Abstract
Background
About one-third of patients with depression fail to achieve remission despite treatment with multiple antidepressants and are considered to have treatment-resistant depression.
Methods
This Phase 3, double-blind, multicenter study enrolled adults with moderate-to-severe depression and nonresponse to ≥2 antidepressants in the current depression episode. Eligible patients (N = 346) were randomized (1:1:1) to twice-weekly nasal spray treatment (esketamine [56 or 84 mg] or placebo) plus a newly initiated, open-label, oral antidepressant taken daily for 4 weeks. The primary efficacy endpoint was change from baseline to day 28 in the Montgomery-Asberg Depression Rating Scale total score, performed by blinded, remote raters. Based on the predefined statistical testing sequence, esketamine 84 mg/antidepressant had to be significant for esketamine 56 mg/antidepressant to be formally tested.
Results
Statistical significance was not achieved with esketamine 84 mg/antidepressant compared with antidepressant/placebo (least squares [LS] means difference [95% CI]: –3.2 [–6.88, 0.45]; 2-sided P value = .088). Although esketamine 56 mg/antidepressant could not be formally tested, the LS means difference was –4.1 [–7.67, –0.49] (nominal 2-sided P value = .027). The most common (>20%) adverse events reported for esketamine/antidepressant were nausea, dissociation, dizziness, vertigo, and headache.
Conclusions
Statistical significance was not achieved for the primary endpoint; nevertheless, the treatment effect (Montgomery-Asberg Depression Rating Scale) for both esketamine/antidepressant groups exceeded what has been considered clinically meaningful for approved antidepressants vs placebo. Safety was similar between esketamine/antidepressant groups and no new dose-related safety concerns were identified. This study provides supportive evidence for the safety and efficacy of esketamine nasal spray as a new, rapid-acting antidepressant for patients with treatment-resistant depression.
Trial Registration
ClinicalTrials.gov identifier: NCT02417064
Keywords: esketamine, s-ketamine, ketamine, treatment-resistant depression
Introduction
Major depressive disorder (MDD), the leading cause of disability worldwide in terms of total years lost due to disability, is associated with excess morbidity and mortality (Baldessarini et al., 2017; World Health Organization, 2018). About one-third of patients with MDD fail to achieve remission despite treatment with multiple biogenic amine (e.g., serotonin, norepinephrine) antidepressants and hence have treatment-resistant depression (TRD) (Fava, 2003). In patients who respond to biogenic amine antidepressants, the time to onset of effect is typically 3 to 7 weeks, during which time patients remain symptomatic and at risk of self-harm (Rush et al., 2006). There is an unmet need to develop novel treatments providing effective, more rapid-acting, and sustained or long-term relief of depressive symptoms, especially in patients with TRD.
Esketamine, the S-enantiomer of racemic ketamine, which has a higher affinity for the N-methyl-D-aspartate receptor than the R-enantiomer, has recently been approved in the United States as a nasal spray formulation for TRD treatment. Phase 2 studies with esketamine, administered adjunctive to an oral antidepressant, demonstrated rapid onset (as early as a few hours) and persistent efficacy compared with placebo nasal spray in patients with TRD (Singh et al., 2016; Daly et al., 2018) as well as in depressed patients at imminent risk for suicide (Canuso et al., 2018). Rapid onset and persistent efficacy compared with an oral antidepressant (active comparator) plus placebo nasal spray was demonstrated in a phase 3 study of flexibly dosed esketamine, administered with a newly initiated oral antidepressant, in patients with TRD (Popova et al., 2019), with evidence of sustained benefit in a phase 3 long-term maintenance of effect study (Daly et al., 2019).
We report findings from a randomized, double-blind, active-controlled phase 3 study comparing the efficacy and safety of fixed doses of esketamine nasal spray plus a newly initiated oral antidepressant to a newly initiated oral antidepressant (active comparator) plus a placebo nasal spray in adult patients with TRD. The inclusion criterion for TRD was based on nonresponse to an adequate therapeutic trial (established by considering dose, duration, and adherence) of at least 2 different antidepressants within the current episode of depression. The study consisted of a 4-week, double-blind treatment phase and up to 24 weeks of follow-up. The 4-week duration of the double-blind treatment phase was considered to be sufficiently long to show the antidepressant effects of the active comparator based on the timing of onset of effect of typical antidepressant treatments (Machado-Vieira et al., 2010).
Materials and Methods
Ethical Practices
An Institutional Review Board or Independent Ethics Committee, depending on the participating country, approved the study protocol and amendments. The study was conducted in accordance with ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. All individuals provided written informed consent before participating in the study.
Study Design
This randomized, double-blind, active-controlled, multicenter study was conducted in outpatient centers between September 2015 and February 2018 and is registered at clinicaltrials.gov, identifier: NCT02417064.
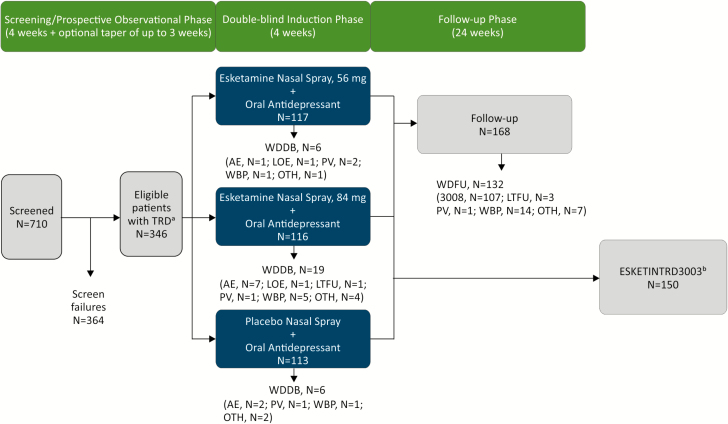
The study consisted of 3 phases: (1) 4-week screening/prospective observation, (2) 4-week double-blind treatment, and (3) up to 24 weeks of follow-up (Figure 1).
Figure 1.
Disposition of patients. AE, adverse event; LOE, lack of efficacy; LTFU, lost to follow-up; MADRS, Montgomery-Asberg Depression Rating Scale; OTH, other reason for withdrawal; PV, protocol violation; TRD, treatment-resistant depression; WBP, withdrawal by patient; WDDB, withdrawal from double-blind phase; WDFU, withdrawn from follow-up phase. 3008 entered Janssen-sponsored Study TRD3008 (NCT02782104). aPatients with nonresponse to ≥2 oral antidepressants, 1 observed prospectively, prior to randomization were eligible to participate in the study. bResponders (defined as ≥50% reduction in MADRS total score from baseline to end of the 28-day double-blind phase) were eligible to continue to Janssen-sponsored Study ESKETINTRD3003 (NCT02493868).
Study Population
Patients were between 18 and 64 years of age with recurrent MDD (per Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria; American Psychiatric Association, 2013) or single-episode MDD (≥2 years), without psychotic features, confirmed by the Mini-International Neuropsychiatric Interview.
At entry, patients had moderate-to-severe depression (Inventory of Depressive Symptomatology [Trivedi, 2004] total score ≥34 and Montgomery-Asberg Depression Rating Scale [MADRS] [Williams and Kobak, 2008] total score ≥28). Based on patient self-report and medical/pharmacy records, the type, dose, and duration of, and response to, antidepressants taken in the current depressive episode were documented on the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (Chandler et al., 2010). In addition to at least 1 antidepressant with nonresponse (≤25% improvement) in the current depressive episode based on historical report, nonresponse to a different antidepressant taken at an adequate dose for a total duration of at least 6 weeks was observed prospectively in the screening/prospective observational phase. At the end of this phase, nonresponders (defined as ≤25% improvement in the MADRS total score from week 1 to week 4 and a MADRS total score ≥28 at weeks 2 and 4) who were eligible to enter the 4-week double-blind treatment phase discontinued all current antidepressant treatment(s). At the time of randomization, patients met the study definition of TRD, which was nonresponse to an adequate trial (dose, duration, adherence) of ≥2 antidepressants in the current episode of depression.
Key exclusion criteria were suicidal ideation with intent to act within the prior 6 months or suicidal behavior within the prior year; diagnosis of psychotic disorder, bipolar or related disorders; recent history (within prior 6 months) of moderate or severe substance use disorder; and, positive test result(s) for specified drugs of abuse. A full list of the exclusion criteria is presented in Supplemental Material.
