ABSTRACT
Background
Whether changes in dairy product consumption are related to subsequent risk of type 2 diabetes (T2D) remains unknown.
Objective
We evaluated the association of long-term changes in dairy product consumption with subsequent risk of T2D among US men and women.
Methods
We followed up 34,224 men in the Health Professionals Follow-Up Study (1986–2012), 76,531 women in the Nurses’ Health Study (1986–2012), and 81,597 women in the Nurses’ Health Study II (1991–2013). Changes in dairy consumption were calculated from consecutive quadrennial FFQs. Multivariable Cox proportional regression models were used to calculate HRs for T2D associated with changes in dairy product consumption. Results of the 3 cohorts were pooled using an inverse variance–weighted, fixed-effect meta-analysis.
Results
During 2,783,210 person-years, we documented 11,906 incident T2D cases. After adjustment for initial and changes in diet and lifestyle covariates, decreasing total dairy intake by >1.0 serving/d over a 4-y period was associated with an 11% (95% CI: 3%, 19%) higher risk of T2D in the subsequent 4 y compared with maintaining a relatively stable consumption (i.e., change in intake of ±1.0 serving/wk). Increasing yogurt consumption by >0.5 serving/d was associated with an 11% (95% CI: 4%, 18%) lower T2D risk, whereas increasing cheese consumption by >0.5 serving/d was associated with a 9% (95% CI: 2%, 16%) higher risk compared with maintaining stable intakes. Substituting 1 serving/d of yogurt or reduced-fat milk for cheese was associated with a 16% (95% CI: 10%, 22%) or 12% (95% CI: 8%, 16%) lower T2D risk, respectively.
Conclusions
Increasing yogurt consumption was associated with a moderately lower risk of T2D, whereas increasing cheese consumption was associated with a moderately higher risk among US men and women. Our study suggests that substituting yogurt or reduced-fat milk for cheese is associated with a lower risk of T2D.
Keywords: cheese, dairy products, milk, type 2 diabetes, yogurt
Introduction
Type 2 diabetes (T2D) affects >380 million adults worldwide and diet undisputedly plays a major role in its prevention (1). In that regard, dairy products contain several bioactive compounds potentially protective against T2D. Calcium, magnesium, whey protein, and vitamin D are suspected to enhance insulin sensitivity (2–4), whereas lactic acid bacteria found in fermented dairy products (e.g., yogurt and cheese) contribute to gut microbial balance, which may reduce the risk of cardiometabolic diseases (5). Dairy products also contain a myriad of fatty acids, mostly saturated, with potential distinct effects on diabetes risk (6). Long, even-chain SFAs [myristic acid (14:0), palmitic acid (16:0), and stearic acid (18:0)] are abundant in dairy fat and have been associated with insulin resistance and incident T2D (6, 7). In contrast, medium- and odd-chain SFAs [caprylic acid (8:0), capric acid (10:0), lauric acid (12:0), pentadecylic acid (15:0), and margaric acid (17:0)] and ruminant trans fatty acids (trans-16:1n–7) are less abundant in dairy fat, but they may improve insulin sensitivity or have been associated with lower diabetes risk (6, 8, 9). Still, how dairy fat, as a whole, influences diabetes risk remains debated (10).
The most recent meta-analysis of prospective cohort studies reported that total dairy product intakes and low-fat dairy product intakes are modestly inversely associated with T2D risk, whereas high-fat dairy consumption appears to be neutral vis-à-vis the risk of T2D (11). Although evidence suggests that the consumption of low-fat dairy products may provide advantages with regard to T2D risk relative to high-fat dairy products, analysis of individual dairy foods showed unclear paradoxical associations (12, 13). Intakes of milk (reduced- or whole-fat) and cheese were not associated with T2D risk, whereas intakes of yogurt and ice cream were inversely associated with T2D risk (12).
Since the 1980s, trends in dairy consumption have markedly changed in the United States. Total milk consumption has been decreasing, whole milk is being replaced by reduced-fat milk, and consumption of yogurt and cheese has increased severalfold (14, 15). Evaluating how changes in dairy consumption are associated with subsequent diabetes risk in prospective cohort studies is likely to provide a more comprehensive understanding of how consuming these foods relates to the risk of diabetes, because it captures the dynamic changes in intakes over time. However, to our knowledge, no study has examined whether changes in dairy intakes over time are associated with subsequent diabetes risk.
We analyzed data from the Health Professionals Follow-Up Study (HPFS), the Nurses’ Health Study (NHS), and the NHS II, in which information on dairy product consumption and diet was collected on a quadrennial basis, for ≤26 y of follow-up. We used these repeated assessments of diet to evaluate how changes in dairy product consumption (total, low-fat, high-fat) are associated with subsequent T2D risk. We also used these data to assess how changes in intakes of individual dairy foods influence subsequent risk of T2D.
Methods
Study population
The HPFS is a prospective cohort study of 51,529 US male health professionals aged 40–75 y at study inception in 1986 (16). The NHS is a prospective cohort study of 121,701 US female registered nurses aged 30–55 y at study inception in 1976 (17). The NHS II is a prospective cohort study that included 116,430 US registered female nurses aged 25–42 y when it was initiated in 1989 (17). In the 3 cohorts, participants were followed using biennial validated questionnaires to update information on medical history, lifestyle, and health conditions. The participant follow-up rate has been ∼90% in each cohort. Detailed descriptions of the 3 studies have been previously published (16, 17).
In the 3 cohorts, diet and dairy product intakes were assessed every 4 y starting in 1986 in the HPFS and the NHS, and in 1991 in the NHS II. Because we used the 4-y change in dairy product consumption to evaluate the risk of T2D in the subsequent 4-y period, the years 1990 in the HPFS and NHS and 1995 in the NHS II (4 y after detailed information on diet was initially collected) were used as baseline for the current analysis. For the current study, we excluded participants with diabetes (type 1 diabetes, T2D, gestational diabetes), cancer, cardiovascular disease, or who died before baseline. We excluded those whose last returned questionnaire was at baseline. We also excluded participants who did not complete 2 consecutive FFQs during follow-up or who always reported implausible calorie intake (<800 or >4200 kcal/d for men and <500 or >3500 kcal/d for women). After exclusions, the current analysis included 34,224 men in the HPFS, 76,531 women in the NHS, and 81,597 women in the NHS II. Supplemental Figure 1 presents the flowchart of participants.
The study was approved by the institutional review boards of Brigham and Women's Hospital and Harvard TH Chan School of Public Health. All participants gave informed consent.
Assessment of dairy product intakes and diet
In the 3 cohorts, dietary information was collected and updated every 4 y using a semiquantitative FFQ. Participants were asked how often on average they consumed a standard portion size of each food in the past year, from “never or less than once per month” to “≥6 times per day.” Questionnaire items on dairy products included skim or 1–2% fat milk (8 oz/237 mL), whole milk (8 oz/237 mL), cream (1 tablespoon/15 mL), sherbet (4 oz/118 mL), ice cream (4 oz/118 mL), yogurt (8 oz/237 mL), cottage or ricotta cheese (4 oz/118 mL), cream cheese (1 oz/28 g), and other cheeses (e.g., American, cheddar, etc.; 1 slice or 1 oz/28 g). For other cheeses, participants were asked whether they usually eat regular or low-fat/non-fat cheese. For the current analysis, skim milk and 1–2% fat milk were grouped as “reduced-fat milk.” “Low-fat dairy products” included reduced-fat milk, yogurt, sherbet, cottage cheese, and other reduced-fat cheeses, whereas “high-fat dairy products” included whole milk, cream, ice cream, cream cheese, and other high-fat cheeses.
