Abstract
Background
Although recent studies provide insight into the molecular mechanisms of the effects of ketamine, the antidepressant mechanism of ketamine enantiomers and their metabolites is not fully understood. In view of the involvement of mechanisms other than the N-methyl-D-aspartate receptor in ketamine’s action, we investigated the effects of (R)-ketamine, (S)-ketamine, (R)-norketamine [(R)-NK], (S)-NK, (2R,6R)-hydroxynorketamine [(2R,6R)-HNK], and (2S,6S)-HNK on monoaminergic neurotransmission in the prefrontal cortex of mice.
Methods
The extracellular monoamine levels in the prefrontal cortex were measured by in vivo microdialysis.
Results
(R)-Ketamine and (S)-ketamine acutely increased serotonin release in a dose-dependent manner, and the effect of (R)-ketamine was greater than that of (S)-ketamine. In contrast, (S)-ketamine caused a robust increase in dopamine release compared with (R)-ketamine. Both ketamine enantiomers increased noradrenaline release, but these effects did not differ. (2R,6R)-HNK caused a slight but significant increase in serotonin and noradrenaline but not dopamine release. (S)-NK increased dopamine and noradrenaline but not serotonin release. Differential effects between (R)-ketamine and (S)-ketamine were also observed in a lipopolysaccharide-induced model of depression. An α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor antagonist, 2,3-dioxo-6-nitro-1,2,3,4- tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), attenuated (S)-ketamine-induced, but not (R)-ketamine-induced serotonin release, whereas NBQX blocked dopamine release induced by both enantiomers. Local application of (R)-ketamine into the prefrontal cortex caused a greater increase in prefrontal serotonin release than that of (S)-ketamine.
Conclusions
(R)-Ketamine strongly activates the prefrontal serotonergic system through an AMPA receptor-independent mechanism. (S)-Ketamine-induced serotonin and dopamine release was AMPA receptor-dependent. These findings provide a neurochemical basis for the underlying pharmacological differences between ketamine enantiomers and their metabolites.
Keywords: (R)-ketamine, (S)-ketamine, monoamine, prefrontal cortex, AMPA receptors
Significance Statement.
Several molecular mechanisms underlying the antidepressant-like effects of (R,S)-ketamine have been proposed, which focus on the glutamatergic system. However, (R)-ketamine has been reported to possess greater potency and longer-lasting antidepressant effects than (S)-ketamine in several animal models of depression despite (R)-ketamine having lower affinity for the N-methyl-D-aspartate receptor than (S)-ketamine. The present study demonstrates the differences between ketamine enantiomers in inducing prefrontal serotonin (5-HT) and dopamine but not noradrenaline release. (R)-Ketamine induced a greater increase in 5-HT release than (S)-ketamine, but this effect was AMPA receptor independent. (S)-Ketamine caused a robust increase in dopamine release compared with (R)-ketamine via an AMPA receptor-dependent mechanism. A ketamine metabolite (2R,6R)-hydroxynorketamine induced a slight increase in 5-HT and noradrenaline release and (S)-norketamine increased dopamine and noradrenaline release. These findings provide a neurochemical basis for understanding the pharmacological differences and mechanisms of (R)-ketamine, (S)-ketamine, and their metabolites.
Introduction
Accumulating evidence has indicated that the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine (racemic ketamine; (R,S)-ketamine) has rapid and potent antidepressant effects in major depressive disorder including treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006a; Murrough et al., 2013; Newport et al., 2015; Su et al., 2017). (R,S)-Ketamine also produces antisuicidal effects in treatment-resistant depression (Price et al., 2009; Murrough et al., 2015; Grunebaum et al., 2018; Wilkinson et al., 2018). Some clinical trials have demonstrated that intranasal administration of esketamine [(S)-ketamine] showed rapid and sustained (>2 months) antidepressant effects in treatment-resistant depression (Daly et al., 2018) and resulted in rapid improvement in depressive symptoms and suicidality in patients at imminent risk for suicide (Canuso et al., 2018). On March 5, 2019, an (S)-ketamine nasal spray was approved as a new antidepressant for treatment-resistant depression by the US Food and Drug Administration (FDA News Release, 2019). Several molecular mechanisms underlying the antidepressant-like effects of ketamine have been proposed, especially focusing on the glutamatergic system such as synaptic or GluN2B-selective extra-synaptic NMDA receptor inhibition, inhibition of NMDA receptors localized on GABAergic interneurons, and the role of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor activation (Maeng et al., 2008; Chaki, 2017; Pałucha-Poniewiera, 2018; Zanos and Gould, 2018; Duman et al., 2019). However, other NMDA antagonists, including memantine and lanicemine, which bind to the receptor at the same site as ketamine, do not exhibit consistent evidence for clinical antidepressant efficacy (Zarate et al., 2006b, 2013; Smith et al., 2013; Newport et al., 2015; Sanacora et al., 2017). Moreover, several animal studies have demonstrated that (R)-ketamine has greater potency and longer lasting antidepressant effects than (S)-ketamine (Zhang et al., 2014a; Yang et al., 2015, 2017a, 2017b, 2018b; Zanos et al., 2016; Fukumoto et al., 2017), while (S)-ketamine (Ki = 0.30 µM) has a higher affinity for the NMDA receptor than (R)-ketamine (Ki = 1.4 µM) (Ebert et al., 1997). Therefore, mechanisms other than the NMDA receptor also play an important role in mediating the antidepressant effects of ketamine.
