Abstract
Neurodegenerative diseases of the central nervous system progressively rob patients of their memory, motor function, and ability to perform daily tasks. Advances in genetics and animal models are beginning to unearth an unexpected role of the immune-system in disease onset and pathogenesis; however, the role of cytokines, growth factors, and other immune signaling pathways in disease pathogenesis is still being examined. Here we review recent genetic risk and genome-wide association studies and emerging mechanisms for three key immune pathways implicated in disease, the growth factor TGF-β, the complement cascade, and the extracellular receptor TREM2. These immune signaling pathways are important under both healthy and neurodegenerative conditions, and recent work has highlighted new functional aspects of their signaling. Finally, we assess future directions for immune-related research in neurodegeneration and potential avenues for immune-related therapies.
Keywords: Microglia, Inflammation, Neuroinflammation, Neurodegeneration, Alzheimer’s disease, Cytokine, Innate Immunity, GWAS, TGF-β, Complement, TREM2, Neuro-immune, Signaling
Etoc blurb:
Hammond, Marsh, and Stevens explore the growing evidence of immune involvement in the initiation and progression of neurodegenerative disease. The authors examine how immune pathways identified by GWAS and histopathological studies contribute to aberrant cellular responses, pathological protein aggregation, and neural deterioration in Alzheimer’s Disease, Parkinson’s Disease and other age-related neurodegenerative diseases.
Introduction
Since the first descriptions of neurodegenerative diseases over a 100 years ago our understanding of the molecular underpinnings of Alzheimer’s disease (AD), Parkinson’s disease (PD), Dementia with Lewy Bodies (DLB), and Frontotemporal Dementia (FTD) have focused largely on gross anatomical changes including protein aggregation and neuronal loss. However, recent genetic, histopathological, and mechanistic evidence also points to immune pathway dysregulation including changes in cytokine signaling, immune cell proliferation and migration, altered phagocytosis, and reactive gliosis as common features of neurodegeneration. These immune changes were long considered a secondary or reactive response to underlying disease processes, but emerging evidence implicates the immune system as a central player in disease onset and progression.
Identification of immune genetic risk loci through genome-wide association studies (GWAS), technological advances in single cell sequencing, molecular pathology, mouse models, and human induced-pluripotent stem cells (iPSCs) have significantly enhanced the ability to study the mechanisms of immune dysfunction (Figure 1). In this Review, we provide an overview of several neurodegenerative diseases for which substantial evidence points toward immune dysregulation and discuss the human genetics that implicate immune signaling in disease risk. We also provide deeper insight into three important immune signaling pathways: the growth factor TGF-β, the complement cascade, and extracellular receptor TREM2, that influence pathological progression in each disease. Finally, we discuss exciting future research directions that could lead to new biomarkers and therapeutic strategies targeting immune signaling pathways.
Figure 1: Dramatic Increase in Immune Related Publications in Alzheimer’s Disease:
Analysis of publications via PubMed reveals dramatic increase in immune-focused Alzheimer’s research since the year 2000 that significantly exceeds overall increase in AD-focused research. Each data point represents the percentage increase in publications normalized to number of publications in year 2000 for their respective publication focus. The most precipitous increases in AD immune-related research have occurred in the last 10 years, that corresponds with the publication of GWASs, which have implicated genes enriched in immune cell expression and immune signaling, and the publication of rare high-risk variants in microglial-specific gene TREM2.
Milestone citations: Reactive Microglia: (McGeer et al., 1987; Rogers et al., 1988); fAD APPV717I: (Goate et al., 1991); ApoE4 Risk: (Corder et al., 1993; Strittmatter et al., 1993); PDAPP & Tg2576: (Games et al., 1995; Hsiao et al., 1996); Aβ immunotherapoy: (Bard et al., 2000); AD GWAS: (Hollingworth et al., 2011; Lambert et al., 2009; Lambert et al., 2013; Naj et al., 2011); Trem2 R47H: (Guerreiro et al., 2013b; Jonsson et al., 2013).
Historically, observations of altered neuroimmune function have been described using broad terms (i.e. neuroinflammation or glial ‘activation’) or binary cell states (i.e. pro- vs. anti-inflammatory signals or M1 vs. M2 myeloid cell phenotypes). However, high-resolution multi-omic studies have confirmed what prior studies had begun to realize: that alterations in immune cell phenotypes, gene and protein expression, and morphology are highly complex processes that exist on a broad spectrum (Hammond et al., 2019; Keren-Shaul et al., 2017; Mathys et al., 2017). New research is beginning to better characterize and link disease-relevant pathways and cell states with specific disease mechanisms and aspects of brain dysfunction. Therefore, for the purposes of this review, where possible we utilize specific annotations to describe the relevant immune changes (i.e., patterns of expression or activation of specific genes or pathways).
Clinical & Pathological Characteristics of Neurodegenerative Disease
Age-related CNS neurodegenerative diseases are characterized by a number of shared and divergent clinical and pathological features including selective vulnerability of brain regions and differential protein aggregation that impact their clinical presentations and immune responses (Figure 2). Alzheimer’s disease is a molecularly and genetically complex disease characterized by extensive synaptic and neuronal loss (subsequently brain volume loss) and leads to a progressive decline in memory and cognitive function that ultimately results in an inability to perform basic daily functions and death. AD was first characterized by Alois Alzheimer in 1907, who noted the hallmark protein accumulations commonly termed plaques and tangles, but importantly also noted the presence of immune cells (likely microglia) in close proximity to the plaques (Alzheimer, 1907, 1911). Today, we know that the extracellular plaques are composed primarily of amyloid-beta (Aβ) and the intracellular tangles are hyperphosphorylated tau (Selkoe, 2001). The “typical” progression of pathology by region is different for amyloid vs. tau, although both eventually heavily impact neocortical, entorhinal cortex, and hippocampal regions (for review and further description see (Braak and Braak, 1991; Montine et al., 2012)). Despite the many co-pathologies, it is the loss of synapses that correlates most closely with cognitive decline (Davies and Maloney, 1976; DeKosky and Scheff, 1990; Terry et al., 1991).
Parkinson’s disease is characterized by motor symptoms including bradykinesia, tremor, and rigidity (For Review see (Moustafa et al., 2016)). Later in the disease course, some PD patients also develop clinical dementia, referred to as Parkinson’s disease dementia (PDD) (Goetz et al., 2008). Pathologically PD is also characterized by hallmark protein aggregates, termed Lewy bodies (Lewy, 1923), which are primarily composed of the protein α-synuclein (α-syn). α-synuclein is also present in AD plaques, as α-syn was originally referred to as the “non-amyloid component” of plaques in AD patient brains, in addition to some AD patients who also have Lewy body pathology (Hamilton, 2000; Hansen et al., 1990; Jakes et al., 1994; Spillantini et al., 1997; Uéda et al., 1993). In many cases Lewy bodies also include pathogenic tau, which has been shown to interact with α-syn (Marsh and Blurton-Jones, 2012). Degeneration and protein aggregation in PD spreads from the brain stem to the substantia nigra and other midbrain regions, then to the neocortex (Braak et al., 2004). The onset of symptoms follows significant loss of dopamine-producing neurons in the substantia nigra that appears to accelerate exponentially as the disease progresses (Cheng et al., 2010; Greffard et al., 2006).
Amidst PD, PDD, and AD sits a disorder known as Dementia with Lewy bodies (DLB) (For Review see (Outeiro et al., 2019; Walker et al., 2015). Clinically, DLB dementia is often distinguished from AD via the presence unique characteristics including of audio and/or visual hallucinations and DLB patients may or may not present with motor symptoms (McKeith et al., 2017) Pathologically, DLB is characterized by the presence of Lewy bodies, similar to PD, although patients may also exhibit amyloid pathology similar to AD (McKeith et al., 2017; Walker et al., 2015). Similar to AD and PD, DLB patients exhibit significant synaptic and neuronal loss, but research indicates that the Lewy bodies themselves are not the first neurotoxic mechanism to arise during either DLB or PD, as neuronal loss is observed prior to Lewy pathology (Milber et al., 2012; Outeiro et al., 2019). The clinical and pathological heterogeneity of DLB suggests that DLB may represent a spectrum of disorders, potentially with differing etiology and/or underlying mechanisms, which complicates the study of many disease mechanisms including the role of immune signaling.
Frontotemporal dementia encompasses a series of likely distinct dementias. Patients under the FTD spectrum are often assigned 1 of 4 primary clinical subtypes and 1 of 3 primary proteinopathy subtypes (For review see (Bang et al., 2015)). Pathologically, the 3 proteinopathy subtypes are characterized by presence of tau pathology, TAR DNA-binding protein with molecular weight 43 kda (TDP-43) pathology, or fused-in sarcoma (FUS) pathology (Bang et al., 2015). As the name implies the neurodegeneration, synaptic loss, and protein aggregation in FTD are concentrated in various regions including the frontal cortex & frontal lobe, anterior temporal lobe, striatum, and more (Bang et al., 2015).
In addition to their hallmark neuropathological and clinical characteristics these disorders also exhibit concurrent and chronic alterations in immune function and signaling. In most neurodegenerative diseases, the primary cellular mediator of the immune response in the brain parenchyma is thought to be microglia, the brain’s resident innate immune cells which are derived from primitive hematopoietic progenitors in the yolk-sac. These progenitors migrate to the developing brain of the embryo and become the resident immune cells of the brain (Ginhoux et al., 2010; Yona et al., 2013). Microglia are highly dynamic cells with diverse homeostatic functions, including roles in synaptic pruning, neurogenesis, and brain homeostasis (Hammond et al., 2018; Hanisch and Kettenmann, 2007; Schafer et al., 2012; Squarzoni et al., 2014). However, at their core, they are innate immune cells and perform classically associated roles like tissue surveillance, removal of pathogens, and response to injury (Hanisch and Kettenmann, 2007; Nimmerjahn et al., 2005). In neurodegenerative disease, microglia respond to their environment, migrating to sites of damage or injury, secreting numerous inflammatory molecules, and phagocytosing debris and aggregated proteins (Heneka et al., 2015; Song and Colonna, 2018). It is becoming increasingly clear that microglia and immune signaling may not just be secondary players in disease processes but actually contribute to synaptic and neuron loss and buildup of pathogenic proteins even at the earliest stages of disease (Figure 2) (Hong et al., 2016; Shi et al., 2017; Sosna et al., 2018).
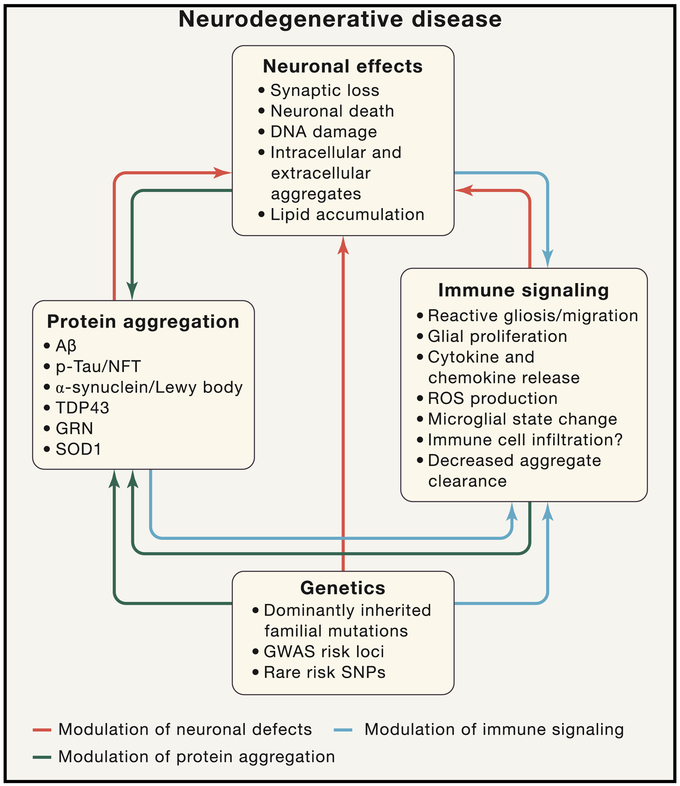
Figure 2: Immune Signaling Is a Complex Multi-Dimensional Process in Neurodegenerative Disease.
Neurodegenerative diseases involve a complex interplay between immune signaling, genetics, and neural damage that result in debilitating cognitive phenotypes. The neuronal and synaptic dysfunction and loss (top) in neurodegenerative disease can be mediated directly by protein accumulation/aggregation (both intracellular and extracellular), by genetic polymorphisms that modulate neuronal function, or by immune cell signaling (red arrows). Immune signaling in neurodegenerative disease can occur, in response to aggregated toxic proteins, in response to neuronal damage, and/or as result of genetic polymorphisms altering immune cell function (blue arrows). Protein aggregation can in turn also be altered by neuronal dysfunction, altered immune function, and/or genetic polymorphisms (black arrows). All of these interconnected effects lead to the memory loss, cognitive decline, motor dysfunction, and death that are the clinical manifestations of these debilitating diseases. Arrow color is indicative of the component being modulated.
While much of the research has focused on the role of the brain resident microglia in the immune response, the peripheral immune system may also play important roles in the progression of neurodegenerative disease. Many studies have focused on potential roles and/or infiltration of peripherally-derived macrophages in the CNS immune response. However, distinguishing peripheral macrophages from microglia within the brain parenchyma is difficult due to high overlap in both phenotype and gene expression. However, two recent studies have identified unique signatures that peripheral macrophages maintain upon infiltration that differentiates them from resident microglia (Bennett et al., 2018; Cronk et al., 2018), providing potential markers and strategies to dissect of the role of peripheral macrophages in the disease-associated immune response. Moreover, while the innate immune system has been the focus of much of the research in neurodegenerative disease, emerging results suggest that adaptive immune system (primarily T & B cells) may also play important roles in disease progression. However, much of the research examining the functional aspects of the adaptive immune system in neurodegenerative disease is relatively recent and requires further study to understand how adaptive immunity fits in the disease process (Baird et al., 2018; Baruch et al., 2015; Marsh et al., 2016; Mosley et al., 2012). Finally, it is important to note that direct peripheral cell infiltration is not necessarily a requirement for peripheral immune impacts on the CNS. There is growing body of literature which suggests that soluble signaling molecules produced in the periphery can enter the brain and effect both microglial phenotype and modulate cognition (Castellano et al., 2017; Derecki et al., 2010; Katsimpardi et al., 2014; Marsh et al., 2016; Villeda et al., 2011; Villeda et al., 2014).