The reproducibility and validity of the FFQ were previously demonstrated (18–20). For both low-fat and high-fat dairy products, the correlation coefficients between multiple dietary records and FFQs were 0.62 (21). For individual dairy foods, correlation coefficients ranged from 0.38 for hard cheese to 0.97 for yogurt (22).
Assessment of T2D
The primary outcome of the current study was incident confirmed T2D. Cases were first identified by self-reporting from participants on the main biennial questionnaire, and subsequently confirmed by the completion of a supplementary questionnaire on the symptoms, diagnostic tests, and treatment of diabetes. Cases before 1998 were defined in accordance with National Diabetes Data Group criteria (23). The report of ≥1 of the following criteria was used to confirm a case of T2D: 1) ≥1 classic symptom (excessive thirst, polyuria, weight loss, hunger) and fasting glucose concentrations ≥7.8 mmol/L or random glucose concentrations ≥11.1 mmol/L; 2) ≥2 elevated glucose concentrations on different occasions (fasting concentrations ≥7.8 mmol/L, random glucose concentrations ≥11.1 mmol/L, and/or concentrations ≥11.1 mmol/L after ≥2 h shown by oral–glucose-tolerance testing) in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). After 1998, cases were defined using the American Diabetes Association criteria, which lowered the threshold for fasting glucose for the diagnosis of diabetes to 7.0 mmol/L, instead of 7.8 mmol/L (24).
The validity of the supplementary questionnaire for T2D diagnosis was previously demonstrated via reconfirmation of cases using medical records in the HPFS and the NHS (25, 26). The prevalence of undiagnosed T2D cases was also previously investigated in the NHS (27). It was observed that only 1 of 200 randomly selected women had fasting glucose or fructosamine concentrations above the diagnostic cutoffs (27). Only incident T2D cases confirmed with the supplementary questionnaire were considered for the current study.
Assessment of covariates
Using the biennial follow-up questionnaires, we collected and updated information on multiple T2D risk factors: age, race, body weight, cigarette smoking, physical activity, and family history of T2D. Information on history of hypercholesterolemia and high blood pressure was also inquired about in the questionnaires. In women, we ascertained menopausal status and the use of postmenopausal hormones and oral contraceptives. Information on alcohol intake was collected via the FFQs. We used the 2010 Alternative Healthy Eating Index (AHEI), calculated with FFQ data, as an overall indicator of diet quality (28, 29).
Statistical analysis
We calculated each participant's person-years from the date of returning the baseline questionnaire to the date of T2D diagnosis, death, or the end of the follow-up (31 January, 2012 for the HPFS; 30 June, 2012 for the NHS; and 30 June, 2013 for the NHS II), whichever came first.
We used changes in dairy product consumption updated every 4 y as a time-varying exposure to estimate the risk of T2D in the subsequent 4-y period (30). For instance, changes in dairy product consumption from 1986 to 1990 were used to evaluate the risk of T2D between 1990 and 1994, and so on.
Participants were divided into 5 categories of change in total dairy product consumption: no change or relatively stable consumption (±1 serving/wk), increase or decrease in consumption ranging from 1 serving/wk to 1 serving/d, and increase or decrease in consumption by >1 serving/d. For analyses with low-fat or high-fat dairy products or individual dairy products as the main exposure, categories of change were adjusted according to the mean consumption as well as the distribution of change of the main exposure. Changes in dairy product consumption were assessed with the use of censoring of data at the 0.5th and 99.5th percentiles to minimize the influence of outliers.
We used time-dependent Cox proportional hazards regression models to calculate HRs of T2D for changes in dairy product intakes. Model 1 was adjusted for age and stratified by calendar year in 4-y intervals. Model 2 was further adjusted for race (Caucasian, non-Caucasian), family history of diabetes, updated history of hypercholesterolemia and high blood pressure, menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator, in the NHS and NHS II), oral contraceptive use (never, current, past, missing indicator, in the NHS II), initial and change in smoking status (never to never, never to current, past to past, current to past, current to current, missing indicator), initial and change in physical activity level (metabolic equivalents of task per week, quintiles), initial BMI (in kg/m2) (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, >32.0), initial and changes in energy and alcohol intakes (quintiles), initial and change in AHEI score (calculated without the alcohol component, in quintiles), and initial intake of the type of dairy product used as the main exposure (quintiles or tertiles, depending on the model). Models with subgroup of dairy products as the main exposure were also adjusted for initial and change in intakes of other dairy products (quintiles). We tested for linear trend across categories of change in dairy product consumption by treating the median value of each category as a continuous variable in the models. We also tested for potential sex interactions (HPFS compared with NHS + NHS II) using the likelihood ratio test. Considering that body weight might represent an intermediate mediator of the association of changes in dairy product consumption and T2D risk (31), we conducted additional exploratory analyses by further adjusting for concurrent 4-y change in body weight (quintiles).
We estimated the effect on T2D risk of increasing the intake of a specific subgroup or type of dairy product while concomitantly decreasing the intake of another subgroup or type of dairy product, using substitution modeling (32, 33). To do so, we calculated the HR from the difference in β-coefficients of changes in intakes of different dairy products and the 95% CIs from the corresponding variances and covariance after including initial and changes in intakes of dairy products (both continuous) in Cox proportional hazard regression models.
Analyses were conducted separately in each cohort. Results of the 3 cohorts were pooled using an inverse variance–weighted, fixed-effect meta-analysis to obtain overall estimates for both sexes. The Q statistic was used to evaluate heterogeneity between the cohorts. Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc.). Statistical significance was considered at P < 0.05 (2-sided).
Results
During a total of 2,783,210 person-years, we documented 11,906 incident cases of T2D (2300 in the HPFS, 5993 in the NHS, and 3613 in the NHS II). Table 1 presents the age-adjusted characteristics of participants according to baseline 4-y changes in total dairy product consumption. Mean ± SD age of the participants at the beginning of the baseline 4-y period was 57.5 ± 9.6 y in the HPFS, 58.1 ± 7.9 y in the NHS, and 41.1 ± 5.4 y in the NHS II. Participants who decreased their total dairy product consumption by >1.00 serving/d were those with the highest initial dairy intake and total energy intake. Those individuals had also the lowest initial AHEI score. Individuals who increased their dairy consumption had a similar initial AHEI score to participants who maintained a stable consumption. During the first 4-y period, AHEI score increased among participants who decreased their total dairy product consumption, whereas it slightly decreased among individuals who increased their dairy product consumption by >1.00 serving/d. Across all categories of changes in total dairy product consumption, milk and cheese were the most consumed dairy products (Supplemental Table 1).
TABLE 1.