Randomization and Blinding
Eligible patients were randomized (1:1:1) based on a computer-generated randomization schedule to double-blind nasal spray treatment with either 1 of 2 fixed doses of esketamine (56 or 84 mg) or placebo. Randomization was balanced by using randomly permuted blocks and stratified by country and class of oral antidepressant (serotonin-norepinephrine reuptake inhibitor [SNRI] or selective serotonin reuptake inhibitor [SSRI]). The newly initiated oral antidepressant was open-label.
Intranasal Study Drug and Administration
Intranasal study drugs were provided in disposable nasal spray devices with identical appearance and packaging. Each device contained 200 μL of solution and delivered 2 sprays of either esketamine (total of 28 mg of esketamine base) or placebo. To maintain blinding, the placebo solution had a bittering agent added to simulate the taste of esketamine solution, and the same number of devices (3 devices) were administered to all patients at all sessions.
Patients self-administered intranasal study drug twice weekly for 4 weeks at the study site under the direct supervision of a site staff member. Dosing occurred on days 1, 4, 8, 11, 15, 18, 22, and 28.
For improved tolerability, patients randomized to esketamine 84 mg started at 56 mg on day 1 and then, in a blinded manner, increased to 84 mg for day 4 and all subsequent intranasal treatment sessions.
Newly Initiated Oral Antidepressant
The open-label antidepressant was assigned by the investigator from 4 choices (duloxetine, escitalopram, sertraline, or venlafaxine extended release) and could not be one that the patient already had nonresponse to (in the current depressive episode) or had not tolerated (lifetime). Dosing of the oral antidepressant began on day 1 and continued daily for 4 weeks based on the mandatory protocol titration schedule (Supplementary Material). The switch to a new oral antidepressant was consistent with clinical treatment guidelines and provided balance between the treatment arms for duration of the concurrent oral antidepressant treatment (Bauer et al., 2015; Kennedy et al., 2016).
Efficacy Assessments
MADRS assessments were performed as primary and secondary efficacy evaluations (the efficacy endpoints are presented below in the Statistical Methods section). Due to transient, dissociative effects observed with esketamine that could potentially cause functional unblinding of site staff, all MADRS assessments were performed via telephone by independent raters who were blinded to the protocol details, including study visit, the patient’s clinical status, and side effects during the trial. MADRS assessments were required to be performed within 2 days prior to a nasal spray dosing.
Investigators rated overall change in severity of depressive illness using the Clinical Global Impression-Severity (CGI-S) (Guy, 1976). Patients rated the impact of the study treatments on socio-occupational disability using the Sheehan Disability Scale (SDS) (Leon et al., 1997), depressive symptoms using the Patient Health Questionnaire 9-item (PHQ-9) (Spitzer et al., 1999), anxiety symptoms using the Generalized Anxiety Disorder 7-item (GAD-7) Scale (Spitzer et al., 2006), and overall health outcome using the EuroQol-5 dimension-5 level (EQ-5D-5L) (EuroQol Group, 2019).
The CGI-S, SDS, and GAD-7 were performed predose on nasal spray dosing days.
Safety Assessments
Adverse events and other safety assessments (e.g., clinical laboratory, physical examination, electrocardiogram, Columbia Suicide Severity Rating Scale; Posner et al., 2007) were monitored throughout the study. Vital signs, Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998), Brief Psychiatric Rating Scale (4-item positive symptom subscale) (Overall and Gorham, 1962), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S), and Global Assessment of Discharge Readiness were assessed at all dosing visits. Investigators were provided guidance on blood pressure monitoring during intranasal treatment days (guidance provided in Supplemental Material).
Local tolerability was assessed via nasal examination, and patients completed a nasal symptom questionnaire. The Physician Withdrawal Checklist (Rickels et al., 2008) was administered to detect potential withdrawal symptoms following cessation of esketamine.
Cognitive testing was performed to measure the potential impact of esketamine on cognition; these data will be reported in a separate manuscript.
Statistical Methods
Sample Size Determination
A maximum sample size of 348 individuals (approximately 116 per treatment group to achieve 90% power) was planned assuming a treatment difference of 6.5 points in MADRS total score between either dose of esketamine/antidepressant and antidepressant/placebo, a SD of 12, a 2-sided significance level of .025, and a drop-out rate of 25%.
Interim Analysis
A prespecified interim analysis was performed 4 weeks after randomizing 121 patients in the study to either reestimate the sample size (to achieve the desired power while maintaining control over type I error) or stop the study due to futility. An independent data monitoring committee recommended continuing the study with a final sample size of 234 patients. The sponsor study team and study site staff were not informed of the adjusted sample size until it had been met to ensure no impact on study conduct.
On notification that 234 patients had been randomized, as an ethical obligation, the sponsor allowed patients in the screening phase or who had a screening visit already scheduled to continue participation in the study if all entry criteria were met, resulting in a total of 346 patients being randomized.
Data Analyses
Data were analyzed based on analysis sets that included all randomized patients who received at least 1 dose of intranasal study medication and (efficacy)/or (safety) 1 dose of oral antidepressant. Analyses were performed using SAS, version 9.2.
Level of Significance
A truncated fixed sequence procedure (Dmitrienko et al., 2008; Dmitrienko and Tamhane, 2011) (supplementary Figure 1) was applied to adjust for multiplicity and control type I error across the primary (change in MADRS total score) and the 3 key secondary efficacy endpoints (tested in the following sequence: onset of clinical response by day 2, change in SDS total score, and change in PHQ-9 total score) and the 2 dose-control comparisons. For each endpoint, testing of the esketamine 56 mg dose group was conducted only if the 84 mg dose group was significant. Sequential testing of the endpoints was performed for both dose groups only if they were significant for the previous endpoint in the hierarchy (84 mg dose group at 2-sided .05 level, 56 mg dose group at 2-sided .0425 level). If only the 84 mg dose group was significant for an endpoint, testing of the other endpoints down the hierarchy was conducted only for this dose group at the 2-sided .0075 level.
Primary Efficacy Endpoint and Analyses
The primary efficacy endpoint, change from baseline (day 1) to day 28 in MADRS total score, was analyzed using a mixed-effects repeated measures model (MMRM) with baseline MADRS total score as a covariate; treatment, region, oral antidepressant class (SNRI or SSRI), day, and day-by-treatment interaction as fixed effects; and a random patient effect. The changes from baseline for all post-baseline MADRS assessments (days 2, 8, 15, 22, and 28) were included in the model as the repeated measure. To account for sample size reassessment, a weighted combination test was used for treatment comparisons, with the test statistic defined as an equally weighted sum of the test statistics determined before and after the interim analysis (Cui et al., 1999; Lehmacher and Wassmer, 1999). A similar MMRM model was used for a post hoc, unweighted analysis of the primary endpoint combining the 2 esketamine dose groups, with the exception that the fixed effect for treatment included pooled esketamine/oral antidepressant and oral antidepressant/placebo groups. Unweighted analyses were also conducted for various subgroups with a similar MMRM model as was used for the primary endpoint.
Secondary Efficacy Endpoints and Analyses
Similar to the primary analysis, estimates were provided for the weighted differences between the 2 esketamine dose groups and active control on the first key secondary efficacy endpoint—onset of clinical response by day 2 (24 hours) that was maintained for the duration of the double-blind phase (day 28) with 1 excursion (i.e., ≥25% reduction relative to baseline MADRS allowed on days 8, 15, or 22).
The second and third key secondary efficacy endpoints, change from baseline in SDS and PHQ-9 total score at day 28, respectively, were analyzed using the MMRM model and weighted combination test described for the primary efficacy analysis but using the respective baseline score (SDS or PHQ-9) as covariate.
The secondary efficacy endpoints of proportion of responders (≥50% reduction from baseline in MADRS total score) and remitters (MADRS ≤12) were summarized. Responders who also remitted were counted in both categories. The number needed to treat (NNT) was estimated for both response and remission at day 28 based on the MADRS total score. Change from baseline in CGI-S score was summarized; the odds of achieving an improved CGI-S score at endpoint (double-blind) were estimated for each esketamine dose/antidepressant group compared with oral antidepressant/placebo. Changes from baseline in GAD-7 total score at day 28 was analyzed using ANCOVA with treatment, region, oral antidepressant class as factors, and the baseline score as a covariate. Change from baseline to day 28 was summarized for EQ-5D-5L health status index and visual analogue scale score.
