Abstract
Few studies have assessed the accuracy of the FreeStyle Libre Pro (FLP) continuous glucose monitor for estimating plasma glucose (PG) in non-diabetic children.
Objective
Determine the accuracy of FLP compared to PG during OGTT in healthy children.
Subjects
Children (7–11.99y) with healthy weight & overweight/obesity (n=33; 52% male).
Methods
Participants wore the FLP before and during a 2-hour OGTT; PG was measured at 30min intervals. Potential systematic- and magnitude-related biases for FLP vs. PG were examined.
Results
FLP 15-minute averages and PG were correlated at most timepoints during OGTT (r2=.35-.69, p’s<0.001 for time point 30–120minutes) and for PG area under the curve (AUC) (r2=0.65, p<0.0001). There were no systematic biases as assessed by Bland-Altman analyses for FLP AUC or for FLP at each OGTT timepoint. However, for fasting glucose, a significant magnitude bias was noted (r2=0.38, p<0.001), such that lower PG was underestimated, and higher PG was overestimated by FLP readings; further, there was poor correlation between fasting PG and FLP (r2=0.06, p=0.22). BMIz was also associated with FLP accuracy: FLP overestimated PG in children with low BMIz and underestimated PG in those with overweight/obesity for OGTT AUC and OGTT PG at baseline, 60, and 120 minutes (all p’s≤0.015). No adverse events occurred with FLP.
Conclusions
Among children without diabetes, the FLP was well tolerated and correlated with post-OGTT glucose, but had magnitude bias affecting fasting glucose and appeared to underestimate plasma glucose in those with overweight/obesity. These results suggest potential limitations for the utility of the FLP for research.
Keywords: Blood Glucose Self-Monitoring, Biosensing Techniques, Glucose Tolerance Test, Body Mass Index, Child
Introduction
Continuous glucose monitor (CGM) devices are valuable in clinical and research settings because CGMs can provide non-invasive high-frequency, adjunctive data.1 CGMs were designed to improve clinical care by providing more frequent glucose measurements than available through conventional testing, by using a sensor inserted into the subcutaneous interstitial space.2,3 Many studies have shown that CGM use can improve the clinical outcomes of children and adults with diabetes.2,4–11 In individuals without diabetes, CGMs could help determine glycemic ranges in specific demographic samples and evaluate changes in glucose tolerance over time.12–16 In most cases, patients with diabetes have found these devices easy to use, relatively painless, and preferential to traditional finger-prick methods of measuring glucose.8,10,17,18 However, some published literature has offered data suggesting that CGM accuracy, relative to venous measurements, may need to be adjusted for time lags and may be affected by meal macronutrient composition.19–22 As new CGMs become increasingly available, it remains important to study each device to understand accuracies and limitations, and to define their scope of use.
Masked glucose monitoring systems are a type of CGM designed to provide patients and care professionals with retrospective data for glycemic patterns that may affect long-term behavior and clinical decisions in patients with diabetes.5,11,23 The FreeStyle Libre Pro (FLP) is a masked glucose monitoring system that can provide information regarding glucose concentration trends and patterns for up to fourteen days.11,23 Unlike early CGM devices, the FLP is factory-calibrated and does not require, or allow, finger-stick calibration by the user at any time.8,11,23 One published report found a mean absolute relative difference of the FLP system and BG reference of only 11.4%.22 Studies have also provided evidence suggesting that the FLP system provides acceptable glucose readings as compared to traditional glucose monitoring methods in samples of children and adults with diabetes.8,9,17,23–25
Limited data are available to assess the accuracy and feasibility for use of the FLP CGM in children without diabetes. If found to measure glucose acceptably, the FLP might be useful in healthy weight and overweight children to describe physiologic profiles and be beneficial for researchers seeking glucose measurements for clinical investigation. We sought to evaluate the accuracy of the FLP in healthy, euglycemic children of all weight strata and determine acceptability and executability of use in such children as a means of obtaining clinical research data.
Materials and Methods
Study Participants
We conducted a secondary data analysis in a convenience sample of pre- and early-pubertal children aged 7–11.99 years old who were of all weight strata (non-overweight: BMI 5th to <85th percentile for age and sex;26 overweight: BMI 85th to <95th percentile; obese: BMI ≥95th percentile), who had agreed to participate in a clinical trial () that was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. All participants and their guardians gave their informed assent/consent prior to their inclusion in the study. Potential participants were excluded if they had significant medical conditions, were prescribed medications that might affect CGM function, glucose homeostasis, or body composition, or were a post-menarchal female. For the current study, only results from participants who were in the control (non-intervention) group were examined.
