Abstract
The goals of a genital herpes vaccine are to prevent painful genital lesions and reduce or eliminate subclinical infection that risks transmission to partners and newborns. We evaluated a trivalent glycoprotein vaccine containing herpes simplex virus type 2 (HSV-2) entry molecule glycoprotein D (gD2) and two immune evasion molecules, glycoprotein C (gC2) that binds complement C3b, and glycoprotein E (gE2) that blocks IgG Fc activities. The trivalent vaccine was administered either as baculovirus proteins with CpG and alum, or the identical amino acids were expressed using nucleoside-modified mRNA in lipid nanoparticles. Both formulations completely prevented genital lesions in mice and guinea pigs. Differences emerged when evaluating subclinical infection. The trivalent protein vaccine prevented dorsal root ganglia infection and day 2 and 4 vaginal cultures were negative in 23/30 (73%) mice compared with 63/64 (98%) in the mRNA group (P=0.0012). In guinea pigs, 5/10 (50%) animals in the trivalent subunit protein group had vaginal shedding of HSV-2 DNA on 19/210 (9%) days compared with 2/10 (20%) animals in the mRNA group that shed HSV-2 DNA on 5/210 (2%) days (P=0.0052). Immunology assays were performed in mice. The trivalent mRNA vaccine was superior to trivalent proteins in stimulating serum and vaginal ELISA IgG antibodies, serum neutralizing antibodies, antibodies that bind to crucial gD2 epitopes involved in entry and cell-to-cell spread, CD4+ T cell responses, and T follicular helper and germinal center B cell responses. The trivalent nucleoside-modified mRNA-LNP vaccine is a promising candidate for human trials.
One Sentence Summary:
Improved efficacy using mRNA for a herpes vaccine
Introduction
Herpes simplex virus type 2 (HSV-2) is a common sexually transmitted infection with 14% prevalence among 15 to 49 year old persons in the USA and 11% globally (1). Individuals remain infected for life, with periodic clinical recurrences that may be painful or subclinical recurrences that risk transmission of infection to intimate partners and newborns (2). The incidence of neonatal herpes is approximately 14,000 cases annually worldwide (3) and results in substantial morbidity and mortality because of encephalitis, pneumonia and hepatitis even with antiviral therapy (4, 5). Genital herpes increases the risk of acquisition and transmission of HIV by 3–4-fold, further highlighting the need for an effective prophylactic vaccine (6).
Previous HSV-2 prophylactic human vaccine efforts included immunizing with two essential virus entry molecules, HSV-2 glycoproteins B (gB2) and D (gD2) (7), or gD2 alone (8, 9). In the gB2/gD2 trial, vaccine recipients had delayed onset of infection, but overall were not protected (7). In the first of two reports using gD2 alone, a subgroup analysis indicated that HSV-1/HSV-2 doubly seronegative women, but not men were protected against HSV-2 genital lesions (8). A repeat study performed in doubly seronegative women failed to confirm this result but noted 58% protection against genital lesions caused by HSV-1. Sixty percent of the cases of genital herpes in the control group were caused by HSV-1 (9). ELISA antibodies to gD2 correlated with protection against genital HSV-1 infection (10). Maternal and neonatal antibodies that neutralize HSV or that mediate antibody-dependent cellular cytotoxicity also correlate with protection against severe neonatal herpes (11–13). These studies suggest that antibodies are important for prevention of herpes infection.
Our vaccine strategy is designed to produce potent antibody responses and includes an entry molecule, gD and two additional HSV glycoproteins, gC and gE. HSV-1 and HSV-2 gC and gE are immune evasion molecules that block the effectiveness of antibody responses (14–16). HSV gC binds complement component C3b to inhibit complement activation, while gE binds the IgG Fc domain of antibodies targeting HSV antigens to block IgG Fc activities, including complement activation and antibody-dependent cellular cytotoxicity (ADCC) (17–20). Antibodies produced to the three glycoproteins perform multiple antiviral activities, including neutralizing virus (gC2 and gD2), blocking cell to cell spread (gD2 and gE2), and preventing immune evasion from antibody and complement (gC2 and gE2) (16, 21–24).
No genital herpes vaccine has prevented both clinical and subclinical infection. Our prior vaccine studies in mice and guinea pigs used baculovirus-produced gC2, gD2, and gE2 subunit proteins with CpG and alum as adjuvants. The vaccine provided strong protection against clinical lesions in mice (100%) and guinea pigs (98%) but it did not prevent vaginal virus replication on days 2 and 4 post-infection in mice and 17/36 (47%) guinea pigs developed subclinical infection detected by vaginal shedding of HSV-2 DNA between days 28 and 49 post-infection (16, 22). Here we evaluated whether an immunization strategy that uses nucleoside-modified mRNA to encode the same three HSV-2 glycoproteins improves vaccine efficacy.
Nucleoside-modified mRNA vaccines have recently emerged as highly promising for infectious disease vaccine development (25). Modifications in mRNA increase translation and reduce inflammatory side effects. Modifications include altering the 5’ cap, the 5’- and 3’-UTRs, poly A tails, replacing uridine with 1-methylpseudouridine, and removing double stranded RNA contaminants. Non-inflammatory mRNA is beneficial because innate immune sensors recognize mRNA, release type I interferons and activate interferon-inducible genes that inhibit translation (26). Various formulations protect nucleoside-modified mRNA from RNAses and facilitate efficient in vivo delivery, including lipid nanoparticles (LNPs) (27, 28). Here we demonstrate that the HSV-2 gC2, gD2 and gE2 trivalent nucleoside-modified mRNA-LNP vaccine outperformed the trivalent protein vaccine given with CpG and alum and achieved outstanding vaccine efficacy in mice and guinea pigs.
Results
The primary goal was to determine whether the trivalent nucleoside-modified mRNA-LNP vaccine provides better protection than the trivalent protein-CpG/alum vaccine. A secondary goal was to determine whether the trivalent mRNA-LNP vaccine outperformed gD2 nucleoside-modified mRNA-LNP alone as support for adding gC2 and gE2 mRNA to the vaccine.
Characterization of the truncated gC2, gD2 and gE2 proteins produced in cells transfected with nucleoside-modified mRNA-LNP
Nucleoside-modified mRNA was prepared to encode gC2 amino acids 27–426, gD2 amino acids 26–331, and gE2 amino acids 24–405, each truncated prior to their transmembrane domains. Expression of the glycoproteins was assessed in HEK 293 cells that were transfected with the mRNAs or with PolyC RNA as a control. The proteins in the mRNA-transfected cells reacted with gC2, gD2 or gE2 antibodies by Western blot and were of the expected size when accounting for different glycosylation patterns in mammalian cells (Figs. S1a–c). Supernatant fluids from the transfected cells contained the glycoprotein antigens when assayed by ELISA (Figs. S1d–f). We conclude that cells transfected with each nucleoside-modified mRNA synthesized and secreted the truncated glycoprotein.
Vaccine efficacy in mice
We infected mice intravaginally with either 5×103 or 5×104 PFU of HSV-2 to assess protection over a 10-fold titer range.
Protection against 5×103 PFU (275 LD50) intravaginal HSV-2 after intradermal immunization with nucleoside-modified mRNA-LNP
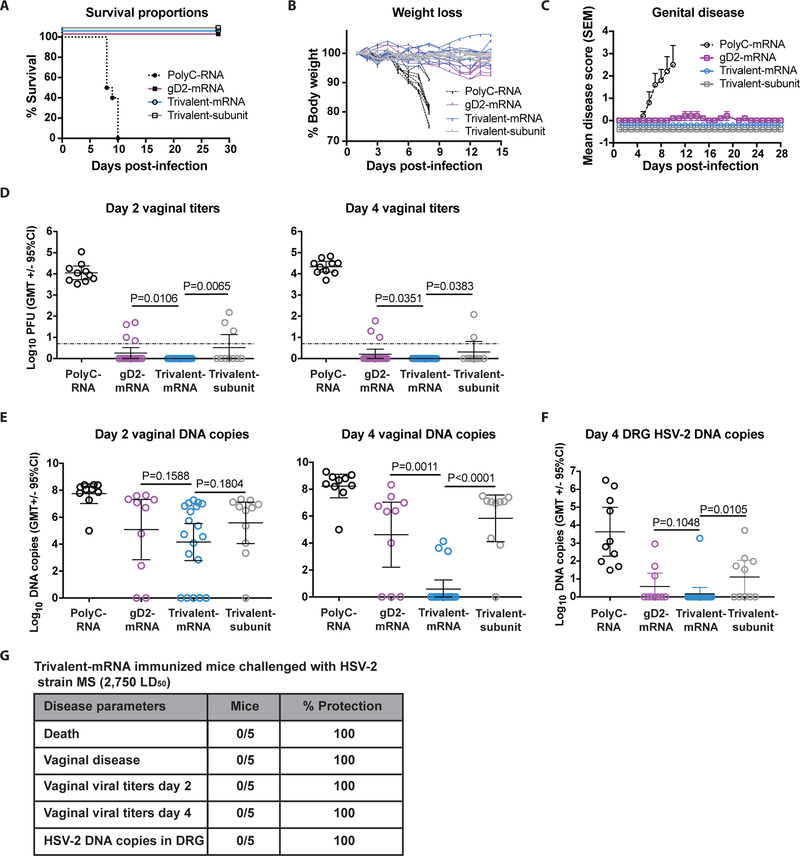
BALB/c mice were immunized intradermally (ID) twice at a 28-day interval with nucleoside-modified mRNA-LNP consisting of 10μg of gD2-LNP mRNA, 10μg each of gC2, gD2 and gE2 mRNA combined in an LNP, or 10μg of PolyC RNA-LNP as a control. For comparison, mice were immunized intramuscularly (IM) three times at two-week intervals with 5μg each of gC2, gD2 and gE2 baculovirus-expressed proteins combined with CpG/alum. These concentrations and dosing interval were chosen based on our prior studies in mice using baculovirus subunit protein antigens and mRNA-LNP studies performed with other pathogen vaccines (21, 22, 29–31). Twenty-eight days after the last immunization, mice were treated with medroxyprogesterone to synchronize and thin the vaginal epithelium and five days later infected intravaginally with 5×103 PFU of HSV-2 strain MS (275 LD50). All mice in the PolyC control group were euthanized by day 10, while no other animals died (Fig. 1a).