Study Results
A total of 710 patients were screened and 346 were randomized (Figure 1). Most randomized patients (315/346, 91.0%) completed the double-blind phase. A total of 6 (5.1%), 19 (16.4%), and 6 (5.3%) patients in the esketamine 56 mg/antidepressant, 84 mg/antidepressant, and antidepressant/placebo groups, respectively, withdrew prior to completing the treatment phase. Of note, 11 of the 19 withdrawn patients in the esketamine 84 mg/antidepressant group were withdrawn after only receiving the first dose, which was 56 mg based on the fixed titration in the study design. The higher withdrawal rate in the esketamine 84 mg/antidepressant group did not appear to be due to any new or dose-related safety finding (Figure 1).
The treatment groups were similar with respect to demographic and baseline characteristics (Table 1). Two-thirds of the patients were female, and mean age was 46 years old. Patients had severe depression; mean duration of the current episode was approximately 4 years.
Table 1.
Demographic and Baseline Characteristics
| Esketamine 56 mg/ oral antidepressant N = 115 | Esketamine 84 mg/ oral antidepressant N = 114 | Oral antidepressant/ placebo N = 113 | Total N = 342 | |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 46.4 (11.18) | 45.7 (11.10) | 46.8 (11.36) | 46.3 (11.19) |
| Range | 22–64 | 18–64 | 18–64 | 18–64 |
| Sex, n (%) | ||||
| Male | 34 (29.6%) | 35 (30.7%) | 32 (28.3%) | 101 (29.5%) |
| Female | 81 (70.4%) | 79 (69.3%) | 81 (71.7%) | 241 (70.5%) |
| Race, n (%) | ||||
| Asian | 2 (1.7%) | 1 (0.9%) | 2 (1.8%) | 5 (1.5%) |
| Black or African American | 7 (6.1%) | 7 (6.1%) | 5 (4.4%) | 19 (5.6%) |
| White | 91 (79.1%) | 85 (74.6%) | 86 (76.1%) | 262 (76.6%) |
| Other | 8 (7.0%) | 12 (10.5%) | 10 (8.8%) | 30 (8.8%) |
| Multiple | 0 | 0 | 1 (0.9%) | 1 (0.3%) |
| Not reported | 7 (6.1%) | 9 (7.9%) | 9 (8.0%) | 25 (7.3%) |
| Baseline body mass index (kg/m2) | ||||
| Mean (SD) | 28.8 (6.70) | 28.4 (5.86) | 29.2 (6.69) | 28.8 (6.42) |
| Range | 18–56 | 17–50 | 19–50 | 17–56 |
| Employment statusa, n (%) | ||||
| Any type of employment | 60 (52.2%) | 67 (58.8%) | 67 (59.3%) | 194 (56.7%) |
| Any type of unemployment | 41 (35.7%) | 41 (36.0%) | 36 (31.9%) | 118 (34.5%) |
| Other | 14 (12.2%) | 6 (5.3%) | 10 (8.8%) | 30 (8.8%) |
| Country, n (%) | ||||
| Belgium | 8 (7.0%) | 9 (7.9%) | 12 (10.6%) | 29 (8.5%) |
| Brazil | 20 (17.4%) | 19 (16.7%) | 18 (15.9%) | 57 (16.7%) |
| Canada | 7 (6.1%) | 7 (6.1%) | 6 (5.3%) | 20 (5.8%) |
| Estonia | 3 (2.6%) | 4 (3.5%) | 3 (2.7%) | 10 (2.9%) |
| France | 11 (9.6%) | 10 (8.8%) | 10 (8.8%) | 31 (9.1%) |
| Hungary | 3 (2.6%) | 1 (0.9%) | 1 (0.9%) | 5 (1.5%) |
| Mexico | 14 (12.2%) | 16 (14.0%) | 15 (13.3%) | 45 (13.2%) |
| Slovakia | 4 (3.5%) | 3 (2.6%) | 3 (2.7%) | 10 (2.9%) |
| United States | 45 (39.1%) | 45 (39.5%) | 45 (39.8%) | 135 (39.5%) |
| Age when diagnosed with MDD, years | ||||
| Mean (SD) | 30.3 (12.34) | 32.1 (12.86) | 31.8 (12.44) | 31.4 (12.54) |
| Range | 11–61 | 9–59 | 10–63 | 9–63 |
| Duration of current episode, weeks | ||||
| Mean (SD) | 202.8 (277.25) | 212.7 (327.62) | 193.1 (264.10) | 202.9 (290.24) |
| Range | 12–1525 | 12–2288 | 6–1720 | 6–2288 |
| No. of previous antidepressant medicationsb,c, n (%) | ||||
| 1 or 2 | 79 (69.9%) | 59 (51.8%) | 67 (59.3%) | 205 (60.3%) |
| ≥3 | 34 (30.1) | 55 (48.2%) | 46 (40.7%) | 135 (39.7%) |
| Class of oral antidepressantd, n (%) | ||||
| SNRI | 65 (56.5%) | 67 (58.8%) | 64 (56.6%) | 196 (57.3%) |
| SSRI | 50 (43.5%) | 47 (41.2%) | 49 (43.4%) | 146 (42.7%) |
| Oral antidepressant, n (%) | ||||
| Duloxetine | 49 (42.6%) | 43 (37.7%) | 44 (38.9%) | 136 (39.8%) |
| Escitalopram | 26 (22.6%) | 23 (20.2%) | 24 (21.2%) | 73 (21.3%) |
| Sertraline | 24 (20.9%) | 24 (21.1%) | 25 (22.1%) | 73 (21.3%) |
| Venlafaxine extended release (XR) | 16 (13.9%) | 24 (21.1%) | 20 (17.7%) | 60 (17.5%) |
| CGI-S | ||||
| Mean (SD) | 5.1 (0.66) | 5.1 (0.73) | 5.1 (0.69) | 5.1 (0.69) |
| PHQ-9 | ||||
| Mean (SD) | 20.3 (4.11) | 20.7 (3.58) | 20.8 (3.69) | 20.6 (3.80) |
Abbreviations: CGI-S, Clinical Global Impression–Severity; MDD, major depressive disorder; PHQ, Patient Health Questionnaire; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
aAny type of employment includes: any category containing “employed”, sheltered work, housewife or dependent husband, and student; any type of unemployment includes: any category containing “unemployed”; other includes: retired and no information available.
bIn accordance with the protocol, patients entering the induction phase had nonresponse to at least 2 oral AD medications prior to randomization. The data presented is the number of AD medications with nonresponse (defined as ≤25% improvement) taken for at least 6 weeks during the current episode as obtained from Massachusetts General Hospital Antidepressant Treatment Response Questionnaire at the beginning of the screening/prospective observational phase.
cNs for the previous antidepressant medications are 113, 114, 113, and 340 for esketamine 56 mg/antidepressant, esketamine 84 mg/antidepressant, antidepressant/placebo, and total, respectively.
dAssigned by the investigator at randomization.