In addition to the glutamatergic system, recent preclinical studies indicate the potential involvement of the monoaminergic system in the antidepressant actions of ketamine (du Jardin et al., 2016b). Furthermore, the monoaminergic system has been implicated in the antidepressant effects of numerous currently used drugs. Regarding the antidepressant-like effects of ketamine, a serotonin (5-HT) synthesis inhibitor, p-chlorophenylalanine (PCPA), attenuated the acute (Fukumoto et al., 2016) and sustained (Pham et al., 2017) antidepressant-like effects of (R,S)-ketamine. The sustained antidepressant effects were also attenuated by intra-medial prefrontal cortex (PFC) injection of a 5-HT1A receptor antagonist, WAY100635 (Fukumoto et al., 2018). Additionally, a recent study shows that activation of Drd1 (the dopamine [DA]-D1 receptor)-expressing pyramidal cells in the medial PFC produces rapid and long-lasting antidepressant responses, and the disruption of Drd1 activity blocked the rapid antidepressant effects of (R,S)-ketamine (Hare et al., 2019). These findings suggest that the monoaminergic system is involved at least partly in the acute and sustained antidepressant-like effects of ketamine. Previous microdialysis studies have shown that acute administration of (R,S)-ketamine in the dose range showing antidepressant activity increased the extracellular levels of 5-HT and DA in the PFC (Lorrain et al., 2003; Pham et al., 2017; Kinoshita et al., 2018). Witkin et al. (2016) reported that (S)-ketamine (10 mg/kg, s.c.) increased the extracellular levels of 5-HT, DA, noradrenaline (NA), histamine, and acetylcholine but not glutamate or γ-aminobutyric acid in rat PFC. However, the neurochemical effects of (R)-ketamine are not fully understood, and there is no comparative study to our knowledge of ketamine enantiomers. In this study, we aimed to clarify the effects of (R)-ketamine and (S)-ketamine on the in vivo release of monoamines in the PFC of both normal mice and a lipopolysaccharide (LPS)-induced mouse model of depression (Zhang et al., 2014b). Since the AMPA receptor is suggested to be involved in the antidepressant-like effects of ketamine (Maeng et al., 2008; Chaki, 2017; Pałucha-Poniewiera, 2018; Zanos and Gould, 2018; Duman et al., 2019), we subsequently examined the effects of 2,3-dioxo-6-nitro-1,2,3,4- tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), an AMPA receptor antagonist, on (R)-ketamine- and (S)-ketamine-induced changes in monoamine release. Some metabolites of ketamine, such as (S)-norketamine [(S)-NK] and (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] have been shown to exert antidepressant-like effects (Zanos et al., 2016; Chou et al., 2018; Pham et al., 2018; Yang et al., 2018a; Fukumoto et al., 2019). Thus, this study also investigated the effects of metabolites of ketamine enantiomers such as (R)-NK, (S)-NK, (2R,6R)-HNK, and (2S,6S)-HNK on central monoaminergic transmission in mice.
Materials and Methods
Animals and Drugs
All animal studies were approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, Osaka University. All experimental procedures were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Every effort was made to minimize animal suffering and to reduce the number of animals used. Eight-week-old male C57BL/6J mice were obtained from SHIMIZU Laboratory Supplies Co., Ltd. (Kyoto, Japan) and housed in cages (28 cm × 17 cm × 12 cm) in groups of 5 or 6 animals under controlled environmental conditions (22 ± 1°C; 50 ± 10% relative humidity; a 12-hour light-dark cycle, lights on at 8:00 am; food and water ad libitum) for at least 1 week before use in the experiments. (R)-Ketamine hydrochloride and (S)-ketamine hydrochloride were prepared by recrystallization of (R,S)-ketamine (Ketalar, ketamine hydrochloride, Daiichi Sankyo Pharmaceutical Ltd., Tokyo, Japan) and D-(-)-tartaric acid and L-(+)-tartaric acid, respectively (Zhang et al., 2014a). (R)-NK, (S)-NK, (2R,6R)-HNK, and (2S,6S)-HNK were purchased from Tocris Bioscience (Bristol, UK). NBQX disodium salt and LPS (serotype O111:B4) were purchased from Abcam (Cambridge, UK) and Sigma-Aldrich (St. Louis, MO), respectively. All drugs were dissolved in saline (0.9% [w/v] solution of NaCl). All drugs except NBQX were administered i.p. at a volume of 10 mL/kg body weight. NBQX was s.c. injected at a volume of 10 mL/kg body weight. The doses of (R)-ketamine, (S)-ketamine, and their metabolites used here were selected according to previous studies (Zhang et al., 2014a; Yang et al., 2015, 2018a; 2017a, 2017b, 2018b; Zanos et al., 2016; Fukumoto et al., 2017, 2019; Pham et al., 2017, 2018; Chou et al., 2018). To induce depression-like models, mice were i.p. injected with LPS (0.5 mg/kg) 24 hours before the microdialysis experiment, as previously described (Zhang et al., 2014b).