Figure 1. ID immunization with nucleoside-modified trivalent mRNA-LNP protects against genital HSV-2 in mice.
(a-f) BALB/c mice were immunized twice with PolyC RNA-LNP, gD2 nucleoside-modified mRNA-LNP, trivalent nucleoside-modified mRNA-LNP, or three times with trivalent subunit protein-CpG/alum and challenged one month after the final immunization intravaginally with 5×103 HSV-2. (a) Survival curves: P<0.001 comparing PolyC RNA with each other group. P values were calculated by the log-rank test. (b) The mean weight loss each day post-infection of animals in the trivalent mRNA, trivalent subunit protein, or gD2 mRNA group was compared with PolyC RNA controls; P<0.0001. (c) Mean genital disease scores post-infection comparing trivalent mRNA, trivalent subunit protein or gD2 mRNA with PolyC RNA, P<0.0001. P value in (b) and (c) was calculated by two-way ANOVA followed by Tukey for significance. Error bars in (c) represent SEM. (d) Day 2 and day 4 vaginal titers. Dotted line indicates the limit of assay detection at 6.7 PFU/ml. (e) Day 2 and day 4 vaginal DNA copy number by qPCR. (f) Day 4 DRG HSV-2 DNA copy number by qPCR. P values in (d-f) are shown on the figures for the primary endpoint comparison between trivalent mRNA and trivalent protein or secondary endpoint comparison between trivalent mRNA and gD2 mRNA and were calculated by the two-tailed Fisher’s exact test. Sample size: (a-c and f), n=10 per group, except trivalent mRNA group, n=20 for (a-c) and n=19 for (f); (d), n=10 in PolyC RNA, n=20 in gD2 mRNA, n=39 in trivalent mRNA and n=10 in trivalent subunit protein; (e), n=10 per group except trivalent mRNA n=20. (g) Challenge of trivalent mRNA-immunized mice with 5×104 PFU (2,750 LD50).
No animal in the trivalent mRNA, trivalent protein, or gD2 mRNA group developed ruffled fur, hunched posture, abnormal gait or lethargy as disease indicators. Weights of animals in the trivalent mRNA, trivalent protein or gD2 mRNA group did not differ significantly from one another, yet each group differed significantly from the PolyC RNA controls (Fig. 1b). No genital disease developed in the trivalent mRNA or trivalent protein group. Mild genital disease occurred in one animal in the gD2 mRNA group. Overall, these three groups did not differ significantly from one another, while extensive genital disease developed in the PolyC RNA mice (Fig. 1c).
Day 2 and 4 vaginal cultures measure acute viral infection and represent a stringent test of vaccine efficacy. Strikingly, 39/39 animals in the mRNA trivalent group had negative vaginal cultures on day 2, which was significantly different from 7/10 in the trivalent protein group and 16/20 in the gD2 mRNA group (Fig. 1d). Significant differences between the trivalent mRNA and the trivalent protein or the gD2 mRNA group were also detected on day 4 post-infection (Fig. 1d). We assessed day 2 and day 4 vaginal HSV-2 DNA copy number by qPCR to further evaluate indicators of virus infection. Approximately 5 log10 HSV-2 DNA was detected in the trivalent mRNA group on day 2 and approximately 4 log10 on day 4 despite recovering no replication competent virus on either day, which suggests that the virus was coated with neutralizing antibodies and/or degraded (Fig. 1e). By day 4, the HSV-2 DNA copy number was significantly lower in the trivalent mRNA mice than the trivalent protein or gD2 mRNA alone mice (Fig. 1e).
Peak HSV-2 DNA copy number in dorsal root ganglia (DRG) develops on day 4 or 5 post-challenge in naïve mice (22). All 10 mice in the PolyC RNA group had HSV-2 DNA detected in DRG on day 4, compared with 3/10 in the gD2 mRNA group, 1/19 in the trivalent mRNA group and 5/10 in the trivalent subunit protein group. Differences between trivalent mRNA and trivalent subunit protein were significant, while trivalent mRNA and gD2 mRNA differences were not significant (Fig. 1f).
Protection against 5×104 PFU (2,750 LD50) intravaginal HSV-2 in mice
As a more stringent test of vaccine efficacy, five mice were immunized ID twice with 10μg each of trivalent mRNA-LNP and infected with a 10-fold higher HSV-2 dose, 5×104 PFU strain MS (2,750 LD50). We did not include the trivalent protein group because breakthrough infections occurred at the lower challenge dose. All 5 mice survived, had no genital disease, had negative vaginal cultures on days 2 and 4 and had no HSV-2 DNA detected in DRG at the end of the experiment on day 28 (Fig. 1g). Therefore, no breakthrough infections occurred even at a 10-fold higher challenge dose.
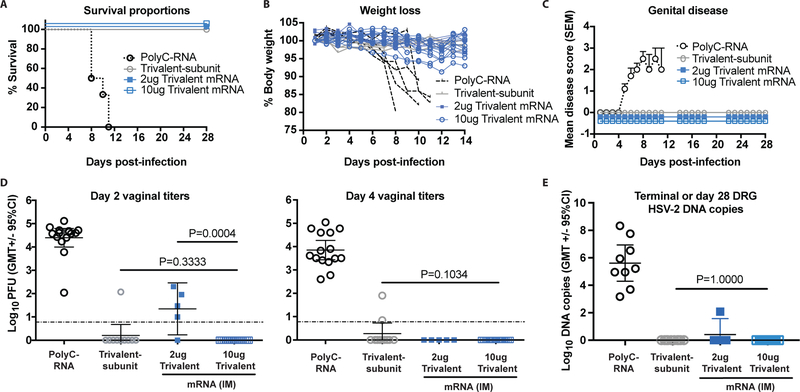
Vaccine efficacy in mice: Protection against intravaginal HSV-2 after IM immunization with nucleoside-modified mRNA-LNP
Our trivalent subunit protein vaccine studies in mice use IM immunization (22). We evaluated the trivalent mRNA vaccine given IM to compare the two vaccine formulations using the same immunization route. Mice were immunized as in Figure 1 except one additional group received trivalent mRNA-LNP using 2μg of each immunogen to assess protection at lower mRNA concentrations. Animals were challenged with 5×103 HSV-2 strain MS (275 LD50). All PolyC control animals were euthanized by day 11, while all other animals survived (Fig. 2a). No animal in the 2μg or 10μg trivalent mRNA or trivalent subunit protein group developed ruffled fur, hunched posture, abnormal gait or lethargy and no significant differences in weight loss were detected comparing these three groups, while the PolyC controls had extensive weight loss (Fig. 2b). The PolyC group developed severe genital disease, while the other groups were totally protected (Fig. 2c). Day 2 vaginal cultures were positive in all 15 mice in the PolyC RNA group, 1/10 in the trivalent subunit protein group, 4/5 in the 2μg trivalent mRNA group and 0/20 in the 10μg trivalent mRNA group (Fig. 2d). All 20 mice in the 10μg trivalent mRNA group also had negative vaginal cultures on day 4, as noted in ID immunized animals (Figs. 2d). DRG were harvested at the time of euthanasia in nine mice from the PolyC group or at the end of the experiment on day 28 in the other groups. All nine animals in the PolyC group had HSV-2 DNA detected in DRG, while 0/10 were positive in the trivalent subunit protein group, 1/5 in the 2μg trivalent mRNA group, and 0/20 in the 10μg trivalent mRNA group (Fig. 2e). We conclude that dose-response protection was apparent in the trivalent mRNA mice immunized IM based on significantly better protection at 10μg than 2μg against vaginal infection on day 2 and slightly better protection of DRG. Although 10μg trivalent mRNA outperformed trivalent subunit protein when evaluating days 2 and 4 vaginal cultures, differences were not statistically significant.
Figure 2. IM immunization with nucleoside-modified trivalent mRNA-LNP protects against genital HSV-2 in mice.