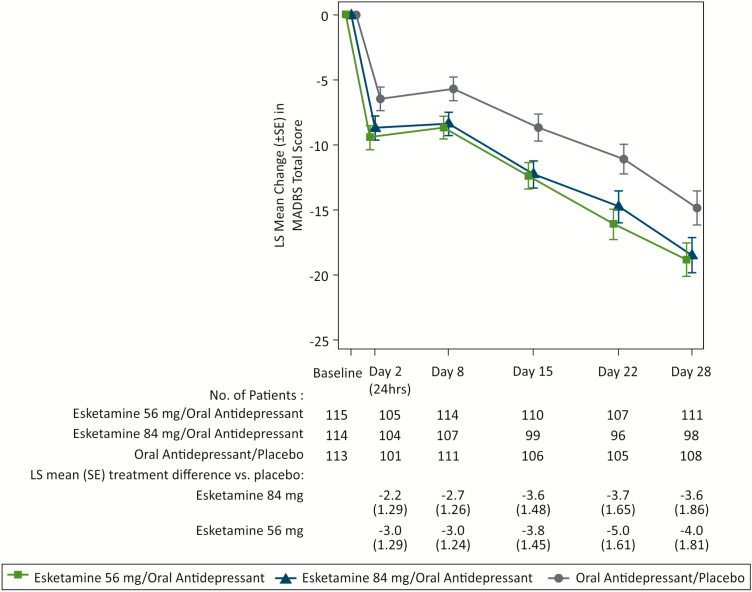
The difference between the esketamine 84 mg/antidepressant and the antidepressant/placebo groups for the change in MADRS total score was not statistically significant (2-sided P = .088) (Table 2); therefore, the esketamine 56 mg/antidepressant group and all key secondary endpoints could not be formally evaluated based on the predefined testing sequence. Although it could not be formally tested for regulatory purposes, statistical testing was performed and reported for esketamine 56 mg/antidepressant – treatment difference in least squares (LS) means (i.e., differences between the treatment groups after adjusting for other factors included in the MMRM model) -4.1 [-7.67, -0.49]; nominal 2-sided P-value: 0.027 – given this data is informative for practitioners. Results of all key secondary efficacy endpoints (onset of clinical response by day 2, SDS total score, and PHQ-9 total score) numerically favored both esketamine/antidepressant treatment groups over antidepressant/placebo (Table 3). Improvement from baseline in MADRS total score numerically favored both esketamine/antidepressant groups over the antidepressant/placebo group at all time points during the 4-week double-blind phase (Figure 2).
Table 2.
MADRS Total Score: Change From Baseline to Day 28 of Double-Blind Phase
| Esketamine 56 mg/oral antidepressant | Esketamine 84 mg/oral antidepressant | Oral antidepressant/ placebo | |
|---|---|---|---|
| Baseline | |||
| N | 115 | 114 | 113 |
| Mean (SD) | 37.4 (4.76) | 37.8 (5.58) | 37.5 (6.16) |
| Change from baseline to day 28 | |||
| N | 111 | 98 | 108 |
| Mean (SD) | –19.0 (13.86) | –18.8 (14.12) | –14.8 (15.07) |
| MMRM analysisa | |||
| Diff. of LS meansb (SE) | –4.1 | –3.2 | |
| 95% CI on differencec | –7.67; –0.49 | –6.88; 0.45 | |
| 2-sided P-valued | .027e | .088 |
Notes: Four randomized patients in the esketamine arms were not included in the efficacy analysis because they did not receive esketamine and/or the oral antidepressant.
MADRS total score ranges from 0 to 60; a higher score indicates a more severe condition. Negative change in score indicates improvement. Negative difference favors esketamine.
Abbreviations: CI, confidence interval; LS, least squares; MADRS, Montgomery-Asberg Depression Rating Scale; MMRM, mixed-effect model using repeated measures; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
aMMRM analysis with change from baseline as the response variable and the fixed effect model terms for treatment (esketamine 56 mg/antidepressant, esketamine 84 mg/antidepressant, antidepressant/placebo) day, region, class of antidepressant (SNRI or SSRI), and treatment-by-day and baseline value as a covariate.
bDifference from placebo is the median unbiased estimate, which is a weighted combination of the LS means of the difference from placebo.
c2-sided flexible CI.
d P value is based on the weighted combination test statistic.
eAs 84 mg was not significant at the 2-sided .05 level, 56 mg could not be formally evaluated, and the 2-sided P value for this dose is considered to be nominal.
Table 3.
Key Secondary Efficacy Endpoints in Double-Blind Phase
| Esketamine 56 mg/oral antidepressant | Esketamine 84 mg/oral antidepressant | Oral antidepressant/ placebo | |
|---|---|---|---|
| Early onset of clinical response by day 2 maintained to day 28a | |||
| N | 115 | 114 | 113 |
| Yes, n (%) | 12 (10.4%) | 10 (8.8%) | 2 (1.8%) |
| Difference in response ratesb | 8.90 | 6.76 | |
| Odds ratioc (95% CI) | 6.47 (1.38, 60.45) | 5.34 (1.09, 50.91) | |
| Sheehan Disability Scale Total Score | |||
| Baseline | |||
| N | 108 | 107 | 105 |
| Mean (SD) | 24.0 (4.12) | 24.7 (4.58) | 24.4 (3.86) |
| Change from baseline to day 28 | |||
| N | 88 | 87 | 90 |
| Mean (SD) | –11.0 (9.32) | –11.1 (10.04) | –8.4 (9.70) |
| MMRM analysis | |||
| Difference of LS meansd | –2.5 | –2.2 | |
| 95% CI on differencee | –5.25; 0.20 | –4.91; 0.53 | |
| Patient Health Questionnaire Total Score | |||
| Baseline | |||
| N | 115 | 114 | 113 |
| Mean (SD) | 20.3 (4.11) | 20.7 (3.58) | 20.8 (3.69) |
| Change from baseline to day 28 | |||
| N | 110 | 99 | 108 |
| Mean (SD) | –11.0 (8.07) | –11.7 (7.74) | –9.1 (8.35) |
| MMRM analysis | |||
| Difference of LS meansd | –2.3 | –2.2 | |
| 95% CI on differencee | –4.34; –0.31 | –4.26; –0.20 |
Abbreviations: CI, confidence interval; LS, least squares; MADRS, Montgomery-Asberg Depression Rating Scale; MMRM, mixed model for repeated measures; SD, standard deviation.
aOnset of clinical response by day 2 defined as ≥50% improvement from baseline in MADRS total score with onset by day 2 that was maintained to day 28. Patients were allowed 1 excursion (non-response) on days 8, 15, or 22, provided the score was ≥25% improvement. Patients with missed assessments or who discontinued early were not considered to have onset of clinical response.
bWeighted difference of the response rates estimated using asymptotic standard error and difference in response rates at each stage.
cUnweighted estimate of the odds of achieving onset of clinical response on esketamine/antidepressant divided by the odds of achieving onset of clinical response on antidepressant/placebo.
dDifference from placebo is the median unbiased estimate, which is a weighted combination of the LS means of the difference from placebo.
e2-sided 95% flexible CI.
Figure 2.
Least squares mean change (±SE) in Montgomery-Asberg Depression Rating Scale (MADRS) total score over time in double-blind phase (observed cases mixed model for repeated measures [MMRM]). LS, least squares; SE, standard error. LS mean and SE were based on MMRM with change from baseline as the response variable and the fixed effect model terms for treatment (esketamine 56 mg/antidepressant, esketamine 84 mg/antidepressant, antidepressant/placebo), day, region, class of oral antidepressant, and treatment-by-day, and baseline value as a covariate. Results are not adjusted for multiple comparisons or sample size reestimation. Negative change in score indicates improvement.
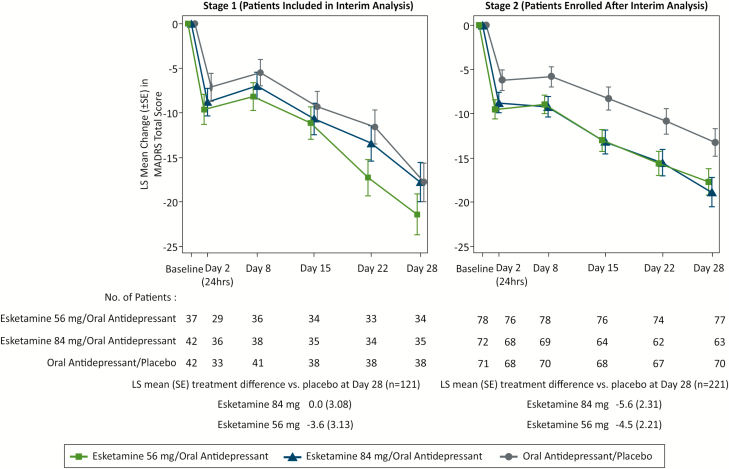
A differential treatment effect was observed for stage 1 (patients enrolled prior to the interim analysis) compared with stage 2 (patients enrolled after interim analysis). The treatment differences in LS means between both esketamine/antidepressant groups and antidepressant/placebo were greater in stage 2 compared with stage 1 (Figure 3).
Figure 3.
Least squares mean changes (±SE) in Montgomery-Asberg Depression Rating Scale (MADRS) total score over time (observed case) by stage in double-blind phase. SE, standard error. LS mean and SE were based on mixed model for repeated measures (MMRM) with change from baseline as the response variable and the fixed effect model terms for treatment (esketamine 56 mg/oral antidepressant, esketamine 84 mg/oral antidepressant, oral antidepressant/placebo), day, region, class of oral AD, stage, treatment-by-day, treatment-by-stage, and treatment-by-day-by-stage, and baseline value as a covariate. Results are not adjusted for multiple comparisons or sample size reestimation. Negative change in score indicates improvement.