In Vivo Microdialysis
Microdialysis experiments were performed as previously reported (Ago et al., 2013; Hara et al., 2016; Tanaka et al., 2017). Briefly, each mouse was anesthetized with a mixture of medetomidine (0.3 mg/kg, i.p.), midazolam (4 mg/kg, i.p.), and butorphanol (5 mg/kg, i.p.) and stereotaxically implanted unilaterally and counterbalanced left or right with a guide-cannula for a dialysis probe (Eicom Corp., Kyoto, Japan) positioned in the PFC (A +1.9 mm, L ±0.5 mm, V –0.8 mm, from the bregma and skull) (Franklin and Paxinos, 1997). The cannula was cemented in place with dental acrylic, and the animal was kept warm and allowed to recover from anesthesia. Postoperative analgesia was performed with a single injection of buprenorphine (0.1 mg/kg, i.p.). The active probe membranes were 3 mm long. Two days after surgery, the probe was perfused with Ringer’s solution (147.2 mM NaCl, 4.0 mM KCl, and 2.2 mM CaCl2; Fuso Pharmaceutical Industries, Ltd., Osaka, Japan) at a constant flow rate of 1 µL/min. A stabilization period of 3 hours was established before the onset of the experiment. Microdialysis samples (20 µL) were collected every 20 minutes and injected immediately onto a high-performance liquid chromatography column for simultaneous assay of 5-HT, DA, and NA (Hara et al., 2016; Tanaka et al., 2017). The concentrations of 5-HT, DA, and NA in brain microdialysates were determined using high-performance liquid chromatography with an electrochemical detector (HTEC-500; Eicom Corp.). After the experiments, Evans Blue dye was microinjected through the cannula to histologically verify the position of the probe, and only data from animals with correct probe placement were used in the analysis.
Statistics
All results are presented as the mean ± SEM. Data from microdialysis were calculated as the percentage of change from dialysate baseline concentrations, with 100% defined as the average of 3 fractions before the drug administration. Data were analyzed using 2- or 3-way ANOVA for treatment or dosage as the inter-subject factor and repeated measures with time as the intra-subject factor, followed by the Tukey-Kramer post hoc test when the interaction was significant. Statistical analyses were performed using the Statview 5.0J software package for Apple Macintosh (SAS Institute Inc., Cary, NC). A value of P < .05 was considered statistically significant.
Results
Effects of (R)-Ketamine, (S)-Ketamine, and Their Metabolites on Extracellular 5-HT, DA, and NA Levels in the PFC of Normal Mice
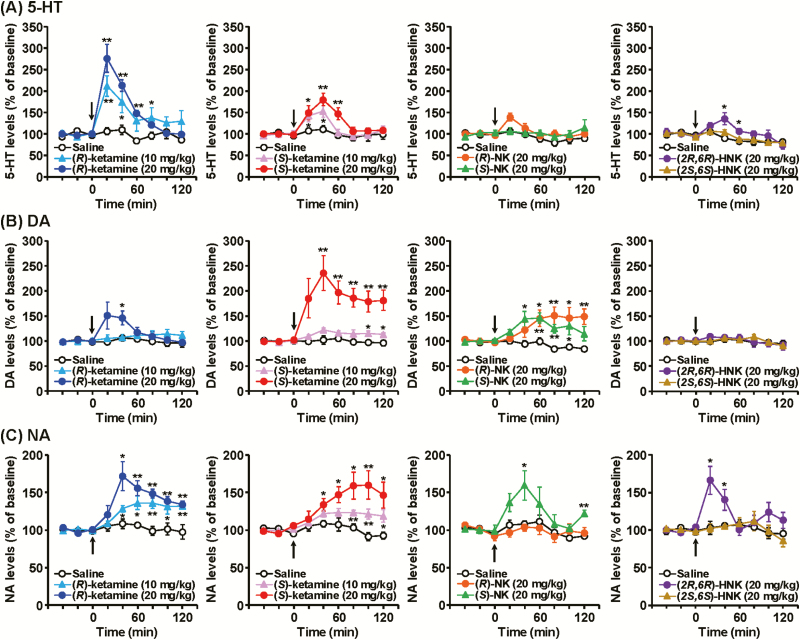
Baseline levels (mean ± SEM) of extracellular 5-HT, DA, and NA in the PFC (not corrected for in vitro probe recovery) were 1.03 ± 0.07, 0.87 ± 0.06, and 1.02 ± 0.05 pg/fraction (20 µL), respectively (n = 95, calculated from Figures 1, 3, and 4). For 5-HT release, a single administration of (R)-ketamine at doses of 10 mg/kg (F8,64 = 3.722, P = .0013) and 20 mg/kg (F8,64 = 17.055, P < .0001) and (S)-ketamine at doses of 10 mg/kg (F8,64 = 2.100, P = .0485) and 20 mg/kg (F8,64 = 5.517, P < .0001) increased extracellular 5-HT levels in a dose-dependent manner (Figure 1A). The increase in 5-HT release by (R)-ketamine was significantly greater than (S)-ketamine (3-way ANOVA with repeated measures: F16,192 = 2.362, P = .0032). (2R,6R)-HNK (20 mg/kg) caused a slight but significant increase in 5-HT release in the PFC (F8,64 = 2.676, P = .0133), whereas (R)-NK (20 mg/kg, F8,64 = 1.582, P = .1480), (S)-NK (20 mg/kg, F8,64 = 0.960, P = .4749), or (2S,6S)-HNK (20 mg/kg, F8,64 = 0.559, P = .8072) did not affect prefrontal 5-HT release.
Figure 1.
The effects of (R)-ketamine, (S)-ketamine, and their metabolites on extracellular serotonin (5-HT) (A), dopamine (DA) (B), and noradrenaline (NA) (C) levels in the prefrontal cortex (PFC) of mice. (R)-ketamine (10, 20 mg/kg), (S)-ketamine (10, 20 mg/kg), (R)-norketamine [(R)-NK] (20 mg/kg), (S)-NK (20 mg/kg), (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] (20 mg/kg), (2S,6S)-HNK (20 mg/kg), or saline was i.p. injected at 0 minutes (arrow). Results are expressed as the mean ± SEM of 5 mice per group. *P < .05, **P < .01, compared with the saline-treated mice at each time point.