BALB/c mice were immunized as in Figure 1 except one group received 2μg trivalent mRNA. Mice were challenged one month after the final immunization intravaginally with 5×103 HSV-2 (275 LD50). (a) Survival curves: P<0.0001 comparing PolyC RNA with each other group. P values were calculated by the log-rank test. (b) Weight loss of individual animals: P<0.0001 comparing PolyC RNA with the other three groups. (c) Genital disease: P<0.0001 comparing PolyC RNA with the other three groups. P values in (b-c) were calculated by two-way ANOVA followed by Tukey for significance. Sample size: (a-c), n=10 in PolyC RNA group for (a) and (c), n=5 for (b), n=10 in trivalent subunit protein, n=5 in 2μg trivalent mRNA, and n=10 in 10μg trivalent mRNA. Error bars in (c) represent SEM. P values in (d-e) are shown on the figures for the primary endpoint comparison between trivalent mRNA and trivalent protein or the comparison between 2μg and 10μg trivalent mRNA. Other P values are noted below. (d) Day 2 vaginal titers: comparing 10μg trivalent mRNA or trivalent subunit protein with PolyC RNA, P<0.0001; comparing 2μg trivalent mRNA with PolyC RNA, P=0.2500. Day 4 vaginal titers: comparing the number of animals with positive day 4 cultures in the 10μg trivalent mRNA, the 2μg trivalent mRNA or the trivalent subunit protein group with PolyC RNA, P<0.0001. Sample size: n=15 in PolyC RNA group, n=10 in trivalent subunit protein group, n=5 in the 2μg trivalent mRNA group and n=20 in the 10μg trivalent mRNA group. Dotted line indicates the limit of assay detection at 6.7 PFU/ml. (e) DRG HSV-2 DNA copy number at time of euthanasia of PolyC RNA group or on day 28 in the other groups; P<0.0001 comparing the number of animals with positive HSV-2 DNA copy number in the PolyC RNA with the 10μg trivalent mRNA or the trivalent subunit protein group; P=0.0050 comparing the number of animals with positive HSV-2 DNA copy in the PolyC RNA with the 2μg trivalent mRNA group. Other comparisons were not significant. Sample size: n=9 in the PolyC group, n=10 in the trivalent subunit protein group, n=5 in the 2μg trivalent mRNA group and n=10 in the 10μg trivalent mRNA group. P values for (d-e) were calculated by the two-tailed Fisher’s exact test.
Combining the ID and IM results in mice
We combined the results of IM and ID for the 10μg trivalent mRNA-immunized mice because both routes produced virtually identical results. The trivalent subunit protein and trivalent mRNA mice were both totally protected against clinical disease as measured by death, genital lesions and signs of generalized illness (Table 1). Differences between the two vaccine formulations emerged when evaluating subclinical infection measured by days 2 and 4 vaginal cultures and HSV-2 DNA in DRG (Table 1). Based on clinical and subclinical infection, immunization ID or IM with 10μg trivalent mRNA achieved sterilizing immunity in 63/64 (98%) mice compared with 23/30 (77%) in the trivalent subunit protein and 15/20 (75%) in the gD2 mRNA group, while 0/25 (0%) in the PolyC RNA group had sterilizing immunity (Table 1). The breakthrough infections in the trivalent subunit protein group included 5/10 animals that had HSV-2 DNA in DRG, which represents more DRG infection than we reported in a prior study (22). In the current study we used a more stringent criterion for a negative result based on normalizing the HSV-2 DNA copy number to 105 adipsin genes rather than 104 previously.
Table 1.
Combined ID and IM efficacy studies
| Outcome | Number of animals with outcome* | |||
|---|---|---|---|---|
| PolyC RNA | gD2 mRNA | Tri mRNA | Tri protein | |
| Clinical disease: | 25/25 | 1/10 | 0/44 | 0/20 |
| Death, genital lesions or generalized illness | P=0.1852 | P=1.0000 | ||
| Subclinical infection: | 25/25 | 4/20 | 0/64 | 4/20 |
| • Day 2 vag. titers | P=0.0025 | P=0.0025 | ||
| • Day 4 vag. titers | 25/25 | 3/20 | 0/64 | 4/20 |
| P=0.0120 | P=0.0025 | |||
| • HSV-2 DNA in DRG | 19/19 | 3/10 | 1/39 | 5/20 |
| P=0.0231 | P=0.0143 | |||
| Sterile immunity: | 0/25 | 15/20 | 63/64 | 23/30 |
| No clinical or subclinical infection | P=0.0025 | P=0.0012 | ||
The primary endpoint is the comparison between trivalent mRNA-LNP and trivalent subunit protein. The secondary endpoint is the comparison between trivalent mRNA-LNP and gD2 mRNA-LNP. P values were calculated by two-tailed Fisher’s exact test.
Transmission studies in mice
Transmission studies were performed using day 2 vaginal secretions collected from 10 mice immunized ID with 10μg trivalent mRNA-LNP or PolyC RNA-LNP as a control and infected with 5×103 PFU (275 LD50). The vaginal secretions (20μl) from each mouse were inoculated intravaginally into a naïve mouse. An additional five naïve mice were inoculated with 5×103 HSV-2 as a positive control and as expected, all five mice became ill and were euthanized. Three of 10 mice inoculated with vaginal secretions from the PolyC RNA group developed disease and were euthanized compared with 0/10 from the trivalent-mRNA group (Fig. S2a). No animal in the trivalent mRNA group lost weight, while 6/10 in the PolyC and 5/5 in the HSV-2 control group lost weight (Fig. S2b). All naïve animals in the HSV-2-inoculated group and 6/10 in the PolyC RNA group developed genital disease compared with 0/10 in the trivalent mRNA group. Differences were also noted comparing days 2 and 4 vaginal titers of mice inoculated with vaginal secretions from trivalent mRNA or PolyC RNA mice with 6/10 mice in the PolyC RNA group positive on both days compared to 0/10 in the trivalent mRNA group (Fig. S2c–e). We did not evaluate transmission using vaginal fluids from subunit vaccine-immunized animals; however, 4/20 mice in this group had positive day 2 titers compared with 0/64 in the modified mRNA group. Therefore, we postulate that more mice in the baculovirus protein group would transmit virus. HSV-2 DNA was detected in the day 2 vaginal fluids of most mice in the trivalent mRNA group yet no replication competent virus was detected and no transmission of infection occurred to naive mice. This result is consistent with the concept that on day 2 the virus was either coated with neutralizing antibody or degraded. Overall, no naïve mouse inoculated with 20μl of vaginal secretions from the 10μg trivalent mRNA group died, developed genital disease, or had positive cultures on days 2 or 4.
Vaccine efficacy in guinea pigs
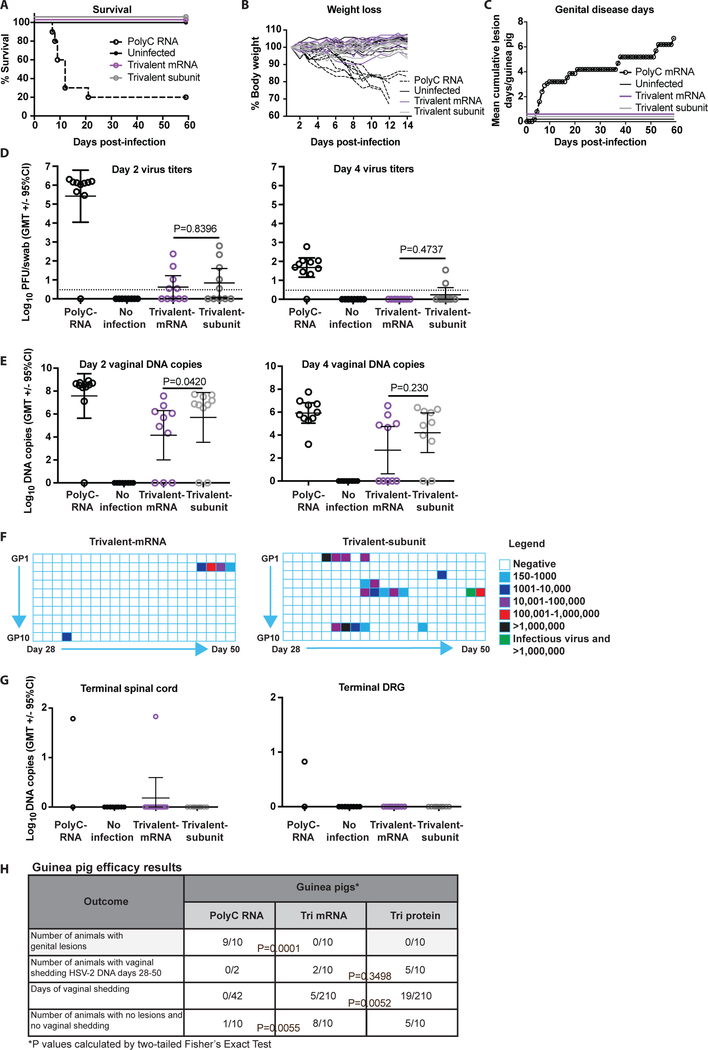
The guinea pig genital infection model is generally viewed as a more stringent test of vaccine efficacy and more biologically relevant in that guinea pigs develop recurrent genital lesions and recurrent vaginal shedding of HSV-2 DNA, while mice do not. Genital HSV-2 shedding indicates reactivation from latency and is a marker of subclinical infection when it occurs in the absence of genital lesions. Nucleoside-modified mRNA-LNP vaccines have not previously been evaluated in guinea pigs; therefore, we decided to use three immunizations, rather than two as in mice. We chose a dose of 20μg per nucleoside-modified mRNA rather than the 10μg dose used in mice based on the larger body size of guinea pigs. Female Hartley strain guinea pigs (10 animals per group) were immunized ID three times at one month intervals with 20μg each of gC2, gD2 and gE2 nucleoside-modified mRNA-LNP; IM with 10μg each of gC2, gD2 and gE2 baculovirus subunit proteins with CpG/alum; IM with 10μg PolyC RNA-LNP as a control; or left unimmunized. One month after the final immunization, animals were infected intravaginally with 5×105 PFU (50 LD50) HSV-2 MS, which is the dose we used in prior studies with the trivalent baculovirus protein vaccine (16). The group that was not immunized were not infected. Animals were observed for survival, weight loss, and genital lesions. Eight of 10 animals in the PolyC RNA-LNP group became ill and were euthanized by day 20 post-infection, while no animal in the trivalent mRNA, trivalent protein, or uninfected group died (Fig. 3a), developed weight loss (Fig. 3b), hind limb weakness or urinary retention. Animals in the PolyC RNA-LNP group had extensive genital lesions, while no genital lesions appeared in the other groups over 60 days (Fig. 3c).