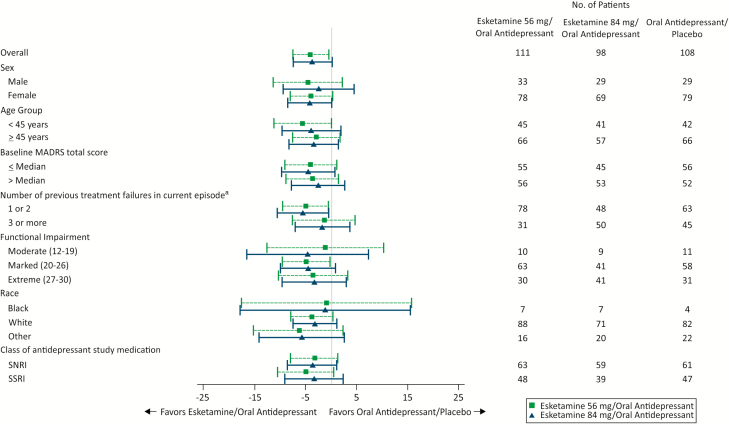
The results of a post hoc analysis on the primary endpoint, with the results of both esketamine dose groups combined, are presented in Table 4. Subgroup analyses on the primary endpoint are presented in Figure 4.
Table 4.
MADRS Total Score: Change from Baseline to Day 28 of the Double-Blind Phase (Combined Esketamine Dose Groups, Unweighted Analysis)
| Esketamine/oral antidepressant | Oral antidepressant/placebo | |
|---|---|---|
| Baseline | ||
| N | 229 | 113 |
| Mean (SD) | 37.6 (5.18) | 37.5 (6.16) |
| Change from baseline to day 28 | ||
| N | 209 | 108 |
| Mean (SD) | –18.9 (13.95) | –14.8 (15.07) |
| MMRM analysisa | ||
| Diff. of LS means (SE) | –3.8 (1.58) | |
| 95% CI on difference | –6.92; –0.70 |
Notes: Four randomized patients in the esketamine arms were not included in the efficacy analysis because they did not receive esketamine and/or the oral antidepressant.
MADRS total score ranges from 0 to 60; a higher score indicates a more severe condition. Negative change in score indicates improvement. Negative difference favors esketamine.
Abbreviations: CI, confidence interval; LS, least squares; MADRS, Montgomery-Asberg Depression Rating Scale; MMRM, mixed-effect model using repeated measures; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
aMMRM analysis with change from baseline as the response variable and the fixed effect model terms for treatment (combined esketamine/antidepressant, antidepressant/placebo) day, region, class of antidepressant (SNRI or SSRI), and treatment-by-day and baseline value as a covariate.
Figure 4.
Forest plot for Montgomery-Asberg Depression Rating Scale (MADRS) total score: least squares mean treatment difference of change from baseline (95% CI) to day 28 mixed model for repeated measures (MMRM) by subgroup in double-blind phase. CI , confidence interval. aNumber of antidepressants taken with nonresponse in addition to 1 prospective antidepressant.
The proportion of patients who were responders and the proportion in remission at any given timepoint generally increased over the double-blind phase in all 3 treatment groups; at day 28, 54.1%, 53.1%, and 38.9% of patients in the esketamine 56 mg/antidepressant, esketamine 84 mg/antidepressant, and antidepressant/placebo groups, respectively, were responders, and 36.0%, 38.8%, and 30.6%, respectively, were in remission. The NNT values for response were 7 and 7 for esketamine 56 mg/antidepressant and esketamine 84 mg/antidepressant, respectively. The NNT values for remission were 18 and 12 for esketamine 56 mg/antidepressant and esketamine 84 mg/antidepressant, respectively.
Median CGI-S scores improved from baseline to day 28 in all 3 treatment groups with median (range) changes of –2.0 (–5; 1) in both esketamine/antidepressant groups and –1.0 (–6; 3) in the antidepressant/placebo group. The odds of a less severe CGI-S score at day 28 in the esketamine/antidepressant groups was 3.2 (56 mg) and 2.5 (84 mg) times that of the antidepressant/placebo group.
Patients in all 3 treatment groups had a decrease in mean GAD-7 total score from baseline to day 28 (mean change [SD] at day 28 from baseline of –7.4 [5.94] from 13.2 for esketamine 56 mg/antidepressant group, –7.7 [5.72] from 13.4 for esketamine 84 mg/antidepressant group, and –6.0 [6.01] from 13.2 for antidepressant/placebo). The LS mean differences (95% CI) vs antidepressant/placebo for the 56 mg and 84 mg esketamine/antidepressant groups were –1.5 (–2.84; –0.20) and −1.4 (–2.77; –0.12), respectively.
Health outcome improved in all 3 treatment groups based on mean [SD] change from baseline to day 28 in the EQ-5D-5L health status index (0.224 [0.2481], 0.243 [0.2395], and 0.181 [0.2495]) and in the EQ-5D-5L visual analogue scale score (20.9 [25.04] 19.1 [26.86], and 14.9 [27.15]) for the esketamine 56 mg/antidepressant, esketamine 84 mg/antidepressant, and antidepressant/placebo groups, respectively.
Treatment-emergent adverse events with an incidence of ≥5% are presented in Table 5. The overall rates of adverse events and severe adverse events were similar for the esketamine 84 mg/antidepressant and esketamine 56 mg/antidepressant groups. Adverse events of dissociation occurred at a higher rate in the esketamine 84 mg/antidepressant group than the esketamine 56 mg/antidepressant group, and severe adverse events of dissociation and nausea occurred at a higher rate in the esketamine 84 mg group. Most adverse events occurred on nasal spray dosing days, were mild or moderate in severity, and resolved the same day.
Table 5.
Most Frequently Reported Adverse Eventsa in the Double-Blind Phase
| Number (%) of patients | ||||
|---|---|---|---|---|
| Adverse event | Esketamine 56 mg/ oral antidepressant N = 115 | Esketamine 84 mg/ oral antidepressant N = 116 | Total esketamine/ oral antidepressant N = 231 | Oral antidepressant/placebo N = 113 |
| Nausea | 31 (27.0%) | 37 (31.9%) | 68 (29.4%) | 12 (10.6%) |
| Dissociation | 30 (26.1%) | 32 (27.6%) | 62 (26.8%) | 4 (3.5%) |
| Dizziness | 32 (27.8%) | 26 (22.4%) | 58 (25.1%) | 10 (8.8%) |
| Vertigo | 24 (20.9%) | 24 (20.7%) | 48 (20.8%) | 2 (1.8%) |
| Headache | 23 (20.0%) | 24 (20.7%) | 47 (20.3%) | 19 (16.8%) |
| Somnolence | 24 (20.9%) | 21 (18.1%) | 45 (19.5%) | 13 (11.5%) |
| Dysgeusia | 17 (14.8%) | 20 (17.2%) | 37 (16.0%) | 17 (15.0%) |
| Hypoesthesia | 14 (12.2%) | 16 (13.8%) | 30 (13.3%) | 2 (1.8%) |
| Paresthesia | 19 (16.5%) | 11 (9.5%) | 30 (13.0%) | 3 (2.7%) |
| Hypoesthesia oral | 16 (13.9%) | 12 (10.3%) | 28 (12.1%) | 2 (1.8%) |
| Vomiting | 7 (6.1%) | 14 (12.1%) | 21 (9.1%) | 2 (1.8%) |
| Fatigue | 12 (10.4%) | 8 (6.9%) | 20 (8.7%) | 5 (4.4%) |
| Anxiety | 10 (8.7%) | 9 (7.8%) | 19 (8.2%) | 7 (6.2%) |
| Blood pressure increased | 8 (7.0%) | 11 (9.5%) | 19 (8.2%) | 5 (4.4%) |
| Insomnia | 10 (8.7%) | 8 (6.9%) | 18 (7.8%) | 11 (9.7%) |
| Vision blurred | 8 (7.0%) | 9 (7.8%) | 17 (7.4%) | 0 |
| Dizziness postural | 7 (6.1%) | 7 (6.0%) | 14 (6.1%) | 0 |
| Sedation | 6 (5.2%) | 8 (6.9%) | 14 (6.1%) | 1 (0.9%) |
| Throat irritation | 5 (4.3%) | 9 (7.8%) | 14 (6.1%) | 4 (3.5%) |
| Diarrhea | 8 (7.0%) | 5 (4.3%) | 13 (5.6%) | 3 (2.7%) |
| Lethargy | 7 (6.1%) | 5 (4.3%) | 12 (5.2%) | 1 (0.9%) |
| Euphoric mood | 8 (7.0%) | 2 (1.7%) | 10 (4.3%) | 2 (1.8%) |
| Feeling drunk | 7 (6.1%) | 3 (2.6%) | 10 (4.3%) | 0 |
| Paresthesia oral | 9 (7.8%) | 1 (0.9%) | 10 (4.3%) | 2 (1.8%) |
| Tremor | 4 (3.5%) | 6 (5.2%) | 10 (4.3%) | 2 (1.8%) |
| Mental impairment | 6 (5.2%) | 3 (2.6%) | 9 (3.9%) | 1 (0.9%) |
| Nasal discomfort | 4 (3.5%) | 5 (4.3%) | 9 (3.9%) | 7 (6.2%) |
| Pollakiuria | 6 (5.2%) | 2 (1.7%) | 8 (3.5%) | 1 (0.9%) |
Notes: Adverse events listed in decreasing order based on incidence within the total esketamine/antidepressant group, and in alphabetical order for events with the same incidence.