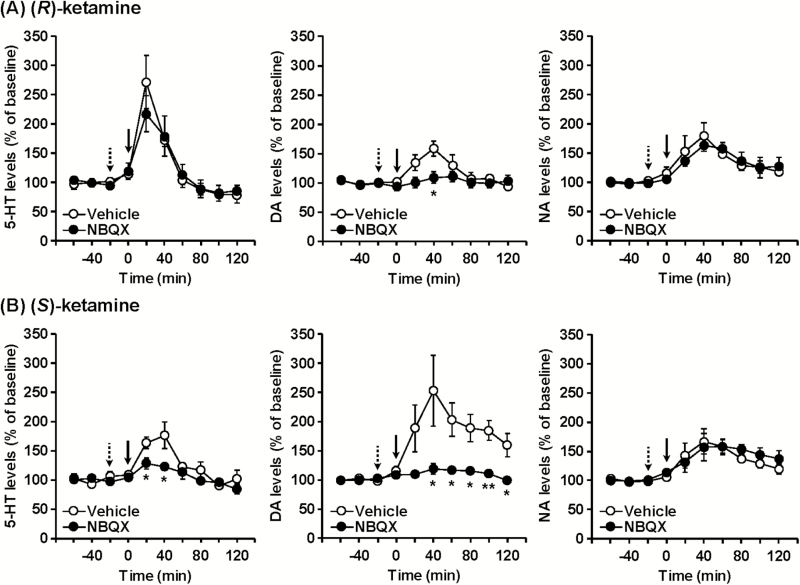
Figure 3.
The effects of NBQX on (R)-ketamine- and (S)-ketamine-induced monoamine release in the prefrontal cortex (PFC) of mice. (R)-Ketamine (20 mg/kg) (A) or (S)-ketamine (20 mg/kg) (B) was i.p. injected at 0 minutes (solid arrow). NBQX (10 mg/kg) or vehicle was s.c. injected 20 minutes before ketamine treatment (dotted arrow). Results are expressed as the mean ± SEM of 5 mice per group. *P < .05, **P < .01, compared with vehicle-pretreated mice at each time point.
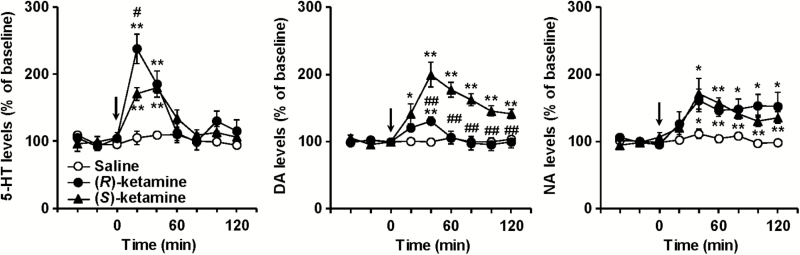
Figure 4.
The effects of local application of (R)-ketamine and (S)-ketamine on extracellular monoamine levels in the prefrontal cortex (PFC) of mice. (R)-Ketamine (50 µM), (S)-ketamine (50 µM), or vehicle was perfused into the PFC via the dialysis probe for the time indicated by the horizontal bar. Results are expressed as the mean ± SEM of 5 mice per group. *P < .05, **P < .01, compared with the vehicle-treated mice at each time point. #P < .05, ##P < .01, compared with the (S)-ketamine-treated mice at each time point.
For DA release, a single administration of (R)-ketamine at a dose of 20 mg/kg (F8,64 = 2.776, P = .0106), but not 10 mg/kg (F8,64 = 1.067, P = .3974), and (S)-ketamine at doses of 10 mg/kg (F8,64 = 2.412, P = .0242) and 20 mg/kg (F8,64 = 5.197, P < .0001) increased extracellular DA levels in the PFC (Figure 1B). The increase in DA release by (S)-ketamine was significantly greater than (R)-ketamine (3-way ANOVA with repeated measures: F16,192 = 2.366, P = .0031). (R)-NK (20 mg/kg, F8,64 = 7.253, P < .0001) and (S)-NK (20 mg/kg, F8,64 = 4.364, P = .0003) caused increases in DA release in the PFC, whereas (2R,6R)-HNK (20 mg/kg, F8,64 = 0.301, P = .9628) or (2S,6S)-HNK (20 mg/kg, F8,64 = 0.651, P = .7316) did not affect prefrontal DA release.
For NA release, a single administration of (R)-ketamine at doses of 10 mg/kg (F8,64 = 5.179, P < .0001) and 20 mg/kg (F8,64 = 7.632, P < .0001) and (S)-ketamine at doses of 10 mg/kg (F8,64 = 3.754, P = .0012) and 20 mg/kg (F8,64 = 5.019, P < .0001) increased extracellular NA levels in a dose-dependent manner (Figure 1C). There was no significant difference between the effects of (R)-ketamine and (S)-ketamine (3-way ANOVA with repeated measures: F16,192 = 1.470, P = .1142). (S)-NK (20 mg/kg, F8,64 = 2.506, P = .0196) and (2R,6R)-NK (20 mg/kg, F8,64 = 4.155, P = .0005) caused increases in NA release in the PFC, whereas (R)-NK (20 mg/kg, F8,64 = 0.640, P = .7411) or (2S,6S)-HNK (20 mg/kg, F8,64 = 0.413, P = .9090) did not affect prefrontal NA release.