Study Design
As part of the protocol, a randomized, parallel clinical trial evaluating the effects of interrupting sedentary behaviors with activity, participants had body composition measured using dual x-ray absorptiometry (Lunar iDXA; GE Healthcare, Madison, WI) and analyzed with GE Encore 15 software. Afterwards, they wore the FreeStyle Libre Pro Flash CGM (Libre Pro, Abbott Laboratories, Lake Bluff, Illinois) for 6 consecutive days, placed on their upper arm per factory instructions. During this time, all energy intake was fixed such that all meals and snacks were prepared by National Institutes of Health (NIH) Clinical Center research dietitians who calculated energy needs using the Institute of Medicine guidelines.27 Food intake was standardized for each participant, such that portions for daily total intake were provided as follows: breakfast 20%, lunch & dinner 30% each, snacks 20% of predicted total daily requirement. Carbohydrates made up 51% of daily participant energy consumption, while fats and proteins made up 37% and 12%, respectively. Each day, the CGM was evaluated for correct placement, skin irritation, functionality, and completeness of data collection. In the case of a lost/prematurely removed device, participants had a replacement device placed. After 6 days, participants took part in a 2-hour oral glucose tolerance test (OGTT; 1.75mg/kg, maximum 75g). Laboratory plasma glucose, collected in tubes containing powdered sodium fluoride that were kept on ice until centrifuged and analyzed, was measured at 30-minute intervals for 120 minutes and compared with the 15-minute average glucose measurements simultaneously obtained from CGM. Baseline CGM values were determined by using the time closest to and prior to the baseline plasma glucose blood draw.
Laboratory Assays
Laboratory plasma glucose was measured by the NIH Clinical Center Laboratory using a Roche instrument (model c502; Roche Diagnostics). For this instrument the analytical sensitivity for glucose was 2 mg/dL, the average intra- and inter-assay coefficients of variation were 0.7% and 1.2% respectively.
Statistical Analyses
Area under the curve (AUC) for each participant’s glucose measurements was calculated via trapezoidal method using GraphPad Prism 6 software (GraphPad Incorporated, San Diego, California). GraphPad Prism 6 software was also used to calculate correlations, paired and unpaired t-tests, regression calculations, and Bland-Altman analyses. SPSS Statistics 25 (IBM Corporation, Armonk, NY) was used for repeated measures analysis of variance (ANOVA) analyses. Glucose at each timepoint and area under the curve (AUC) for CGM and lab-measured glucose were compared using paired t-tests. Repeated measures ANOVA compared log transformed glucose values from each measurement modality across all time points controlling for age (years), sex, and BMIz. To examine potential systematic and magnitude biases, Bland-Altman analyses28 of plasma and CGM glucose were performed for individual time points and for AUC. Regression was performed to determine magnitude biases and paired t-tests were used to determine systematic biases at each time point and for AUC.
Results
Thirty-three participants were potential subjects of this study. Mean was age 9.9±1.4 years, 52% were male and 76% were of healthy weight; mean BMIz score was 0.30±1.05 (Table 1). All thirty-three participants were included in qualitative feasibility and safety analyses. Of the thirty-three potential participants, two participants discontinued protocol participation before they underwent OGTT, four were unable to complete the OGTT due to failed intravenous access or inability to tolerate the glucose solution, and one did not have sufficient time wearing the CGM before the OGTT. Therefore, twenty-six participants were included in the accuracy analyses. There were no significant differences in age, BMIz, or fasting glucose between participants with data for accuracy analyses and those not included (Table 1). All twenty-six of the included participants wore the FLP CGM for six consecutive days prior to OGTT.
Table 1:
Sample characteristics. Of 33 children who were enrolled in the study, 26 had sufficient data to be included in this analysis. Children who were excluded (primarily because of difficulties maintaining intravenous access during the OGTT) did not differ significantly in any sociodemographic characteristic from those who were included.
