Abstract
Background
Previous meta-analyses of randomized controlled trials (RCTs) of vitamin D supplementation and total cancer incidence and mortality found inconsistent results, and most included trials administered generally low doses of vitamin D (≤1100 IU/day). We updated the meta-analysis by incorporating recent RCTs that have tested higher doses of vitamin D supplements.
Materials and methods
PubMed and Embase were searched from the inception to November 2018. Summary relative risks (RRs) and 95% confidence intervals (CIs) were estimated using a random-effects model.
Results
For total cancer incidence, 10 trials were included [6537 cases; 3–10 years of follow-up; 54–135 nmol/l of attained levels of circulating 25(OH) vitamin D [25(OH)D] in the intervention group]. The summary RR was 0.98 (95% CI, 0.93–1.03; P = 0.42; I2 = 0%). The results remained null across subgroups tested, including even when attained 25(OH)D levels exceeded 100 nmol/l (RR, 0.95; 95% CI, 0.83–1.09; P = 0.48; I2 = 26%). For total cancer mortality, five trials were included [1591 deaths; 3–10 years of follow-up; 54–135 nmol/l of attained levels of circulating 25(OH)D in the intervention group]. The summary RR was 0.87 (95% CI, 0.79–0.96; P = 0.005; I2 = 0%), which was largely attributable to interventions with daily dosing (as opposed to infrequent bolus dosing). No statistically significant heterogeneity was observed by attained levels of circulating 25(OH)D (Pheterogeneity = 0.83), with RR being 0.88 (95% CI, 0.78–0.98; P = 0.02; I2 = 0%) for ≤100 nmol/l and 0.85 (95% CI, 0.70–1.03; P = 0.11; I2 = 0%) for >100 nmol/l.
Conclusions
In an updated meta-analysis of RCTs, vitamin D supplementation significantly reduced total cancer mortality but did not reduce total cancer incidence.
Keywords: vitamin D supplements, circulating 25(OH)D, cancer incidence, cancer mortality, meta-analysis, randomized controlled trial
Key message
In this meta-analysis of randomized controlled trials, compared with controls, vitamin D supplementation was not associated with total cancer incidence, but was associated with a statistically significant reduction in total cancer death. Risk reductions were apparent in trials testing daily dosing of vitamin D but not in trials testing bolus dosing.
Introduction
In 1980, vitamin D was hypothesized to lower risk of cancer incidence and mortality [1]. Subsequently in various animal models, vitamin D has demonstrated promotion of cell differentiation and apoptosis, and inhibition of cancer cell proliferation and angiogenesis, as well as anti-inflammatory and immunomodulatory properties [2]. As a whole, these data support an effect of vitamin D on cancer development and progression.
Observational epidemiologic studies, however, suggest divergent patterns, with evidence for a broad benefit of vitamin D weakening for cancer incidence but strengthening for cancer mortality. Studies based on circulating levels of 25(OH) vitamin D [25(OH)D] (approximate range: ≤13–≥150 nmol/l) have generally not confirmed associations with the risk of most cancers [3–11], except for colorectal cancer [12]. In addition, Mendelian randomization studies have not found that genetic variation in 25(OH)D levels is associated with cancer incidence [13–15], with the possible exception of ovarian cancer [16]. In contrast, studies of circulating 25(OH)D (approximate range: 5.7–188 nmol/l), measured either during the prediagnostic period or shortly after diagnosis of cancer, have demonstrated superior survival in cancer patients with higher circulating 25(OH)D levels [17–20]. An inverse association between 25(OH)D status and cancer mortality has also been supported by at least some Mendelian randomization data [14]. Moreover, the geographical association between solar UV-B exposure and cancer was stronger for mortality than for incidence for many cancers in the United States and China [21, 22].
In testing the hypothesis that enhancing vitamin D status may lower cancer incidence or mortality, randomized controlled trials (RCTs) provide high-level evidence. Two meta-analyses of RCTs published in 2014 did not support an effect of vitamin D3 on cancer incidence, but did find an approximately 12% reduced cancer mortality [23, 24], which were limited by number (n ≤ 4) and administration of generally low dose of vitamin D (≤1100 IU/day). In 2014, the U.S. Preventive Services Task Force concluded that data were insufficient to evaluate the effectiveness of vitamin D supplementation for cancer or cardiovascular disease prevention [25]. The most recent meta-analysis including RCTs published up to 2016 did not find evidence to suggest that vitamin D supplementation reduce cancer incidence or mortality [26]. Subsequently, a number of large RCTs have been published, generally utilizing a higher dose of vitamin D (2000 IU/day or 100 000/month) [27–29]. We thus updated the meta-analysis of RCTs on cancer incidence and mortality to resolve inconsistency in previous findings, and to derive insights on the potential effects of dose or attained 25(OH)D levels on the efficacy of vitamin D.