Evidence from Human Genetics Highlights Immune Signaling in Disease Risk
AD, PD, DLB, and FTD all have strong heritable components. Each of these disorders have rare familial forms linked to mutations in the genes that code for the protein aggregating in the specific disease (APP, MAPT [Tau], SNCA [α-synuclein]), in the genes processing APP into Aβ (PSEN1, PSEN2), or in other genes such as GRN and C9ORF72 (For Review see (Billingsley et al., 2018; Hinz and Geschwind, 2017; Orme et al., 2018; Selkoe and Podlisny, 2002; Tanzi, 2012). In addition to familial forms of these diseases, advances in human genetics have identified a number of genes and loci associated with altered disease risk in each of these disorders, many of which exhibit highly enriched expression in myeloid and other immune cells or take part in immune signaling pathways, especially in the case of AD, which appears to have the most genetically-driven immune component (Figure 2). However, more evidence is emerging that links the genetics of PD, DLB, and FTD to altered immune signaling as well. Here, we summarize some of findings from each disease that link altered disease risk with modulation of immune function and signaling.
More than half of the AD risk loci discovered in GWAS are significantly enriched or, in some cases, uniquely expressed in immune cells – genes including CR1, CD33, members of the MS4A family, the HLA locus, and MEF2C (Bennett et al., 2016; GTEx Consortium, 2013; Hammond et al., 2019; Hollingworth et al., 2011; Jansen et al., 2019; Lambert et al., 2009; Lambert et al., 2013; Marioni et al., 2018; Saunders et al., 2018; Zhang et al., 2014; Zhang et al., 2016). Specifically, there is significant enrichment of immune signaling and expression within cells of the myeloid lineage, including microglia and macrophages (Gjoneska et al., 2015; International Genomics of Alzheimer's Disease Consortium, 2015; Jones et al., 2010; Raj et al., 2014); however the specific myeloid population(s) that contribute to AD risk and pathogenesis is an important open question in the field.
The myeloid component is supported by recent analyses that go further than simple GWASs. Researchers have used GWASs and genotyping results combined with gene expression data to perform an analysis of expression quantitative trait loci (eQTLs), to determine whether a single nucleotide polymorphism (SNP) from a GWAS is associated with altered expression of either local (cis-eQTL) or distant (trans-eQTL) genes (Michaelson et al., 2009; Rockman and Kruglyak, 2006). Cis-eQTL analysis recently resulted in the discovery of a cis-eQTL for the myeloid lineage determining transcription factor PU.1 (gene SPI1) within the CELF1 GWAS locus. However, it was the discovery in 2013 of a rare SNP in Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) that increased AD risk on par with possessing a single copy of the AD risk allele APOE-ε4, that dramatically increased the focus on microglia in AD pathogenesis (Guerreiro et al., 2013a; Jonsson et al., 2013; Sims et al., 2017) (see in-depth discussion later in this review). More recently rare variant analyses have also uncovered AD risk variants within the microglia-specific genes PLCG2 and ABI3 (Huang et al., 2017; Sims et al., 2017). Finally, the most-well known AD risk gene is APOE, which has long been studied in the context of its basal expression by astrocytes in the brain (Huang et al., 2004; Liu et al., 2013). However, the power of single-cell sequencing to separate gene expression at unprecedented resolution revealed a massive microglial-specific upregulation in Apoe in mouse models of AD (Keren-Shaul et al., 2017; Krasemann et al., 2017). These studies highlight the need to study gene expression of risk variants not just under basal conditions, but also in disease-relevant contexts.
Newer studies are beginning to examine not just the links between genetics and disease risk, but also those between disease variables such as age of onset. There is a significant association between the age of onset in sporadic AD and APOE, plus other immune-enriched loci like CR1, PICALM, and BIN1 (Corder et al., 1993; Naj et al., 2014). Furthermore, a recent study in early onset familial AD found a haplotype of SNPs within a chemokine gene cluster (that included a coding variant in CCL11) that altered age of onset by 10 years in carriers (Lalli et al., 2015). Unlike some of the other immune-related genes CCL11 has virtually no expression in the brain parenchyma. Instead the greatest tissue expression, in humans, is found in the gut, indicating a potential link between peripheral immunity and disease onset (GTEx Consortium, 2013; Hammond et al., 2019; Heng et al., 2008; Saunders et al., 2018; Zhang et al., 2014; Zhang et al., 2016). Research in mice which linked increased peripheral Ccl11 in blood from aged mice was associated with cognitive deficits and decreased neurogenesis (Villeda et al., 2011). While there are certainly caveats to the applicability of this to sporadic AD (i.e., it was found in a single family, in familial AD which accounts for only 1-2% of total AD cases), it reinforces how altered immune system function can drastically alter the course of disease. Taken together, these results provide further evidence that immune signaling in AD may not just be a response to pathology but a driving factor in disease development or onset.
While not as heavily biased toward immune pathways, results from several GWAS studies in Parkinson’s disease also demonstrate a link to immune dysfunction (Dzamko et al., 2015). Relevant immune-enriched loci include the HLA locus, BST1, LRRK2, and CTSD (Chang et al., 2017; Hamza et al., 2010; Nalls et al., 2014). However, these loci are classified as ‘immune enriched’ based on the basal gene expression patterns of healthy adults. There could be other PD loci not classically considered to be immune genes that are upregulated in immune cells only during disease. One potential candidate gene is GPNMB (Nalls et al., 2014), whose expression is significantly upregulated in microglia in AD models (Keren-Shaul et al., 2017), but has nearly undetectable microglial expression through healthy adulthood and even in very old wild-type mice (Hammond et al., 2019).
Pathway analysis of risk genes in PD found significant enrichment in certain adaptive immune cell populations and adaptive immune signaling GO pathways (Gagliano et al., 2016; Holmans et al., 2013). This fits with observational studies of T cell infiltration in PD patients and animal models (Brochard et al., 2009; Castellani et al., 2011; McGeer et al., 1988). The role of T cells in PD pathogenesis is supported by a recent report which found both that fragments of α-synuclein bound to HLA variants implicated in PD and that native and pathogenic forms of α-synuclein induced responses in T cells isolated from PD patients (Sulzer et al., 2017). While this study implicates a functional role for T cells in PD more research is needed to better understand their function in disease.
In addition to enrichment in immune cells and pathways, genetic studies have also begun to examine more complex interactions between multiple genes both at gene and protein level. To perform these studies researchers have utilized protein quantitative trait loci (pQTL) analysis to determine whether a genetic variant is associated with altered expression of a protein not coded for by the genetic variant. Using this methodology, a trans-pQTL analysis found an association between the PD risk locus RIT2 and higher expression of the protein produced by AD risk gene CD33 in peripheral monocytes (Chan et al., 2016). RIT2 is expressed in neurons and oligodendrocytes and has very low expression in the rest of the body (GTEx Consortium, 2013; Saunders et al., 2018; Zhang et al., 2016). This result shows that non-immune genes can still impact immune function. Higher expression of CD33 in peripheral monocytes was also found to be associated with increased incidence of parkinsonism in older patients (Chan et al., 2016), showing how the mechanisms of neurodegenerative disease may overlap. Furthermore, this result highlights the need to study immune signaling at the protein level, as this trans association did not occur at the mRNA level.
The genetics of sporadic DLB is still a very new field (Orme et al., 2018). The first large-scale GWAS in DLB was just published in 2018 (Guerreiro et al., 2018). Of the five loci that reached genome-wide significance, the only one considered immune-enriched by gene expression was APOE. While some of the significance of APOE in DLB could be partially the result of neuropathological overlap between DLB and AD with Lewy bodies, APOE-ε4 also increases dementia risk in “pure” DLB cases that lack or have low Aβ pathology (Dickson et al., 2018; Orme et al., 2018; Tsuang et al., 2013). The GWAS in DLB is the most underpowered of all the neurodegenerative diseases discussed in this review, containing an analysis of just over 1,700 patients. The addition of more patients to future studies will likely aid in finding more loci.
Like DLB, the genetics of FTD is still a new field, however several studies have still identified links between FTD genetics and altered immune function and signaling (Broce et al., 2018; Carmona et al., 2018; Pottier et al., 2019). As previously discussed, the study of FTD is complicated by the significant pathological and clinical heterogeneity of the disorder. Therefore, recent studies have either used neuropathological or clinical stratifications to guide their search for risk variants (Ferrari et al., 2014; Mishra et al., 2017; Van Deerlin et al., 2010). After stratifying the patients by clinical phenotype, Ferrari et al. (2014) found immune-related hits, with the HLA locus reaching genome-wide significance across combined analysis of all FTD cases. Another study using clinical stratification identified several more immune-related loci including APOE in the behavioral variant of FTD and SERPINA1 in progressive non-fluent aphasia (Mishra et al., 2017). Finally, in 2012, two simultaneous studies found that expanded repeats in C9ORF72 is the most common cause of familial FTD and ALS (DeJesus-Hernandez et al., 2011; Renton et al., 2011). While more research needs to be done to further understand the biological functions of C9ORF72 and these repeats, gene expression analysis indicates significant enrichment in immune populations including microglia (Bennett et al., 2016; Zhang et al., 2014; Zhang et al., 2016).
There are a few caveats to discuss with regards to current genetic results and future directions. As mentioned in the context of APOE in AD, when examining gene expression profiles of risk variants, it is critical to examine results from both homeostatic and disease-specific contexts. While recent single-cell sequencing studies in AD mouse models (Keren-Shaul et al., 2017) have provided new insights, the same is needed in animal models of PD, FTD, and DLB. Finally, these profiles need to be verified at the single-cell or -nuclei level in human patients to determine whether observations in the mouse are translatable to humans. While GWASs have been instrumental in driving new research focuses, there are some caveats to GWASs that should be addressed, as possible, going forward. These caveats includea need for stratification of populations using more than clinical diagnosis, such as retrospective autopsy verified GWASs, analysis of genetic risk in disease traits like rate of decline or age at onset, and increased diversity in ethnicity of patients analyzed. Encouragingly, many recent efforts have been moving to address some of these issues (Farfel et al., 2016; Naj et al., 2014) and results in the next few years will be especially interesting.
Modeling the Immune System in Neurodegeneration
Research using animal models and human post-mortem tissue have identified a host of cellular players, including microglia, neurons, astrocytes, oligodendrocytes, vessel-associated cells, and infiltrating immune cells, that produce signals that contribute to the pathological brain environment in neurodegenerative disease. Thus, it is becoming increasingly clear that there is a complex network of communication that drives both beneficial and pathological cell interactions and outcomes.
In this Review, there is not space to delve into the litany of signaling molecules involved. We therefore focused on three pathways (TGF-β, Complement, and TREM2) to describe in greater detail including (1) their importance in immune signaling under both healthy and neurodegenerative conditions, (2) human genetic studies implicating these factors in disease, (3) recent publications highlighting new functional aspects of their signaling, and (4) a large body of literature studying the actions of these signals in vivo.
Dozens of mouse models have been created to understand the pathological mechanisms of neurodegenerative disease including cellular responses to protein aggregates, alterations in the brain signaling environment, and how other pathological features arise (Dawson et al., 2018; Götz et al., 2018). However, teasing apart the involvement of specific immune pathways using these models has been difficult and requires the generation of transgenic or knock-in mice that both express disease mutations and disrupt the pathway of interest. This complexity is compounded by immense variability in the models themselves, most of which fail to fully recapitulate human disease etiology and progression (Ransohoff, 2018). Here we will outline the most common models of AD, PD, and FTD and their associated pathological features to give context to the subsequent in-depth analysis of the TGF-β, Complement, and Trem2 pathways.
The most commonly used AD mouse models are based on pathological amyloid protein buildup and familial mutations in amyloid precursor protein (APP) or in presenilin 1 (PSEN1) or presenilin 2 (PSEN2), which are part of the pathogenic processing pathway of APP (Sasaguri et al., 2017). Transgenic expression of the mutated forms of the human genes leads to toxic oligomeric Aβ accumulation and plaque formation (Games et al., 1995; Hsiao et al., 1996; Jawhar et al., 2012; Oakley et al., 2006). Similar effects can be achieved by knock-in of multiple concurrent fAD mutations or focal injection of oligomeric Aβ in mice, which offer similar – but not completely overlapping – phenotypes as the transgenic models (Saito et al., 2014; Götz et al., 2018). Models of amyloidosis, however, lack buildup of hyperphosphorylated tau and neurofibrillary tangles, do not have extensive neurodegeneration, and have behavioral deficits that are generally mild and not reflective of disease progression in AD. The lack of tau pathology may be due, in part, to differences in the number of tau isoforms between humans and mice (Götz et al., 2018).