Figure 3. ID immunization with nucleoside-modified trivalent mRNA-LNP protects against genital HSV-2 in guinea pigs.
(a-f) Guinea pigs were immunized three times monthly IM with 10μg PolyC RNA-LNP; ID with 20μg each trivalent nucleoside-modified mRNA-LNP; IM with 10μg each trivalent subunit protein-CpG/alum; or left unimmunized (n=10/group). One month after the final immunization, all immunized animals were infected intravaginally with 5×105 HSV-2 (50 LD50), while unimmunized animals remained uninfected. (a) Survival curves: P<0.0001 comparing PolyC RNA with each other group. P values calculated by the log-rank test. (b) The mean weight loss each day post-infection of animals in the trivalent mRNA, trivalent subunit protein, or uninfected group was compared with PolyC RNA controls; P<0.0001. (c) Cumulative mean genital disease score per guinea pig is shown for each day post-infection. No animal developed genital lesions in the trivalent mRNA, trivalent subunit protein or uninfected group. Comparing trivalent mRNA, trivalent subunit protein or uninfected with PolyC RNA, P<0.0001. The P value in (b) and (c) was calculated by two-way ANOVA followed by Tukey for significance. (d-e) P values shown on the figures are for the primary endpoint comparison between trivalent mRNA and trivalent protein. Other P values are noted below. (d) Day 2 and day 4 vaginal titers: Day 2, P=0.0013 and day 4 P=0.0010 comparing PolyC RNA with trivalent mRNA. Dotted line indicates the limit of assay detection at 3.3 PFU/ml. (e) Day 2 and day 4 vaginal DNA copy number by qPCR. P value in (d) and (e) was calculated by the two-tailed Mann-Whitney, except the day 4 comparison between trivalent mRNA and trivalent subunit proteins was performed by the two-tailed Fisher’s exact test. (f) Vaginal shedding of HSV-2 DNA over 21 days from day 28–50 post-infection. One animal in the trivalent subunit protein group had replication competent virus recovered on a day HSV-2 DNA shedding was detected (marked in green). Comparing days of shedding in the trivalent mRNA and trivalent subunit protein group, P=0.0052, calculated by the two-tailed Fisher’s exact test. (g) HSV-2 DNA copy number/106 copies of GAPDH in spinal cord and DRG at the end of the experiment; n=2 in PolyC RNA and n=10 in the 3 other groups. (h) Table summarizing key results of guinea pig studies.
We performed vaginal cultures on days 2 and 4 post-infection. Nine of 10 guinea pigs in the PolyC RNA-LNP group had positive titers on day 2. The negative day 2 titer in one animal indicates that animal did not get infected, which is consistent with the observation that one animal in the PolyC RNA group did not lose weight or develop genital lesions. In the trivalent mRNA and trivalent subunit protein groups, 5/10 animals had positive vaginal titers on day 2. The positive vaginal cultures represent the mean of two determinations in a total of 300μl of vaginal fluid. Two animals in the trivalent mRNA group and one animal in the trivalent subunit protein group had 1 virus plaque detected (Fig. 3d). Vaginal titers remained positive on day 4 in 9/10 animals in the PolyC group and 2/10 in the trivalent subunit protein group. In contrast, all 10 animals in the trivalent mRNA group had negative vaginal cultures on day 4 (Fig. 3d). Therefore, no animal in the trivalent mRNA group had a positive vaginal virus titer beyond day 2. We evaluated day 2 and day 4 HSV-2 DNA copy number by qPCR. No significant differences were detected comparing trivalent mRNA with trivalent subunit protein animals although DNA copy numbers were generally lower in the trivalent mRNA group (Fig. 3e). Some guinea pigs had high copy numbers of HSV-2 DNA despite negative virus cultures, suggesting that the virus was neutralized and/or degraded, as in the murine studies.
The inoculation titer used for the guinea pig experiments was 5×105 PFU, which is 10-fold to100-fold higher than the inoculum used in mice. The low positive day 2 titers in the trivalent mRNA and trivalent protein groups may represent residual input virus and/or local replication of virus in genital tissues. An important goal of the vaccine is to prevent the virus from reaching sites where virus establishes latency in ganglia that serve as a source for reactivation infection. We performed daily vaginal swabs for 21 days starting on day 28 post-infection to detect vaginal shedding of HSV-2 DNA as a marker of reactivation infection. Two animals in the PolyC RNA group survived until day 21, but only one was infected based on day 2 titers. We did not detect HSV-2 DNA in vaginal swabs from either the infected or uninfected animal. No animal in the uninfected group shed HSV-2 DNA. In the trivalent subunit protein group, 5/10 animals shed HSV-2 DNA on 19/210 (9.0%) days. In contrast, 2/10 guinea pigs shed HSV-2 DNA in the trivalent nucleoside-modified mRNA-LNP group on a total of 5/210 (2.4%) days (P=0.0052) (Fig. 3f). Virus cultures were performed to detect replication competent virus on days that animals had vaginal shedding of HSV-2 DNA. We performed virus cultures only on samples that were positive for HSV-2 DNA because we consistently fail to recover replication competent virus on days animals are not shedding HSV-2 DNA (32). In the trivalent mRNA group, 0/5 HSV-2 DNA positive samples yielded replication competent virus, compared with 1/19 in the trivalent protein group. Spinal cord and DRG were evaluated for HSV-2 DNA at the end of the experiment. One of two animals in the PolyC RNA group was positive for HSV-2 DNA in DRG and spinal cord (the animal with disease), while 1/10 in the trivalent mRNA group was positive for HSV-2 DNA in spinal cord, but not DRG. The positive animal was the guinea pig that had vaginal shedding of HSV-2 DNA on 4 days from days 47 to 50. In the trivalent subunit protein group, 0/10 animals was positive for HSV-2 DNA in spinal cord or DRG, suggesting that detecting HSV-2 DNA in vaginal secretions is a more sensitive assay for subclinical infection than assays performed at the end of the experiment to detect HSV-2 DNA in DRG or spinal cord (Fig. 3g). Key guinea pig results are summarized in Fig. 3h.
Immunology Assays in Mice
In both mice and guinea pigs, vaccine efficacy was better with the trivalent nucleoside-modified mRNA-LNP formulation than with the trivalent subunit protein vaccine. We performed immunologic analyses in mice to evaluate the mechanisms of the enhanced protection.
Serum IgG antibody responses in mice
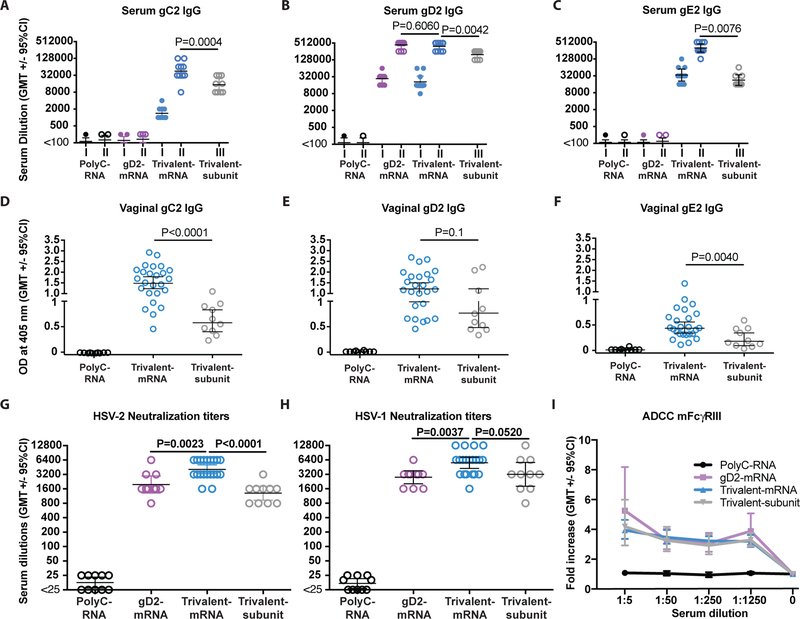
ELISA endpoint IgG titers were evaluated prior to infection on sera collected 28 days after the first and second (final) mRNA ID immunization or 28 days after the third (final) trivalent subunit protein IM immunization. ELISA titers increased comparing the first and second immunization for each nucleoside-modified mRNA (Fig. 4a–c). The nucleoside-modified mRNA produced significantly higher ELISA endpoint titers to each immunogen after the second immunization compared to the baculovirus-expressed subunit proteins after the third immunization (Fig. 4a–c). After the final immunization, the ratio of IgG2a to IgG1 in mice immunized with trivalent mRNA was 0.89, while the ratio in animals immunized with trivalent subunit proteins was 1.04, suggesting a balanced TH1/TH2 response for both (Fig. S3) (33).