Methods
This meta-analysis was conducted and reported in accordance with the PRISMA guideline [30]. Two authors (DL and NK) participated in the literature search, study selection, data abstraction independently and resolved any discrepancy through discussion.
Study selection
PubMed and Embase were searched for relevant articles published up to November 2018. Detailed search terms are provided (supplementary Table S1, available at Annals of Oncology online). Except for English language and human subjects, no other restrictions were imposed. Abstracts and unpublished results were not included. To identify additional papers, the reference lists of previous systematic reviews and meta-analyses were reviewed.
To be included, studies had to be a RCT that tested the effect of vitamin D supplementation (provided as cholecalciferol or ergocalciferol, with or without other nutrients) on total cancer incidence or mortality, with the results as relative risk (RR) (risk ratio or hazard ratio) and 95% CI (confidence interval); or as the number of incident cases of total cancer and/or total cancer death in each arm. We excluded RCTs when the total number of outcomes is ≤10, because effect size is unreliable and these were mostly small short-term RCTs (e.g. 1 year or less) in specialized populations (e.g. at risk for fractures). RCTs with ≤1 year of follow-up were also excluded because (1) latent cancers may be undiagnosed and a sizable proportion of cancer incidence in year 1 consists of undiagnosed cancers present at baseline; (2) cancer mortality within 1 year of follow-up is likely mostly from undiagnosed cancers that had metastasized already at the time of study inception; (3) it may take 3 to 6 months for 25(OH)D levels to reach homeostasis after initiation of supplementation. When there were several publications from the same trial, the publication fully covering the intervention period and directly reporting RR rather than the number of events alone was selected. This study selection process is summarized in Figure 1.
Figure 1.
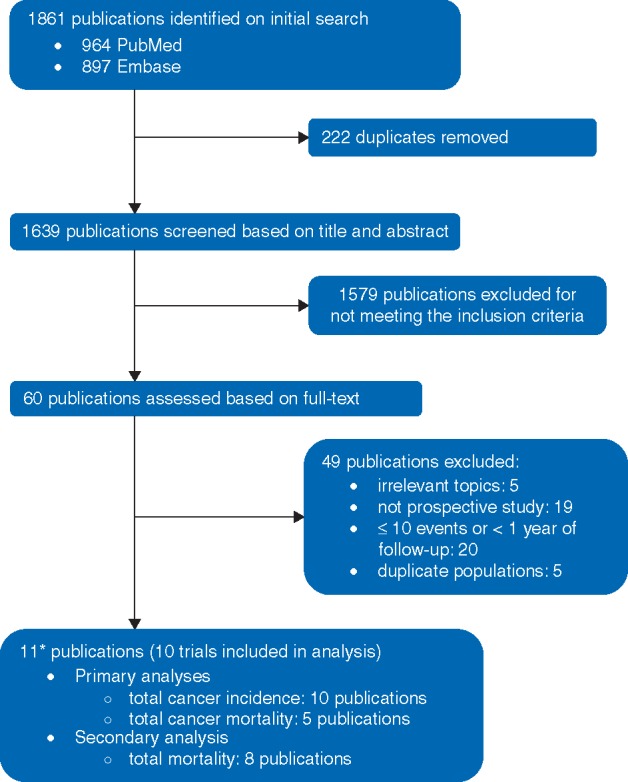
Flowchart for study selection. *Two publications are from the same trial, Calcium plus vitamin D trial [34,35].
Data abstraction
The following information was extracted: definition of intervention and control, RR and corresponding 95% CI or the number of participants and events in each arm, level of circulating 25(OH)D (at baseline, at follow-up), and important characteristics of the study population (Table 1). To conduct secondary analyses on total mortality, a robust end point that answers the ultimate question of whether vitamin D supplementation improves overall survival, we also extracted RR and corresponding 95% CI for all-cause death or the number of participants and total deaths in each arm.
Table 1.
Main characteristics of the RCTs included in meta-analyses
| First author, year, country, reference | Trial name, population (%M), age at baseline | Primary end point of trial, follow-up period | Contrast for RR | Cancer incidence: RR (95% CI), (n case/n total) | Cancer mortality: RR (95% CI), (n case/n total), total mortality: RR (95% CI), (n case/n total) | 25(OH)D level (nmol/l)a: baseline--> follow-up | Inclusion/ exclusion criteria regarding Vit D supplement/ treatment |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NA |
|
Not specified |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
To convert to ng/ml, divide by 2.5.
Excluded events occurring within the first year of randomization.
Excluded events occurring within the first 2 years of randomization.