Hyperphosphorylated and misfolded tau is found extensively in AD patients (Köpke et al., 1993) and increased pathological tau plays an important role in neuronal health and dysfunction, based on mechanistic studies (DeVos et al., 2018; Fu et al., 2017; Lewis et al., 2000) and analysis from patients demonstrating tau pathology correlates with both neuronal loss and cognitive decline (Giannakopoulos et al., 2003; Gómez-Isla et al., 1997; Nelson et al., 2012), Importantly, hyperphosphorylated tau is also found in Huntington’s disease, DLB, and FTD, among others (Iqbal et al., 2016). Tau, a protein involved in microtubule assembly and found in the axons and dendrites of neurons, is heavily post-translationally modified with approximately 85 phosphorylation sites (Wang and Mandelkow, 2016). Several mutations in the human tau gene (MAPT) have been found in patients with frontotemporal dementia with parkinsonism-17 (FTDP), indicating a causal role in neurodegeneration. Tauopathy can be modeled by transgenic overexpression of different human familial FTDP mutations (Denk and Wade-Martins, 2009), and common features of these mutant mouse strains include neurofibrillary tangles that increase in number with age and progressive neurodegeneration (Lewis et al., 2000; Yoshiyama et al., 2007). Combinations of the MAPT and APP or PSEN1/2 mouse mutants leads to tau and amyloid pathology that more closely recapitulates AD (Lewis 2001; Oddo et al., 2003; Saul et al., 2013), but the translational relevance of this approach has been questioned, as the tau mutations are not found in human AD patients and may confer a different phenotype. Interestingly, hyperphosphorylated tau can also be ‘seeded’ in mouse brains by injecting purified forms of the protein (Clavaguera et al., 2013; Clavaguera et al., 2009), which leads to spreading between neurons in a prion-like fashion, and causes neurotoxicity (Kaufman et al., 2016).
Several different approaches are used to model different aspects of PD including chemical agents to trigger the loss of dopaminergic neurons and overexpression of mutated human α-synuclein linked to familial forms of the disease (Tieu, 2011). Chemical agents include 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which are injected into the striatum or substantia nigra or systemically to induce varying degrees of dopaminergic neuron loss (Meredith and Rademacher, 2011). These models, however, fail to faithfully recreate the α-synuclein fibrils, Lewy bodies, and associated proteinopathy found in PD. Transgenic overexpression of familial mutations of the human α-synuclein gene in mice can cause toxic fibril buildup and Lewy body formation, but does not specifically target dopaminergic neurons and leads to widespread neuronal pathology outside nigrostriatal circuits (Visanji et al., 2016). Other PD models including mutations in other genetic risk factors and seeding of α-synuclein have been useful for teasing apart other mechanisms of disease pathogenesis and have been reviewed in detail elsewhere (Bobela et al., 2014; Lee et al., 2012; Tieu, 2011; Visanji et al., 2016).
These mouse models of neurodegenerative disease have been combined with manipulations of immune pathways to uncover the in vivo roles of each signaling cascade using genetic ablation or overexpression of pathway elements. These models are complex and have many caveats including the timing and method of manipulation, so we have focused on three pathways with the most extensive evidence thus far.
Transforming Growth Factor Beta
TGF-beta is a cytokine important for both innate (Kelly et al., 2017) and adaptive (Travis and Sheppard, 2014) immune function. It is also essential for brain development and function, as it regulates neural patterning (Bond et al., 2012), neurogenesis (Dias et al., 2014), gliogenesis (Stipursky et al., 2014), microglia development (Butovsky et al., 2014), myelination (Palazuelos et al., 2014), and brain wiring (Yi et al., 2010). Tgfb1-knockout mice, which die early in life due to runaway peripheral inflammation, exhibit markedly reduced synapse numbers, thinned cerebral cortex, and an increase in dying neurons (Brionne et al., 2003). Three TGF isoforms exist in the brain, TGF-β1, TGF-β2, and TGF-β3, which are transcribed and cleaved before being released and sequestered by milieu molecules on the cell surface or in the extracellular matrix (Figure 3A) (Dobolyi et al., 2012; Kelly et al., 2017). Latent TGF-β, the final active form of the peptide, is then released and binds to the homodimeric TGF beta type II receptor (TGFBRII), a serine threonine kinase receptor. Then TGFBRII forms a tetrameric complex with the TGF beta type I receptor (TGFBRI). The TGFBRI is then phosphorylated, and a cascade of SMAD proteins propagate the intracellular signaling pathway, thus regulating gene transcription (Kelly et al., 2017).
Figure 3: TGF-β Signaling and the Complement Cascade in Neurodegeneration:
A. TGF-β is a secreted signal that binds to TGFBRI and TGFBRII complexes on the cell surface of neurons and microglia. TGF-β levels are elevated in the brain and CSF of AD, PD, and FTD patients. In mouse models of AD, PD, and FTD, TGF-β is neuroprotective and decreases amyloid plaques in AD models, acting in part through interleukin-like epithelial-mesenchymal transition inducer (ILEI) in neurons to reduce Aβ production. TGF-β is also necessary for normal microglia maturation and for maintaining their unique brain profile. by Microglia in TGF-β receptor knockout mice or mice lacking the TGF-β associated milieu molecule LRRC33 exhibit increased lysosomal content, decreased ramification, and altered transcriptional profile. Altered TGF-β levels could modulate microglial state and function in neurodegenerative disease.
B. The complement pathway is upregulated in AD, PD, and FTD patients. In AD and FTD mouse models, heterotrimeric C1q molecules (made up of C1qa, C1qb, C1qc) bind to vulnerable synapses, leading to opsonization by C3 and phagocytosis of the structure by microglia which express the C3 receptor (CR3/CD11b). This process could contribute to synapse loss found in early stages of neurodegenerative disease. Blocking complement activation limits neuroimmune alterations and ameliorates neurocognitive deficits in mouse models of AD and FTD. C1q molecules (produced by microglia) also bind to amyloid plaques to trigger Aβ phagocytosis. ApoE can limit complement pathway activation by binding to C1q, and an association found in AD plaques and is reduced in the presence of the APOE-ε4 risk allele.
In the healthy mouse brain, the Tgfbr1 and Tgfbr2 genes are expressed at the highest levels in microglia, endothelial cells, and oligodendrocyte precursor cells (Bennett et al., 2016; Saunders et al., 2018; Zhang et al., 2014), and their receptors are found in most brain cell types, including neurons (Tesseur et al., 2006) (Figure 3A). TGF-β1 is essential for normal microglial development and modulates their homeostatic state. In the absence of TGF-β1, microglia have an altered transcriptional profile (Butovsky et al., 2014), which includes downregulation of microglial homeostatic markers, such as Tmem119 and P2ry12, and upregulation of the injury-associated markers Axl and Apoe (Hammond et al., 2019; Keren-Shaul et al., 2017). These transcriptional changes are phenocopied in mice lacking the milieu molecule LRRC33, which is necessary for microglial TGF-β1 signaling (Qin et al., 2018; Wong et al., 2017). Microglia in the brains of LRRC33 (Nrros) knockout mice resemble brain border macrophages and are less ramified and have increased lysosomal content, an indicator of increased phagocytic activity (Schafer et al., 2012).
Increased expression of TGF-β1 protein has been found in the CSF and serum of AD patients (Chao et al., 1994; Zetterberg et al., 2004) and in the brains of individuals with PD (Mogi et al., 1995). Increased expression of TGF-β2 protein has also been found in the brains of FTD and AD patients (Chong et al., 2017). Given the extensive evidence of microglial dysfunction in neurodegeneration, including changes in microglial phagocytic activity, it is possible that changes in brain TGF-β signaling in AD and PD could alter microglial state and trigger their pathogenic functions (Salter and Stevens, 2017). Along these lines, ablating TGFBRII in CD11c+ immune cells in APP mutant mice drastically reduces plaques. It was originally thought that the CD11c+ cells were infiltrating immune cells, but given that plaque-associated microglia express CD11c (gene: Itgax) (Keren-Shaul et al., 2017), it is possible that these unique microglia produced the observed phenotype.
TGF-β signaling exerts anti-inflammatory effects and is largely neuroprotective (Dobolyi et al., 2012). In mice, infusing TGF-β1 into the ventricles prevents Aβ oligomer-induced synapse loss (Diniz et al., 2017), and intracerebroventricular (ICV) injection or nasal administration of TGF-β1 prevents neuron death and limits microglial state change in rats injected with Aβ oligomers (Chen et al., 2015). The neuroprotective effects of TGF-β are not limited to amyloid pathology. TGF-β1 reduces MPTP-induced degeneration of dopaminergic neurons in the substantia nigra, causing a higher survival rate and lowered levels of inflammatory cytokines and decreased microglial lysosomal content (Tesseur et al., 2017).
The exact mechanism underlying TGF-β-mediated neuroprotection is unknown, but the reduction of toxic protein load could play a major role. Transgenic mice expressing a non-functional kinase-deficient TGFBRII under a broadly expressed Prnp promoter exhibit age-related neurodegeneration and increased amyloid beta accumulation and plaque formation (Tesseur et al., 2006). Conversely, overexpressing TGF-β1 in the astrocytes of hAPP mice causes a major reduction in Aβ load and plaque formation, large increases in the number of microglia, and altered microglia morphology (Wyss-Coray et al., 2001). Reduced Aβ load could be neuron-intrinsic mechanism, as TGF-β1 induces expression of interleukin-like epithelial-mesenchymal transition inducer (ILEI) – a factor that reduces Aβ production and plaque load in mice (Hasegawa et al., 2014). TGF-β also prevents Aβ-induced neuronal cell cycle reactivation and tau hyperphosphorylation (Caraci et al., 2008). TGF-β may indirectly stimulate microglial phagocytosis of Aβ, which happens in cultured cells (Wyss-Coray et al., 2001). In summary, the neuroprotective effects of TGF-β signaling are likely due to a synergistic effect of improved neuron function, reduced the production of inflammatory molecules in the brain and changes in microglia cell state.
Complement Cascade
Complement factors are highly upregulated in the aged brain and in several CNS neurodegenerative diseases (Stephan et al., 2012; Stephan et al., 2013). Increased complement activation and deposition of pathway components has been found in AD (Afagh et al., 1996; Dejanovic et al., 2018; Yasojima et al., 1999; Zanjani et al., 2005), PD (Loeffler et al., 2006; Yamada et al., 1992), glaucoma (Stevens et al., 2007), and many other diseases. Human evidence also points toward complement cascade activation on amyloid plaques and Lewy bodies in these diseases, but recent evidence in animal models indicate that vulnerable synapses are also a target in early disease stages, particularly in models of amyloidosis and tau pathology (Lui et al., 2016).
Activation of the complement pathway triggers a proteolytic cascade leading to deposition (also called opsonization) of activated protein fragments on the surface of cells or structures that need to be removed. The pathway is typically known for opsonizing pathogens and infected cells, but it is also important for removing neural structures in the brain. There are three different complement pathways: the classical cascade, the lectin pathway, and the alternative pathway. The cascade involves more than 30 proteins and the sequence of events have been reviewed in great detail elsewhere (Orsini et al., 2014; Sarma and Ward, 2011). In the developing brain, the classical complement pathway proteins localize to developing axons and synapses during refinement of the visual system, leading to their removal by microglia expressing complement receptor 3 (CR3/CD11b) (Schafer et al., 2012; Stephan et al., 2012; Stevens et al., 2007). Complement-knockout mice display altered synaptic connectivity (Chu et al., 2010).
During early disease onset in mouse models of AD, aberrant upregulation and deposition of complement contributes to synapse loss and cognitive dysfunction largely before widespread protein aggregates form and neuronal death occurs (Figure 3b). For example, excess C1qa is deposited on synapses in the hippocampus of several transgenic mouse models of amyloidosis even before the appearance of plaques (Hong et al., 2016). C1qa is also enriched on postsynaptic dendritic spines in the PS19 (TauP301S) mouse model (Dejanovic et al., 2018) and at synapses in a model of FTD caused by progranulin deficiency (Lui et al., 2016). Elimination of CR3 is sufficient to block synapse loss after systemic injection of oligomeric Aβ, showing that microglial phagocytosis is one of the major causes of complement-mediated synapse loss (Hong et al., 2016). In agreement with these results, ablation of C3a receptor 1 (C3ar1) also rescued synapse loss and neuron death in the PS19 tauopathy model, suggesting that recognition of deposited C3 is a major driver of pathology (Litvinchuk et al., 2018).
Inhibiting the complement pathway is effective for limiting the effects of neurodegeneration-associated pathology. Anti-C1q blocking antibodies prevented synapse loss in PS19 mice (Dejanovic et al., 2018), and synapse loss and cognitive decline in two mouse models of amyloidosis are reversed in C3 KO mice (Maier et al., 2008; Shi et al., 2017). C3-KO/APP-transgenic mice and C3ar1-KO/PS19-transgenic mice also exhibit reduced microglial lysosomal content and astrocyte GFAP levels, indicating a possible role for C3 in modulating immune cell function (Litvinchuk et al., 2018; Shi et al., 2017). Interestingly despite upregulation of complement components, in PD models there appears to be little involvement of the complement system in dopaminergic neuron loss in mouse models of MPTP-toxicity (Depboylu et al., 2011; Liang et al., 2007). Loss of C1q or C3 has no effect on survival of nigrostriatal neurons, but synapse loss has not been examined in these knockout mice and warrants further investigation following MPTP-induced injury and in other PD mouse models.
In addition to binding to synapses, C1q directly binds to protein aggregates in AD, FTD, and PD. C1q binds to Aβ and is localized to plaques in AD patients and in APP mouse models (Afagh et al., 1996; Jiang et al., 1994; Reichwald et al., 2009; Rogers et al., 1992) (Figure 3B). Classical cascade components colocalize with Lewy bodies in PD (Loeffler et al., 2006; Yamada et al., 1992). Aβ is also a potent stimulator of the complement pathway, triggering C1q and C3 upregulation (Hong et al., 2016; Lian et al., 2016). Yet, it is still unclear whether complement tagging reduces plaque load. Genetic loss of C3 or injection of C5ar1 receptor antagonist in APP mice led to increased plaque load (Fonseca et al., 2009; Maier et al., 2008), but no difference was seen in C1qa knockout/APP mice which could indicate a role for the alternative complement or lectin pathway, which do not require C1q.