| Characteristic | Included in analyses | Excluded (missing data) | Total sample |
|---|---|---|---|
| Sample size | 26 | 7 | 33 |
| Race, % | |||
| White | 76 | 57 | 73 |
| African American | 4 | 43 | 12 |
| Multi-racial | 8 | 0 | 6 |
| Asian | 12 | 0 | 9 |
| Ethnicity, % | |||
| Hispanic or Latino | 0 | 14 | 3 |
| Non Hispanic or Latino | 100 | 86 | 97 |
| Sex, % | |||
| Male | 50 | 57 | 52 |
| Female | 50 | 43 | 48 |
| Age, Mean±SD | 10.15±1.12 | 9.00±2.00 | 9.90±1.40 |
| BMI-z Score, Mean±SD | 0.33±1.09 | 0.14±0.99 | 0.30±1.05 |
| Cohort, % | |||
| Non-overweight Weight | 77 (n=20) | 86 (n=6) | 76 (n=26) |
| Overweight or Obesity | 23 (n=6) | 14 (n=1) | 24 (n=7) |
| Fasting Glucose, Mean±SD | 88.1±5.70 | 80.9±9.39 | 86.5±7.13 |
Accuracy
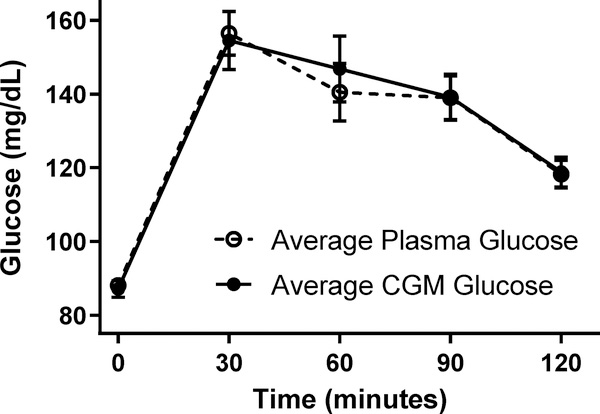
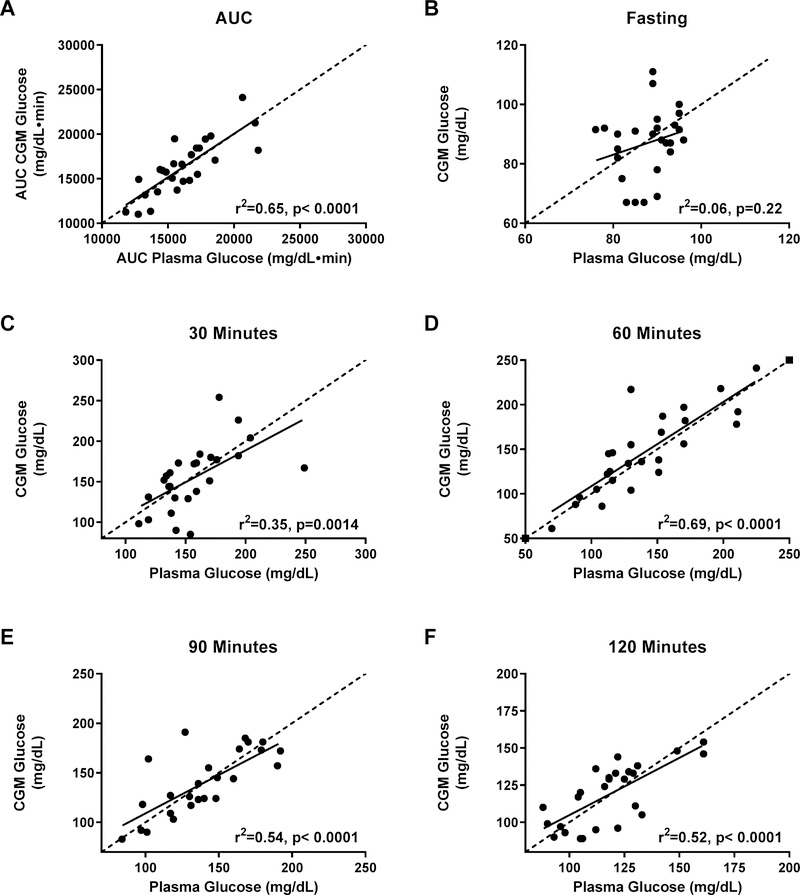
The average plasma and FLP CGM glucose readings were not significantly different at any time point (all p’s>0.212; Figure 1). AUC for plasma glucose was well correlated with AUC for FLP CGM glucose (r2=0.65, p<.0001) (Figure 2A) and glucose measurements were moderately- to well-correlated at times 30, 60, 90, and 120 minutes (all p<0.001, Figures 2C–2F). However, plasma glucose and FLP CGM glucose concentrations were not significantly correlated for fasting glucose (p=0.22, r2=0.06; Figure 2B). Given that the FLP sensor 15-minute averages were imperfectly aligned with blood sampling times, we sought to find the difference in times between the baseline FLP time and the baseline plasma time. We subtracted the time of baseline blood draw from the closest 15-minute FLP interval recorded from the sensor. We determined there was a mean 7.0±0.003 minute difference between the FLP CGM baseline time and the plasma baseline time. Shifting to use the data to the next 15-minute phase interval for the FLP did not improve accuracy analysis results at any timepoint or for AUC.
Figure 1.