Figure 4. ELISA, neutralizing and ADCC antibody responses.
Mice were immunized ID with PolyC RNA-LNP, gD2 mRNA-LNP, 10μg trivalent mRNA-LNP, or IM with trivalent subunit protein-CpG/alum. Sera were obtained 4 weeks after the first and second immunizations in the mRNA-LNP groups and 4 weeks after the third immunization in the subunit protein group. P values are shown on the figures for the primary endpoint comparison between trivalent mRNA and trivalent protein or secondary endpoint comparison between trivalent mRNA and gD2 mRNA. Other P values are noted below. (a) Serum gC2 IgG: P=0.0058 comparing first and second trivalent mRNA immunization. (b) Serum gD2 IgG: P=0.6060 comparing final immunizations of trivalent mRNA with gD2 mRNA; P=0.0085 comparing first and second gD2 mRNA immunization; P=0.0056 comparing first and second trivalent mRNA immunization. (c) Serum gE2 IgG: P=0.0058 comparing first and second trivalent mRNA immunization. n=9 animals per group. (a-c) P values comparing first with second immunization were calculated by the two-tailed Wilcoxon signed-rank test. P values comparing final immunizations were performed by the two-tailed Mann-Whitney test. (d-f) Vaginal gC2 IgG, gD2 IgG or gE2 IgG ELISA titers: n=10 for PolyC and gD2 mRNA, n=25 for trivalent mRNA, n=10 for trivalent subunit protein. (g) HSV-2 strain MS neutralizing titers in the presence of 10% HSV-1/HSV-2 seronegative human serum as a source of complement. (h) HSV-1 strain NS neutralizing antibody titers in the presence of 10% seronegative human serum as a source of complement. n=10 in PolyC, n=10 in gD2, n=20 in trivalent mRNA and n=10 in trivalent subunit groups in (g-h). (d-h) P values comparing trivalent mRNA with trivalent subunit protein or with gD2 mRNA were calculated by the two-tailed Mann-Whitney test. (i) ADCC using sera from PolyC RNA, gD2 mRNA, trivalent mRNA and trivalent subunit mice. Results shown represent 5–6 separate sera tested per group. P<0.001 comparing each curve with PolyC RNA as calculated by two-way ANOVA with Tukey for significance.
Vaginal wash IgG antibody responses in mice
Vaginal mucosa fluids were obtained prior to infection at four weeks after the final immunization. IgG vaginal mucosa antibody responses to gC2 and gE2 were significantly higher in mice immunized with trivalent mRNA-LNP than trivalent subunit proteins (Figs. 4d, f). Titers were also higher to gD2 in the trivalent mRNA mice, although differences were not significant (Fig. 4e).
Serum neutralizing antibodies in mice
HSV-2 neutralizing antibody titers in the presence of complement were evaluated after the second (final) mRNA immunization or third (final) subunit protein immunization. The neutralizing antibody titers in the trivalent mRNA group were higher than in the trivalent subunit protein group (Fig. 4g). Neutralizing titers were also higher in the trivalent mRNA group than in the gD2 mRNA group, indicating that gC2 and/or gE2 antibodies enhance neutralization (Fig. 4g) (16, 21, 22). HSV-1 is a frequent cause of genital herpes (9); therefore, we evaluated the same sera for neutralizing antibody titers against HSV-1. Each of the vaccine formulations produced high titers of neutralizing antibodies against HSV-1 (Fig. 4h). The trivalent mRNA vaccine produced higher HSV-1 neutralizing antibody titers than trivalent subunit proteins or gD2 mRNA, although the differences did not reach statistical significance (Fig. 4h).
Antibody-dependent cellular cytotoxicity responses in mice
The control PolyC RNA sera failed to stimulate ADCC activity mediated by murine FcγRIII, while sera from the trivalent mRNA, the trivalent subunit protein and the gD2 mRNA mice produced significantly greater ADCC responses (Fig. 4i). ADCC responses did not differ comparing trivalent mRNA and trivalent subunit protein, suggesting that ADCC does not explain the superior performance of the trivalent mRNA vaccine (Fig. 4i).
Antibodies that block immune evasion domains on gC2 and gE2 in mice
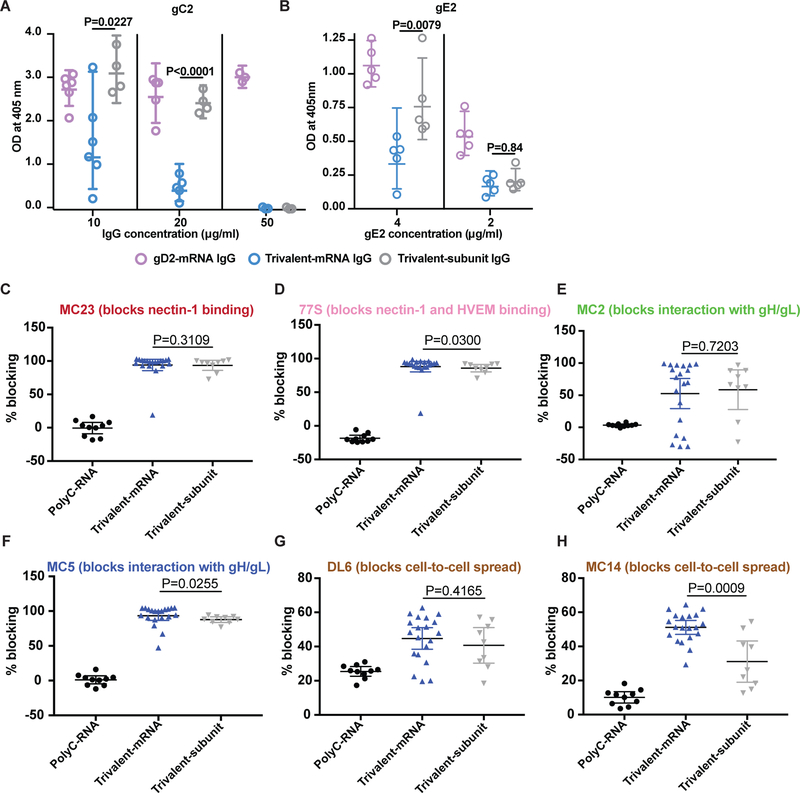
We reported that immunization with trivalent subunit proteins produced higher titers of antibodies that blocked gC2 binding to C3b than produced by HSV-2 infection (34). Here we assessed whether trivalent mRNA immunization produces higher titers of blocking antibodies than trivalent subunit proteins. We compared multiple concentrations of IgG purified from sera obtained four weeks after the final immunization with trivalent mRNA-LNP or trivalent subunit proteins. The IgG was incubated with 40ng of gC2 to determine whether the antibodies block gC2 binding to C3b on an ELISA plate (16, 21). IgG from gD2 mRNA-immunized mice was included as a negative control. At 10μg/ml or 20μg/ml IgG, the trivalent mRNA-LNP significantly outperformed trivalent subunit proteins in blocking gC2 binding to C3b (Fig. 5a). At 50μg/ml IgG, both trivalent mRNA and trivalent subunit protein totally blocked gC2 binding to C3b (Fig. 5a). The results indicate that both trivalent mRNA and trivalent subunit proteins produce antibodies that block gC2 binding to C3b, but that the trivalent mRNA produces higher titers of blocking antibodies.
Figure 5. Antibodies that block gC2 and gE2 immune evasion domains and that bind gD2 epitopes that mediate crucial functions.
P values are shown on the figures for the primary endpoint comparison between trivalent mRNA and trivalent protein. Other P values are noted below. (a) Antibodies produced by trivalent mRNA and trivalent subunit protein block C3b binding to gC2. Error bars represent geometric mean and 95% confidence intervals. Comparing trivalent mRNA with gD2 mRNA, P=0.0220 at 10μg/ml; P<0.0001 at 20 and 50μg/ml. Comparing trivalent subunit protein with gD2 mRNA, P<0.0001 at 50μg/ml; P not significant at other IgG concentrations. (b) Blocking gE2 binding to IgG Fc. Comparing trivalent mRNA with gD2 mRNA at 4μg/ml, P=0.0079. Comparing trivalent subunit protein with gD2 mRNA at 4μg/ml, P=0.1508; at 2μg/ml, P=0.0079. Results shown in (a-b) are geometric means with 95% CI. IgG was purified from pooled sera of 10 animals/group in (a-b). Each entry in (a-b) represents results from a single assay. P values in (a-b) were determined by the two-tailed Mann-Whitney test. (c-h) Sera were evaluated for blocking gD2 binding to prototype MAbs that recognize crucial gD2 epitopes. n= 10 for PolyC RNA, n=20 for trivalent mRNA, n=9 for trivalent subunit protein. Results in (c-g) represent means with 95% CI. P values comparing trivalent mRNA with trivalent subunit protein were calculated using the two-tailed Mann-Whitney test.