While Aβ is a potent stimulator of the complement pathway, it is also possible that underlying genetics could modulate pathway activation. A recent study reported that activation of the classical complement cascade is inhibited by ApoE through selective binding to C1q, an interaction that is suppressed in mice expressing the human AD risk allele APOE-ε4 (Yin et al., 2019). Furthermore, C1q-ApoE complexes are found in plaques and the choroid plexus of individuals with AD (Yin et al., 2019) (Bonham et al., 2016). Further studies will be needed to determine the extent to which complement pathway activation is a response to synapse toxicity, amyloid buildup, or cell death and how it acts synergistically with other inflammatory factors to exacerbate disease progression.
Altogether, the complement pathway influences a wide range of phenotypes associated with neurodegeneration by contributing to neuron and synapse loss and modulating glial responses. It is still unclear whether complement plays a primary role in pathology or whether activation of the pathway occurs in response to neuronal dysfunction or buildup of protein aggregates. The recent identification of complement receptor 1 (CR1) as a genetic risk factor AD (Lambert et al., 2009) has provided the first evidence for a primary role in disease, but a lack of evidence demonstrating CR1 expression and function in the brain have limited our understanding of this genetic connection (Johansson et al., 2018).
TREM2 Signaling Network
TREM2 is an extracellular receptor uniquely expressed in cells of the myeloid lineage. TREM2 is highly expressed by osteoclasts in the bone and, within the CNS parenchyma, its expression is unique to microglia (Cella et al., 2003; Heng et al., 2008; Saunders et al., 2018; Zhang et al., 2016). It was first cloned in 2000 by Marco Colonna and colleagues (Bouchon et al., 2000) and is part of a series of Trem and Trem-like genes on human chromosome 6 (mouse chr. 17). Shortly after its discovery, a set of homozygous TREM2 loss-of-function variants were found to be one cause of a very rare genetic disorder known as Polycystic Lipomembranous Osteodysplasia with Sclerosing Leukoencephalopathy (PLOSL) or Nasu-Hakola Disease (Klünemann et al., 2005; Paloneva et al., 2002). The symptoms of this disease parallel what we now know about the gene expression of TREM2, as PLOSL presented with brain symptoms (as aggressive frontotemporal dementia-like syndrome) and bone-related symptoms (Paloneva et al., 2001). However, PLOSL is extremely rare and only a handful of studies examined TREM2 in AD until 2013. It was then that a pair of reports published back-to-back detailed findings that a rare SNP in TREM2 (resulting in amino acid change R47H) altered AD risk with the highest odds ratio of any AD risk gene outside of APOE (Figure 1) (Guerreiro et al., 2013a; Jonsson et al., 2013). More recent analysis has found an additional SNP variant in TREM2 that also reached statistical significance (R62H; (Sims et al., 2017)), and several other variants are more prevalent in AD patients than controls but have yet to reach significance or be replicated, likely in part due to their scarcity in the population (Carmona et al., 2018). In addition, homozygous TREM2 variants were also found to be potentially casual for a form of FTD (Carmona et al., 2018; Chouery et al., 2008; Guerreiro et al., 2013a), while other variants significantly elevated FTD risk (Carmona et al., 2018; Giraldo et al., 2013). While an initial report suggested an association between TREM2 R47H and Parkinson’s disease (Rayaprolu et al., 2013), a more recent meta-analysis including those original results failed to replicate the findings (Lill et al., 2015). Taken together, these results indicate that altered microglial TREM2 signaling is sufficient to accelerate neurodegeneration or, in the case of homozygous complete LOF variants, are sufficient to cause neurodegeneration.
Current evidence suggests that R47H and R62H are both partial loss of function variants, primarily due to decreased affinity for TREM2 ligands (Kober et al., 2016; Kober and Brett, 2017; Song et al., 2017). While the results from initial studies with crosses of Trem2-KO mice to amyloid mouse models have been slightly controversial with conflicting results, recent studies have found this may have been due to differences in the time points and assays used to assess pathology, the transgenic mouse models used, and differences in the Trem2-KO models (Ulland and Colonna, 2018; Yeh et al., 2017). Early research in the impact of TREM2 on tau pathology has had similar issues. While one report found that Trem2-KO reduced brain atrophy but had no impact on tau pathology when crossed to the PS19 transgenic tau model (Leyns et al., 2017), another report found that a different Trem2-KO mouse crossed with a different tau model exacerbated tau pathology (Bemiller et al., 2017). A third study, again using the PS19 model, found that while homozygous Trem2-KO had slight to no effect on tau pathology. However, they found that haploinsufficiency, which the authors argued better mimics a partial loss of function, increased tau pathology (Sayed et al., 2018). More comprehensive studies at multiple time points in disease pathogenesis will be critical to understanding the functional consequences various risk variants or knockout of TREM2. More information on TREM2 function and dysfunction in AD models and beyond is covered in several excellent reviews including (Ulland and Colonna, 2018; Yeh et al., 2017).
Here we highlight a hypothetical model of potential points of convergence of a number of different AD risk genes with TREM2 signaling as individual results from several studies provide evidence for interactions between TREM2 and other AD risk genes (Figure 4). Much of what is known about intracellular signaling following TREM2 activation comes from research on its adaptor protein binding partner Tyro Protein Kinase Binding Protein (TYROBP, commonly known as DAP12). DAP12 is a promiscuous adaptor protein that binds to a large number of immune receptors including TREM1, SIRPβ, PILRβ, and NKp44 (for in depth review of DAP12 see (Hamerman et al., 2009; Turnbull and Colonna, 2007). TREM2 requires binding to DAP12 in order to function as TREM2 lacks an intracellular signaling domain. To mediate its intracellular signaling, DAP12 contains an immunoreceptor tyrosine-based activation motif (ITAM). Phosphorylation of this domain leads to the recruitment of spleen tyrosine kinase (Syk), which in turn activates PI3K and PLCγ, which activate downstream signaling cascades that include ERK, AKT, NF-κB, and NFAT (Kober and Brett, 2017; McVicar et al., 1998; Mócsai et al., 2006; Mócsai et al., 2010; Peng et al., 2010; Takahashi et al., 2005; Turnbull and Colonna, 2007). A recent report found that a rare variant (P522R) in PLCγ2 is protective in AD patients (Sims et al., 2017). Further mechanistic research is needed to examine whether this protective mechanism is dependent or independent of TREM2, as PLCγ2 activation by Syk is not unique to TREM2.
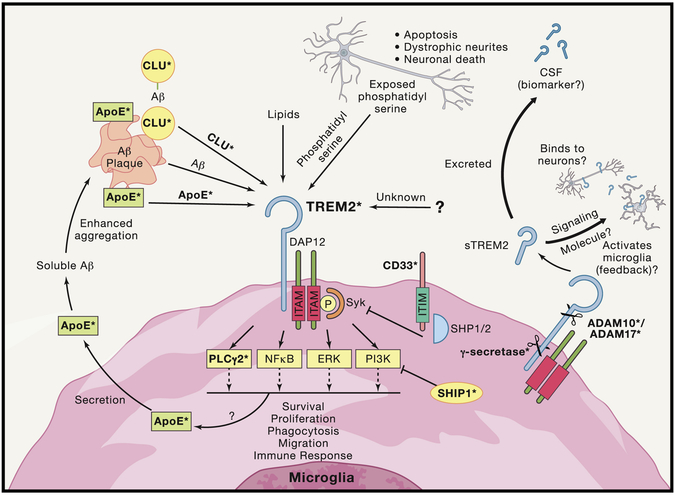
Figure 4: Convergence of Multiple AD Risk Genes/Loci on TREM2 Signaling.
TREM2 signaling pathways involve potential complex signaling interactions with other AD genetic risk factors with a diverse array of functional consequences. Studies of TREM2 have begun to uncover a number of possible ligands for TREM2 that are highly expressed in the CNS and increased in AD brain, and more ligands potentially await discovery. Among the proposed ligands for TREM2 are ApoE, Clu/ApoJ, various lipids, phosphatidyl serine, and even potentially Aβ itself. TREM2 lacks its own signal transduction component and must interact with ITAM adaptor protein DAP12 in order to mediate downstream signaling. DAP12 activation and Syk phosphorylation led to a number of potential intracellular signals including NF-kB, PI3K, ERK, and newly identified AD risk gene PLCG2. Recent evidence also indicates that activation of TREM2 increases microglial production of ApoE which may then act in reciprocal cycle binding to amyloid and then binding to TREM2. The natural inhibitor of ITAM signaling comes from ITIM signaling. Interestingly, another AD risk gene CD33 contains an ITIM signaling domain, creating a potential link between TREM2 and CD33. Additionally, the gene which encodes for the protein SHIP1 has been identified as potential risk allele in AD and studies have shown that SHIP1 inhibition of PI3K activation exerts an inhibitory effect on TREM2 signaling. Ultimately, activation of TREM2 signaling results in several potential phenotypic alterations including altered migration, survival, proliferation, cytokine release, and other functions. Which intracellular pathways are directly responsible for the different functional consequences are still being worked out and may be ligand specific. TREM2 is also cleaved into a soluble fragment (sTREM2) by α-secretase (ADAM10/17, which are also implicated in AD risk and play key roles in APP processing). The function of sTREM2 is still not fully understood but sTREM2 can be detected in CSF and is being examined as potential biomarker of disease state in AD and other CNS disorders. sTREM2 has also been recently proposed to potentially bind to neurons, as well as potentially binding to an unknown receptor reciprocally on microglial. Finally, after α-secretase cleavage the remaining membrane fragment of TREM2 is cleaved by γ-secretase (components of which, PSEN1, and PSEN2, are known to cause early onset fAD). Inhibition of this cleavage results in inability of DAP12 to release from non-functional TREM2 and therefore sequestration of DAP12. Bold terms with asterisks indicate proteins coded for by genes that alter risk of AD.
Activation of TREM2 downstream signaling results in a number of different cellular effector functions including alterations in proliferation, altered survival, cytokine release, phagocytosis, and migration (for review see (Kober and Brett, 2017; Ulland and Colonna, 2018; Yeh et al., 2017)). Exactly how different ligands may activate different downstream aspects of the TREM2 response remains to be elucidated. Current research has identified several potential TREM2 ligands including bacteria (Daws et al., 2003; N'Diaye et al., 2009) anionic lipids (Daws et al., 2003; N'Diaye et al., 2009; Wang et al., 2015), phosphatidyl serine (P/S) (Cannon et al., 2012; Wang et al., 2015), proteins coded for by AD risk genes ApoE, Clusterin (CLU/APOJ) (Atagi et al., 2015; Bailey et al., 2015; Yeh et al., 2016), and potentially Aβ (Zhao et al., 2018). In addition to risk variants which serve as ligands for TREM2 signaling, other risk genes may inhibit TREM2. GWAS risk loci, INPP5D (SHIP1), was shown to inhibit TREM2 signaling via binding to DAP12, preventing recruitment of PI3K (Peng et al., 2010). Additionally, ITAM signaling is classically inhibited by ITIM signaling (immunoreceptor tyrosine-based inhibition motif) (Linnartz et al., 2010; Lowell, 2011) and another AD risk gene CD33 codes for an immune cell receptor which contains a classical ITIM domain (Crocker and Varki, 2001; Hollingworth et al., 2011; Naj et al., 2014), and could therefore potentially exert an inhibitory effect on TREM2 signaling. There have been a few studies which have examined potential interactions between CD33 and TREM2; however, much of that work has been done in either monocytes or mouse models, which have important caveats. Mouse Cd33 is not homologous to human CD33, because it lacks the ITIM domain and has differing ligand binding affinities and therefore results from mouse work involving deletion of Cd33 or altered Cd33 gene expression should be interpreted with caution (Brinkman-Van der Linden et al., 2003). Additionally, while the observed monocyte biology may be important to the pathogenesis of AD, there is a real need to study potential TREM2-CD33 interactions in human microglia, using either primary or iPSC-derived cells to better understand how these two risk factors may interact with a more physiologically-relevant cell type.
Degeneration of neurons, axons and synapses are hallmarks of neurodegenerative disease. As the resident innate immune cell and phagocytes in the brain, microglia are responsible for sensing and clearing dead or dying cells and debris. One mechanism by which microglia sense neuronal damage is binding to exposed P/S, which flips to the extracellular side of neuronal membranes during apoptosis and damage. Blocking P/S with AnnexinV decreases microglial phagocytosis of damaged neurons (Krasemann et al., 2017). Several receptors have been found to directly bind P/S, including TIM4, BAI1, and TREM2 (Cannon et al., 2012; Miyanishi et al., 2007; Park et al., 2007; Wang et al., 2015), as well as other which bind P/S via indirect mechanisms to other receptors including MERTK and AXL (Grommes et al., 2008; Meyer et al., 2015). Of all of the proposed receptors, TREM2 exhibits the highest gene expression in microglia (Hammond et al., 2019; Zhang et al., 2016) and TREM2 recognition of exposed P/S on damaged neurons fits with the idea that TREM2, among other receptors on microglia, function as damage sensors that are activated by neurodegeneration. In line with the model of TREM2 as a sensor is the consistent finding that in amyloid mouse models crossed to Trem2-KO mice microglia no longer cluster in close proximity to amyloid plaques (Jay et al., 2015; Wang et al., 2015; Yuan et al., 2016). Recent evidence has also found that human AD patients carrying AD risk variants in TREM2 also exhibit a decrease in microglia association with plaque pathology (Parhizkar et al., 2019; Yuan et al., 2016). However, the question remains as to what exactly TREM2 is sensing in or around the plaques that mediates microglial migration. It is possible that microglia are detecting ApoE or Clusterin, which have both been found to associate with Aβ and are found within plaques in human AD patients and mouse models (Calero et al., 2000; Holtzman, 2004; Namba et al., 1991).