Age-adjusted characteristics of subjects according to baseline 4-y changes in total dairy product consumption1
| Changes in total dairy product consumption | |||||
|---|---|---|---|---|---|
| Decrease | No change or relatively stable | Increase | |||
| >1.00 serving/d | >0.14 to 1.00 serving/d | ±0.14 serving/d | >0.14 to 1.00 serving/d | >1.00 serving/d | |
| HPFS (n = 34,224) | |||||
| Participants, n | 5399 | 9834 | 5624 | 8792 | 4575 |
| Initial dairy intake, serving/d | 3.70 ± 1.69 | 1.89 ± 1.07 | 1.38 ± 1.10 | 1.41 ± 1.04 | 1.70 ± 1.13 |
| Change in dairy intake, serving/d | −2.01 ± 0.97 | −0.49 ± 0.24 | 0.00 ± 0.08 | 0.49 ± 0.24 | 1.96 ± 0.93 |
| Age,2 y | 57.5 ± 9.9 | 57.3 ± 9.6 | 57.3 ± 9.4 | 57.4 ± 9.6 | 58.2 ± 9.9 |
| Initial BMI, kg/m2 | 25.4 ± 3.1 | 25.4 ± 3.1 | 25.2 ± 3.3 | 25.5 ± 3.0 | 25.6 ± 3.3 |
| Weight change, kg | 0.6 ± 4.4 | 0.6 ± 4.0 | 0.8 ± 4.0 | 0.8 ± 4.1 | 0.7 ± 4.4 |
| Current smoker, % | 8.5 | 7.9 | 8.5 | 8.2 | 9.9 |
| High blood pressure, % | 23.7 | 23.0 | 22.6 | 23.7 | 23.8 |
| Hypercholesterolemia, % | 29.6 | 30.9 | 28.9 | 28.5 | 25.2 |
| Family history of diabetes, % | 25.9 | 26.6 | 24.8 | 25.5 | 26.5 |
| Initial physical activity, MET-h/wk | 20.9 ± 28.0 | 20.2 ± 25.5 | 19.3 ± 25.8 | 19.1 ± 26.1 | 20.4 ± 28.2 |
| Change in physical activity, MET-h/wk | 1.0 ± 25.6 | 1.3 ± 24.1 | 2.2 ± 23.5 | 2.4 ± 25.3 | 2.1 ± 27.3 |
| Initial total energy intake, kcal/d | 2306 ± 648 | 1987 ± 587 | 1878 ± 589 | 1906 ± 598 | 2029 ± 636 |
| Change in total energy intake, kcal/d | −348 ± 535 | −152 ± 464 | −54 ± 463 | 35 ± 483 | 211 ± 545 |
| Initial AHEI score | 52.0 ± 11.4 | 53.3 ± 11.4 | 53.4 ± 11.8 | 53.4 ± 11.7 | 53.0 ± 11.5 |
| Change in AHEI score | 2.1 ± 9.0 | 1.4 ± 8.8 | 0.9 ± 8.7 | 0.8 ± 8.8 | −0.5 ± 9.0 |
| NHS (n = 76,531) | |||||
| Participants, n | 13,641 | 21,225 | 10,366 | 19,562 | 11,737 |
| Initial dairy intake, serving/d | 3.72 ± 1.54 | 2.09 ± 1.07 | 1.60 ± 1.11 | 1.56 ± 1.03 | 1.69 ± 1.04 |
| Change in dairy intake, serving/d | −2.01 ± 0.95 | −0.50 ± 0.24 | 0.00 ± 0.08 | 0.50 ± 0.24 | 1.94 ± 0.90 |
| Age,2 y | 58.1 ± 7.9 | 58.1 ± 7.9 | 58.1 ± 8.0 | 58.1 ± 7.8 | 58.2 ± 7.8 |
| Initial BMI, kg/m2 | 25.4 ± 4.9 | 25.3 ± 4.7 | 25.2 ± 4.7 | 25.4 ± 4.7 | 25.6 ± 4.8 |
| Weight change, kg | 1.1 ± 5.7 | 1.2 ± 5.2 | 1.3 ± 5.0 | 1.2 ± 5.2 | 1.1 ± 5.4 |
| Current smoker, % | 19.0 | 18.1 | 19.7 | 18.1 | 18.5 |
| Postmenopausal, % | 19.1 | 20.2 | 20.6 | 20.39 | 19.8 |
| High blood pressure, % | 31.5 | 31.8 | 31.3 | 32.1 | 31.6 |
| Hypercholesterolemia, % | 42.0 | 41.3 | 39.9 | 40.7 | 40.0 |
| Family history of diabetes, % | 28.0 | 27.1 | 27.1 | 27.8 | 27.6 |
| Initial physical activity, MET-h/wk | 15.7 ± 23.3 | 14.6 ± 20.4 | 14.2 ± 21.0 | 14.5 ± 20.8 | 14.8 ± 20.3 |
| Change in physical activity, MET-h/wk | 1.3 ± 23.3 | 1.8 ± 22.3 | 1.6 ± 21.2 | 2.0 ± 23.1 | 2.4 ± 22.1 |
| Initial total energy intake, kcal/d | 1998 ± 540 | 1764 ± 507 | 1639 ± 506 | 1665 ± 508 | 1724 ± 519 |
| Change in total energy intake, kcal/d | −274 ± 467 | −96 ± 410 | 0 ± 401 | 83 ± 416 | 253 ± 462 |
| Initial AHEI score | 52.0 ± 11.0 | 52.5 ± 11.1 | 52.6 ± 11.3 | 52.9 ± 11.2 | 53.4 ± 11.4 |
| Change in AHEI score | 2.0 ± 8.9 | 1.2 ± 8.6 | 0.7 ± 8.5 | 0.7 ± 8.6 | −0.6 ± 8.9 |
| NHS II (n = 81,597) | |||||
| Participants, n | 25,820 | 23,573 | 9051 | 14,269 | 8884 |
| Initial dairy intake, serving/d | 3.51 ± 1.48 | 1.89 ± 1.02 | 1.46 ± 1.09 | 1.55 ± 1.09 | 1.71 ± 1.09 |
| Change in dairy intake, serving/d | −2.18 ± 1.02 | −0.54 ± 0.25 | −0.01 ± 0.08 | 0.48 ± 0.24 | 2.03 ± 1.01 |
| Age,2 y | 40.5 ± 5.2 | 41.2 ± 5.3 | 41.6 ± 5.4 | 41.6 ± 5.6 | 41.7 ± 5.9 |
| Initial BMI, kg/m2 | 24.6 ± 5.2 | 24.6 ± 5.3 | 24.5 ± 5.1 | 24.6 ± 5.3 | 24.8 ± 5.5 |
| Weight change, kg | 2.9 ± 6.6 | 3.2 ± 6.2 | 3.3 ± 6.1 | 3.1 ± 6.5 | 2.9 ± 7.0 |
| Current smoker, % | 10.9 | 11.1 | 11.9 | 11.3 | 12.8 |
| High blood pressure, % | 8.3 | 9.3 | 9.3 | 8.9 | 8.3 |
| Hypercholesterolemia, % | 19.2 | 20.7 | 21.3 | 20.8 | 20.4 |
| Family history of diabetes, % | 34.2 | 35.1 | 34.8 | 34.5 | 34.0 |
| Initial physical activity, MET-h/wk | 24.1 ± 35.3 | 23.0 ± 33.3 | 22.7 ± 33.0 | 24.5 ± 35.4 | 25.9 ± 36.4 |
| Change in physical activity, MET-h/wk | −2.9 ± 33.0 | −2.5 ± 31.4 | −2.7 ± 31.2 | −3.9 ± 34.0 | −3.5 ± 34.0 |
| Initial total energy intake, kcal/d | 1992 ± 537 | 1725 ± 514 | 1617 ± 518 | 1656 ± 523 | 1733 ± 547 |
| Change in total energy intake, kcal/d | −153 ± 497 | 11 ± 459 | 87 ± 449 | 167 ± 466 | 276 ± 514 |
| Initial AHEI score | 47.8 ± 10.9 | 49.3 ± 11.2 | 49.4 ± 11.3 | 50.4 ± 11.3 | 50.7 ± 11.3 |
| Change in AHEI score | 1.9 ± 9.0 | 0.8 ± 8.9 | 0.6 ± 8.9 | 0.2 ± 9.2 | 0.0 ± 9.9 |
1Values are means ± SDs or percentages and are standardized to the age distribution of the study population. AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; MET-h, metabolic equivalents of task; NHS, Nurses’ Health Study.
