated STAT1 phosphorylation upon stimulation with IFNγ (Fig. 1D). She was started on IVIg, pulse methylprednisolone, and MMF, but developed worsening digital ulcerations, arthritis, and calcification. Abatacept was added, after which she had improvement in rashes, ulcerations, and arthritis; however, her ILD worsened with DLCO of 41%. She was started on cyclophosphamide biweekly for 6 months with little improvement. Rituximab and sildenafil were added, but due to persistently active disease she was started on tofacitinib 5 mg twice a day. Within 6 months following tofacitinib initiation, she had significant improvement in her disease activity, which improved further at the 1-year post-tofacitinib evaluation: PGA improved from 5 to 1.8, CMAS from 21 to 49, MMT8 from 142 to 149, CDASI from 21 to 7, DAS from 15 to 7.5, extramuscular activity from 5.3 to 1.6, and CHAQ from 1.6 to 0. Aldolase decreased from 10.1 to 4.9 U/l. She achieved moderate improvement in disease activity by the ACR/EULAR Response Criteria for JDM (Rider et al., 2017), with a Total Improvement Score of 60. She had improved pulmonary function tests with DLCO of 65% predicted value, along with improved reticular and ground glass opacities and increased lung aeration on high-resolution CT. Repeat MRI showed resolution of myofascial oedema. Arthritis and digital ulcers resolved (Fig. 2D), and the extent of calcinosis decreased from 14.4% of involved body surface area to 7% body surface area 12 months post-treatment. A repeat 28-gene IFN score 12 months post-tofacitinib treatment decreased to 117 (Fig. 1C). On tofacitinib therapy, the upregulation of STAT1 phosphorylation in patient’s CD4+ T cells stimulated with IL-6 and IL-21 and monocytes stimulated with IFNγ reverted to the healthy control level (Fig. 1D). Daily prednisone was decreased from 30 to 10 mg and cyclophosphamide was discontinued. There were no notable adverse events while on tofacitinib.
Discussion
We report two patients with anti-MDA5 autoantibody-positive refractory JDM who received the JAK inhibitor, tofacitinib, and improved clinically in the disease activity of their muscles, skin and other target organs, achieving moderate improvement by the ACR/EULAR Myositis Response Criteria for JDM (Rider et al., 2017). Both patients had remarkable improvement of ILD, including improvement in pulmonary function tests and high-resolution CT findings, and peripheral calcinosis improved in one patient (Patient 2). Both patients demonstrated a significant decrease of a 28-gene IFN score from levels comparable to patients with monogenic type I interferonopathies to levels closer to those seen in healthy controls (Fig. 1A and C). Tofacitinib inhibits signalling of type I and II cytokine receptors which bind to Tyk2, Jak1, Jak2 and Jak3 and block signalling by IL-2, IL-4, IL-15, IL-21, IFN-γ, IL-6, and to a lesser extent IL-12, and IL-23 (Leonard and O'Shea, 1998). As a result of this broader activity, tofacitinib impairs differentiation of CD4+ T helper cells (Th1 and Th2), and limits the generation of pathogenic Th17 cells. Our data show that in both patients treated with tofacitinib, STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with multiple cytokines improved to levels comparable to healthy controls, underscoring that the immune modulating effect of tofacitinib is broader than inhibition of type I IFNs (Fig. 1B and D). Both treatment refractory patients were able to wean or discontinue prednisone therapy and/or other immunosuppressive medications. JDM patients with anti-MDA5 autoantibody associated ILD are often refractory to therapy. The disease course is associated with significant morbidity and risk of fatality (Kobayashi et al., 2015) and therapeutic options are currently lacking. These cases underscore the potential utility of JAK inhibition in anti-MDA5 autoantibody positive JDM patients.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its Supplementary material.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, NIEHS, NIAID, NIAMS and the Clinical Center.
Competing interests
R.G-B. received grant support under a government CRADA from SOBI, Lilly and Regeneron. L.G.R. reports Grant/Research support: Cure JM Foundation; Bristol Myers Squibb; Hope Pharmaceuticals. Other support: Lilly- drug; MedImmune/AstraZeneca- research collaboration; aTyr- research collaboration. All other authors report no competing interests.
Supplementary Material
References
- Kato M, Ikeda K, Kageyama T, Kasuya T, Kumagai T, Furuya H, et al. Successful treatment for refractory interstitial lung disease and pneumomediastinum with multidisciplinary therapy including tofacitinib in a patient with anti-MDA5 antibody-positive dermatomyositis. J Clin Rheumatol 2019. doi: 10.1097/RHU.0000000000000984. [DOI] [PubMed] [Google Scholar]
- Kim H, de Jesus AA, Brooks SR, Liu Y, Huang Y, VanTries R, et al. Development of a validated interferon score using nanostring technology. J Interferon Cytokine Res 2018; 38: 171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Takezaki S, Kobayashi I, Iwata N, Mori M, Nagai K, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology (Oxford) 2015; 54: 784–91. [DOI] [PubMed] [Google Scholar]
- Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford) 2018; 57: 2114–9. [DOI] [PubMed] [Google Scholar]
- Kurtzman DJ, Wright NA, Lin J, Femia AN, Merola JF, Patel M, et al. Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol 2016; 152: 944–5. [DOI] [PubMed] [Google Scholar]
- Ladislau L, Suarez-Calvet X, Toquet S, Landon-Cardinal O, Amelin D, Depp M, et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain 2018; 141: 1609–21. [DOI] [PubMed] [Google Scholar]
- Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol 1998; 16: 293–322. [DOI] [PubMed] [Google Scholar]
- Moghadam-Kia S, Charlton D, Aggarwal R, Oddis CV. Management of refractory cutaneous dermatomyositis: potential role of Janus kinase inhibition with tofacitinib. Rheumatology (Oxford) 2019; 58: 1011–5. [DOI] [PubMed] [Google Scholar]
- Papadopoulou C, Hong Y, Omoyinmi E, Brogan PA, Eleftheriou D. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain 2019; 142: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AM, Crowson CS, Dvergsten JA. A path to prediction of outcomes in juvenile idiopathic inflammatory myopathy. Front Immunol 2019; 10: 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider LG, Aggarwal R, Pistorio A, Bayat N, Erman B, Feldman BM, et al. 2016 American College of Rheumatology/European League against rheumatism criteria for minimal, moderate, and major clinical response in juvenile dermatomyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol 2017; 69: 911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its Supplementary material.