Abbreviations: Ca, calcium; d, day; IU, international unit, M, male; m, month; n, number; NA, not available; Vit, vitamin; w, with; w/o, without.
Statistical analyses
The summary RR and 95% CI on primary end points (total cancer incidence, total cancer death) and secondary end point (total mortality) were calculated using the DerSimonian–Laird random effects model [31]. Potential for small study effects, such as publication bias, was assessed using Egger’s test [32]. For primary meta-analyses, several sensitivity analyses were performed. In some trials [27, 29, 33], the benefit of vitamin D emerged approximately 1 year after randomization. To explore the potential for a latent effect, meta-analyses were conducted after replacing the results including all outcomes during the follow-up with the results excluding cases during the first year after randomization when reported. Two trials compared the intervention of vitamin D and calcium combined against placebo [27, 34, 35], and a meta-analysis was conducted after excluding these trials. To understand the degree to which the effect of vitamin D supplementation on total mortality may be explained by its effect through total cancer mortality, we conducted a meta-analysis by including only trials that reported outcomes for both total mortality and total cancer mortality.
Heterogeneity in the relationship across trials was assessed by I2 [36]. To explore potential sources of heterogeneity, subgroup analyses and meta-regression were conducted using a priori selected variables: potential effect modifiers [regimen of vitamin D supplementation, baseline and attained circulating 25(OH)D levels, contrast in circulating 25(OH)D levels between the intervention and control group, age, sex, body mass index of the population] and methodological characteristics (duration of follow-up, total number of cases, exclusion of active cancer at baseline, primary end point).
For statistical significance, two-sided α was set at P = 0.05. All statistical analyses were conducted using STATA 12 (StataCorp, College Station, TX).
Results
Characteristics of included RCTs
After screening 1861 articles, a total of 10 RCTs were included in the meta-analysis of total cancer incidence (6537 cases) [27–29, 33, 34, 37–41], of which five RCTs were included in the meta-analysis of total cancer mortality (1591 deaths) (Table 1) [28, 29, 34, 37, 41]. Five RCTs were conducted in the United States [27, 28, 33, 34, 40], three RCTs in Europe [37, 39, 41], and one in Australia and one in New Zealand [29, 38]. Six RCTs provided vitamin D3 daily (400–2000 IU/day) [27, 28, 33, 34, 40, 41]. Four RCTs provided a large bolus of vitamin D3 nondaily (20 000 IU/week to 500 000 IU/year) [29, 37–39]. Circulating levels of 25(OH)D of the included trials ranged approximately between 38 and 83 nmol/l at baseline, and the range of the intervention group reached between 54 and 135 nmol/l at a point during the follow-up. The contrast in the attained blood 25(OH)D level between intervention and control group ranged between 12 and 50 nmol/l. The durations of follow-up period, including intervention and post-intervention follow-up, were approximately between 3 and 10 years.
Primary meta-analysis: vitamin D supplementation and total cancer incidence
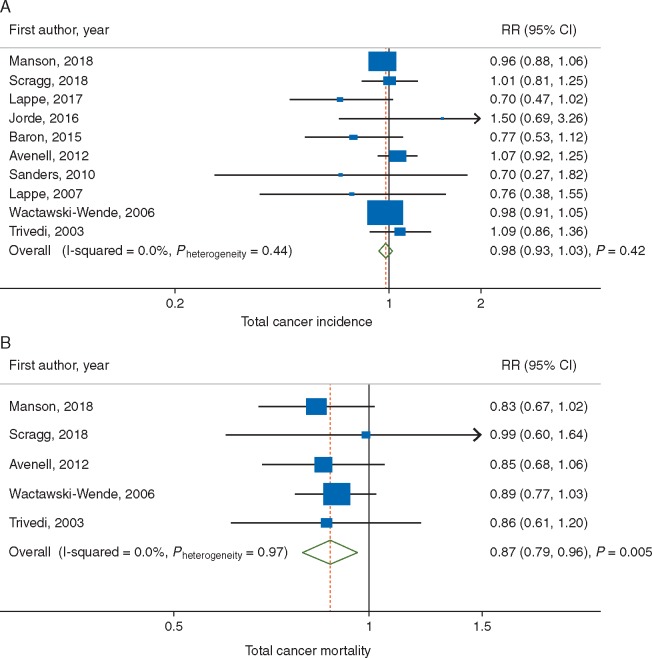
The summary RR for intervention versus control group was 0.98 (95% CI, 0.93–1.03; P = 0.42) with no evidence of heterogeneity (I2 = 0%) (Figure 2A). In sensitivity analyses, the summary RR was 0.97 (95% CI = 0.91–1.04, P = 0.43, I2 = 18%) after replacing the results [27, 29, 33] that included all cases with the results that excluded cases during the first year of follow-up when provided; and 0.99 (95% CI, 0.92–1.06; P = 0.77; I2 = 0%) after excluding two studies that tested the combined effect of vitamin D3 and calcium against placebo [27, 34]. Small study effects, such as publication bias, were not indicated in either primary (PEgger = 0.53) or sensitivity analyses (PEgger = 0.32 and 0.84, respectively).