2Value is not age-adjusted.
Table 2 presents HRs for T2D according to categories of updated 4-y changes in total, low-fat, and high-fat dairy product consumption. In the pooled analysis, participants who decreased their total dairy intake by >1.00 serving/d had an 11% (95% CI: 3%, 19%) higher risk of T2D than did those who maintained a relatively stable total dairy consumption (±1.00 serving/wk). Participants who increased their total dairy product consumption by >1.00 serving/d did not have a lower or higher risk of T2D than those who maintained a stable consumption. Decreasing low-fat dairy consumption by >0.50 serving/d was associated with a marginally significant 8% (95% CI: 0%, 15%) higher risk of T2D than maintaining a stable consumption (±0.50 serving/wk). Increasing low-fat dairy intake was not associated with T2D risk. Changes in high-fat dairy consumption were not associated with T2D risk.
TABLE 2.
HRs (95% CIs) for type 2 diabetes according to categories of updated 4-y changes in total, low-fat, and high-fat dairy product consumption1
| Changes in dairy product consumption frequency | ||||||
|---|---|---|---|---|---|---|
| Decrease | No change or relatively stable | Increase | P values for trend | |||
| Total dairy | >1.00 serving/d | >0.14 to 1.00 serving/d | ±0.14 serving/d | >0.14 to 1.00 serving/d | >1.00 serving/d | |
| HPFS | ||||||
| Median change | −1.67 | −0.43 | 0.00 | 0.43 | 1.65 | |
| Cases/person-years | 332/71,591 | 606/139,354 | 342/85,151 | 665/139,466 | 355/69,219 | |
| Model 1 | 1.19 (1.02, 1.38) | 1.10 (0.97, 1.26) | 1.00 | 1.17 (1.03, 1.34) | 1.27 (1.09, 1.47) | 0.23 |
| Model 2 | 1.13 (0.95, 1.35) | 1.09 (0.95, 1.25) | 1.00 | 1.10 (0.97, 1.26) | 1.12 (0.96, 1.31) | 0.86 |
| NHS | ||||||
| Median change | −1.71 | −0.49 | 0.00 | 0.49 | 1.65 | |
| Cases/person-years | 536/198,684 | 1535/319,264 | 773/161,349 | 1598/309,624 | 1022/189,919 | |
| Model 1 | 1.13 (1.03, 1.24) | 1.01 (0.93, 1.10) | 1.00 | 1.06 (0.98, 1.16) | 1.07 (0.97, 1.17) | 0.47 |
| Model 2 | 1.08 (0.97, 1.19) | 1.00 (0.92, 1.10) | 1.00 | 1.04 (0.95, 1.13) | 0.97 (0.89, 1.07) | 0.13 |
| NHS II | ||||||
| Median change | −1.82 | −0.50 | 0.00 | 0.50 | 1.82 | |
| Cases/person-years | 742/237,596 | 817/267,867 | 420/121,069 | 912/250,598 | 722/222,459 | |
| Model 1 | 1.01 (0.89, 1.13) | 0.92 (0.81, 1.03) | 1.00 | 1.04 (0.93, 1.17) | 0.97 (0.85, 1.09) | 0.98 |
| Model 2 | 1.14 (1.00, 1.31) | 0.94 (0.84, 1.06) | 1.00 | 1.03 (0.91, 1.15) | 0.92 (0.81, 1.04) | 0.006 |
| Pooled2 | ||||||
| Model 2 | 1.11 (1.03, 1.19) | 1.00 (0.94, 1.07) | 1.00 | 1.05 (0.98, 1.11) | 0.98 (0.92, 1.05) | 0.008 |
| P for heterogeneity | 0.75 | 0.31 | — | 0.66 | 0.13 | 0.22 |
| Low-fat dairy | >0.50 serving/d | >0.07 to 0.50 serving/d | ±0.07 serving/d | >0.07 to 0.50 serving/d | >0.50 serving/d | |
| HPFS | ||||||
| Median change | −1.02 | −0.28 | 0.00 | 0.28 | 1.00 | |
| Cases/person-years | 400/87,965 | 480/100,820 | 434/104,515 | 475/109,289 | 511/102,194 | |
| Model 1 | 1.06 (0.92, 1.21) | 1.12 (0.98, 1.27) | 1.00 | 1.03 (0.91, 1.18) | 1.18 (1.04, 1.34) | 0.17 |
| Model 2 | 1.07 (0.91, 1.25) | 1.13 (0.98, 1.29) | 1.00 | 1.05 (0.92, 1.20) | 1.13 (0.98, 1.29) | 0.53 |
| NHS | ||||||
| Median change | −1.07 | −0.28 | 0.00 | 0.28 | 1.07 | |
| Cases/person-years | 1469/260,811 | 1088/219,594 | 789/163,960 | 1137/236,281 | 1510/298,196 | |
| Model 1 | 1.06 (0.97, 1.16) | 0.97 (0.89, 1.07) | 1.00 | 0.97 (0.89, 1.06) | 0.99 (0.91, 1.08) | 0.07 |
| Model 2 | 1.05 (0.96, 1.16) | 0.99 (0.90, 1.09) | 1.00 | 0.99 (0.90, 1.09) | 0.95 (0.87, 1.04) | 0.02 |
| NHS II | ||||||
| Median change | −1.14 | −0.28 | 0.00 | 0.28 | 1.14 | |
| Cases/person-years | 964/301,854 | 598/188,203 | 454/132,606 | 664/186,166 | 933/290,759 | |
| Model 1 | 0.98 (0.87, 1.09) | 0.92 (0.81, 1.04) | 1.00 | 1.01 (0.90, 1.14) | 0.94 (0.84, 1.05) | 0.59 |
| Model 2 | 1.12 (0.98, 1.28) | 1.00 (0.88, 1.14) | 1.00 | 1.05 (0.93, 1.19) | 0.97 (0.86, 1.09) | 0.02 |
| Pooled3 | ||||||
| Model 2 | 1.08 (1.00, 1.15) | 1.02 (0.95, 1.09) | 1.00 | 1.02 (0.96, 1.09) | 0.99 (0.93, 1.06) | 0.006 |
| P for heterogeneity | 0.74 | 0.29 | — | 0.66 | 0.11 | 0.16 |
| High-fat dairy | >0.50 serving/d | >0.07 to 0.50 serving/d | ±0.07 serving/d | >0.07 to 0.50 serving/d | >0.50 serving/d | |
| HPFS | ||||||
| Median change | −0.93 | −0.29 | 0.00 | 0.29 | 0.93 | |
| Cases/person-years | 372/78,329 | 505/123,807 | 630/135,625 | 475/105,265 | 318/61,755 | |
| Model 1 | 1.12 (0.99, 1.28) | 0.94 (0.83, 1.06) | 1.00 | 0.98 (0.87, 1.10) | 1.13 (0.99, 1.30) | 0.85 |
| Model 2 | 1.07 (0.91, 1.26) | 0.91 (0.80, 1.04) | 1.00 | 0.91 (0.80, 1.02) | 0.99 (0.86, 1.14) | 0.48 |
| NHS | ||||||
| Median change | −0.93 | −0.29 | 0.00 | 0.29 | 0.93 | |
| Cases/person-years | 904/201,659 | 1396/298,611 | 1428/283,095 | 1398/245,361 | 867/150,116 | |
| Model 1 | 1.01 (0.93, 1.10) | 1.00 (0.92, 1.07) | 1.00 | 1.11 (1.03, 1.19) | 1.12 (1.03, 1.22) | 0.003 |
| Model 2 | 0.88 (0.79, 0.97) | 0.91 (0.84, 0.99) | 1.00 | 1.03 (0.96, 1.11) | 0.99 (0.91, 1.08) | 0.008 |
| NHS II | ||||||
| Median change | −0.94 | −0.29 | 0.00 | 0.29 | 1.00 | |
| Cases/person-years | 630/198,992 | 780/264,081 | 752/236,377 | 767/220,593 | 684/179,546 | |
| Model 1 | 1.13 (1.02, 1.26) | 1.03 (0.93, 1.14) | 1.00 | 1.13 (1.02, 1.25) | 1.23 (1.11, 1.37) | 0.04 |