aIncidence ≥5% any treatment group.
Present-state dissociative symptoms and transient perceptual effects were assessed by the CADSS total score. These symptoms emerged shortly after the start of esketamine dosing, peaked at 40 minutes, and resolved by 1.5 hours (Figure 5). In Brief Psychiatric Rating Scale-positive symptom subscale assessments, no symptoms or adverse events of psychosis were reported.
Figure 5.
Mean (±SE) Clinician-Assessed Dissociative Symptom Scale (CADSS) total score over time in the double-blind phase. SE, standard error.
There were no deaths in the study. Two patients in the esketamine 56 mg/antidepressant group each experienced 1 serious adverse event during the double-blind phase that the investigator classified as possibly related (worsening of depression on day 15) and probably related (headache on day 12) to the intranasal study drug.
Ten patients experienced 1 or more adverse events during the double-blind phase leading to discontinuation of intranasal study drug, 1 (0.9%) in the esketamine 56 mg/antidepressant group (event of depression, as noted above), 7 (6.0%) in the esketamine 84 mg/antidepressant group (single events of anxiety, disturbance in attention, extrasystoles, headache, mania, motion sickness, panic attack, and tachycardia, and 2 events each of dizziness, nausea, and vomiting), and 2 (1.8%) in the antidepressant/placebo group (single events of erectile dysfunction and worsening insomnia). Notably, the majority of the patients in the esketamine 84 mg/antidepressant group (i.e., 5 of 7 patients) who discontinued the intranasal study drug due to an adverse event did so after receiving only the starting dose of intranasal study medication on day 1, which was 56 mg.
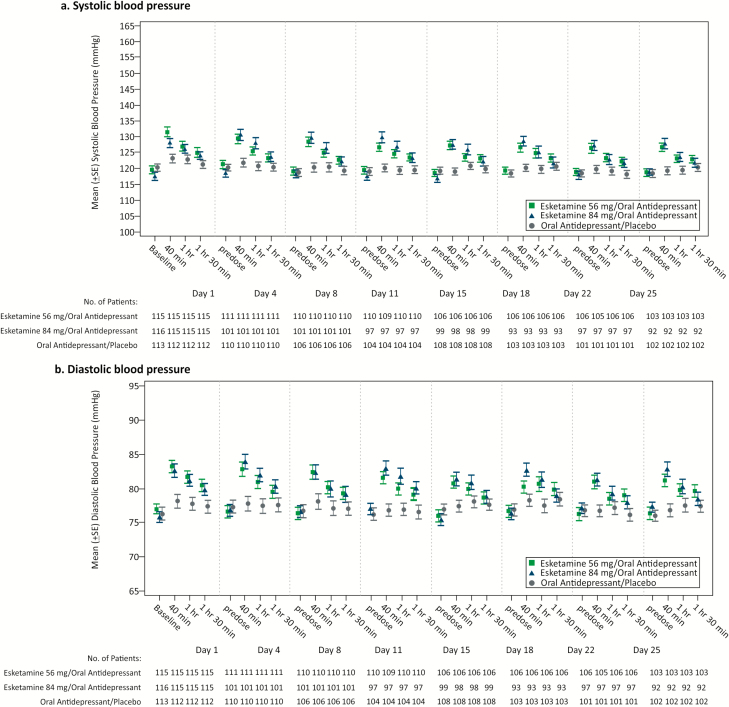
In both esketamine/antidepressant dose groups, after each nasal spray dose, transient blood pressure increases from baseline of a similar magnitude occurred at 40-minute postdose and subsequently returned close to predose values at the 1.5-hour postdose timepoint (Figure 6). The largest mean maximum increase (across all dosing days) for systolic blood pressure was +14.3 and +15.0 mmHg for the esketamine 56 mg/antidepressant and esketamine 84 mg/antidepressant groups, respectively, and +7.2 mmHg for antidepressant/placebo, and for diastolic blood pressure was +8.9, +9.4, and +5.3 mmHg for the respective treatment groups. There was no case of respiratory depression, as assessed by respiratory rate and oxygen saturation.
Figure 6.
Mean (±SE) blood pressure over time in the double-blind phase. SE, standard error.
Based on the pattern of responses on the MOAA/S scale, sedative effects of esketamine were generally mild (corresponding to a MOAA/S score of 4 [lethargic response to name spoken in normal tone]), had onset shortly after nasal spray dose administration, typically peaked at 30 to 45 minutes postdose, and resolved by 1 to 1.5 hours postdose. A greater proportion of patients treated with esketamine (56 mg: 9.6% [11/115]; 84 mg: 12.1% [14/116]) had moderate or greater sedation (MOAA/S score of ≤3) at any time during the double-blind phase compared with antidepressant/placebo (0.9% [1/113]).
Twelve (10.4%) patients in the esketamine 56 mg/antidepressant group, 8 (7.1%) patients in the esketamine 84 mg/antidepressant group, and 13 (11.5%) patients in the antidepressant/placebo group had treatment-emergent post-baseline suicidal ideation assessed by the Columbia Suicide Severity Rating Scale. One patient (treated with esketamine 56 mg/antidepressant) reported postbaseline preparatory acts/behavior (i.e., patient placed sleeping medications under pillow to use for sleep and to forget past), which was reported as an adverse event that resolved on the same day (day 7) without additional intervention.
Few patients had evidence of symptoms on nasal examination (2 [1.7%] and 3 [2.6%] in the esketamine 56 mg/antidepressant and 84 mg/antidepressant groups, respectively, and 2 [1.8%] in the antidepressant/placebo group). Most patients (83 [72.2%], 87 [75.0%], and 83 [73.5%] in the respective groups) reported no nasal symptoms or mild symptoms for individual items on the Nasal Symptom Questionnaire.
On each intranasal treatment day, approximately one-half of patients treated with esketamine/antidepressant (≥56% for 56 mg and ≥44% for 84 mg) and ≥88% of patients treated with antidepressant/placebo were considered ready for discharge by 1 hour after dosing based on the Global Assessment of Discharge Readiness, and approximately ≥90%, ≥87%, and ≥97%, respectively, were considered ready for discharge by 1.5 hours postdose.
No evidence of withdrawal symptoms was observed during the 2 weeks after cessation of treatment with esketamine/antidepressant assessed by Physician Withdrawal Checklist-20.