Figure 2.
Meta-analyses of vitamin D supplementation and (A) total cancer incidence; and (B) total cancer mortality.
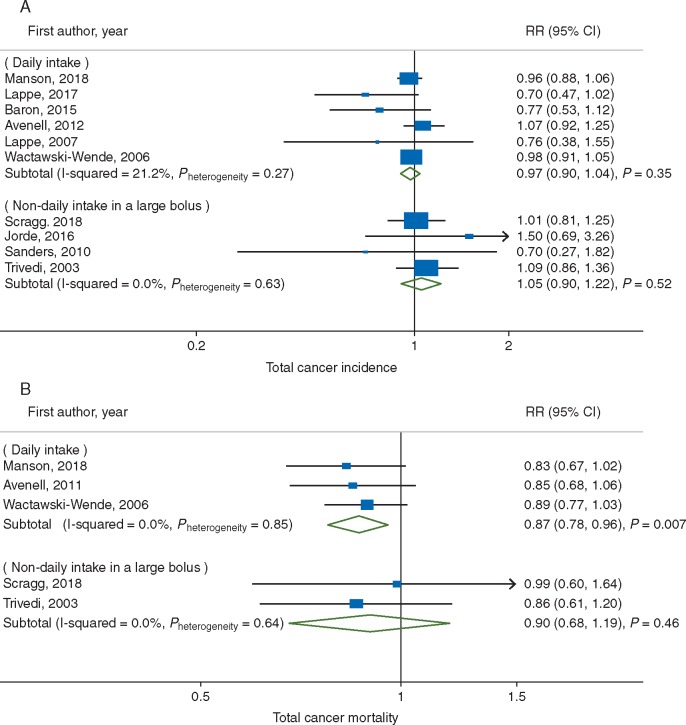
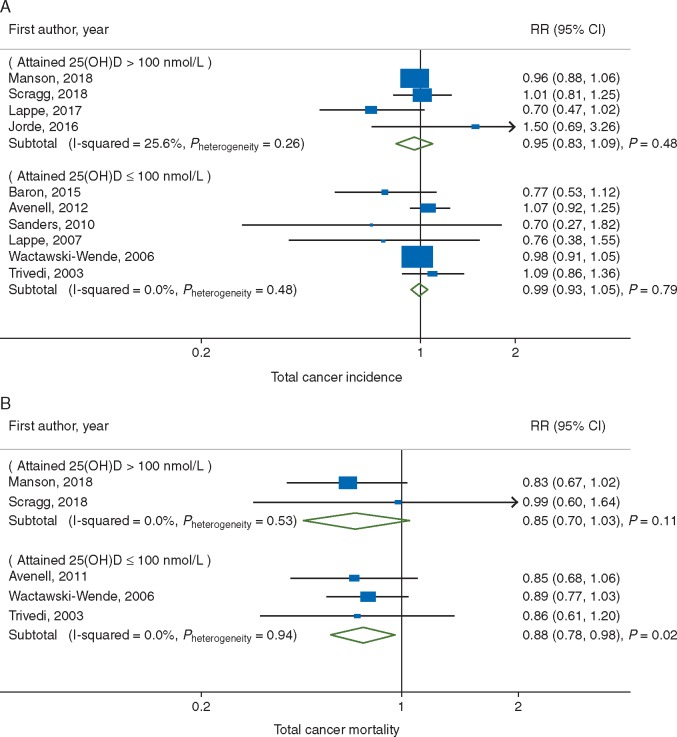
The association between vitamin D supplementation and total cancer incidence was not statistically significantly heterogeneous with respect to regimen of intake (Pheterogeneity = 0.37, Figure 3A), attained 25(OH)D level (Pheterogeneity = 0.55, Figure 4A), and other stratifying factors (supplementary Table S2, available at Annals of Oncology online).
Figure 3.
Subgroup meta-analyses of vitamin D supplementation and (A) total cancer incidence; and (B) total cancer mortality by regimen of vitamin D intake.
Figure 4.
Subgroup meta-analyses of vitamin D supplementation and (A) total cancer incidence; and (B) total cancer mortality by attained 25(OH)D level.