| Model 2 | 1.14 (1.00, 1.30) | 1.01 (0.91, 1.13) | 1.00 | 1.05 (0.95, 1.16) | 1.09 (0.98, 1.22) | 0.82 |
| Pooled4 | ||||||
| Model 2 | 0.99 (0.92, 1.06) | 0.94 (0.89, 1.00) | 1.00 | 1.01 (0.96, 1.07) | 1.02 (0.96, 1.09) | 0.09 |
| P for heterogeneity | 0.006 | 0.27 | — | 0.14 | 0.33 | 0.09 |
1Cox proportional hazards regression models were adjusted as follows. Model 1: adjusted for age and stratified by calendar year in 4-y intervals. Model 2: model 1 + race (Caucasian, non-Caucasian), family history of diabetes, updated history of hypercholesterolemia and high blood pressure, initial and change in smoking status (never to never, never to current, past to past, current to past, current to current, missing indicator), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator, in the NHS and NHS II), oral contraceptive use (never, current, past, missing indicator, in the NHS II), initial and change in physical activity level (metabolic equivalents of task per week, quintiles), initial BMI (in kg/m2) (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, >32.0), initial and changes in energy and alcohol intakes (quintiles), initial and change in Alternative Healthy Eating Index score (calculated without the alcohol component, quintiles), and initial total dairy product intake or initial intake of low-fat and high-fat dairy products (depending on the model, quintiles). Analyses on changes in low-fat and high-fat dairy product consumption were mutually adjusted for changes in high-fat or low-fat dairy product intake. Results of the 3 cohorts were pooled using an inverse variance–weighted, fixed-effect meta-analysis. P for heterogeneity was assessed using the Q statistic. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
2Total dairy P value for sex interaction = 0.34.
3Low-fat dairy P value for sex interaction = 0.04.
4High-fat dairy P value for sex interaction = 0.18.
Decreasing reduced-fat milk consumption by >0.50 serving/d was associated with a marginally significant 7% (95% CI: 0%, 13%) higher risk of T2D (Table 3). Increasing reduced-fat milk was not differentially associated with T2D risk compared with maintaining a stable consumption. The association of changes in reduced-fat milk consumption with T2D risk was marked by heterogeneity among the 3 cohorts (P for heterogeneity for trend analysis = 0.04) and a significant sex interaction was found (P = 0.0007). To that extent, the prevalence of hypercholesterolemia and high blood pressure according to changes in reduced-fat milk intake differed between the 3 cohorts. Compared with individuals who maintained a stable consumption, the hypercholesterolemia rate was higher among individuals who increased their consumption by >0.50 serving/d in the HPFS (31.1% compared with 27.7%) and the NHS (42.9% compared with 39.1%), but not in the NHS II (20.8% compared with 20.6%). High blood pressure prevalence was also higher among the same individuals in the HPFS (24.3% compared with 22.7%), but not in the NHS (31.8% compared with 31.3%) or the NHS II (8.5% compared with 9.1%). Changes in intakes of whole milk were not associated with T2D risk (Supplemental Table 2).
TABLE 3.
HRs (95% CIs) for type 2 diabetes according to categories of updated 4-y changes in individual dairy product consumption1
| Changes in dairy product consumption frequency | ||||||
|---|---|---|---|---|---|---|
| Decrease | No change or relatively stable | Increase | P values for trend | |||
| Reduced-fat milk (0–2%) | >0.50 serving/d | >0.07 to 0.50 serving/d | ±0.07 serving/d | >0.07 to 0.50 serving/d | >0.50 serving/d | |
| HPFS | ||||||
| Median change | −1.00 | −0.29 | 0.00 | 0.29 | 1.00 | |
| Cases/person-years | 287/66,644 | 343/65,316 | 996/236,750 | 338/67,697 | 336/68,374 | |
| Model 1 | 1.01 (0.89, 1.15) | 1.22 (1.08, 1.38) | 1.00 | 1.18 (1.04, 1.34) | 1.17 (1.04, 1.33) | 0.09 |
| Model 2 | 0.97 (0.84, 1.12) | 1.23 (1.08, 1.40) | 1.00 | 1.16 (1.02, 1.31) | 1.10 (0.97, 1.25) | 0.30 |
| NHS | ||||||
| Median change | −1.00 | −0.29 | 0.00 | 0.29 | 1.00 | |
| Cases/person-years | 1067/178,944 | 740/148,073 | 2536/517,261 | 761/151,760 | 889/182,804 | |
| Model 1 | 1.17 (1.09, 1.26) | 0.98 (0.90, 1.06) | 1.00 | 1.02 (0.94, 1.10) | 1.00 (0.93, 1.08) | 0.001 |
| Model 2 | 1.10 (1.01, 1.19) | 0.94 (0.87, 1.03) | 1.00 | 0.98 (0.90, 1.06) | 0.91 (0.84, 0.99) | 0.001 |
| NHS II | ||||||
| Median change | −1.07 | −0.33 | 0.00 | 0.29 | 1.25 | |
| Cases/person-years | 777/257,149 | 558/164,838 | 1141/336,767 | 484/135,955 | 653/204,880 | |
| Model 1 | 0.98 (0.89, 1.08) | 1.00 (0.90, 1.11) | 1.00 | 1.01 (0.90, 1.12) | 1.00 (0.90, 1.11) | 0.79 |
| Model 2 | 1.07 (0.95, 1.20) | 1.01 (0.90, 1.13) | 1.00 | 0.98 (0.88, 1.10) | 0.98 (0.88, 1.09) | 0.21 |
| Pooled2 | ||||||
| Model 2 | 1.07 (1.00, 1.13) | 1.02 (0.96, 1.08) | 1.00 | 1.02 (0.96, 1.08) | 0.97 (0.91, 1.02) | 0.01 |
| P for heterogeneity | 0.35 | 0.005 | — | 0.07 | 0.04 | 0.04 |
| Cheese | >0.50 serving/d | >0.07 to 0.50 serving/d | ±0.07 serving/d | >0.07 to 0.50 serving/d | >0.50 serving/d | |
| HPFS | ||||||
| Median change | −0.79 | −0.29 | 0.00 | 0.29 | 0.72 | |
| Cases/person-years | 215/41,566 | 514/123,189 | 746/175,732 | 588/123,236 | 237/41,059 | |
| Model 1 | 1.28 (1.10, 1.49) | 1.02 (0.91, 1.14) | 1.00 | 1.11 (0.99, 1.23) | 1.33 (1.14, 1.53) | 0.30 |