Mean plasma glucose and mean CGM glucose concentrations during an oral glucose tolerance test. There were no significant differences between mean plasma and CGM glucose values at any time point (all p’s>0.21).
Figure 2.
Correlations (solid lines) between simultaneously-obtained plasma glucose and CGM glucose during an oral glucose tolerance test. (A) Glucose area under the curve (AUC); (B) fasting glucose; (C) time 30 minutes; (D) time 60 minutes; (E) time 90 minutes; (F) time 120 minutes of the glucose tolerance test. Dotted lines show the line of identity (slope =1, intercept=0).
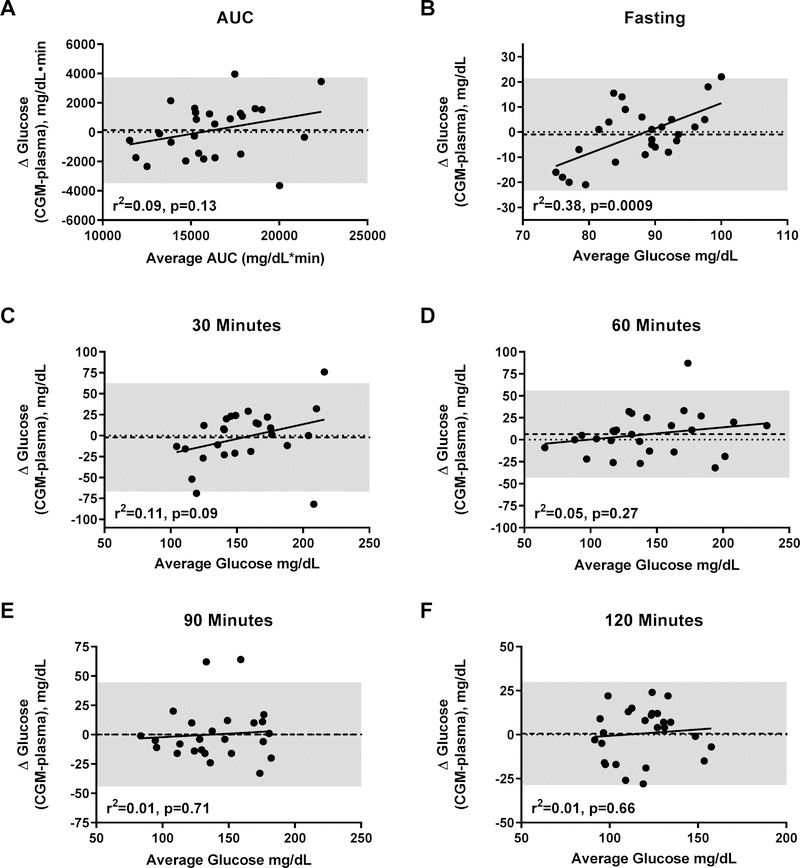
Interestingly, Bland-Altman analyses for fasting glucose (Figure 3B) found a significant magnitude bias (r2=0.038, p=0.0009), such that, within the physiologic range, lower plasma glucose measurements were underestimated, and higher readings overestimated; there was no systematic bias (p=0.671). The limits of agreement (defined as ±2 SD) at baseline were ±22.8 mg/dL. There were no significant systematic or magnitude biases in the estimation of glucose AUC (Figure 3A), or for glucose at any other timepoint (Figures 3C–F). Nevertheless, for all OGTT timepoints, the ±2 SD limits of agreement were wide, ranging from ±29.9 to ±66.0 mg/dL.
Figure 3.
Bland-Altman plots for systematic and magnitude bias in the measurement of glucose by CGM using plasma glucose as criterion method during an oral glucose tolerance test. (A) Area under the curve (AUC); (B); fasting glucose; (C) time 30 minutes; (D); time 60 minutes; (E); time 90 minutes; (F) time 120 minutes of the glucose tolerance test. Shaded area represents ±2 SD of mean difference. Linear regression lines (magnitude biases) are shown for each graph. The heavy dashed lines show mean difference between CGM and plasma glucose readings (systematic biases) and the shaded area indicates the limits of agreement. No significant systematic biases were found (all p’s>0.20).