Primary meta-analysis: vitamin D supplementation and total cancer mortality
The summary RR for intervention versus control groups was 0.87 (95% CI, 0.79–0.96; P = 0.005) with no evidence of heterogeneity (I2 = 0%) (Figure 2B). An inverse association became stronger in sensitivity analyses accounting for potential latent effect (utilizing studies that excluded the first year of follow-up) (RR, 0.86; 95% CI, 0.78–0.95; P = 0.004; I2=0%) [28]; and excluding a study that compared concomitant supplementation of vitamin D and calcium with placebo [34] (RR, 0.85; 95% CI, 0.75–0.97; P = 0.02, I2 = 0%). There was no evidence of small study effects, such as publication bias, in either primary (PEgger = 0.76) or sensitivity analyses (PEgger = 0.91 and 0.08, respectively).
Given the small number of trials, the meta-analysis of total cancer mortality had limited statistical power to explore potential sources of heterogeneity. Indeed, the association between vitamin D supplementation and total cancer mortality did not vary statistically significantly by any of the stratifying variables tested (Pheterogeneity ≥ 0.64 for all) (Figures 3B and 4B, supplementary Table S2, available at Annals of Oncology online). Yet, it is notable that a statistically significantly reduced cancer mortality was observed in response to daily dose (RR, 0.87; 95% CI, 0.78–0.96; P = 0.007; I2 = 0%) but not to infrequent bolus dose (Figure 3B). Additionally, the protective benefit of vitamin D supplementation against total cancer mortality was evident even when attained levels of circulating 25(OH)D were ≤100 nmol/l (RR, 0.88; 95% CI, 0.78–0.98; P = 0.02; I2 = 0%) (Figure 4B). Yet, although not statistically significant, a similar magnitude reduction was also observed in trials where the attained 25(OH)D levels were >100 nmol/l (RR, 0.85; 95% CI, 0.70–1.03; P = 0.11; I2 = 0%). An inverse association was manifest among trials with >5 years of follow-up (RR, 0.87; 95% CI, 0.78–0.96; P = 0.005; I2 = 0%) but not in a trial with ≤5 years (RR, 0.99; 95% CI, 0.60–1.64; P = 0.97; I2 = not applicable) (supplementary Table S2, available at Annals of Oncology online) adds to evidence for a requirement of a latency period for an effect of vitamin D to emerge as suggested by previous trials [27, 29, 33].
Secondary meta-analysis: vitamin D supplementation and total mortality
Based on eight trials [27–29, 35, 37, 38, 40, 41] including 5002 total deaths, the summary RR for intervention versus control groups was 0.93 (95% CI, 0.88–0.98; P = 0.009) with no evidence of heterogeneity (I2 = 0%) and small study effects (PEgger > 0.99) (supplementary Figure S1, available at Annals of Oncology online). When the analysis was restricted to five trials (4872 total deaths) [28, 29, 35, 37, 41] included in total cancer mortality, an inverse association with total mortality remained virtually unchanged (RR, 0.93; 95% CI, 0.88–0.98; P = 0.01; I2 = 0%), which is weaker than an association with total cancer mortality.
Discussion
In this meta-analysis of RCTs, we found that vitamin D supplementation was associated with 13% reduced cancer mortality over 3–10 years of follow-up, which confirms findings in the previous meta-analysis in 2014 [24]. Corresponding to the 13% reduction in cancer mortality, there was a statistically significant 7% reduction in total mortality in our meta-analysis where cancer death (n = 1591) accounted for 33% of total death (n = 4872). The VITAL study was the only trial specifically powered to examine cancer mortality as a prespecified end point, yielding a contrast of nearly 30 nmol/l between intervention (105 nmol/l) and control group (73 nmol/l) with a vitamin D dose of 2000 IU/day [28]. Interestingly, in VITAL, increasing benefit over time was indicated, with RR reducing to 0.75 (95% CI, 0.59–0.96) upon excluding deaths occurring during the first 2 years of follow-up [28]. With additional restriction of analysis to cancer deaths confirmed by medical records or other adjudication beyond the National Death Index coding, RR further reduced to 0.63 (95% CI, 0.43–0.92) [28].
In contrast to the results for mortality, we found that supplementation of vitamin D did not lower cancer incidence compared to the control groups. The results remained null across diverse subgroups tested. Of note, while statistically insignificant, the direction of association slightly favored vitamin D supplementation (RR, 0.95; 95% CI, 0.83–1.09; P = 0.48) when attained 25(OH)D levels were > 100 nmol/l. Current epidemiologic evidence based on circulating 25(OH)D levels suggests a potential benefit only for colorectal and ovarian cancer incidence [12, 16]. If any anticancer benefit of vitamin D is truly limited to specific cancer sites, then estimates for total cancer incidence may be weak and statistical significance lost. Alternatively, adult cancers typically develop over several decades and vitamin D could act on early stages of carcinogenesis. In this case, 3–10 years of follow-up periods of RCTs in this meta-analysis may have been insufficient to observe an effect. Specifically for colorectal carcinogenesis, vitamin D may act early given that 25(OH)D levels have been shown to be inversely associated with incidence of colorectal adenoma, a likely precursor for most colorectal cancers [42].