HSV-2 gE2 functions as an IgG Fc receptor (22, 35). We evaluated whether gE2 antibody produced by immunization with trivalent mRNA is more effective than trivalent subunit proteins at blocking gE2 protein binding to non-immune IgG Fc (16, 22). As a control, we assessed whether gE2 antibodies are more effective that gD2 antibodies at blocking gE2 binding to non-immune IgG. This control takes into consideration that gE2 antibodies can bind to gE2 protein by their F(ab’)2 domain and/or Fc domain, while antibodies to gD2 protein can only bind by their Fc domain. We used 4 or 2μg/ml of gE2 protein that was incubated with 62.5μg/ml of IgG from immunized mice. Comparing trivalent mRNA with trivalent subunit proteins at a gE2 protein concentration of 4μg/ml, IgG from the trivalent mRNA mice blocked gE2 binding better than trivalent subunit proteins, suggesting that the trivalent mRNA produced higher titers of gE2 IgG Fc-blocking antibodies (Fig. 5b). These differences were not apparent at a lower gE2 concentration of 2μg/ml, although both vaccine formulations blocked gE2 protein binding to IgG Fc significantly better than gD2 antibody (Fig. 5b). These results indicate that immunization with both trivalent mRNA and trivalent subunit proteins produce antibodies that block gE2 binding to IgG Fc, but higher titers of blocking antibodies are produced by trivalent mRNA immunization.
Antibodies to gD2 epitopes involved in virus entry and cell-to-cell spread in mice
We recently evaluated epitope-specific antibody responses to 3 linear and 4 conformational gD2 epitopes that are crucial for gD2 function (23). The gD2 epitopes are involved in virus binding to herpesvirus entry mediator (HVEM) and/or nectin-1 receptors, gD2 interaction with HSV-2 downstream entry molecules glycoproteins H and L (gH2/gL2), or cell-to-cell spread (36–38). We reported that guinea pigs immunized with gD2-CpG/alum produced a range of responses to crucial epitopes. Some animals produced antibodies to few epitopes, while others produced antibodies to most or all epitopes. The more crucial epitopes blocked, the better the protection was against intravaginal HSV-2 challenge (23).
Here we evaluated antibody responses in mice to six gD2 epitopes that are involved in receptor binding, interaction with gH2/gL2 or cell-to-cell spread. The assay places monoclonal antibodies (MAbs) that recognize each of the gD2 crucial epitopes on a biosensor chip. Individual sera obtained after the second immunization with trivalent mRNA or third immunization with trivalent subunit proteins were incubated with gD2 protein and floated over the MAb-coated chip. Sera from mice immunized with PolyC RNA served as a control. Blocking gD2 protein binding to MAb on the biosensor chip indicates that antibody is present in the mouse serum to the epitope recognized by the MAb. The percent blocking of gD2 binding to prototype MAbs that recognize the six crucial epitopes is shown in Figs.5c–h. The trivalent mRNA immunized mice produced antibodies that blocked gD2 binding significantly better than trivalent subunit proteins to 3/6 crucial epitopes. Although significant, the differences were fairly small for two of these three epitopes, 77S and MC5, but more impressive for the MC14 epitope (blocks cell-to-cell spread) (Figs.5d, f, h). These results indicate that most mice immunized with either vaccine formulation produced antibodies to the gD2 epitopes, although trivalent mRNA outperformed trivalent subunit proteins by producing higher titers of blocking antibodies to 3/6 epitopes.
CD4+ and CD8+ T cell responses in mice
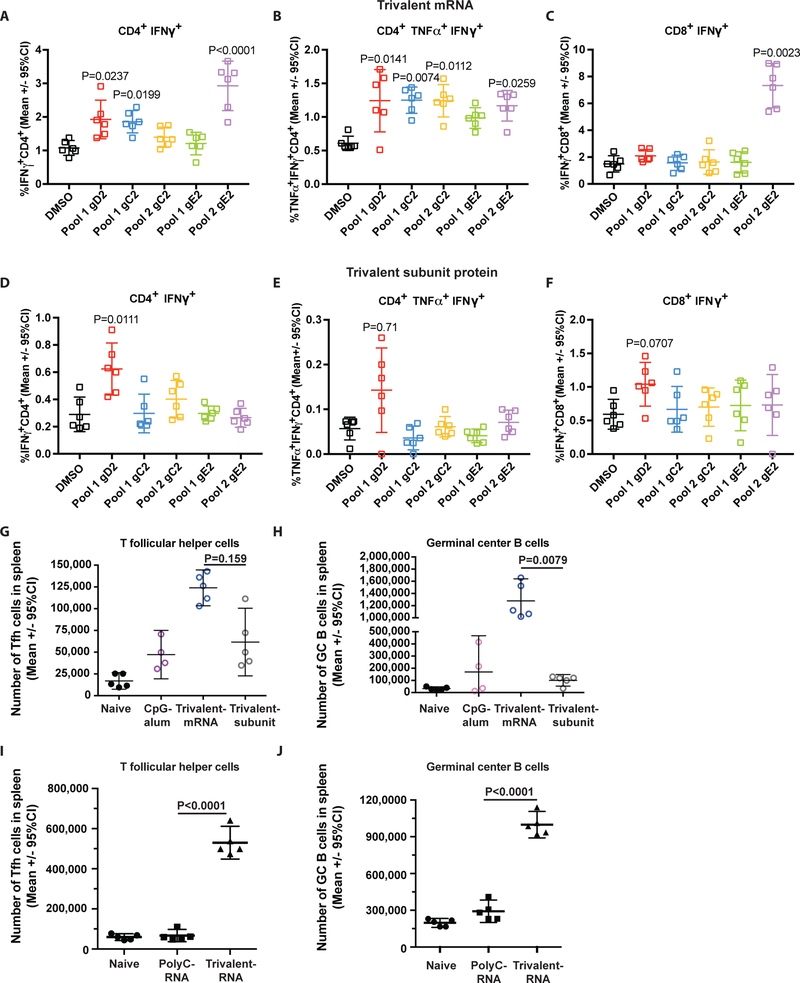
Splenocytes were harvested two weeks after the second trivalent mRNA or third trivalent subunit protein immunization. CD4+ and CD8+ T cells were stimulated in vitro with a single gD2 overlapping peptide pool, and gC2 or gE2 overlapping peptides each divided into two pools. The peptide pools span the entire amino acid sequences of the subunit proteins. As a control, T cells were stimulated with DMSO, the vehicle used to dissolve the overlapping peptides. CD4+ T cells in mice immunized with trivalent mRNA significantly increased production of IFNγ alone or both IFNγ and TNFα when stimulated with gD2, gC2 or gE2 peptide pools, compared with DMSO alone (Figs.6a, b). Trivalent subunit protein immunization was less effective at stimulating CD4+ T cell cytokine production (Figs.6d, e). The trivalent mRNA and subunit protein groups did not produce robust CD8+ T cell responses. In the mRNA mice, only gE2 stimulated a significant increase in CD8+ IFNγ production, while in the protein subunit group, gD2 pool 1 stimulated an increase in CD8+ IFNγ production that approached statistical significance (Fig. 6c, f). We conclude that mice immunized with trivalent mRNA stimulated a more robust CD4+ T cell response than mice immunized with trivalent subunit proteins, and a marginally better CD8+ T cell response.
Figure 6. CD4+, CD8+, Tfh+ T cell responses and germinal center B cell responses.
Splenocytes for CD4+ and CD8+ T cell assays were pooled from 5 animals and the responses of 6 replicate wells are shown. (a) CD4+ T cell IFNγ responses in mice immunized with trivalent mRNA or (b) CD4+ polyfunctional IFNγ and TNFα responses in mice immunized with trivalent mRNA. (c) CD8+ T cell IFNγ responses in mice immunized with trivalent mRNA. (d) CD4+ T cell IFNγ responses in mice immunized with trivalent subunit protein or (e) CD4+ polyfunctional IFNγ and TNFα responses in mice immunized with trivalent subunit protein. (f) CD8+ T cell IFNγ responses in mice immunized with trivalent subunit protein. (a-f) Each peptide-stimulated CD4+ or CD8+ T cell response was compared with DMSO vehicle-treated controls. The CD4+ and CD8+ T cell assays for trivalent mRNA and trivalent subunit protein were run on separate days, which accounts for the variability in the DMSO control results. The percent of cytokine positive cells is based on the total number of CD4+ T cells counted in the trivalent mRNA and subunit protein assays. The mean number of CD4+ T cells counted in the trivalent mRNA experiments was 4680 ± 714, and in the trivalent subunit protein was 7850 ± 960. The mean number of CD8+ T cells counted in the trivalent mRNA experiments was 981 ± 232, and in the trivalent subunit protein 1118 ± 206. All values with P≤0.05 are shown in the figures. P values were calculated by the Kruskal-Wallis test with Dunn’s for multiple comparisons. (g-h) Tfh and GC B cell responses that include CpG/alum as a control. Splenocytes were harvested for Tfh and GC B cell assays from individual mice 10 days after a single immunization with CpG/alum alone, trivalent mRNA-LNP, trivalent subunit protein-CpG/alum, or no immunization. (g-h) P values are shown on the figures for the primary endpoint comparison between trivalent mRNA and trivalent protein. Other P values are noted below. (g) Tfh cells: P=0.532 comparing CpG/alum with trivalent subunit protein-CpG/alum. (h) Germinal center B cells: P=0.90 comparing CpG/alum with trivalent subunit protein. (g-h): n=5 animals per group, except n=4 for CpG/alum. P values were calculated using the two-tailed Mann-Whitney test. Error bars represent geometric means and 95% confidence intervals. (i-j) Tfh and GC B cell responses that include PolyC RNA-LNP as a control. Splenocytes were harvested for Tfh and GC B cell assays from individual mice 10 days after the second immunization with PolyC RNA-LNP, trivalent mRNA-LNP, or no immunization. P values are shown on the figures and were calculated by the two-tailed Mann-Whitney test. (i-j): n=5 animals per group.