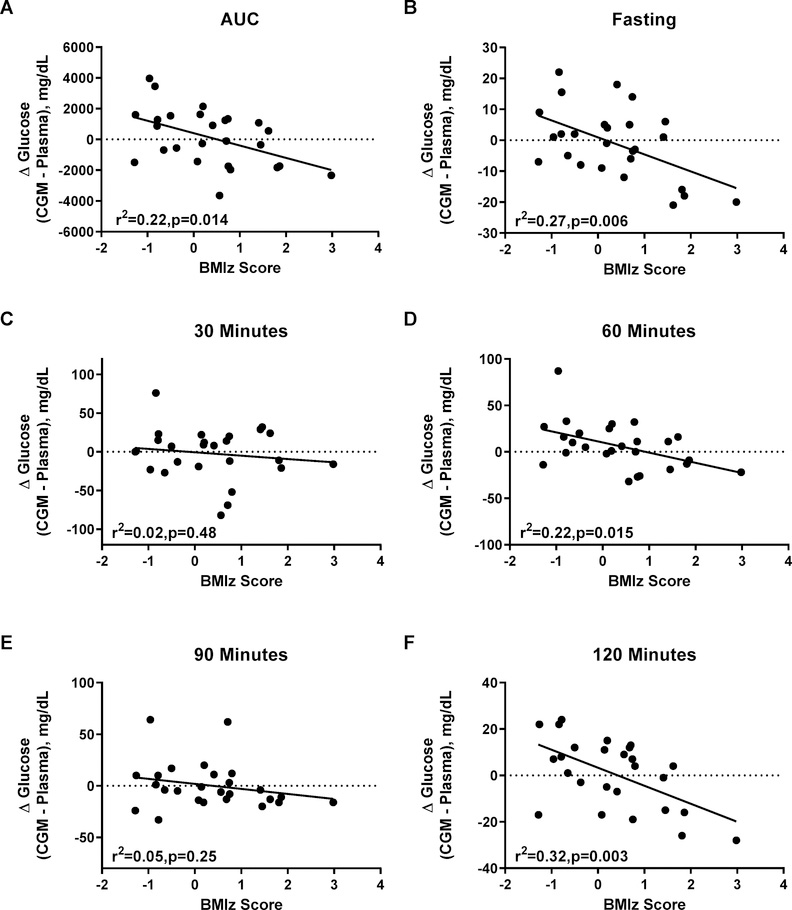
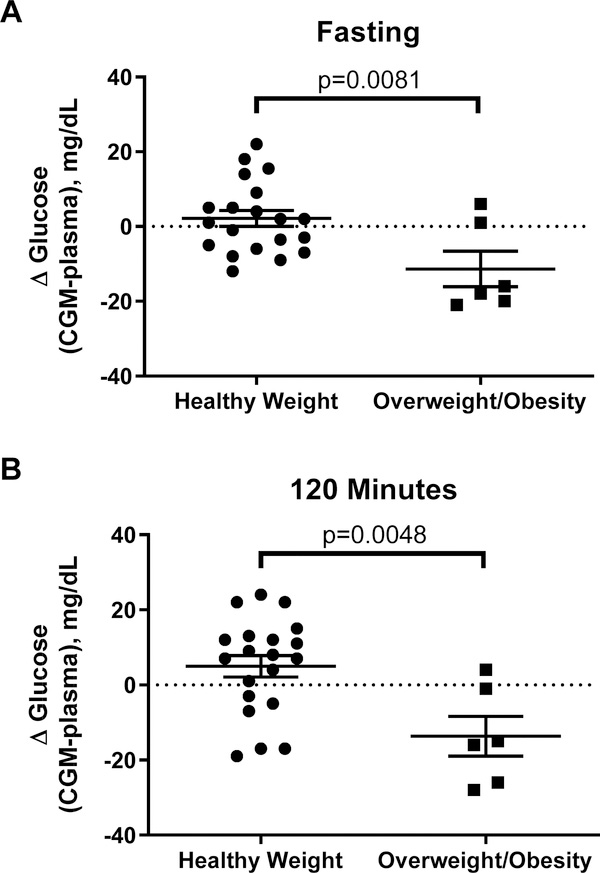
Unexpectedly, BMIz was significantly associated with the difference in glucose measurements by FLP versus the central laboratory at many OGTT time points. Specifically, higher BMIz was associated with significant underestimation of plasma glucose by FLP CGM for OGTT AUC (r2=0.22, p=0.014; Figure 4A), fasting glucose, time 60, and time 120 glucose (all p<0.003, Figures 4B, 4D, and 4F). Similarly, higher total fat mass and body fat percentage was associated with significant underestimation of plasma glucose by the FLP CGM for OGTT AUC, fasting glucose, time 60, and time 120 glucose (Supplemental Figures 1 and 2). Of note, BMIz and Fat Mass associations remained generally consistent after removal of an extreme outlier (Supplemental Figures 1b, 2b, & 3). On average, in the children with overweight/obesity the FLP CGM underestimated fasting glucose by 11.3±4.8 mg/dL (between group difference p=0.008) and underestimated time 120 glucose by13.7±5.3 mg/dL (between group difference p=0.005) (Figure 5A–B).