Discussion
Although esketamine 84 mg/antidepressant was not statistically significant relative to antidepressant/placebo, the treatment differences at day 28 of –3.2 and –4.1 for the esketamine 84 mg/antidepressant and esketamine 56 mg/antidepressant groups, respectively, appear consistent with the positive findings in a similar, Phase 3 flexible-dose esketamine study in adults with TRD (Popova et al., 2019). To put these results in perspective, a 2-point difference between antidepressant and placebo treatment on the MADRS is considered a clinically meaningful treatment difference for biogenic amine antidepressants with proven efficacy (Montgomery and Moller, 2009). Notably, the treatment difference at day 28 in this study was based on comparison to a newly initiated biogenic amine antidepressant rather than placebo alone. The key secondary efficacy endpoints, response and remission rates, and subgroup analyses of the primary endpoint, assessed from both the clinician and patient perspective, were also all consistent with the primary endpoint analyses in that the results numerically favored both esketamine/antidepressant groups over antidepressant/placebo. The data from the esketamine phase 3 studies, including this study, provided evidence of clinically meaningful efficacy when esketamine is used in combination with a newly initiated oral antidepressant (Kim et al., 2019).
There are several factors that may have contributed to the lack of statistical significance for esketamine 84 mg/antidepressant. First, the withdrawal rate in the esketamine 84 mg/antidepressant group was 3-fold higher compared with the other treatment groups, with many patients withdrawing after the first dose (56 mg). No clear pattern or trend in the reasons for discontinuation was identified and this does not appear to be due to a new or dose-related safety finding.
Second, although rigorous eligibility criteria were used to ensure enrollment of TRD patients, including independent raters to confirm diagnosis, severity, and antidepressant nonresponse at entry, the antidepressant/placebo group had greater improvement in depressive symptoms than had been anticipated based on the STAR*D and olanzapine/fluoxetine combination studies (Fava, 2003; Thase et al., 2007). The demographics were similar to the STAR*D Step 3 population in age, gender, illness duration, and previous treatment failures (Fava, 2003). It is conceivable that the higher response and remission rates observed in the antidepressant/placebo group may reflect high patient expectation of receiving a novel drug and/or by the frequent, lengthy (i.e., in some cases up to 4 hours twice weekly) clinical encounters that exceed the duration of routine office visits. Increases from baseline in the CADSS total score were observed in 28.3% of patients receiving antidepressant/placebo treatment, where dissociative symptoms are not anticipated, showing a nocebo effect (i.e., adverse effect following an “inert” treatment) was present. These factors may have contributed to a lower effect size than was observed in a phase 2 study (Daly et al., 2018) (on which sample size for the current study was based), which compared esketamine nasal spray with placebo nasal spray as adjunctive treatment to an existing oral antidepressant(s). The assumptions made in the analysis method selected to adjust for multiplicity (i.e., fixed sequence approach starting with the 84 mg dose) were based on the results of the phase 2 study, specifically that the efficacy of the 84 mg dose would be at least similar to or exceed that of 56 mg.
Third, there were differences in efficacy observed between the 2 stages of the interim analysis, specifically less improvement in the antidepressant/placebo group, resulting in a greater treatment difference being observed in stage 2. While the study was ongoing, prior to and independent of the interim analysis, several initiatives were implemented to further enhance the quality of study conduct, including the implementation of audio-recording for independent MADRS assessments, addition of new, experienced clinical sites, enhanced site education and training, and the availability of a long-term esketamine open-label safety extension study, all of which were considered to have an impact on patients enrolled in stage 2.
Fourth, the study design randomized only one-third of patients into the placebo arm, a design element associated with exaggerated placebo effect sizes and lower drug vs placebo differences in antidepressant trials (Papakostas and Fava, 2009). Safety and tolerability findings were consistent with the phase 2 and 3 studies, with no new or unexpected safety concerns observed in this study.
The generalizability of the study findings is limited by the exclusion of patients with significant psychiatric or medical co-morbidities or moderate/severe substance use disorder, and patients with MDD at imminent risk of suicide (who are being studied in a separate research program), the greater proportion of female to male patients, and the low proportion of non-white patients. While independent remote MADRS raters were used, it is possible that the specific adverse event profile of esketamine may have affected the blind for the study patients. Finally, this study assessed only the short-term efficacy and safety of esketamine and thus did not provide information on maintenance of effect and long-term safety, which were evaluated in other studies (Daly et al., 2019; Wajs et al., 2018).
In summary, statistical significance was not achieved with esketamine 84 mg/antidepressant compared with antidepressant/placebo; therefore, esketamine 56 mg/antidepressant could not be formally evaluated for regulatory purposes. However, the data for esketamine 56 mg/antidepressant is provided as it is informative to practitioners. Results for the primary endpoint and key secondary endpoints numerically favored both esketamine/ antidepressant groups over the antidepressant/placebo group. Notably, the treatment effect for both esketamine/antidepressant treatment groups in this study exceeded what has been observed and considered clinically meaningful with approved antidepressants when compared with placebo. Esketamine appeared to be safe and tolerable, with no meaningful differences in safety and tolerability between doses. This study provides supportive evidence for the safety and efficacy of esketamine nasal spray as a new, rapid-acting antidepressant for patients with TRD.
Supplementary Material
Acknowledgments
We acknowledge Sandra Norris, PharmD, of the Norris Communications Group LLC, supported by Janssen Research and Development, LLC, for medical writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) for additional editorial support. The authors thank the study patients for their participation in this study.
This work was supported by Janssen Research and Development, LLC, Titusville, NJ.
Sites and Principal Investigators
The following investigators participated in the study: BELGIUM: G. DeBruecker, M. Desseilles, S. Estercam, S. Geerts, W. Pitchot, J. Raemdonck, C. Reynaert, H. van den Ameele, D. Zeeuws; BRAZIL: F. Garcia, H. Grabowski, J. Hubner, A. Nardi, C. Perico, F. Lopes Rocha, S. Ruschel, F. Souza; CANADA: P. Blier, R. Lam, R. Milev, A. Ravindran, S. Richard-Devantoy; ESTONIA: A. Arold, I. Toru; FRANCE: M. Abbar, J. Bartoli, P. Desbonnet, W. El-Hage, R. Gaillard, M. Garnier, E. Haffen, N. Jaafari, A. Sauvaget; HUNGARY: Z. Kiss, Z. Makkos, J. Rethelyi; MEXICO: S.J. Villasenor Bayardo, G. Bernal, F. Brandi, M. Caceres, V. Escaname, M.A. Herrera Estrella, G. Alejo Galarza, E. Saucedo, J.L. Vazquez; SLOVAKIA: J. Greskova, Z. Janikova, R. Korba, A. Shinwari, L Vavrusova; UNITED STATES: Alabama: R. Shelton; Arkansas: P. Wylie; California: J. Bermak, D. Chueh, M. Geromi/I. Kovacs, V. Mehra, M. Plopper, D. Walling; Florida: E. Arocha, J. Benito, L. Harper; S. Rente, J. Zaglul; Pennsylvania: S. Hatti, M. Thase; Massachusetts: G. Labun/B. Lal, I. Mezhebovsky, A. Rothschild; D. Rutrick; Maryland: R. Litman; Illinois: A. Halaris, A. Hirsch, A. Kim, B. Plyler, J. Sonnenberg, C. Goldstein/B. Hesler; Kansas: M. Macaluso; Missouri: H. Ilivicky, G. Mattingly; New York: M. Liebowitz; North Carolina: S. Szabo; Oklahoma: C. Nixon, S. Randhawa; Nebraska: S. Ramaswamy; Rhode Island: J. Whalen; Texas: D. Brown, M. Downing, M. Jha, D. Patel; Vermont: A. Groft; Washington: S. Mustafa; Wisconsin: A. Koplin.
Contributors
Maggie Fedgchin, Ella Daly, Jaskaran Singh, Wayne Drevets, Rosanne Lane, Pilar Lim, Rama Melkote, Dawn Vitagliano, Madhukar Trivedi, Pierre Blier, Maurizio Fava, Michael Liebowitz, Arun Ravindran, Raphael Gaillard, Hans van den Ameele, and Sheldon Preskorn were involved in study design and/or data collection. Rama Melkote conducted the statistical analyses together with Rosanne Lane and Pilar Lim. All authors were involved in interpretation of the results and review of the manuscript. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors.