In the case of ApoE, new data implies that ApoE observed in plaques may actually be the result of microglial TREM2 activation. Microglia in an AD mouse massively upregulate Apoe in response to amyloid (Keren-Shaul et al., 2017), and both Trem2-KO and a loss-of-function TREM2 variant from FTD (T66M) both dramatically reduce plaque-associated ApoE in a mouse model (Parhizkar et al., 2019). Thus, a potential cycle involves initial microglial reaction, which results in upregulation and secretion of ApoE, leading to altered amyloid deposition with ApoE incorporated into the plaque, which binds to TREM2 and continues the cycle. This potential cycle is supported by work demonstrating that APP/PS1 mice crossed to Apoe-KO mice have significant reductions in the number of plaque-associated microglia (Ulrich et al., 2018). Furthermore, treatment of an AD mouse model with antibody specifically against non-lipidated ApoE (including that found in plaques) reduced amyloid deposition (Liao et al., 2018).
One aspect of TREM2 signaling that needs further examination is the importance of soluble TREM2 (sTREM2) and TREM2 C-terminal fragments (CTFs). sTREM2 is generated through alpha secretase cleavage of the extracellular domain at histidine 157 by ADAM10/17 (Schlepckow et al., 2017; Thornton et al., 2017). This cleavage site on TREM2 is also the site of an AD risk SNP in the Han Chinese population (Jiang et al., 2014). Alpha-secretase cleavage is also a critical part of the amyloid processing pathway of APP, and both rare and common variants in ADAM10 and ADAM17 have been found to increase AD risk (Hartl et al., 2018; Kim et al., 2009; Kunkle et al., 2019). The TREM2 H157Y variants is thought to result in loss of function of TREM2 via increased cleavage of TREM2 into sTREM2 (Schlepckow et al., 2017; Thornton et al., 2017). However, what function sTREM2 might exert after cleavage is currently unknown. One recent report in humanized TREM2 mice crossed to an AD model found that, in the presence of AD pathology, human sTREM2 binds to the surface of neurons in both the cortex and hippocampus and that mice carrying human TREM2 with the R47H variant exhibited decreased neuronal associated sTREM2 (Song et al., 2018). Although this finding will need further validation and research into what the functional role of such binding to neurons may be, it is intriguing. Others have found that sTREM2 induces inflammatory response in microglia and therefore may be acting as part of a feedback mechanism (Zhong et al., 2017). Finally, several groups are studying the applicability of sTREM2 as a biomarker in various neurodegenerative disorders (Piccio et al., 2008; Piccio et al., 2016; Suárez-Calvet et al., 2016; Suárez-Calvet et al., 2019; Woollacott et al., 2018), but it is still unclear whether the sTREM2 in CSF has any active biological function or whether it is simply being cleared from the brain. While most of the research has focused on sTREM2 following cleavage, more work is needed to understand the impact of TREM2 CTF and CTF cleavage. The TREM2 CTF that remains after alpha-secretase cleavage is still bound to DAP12 and is subsequently cleaved by γ-secretase (Wunderlich et al., 2013). Two of the components of the γ-secretase enzyme complex are presenilin-1 and presenilin-2, mutations in which cause early-onset familial AD (Selkoe 2001; Tanzi, 2012). Those mutations have been shown to modulate γ-secretase’s cleavage of APP, but it is unknown whether those variants may also affect γ-secretase cleavage of TREM2. In vitro research has found that γ-secretase cleavage of TREM2 is critical, as inhibition of γ-secretase resulted in loss of downstream TREM2 signaling (Glebov et al., 2016) and that overexpression of TREM2 CTFs resulted in decreased response to lipopolysaccharide exposure (Zhong et al., 2015). This research will need further validation in more physiologically relevant human cells and in vivo mouse models and adds another layer to the complexities of TREM2 signaling. Collectively, this recent work provides intriguing links between TREM2 and other AD-associated risk genes and suggests that part of the risk conferred by other AD variants may be due to their modulation of TREM2 signaling.
Concluding Remarks
Due to widespread alterations in neuroimmune signaling during neurodegenerative disease, it is likely that components of specific immune pathways could be used to track or limit disease progression. Analysis of TGF-β, complement, sTREM2, and other immune signals in the CSF of patients has already yielded informative results about the extent of immune-related changes at each disease stage. As we gain more insight into the cellular players and mechanisms that contribute to pathology, a new suite of important pathways and targets will undoubtedly emerge. This process has been accelerated by recent advancements in genomic analysis and tools like single-cell RNA-seq, which are being used to investigate “normal” and pathological microglia subpopulations in disease (some recent examples include (Hammond et al., 2019; Keren-Shaul et al., 2017; Mathys et al., 2017). These new datasets are providing important insights into microglial phenotypes during normal development and within amyloid mouse models. However, similar analyses need to be undertaken in animal models of other neurodegenerative diseases. It is also important for single-nucleus RNA-seq studies to be performed on human samples to assess both patient variability and differences between mouse models and human disease states. Finally, advances in single cell and bulk-epigenetic analyses will also enable better exploration of the role of disease risk variants in which occur in non-coding regions and decode potential cell-type specific promoters or enhancers (Gosselin et al., 2017).
While single-cell analysis is useful for static measurements of inflammatory states, we also need better ways to track inflammatory cell states in real-time in live animals and human patients with approaches like PET imaging. The most common PET ligand used to track altered immune cell function binds to translocator protein (TSPO), but is not specific to any cell type and cannot distinguish between beneficial and pathological inflammatory events (Notter et al., 2018). Development of new biomarkers that distinguish between different subpopulations of microglia, astrocytes, and immune cells will give real-time insight into the progression of disease, revealing the location of plaques, foci of dying neurons, and other pathologies. The recent discovery that factors from the brain drain into the lymphatic system suggests that changes in the brain-immune signaling environment could be tracked less invasively (Da Mesquita et al., 2018; Louveau et al., 2015). Many groups are currently examining a variety of potentially immune-related biomarkers, including sTREM2, YKL-40, complement, and IP-10 in CSF to determine whether they correlate with diagnosis and/or disease progression (For recent review on biomarkers in neurodegenerative diseases see (Magdalinou et al., 2014; Molinuevo et al., 2018; Zetterberg et al., 2019)). While some of these markers are showing promise in regards to their correlation with diagnosis/disease states, more research will be required to understand how immune signaling leads to alterations in biomarker concentrations in CSF and whether those molecules in the CSF still play a biologically active role in disease-related immune signaling.
Targeting immune signaling pathways to limit neurodegenerative disease progression should also be a major research focus moving forward. Given the conserved disease mechanisms of the immune system across of neurodegenerative disease, dedicating resources to identifying and targeting key triggers of immune dysfunction and pathological functions of immune cells, including phagocytosis and synaptic pruning, will be important. However, it is still unclear whether targeting pathways like TGF-β, complement, and TREM2 will be effective, and caution must be taken in modulating critical systems involved in the function of the immune system outside the brain.
Much of the functional work in the field, including those we highlighted in this Review, have been performed using rodent models or cells, thus there is need to examine these aspects using more physiologically relevant human cells and chimeric humanized models. Recent advances in the ability to generate microglia-like cells from induced pluripotent stem cells (iPSC) offer a novel tool for the assessment of altered immune signaling and function in cells derived from patients with and without specific disease variants. Issues generating microglia and maintaining them in a state that resembles their in vivo form have plagued the field, but with the creation of fully-defined media and stepwise differentiation protocols that mimic the in vivo microglia development, the field now has more relevant tools to examine human microglial function (Abud et al., 2017). Importantly, combining iPSC microglia into complex cultures with other brain cell types, 3D brain organoids, and humanized chimeric mouse models will allow for the dissection of complex cell interactions that are not observed in monoculture conditions (Abud et al., 2017; Mancuso et al., 2019). However, caution should be used in both the design and interpretation of these experiments making sure to use iPSCs from many different patients while accounting for human genetic variation that could skew results. Ultimately, these models will never completely recapitulate the in vivo setting but allow a closer examination of human disease mechanisms.
One idea that has gained traction in the past few decades is the use of immunotherapy: harnessing the immune system to clear protein aggregates and remove neurotoxic stimuli. Several immunotherapy phase 3 trials have been completed in AD, all ending in failure. However, the failure was based on not meeting the primary endpoint of improved cognition. Biomarker data emerging from some of these trials has shown that treatments appeared to lower brain amyloid levels (Liu et al., 2015). The failure to improve cognition likely represents the fact that amyloid is thought to be the initiating factor in the disease and therefore, the downstream neurotoxic processes might have been too far along by the time the trials were conducted (Jack et al., 2013). New trials with more promising antibodies are currently being run with patients at the earliest signs of memory loss, with the hope that earlier treatment will slow cognitive decline (Sevigny et al., 2016). Additionally, prevention trials, such as the A4 trial and those using familial AD cohorts, will also provide valuable data on the timing necessary for anti-amyloid therapies to potentially impact cognition (Sperling et al., 2014). In addition to amyloid, several preclinical and clinical trials are currently underway with immunotherapies targeting a range of other pathogenic protein aggregates including tau and α-synuclein (Brys et al., 2018; Qureshi et al., 2018; Schenk et al., 2017; Weihofen et al., 2019). Only time will tell if any of these approaches is sufficient to improve cognition, but results indicate that, when properly targeted or activated, the immune system is capable of clearing these neurotoxic aggregates. However, how antibodies exert their function to clear protein aggregates in neurodegenerative diseases still requires further research to understand the mechanisms and signaling pathways involved. Some AD immunotherapies have been found to be dependent on standard Fc receptor-based mechanisms (Bard et al., 2000; Koenigsknecht-Talboo et al., 2008; Liao et al., 2018), whereas other AD immunotherapies have shown ability to clear their protein targets independent of the Fc receptor (Bacskai et al., 2002; Das et al., 2003; Lee et al., 2016). Furthermore, both ApoE isoform and TREM2 function have been found to modulate the efficacy of anti-amyloid immunotherapy (Pankiewicz et al., 2017; Xiang et al., 2016). These results likely mean that a combination of both the correct protein target (or correct conformation of the protein), along with optimization of other properties of the antibody, are required both for the correct signaling pathways to be activated and the therapy to be truly effective. Furthermore, timing of potential immune-based treatments may be critical; a therapy that improves disease pathology at one stage may exacerbate disease at another. Striking a balance between maintaining the beneficial functions of these pathways (i.e., removing protein aggregates), while preventing the pathological ones (i.e., depletion of synapses) will also be tricky.
Taken together, the immune system is front and center in the fight against neurodegenerative disease. Dissecting these signaling circuits in health and disease contexts will take time to fully understand. What is clear is that disease progression and outcome are driven by a complex interplay between dysfunctional neurons and signals from microglia and the periphery and a highly collaborative, interdisciplinary effort is needed to move this research forward in the field.
Acknowledgments
This work was supported in part by: Cure Alzheimer’s Fund (B.S.), NIH/NIA 1RF1AG051496 (B.S.), and NICHD U54HD090255 (B.S.).
We wish to thank Christina Usher for her helpful feedback and editing. We also wish to thank the authors of the following papers for their commitment to open science for their creation of a series of excellent online databases which were extremely helpful in examination of immune cell enrichment for novel genes/loci: Dropviz.org (Saunders et al., 2018); Microgliasinglecell.com (Hammond et al., 2019); Brainrnaseq.org (Bennett et al., 2016; Zhang et al., 2014; Zhang et al., 2016); Immgen.org (Immunological Genome Project; (Heng et al., 2008)); gtexportal.org (Gene-Tissue Expression Project; Gtex 2013).
The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used in reference to tissue gene expression enrichment in this manuscript were obtained from: GTEx Gene Expression Portal on 02/05/19 https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000424.v7.p2
Footnotes
Conflicts of Interest:
No conflicts of interest to declare.