| Model 2 | 1.13 (0.94, 1.36) | 0.98 (0.86, 1.11) | 1.00 | 1.04 (0.93, 1.16) | 1.14 (0.98, 1.32) | 0.38 |
| NHS | ||||||
| Median change | −0.79 | −0.29 | 0.00 | 0.29 | 0.79 | |
| Cases/person-years | 509/115,369 | 1556/308,605 | 1885/371,948 | 1462/281,737 | 581/101,183 | |
| Model 1 | 0.94 (0.85, 1.04) | 1.02 (0.95, 1.09) | 1.00 | 1.01 (0.94, 1.08) | 1.09 (1.00, 1.20) | 0.05 |
| Model 2 | 0.89 (0.79, 1.00) | 0.96 (0.89, 1.03) | 1.00 | 0.96 (0.90, 1.03) | 1.03 (0.93, 1.13) | 0.11 |
| NHS II | ||||||
| Median change | −0.79 | −0.29 | 0.00 | 0.29 | 0.79 | |
| Cases/person-years | 360/107,041 | 802/274,355 | 1038/325,496 | 922/276,632 | 491/116,064 | |
| Model 1 | 1.08 (0.96, 1.22) | 0.95 (0.86, 1.04) | 1.00 | 1.04 (0.95, 1.13) | 1.27 (1.14, 1.41) | 0.001 |
| Model 2 | 1.00 (0.86, 1.16) | 0.95 (0.86, 1.05) | 1.00 | 1.02 (0.93, 1.12) | 1.16 (1.04, 1.29) | 0.006 |
| Pooled3 | ||||||
| Model 2 | 0.97 (0.89, 1.05) | 0.96 (0.91, 1.01) | 1.00 | 1.00 (0.95, 1.05) | 1.09 (1.02, 1.16) | 0.002 |
| P for heterogeneity | 0.09 | 0.93 | — | 0.40 | 0.21 | 0.52 |
| Yogurt | >0.50 serving/d | >0.07 to 0.50 serving/d | ±0.07 serving/d | >0.07 to 0.50 serving/d | >0.50 serving/d | |
| HPFS | ||||||
| Median change | −0.79 | −0.28 | 0.00 | 0.27 | 0.79 | |
| Cases/person-years | 38/9394 | 196/44,806 | 1799/385,943 | 206/52,476 | 61/12,162 | |
| Model 1 | 0.88 (0.64, 1.22) | 0.94 (0.81, 1.10) | 1.00 | 0.82 (0.71, 0.95) | 1.04 (0.80, 1.34) | 0.80 |
| Model 2 | 1.00 (0.72, 1.41) | 1.05 (0.88, 1.25) | 1.00 | 0.87 (0.75, 1.00) | 1.10 (0.85, 1.42) | 0.50 |
| NHS | ||||||
| Median change | −0.86 | −0.29 | 0.00 | 0.29 | 0.86 | |
| Cases/person-years | 351/68,409 | 762/158,475 | 3505/682,269 | 919/185,517 | 456/84,172 | |
| Model 1 | 0.91 (0.81, 1.02) | 0.92 (0.85, 0.99) | 1.00 | 0.91 (0.84, 0.98) | 0.86 (0.77, 0.95) | 0.24 |
| Model 2 | 1.04 (0.90, 1.19) | 1.04 (0.94, 1.14) | 1.00 | 0.96 (0.89, 1.03) | 0.86 (0.78, 0.95) | 0.002 |
| NHS II | ||||||
| Median change | −0.74 | −0.29 | 0.00 | 0.29 | 0.79 | |
| Cases/person-years | 165/45,690 | 483/155,037 | 2110/626,550 | 639/204,215 | 216/68,096 | |
| Model 1 | 0.95 (0.81, 1.12) | 0.85 (0.77, 0.94) | 1.00 | 0.86 (0.79, 0.94) | 0.83 (0.72, 0.95) | 0.14 |
| Model 2 | 1.20 (1.00, 1.44) | 1.05 (0.93, 1.19) | 1.00 | 0.95 (0.87, 1.04) | 0.89 (0.77, 1.02) | 0.003 |
| Pooled4 | ||||||
| Model 2 | 1.09 (0.98, 1.21) | 1.04 (0.97, 1.12) | 1.00 | 0.94 (0.89, 0.99) | 0.89 (0.82, 0.96) | <0.0001 |
| P for heterogeneity | 0.40 | 0.98 | — | 0.49 | 0.23 | 0.64 |
1Cox proportional hazards regression models were adjusted as follows. Model 1: adjusted for age and stratified by calendar year in 4-y intervals. Model 2: model 1 + race (Caucasian, non-Caucasian), family history of diabetes, updated history of hypercholesterolemia and high blood pressure, initial and change in smoking status (never to never, never to current, past to past, current to past, current to current, missing indicator), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator, in the NHS and NHS II), oral contraceptive use (never, current, past, missing indicator, in the NHS II), initial BMI (in kg/m2) (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, >32.0), initial and change in physical activity level (metabolic equivalents of task per week, quintiles), initial and changes in energy and alcohol intakes (quintiles), initial and change in Alternative Healthy Eating Index score (calculated without the alcohol component, quintiles), initial intake of model-specific dairy product (quintiles or tertiles), and initial and change in other dairy product intakes (quintiles). Results of the 3 cohorts were pooled using an inverse variance–weighted, fixed-effect meta-analysis. P for heterogeneity was assessed using the Q statistic. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
2Reduced-fat milk P value for sex interaction = 0.0007.
3Cheese P value for sex interaction = 0.26.
4Yogurt P value for sex interaction = 0.29.
Decreasing total cheese consumption was not associated with T2D risk, but increasing total cheese consumption by >0.50 serving/d was associated with a 9% (95% CI: 2%, 16%) higher risk than maintaining a stable consumption (Table 3). Similar associations with T2D risk were observed when changes in low-fat cheese and high-fat cheese consumption were analyzed separately, with the exception that decreasing high-fat cheese consumption was associated with a marginally significant 6–8% lower risk of T2D (Supplemental Table 3). Compared with maintaining a stable yogurt consumption, decreasing yogurt intake was not differentially associated with T2D risk (Table 3). However, participants who increased their daily yogurt intake by >0.50 serving/d had an 11% (95% CI: 4%, 18%) lower risk of T2D than those who maintained a stable consumption. Changes in intakes of cream and sherbet were not associated with T2D risk (Supplemental Table 2). Participants who decreased their ice cream consumption had an 8% (95% CI: 1%, 16%) higher risk of T2D, but those who increased their consumption had a similar T2D risk to those who maintained a stable consumption (Supplemental Table 2). In all “change-to-risk” analyses, further adjustment for concurrent 4-y change in body weight had little or no effect on the HRs and did not change the significance of the results (Supplemental Table 4).