Concern has been raised over the safety of ‘high’ circulating levels of 25(OH)D, generally considered to be those exceeding 100 nmol/l. In our meta-analysis conducted among trials whose achieved 25(OH)D in the intervention group exceeded 100 nmol/l, there was no statistically significant evidence for an increased risk of cancer incidence and mortality. In contrast, a decreased risk was suggested for cancer incidence (RR, 0.95) and cancer mortality (RR, 0.85), albeit not statistically significant. Particularly, two of the trials had baseline levels around 80 nmol/l and the intervention groups achieved average levels around 105 nmol/l with daily supplementation of 2000 IU vitamin D [27, 28]. One of these, a relatively small study [27], found a reduction in incidence (RR, 0.65; 95% CI, 0.42–1.00) and did not report on mortality. The other study (VITAL) showed a borderline nonsignificant lower incidence and an increasing reduction in mortality over time. These findings provide reassurance for the safety of attaining levels at least in the range of 100–120 nmol/l. This level is much higher than 50 nmol/l, a level currently considered sufficient by the Institute of Medicine but lower than the tolerable upper level of 125 nmol/l [43]. According to the Endocrine Society, vitamin D intoxication including hypercalcemia and kidney stones rarely occurs with levels below 375 nmol/l [44].
Not only the attained levels of 25(OH)D but also supplementation regimen to reach the level may modify the effect of vitamin D supplementation on cancer outcomes. Six of the trials in this meta-analysis used daily dosing [27, 28, 33, 34, 40, 41], but one used weekly dosing [39], two used monthly dosing [29, 37], and one used yearly dosing [38]. In a trial that tested a bolus of 500 000 IU of vitamin D3 per year, the median 25(OH)D level in the intervention group rose to approximately 120 nmol/l 1 month after dose, with ≥25% of the group reaching 150 nmol/l [38]. This trial was small and there were only 7 incident cancers in the intervention group versus 10 in the control group. Intermittent bolus dosing might yield nonphysiologic fluctuations in vitamin D [45]. Additionally, vitamin D intoxication only results from taking very large doses (e.g. 50 000–1 000 000 IU/day) of vitamin D for several months to years [46]. In this meta-analysis, the significant findings for cancer mortality were largely driven by trials that assigned daily intake of more modest levels of vitamin D supplements (Figure 3B).
The reason for the divergent findings for incidence and mortality of total cancer is not clear. Nonetheless, there are plausible mechanisms for vitamin D operating at multiple stages of carcinogenesis. Most relevant to the findings on cancer mortality, vitamin D may decrease tumor invasiveness and propensity to metastasize and influence immunomodulatory properties [2, 47]. Although some of the RCTs had some participants already diagnosed with cancer at baseline [29, 38], the vast majority of the cancer mortality cases were those that were diagnosed and became fatal over the course of the study period. Thus, the potential benefit for vitamin D status on cancer mortality could operate during the prediagnostic stages by influencing late-stage tumor progression and metastatic seeding, during treatment by complementing or enhancing effects of therapies, or in post-diagnostic stages by improving survival. It is rational to design studies testing the survival benefit of enhancing vitamin D status after cancer diagnosis, though such designs may underestimate any benefit if some or all of the effects of vitamin D are in the prediagnostic stages.
Our study confirms the findings of the previous meta-analyses by our group [24] and Bjelakovic et al. [23]. Our studies excluded trials of small size (≤10 participants with outcomes) and/or short term (≤1 year of follow-up) as explained in the Methods section. In contrast, the meta-analysis by Bjelakovic et al. included trials of any size and any duration. Despite differential inclusion/exclusion criteria, all meta-analyses consistently concluded that vitamin D supplements had no effect on cancer incidence but reduced cancer mortality by 12%–13%. Bjelakovic et al. noted that the positive finding with cancer mortality could have been due to random errors given too few participants examined (4 trials, 1192 cancer deaths, 44 492 participants) and the evidence of low quality. Our meta-analysis including recent trials of large size, testing higher vitamin D doses, and/or with cancer mortality set as a prespecified end point (5 trials, 1591 cancer deaths, 75 239 participants), provides more robust confirmation of these relationships and alleviates the earlier concerns.