B.S. is a member of the Scientific Advisory Board and minor shareholder in Annexon Biosciences. B.S. is an inventor on multiple patents: “Modulation of Synaptic Maintenance” (US8148330B2, US9149444B2) and is inventor on pending patent “Biomarkers for dementia and dementia related neurological disorders” (WO2015103594A1/US20160327572A1). T.R.H. & S.E.M. have no interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abud E, Ramirez R, Martinez E, Healy L, Nguyen C, Newman S, Yeromin A, Scarfone V, Marsh S, Fimbres C, et al. (2017). iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94, 278–293.e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afagh A, Cummings BJ, Cribbs DH, Cotman CW, and Tenner AJ (1996). Localization and cell association of C1q in Alzheimer's disease brain. Exp Neurol 138, 22–32. [DOI] [PubMed] [Google Scholar]
- Alzheimer A (1907). Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift für Psychiatrie und psychisch-gerichtliche Medizin 64. [Google Scholar]
- Alzheimer A (1911). Über eigenartige Krankheitsfälle des späteren Alters. [Google Scholar]
- Atagi Y, Liu C, Painter M, Chen X, Verbeeck C, Zheng H, Li X, Rademakers R, Kang S, Xu H, et al. (2015). Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J Biol Chem 290, 26043–26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai B, Kajdasz S, McLellan M, Games D, Seubert P, Schenk D, and Hyman B (2002). Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci 22, 7873–7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C, DeVaux L, and Farzan M (2015). The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J Biol Chem 290, 26033–26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J, Bourdette D, Meshul C, and Quinn J (2018). The key role of T cells in Parkinson's disease pathogenesis and therapy. Parkinsonism Relat Disord. [DOI] [PubMed] [Google Scholar]
- Bang J, Spina S, and Miller B (2015). Frontotemporal dementia. Lancet 386, 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke R, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. (2000). Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6, 916–919. [DOI] [PubMed] [Google Scholar]
- Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif A, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, and Schwartz M (2015). Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat Commun 6, 7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemiller S, McCray T, Allan K, Formica S, Xu G, Wilson G, Kokiko-Cochran O, Crish S, Lasagna-Reeves C, Ransohoff R, et al. (2017). TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol Neurodegener 12, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett F, Bennett M, Yaqoob F, Mulinyawe S, Grant G, Hayden Gephart M, Plowey E, and Barres B (2018). A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 98, 1170–1183.e1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, et al. (2016). New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113, E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley KJ, Bandres-Ciga S, Saez-Atienzar S, and Singleton AB (2018). Genetic risk factors in Parkinson’s disease. Cell and Tissue Research 373, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobela W, Zheng L, and Schneider BL (2014). Overview of mouse models of Parkinson's disease. Curr Protoc Mouse Biol 4, 121–139. [DOI] [PubMed] [Google Scholar]
- Bond AM, Bhalala OG, and Kessler JA (2012). The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol 72, 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham LW, Desikan RS, Yokoyama JS, and Alzheimer's Disease Neuroimaging I (2016). The relationship between complement factor C3, APOE epsilon4, amyloid and tau in Alzheimer's disease. Acta Neuropathol Commun 4, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon A, Dietrich J, and Colonna M (2000). Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. The Journal of Immunology 164, 4991–4995. [DOI] [PubMed] [Google Scholar]
- Braak H, and Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, and Del Tredici K (2004). Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 318, 121–134. [DOI] [PubMed] [Google Scholar]
- Brinkman-Van der Linden ECM, Angata T, Reynolds SA, Powell LD, Hedrick SM, and Varki A (2003). CD33/Siglec-3 Binding Specificity, Expression Pattern, and Consequences of Gene Deletion in Mice. Molecular and Cellular Biology 23, 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, and Wyss-Coray T (2003). Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145. [DOI] [PubMed] [Google Scholar]
- Broce I, Karch C, Wen N, Fan C, Wang Y, Tan C, Kouri N, Ross O, Höglinger G, Muller U, et al. (2018). Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies. PLoS Med 15, e1002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay J, et al. (2009). Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys M, Fanning L, Penner N, Yang M, Welch M, Koenig E, David E, Fox T, Makh S, Graham D, et al. (2018). Randomized, double-blind, placebo-controlled, single ascending dose study of anti-alpha-synuclein antibody BIIB054 in patients with Parkinson's disease. Paper presented at: 70th Annual Meeting of the American Academy of Neurology, AAN 2018 (Los Angeles, CA). [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. (2014). Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero M, Rostagno A, Matsubara E, Zlokovic B, Frangione B, and Ghiso J (2000). Apolipoprotein J (clusterin) and Alzheimer's disease. Microsc Res Tech 50, 305–315. [DOI] [PubMed] [Google Scholar]
- Cannon J, O'Driscoll M, and Litman G (2012). Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics 64, 39–47. [DOI] [PubMed] [Google Scholar]
- Caraci F, Battaglia G, Busceti C, Biagioni F, Mastroiacovo F, Bosco P, Drago F, Nicoletti F, Sortino MA, and Copani A (2008). TGF-beta 1 protects against Abeta-neurotoxicity via the phosphatidylinositol-3-kinase pathway. Neurobiol Dis 30, 234–242. [DOI] [PubMed] [Google Scholar]
- Carmona S, Zahs K, Wu E, Dakin K, Bras J, and Guerreiro R (2018). The role of TREM2 in Alzheimer's disease and other neurodegenerative disorders. Lancet Neurol 17, 721–730. [DOI] [PubMed] [Google Scholar]
- Castellani R, Nugent S, Morrison A, Zhu X, Lee H, Harris P, Bajić V, Sharma H, Chen S, Oettgen P, et al. (2011). CD3 in Lewy pathology: does the abnormal recall of neurodevelopmental processes underlie Parkinson's disease. J Neural Transm (Vienna) 118, 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano J, Mosher K, Abbey R, McBride A, James M, Berdnik D, Shen J, Zou B, Xie X, Tingle M, et al. (2017). Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, and Colonna M (2003). Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med 198, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, White CC, Winn PA, Cimpean M, Replogle JM, Glick LR, Cuerdon NE, Ryan KJ, Johnson KA, Schneider JA, et al. (2016). Trans-pQTL study identifies immune crosstalk between Parkinson and Alzheimer loci. Neurology Genetics 2, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Nalls M, Hallgrímsdóttir I, Hunkapiller J, van der Brug M, Cai F, International, P.s.D.G.C., 23andMe, R.T., Kerchner G, Ayalon G, et al. (2017). A meta-analysis of genomewide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Frey WH 2nd, Ala TA, Tourtellotte WW, and Peterson PK (1994). Transforming growth factor beta in Alzheimer's disease. Clin Diagn Lab Immunol 1, 109–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Ke KF, Lu JH, Qiu YH, and Peng YP (2015). Protection of TGF-beta1 against neuroinflammation and neurodegeneration in Abeta1-42-induced Alzheimer's disease model rats. PLoS One 10, e0116549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Ulane C, and Burke R (2010). Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67, 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JR, Chai YL, Lee JH, Howlett D, Attems J, Ballard CG, Aarsland D, Francis PT, Chen CP, and Lai MK (2017). Increased Transforming Growth Factor beta2 in the Neocortex of Alzheimer's Disease and Dementia with Lewy Bodies is Correlated with Disease Severity and Soluble Abeta42 Load. J Alzheimers Dis 56, 157–166. [DOI] [PubMed] [Google Scholar]
- Chouery E, Delague V, Bergougnoux A, Koussa S, Serre J, and Mégarbané A (2008). Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat 29, E194–204. [DOI] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, and Prince DA (2010). Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A 107, 7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, et al. (2013). Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci U S A 110, 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. (2009). Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11, 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses A, Haines J, and Pericak-Vance M (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Crocker P, and Varki A (2001). Siglecs, sialic acids and innate immunity. Trends Immunol 22, 337–342. [DOI] [PubMed] [Google Scholar]
- Cronk J, Filiano A, Louveau A, Marin I, Marsh R, Ji E, Goldman D, Smirnov I, Geraci N, Acton S, et al. (2018). Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med 215, 1627–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, et al. (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 560, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy M, and Golde T (2003). Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. J Neurosci 23, 8532–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, and Maloney A (1976). Selective loss of central cholinergic neurons in Alzheimer's disease. In Lancet, pp. 1403. [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, and Seaman WE (2003). Pattern Recognition by TREM-2: Binding of Anionic Ligands. The Journal of Immunology 171, 594–599. [DOI] [PubMed] [Google Scholar]
- Dawson T, Golde T, and Lagier-Tourenne C (2018). Animal models of neurodegenerative diseases. Nat Neurosci 21, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Huntley MA, De Maziere A, Meilandt WJ, Wu T, Srinivasan K, Jiang Z, Gandham V, Friedman BA, Ngu H, et al. (2018). Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 100, 1322–1336 e1327. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie I, Boeve B, Boxer A, Baker M, Rutherford N, Nicholson A, Finch N, Flynn H, Adamson J, et al. (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S, and Scheff S (1990). Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol 27, 457–464. [DOI] [PubMed] [Google Scholar]
- Denk F, and Wade-Martins R (2009). Knock-out and transgenic mouse models of tauopathies. Neurobiol Aging 30, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depboylu C, Schorlemmer K, Klietz M, Oertel WH, Weihe E, Hoglinger GU, and Schafer MK (2011). Upregulation of microglial C1q expression has no effects on nigrostriatal dopaminergic injury in the MPTP mouse model of Parkinson disease. J Neuroimmunol 236, 39–46. [DOI] [PubMed] [Google Scholar]
- Derecki N, Cardani A, Yang C, Quinnies K, Crihfield A, Lynch K, and Kipnis J (2010). Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 207, 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVos S, Corjuc B, Commins C, Dujardin S, Bannon R, Corjuc D, Moore B, Bennett R, Jorfi M, Gonzales J, et al. (2018). Tau reduction in the presence of amyloid-β prevents tau pathology and neuronal death in vivo. Brain 141, 2194–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias JM, Alekseenko Z, Applequist JM, and Ericson J (2014). Tgfbeta signaling regulates temporal neurogenesis and potency of neural stem cells in the CNS. Neuron 84, 927–939. [DOI] [PubMed] [Google Scholar]
- Dickson D, Heckman M, Murray M, Soto A, Walton R, Diehl N, van Gerpen J, Uitti R, Wszolek Z, Ertekin-Taner N, et al. (2018). APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 91, e1182–e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LP, Tortelli V, Matias I, Morgado J, Bergamo Araujo AP, Melo HM, Seixas da Silva GS, Alves-Leon SV, de Souza JM, Ferreira ST, et al. (2017). Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Abeta Oligomers in Alzheimer's Disease Model. J Neurosci 37, 6797–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Vincze C, Pal G, and Lovas G (2012). The neuroprotective functions of transforming growth factor beta proteins. Int J Mol Sci 13, 8219–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Geczy C, and Halliday G (2015). Inflammation is genetically implicated in Parkinson's disease. Neuroscience 302, 89–102. [DOI] [PubMed] [Google Scholar]
- Farfel J, Yu L, Buchman A, Schneider J, De Jager P, and Bennett D (2016). Relation of genomic variants for Alzheimer disease dementia to common neuropathologies. Neurology 87, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Hernandez D, Nalls M, Rohrer J, Ramasamy A, Kwok J, Dobson-Stone C, Brooks W, Schofield P, Halliday G, et al. (2014). Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol 13, 686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, and Tenner AJ (2009). Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J Immunol 183, 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Rodriguez G, Herman M, Emrani S, Nahmani E, Barrett G, Figueroa H, Goldberg E, Hussaini S, and Duff K (2017). Tau Pathology Induces Excitatory Neuron Loss, Grid Cell Dysfunction, and Spatial Memory Deficits Reminiscent of Early Alzheimer's Disease. Neuron 93, 533–541.e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano SA, Pouget JG, Hardy J, Knight J, Barnes MR, Ryten M, and Weale ME (2016). Genomics implicates adaptive and innate immunity in Alzheimer's and Parkinson's diseases. Annals of Clinical and Translational Neurology 3, 924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, and Gillespie F (1995). Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373, 523–527. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann F, Bussière T, Bouras C, Kövari E, Perl D, Morrison J, Gold G, and Hof P (2003). Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60, 1495–1500. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler M, Conway S, Ng L, Stanley E, et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo M, Lopera F, Siniard A, Corneveaux J, Schrauwen I, Carvajal J, Muñoz C, Ramirez-Restrepo M, Gaiteri C, Myers A, et al. (2013). Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer's disease. Neurobiol Aging 34, 2077.e2011–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjoneska E, Pfenning A, Mathys H, Quon G, Kundaje A, Tsai L, and Kellis M (2015). Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer's disease. Nature 518, 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov K, Wunderlich P, Karaca I, and Walter J (2016). Functional involvement of γ-secretase in signaling of the triggering receptor expressed on myeloid cells-2 (TREM2). J Neuroinflammation 13, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin M, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, and James L (1991). Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349, 704–706. [DOI] [PubMed] [Google Scholar]
- Goetz C, Emre M, and Dubois B (2008). Parkinson's disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol 64 Suppl 2, S81–92. [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Hollister R, West H, Mui S, Growdon J, Petersen R, Parisi J, and Hyman B (1997). Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol 41, 17–24. [DOI] [PubMed] [Google Scholar]
- Götz J, Bodea L, and Goedert M (2018). Rodent models for Alzheimer disease. Nat Rev Neurosci 19, 583–598. [DOI] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet A, Beinis J, Gallinari C, Meaume S, Piette F, Hauw J, and Duyckaerts C (2006). Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63, 584–588. [DOI] [PubMed] [Google Scholar]
- Grommes C, Lee C, Wilkinson B, Jiang Q, Koenigsknecht-Talboo J, Varnum B, and Landreth G (2008). Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol 3, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium (2013). The Genotype-Tissue Expression (GTEx) project. Nat Genet 45, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Lohmann E, Brás J, Gibbs J, Rohrer J, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, et al. (2013a). Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol 70, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Ross O, Kun-Rodrigues C, Hernandez D, Orme T, Eicher J, Shepherd C, Parkkinen L, Darwent L, Heckman M, et al. (2018). Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol 17, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe J, Younkin S, et al. (2013b). TREM2 variants in Alzheimer's disease. N Engl J Med 368, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman J, Ni M, Killebrew J, Chu C, and Lowell C (2009). The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev 232, 42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R (2000). Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 10, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond T, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker A, Gergits F, Segel M, Nemesh J, et al. (2019). Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50, 253–271.e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Robinton D, and Stevens B (2018). Microglia and the Brain: Complementary Partners in Development and Disease. Annu Rev Cell Dev Biol. [DOI] [PubMed] [Google Scholar]