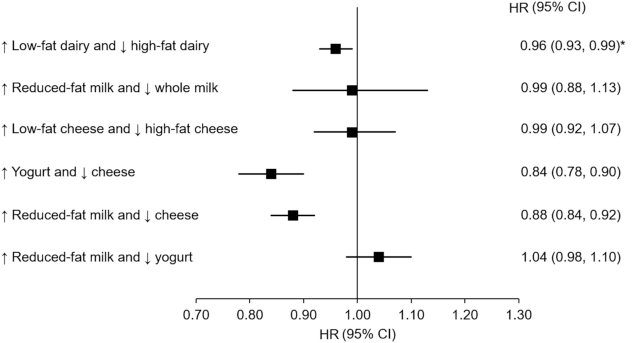
Figure 1 presents pooled HRs from substitution models for T2D associated with increasing intake of a specific dairy product and concomitantly decreasing intake of another dairy product by 1 serving/d. We estimated that increasing overall low-fat dairy consumption while decreasing high-fat dairy consumption was associated with a 4% (95% CI: 1%, 7%) lower risk of T2D. However, substituting reduced-fat milk for whole milk or low-fat cheese for high-fat cheese was not associated with subsequent T2D risk. Increasing intake of yogurt and concomitantly decreasing cheese intake by 1 serving/d was associated with a 16% (95% CI: 10%, 22%) lower risk of T2D, whereas substituting 1 serving/d of reduced-fat milk for cheese was associated with a 12% (95% CI: 8%, 16%) lower risk.
FIGURE 1.
HRs (95% CIs) from substitution models for type 2 diabetes associated with increasing intake of a specific dairy product by 1 serving/d and concomitantly decreasing intake of another dairy product by 1 serving/d during a 4-y period. Pooled results from the Health Professionals Follow-Up Study (n = 34,224), the NHS (n = 76,531), and the NHS II (n = 81,597). Cox proportional hazards regression models were stratified by calendar year in 4-y intervals and adjusted for age, race (Caucasian, non-Caucasian), family history of diabetes, history of hypertension and hypercholesterolemia, initial and change in smoking status (never to never, never to current, past to past, current to past, current to current, missing indicator), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator), oral contraceptive use (never, current, past, missing indicator), initial BMI (in kg/m2) (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, >32.0), initial and change in physical activity level (metabolic equivalents of task per week, quintiles), initial energy intake (quintiles), initial and change in alcohol intake (quintiles), and initial and change in Alternative Healthy Eating Index score (calculated without the alcohol component, quintiles). Initial and changes (both continuous) in intakes of dairy products were included in the models. HRs were calculated using the difference in β coefficients of changes in intakes of dairy products. Results of the 3 cohorts were pooled using an inverse variance–weighted, fixed-effect meta-analysis. *P-heterogeneity < 0.05. NHS, Nurses’ Health Study.
Discussion
In these 3 large cohort studies of US individuals, we observed that increasing yogurt consumption by >0.50 serving/d was associated with an 11% lower risk of T2D, and increasing cheese consumption by >0.50 serving/d was associated with a 9% higher risk, compared with maintaining a stable consumption of these foods. We estimated that substituting 1 serving/d of yogurt or reduced-fat milk for cheese was associated with a 16% or 12% lower risk of T2D, respectively. Our study suggests that increasing yogurt consumption to the detriment of cheese consumption is favorably associated with T2D risk among US women and men.
To a large extent, the higher risk of T2D associated with decreasing total dairy and low-fat dairy intakes, and the lack of association between changes in total high-fat dairy product consumption and T2D risk are both consistent with previous studies. Indeed, most meta-analyses of prospective cohort studies reported that total and low-fat dairy product consumption are associated with a slightly lower risk of T2D, and that high-fat dairy product intakes are not significantly associated with T2D risk (13, 34).
Mendelian randomization studies on genetically predicted milk consumption and most meta-analyses of prospective cohort studies reported that intakes of milk, independent of milk-fat content, are not associated with the risk of T2D (13, 35–37). Accordingly, changes in consumption of milk were not appreciably associated with T2D risk in the current analysis. Although we observed that decreasing reduced-fat milk by >0.50 serving/d was associated with a marginally higher risk, the heterogeneity and the sex interaction in the overall association of changes in reduced-fat milk intake with T2D risk raise uncertainty on the true direction of the association, if there is any (34). The heterogeneity and the sex difference observed in this analysis may be due to the large number of cases in each cohort. Reverse causation is another possibility. Indeed, relative to individuals who maintained a stable consumption, individuals who increased their reduced-fat milk intake appeared to be at higher risk of T2D (higher prevalence of hypercholesterolemia and high blood pressure) in the HPFS, but not in the NHS and the NHS II (34). Still, it is unclear why reduced-fat milk consumption changed in opposite directions among individuals at higher T2D risk across the 3 cohorts. Regarding whole milk, consumption is very low in our cohorts and only a small proportion of individuals reported marked changes in consumption. Hence, we cannot exclude the possibility that the neutral association we observed between changes in whole milk intakes and T2D risk resulted from a lack of statistical power.
Compared with milk, the consumption of yogurt and cheese, both fermented dairy products, may provide further effects on cardiometabolic health, because of their probiotic lactic acid bacteria content (38, 39). All meta-analyses of prospective cohort studies on yogurt consumption and diabetes risk published to date reported a protective association (11, 13). Accordingly, we observed that increasing yogurt consumption was associated with a lower subsequent risk of T2D. For cheese, we observed that increasing consumption was associated with a higher T2D risk. On the one hand, this result is consistent with previous data from the HPFS, NHS, and NHS II, where a modest positive association between long-term cheese consumption and diabetes risk was reported (34). On the other hand, it contrasts with data from meta-analyses of prospective cohort studies associating cheese consumption with a lower or unchanged risk of diabetes mellitus (13, 34). One potential explanation for this discrepancy may be related to cheese consumption habits. In the US population, cheese is most often consumed as an ingredient in mixed dishes (e.g., pizza, hamburgers, sandwiches), which may influence the association between cheese consumption and T2D risk, especially because these are food sources high in refined carbohydrates (10). Thus, residual confounding may be of concern, although we carefully adjusted for initial and changes in diet quality.
Dairy fat contains several different fatty acids that may exert opposite effects on insulin sensitivity and T2D risk (6, 10). To date, it remains debated whether the consumption of dairy fat is detrimental for cardiometabolic health, and whether the consumption of low-fat dairy products provides advantages with regard to T2D risk relative to high-fat dairy products (13, 40). We estimated that shifting overall consumption from high-fat to low-fat dairy products was associated with a slightly lower risk of T2D. This observation was further supported by the lower risk of T2D associated with replacing dairy products with high fat content, like cheese, with dairy products with lower fat content, like yogurt or reduced-fat milk. On the other hand, we also estimated that substituting reduced-fat milk for whole milk or low-fat cheese for high-fat cheese was not associated with a lower risk of T2D. Still, the low consumption of whole milk is likely to have limited the statistical power of the milk substitution analysis. Also, it is plausible that the difference in fat content between reduced-fat milk and whole milk may be too small to affect the risk. The same may apply for cheese because the fat content of most low-fat cheeses remains relatively high, and low-fat cheese consumption may provide high amounts of dairy fat. Also, because of the challenges associated with reporting cheese intakes from mixed dishes and other sources of measurement errors, our ability to estimate the effect of substituting low-fat for high-fat cheeses was considerably limited. The foregoing would explain why we observed a reduced risk only when we estimated the effect of substituting composites of low-fat and high-fat dairy products. Overall, our results suggest that replacing dairy products with high fat content, like cheese, with dairy products with lower fat content, like yogurt or reduced-fat milk, is associated with a lower risk of T2D. Thus, our observations do not support that high-fat dairy products are superior to low-fat dairy products with regard to diabetes risk, albeit that previous studies suggested the latter (9, 41).