In contrast, in the meta-analysis by Goulao et al., an inverse association with cancer mortality was suggested but not statistically significant (RR, 0.85; 95% CI, 0.70–1.04; 17 trials, 407 cancer deaths, 15 893 participants) [26]. Unlike ours, Goulao et al. included trials of any size and testing any form of vitamin D supplementation (cholecalciferol, ergocalciferol, calcitriol, and vitamin D analog), and excluded trials if supplements (e.g. calcium) co-administered with vitamin D supplements were not provided in the control groups (e.g. the Women’s Health Initiative [34]). They also included data on cancer incidence (e.g. Sanders et al. [38]) in the analysis of cancer mortality by counting cancer incidence as cancer death. Collectively, these may have affected statistical significance of the findings. Yet, in their sensitivity analysis additionally including after-study events (n = 160 deaths) from the RECORD trial, the results became marginally significant (RR, 0.85; 95% CI, 0.72–1.00) [26].
Our meta-analysis has several strengths. Although the time required to see an effect of vitamin D supplementation on cancer, if present, is not known, we were able to assess the influence on total cancer incidence and mortality over a period ranging from 3 to 10 years. At least for cancer mortality, which possibly may be influenced over a relatively short period, this length of time may be adequate. In addition, the studies varied in doses, but this allowed us to examine a potentially interesting and wide range of attained 25(OH)D levels, up to around 120–135 nmol/l of 25(OH)D. Unlike our earlier meta-analysis, we were able to separate effects of vitamin D from those of calcium and to evaluate higher doses such as 2000 IU/day of vitamin D.
Limitations of our meta-analysis also warrant considerations. Most of the trials were not initially designed to test the hypothesis that vitamin D influenced the risk of cancer incidence or mortality. Most of the study populations were composed of whites. Notably, in VITAL, which prespecified cancer end points and oversampled African-Americans, a suggestive inverse association was observed for cancer incidence in this racial subgroup (RR, 0.77; 95% CI, 0.59–1.01, 224 total cases), which is consistent with prior epidemiologic data [48]. Finally, because many trials did not provide data on site-specific cancers, we could not assess if a benefit of vitamin D supplementation was differential across cancer sites. Nevertheless, the epidemiologic data suggest potentially broad effects against many cancer types, though perhaps with stronger effects against some cancer types such as digestive tract malignancies [49].
Despite the globally declining trend in cancer mortality over the past decades [50], approximately 9.6 million cancer deaths were projected in 2018 worldwide [51]. In addition, a substantial percentage of the world population has vitamin D levels below 50 nmol/l, at least in winter [52]. Given the results from our meta-analysis, efforts to achieve circulating levels of 25(OH)D around 54–135 nmol/l may contribute to reducing cancer mortality. To consistently raise the level above 75 nmol/l, at least 1500–2000 IU/day intake of vitamin D may be required for adults as suggested by the Endocrine Society [44]. This requirement is higher than the recommended dietary allowance for vitamin D, which was established to avoid poor bone health: 600 IU for individuals aged 19–70 years and 800 IU for those aged over 70 years [43]. To determine the optimal level of vitamin D intake to reduce cancer mortality, future studies are warranted that examine the dose–response relationship between vitamin D supplementation and cancer death beyond the level of recommended dietary allowance [43].
Funding
NK was supported by a funding from the National Research Foundation of Korea (NRF-2018R1C1B6008822; NRF-2018R1A4A1022589).
Disclosure
The authors declare no conflict of interest.