Figure 4.
Linear regressions between BMIz score and difference between CGM glucose and plasma glucose during an oral glucose tolerance test. (A) Area under the curve (AUC); (B) fasting glucose; (C) time 30 minutes; (D) time 60 minutes; (E) time 90 minutes; (F) time 120 minutes of the glucose tolerance test.
Figure 5.
Differences in glucose as measured by CGM and in plasma in children with healthy weight versus children with overweight/obesity at: (A) baseline; (B) time 120 minutes of oral glucose tolerance test. The CGM significantly underestimated plasma glucose in children with overweight or obesity.
Safety and Feasibility
There were no significant adverse events associated with FLP CGM use. Two of the thirty-three participants experienced early (before 6th day of wear) sensor removal, both of which were attributed to accidental removal due to decreased CGM sensor adhesion. No participants removed the CGM due to pain or complained of significant inconvenience during placement or use.
Discussion
We sought to evaluate the accuracy and feasibility of the FLP glucose monitor in children without diabetes. We examined the accuracy of the FLP CGM in estimating glucose in children with healthy weight, overweight, and obesity using simultaneously-timed serial collections of plasma glucose. Comprehensively, these data identified notable areas of inaccuracy in the FLP CGM device as compared to intravenously obtained plasma glucose in healthy children. Most notably, the FLP CGM was poorly correlated with fasting plasma glucose in children of all weight strata and underestimated fasting and OGTT glucose concentrations in children with elevated BMIz.
Replacing or supplementing self-measured blood glucose with CGM data can be useful in mitigating inaccuracies in self-measured blood glucose due to human error, physiological discrepancies, medication interference, or extreme environmental conditions29–31 and may be deemed more acceptable to patients and research volunteers. If found to read glucose accurately, the FLP system would seem a valuable CGM for pediatric research use due to the extended, 14-day sensor wear time, factory calibration, and usability of the device.2,7,8,10,17,18 When FLP readings are compared to self-measured capillary blood glucose (i.e. results obtained using bedside glucometers) in children with T1DM, the FLP has been reported to provide acceptable glucose measurements, with an increase in accuracy at night.8,17,24,25 Contrary to these findings, the data presented here identified that in fasted states, the FLP CGM glucose readings were not significantly correlated with plasma glucose readings. Furthermore, we found a magnitude bias in the fasting FLP readings such that the CGM overestimated higher plasma glucose concentrations and underestimated lower glucose readings, while all readings remained within the spectrum of the physiologic range. We also found surprisingly wide limits of agreement between plasma glucose and FLP CGM glucose measurements during OGTT, with some measurements differing by as much as 70 mg/dL. At least one additional study in adults, published by Fokkert et al, identified a similar pattern of underestimation of glucose by the FLP CGM when actual glucose is either frankly hypoglycemic or within the lower spectrum of the physiologic fasting state along with overestimation at higher glucose values.32
Prior research has reported short lags in reading times in the FLP CGM, which may be one reason for the inaccuracies that we noted,22,32 however, mathematically adjusting for the lag time seen in the data presented here did not improve accuracy. Another related possible explanation for inconsistencies in glucose readings may stem from the difference between in intravenous glucose from interstitial fluid measurement. Physiologically, in a dynamic glucose state, there may be a delay in equilibrium between the intravenous space and interstitial spaces. This has been described to lead to lower glucose reading when taken from the interstitial fluid rather than the blood plasma.19 However, because we obtained data during fasting, baseline, non-dynamic states, this cannot fully explain the inaccuracies in the FLP compared to plasma glucose values. Nonetheless, the data presented here add to previous data identifying concern for FLP accuracy in the fasting state and at each extreme end of the spectrum of the glucose range. This nuance regarding FLP accuracy may be intrinsic to the technology’s approach or may indicate that the technology’s accuracy depends in part on subject characteristics not accounted for by the FLP. Interestingly, the data here suggest a potential interaction with subject BMI.
We identified an association between body composition and CGM accuracy. On average, the FLP significantly underestimated glucose concentrations in children with higher BMIz. For example, an increase of 1 BMIz unit corresponded with an average FLP glucose measurement 13 mg/dL lower than the simultaneously-obtained plasma glucose value at time 120 minutes. The FLP CGM significantly underestimated glucose by an average of 11–14 mg/dL in children with overweight or obesity, compared to a small mean overestimation of 2–5 mg/dL in the healthy weight volunteers. Increased total fat mass and fat percentage was similarly associated with FLP accuracy, thus suggesting that differences in subcutaneous fat, potentially altering diffusion or utilization rates of glucose, may be one explanation for changes in accuracy of the FLP CGM for those with lower and higher BMIz scores.