Effects of (R)-Ketamine and (S)-Ketamine on Prefrontal Monoamine Release in an LPS-Induced Mouse Model of Depression
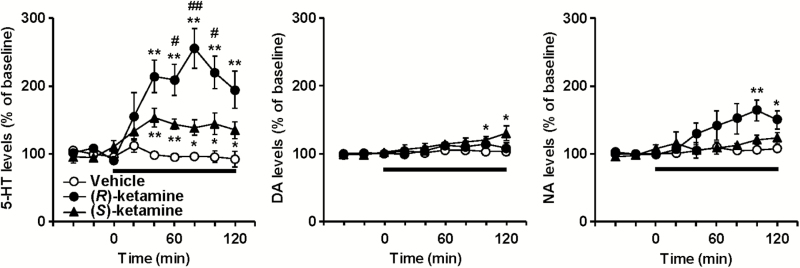
Baseline levels of extracellular 5-HT, DA, and NA in the PFC of LPS-treated mice were 0.73 ± 0.08, 0.67 ± 0.13, and 0.76 ± 0.09 pg/fraction (20 µL), respectively (n = 15, calculated from Figure 2). (R)-Ketamine (20 mg/kg, F8,64 = 10.460, P < .0001) and (S)-ketamine (20 mg/kg, F8,64 = 7.205, P < .0001) increased prefrontal 5-HT release in LPS-treated mice, and the increase by (R)-ketamine in 5-HT release was significantly greater than (S)-ketamine (F8,64 = 2.400, P = .0249) (Figure 2). Conversely, (R)-ketamine (20 mg/kg, F8,64 = 3.672, P = .0014) and (S)-ketamine (20 mg/kg, F8,64 = 13.957, P < .0001) increased prefrontal DA release in LPS-treated mice, and the increase by (S)-ketamine in DA release was significantly greater than (R)-ketamine (F8,64 = 7.909, P < .0001). (R)-Ketamine (20 mg/kg, F8,64 = 3.121, P = .0049) and (S)-ketamine (20 mg/kg, F8,64 = 4.696, P = .0001) increased prefrontal NA release in LPS-treated mice, and there was no significant difference between the effects of (R)-ketamine and (S)-ketamine (F8,64 = 0.581, P = .7898).
Figure 2.
The effects of (R)-ketamine and (S)-ketamine on extracellular monoamine levels in the prefrontal cortex (PFC) of lipopolysaccharide (LPS)-treated mice. Mice were i.p. injected with LPS (0.5 mg/kg) 24 hours before the experiment. (R)-Ketamine (20 mg/kg), (S)-ketamine (20 mg/kg), or saline was i.p. injected at 0 minutes (arrow). Results are expressed as the mean ± SEM of 5 mice per group. *P < .05, **P < .01, compared with saline-treated mice at each time point. #P < .05, ##P < .01, compared with (S)-ketamine-treated mice at each time point.
Involvement of the AMPA Receptor in the (R)-Ketamine- and (S)-Ketamine-Induced Increases in Prefrontal Monoamine Release
Pretreatment with the AMPA receptor antagonist NBQX (10 mg/kg) attenuated (S)-ketamine-induced prefrontal 5-HT release (F9,72 = 2.152, P = .0356), whereas it did not affect (R)-ketamine-induced 5-HT release (F9,72 = 0.700, P = .7066) (Figure 3). NBQX blocked both (R)-ketamine-induced (F9,72 = 3.074, P = .0036) and (S)-ketamine-induced (F9,72 = 4.355, P < .001) DA release. NBQX did not affect either (R)-ketamine-induced (F9,72 = 0.451, P = .9019) or (S)-ketamine-induced (F9,72 = 0.552, P = .8310) NA release.
Effects of the Local PFC Application of (R)-Ketamine and (S)-Ketamine on the Extracellular Monoamine Levels in the PFC
Local application of (R)-ketamine and (S)-ketamine at a dose of 10 μM had minimal effects on monoamine release in the PFC (data not shown). Local application of (R)-ketamine (F8,64 = 9.785, P < .0001) and (S)-ketamine (F8,64 = 2.979, P = .0067) at a dose of 50 µM increased extracellular 5-HT levels in the PFC, and the increase by (R)-ketamine in 5-HT release was significantly greater than (S)-ketamine (F8,64 = 3.469, P = .0022) (Figure 4). Local application of (S)-ketamine (F8,64 = 2.512, P = .0193), but not of (R)-ketamine (F8,64 = 0.950, P = .4826), caused a slight increase in DA release. Conversely, the local application of (R)-ketamine (F8,64 = 4.647, P = .0002), but not (S)-ketamine (F8,64 = 0.742, P = .6539), increased NA release.