T follicular helper (Tfh) and germinal center (GC) B cell responses in mice
Tfh cells are important for forming and maintaining GCs, stimulating class switching and B cell affinity maturation, and developing memory B cells and long-lived plasma cells (39, 40). Nucleoside-modified mRNA-LNP vaccines are potent inducers of CD4+ Tfh and GC B cell responses (30). We evaluated the number of splenic CD4+ Tfh cells and GC B cells 10 days after a single immunization with trivalent mRNA-LNP or trivalent subunit proteins-CpG/alum. The flow cytometer gating strategy for Tfh and GC B cells is shown in Figs. S4a,b. Tfh cell responses were significantly higher in animals immunized with trivalent mRNA than trivalent subunit proteins (Fig. 6g). The Tfh response in the trivalent subunit protein group was not significantly higher than in the CpG/alum controls. Germinal center B cell responses were also significantly higher in the trivalent mRNA than subunit protein mice, while differences between CpG/alum and trivalent subunit proteins were not significant (Fig. 6h). We performed an additional experiment that included PolyC RNA-LNP as a control to assess the effects of LNP. Mice were immunized with PolyC RNA-LNP and responses compared with naïve mice or mice immunized with trivalent mRNA-LNP. Splenocytes were harvested 10 days after the second immunization. Trivalent mRNA-LNP produced significantly higher Tfh and GC B cell responses than PolyC RNA-LNP mice (Fig. 6i, j). These results indicate that trivalent mRNA-LNP induces a more potent Tfh and GC B cell response than trivalent subunit proteins with CpG/alum. Overall, where differences in immune responses were detected comparing trivalent mRNA with trivalent subunit protein, responses favored the trivalent mRNA vaccine.
Discussion
The virus titer that results in genital herpes infection in humans is unknown making it difficult to choose the optimal challenge dose in animals. We selected a high challenge dose by using 275 or 2,750 LD50 of HSV-2 in mice and 50 LD50 in guinea pigs. Despite these high doses, no mouse immunized ID or IM with 10μg trivalent nucleoside-modified mRNA-LNP and no guinea pig immunized with 20μg trivalent nucleoside-modified mRNA-LNP developed genital disease, although it is possible that at even higher challenge doses some breakthrough infection may occur. Mice and guinea pigs were also well-protected against subclinical infection. Commonly used indicators of subclinical infection in mice include vaginal swab cultures on days 2 and 4 and HSV-2 DNA in DRG, while a useful marker for subclinical infection in guinea pigs is vaginal shedding of HSV-2 DNA after resolution of the acute infection (41–43). By these criteria, only 1/64 mice and 2/10 guinea pigs developed subclinical infection. Overall, guinea pigs shed HSV-2 DNA on 2.4% of days and no replication competent virus was recovered in the vaginal secretions, suggesting that the risk of transmission is very low in these animals. Our decision to immunize with three antigens was based on our prior studies that reported adding gC2 to gD2 subunit protein improved protection in mice and guinea pigs, and although performed in different studies, we noted that adding gE2 to gC2/gD2 subunit proteins further improved protection in mice (16, 21, 22). These results are consistent with our current study demonstrating that trivalent mRNA-LNP provides better protection than gD2 mRNA-LNP alone.
Trivalent nucleoside-modified mRNA-LNP immunization outperformed the same immunogens administered as subunit proteins with CpG/alum in mice and guinea pigs. Our choice of 10μg of each nucleoside-modified mRNA in mice was partially guided by dose-response studies using nucleoside-modified mRNA to express firefly luciferase and vaccine studies of Zika, influenza and HIV antigens in mice (29, 30, 44). The choice of 10μg seems appropriate based on the nearly complete protection provided by trivalent mRNA at this concentration and lower efficacy at 2μg. Immunization with 20μg of each trivalent nucleoside-modified mRNA in guinea pigs was based on the larger body mass of guinea pigs than mice. Our choice of 5μg for subunit proteins in mice and 10μg in guinea pigs was based on our prior results demonstrating excellent protection in mice and guinea pigs against genital lesions at these concentrations (16, 22). A limitation of our study is that we did not evaluate whether higher concentrations of trivalent modified mRNA-LNP or trivalent subunit proteins will further improve vaccine efficacy. We also did not assess the durability of immunity and protection. The potent Tfh cell and GC B cell responses produced by the trivalent mRNA immunogens suggest that durable protection is likely. Impressive durability was demonstrated for HIV, influenza and Zika when animals were immunized by the same nucleoside-modified mRNA-LNP platform as used here (29, 30). The high antibody titers and potent Tfh and GC B cell responses noted with other nucleoside modified mRNA-LNP immunogens, including influenza, Zika, and HIV was a key reason we chose to evaluate nucleoside-modified mRNA as a delivery mechanism for our candidate HSV-2 vaccine (25, 30).
We performed multiple immunology assays to assess mechanisms by which the trivalent nucleoside-modified mRNA outperformed the trivalent protein vaccine. Where differences emerged, mRNA was superior including: i) serum and vaginal IgG ELISA titers; ii) HSV-2 neutralizing antibody titers; iii) antibodies that block gC2 and gE2 immune evasion domains; iv) antibody responses to gD2 epitopes involved in binding to receptors, interaction with gH2/gL2 and cell-to-cell spread; v) CD4+ T cell responses; and vi) Tfh cell and GC B cell responses. It seems likely that a combination of these immune responses accounts for the enhanced performance of the trivalent mRNA vaccine. The prolonged expression of antigens using nucleoside-modified mRNA-LNP delivery and the Tfh adjuvant activity of LNPs may explain the more potent immune responses (44, 45). Although trivalent nucleoside-modified mRNA outperformed trivalent subunit protein CpG/alum, it is possible that new adjuvants may improve the efficacy of subunit protein vaccines.
Few preclinical HSV-2 vaccine studies have been published that evaluated the same antigens and adjuvants that were subsequently used in human trials. One exception is the gD2 subunit protein vaccine administered with MPL/alum that was evaluated in a guinea pig model of genital herpes. The gD2-MPL/alum vaccine prevented genital lesions in 9/10 guinea pigs in one study and 10/12 in another, but all animals developed subclinical infection as measured by vaginal shedding of HSV-2 DNA (46, 47). In our current study, the trivalent nucleoside modified mRNA-LNP vaccine prevented genital lesions in 100% of guinea pigs and subclinical genital shedding of HSV-2 DNA in 80%, which surpasses the results obtained using an immunogen that entered phase 3 human trials. We conclude that the trivalent nucleoside-modified mRNA-LNP vaccine is a promising candidate for future human trials.
Materials and Methods
Study Design
The primary goal was to assess whether the trivalent nucleoside-modified mRNA-LNP vaccine outperformed the same antigens expressed as baculovirus subunit proteins administered with CpG/alum. A secondary goal was to evaluate whether adding gC2 and gE2 nucleoside-modified mRNA-LNP to gD2 nucleoside-modified mRNA-LNP improved the protection provided by gD2. Mice and guinea pigs were immunized with nucleoside-modified mRNA-LNP immunogens or baculovirus subunit protein-CpG/alum antigens. Serum and vaginal wash fluids were obtained approximately one month after the final immunization and animals were then infected Ivag with HSV-2 strain MS. Mice: Vaginal swabs were obtained for cultures on days 2 and 4 post-infection; animals were weighed daily for 2 weeks and scored for genital disease for 4 weeks; DRG were obtained either on day 4 post-infection or at the end of the experiment. Guinea pigs: Vaginal swabs were obtained for cultures on days 2 and 4 post-infection; animals were scored for genital disease for 60 days; swabs were obtained for vaginal shedding of HSV-2 DNA for 21 days beginning 28 days post-infection; DRG and spinal cord were harvested for HSV-2 DNA at the end of the experiment. Scoring for genital disease in mice was performed without blinding, while scoring in guinea pigs was done blinded.
Ethics statement
All animal studies were conducted under protocol 805187 approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Production of bac-gC2, bac-gD2 and bac-gE2 subunit proteins, production of mRNA, formulation in LNPs, and Western blots
The baculovirus proteins were produced as previously described (22, 48, 49). The methods used to synthesize trivalent nucleoside-modified mRNA-LNP and to perform Western blots are described in the legend to Fig. S1.
ELISA, neutralizing antibody, and ADCC titers
Serum IgG ELISA titers and IgG1/IgG2a subtype titers were evaluated as previously described (16, 50). ELISA vaginal wash fluid titers were assessed by adding 30μl of DMEM with 5% fetal bovine serum to the vaginal cavity and retrieving the fluid several seconds later. This procedure was repeated once. Mucosal IgG antibody in vaginal fluid was measured at a 1:50 dilution. Neutralizing antibody titers were reported as the serum dilution that reduced the number of virus plaques by 50% (21). ADCC was measured using the murine FcγRIII ADCC Reporter Bioassay (Promega Corporation) that binds murine IgG1 and IgG2a (23, 51).