Potential Conflict of Interest
MF, ED, RM, RL, PL, DV, HM, DH, WD, and JS are employees of Janssen Research & Development, LLC and hold company equity. MT has consulted for or served on the advisory board of Alkermes Inc., Akili Interactive, Allergan Pharmaceuticals, Arcadia Pharmaceuticals, Avanir Pharmaceuticals, Brintellix Global, Bristol Myers Squibb, Caudex, Cerecor, Forest Pharmaceuticals, Global Medical Education Inc., Health Research Associates, Insys, Johnson & Johnson Pharmaceutical Research & Development, Lilly Research Laboratories, Lundbeck Research USA, Medscape, Merck & Co. Inc., Mitsubishi Pharma, MSI Methylation Sciences – Pamlab Inc., Navitor, Otsuka America Pharmaceutical Inc., One Carbon Therapeutics, Otsuka America Pharmaceutical Inc., Pfizer Inc., Takeda Global Research; he has received royalties from Janssen Research and Development, LLC; he has author agreements from Janssen Asia Pacific and the Oxford University Press; and he has received grants from Agency for Healthcare Research and Quality (AHRQ), Cancer Prevention and Research Institute of Texas (CPRIT), National Institute of Mental Health (NIMH), National Institute of Drug Abuse (NIDA), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Center for Advancing Translational Sciences (NCATS), Johnson & Johnson, and the Patient-Centered Outcomes Research Institute (PCORI). PB has served as a consultant and/or received research grants from Allergan, Bristol Myers Squibb, Janssen, Lundbeck, Meda-Valeant, Otsuka, Pfizer, Sunovion, and Takeda. Disclosures for MF (Fava) are listed at: https://mghcme.org/faculty/faculty-detail/maurizio_fava. ML has consulted for Pherin, Otsuka, Allergan, and Edgemont; has recent or current clinical trial contracts with Pfizer, Forest, Tikvah, Allergan, Takeda, Lundbeck, Otsuka, Naurex, Teva, Cerecor, MSI, Sunovion, Janssen, Vistagen, Gruenthal, Toche, Purdue Pharma, Alcobra, Daiichi Sankyo, Alkermes, Edgemont, Enzymotec, Indivior, Pherin, and Tonix; has recent or current investigator-initiated trial support from Pfizer, Forest, and Pherin; is a speaker/poster presenter for Wyeth, Pfizer, Pherin, and Forest; holds licensing LSAS: Janssen; and equity ownership/options with Pherin Pharmaceutical and Liebowitz Social Anxiety Scale. AR has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: Allergan, Brain Canada, CAMH Foundation, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Grand Challenges Canada, Janssen, Otsuka, and Pfizer. AR does not own any stocks or shares in companies in the pharmaceutical industry. RG has received compensation as a member of the scientific advisory board of Janssen, Lundbeck, Roche, SOBI, and Takeda. He has served as consultant and/or speaker for Astra Zeneca, Boehringer-Ingelheim, Pierre Fabre, Lilly, Lundbeck, MAPREG, Otsuka, Pileje, SANOFI, Servier, and LVMH for which he has received compensation, and he has received research support from Servier. He is co-founder and stock shareholder of Regstem. H vd A has received research grants from Janssen. Within the last year, SP has received research grants (with payments made to Kansas University Medical Center Research Institute) from Acadia, Allergan, and Janssen. He has received compensation as a consultant to Alkermes, Allergan, BioXcel, Janssen, Merck, Novartis, and Perception, and as a speaker for Sunovion and over his career has been a principal investigator or consultant for 140 pharmaceutical, biotechnology, device, and diagnostic companies.
Role of the Sponsor
Employees of the sponsor, as noted in author contributions, were involved in trial design; patient recruitment; data collection, analysis, or interpretation; and/or other aspects pertinent to the study. Authors had full access to all of the data in the study, were involved in writing and/or revising the manuscript, and had final responsibility for the decision to submit for publication.
Previous Presentations
Data from this study were presented in oral and poster sessions at the 9th Biennial Conference of the International Society for Affective Disorders (ISAD) and the Houston Mood Disorders Conference, September 21, 2018; and the Psych Congress 2018, Orlando, FL, October 26 2018.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed.Washington, DC: American Psychiatric Association. [Google Scholar]
- Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, Vázquez GH (2017) Morbidity in depressive disorders. Psychother Psychosom 86:65–72. [DOI] [PubMed] [Google Scholar]
- Bauer M, Severus E, Köhler S, Whybrow PC, Angst J, Möller HJ; Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders (2015) World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry 16:76–95. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175:620–630. [DOI] [PubMed] [Google Scholar]
- Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M (2010) RESEARCH: validation of the Massachusetts general hospital antidepressant treatment history questionnaire (ATRQ). CNS Neurosci Ther 16:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Hung HM, Wang SJ (1999) Modification of sample size in group sequential clinical trials. Biometrics 55:853–857. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, Lane R, Lim P, Duca AR, Hough D, Thase ME, Zajecka J, Winokur A, Divacka I, Fagiolini A, Cubała WJ, Bitter I, Blier P, Shelton RC, Molero P, Manji H, Drevets WC, Singh JB (2019) A double-blind, randomized withdrawal, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression (SUSTAIN-1). JAMA Psychiatry 2019. Published online June 5, 2019. doi: 10.1001/jamapsychiatry.2019.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrienko A, Tamhane AC (2011) Mixtures of multiple testing procedures for gatekeeping applications in clinical trials. Stat Med 30:1473–1488. [DOI] [PubMed] [Google Scholar]
- Dmitrienko A, Tamhane AC, Wiens BL (2008) General multistage gatekeeping procedures. Biom J 50:667–677. [DOI] [PubMed] [Google Scholar]
- EuroQol Group About EQ-5D Available at: http://www.euroqol.org/about-eq-5d.html. Accessed 5 March 2019.
- Fava M. (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53:649–659. [DOI] [PubMed] [Google Scholar]
- Guy WM, (1976) ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare. [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R; CANMAT Depression Work Group (2016) Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry 61:540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Farchione T, Potter A, Chen Q, Temple R (2019) Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N Engl J Med 381:1–4. [DOI] [PubMed] [Google Scholar]
- Lehmacher W, Wassmer G (1999) Adaptive sample size calculations in group sequential trials. Biometrics 55:1286–1290. [DOI] [PubMed] [Google Scholar]
- Leon AC, Olfson M, Portera L, Farber L, Sheehan DV (1997) Assessing psychiatric impairment in primary care with the Sheehan disability scale. Int J Psychiatry Med 27:93–105. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA (2010) The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals 3:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Möller HJ (2009) Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol 24:111–118. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Papakostas GI, Fava M (2009) Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 19:34–40. [DOI] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB (2019) Efficacy and safety of flexibly-dosed esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study. Am J Psychiatry 176:428–438. doi: 10.1001/jamapsychiatry.2019.1189. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M (2007) Columbia classification algorithm of suicide assessment (C-CASA): classification of suicidal events in the FDA’S pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels K, Garcia-Espana F, Mandos LA, Case GW (2008) Physician withdrawal checklist (PWC-20). J Clin Psychopharmacol 28:447–451. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L (2016) Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80:424–431. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB (1999) Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. Jama 282:1737–1744. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Löwe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166:1092–1097. [DOI] [PubMed] [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, Watson SB, Dubé S (2007) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry 68:224–236. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM (2004) The inventory of depressive symptomatology, clinician rating (IDS-C) and self-report (IDS-SR), and the quick inventory of depressive symptomatology, clinician rating (QIDS-C) and self-report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med 34:73–82. [DOI] [PubMed] [Google Scholar]
- Wajs E, Aluisio L, Morrison R, Daly E, Lane R, Lim P, Holder R, Sanacora G, Young AH, Kasper S, Sulaiman AH, Li C-T, Paik J-W, Manji H, Hough D, Drevets W, Singh J (2018) Long-term safety of intranasal esketamine plus oral antidepressant in patients with treatment-resistant depression: phase 3, open-label, safety and efficacy study (SUSTAIN-2) (abstract T67). Presented at the 2018 Annual Meeting of the American Society of Clinical Psychopharmacology (ASCP), May 29-June 1, 2018, Miami, FL: Available at: https://pmg.joynadmin.org/documents/1005/5afde1ec68ed3f2e245822b9.pdf. [Google Scholar]
- Williams JB, Kobak KA (2008) Development and reliability of a structured interview guide for the Montgomery Asberg depression rating scale (SIGMA). Br J Psychiatry 192:52–58. [DOI] [PubMed] [Google Scholar]
- World Health Organization Depression Fact Sheet, Updated March 2018. Available at: http://www.who.int/en/news-room/fact-sheets/detail/depression. Accessed March 5, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.