Discussion
In this study, we identified differences between (R)-ketamine and (S)-ketamine in their abilities to induce prefrontal 5-HT and DA but not NA release. Both (R)-ketamine and (S)-ketamine caused an increase in 5-HT release, and the effect of (R)-ketamine was significantly greater than that of (S)-ketamine. In contrast, (S)-ketamine caused a robust increase in DA release compared with (R)-ketamine. Both (R)-ketamine and (S)-ketamine increased NA release, but these have similar effects. Although it is unclear exactly how these differences would contribute to the pharmacological differences between (R)-ketamine and (S)-ketamine, several reports show differences in the effects of ketamine enantiomers on antidepressant-like activity and psychosis- or addiction-related behaviors. (R)-Ketamine exhibits more potent and longer acting antidepressant-like effects than (S)-ketamine (Zhang et al., 2014a; Yang et al., 2015, 2017a). Pham et al. (2017) previously reported that (R,S)-ketamine-induced increases in 5-HT release in the medial PFC were positively correlated with its antidepressant-like activity in BALB/cJ mice. Additionally, local injection of (R,S)-ketamine into the PFC induces sustained antidepressant-like effects (Pham et al., 2017; Fukumoto et al., 2018), and this effect was mediated by the local activation of 5-HT1A receptors in the PFC (Fukumoto et al., 2018). These findings suggest that enhanced prefrontal serotonergic activity by (R)-ketamine could contribute to its potent and sustained antidepressant-like effect. The ketamine metabolites (R)-NK, (S)-NK, and (2S,6S)-HNK did not affect 5-HT release in the PFC, although (2R,6R)-HNK caused a slight increase in 5-HT release. Thus, the serotonergic system might not be involved mainly in the antidepressant-like effects of ketamine metabolites, except for (2R,6R)-HNK. Interestingly, like (R,S)-ketamine, both systemic and local injection of (2R,6R)-HNK caused an increase in baseline 5-HT release in the PFC 24 hours after injection (Pham et al., 2018), although (2R,6R)-HNK does not bind to NMDA receptors at antidepressant-relevant concentrations (Gould et al., 2017). In this study, (R)-ketamine, (S)-ketamine, (S)-NK, and (2R,6R)-HNK increased prefrontal NA release. (S)-Ketamine, (S)-NK, and (R)-NK, but not (2R,6R)-HNK, increased prefrontal DA release. (R)-NK at 20 mg/kg, but not at lower doses (5 and 10 mg/kg), significantly attenuates increased immobility time in the forced swim test in LPS-treated mice, although (S)-NK is more potent than (R)-NK (Yang et al., 2018a). These findings raise the possibility that increases in NA and DA release in the PFC might contribute at least partly to the antidepressant-like effects of ketamine and its metabolites. Of note, (R,S)-ketamine-induced antidepressant-like effects are blocked by the disruption of DA-D1 receptor activity in the PFC (Hare et al., 2019). The prefrontal DA system has been implicated in playing a pivotal role in depression and antidepressant actions (Ago et al., 2005, 2017; Furuyashiki, 2012; Rogóż, 2013; Watt et al., 2014). Therefore, the enhanced activity of the prefrontal dopaminergic system by (S)-ketamine would contribute to its antidepressant-like activity, whereas DA-D1 receptors might not play a major role in the antidepressant actions of (R)-ketamine (Chang et al., 2019). Conversely, (S)-ketamine might have a higher potential for inducing psychotomimetic effects such as hyperactivity, pre-pulse inhibition deficits, and rewarding effects compared with (R)-ketamine (Yang et al., 2015), probably due to the affinity for the NMDA receptor. In addition, a positron emission tomography study showed that a single infusion of (S)-ketamine, but not (R)-ketamine, causes a reduction in the binding availability of DA-D2/3 receptors in the striatum of conscious monkeys, suggesting that (S)-ketamine induces DA release (Hashimoto et al., 2017). Similar to this observation, in this study, (S)-ketamine caused a robust increase in DA release in the PFC, while (R)-ketamine had a small effect. Thus, the exact role of DA release induced by ketamine enantiomers and their metabolites remains unclear. Further studies investigating the effects of ketamine and its metabolites in other brain regions such as the striatum, nucleus accumbens, and hippocampus are required for full elucidation.
Previous studies have suggested the involvement of AMPA receptor activation in the antidepressant-like action of (R)-ketamine, (S)-ketamine, and (R,S)-ketamine (Maeng et al., 2008; Koike et al., 2011; Walker et al., 2013; Koike and Chaki, 2014; Zhou et al., 2014; Yang et al., 2015; Zhang et al., 2016; Fukumoto et al., 2017; Kinoshita et al., 2018). Microdialysis studies also showed that raphe AMPA receptors mediate at least in part (R,S)-ketamine-induced 5-HT release in the rat PFC (Nishitani et al., 2014; Pham et al., 2017). Moreover, AMPA itself induces 5-HT and DA release in the PFC (Araki et al., 2014). These observations suggest that AMPA receptors might be involved in the behavioral and neurochemical effects of ketamine. In the present study, the AMPA receptor antagonist NBQX blocked (R)-ketamine- and (S)-ketamine-induced DA, but not NA release in the PFC, suggesting the involvement of AMPA receptors in regulating the dopaminergic system by both ketamine enantiomers. Interestingly, NBQX partially blocked (S)-ketamine-induced prefrontal 5-HT release, while it did not affect (R)-ketamine-induced prefrontal 5-HT release. This finding suggests that AMPA receptor activation is not involved in (R)-ketamine-induced 5-HT release. A positron emission tomography study showed that subanesthetic doses of ketamine transiently decrease 5-HT transporter (SERT) binding in conscious monkeys, and ketamine infusion transiently increased 5-HT but not DA levels in the extracellular fluid of the PFC of conscious monkeys (Yamamoto et al., 2013). These findings suggest that subanesthetic ketamine might enhance serotonergic transmission by the inhibition of SERT activity. In vitro studies have reported that ketamine at concentrations >10–6 M inhibited the uptake of [3H]5-HT by the SERT transfected into human embryonic kidney 293 cells in a dose-dependent manner (Nishimura et al., 1998; Zhao and Sun, 2008). We also observed that local application of (R)-ketamine and (S)-ketamine into the PFC induced increases in prefrontal 5-HT release, and the effect of (R)-ketamine was significantly greater than that of (S)-ketamine. These phenomena might be related to a greater increase in 5-HT release by systemic administration of (R)-ketamine than (S)-ketamine, although (R)-ketamine (Ki = 148 µM) and (S)-ketamine (Ki = 156 µM) have similar affinities for the SERT (Nishimura and Sato, 1999). Therefore, the mechanism of (R)-ketamine-induced prefrontal 5-HT release remains unknown.
