Abstract
Motivation
Objective assessment of bioimage analysis methods is an essential step towards understanding their robustness and parameter sensitivity, calling for the availability of heterogeneous bioimage datasets accompanied by their reference annotations. Because manual annotations are known to be arduous, highly subjective and barely reproducible, numerous simulators have emerged over past decades, generating synthetic bioimage datasets complemented with inherent reference annotations. However, the installation and configuration of these tools generally constitutes a barrier to their widespread use.
Results
We present a modern, modular web-interface, CytoPacq, to facilitate the generation of synthetic benchmark datasets relevant for multi-dimensional cell imaging. CytoPacq poses a user-friendly graphical interface with contextual tooltips and currently allows a comfortable access to various cell simulation systems of fluorescence microscopy, which have already been recognized and used by the scientific community, in a straightforward and self-contained form.
Availability and implementation
CytoPacq is a publicly available online service running at https://cbia.fi.muni.cz/simulator. More information about it as well as examples of generated bioimage datasets are available directly through the web-interface.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Ceaseless advances in optical microscopy and fluorescent labelling facilitate in vivo studies of cells and their populations. Consequently, as the amounts of acquired bioimage data increase, the need for accurate and robust bioimage analysis raises. This motivates the development of automated image analysis methods to process vast amount of data without the need of manual intervention (Cardona and Tomancak, 2012; Carpenter et al., 2012).
A high demand for automated methods in quantitative microscopy calls for defining objective criteria for their evaluation. To properly evaluate results and performance of bioimage analysis algorithms, suitable benchmark datasets are needed together with their reference annotations (Ljosa et al., 2012). One can obtain such datasets by carefully selecting a reasonable number of naturally varying real bioimages and letting the experts manually annotate them. This is, however, a laborious task with a potential incoherence of results between various specialists (Coutu and Schroeder, 2013), limiting the public availability of such datasets (Kozubek, 2016). To alleviate the existing difficulties, there is an ongoing development of simulators capable of generating synthetic bioimage datasets with accompanying reference annotations as a natural by-product of the underlying simulation processes (Murphy, 2016; Ulman et al., 2016). These simulators often implement complex computational models, allowing in silico generation of multi-dimensional bioimage data of a high resemblance to real biological specimens acquired using an optical microscope (Chenouard et al., 2014; Sage et al., 2015; Sorokin et al., 2018; Svoboda and Ulman, 2017).
As the internal complexity of simulation systems naturally increases, working with them becomes progressively more demanding. Setting up these tools generally involves a laborious installation process, often coupled with requirements for a specific operating system and third-party libraries of specific versions. Furthermore, configuration and operation of these tools usually implicate arduous work with a particular programming language or command line interface and a detailed knowledge of complex configuration parameters related to the employed computational models.
In this paper, we present CytoPacq, a modular web-interface for generating synthetic benchmark datasets of various cytology-centric 3D digital phantoms (Svoboda et al., 2009), supporting different configurations of the optical system and acquisition devices, including time-lapse imaging. The web-interface allows users to conveniently configure the whole simulation process by controlling the most important parameters only and by navigating their steps using informative tooltips. Furthermore, advanced options are also available for experienced users, allowing for a detailed configuration of the underlying computational models. The modular architecture built from the ground up with the future-proofing in mind allows the integration of new simulation modules as they become available. The currently implemented simulation modules have already been published and utilized for the generation of bioimage data in multiple on-going, well-established and open-science-supporting research projects.
Datasets, generated using the simulation modules in CytoPacq, have already been used for benchmarking of segmentation and tracking algorithms submitted to the Cell Tracking Challenge (Maška et al., 2014; Ulman et al., 2017), evaluation of image analysis algorithms during development of CellProfiler (McQuin et al., 2018), quantitative assessment of segmentation quality (Stegmaier et al., 2014), validation of image reconstruction via blind deconvolution estimating an unknown point spread function (Keuper et al., 2012) as well as for training of widespread deep-learning approaches (Castilla et al., 2019). Furthermore, the datasets are also included in the well-recognized Broad Bioimage Benchmark Collection (https://data.broadinstitute.org/bbbc).
2 Methods and implementation
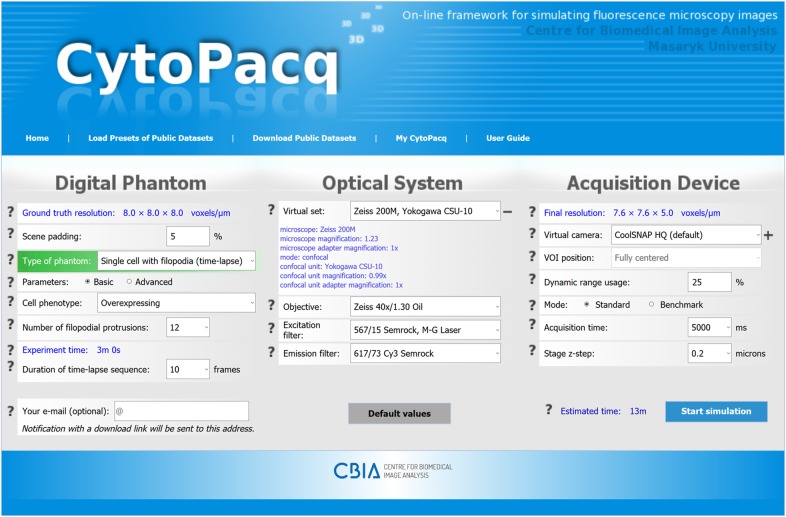
The CytoPacq web-interface separates configuration parameters into three groups (Fig. 1), depending on the functionality of underlying modules: 3D digital phantom simulation that generates spatial objects of interest and their structure; optical system simulation that models image formation in the optical system; and finally acquisition device simulation that mimics the phenomena occurring during image capture when using digital image detectors.