- Hamza T, Zabetian C, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay D, Doheny K, Paschall J, Pugh E, et al. (2010). Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet 42, 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U, and Kettenmann H (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay M, Hofstetter R, and Klauber M (1990). The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 40, 1–8. [DOI] [PubMed] [Google Scholar]
- Hartl D, AESG, May P, Gu W, Mayhaus M, Pichler S, Spaniol C, Glaab E, Bobbili DR, Antony P, et al. (2018). A rare loss-of-function variant of ADAM17 is associated with late-onset familial Alzheimer disease. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Liu L, Tooyama I, Murayama S, and Nishimura M (2014). The FAM3 superfamily member ILEI ameliorates Alzheimer's disease-like pathology by destabilizing the penultimate amyloid-beta precursor. Nat Commun 5, 3917. [DOI] [PubMed] [Google Scholar]
- Heneka M, Carson M, Khoury J, Landreth G, Brosseron F, Feinstein D, Jacobs A, Wyss-Coray T, Vitorica J, Ransohoff R, et al. (2015). Neuroinflammation in Alzheimer's disease. In Lancet Neurol, pp. 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng T, Painter M, and Immunological GPC (2008). The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9, 1091–1094. [DOI] [PubMed] [Google Scholar]
- Hinz F, and Geschwind D (2017). Molecular Genetics of Neurodegenerative Dementias. Cold Spring Harb Perspect Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J, Carrasquillo M, Abraham R, Hamshere M, Pahwa J, Moskvina V, et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 43, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F, Sadd M, Bras JM, Bettella F, Nicolaou N, et al. (2013). A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson's disease. Human Molecular Genetics 22, 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D (2004). In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci 23, 247–254. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, and Cole G (1996). Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102. [DOI] [PubMed] [Google Scholar]
- Huang K, Marcora E, Pimenova A, Di Narzo A, Kapoor M, Jin S, Harari O, Bertelsen S, Fairfax B, Czajkowski J, et al. (2017). A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer's disease. Nat Neurosci 20, 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Weisgraber K, Mucke L, and Mahley R (2004). Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer's disease. J Mol Neurosci 23, 189–204. [DOI] [PubMed] [Google Scholar]
- International Genomics of Alzheimer's Disease Consortium, I. (2015). Convergent genetic and expression data implicate immunity in Alzheimer's disease. Alzheimers Dement 11, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, and Gong CX (2016). Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 12, 15–27. [DOI] [PubMed] [Google Scholar]
- Jack CJ, Knopman D, Jagust W, Petersen R, Weiner M, Aisen P, Shaw L, Vemuri P, Wiste H, Weigand S, et al. (2013). Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes R, Spillantini M, and Goedert M (1994). Identification of two distinct synucleins from human brain. FEBS Lett 345, 27–32. [DOI] [PubMed] [Google Scholar]
- Jansen I, Savage J, Watanabe K, Bryois J, Williams D, Steinberg S, Sealock J, Karlsson I, Hägg S, Athanasiu L, et al. (2019). Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, Bayer T, and Wirths O (2012). Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging 33, 196.e129–140. [DOI] [PubMed] [Google Scholar]
- Jay T, Miller C, Cheng P, Graham L, Bemiller S, Broihier M, Xu G, Margevicius D, Karlo J, Sousa G, et al. (2015). TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med 212, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Burdick D, Glabe CG, Cotman CW, and Tenner AJ (1994). beta-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J Immunol 152, 5050–5059. [PubMed] [Google Scholar]
- Jiang T, Tan L, Zhu X, Zhang Q, Cao L, Tan M, Gu L, Wang H, Ding Z, Zhang Y, et al. (2014). Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer's disease. Neuropsychopharmacology 39, 2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JU, Brubaker WD, Javitz H, Bergen AW, Nishita D, Trigunaite A, Crane A, Ceballos J, Mastroeni D, Tenner AJ, et al. (2018). Peripheral complement interactions with amyloid beta peptide in Alzheimer's disease: Polymorphisms, structure, and function of complement receptor 1. Alzheimers Dement 14, 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Holmans P, Hamshere M, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, et al. (2010). Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PLoS One 5, e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson P, Snaedal J, Bjornsson S, Huttenlocher J, Levey A, Lah J, et al. (2013). Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 368, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman N, Schein P, Miller C, Loffredo F, Wojtkiewicz G, Chen J, Lee R, Wagers A, and Rubin L (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM, Miller TM, and Diamond MI (2016). Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 92, 796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Houston SA, Sherwood E, Casulli J, and Travis MA (2017). Regulation of Innate and Adaptive Immunity by TGFbeta. Adv Immunol 134, 137–233. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland T, David E, Baruch K, Lara-Astaiso D, Toth B, et al. (2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell 169, 1276–1290.e1217. [DOI] [PubMed] [Google Scholar]
- Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, Norton D, Tesco G, Elliott K, Wagner SL, et al. (2009). Potential late-onset Alzheimer's disease-associated mutations in the ADAM10 gene attenuate α-secretase activity. Human Molecular Genetics 18, 3987–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klünemann H, Ridha B, Magy L, Wherrett J, Hemelsoet D, Keen R, De Bleecker J, Rossor M, Marienhagen J, Klein H, et al. (2005). The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology 64, 1502–1507. [DOI] [PubMed] [Google Scholar]
- Kober DL, Alexander-Brett JM, Karch CM, Cruchaga C, Colonna M, Holtzman MJ, and Brett TJ (2016). Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. Elife 5, e20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober DL, and Brett TJ (2017). TREM2-Ligand Interactions in Health and Disease. Journal of Molecular Biology 429, 1607–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn M, Hyman B, Bacskai B, and Holtzman D (2008). Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci 28, 14156–14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, and Grundke-Iqbal I (1993). Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268, 24374–24384. [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z, et al. (2017). The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle BW, Alzheimer DGCA, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, et al. (2019). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nature Genetics 51, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli M, Bettcher B, Arcila M, Garcia G, Guzman C, Madrigal L, Ramirez L, Acosta-Uribe J, Baena A, Wojta K, et al. (2015). Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer's disease. Mol Psychiatry 20, 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido M, Tavernier B, et al. (2009). Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet 41, 1094–1099. [DOI] [PubMed] [Google Scholar]
- Lambert J, Ibrahim-Verbaas C, Harold D, Naj A, Sims R, Bellenguez C, DeStafano A, Bis J, Beecham G, Grenier-Boley B, et al. (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 45, 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Le Pichon C, Adolfsson O, Gafner V, Pihlgren M, Lin H, Solanoy H, Brendza R, Ngu H, Foreman O, et al. (2016). Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement. Cell Rep 16, 1690–1700. [DOI] [PubMed] [Google Scholar]
- Lee Y, Dawson VL, and Dawson TM (2012). Animal models of Parkinson's disease: vertebrate genetics. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, et al. (2000). Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25, 402–405. [DOI] [PubMed] [Google Scholar]
- Leyns C, Ulrich J, Finn M, Stewart F, Koscal L, Remolina Serrano J, Robinson G, Anderson E, Colonna M, and Holtzman D (2017). TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A 114, 11524–11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, and Zheng H (2016). Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer's Disease. J Neurosci 36, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Li S, Guo Q, Zhang Y, Wen C, Zou Q, and Su B (2007). Complement 3-deficient mice are not protected against MPTP-induced dopaminergic neurotoxicity. Brain Res 1178, 132–140. [DOI] [PubMed] [Google Scholar]
- Liao F, Li A, Xiong M, Bien-Ly N, Jiang H, Zhang Y, Finn MB, Hoyle R, Keyser J, Lefton KB, et al. (2018). Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation. Journal of Clinical Investigation 128, 2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill C, Rengmark A, Pihlstrøm L, Fogh I, Shatunov A, Sleiman P, Wang L, Liu T, Lassen C, Meissner E, et al. (2015). The role of TREM2 R47H as a risk factor for Alzheimer's disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson's disease. Alzheimers Dement 11, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz B, Wang Y, and Neumann H (2010). Microglial immunoreceptor tyrosine-based activation and inhibition motif signaling in neuroinflammation. Int J Alzheimers Dis 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinchuk A, Wan YW, Swartzlander DB, Chen F, Cole A, Propson NE, Wang Q, Zhang B, Liu Z, and Zheng H (2018). Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer's Disease. Neuron 100, 1337–1353 e1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kanekiyo T, Xu H, and Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. In Nat Rev Neurol, pp. 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Schmidt M, Margolin R, Sperling R, Koeppe R, Mason N, Klunk W, Mathis C, Salloway S, Fox N, et al. (2015). Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology 85, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler DA, Camp DM, and Conant SB (2006). Complement activation in the Parkinson's disease substantia nigra: an immunocytochemical study. J Neuroinflammation 3, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell C (2011). Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui H, Zhang J, Makinson S, Cahill M, Kelley K, Huang H, Shang Y, Oldham M, Martens L, Gao F, et al. (2016). Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 165, 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalinou N, Lees A, and Zetterberg H (2014). Cerebrospinal fluid biomarkers in parkinsonian conditions: an update and future directions. J Neurol Neurosurg Psychiatry 85, 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook T, Carroll M, and Lemere C (2008). Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. In J Neurosci, pp. 6333–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R, Van Den Daele J, Fattorelli N, Wolfs L, Balusu S, Burton O, Sierksma A, Fourne Y, Poovathingal S, Arranz-Mendiguren A, et al. (2019). Stem cell derived human microglia transplanted in mouse brain to study genetic risk of Alzheimer's Disease. bioRxiv, 562561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Harris SE, Zhang Q, McRae AF, Hagenaars SP, Hill WD, Davies G, Ritchie CW, Gale CR, Starr JM, et al. (2018). GWAS on family history of Alzheimer’s disease. Translational Psychiatry 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S, Abud E, Lakatos A, Karimzadeh A, Yeung S, Davtyan H, Fote G, Lau L, Weinger J, Lane T, et al. (2016). The adaptive immune system restrains Alzheimer's disease pathogenesis by modulating microglial function. Proc Natl Acad Sci U S A 113, E1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S, and Blurton-Jones M (2012). Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimers Res Ther 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Adaikkan C, Gao F, Young J, Manet E, Hemberg M, De Jager P, Ransohoff R, Regev A, and Tsai L (2017). Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep 21, 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P, Itagaki S, Boyes B, and McGeer E (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291. [DOI] [PubMed] [Google Scholar]
- McGeer P, Itagaki S, Tago H, and McGeer E (1987). Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett 79, 195–200. [DOI] [PubMed] [Google Scholar]
- McKeith I, Boeve B, Dickson D, Halliday G, Taylor J, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard C, et al. (2017). Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicar DW, Taylor LS, Gosselin P, Willette-Brown J, Mikhael AI, Geahlen RL, Nakamura MC, Linnemeyer P, Seaman WE, Anderson SK, et al. (1998). DAP12-mediated Signal Transduction in Natural Killer Cells: A DOMINANT ROLE FOR THE Syk PROTEIN-TYROSINE KINASE. Journal of Biological Chemistry 273, 32934–32942. [DOI] [PubMed] [Google Scholar]
- Meredith GE, and Rademacher DJ (2011). MPTP mouse models of Parkinson's disease: an update. J Parkinsons Dis 1, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Zweemer A, and Lauffenburger D (2015). The AXL Receptor is a Sensor of Ligand Spatial Heterogeneity. Cell Syst 1, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson J, Loguercio S, and Beyer A (2009). Detection and interpretation of expression quantitative trait loci (eQTL). Methods 48, 265–276. [DOI] [PubMed] [Google Scholar]
- Milber JM, Noorigian JV, Morley JF, Petrovitch H, White L, Ross GW, and Duda JE (2012). Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology 79, 2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Ferrari R, Heutink P, Hardy J, Pijnenburg Y, Posthuma D, and International F-GC (2017). Gene-based association studies report genetic links for clinical subtypes of frontotemporal dementia. Brain 140, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, and Nagata S (2007). Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439. [DOI] [PubMed] [Google Scholar]
- Mócsai A, Abram C, Jakus Z, Hu Y, Lanier L, and Lowell C (2006). Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol 7, 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A, Ruland J, and Tybulewicz V (2010). The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 10, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P, and Nagatsu T (1995). Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson's disease. Neurosci Lett 193, 129–132. [DOI] [PubMed] [Google Scholar]
- Molinuevo J, Ayton S, Batrla R, Bednar M, Bittner T, Cummings J, Fagan A, Hampel H, Mielke M, Mikulskis A, et al. (2018). Current state of Alzheimer's fluid biomarkers. Acta Neuropathol 136, 821–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine T, Phelps C, Beach T, Bigio E, Cairns N, Dickson D, Duyckaerts C, Frosch M, Masliah E, Mirra S, et al. (2012). National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R, Hutter-Saunders J, Stone D, and Gendelman H (2012). Inflammation and adaptive immunity in Parkinson's disease. Cold Spring Harb Perspect Med 2, a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AA, Chakravarthy S, Phillips JR, Gupta A, Keri S, Polner B, Frank MJ, and Jahanshahi M (2016). Motor symptoms in Parkinson’s disease: A unified framework. Neuroscience & Biobehavioral Reviews 68, 727–740. [DOI] [PubMed] [Google Scholar]
- N'Diaye E, Branda C, Branda S, Nevarez L, Colonna M, Lowell C, Hamerman J, and Seaman W (2009). TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol 184, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj A, Jun G, Beecham G, Wang L, Vardarajan B, Buros J, Gallins P, Buxbaum J, Jarvik G, Crane P, et al. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet 43, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Reitz C, Kunkle BW, Perry W, Park YS, Beecham GW, Rajbhandary RA, Hamilton-Nelson KL, Wang L-S, et al. (2014). Effects of Multiple Genetic Loci on Age at Onset in Late-Onset Alzheimer Disease. JAMA Neurology 71, 1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls M, Pankratz N, Lill C, Do C, Hernandez D, Saad M, DeStefano A, Kara E, Bras J, Sharma M, et al. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet 46, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, and Ikeda K (1991). Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 541, 163–166. [DOI] [PubMed] [Google Scholar]