Previous studies showed that the depletion of 5-HT by PCPA abolished the acute antidepressant effects of (R,S)-ketamine (30 mg/kg, 30 minutes prior to the forced swim test) in control C57BL/6J mice (Fukumoto et al., 2016), and the sustained antidepressant effects of (R,S)-ketamine (10 mg/kg, 24 hours prior to the forced swim test) in highly anxious BALB/cJ mice (Pham et al., 2017). Additionally, the acute (1 hour) and sustained (24 hours) antidepressant effects of (S)-ketamine (15 mg/kg) in Flinders Sensitive Line rats (a genetic model of depression) were abolished by 5-HT depletion, suggesting that the acute and sustained antidepressant-like effects of (S)-ketamine in Flinders Sensitive Line rats depend on the endogenous 5-HT concentration (du Jardin et al., 2016a). In contrast, depletion of 5-HT by PCPA did not abolish the acute antidepressant effects of (R,S)-ketamine (25 mg/kg, 1 hour prior to the forced swim test) in control Sprague–Dawley rats (Gigliucci et al., 2013). Furthermore, PCPA did not abolish the acute (4 hours prior to the tail-suspension test) and long-lasting (2 or 5 days after a single dose in the sucrose preference test) antidepressant effects of (R)-ketamine (10 mg/kg) in a chronic social defeat stress model in male C57BL/6 mice (Zhang et al., 2018). Although the reasons for these discrepancies are currently unknown, several factors such as different animal models used (normal vs stress-induced or genetic models of depression), behavioral tests, and different doses and isomers of ketamine could account for them. Thus, the role of 5-HT in the acute and sustained antidepressant effects of (R,S)-ketamine and its enantiomers might differ depending on the experimental conditions. In this regard, it might be important to see whether the differential effects of (R)-ketamine and (S)-ketamine on prefrontal monoaminergic transmission in normal animals are also observed in depression-like models. LPS is known to cause depression-like behaviors in the forced swim and tail-suspension tests (Zhang et al., 2014b). Both (R)-ketamine and (S)-ketamine show antidepressant-like effects in an LPS-induced depression model, but the potency of (R)-ketamine is higher than that of (S)-ketamine (Yang et al., 2017a; Yamaguchi et al., 2018). In this study, we found that (R)-ketamine and (S)-ketamine enhanced serotonergic and dopaminergic neurotransmission, respectively, in LPS-treated mice as seen in normal mice. This finding implies that (R)-ketamine would produce pronounced 5-HT release, leading to antidepressant effects under some conditions of depression.
In conclusion, our study showed that (R)-ketamine and (S)-ketamine differentially affect serotonergic and dopaminergic neurotransmission in the PFC in particular. (R)-Ketamine caused a greater increase in 5-HT release than (S)-ketamine, but this effect was AMPA receptor-independent. Ketamine-induced DA but not NA release was AMPA receptor-dependent. (2R,6R)-HNK acutely induced a slight increase in 5-HT release. (S)-NK, which potentially has antidepressant activity, enhances DA and NA, but not 5-HT release. These findings provide a neurochemical basis for understanding the pharmacological differences and the mechanisms of action of (R)-ketamine, (S)-ketamine, and their metabolites.
Acknowledgments
This research was supported in part by AMED under grant number JP19am0101084 (Kazutake Tsujikawa, PhD, Graduate School of Pharmaceutical Sciences, Osaka University). We also thank Trent Rogers, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was also supported in part by JSPS KAKENHI, grant numbers JP16K08268 (Y.A.), JP17K19488 (H.H.), and JP17H03989 (H.H.); MEXT KAKENHI, grant number JP18H05416 (H.H.); AMED, grant numbers JP19dm0107122 (H.H.), JP19dm0207061 (H.H.), and JP19dm0107119 (K.H.); grants from the Takeda Science Foundation, Japan (Y.A.), and the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Japan (Y.A.).
Statement of Interest
Dr. Kenji Hashimoto is an inventor on a filed patent application on “The use of (R)-ketamine in the treatment of psychiatric diseases” by Chiba University. Dr. Kenji Hashimoto has received research support from Sumitomo Dainippon Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Taisho Pharmaceutical Co., Ltd. The other authors declare no conflicts of interest.
References
- Ago Y, Nakamura S, Baba A, Matsuda T (2005) Sulpiride in combination with fluvoxamine increases in vivo dopamine release selectively in rat prefrontal cortex. Neuropsychopharmacology 30:43–51. [DOI] [PubMed] [Google Scholar]
- Ago Y, Araki R, Tanaka T, Sasaga A, Nishiyama S, Takuma K, Matsuda T (2013) Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacology 38:1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Hasebe S, Hiramatsu N, Hashimoto H, Takuma K, Matsuda T (2017) Psychopharmacology of combined activation of the serotonin1a and σ1 receptors. Eur J Pharmacol 809:172–177. [DOI] [PubMed] [Google Scholar]
- Araki R, Ago Y, Hasebe S, Nishiyama S, Tanaka T, Oka S, Takuma K, Matsuda T (2014) Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int J Neuropsychopharmacol 17:883–893. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175:620–630. [DOI] [PubMed] [Google Scholar]
- Chaki S. (2017) Beyond ketamine: new approaches to the development of safer antidepressants. Curr Neuropharmacol 15:963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Shirayama Y, Hashimoto K (2019) Lack of dopamine D1 receptors in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Eur Arch Psychiatry Clin Neurosci. (Epub ahead of print). doi: 10.1007/s00406-019-01012-1. [DOI] [PubMed] [Google Scholar]
- Chou D, Peng HY, Lin TB, Lai CY, Hsieh MC, Wen YC, Lee AS, Wang HH, Yang PS, Chen GD, Ho YC (2018) (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 139:1–12. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Müller HK, Elfving B, Sanchez C, Wegener G (2016a) Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology 233:2813–2825. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Müller HK, Elfving B, Dale E, Wegener G, Sanchez C (2016b) Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: a critical review. Prog Neuropsychopharmacol Biol Psychiatry 71:27–38. [DOI] [PubMed] [Google Scholar]
- Duman RS, Shinohara R, Fogaça MV, Hare B (2019) Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. (Epub ahead of print). doi: 10.1038/s41380-019-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM (1997) Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol 333:99–104. [DOI] [PubMed] [Google Scholar]
- FDA News Release on March 5 (2019) FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic.https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm. Accessed March 5, 2019.
- Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press, Inc. [Google Scholar]
- Fukumoto K, Iijima M, Chaki S (2016) The antidepressant effects of an mglu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mpfc and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S (2017) Antidepressant potential of ®-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361:9–16. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Funakoshi T, Chaki S (2018) Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 21:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaça MV, Liu RJ, Duman C, Kato T, Li XY, Duman RS (2019) Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A 116:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyashiki T. (2012) Roles of dopamine and inflammation-related molecules in behavioral alterations caused by repeated stress. J Pharmacol Sci 120:63–69. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Gould TD, Zanos P, Zarate CA Jr (2017) Ketamine mechanism of action: separating the wheat from the chaff. Neuropsychopharmacology 42:368–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ (2018) Ketamine for rapid reduction of suicidal thoughts in major depression: A midazolam-controlled randomized clinical trial. Am J Psychiatry 175:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Ago Y, Taruta A, Katashiba K, Hasebe S, Takano E, Onaka Y, Hashimoto H, Matsuda T, Takuma K (2016) Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid-induced autism. Autism Res 9:926–939. [DOI] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS (2019) Optogenetic stimulation of medial prefrontal cortex drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun 10:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H (2017) Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Nishitani N, Nagai Y, Andoh C, Asaoka N, Kawai H, Shibui N, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S (2018) Ketamine-induced prefrontal serotonin release is mediated by cholinergic neurons in the pedunculopontine tegmental nucleus. Int J Neuropsychopharmacol 21:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Chaki S (2014) Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res 271:111–115. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S (2011) Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224:107–111. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS (2015) Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med 45:3571–3580. [DOI] [PubMed] [Google Scholar]
- National Research Council (1996) Guide for the care and use of laboratory animals. Washington, DC: National Academy Press. [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K (1999) Ketamine stereoselectively inhibits rat dopamine transporter. Neurosci Lett 274:131–134. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M (1998) Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 88:768–774. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S (2014) Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17:1321–1326. [DOI] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A. (2018) The role of glutamatergic modulation in the mechanism of action of ketamine, a prototype rapid-acting antidepressant drug. Pharmacol Rep 70:837–846. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM (2017) Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cj mice. Neuropharmacology 112:198–209. [DOI] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM (2018) Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84:e3–e6. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogóż Z. (2013) Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep 65:1535–1544. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ, Pathak S (2017) Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology 42:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, Rothschild AJ (2013) Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 74:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, Tu PC, Bai YM, Cheng CM, Krystal JH (2017) Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42:2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Ago Y, Umehara C, Imoto E, Hasebe S, Hashimoto H, Takuma K, Matsuda T (2017) Role of prefrontal serotonergic and dopaminergic systems in encounter-induced hyperactivity in methamphetamine-sensitized mice. Int J Neuropsychopharmacol 20:410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R (2013) NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Roberts CL, Scholl JL, Meyer DL, Miiller LC, Barr JL, Novick AM, Renner KJ, Forster GL (2014) Decreased prefrontal cortex dopamine activity following adolescent social defeat in male rats: role of dopamine D2 receptors. Psychopharmacology 231:1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr, Sanacora G (2018) The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 175:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM (2016) The rapidly acting antidepressant ketamine and the mglu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. [DOI] [PubMed] [Google Scholar]
- Yamaguchi JI, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, Mizuno-Yasuhira A, Chaki S (2018) (2R,6R)-hydroxynorketamine is not essential for the antidepressant actions of ®-ketamine in mice. Neuropsychopharmacology 43:1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H, Domino EF (2013) Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology 38:2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K (2017a) ®-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82:e43–e44. [DOI] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017b) Possible role of the gut microbiota-brain axis in the antidepressant effects of ®-ketamine in a social defeat stress model. Transl Psychiatry 7:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kobayashi S, Nakao K, Dong C, Han M, Qu Y, Ren Q, Zhang JC, Ma M, Toki H, Yamaguchi JI, Chaki S, Shirayama Y, Nakazawa K, Manabe T, Hashimoto K (2018a) AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol Psychiatry 84:591–600. [DOI] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018b) Mechanistic target of rapamycin-independent antidepressant effects of ®-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28. [DOI] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006a) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS (2006b) A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 163:153–155. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA (2013) A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry 74:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K (2014a) R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K (2014b) Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 18:pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, Huganir RL, Cai X (2016) Essential roles of AMPA receptor glua1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal 9:ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Dong C, Fujita Y, Fujita A, Hashimoto K (2018) 5-hydroxytryptamine-independent antidepressant actions of ®-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol 21:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun L (2008) Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci 15:1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mtor and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–423. [DOI] [PubMed] [Google Scholar]