The strengths of our study include the study design, the large sample size, and the high follow-up rate. Indeed, the HPFS, the NHS, and the NHS II are among the few studies with long-term repeated assessments of diet. This allowed us to design the current study in order to simulate a large nutritional intervention in which individuals would modify their consumption of dairy products in real-world settings (30, 42). Moreover, the similar design of the 3 cohorts allowed us to increase the statistical power of the study by combining results. On the other hand, the study population is mainly composed of Caucasian educated health professionals, which may limit the generalizability of our results. Also, measurement errors in dairy consumption are expected. The latter particularly applies to cheese because it is often consumed in mixed meals and prepared food. Finally, because we do not know why and when the changes occurred within the 4-y periods, the presence of unmeasured confounding from other lifestyle factors might have led to some of the between-cohort discrepancies observed in our study, even though we carefully accounted for initial and changes in diet and lifestyle.
In conclusion, this study suggests that increasing yogurt consumption is associated with a lower risk of T2D, whereas increasing cheese consumption is associated with a higher risk among US women and men. Our analysis also suggests that substituting yogurt or reduced-fat milk for cheese is associated with a lower risk of T2D.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—J-PD-C and FBH: designed the research; J-PD-C: performed the statistical analyses and drafted the manuscript; YL and AVAK: contributed to the statistical analyses; J-PD-C and FBH: share the responsibility for the integrity of the data and the accuracy of the analysis; and all authors: read, edited, and approved the final manuscript. J-PD-C received speaker and consulting honoraria from the Dairy Farmers of Canada in 2016 and 2018, outside the current work. BL has received investigator-initiated and peer-reviewed research funding from the Dairy Farmers of Canada and National Dairy Council for research outside of the current work over the last 5 y. BL has also received speaker honoraria from the Dairy Farmers of Canada over the same period. None of the other authors reported a conflict of interest related to the study.
Notes
Supported by NIH research grants UM1 CA186107, UM1 CA176726, UM1 CA167552, and DK112940; Canadian Institutes of Health Research Banting Postdoctoral Fellowship BPF-156628 (to J-PD-C); and NIH training grant T32CA009001 (to AVAK).
The funders had no role to play in the design of the study and in the interpretation of the results.
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will not be made publicly available. Further information including the procedures to obtain and access data from the Nurses’ Health Studies and Health Professionals’ Follow-up Study is described at http://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu) and http://sites.sph.harvard.edu/hpfs/for-collaborators.
Abbreviations used: AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
References
- 1. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jakubowicz D, Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J Nutr Biochem. 2013;24(1):1–5. [DOI] [PubMed] [Google Scholar]
- 3. Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Guerrero-Romero F. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res. 2016;111:272–82. [DOI] [PubMed] [Google Scholar]
- 4. Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2(7):687–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez MA, Marette A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr Rev. 2018;76(Supplement_1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122(2):369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tremblay BL, Rudkowska I. Nutrigenomic point of view on effects and mechanisms of action of ruminant trans fatty acids on insulin resistance and type 2 diabetes. Nutr Rev. 2017;75(3):214–23. [DOI] [PubMed] [Google Scholar]
- 9. Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Wong K et al.. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15(10):e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu E, Hu FB. Dairy products, dairy fatty acids, and the prevention of cardiometabolic disease: a review of recent evidence. Curr Atheroscler Rep. 2018;20(5):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soedamah-Muthu SS, de Goede J. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analyses of prospective cohort studies. Curr Nutr Rep. 2018;7(4):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose–response meta-analysis of observational studies. Am J Clin Nutr. 2016;103(4):1111–24. [DOI] [PubMed] [Google Scholar]
- 13. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté M-È, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Li S. Worldwide trends in dairy production and consumption and calcium intake: is promoting consumption of dairy products a sustainable solution for inadequate calcium intake?. Food Nutr Bull. 2008;29(3):172–85. [DOI] [PubMed] [Google Scholar]
- 15. Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 17. Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–99. [DOI] [PubMed] [Google Scholar]
- 19. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 20. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 21. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 22. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 23. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57. [DOI] [PubMed] [Google Scholar]
- 24. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. [DOI] [PubMed] [Google Scholar]
- 25. Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–8. [DOI] [PubMed] [Google Scholar]
- 26. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–8. [DOI] [PubMed] [Google Scholar]
- 27. Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–6. [DOI] [PubMed] [Google Scholar]
- 28. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ley SH, Pan A, Li Y, Manson JE, Willett WC, Sun Q, Hu FB. Changes in overall diet quality and subsequent type 2 diabetes risk: three U.S. prospective cohorts. Diabetes Care. 2016;39(11):2011–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med. 2013;173(14):1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwingshackl L, Hoffmann G, Schwedhelm C, Kalle-Uhlmann T, Missbach B, Knuppel S, Boeing H. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: a systematic review and meta-analysis of cohort studies. PLoS One. 2016;11(6):e0157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond). 2013;37(10):1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 34. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Q, Lin SL, Au Yeung SL, Kwok MK, Xu L, Leung GM, Schooling CM. Genetically predicted milk consumption and bone health, ischemic heart disease and type 2 diabetes: a Mendelian randomization study. Eur J Clin Nutr. 2017;71(8):1008–12. [DOI] [PubMed] [Google Scholar]
- 36. Bergholdt HK, Nordestgaard BG, Ellervik C. Milk intake is not associated with low risk of diabetes or overweight-obesity: a Mendelian randomization study in 97,811 Danish individuals. Am J Clin Nutr. 2015;102(2):487–96. [DOI] [PubMed] [Google Scholar]
- 37. Vissers LET, Sluijs I, van der Schouw YT, Forouhi NG, Imamura F, Burgess S, Barricarte A, Boeing H, Bonet C, Chirlaque MD et al.. Dairy product intake and risk of type 2 diabetes in EPIC-InterAct: a Mendelian randomization study. Diabetes Care. 2019;42(4):568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thorning TK, Bertram HC, Bonjour JP, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC et al.. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105(5):1033–45. [DOI] [PubMed] [Google Scholar]
- 39. Fernandez MA, Panahi S, Daniel N, Tremblay A, Marette A. Yogurt and cardiometabolic diseases: a critical review of potential mechanisms. Adv Nutr. 2017;8(6):812–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drouin-Chartier J-P, Côté JA, Labonté M-È, Brassard D, Tessier-Grenier M, Desroches S, Couture P, Lamarche B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7(6):1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ericson U, Hellstrand S, Brunkwall L, Schulz CA, Sonestedt E, Wallstrom P, Gullberg B, Wirfalt E, Orho-Melander M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr. 2015;101(5):1065–80. [DOI] [PubMed] [Google Scholar]
- 42. Satija A, Stampfer MJ, Rimm EB, Willett W, Hu FB. Perspective: are large, simple trials the solution for nutrition research?. Adv Nutr. 2018;9(4):378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.