The effect of BMIz on FLP accuracy is a finding that has been described with mixed results previously. A few other studies have explored the relationship between FLP sensor accuracy and body weight in children and found no association. One 2017 study by Edge et. al. analyzed FLP CGM accuracy in 89 adolescents with T1DM.8 This study states that although about 16% of CGM values were sufficiently discordant with plasma glucose to elicit clinical concern, FLP accuracy did not change with body weight. However, the study did not provide a weight or BMI distribution for the participants. Given that the cohort analyzed had T1DM, we expect that many of the participants in the Edge et al. study may have been within the healthy weight category and may have had few with obesity. Bailey et. al. reported no significant effect of BMI on FLP accuracy in their 2015 study of an adult cohort and Szadkowska et al. identified no association with BMIz in a small cohort children with Type 1 Diabetes (only 6 of which had overweight or obesity).22,25, However, Massa et al. identified a significant association between FLP accuracy and BMI in youth with Type 1 Diabetes and of healthy weight.24 In this healthy weight cohort, Massa et al. identified overestimation of glucose in individuals with lower BMI (Mean Difference inversely related with r=−0.261, p<0.05).24
If the effect of body composition on sensor accuracy is confirmed in a larger sample, this might be a vital consideration when using FLP CGM in pediatric clinical care or research, as inaccuracies in glucose measurements in children with extreme BMIz scores could significantly alter clinical action. For example, for children with overweight or obesity, an underestimated glucose reading might lead to a missed diagnosis of metabolic impairment and potentially delay treatment. The extremely inaccurate glucose readings from this CGM we observed (errors up to 66mg/dL) might result in non-optimal insulin dosing in children with obesity and diabetes, although errors of 10–15 mg/dL are unlikely to cause serious under- or over-administration of insulin.
In general, the FLP demonstrated acceptable safety in this study, with no reports of CGM-related AEs. FLP also showed usability for 7–11 year old children. In this sample, two children (n=2, 6%) experienced early sensor removal, both of which were accidental removals because of decreased sensor adhesion to the skin.
This analysis is limited given the relatively few OGTT participants with BMIz scores ≥1.0 (n=6, 24%). Greater representation of children with higher BMIz scores is needed to verify the potential effect of BMIz on FLP CGM accuracy. Additionally, other studies have shown increased accuracy with longer FLP sensor wear by measuring glucose accuracy of the FLP sensor at different timespans.22 Although all participants wore FLP CGM for a standardized 6 days, it is possible that accuracy might further improve during the second week of wear. One strength of the study was the inclusion of a wide range of participant BMIz scores, which allowed us to assess how weight may contribute to FLP accuracy. Additionally, use of the OGTT as a means of comparison uniquely captured plasma glucose throughout a broad range of glycemia. Importantly, we collected plasma glucose samples in fluoride-containing tubes and kept samples cold until they were processed (within 30 minutes), thus decreasing inaccuracies due to lag time between drawing the samples and analysis. Finally, the participants all wore the FLP CGM for exactly 6 days, which obviated the influence of variable sensor wear time.
Overall, the general accuracy of FLP CGM for children without diabetes has been demonstrated in this study but with some limitations: 1. magnitude biases in fasting glucose measurements were identified and 2. body composition (high body weight/adiposity) impacted accuracy. These findings suggest that the FLP may provide erroneous glucose measurement in children with overweight or obesity, and may be less accurate in the fasting, euglycemic state regardless of weight status. Thus, the glucose measurements from this device should be used with caution in children.
Supplementary Material
Acknowledgements
This work was supported by grant 1ZIAHD000641 (to JAY) from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development with supplemental funding from The Thrasher Foundation and The Pediatric Endocrine Society (to MMB). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Potential Conflicts of Interest: The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Disclaimer: J.A. Yanovski is a Commissioned Officer in the United States Public Health Service (PHS). The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS.