- Nelson P, Alafuzoff I, Bigio E, Bouras C, Braak H, Cairns N, Castellani R, Crain B, Davies P, Del Tredici K, et al. (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, and Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A, et al. (2018). Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Molecular Psychiatry 23, 323–334. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole S, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, et al. (2006). Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci 26, 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme T, Guerreiro R, and Bras J (2018). The Genetics of Dementia with Lewy Bodies: Current Understanding and Future Directions. Current Neurology and Neuroscience Reports 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini F, De Blasio D, Zangari R, Zanier ER, and De Simoni MG (2014). Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci 8, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro T, Koss D, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor J, Thomas A, et al. (2019). Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Klingener M, and Aguirre A (2014). TGFbeta signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci 34, 7917–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloneva J, Autti T, Raininko R, Partanen J, Salonen O, Puranen M, Hakola P, and Haltia M (2001). CNS manifestations of Nasu-Hakola disease: A frontal dementia with bone cysts. Neurology 56, 1552–1558. [DOI] [PubMed] [Google Scholar]
- Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. (2002). Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiewicz J, Baquero-Buitrago J, Sanchez S, Lopez-Contreras J, Kim J, Sullivan P, Holtzman D, and Sadowski M (2017). APOE Genotype Differentially Modulates Effects of Anti-Aβ, Passive Immunization in APP Transgenic Mice. Mol Neurodegener 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, Nuscher B, Xiong M, Ghasemigharagoz A, Katzmarski N, et al. (2019). Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nature Neuroscience 22, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont A, Elliott M, Lu M, Haney L, Ma Z, Klibanov A, Mandell J, and Ravichandran K (2007). BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434. [DOI] [PubMed] [Google Scholar]
- Peng Q, Malhotra S, Torchia J, Kerr W, Coggeshall K, and Humphrey M (2010). TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal 3, ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt R, Fenoglio C, Rinker J, Naismith R, Panina-Bordignon P, Passini N, et al. (2008). Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 131, 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Deming Y, Del-Águila J, Ghezzi L, Holtzman D, Fagan A, Fenoglio C, Galimberti D, Borroni B, and Cruchaga C (2016). Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol 131, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C, Ren Y, Perkerson R, Baker M, Jenkins G, van Blitterswijk M, DeJesus-Hernandez M, van Rooij J, Murray M, Christopher E, et al. (2019). Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Garrison BS, Ma W, Wang R, Jiang A, Li J, Mistry M, Bronson RT, Santoro D, Franco C, et al. (2018). A Milieu Molecule for TGF-beta Required for Microglia Function in the Nervous System. Cell 174, 156–171 e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi I, Tirucherai G, Ahlijanian M, Kolaitis G, Bechtold C, and Grundman M (2018). A randomized, single ascending dose study of intravenous BIIB092 in healthy participants. Alzheimers Dement (N Y) 4, 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj T, Rothamel K, Mostafavi S, Ye C, Lee M, Replogle J, Feng T, Lee M, Asinovski N, Frohlich I, et al. (2014). Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM (2018). All (animal) models (of neurodegeneration) are wrong. Are they also useful? J Exp Med 215, 2955–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley W, Hatanpaa K, Lomen-Hoerth C, Kertesz A, Bigio E, et al. (2013). TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson's disease. Mol Neurodegener 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichwald J, Danner S, Wiederhold KH, and Staufenbiel M (2009). Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs J, Schymick J, Laaksovirta H, van Swieten J, Myllykangas L, et al. (2011). A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman M, and Kruglyak L (2006). Genetics of global gene expression. Nat Rev Genet 7, 862–872. [DOI] [PubMed] [Google Scholar]
- Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, et al. (1992). Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 89, 10016–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren S, and Civin W (1988). Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging 9, 339–349. [DOI] [PubMed] [Google Scholar]
- Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, and Saido TC (2014). Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci 17, 661–663. [DOI] [PubMed] [Google Scholar]
- Salter MW, and Stevens B (2017). Microglia emerge as central players in brain disease. Nat Med 23, 1018–1027. [DOI] [PubMed] [Google Scholar]
- Sarma JV, and Ward PA (2011). The complement system. Cell Tissue Res. 343, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, Hardy J, Vassar R, Winblad B, and Saido TC (2017). APP mouse models for Alzheimer's disease preclinical studies. EMBO J 36, 2473–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, et al. (2018). Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015–1030 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed FA, Telpoukhovskaia M, Kodama L, Li Y, Zhou Y, Le D, Hauduc A, Ludwig C, Gao F, Clelland C, et al. (2018). Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proceedings of the National Academy of Sciences, 201811411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D, Lehrman E, Kautzman A, Koyama R, Mardinly A, Yamasaki R, Ransohoff R, Greenberg M, Barres B, and Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Koller M, Ness D, Griffith S, Grundman M, Zago W, Soto J, Atiee G, Ostrowitzki S, and Kinney G (2017). First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov Disord 32, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlepckow K, Kleinberger G, Fukumori A, Feederle R, Lichtenthaler S, Steiner H, and Haass C (2017). An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D (2001). Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Selkoe D, and Podlisny M (2002). Deciphering the genetic basis of Alzheimer's disease. Annu Rev Genomics Hum Genet 3, 67–99. [DOI] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et al. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 537, 50–56. [DOI] [PubMed] [Google Scholar]
- Shi Q, Chowdhury S, Ma R, Le K, Hong S, Caldarone B, Stevens B, and Lemere C (2017). Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R, van der Lee S, Naj A, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle B, Boland A, Raybould R, Bis J, et al. (2017). Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet 49, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Hooli B, Mullin K, Jin SC, Cella M, Ulland TK, Wang Y, Tanzi RE, and Colonna M (2017). Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement 13, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, and Colonna M (2018). The Microglial Response to Neurodegenerative Disease. Adv Immunol 139, 1–50. [DOI] [PubMed] [Google Scholar]
- Song W, Joshita S, Zhou Y, Ulland T, Gilfillan S, and Colonna M (2018). Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosna J, Philipp S, Albay R, Reyes-Ruiz J, Baglietto-Vargas D, LaFerla F, and Glabe C (2018). Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Rentz D, Johnson K, Karlawish J, Donohue M, Salmon D, and Aisen P (2014). The A4 study: stopping AD before symptoms begin. Sci Transl Med 6, 228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M, Schmidt M, Lee V, Trojanowski J, Jakes R, and Goedert M (1997). Alpha-synuclein in Lewy bodies. In Nature, pp. 839–840. [DOI] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, and Garel S (2014). Microglia modulate wiring of the embryonic forebrain. Cell Rep 8, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, and Stevens B (2012). The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35, 369–389. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, et al. (2013). A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci 33, 13460–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Stipursky J, Francis D, Dezonne RS, Bergamo de Araujo AP, Souza L, Moraes CA, and Alcantara Gomes FC (2014). TGF-beta1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci 8, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W, Saunders A, Schmechel D, Pericak-Vance M, Enghild J, Salvesen G, and Roses A (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Calvet M, Araque Caballero M, Kleinberger G, Bateman R, Fagan A, Morris J, Levin J, Danek A, Ewers M, Haass C, et al. (2016). Early changes in CSF sTREM2 in dominantly inherited Alzheimer's disease occur after amyloid deposition and neuronal injury. Sci Transl Med 8, 369ra178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Calvet M, Morenas-Rodríguez E, Kleinberger G, Schlepckow K, Araque Caballero M, Franzmeier N, Capell A, Fellerer K, Nuscher B, Eren E, et al. (2019). Early increase of CSF sTREM2 in Alzheimer's disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol Neurodegener 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay R, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand W, Mao X, et al. (2017). T cells from patients with Parkinson's disease recognize α-synuclein peptides. Nature 546, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford C, and Neumann H (2005). Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201, 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R (2012). The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R, Masliah E, Salmon D, Butters N, DeTeresa R, Hill R, Hansen L, and Katzman R (1991). Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30, 572–580. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Nguyen A, Chang B, Li L, Woodling NS, Wyss-Coray T, and Luo J (2017). Deficiency in Neuronal TGF-beta Signaling Leads to Nigrostriatal Degeneration and Activation of TGF-beta Signaling Protects against MPTP Neurotoxicity in Mice. J Neurosci 37, 4584–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, et al. (2006). Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest 116, 3060–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton P, Sevalle J, Deery M, Fraser G, Zhou Y, Ståhl S, Franssen E, Dodd R, Qamar S, Gomez Perez-Nievas B, et al. (2017). TREM2 shedding by cleavage at the H157-S158 bond is accelerated for the Alzheimer's disease-associated H157Y variant. EMBO Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K (2011). A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harb Perspect Med 1, a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, and Sheppard D (2014). TGF-beta activation and function in immunity. Annu Rev Immunol 32, 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, et al. (2013). APOE ϵ4 Increases Risk for Dementia in Pure Synucleinopathies. JAMA Neurology 70, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull I, and Colonna M (2007). Activating and inhibitory functions of DAP12. Nat Rev Immunol 7, 155–161. [DOI] [PubMed] [Google Scholar]
- Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero D, Kondo J, Ihara Y, and Saitoh T (1993). Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 90, 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, and Colonna M (2018). TREM2 — a key player in microglial biology and Alzheimer disease. Nature Reviews Neurology. [DOI] [PubMed] [Google Scholar]
- Ulrich J, Ulland T, Mahan T, Nyström S, Nilsson K, Song W, Zhou Y, Reinartz M, Choi S, Jiang H, et al. (2018). ApoE facilitates the microglial response to amyloid plaque pathology. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin V, Sleiman P, Martinez-Lage M, Chen-Plotkin A, Wang L, Graff-Radford N, Dickson D, Rademakers R, Boeve B, Grossman M, et al. (2010). Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42, 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S, Luo J, Mosher K, Zou B, Britschgi M, Bieri G, Stan T, Fainberg N, Ding Z, Eggel A, et al. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S, Plambeck K, Middeldorp J, Castellano J, Mosher K, Luo J, Smith L, Bieri G, Lin K, Berdnik D, et al. (2014). Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, and Lang AE (2016). alpha-Synuclein-Based Animal Models of Parkinson's Disease: Challenges and Opportunities in a New Era. Trends Neurosci 39, 750–762. [DOI] [PubMed] [Google Scholar]
- Walker Z, Possin K, Boeve B, and Aarsland D (2015). Lewy body dementias. Lancet 386, 1683–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich J, Young K, Robinette M, Gilfillan S, Krishnan G, Sudhakar S, Zinselmeyer B, et al. (2015). TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell 160, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, and Mandelkow E (2016). Tau in physiology and pathology. Nat Rev Neurosci 17, 5–21. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Liu Y, Arndt J, Huy C, Quan C, Smith B, Baeriswyl J, Cavegn N, Senn L, Su L, et al. (2019). Development of an aggregate-selective, human-derived α-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson's disease models. Neurobiol Dis 124, 276–288. [DOI] [PubMed] [Google Scholar]
- Wong K, Noubade R, Manzanillo P, Ota N, Foreman O, Hackney JA, Friedman BA, Pappu R, Scearce-Levie K, and Ouyang W (2017). Mice deficient in NRROS show abnormal microglial development and neurological disorders. Nat Immunol 18, 633–641. [DOI] [PubMed] [Google Scholar]
- Woollacott I, Nicholas J, Heslegrave A, Heller C, Foiani M, Dick K, Russell L, Paterson R, Keshavan A, Fox N, et al. (2018). Cerebrospinal fluid soluble TREM2 levels in frontotemporal dementia differ by genetic and pathological subgroup. Alzheimers Res Ther 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich P, Glebov K, Kemmerling N, Tien N, Neumann H, and Walter J (2013). Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J Biol Chem 288, 33027–33036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, and Mucke L (2001). TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med 7, 612–618. [DOI] [PubMed] [Google Scholar]
- Xiang X, Werner G, Bohrmann B, Liesz A, Mazaheri F, Capell A, Feederle R, Knuesel I, Kleinberger G, and Haass C (2016). TREM2 deficiency reduces the efficacy of immunotherapeutic amyloid clearance. EMBO Mol Med 8, 992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, and McGeer EG (1992). Lewy bodies in Parkinson's disease are recognized by antibodies to complement proteins. Acta Neuropathol 84, 100–104. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, and McGeer PL (1999). Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am J Pathol 154, 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F, Hansen D, and Sheng M (2017). TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med 23, 512–533. [DOI] [PubMed] [Google Scholar]
- Yeh F, Wang Y, Tom I, Gonzalez L, and Sheng M (2016). TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron 91, 328–340. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Barnes AP, Hand R, Polleux F, and Ehlers MD (2010). TGF-beta signaling specifies axons during brain development. Cell 142, 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, Nietzsche S, Westermann M, Peng L, Hu D, et al. (2019). ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim K, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. (2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang S, Iwata N, Saido T, Maeda J, Suhara T, Trojanowski J, and Lee V (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351. [DOI] [PubMed] [Google Scholar]
- Yuan P, Condello C, Keene C, Wang Y, Bird T, Paul S, Luo W, Colonna M, Baddeley D, and Grutzendler J (2016). TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 90, 724–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani H, Finch CE, Kemper C, Atkinson J, McKeel D, Morris JC, and Price JL (2005). Complement activation in very early Alzheimer disease. Alzheimer Dis Assoc Disord 19, 55–66. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Andreasen N, and Blennow K (2004). Increased cerebrospinal fluid levels of transforming growth factor-beta1 in Alzheimer's disease. Neurosci Lett 367, 194–196. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, van Swieten J, Boxer A, and Rohrer J (2019). Review: Fluid biomarkers for frontotemporal dementias. Neuropathol Appl Neurobiol 45, 81–87. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan S, Bennett M, Scholze A, O'Keeffe S, Phatnani H, Guarnieri P, Caneda C, Ruderisch N, et al. (2014). An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan S, Clarke L, Caneda C, Plaza C, Blumenthal P, Vogel H, Steinberg G, Edwards M, Li G, et al. (2016). Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li X, Jiang L-L, Gui X, Liu Y, Sun Y, Zhu B, Piña-Crespo JC, Zhang M, et al. (2018). TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron 97, 1023–1031.e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Chen X, Wang T, Wang Z, Liao C, Wang Z, Huang R, Wang D, Li X, Wu L, et al. (2017). Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med 214, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Chen X, Zhang Z, Wang Z, Shi X, Xu K, Zhang Y, Xu H, and Bu G (2015). DAP12 Stabilizes the C-terminal Fragment of the Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) and Protects against LPS-induced Pro-inflammatory Response. J Biol Chem 290, 15866–15877. [DOI] [PMC free article] [PubMed] [Google Scholar]