Supplementary Material
References
- 1. Garland CF, Comstock GW, Garland FC. et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet 1989; 2(8673): 1176–1178. [DOI] [PubMed] [Google Scholar]
- 2. Feldman D, Krishnan AV, Swami S. et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014; 14(5): 342–357. [DOI] [PubMed] [Google Scholar]
- 3. Gandini S, Boniol M, Haukka J. et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 2011; 128(6): 1414–1424. [DOI] [PubMed] [Google Scholar]
- 4. Kim Y, Je Y.. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer 2014; 110(11): 2772–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallicchio L, Helzlsouer KJ, Chow WH. et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1): 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helzlsouer KJ; VDPP Steering Committee. Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1): 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abnet CC, Chen Y, Chow WH. et al. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1): 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeleniuch-Jacquotte A, Gallicchio L, Hartmuller V. et al. Circulating 25-hydroxyvitamin D and risk of endometrial cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1): 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng W, Danforth KN, Tworoger SS. et al. Circulating 25-hydroxyvitamin D and risk of epithelial ovarian cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purdue MP, Freedman DM, Gapstur SM. et al. Circulating 25-hydroxyvitamin D and risk of non-Hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1): 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCullough ML, Zoltick ES, Weinstein SJ. et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst 2019; 111(2): 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He Y, Timofeeva M, Farrington SM. et al. Exploring causality in the association between circulating 25-hydroxyvitamin D and colorectal cancer risk: a large Mendelian randomisation study. BMC Med 2018; 16: 142.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG.. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014; 349: g6330.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimitrakopoulou VI, Tsilidis KK, Haycock PC. et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017; 359: j4761.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ong JS, Cuellar-Partida G, Lu Y. et al. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int J Epidemiol 2016; 45: 1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song ZY, Yao Q, Zhuo Z. et al. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect 2018; 7: R294–R303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maalmi H, Ordóñez-Mena JM, Schöttker B, Brenner H.. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer 2014; 50(8): 1510–1521. [DOI] [PubMed] [Google Scholar]
- 19. Maalmi H, Walter V, Jansen L. et al. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients 2018; 10: pii: E896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vrieling A, Seibold P, Johnson TS. et al. Circulating 25-hydroxyvitamin D and postmenopausal breast cancer survival: influence of tumor characteristics and lifestyle factors? Int J Cancer 2014; 134(12): 2972–2983. [DOI] [PubMed] [Google Scholar]
- 21. Boscoe FP, Schymura MJ.. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer 2006; 6: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen W, Clements M, Rahman B. et al. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control 2010; 21(10): 1701–1709. [DOI] [PubMed] [Google Scholar]
- 23. Bjelakovic G, Gluud LL, Nikolova D. et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev 2014;23(6): CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keum N, Giovannucci E.. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer 2014; 111(5): 976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moyer VA; U.S. Preventive Services Task Force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160(8): 558–564. [DOI] [PubMed] [Google Scholar]
- 26. Goulao B, Stewart F, Ford JA. et al. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr 2018; 107(4): 652–663. [DOI] [PubMed] [Google Scholar]
- 27. Lappe J, Watson P, Travers-Gustafson D. et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA 2017; 317(12): 1234–1243. [DOI] [PubMed] [Google Scholar]
- 28. Manson JE, Cook NR, Lee IM. et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019; 380(1): 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scragg R, Khaw KT, Toop L. et al. Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol 2018; 4(11): e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lappe JM, Travers-Gustafson D, Davies KM. et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007; 85(6): 1586–1591. [DOI] [PubMed] [Google Scholar]
- 34. Wactawski-Wende J, Kotchen JM, Anderson GL. et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006; 354(7): 684–696. [DOI] [PubMed] [Google Scholar]
- 35. LaCroix AZ, Kotchen J, Anderson G. et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 2009; 64: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 37. Trivedi DP, Doll R, Khaw KT.. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003; 326(7387): 469.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanders KM, Stuart AL, Williamson EJ. et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010; 303(18): 1815–1822. [DOI] [PubMed] [Google Scholar]
- 39. Jorde R, Sollid ST, Svartberg J. et al. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab 2016; 101(4): 1647–1655. [DOI] [PubMed] [Google Scholar]
- 40. Baron JA, Barry EL, Mott LA. et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med 2015; 373(16): 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Avenell A, MacLennan GS, Jenkinson DJ. et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab 2012; 97(2): 614–622. [DOI] [PubMed] [Google Scholar]
- 42. Wei MY, Garland CF, Gorham ED. et al. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2008; 17(11): 2958–2969. [DOI] [PubMed] [Google Scholar]
- 43. Ross AC, Manson JE, Abrams SA. et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1): 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holick MF, Binkley NC, Bischoff-Ferrari HA. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7): 1911–1930. [DOI] [PubMed] [Google Scholar]
- 45. Hollis BW, Wagner CL, Clinical R.. the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013; 98(12): 4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holick MF. Vitamin D is not as toxic as was once thought: a historical and an up-to-date perspective. Mayo Clin Proc 2015; 90(5): 561–564. [DOI] [PubMed] [Google Scholar]
- 47. Dou R, Ng K, Giovannucci EL. et al. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br J Nutr 2016; 115(09): 1643–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giovannucci E, Liu Y, Willett WC.. Cancer incidence and mortality and vitamin D in Black and White male health professionals. Cancer Epidemiol Biomarkers Prev 2006; 15(12): 2467–2472. [DOI] [PubMed] [Google Scholar]
- 49. Giovannucci E, Liu Y, Rimm EB. et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006; 98(7): 451–459. [DOI] [PubMed] [Google Scholar]
- 50. Hashim D, Boffetta P, La Vecchia C. et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol 2016; 27(5): 926–933. [DOI] [PubMed] [Google Scholar]
- 51. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 52. van Schoor N, Lips P.. Global overview of vitamin D status. Endocrinol Metab Clin North Am 2017; 46(4): 845–870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.