References
- 1.Coss-Bu JA, Jefferson LS, Stone-McCord S, et al. Evaluation of a real-time blood glucose monitor in children with diabetic ketoacidosis. Diabetes Res Clin Pract 1999;44:175–81. [DOI] [PubMed] [Google Scholar]
- 2.Lal RA, Maahs DM. Clinical Use of Continuous Glucose Monitoring in Pediatrics. Diabetes Technol Ther 2017;19:S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Fox LA, Beck RW, Xing D Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care 2010;33:1297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block JM. Continuous glucose monitoring: changing diabetes behavior in real time and retrospectively. J Diabetes Sci Technol 2008;2:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derosa G, Salvadeo SA, Mereu R, et al. Continuous glucose monitoring system in free-living healthy subjects: results from a pilot study. Diabetes Technol Ther 2009;11:159–69. [DOI] [PubMed] [Google Scholar]
- 7.Dovc K, Bratina N, Battelino T. A new horizon for glucose monitoring. Horm Res Paediatr 2015;83:149–56. [DOI] [PubMed] [Google Scholar]
- 8.Edge J, Acerini C, Campbell F, et al. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Archives of disease in childhood 2017;102:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesavadev J, Shankar A, Ashok AD, et al. Our First 825 T2DM Patients on 14-Day Factory-Calibrated Glucose Monitoring System: Clinical Utility and Challenges. J Diabetes Sci Technol 2018;12:230–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laffel L Improved Accuracy of Continuous Glucose Monitoring Systems in Pediatric Patients with Diabetes Mellitus: Results from Two Studies. Diabetes Technol Ther 2016;18 Suppl 2:S223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palylyk-Colwell E, Ford C. Flash Glucose Monitoring System for Diabetes. CADTH Issues in Emerging Health Technologies; Ottawa (ON)2016:1–13. [PubMed] [Google Scholar]
- 12.Mauras N, Beck RW, Ruedy KJ, et al. Lack of accuracy of continuous glucose sensors in healthy, nondiabetic children: results of the Diabetes Research in Children Network (DirecNet) accuracy study. J Pediatr 2004;144:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazze RS, Strock E, Wesley D, et al. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther 2008;10:149–59. [DOI] [PubMed] [Google Scholar]
- 14.Okada J, Yamada E, Saito T, Okada S, Yamada M. Variation of measured values associated with different lots of sensors for the FreeStyle Libre. J Diabetes 2018;10:986–7. [DOI] [PubMed] [Google Scholar]
- 15.Rasbach LE, Volkening LK, Markowitz JT, Butler DA, Katz ML, Laffel LM. Youth and parent measures of self-efficacy for continuous glucose monitoring: survey psychometric properties. Diabetes Technol Ther 2015;17:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Li H, Ran X, et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care 2009;32:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai S, Hulse A, Kumar P. Feasibility and acceptability of ambulatory glucose profile in children with Type 1 diabetes mellitus: A pilot study. Indian J Endocrinol Metab 2016;20:790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deja G, Kleczek M, Chumiecki M, Strzala-Kleczek A, Deja R, Jarosz-Chobot P. The usefulness of the FlashStyle Libre system in glycemic control in children with type 1 diabetes during summer camp. Pediatr Endocrinol Diabetes Metab 2018;24:11–9. [DOI] [PubMed] [Google Scholar]
- 19.Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther 2009;11 Suppl 1:S11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheyne EH, Cavan DA, Kerr D. Performance of a continuous glucose monitoring system during controlled hypoglycaemia in healthy volunteers. Diabetes Technol Ther 2002;4:607–13. [DOI] [PubMed] [Google Scholar]
- 21.Freckmann G, Link M, Pleus S, Westhoff A, Kamecke U, Haug C. Measurement Performance of Two Continuous Tissue Glucose Monitoring Systems Intended for Replacement of Blood Glucose Monitoring. Diabetes Technol Ther 2018;20:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther 2015;17:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodbard D Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol Ther 2016;18 Suppl 2:S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massa GG, Gys I, Op ’t Eyndt A, et al. Evaluation of the FreeStyle(R) Libre Flash Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. Horm Res Paediatr 2018;89:189–99. [DOI] [PubMed] [Google Scholar]
- 25.Szadkowska A, Gawrecki A, Michalak A, Zozulinska-Ziolkiewicz D, Fendler W, Mlynarski W. Flash Glucose Measurements in Children with Type 1 Diabetes in Real-Life Settings: To Trust or Not to Trust? Diabetes Technol Ther 2018;20:17–24. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002:1–190. [PubMed] [Google Scholar]
- 27.Otten JJ, Hellwig JP, Meyers LD. DRI, dietary reference intakes : the essential guide to nutrient requirements. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 29.Erbach M, Freckmann G, Hinzmann R, et al. Interferences and Limitations in Blood Glucose Self-Testing: An Overview of the Current Knowledge. J Diabetes Sci Technol 2016;10:1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol 2009;3:903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kermani SK, Khatony A, Jalali R, Rezaei M, Abdi A. Accuracy and Precision of Measured Blood Sugar Values by Three Glucometers Compared to the Standard Technique. J Clin Diagn Res 2017;11:OC05–OC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fokkert MJ, van Dijk PR, Edens MA, et al. Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care 2017;5:e000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.