Fig. 1.
A screenshot of the CytoPacq web-interface. The configuration form is divided into three sections, corresponding to the underlying simulation modules
The currently available simulation modules for fluorescently labelled 3D digital phantoms are CytoGen (Svoboda et al., 2009), MitoGen (Svoboda and Ulman, 2017) and FiloGen (Sorokin et al., 2018), generating 3D static digital phantoms of microspheres, HL-60 cell nuclei, granulocyte nuclei and colon tissues, 3D time-lapse digital phantoms of mitotically dividing HL-60 cells, and 3D time-lapse digital phantoms of single lung cancer cells with growing and branching filopodial protrusions, respectively. The simulation of a blurring process occurring in the optical system is realized in the OptiGen module (Svoboda et al., 2009) via an experimentally measured point spread function. And finally, an image acquisition process is mimicked by the AcquiGen module (Svoboda et al., 2009) by including various types of noise and phenomena like sampling and quantization. At present, the web-interface offers a selection of 40 distinct virtual optical system configurations and 6 virtual acquisition devices, available through the internal database.
CytoPacq is an online service, running all simulations on the dedicated computational servers, thus circumventing the requirement for a powerful workstation on the user side. However, we also endorse the possibility of locally installing the underlying simulation modules on private workstations, thus giving more experienced users complete control over the simulation process, as detailed in the Supplementary Material.
3 Concluding remarks
CytoPacq facilitates the generation of theoretically unlimited amount of heterogeneous, completely annotated bioimage data, thus being a valuable resource for objective benchmarking of bioimage analysis methods. Being developed and maintained as a part of the Euro-BioImaging infrastructure (http://www.eurobioimaging.eu) as well as in the frame of COST NEUBIAS networking project (http://neubias.org), its continuous development is well motivated to reflect the needs of the bioimaging community. The modular architecture of CytoPacq opens avenues for integrating new features into it, such as the ability to render multi-channel bioimage data, the support for label-free microscopy modalities, or the possibility of adding new digital phantoms.
Funding
This work was supported by the Czech Science Foundation under the grant No. GA17-05048S (M. M., D. S.), and by the Czech Ministry of Education, Youth and Sports under the grants No. LM2015062 (D. W.), No. CZ.02.1.01/0.0/0.0/16_013/0001775 (M. K.), and No. LTC17016 (D. S.).
Conflict of Interest: none declared.
Supplementary Material
References
- Cardona A., Tomancak P. (2012) Current challenges in open-source bioimage informatics. Nat. Methods, 9, 661–665. [DOI] [PubMed] [Google Scholar]
- Carpenter A.E. et al. (2012) A call for bioimaging software usability. Nat. Methods, 9, 666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla C. et al. (2019) 3-D quantification of filopodia in motile cancer cells. IEEE Trans. Med. Imaging, 38, 862–872. [DOI] [PubMed] [Google Scholar]
- Chenouard N. et al. (2014) Objective comparison of particle tracking methods. Nat. Methods, 11, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu D.L., Schroeder T. (2013) Probing cellular processes by long-term live imaging – historic problems and current solutions. J. Cell Sci., 126, 3805–3815. [DOI] [PubMed] [Google Scholar]
- Keuper M. et al. (2012). Blind deconvolution with PSF regularization for wide-field microscopy. In: 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI), pp. 1292–1295.
- Kozubek M. (2016) Challenges and benchmarks in bioimage analysis. In: Focus on Bio-Image Informatics, Chapter 9. Springer, Cham, pp. 231–262. [DOI] [PubMed] [Google Scholar]
- Ljosa V. et al. (2012) Annotated high-throughput microscopy image sets for validation. Nat. Methods, 9, 637.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maška M. et al. (2014) A benchmark for comparison of cell tracking algorithms. Bioinformatics, 30, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuin C. et al. (2018) CellProfiler 3.0: next-generation image processing for biology. PLoS Biol., 16, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R.F. (2016) Building cell models and simulations from microscope images. Methods, 96, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage D. et al. (2015) Quantitative evaluation of software packages for single-molecule localization microscopy. Nat. Methods, 12, 717–724. [DOI] [PubMed] [Google Scholar]
- Sorokin D.V. et al. (2018) FiloGen: a model-based generator of synthetic 3-D time-lapse sequences of single motile cells with growing and branching filopodia. IEEE Trans. Med. Imaging, 37, 2630–2641. [DOI] [PubMed] [Google Scholar]
- Stegmaier J. et al. (2014) Fast segmentation of stained nuclei in terabyte-scale, time resolved 3D microscopy image stacks. PLoS One, 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D. et al. (2009) Generation of digital phantoms of cell nuclei and simulation of image formation in 3D image cytometry. Cytometry A, 75, 494–509. [DOI] [PubMed] [Google Scholar]
- Svoboda D., Ulman V. (2017) MitoGen: a framework for generating 3D synthetic time-lapse sequences of cell populations in fluorescence microscopy. IEEE Trans. Med. Imaging, 36, 310–321. [DOI] [PubMed] [Google Scholar]
- Ulman V. et al. (2016) Virtual cell imaging: a review on simulation methods employed in image cytometry. Cytometry A, 89, 1057–1072. [DOI] [PubMed] [Google Scholar]
- Ulman V. et al. (2017) An objective comparison of cell-tracking algorithms. Nat. Methods, 14, 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.