Blocking C3b binding to gC2 and IgG Fc binding to gE2
Serum (10μl) from each mouse in a group was pooled and the IgG purified on a NAb Protein G Spin Column (Pierce Biotechnology). Blocking gC2 binding to C3b was performed by coating ELISA plates with 200ng of purified C3b protein (Complement Technologies), incubating 40ng of purified gC2 protein with varying concentrations of purified IgG, and detecting bound gC2 (34). Blocking gE2 binding to IgG Fc was evaluated using HSV-1/HSV-2 seronegative human IgG (1mg/well) to coat ELISA wells, and incubating 62.5μg/ml of purified IgG from immunized mice with 2 or 4μg/ml bac-gE2(24–405t) for 15 min at room temperature, and detecting bound gE2 (16).
Biosensor-based antibody competition assay
The Carterra Microfluidics continuous flow microspotter surface plasmon resonance imaging (CFM/SPRi) system was used to detect epitope-specific antibody responses to crucial gD2 epitopes involved in virus entry and cell-to-cell spread (23, 24).
CD4+ and CD8+ T cell responses and Tfh and GC B cell responses
Spleens were pooled from 5 animals per group and 106 splenocytes were stimulated at 37°C with gC2, gD2 or gE2 peptide pools consisting of 15 amino acids with 11 overlapping amino acids (JPT Innovative Peptide Solutions). After 1 h, brefeldin A (10μg/ml) (BD Pharmingen) was added for 16 h at 37°C. Splenocytes were stained with aqua blue (Invitrogen) to distinguish live-dead cells, Pacific blue-conjugated anti-CD8 mouse MAb (Biolegend) and R-phycoerythrin–cyanine 5.5 (PE-Cy5.5) anti-CD4 mouse MAb (BD Pharmingen). Cells were permeabilized with Cytofix/Cytoperm (BD Pharmingen), stained with Alexa Fluor 700-conjugated anti-IFNγ mouse MAb, PE-Cy7 anti-TNFα mouse MAb, and allophycocyanin-Cy7 anti-CD3 mouse MAb. Splenocytes were fixed with 1% paraformaldehyde and analyzed by FACS using an 18-color LSR II flow cytometer and FlowJo flow cytometry analytic software (21, 52, 53). Details of the staining and gating strategy for Tfh and GC B cells are presented in the legend to Fig. S4.
Immunizations, vaginal infections and scoring
BALB/c mice (Charles River): The gC2, gD2, and gE2 baculovirus subunit proteins were individually incubated at RT for two h with 16.7μg of CpG oligonucleotide 5’-TCCATGACGTTCCTGACGTT-3’ (Coley Pharmaceutical) and 25μg of alum/μg protein (Alhydragel; Accurate Chemical and Scientific Corp.) and combined prior to immunization in a volume of 50μl (22). Mock IM immunizations were performed with CpG and alum without proteins. The trivalent (gC2, gD2, and gE2) mRNAs were combined prior to LNP encapsulation. ID immunizations with trivalent mRNA-LNP, polyC RNA-LNP or gD2 mRNA-LNP were administered in four sites on the flank using a 29-gauge insulin syringe containing 30μl for each site, while IM immunizations with LNP-containing immunogens were given in 50μl into the gastrocnemius muscle. Mice were infected Ivag one month after the final immunization and scored for genital disease (22).
Hartley strain guinea pigs (Charles River): 10μg of gC2, gD2, gE2 subunit proteins were mixed individually and then combined to form a final concentration of 100μg CpG (5’-TCGTCGTTGTCGTTTTGTCGTT-3’) (Trilink Inc.) per guinea pig and 150μg of alum (16). ID immunizations and Ivag infections one month after the final immunization were performed as in mice, except 40μl was inoculated ID at each site (16).
Virus cultures from vaginal swabs
Vaginal swabs were placed in one ml Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum supplemented with 25μg/ml vancomycin. For viral titers, 150μl was evaluated in mice and 300μl in guinea pigs. Serial 10-fold dilutions were added to Vero cells to assess virus titers by plaque assay (21).
HSV-2 DNA isolation, real-time qPCR for HSV-2 DNA copy number in DRG, spinal cord and in vaginal swab samples
Mouse DRG samples were analyzed in duplicate for HSV-2 DNA as previously described, except separate reactions were used to amplify HSV-2 DNA and the mouse adipsin gene and DRG HSV-2 DNA copy number was expressed as log10 DNA copies per 105 adipsin genes instead of 104 (22). HSV-2 DNA copy number in guinea pig DRG and spinal cord samples were calculated as log10 DNA copies per 106 GAPDH genes (54). HSV-2 DNA in day 2 and 4 vaginal swab samples in mice and guinea pigs and in vaginal samples obtained on 21 days between days 28–50 in guinea pigs were run as single samples and expressed as HSV-2 DNA copies per ml (16). Samples with <1 copy by 40 cycles were considered negative.
Statistical analysis
P values are displayed in the figures for primary endpoints (trivalent mRNA-LNP compared with trivalent subunit protein-CpG/alum) and secondary endpoints (trivalent mRNA-LNP compared with gD2 mRNA-LNP). We used the Mann-Whitney test for these two-group comparisons. Some P values are also noted in the figure legends. For some comparisons, the mice in one treatment group all had the same response (e.g. zero as the lower limit of quantification) and the two-tailed Fisher’s exact test was used to compare the values. For comparisons looking across several time points, we used two-way ANOVA followed by Tukey adjustment for significance. We used the log-rank test to compare survival distributions between groups. We used Wilcoxon signed-rank test to compare samples from the same animal at different time points. All significance tests were two-sided and performed at P<0.05. Analyses were done using GraphPad Prism version 7.0 (GraphPad Software, Inc.).
Supplementary Material
Acknowledgements
Funding: H.M.F. and S.A. were supported by NIH NIAID grants RO1 AI104854 and AI139618. G.H.C. was supported by NIH NIAID grant RO1 AI18289. D.W. was supported by NIH NIAID grants R01 AI050484, R01 AI124429 and R01 AI084860, a Gates Foundation CAVD 0PP1033102 and the New Frontier Sciences division of Takeda Pharmaceuticals. Pamela Shaw from the Department of Biostatistics, Epidemiology and Informatics at the Perelman School of Medicine provided biostatistical support through core services from the Penn Center for AIDS Research (CFAR) NIH grant P30 AI045008. We thank Barbara L. Mui and Ying K. Tam from Acuitas Therapeutics, Vancouver, BC, Canada for their role in formulating the nucleoside-modified mRNA into LNPs.
Footnotes
Competing Interests: In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that Harvey Friedman, Sita Awasthi, and Gary Cohen are named on patents that describe the use of multiple subunit glycoprotein antigens for HSV vaccines. Harvey Friedman, Sita Awasthi and Drew Weissman have submitted a patent claim that uses nucleoside-modified mRNA as a vaccine for HSV. Drew Weissman is named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins. Drew Weissman and Norbert Pardi are also named on a patent describing the use of nucleoside-modified mRNA in lipid nanoparticles as a vaccine platform. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from licensing of our patents.
Data and materials availability
All data are available within the article and will be made available upon request.
Access to subunit antigens must be obtained through a MTA.
References
- 1.Looker KJ, Garnett GP, Schmid GP, An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 86, 805–812, A (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, Watts DH, Berry S, Herd M, Corey L, The acquisition of herpes simplex virus during pregnancy. N Engl J Med 337, 509–515 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Newman LM, Gottlieb SL, First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health 5, e300–e309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, Arvin AM, Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Weller S, Soong SJ, Kiell J, Lakeman FD, Whitley RJ, Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 108, 230–238 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Poole CL, Kimberlin DW, Antiviral Approaches for the Treatment of Herpes Simplex Virus Infections in Newborn Infants. Annu Rev Virol 5, 407–425 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Wald A, Link K, Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 185, 45–52 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr., Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE, Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama 282, 331–340 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G, GlaxoSmithKline G Herpes Vaccine Efficacy Study, Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347, 1652–1661 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366, 34–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD, Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 209, 828–836 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prober CG, Sullender WM, Yasukawa LL, Au DS, Yeager AS, Arvin AM, Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. The New England journal of medicine 316, 240–244 (1987). [DOI] [PubMed] [Google Scholar]
- 12.Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM, Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis 160, 770–776 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Yeager AS, Arvin AM, Urbani LJ, Kemp JA 3rd, Relationship of antibody to outcome in neonatal herpes simplex virus infections. Infect Immun 29, 532–538 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagashunmugam T, Lubinski J, Wang L, Goldstein LT, Weeks BS, Sundaresan P, Kang EH, Dubin G, Friedman HM, In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J Virol 72, 5351–5359 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris JD, Friedman HM, In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J Exp Med 190, 1637–1646 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awasthi S, Hook LM, Shaw CE, Pahar B, Stagray JA, Liu D, Veazey RS, Friedman HM, An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog 13, e1006141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB, Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309, 633–635 (1984). [DOI] [PubMed] [Google Scholar]
- 18.Frank I, Friedman HM, A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol 63, 4479–4488 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubin G, Socolof E, Frank I, Friedman HM, Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol 65, 7046–7050 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostavasili I, Sahu A, Friedman HM, Eisenberg RJ, Cohen GH, Lambris JD, Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J Immunol 158, 1763–1771 (1997). [PubMed] [Google Scholar]
- 21.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM, Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J Virol 85, 10472–10486 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awasthi S, Huang J, Shaw C, Friedman HM, Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol 88, 8421–8432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hook LM, Cairns TM, Awasthi S, Brooks BD, Ditto NT, Eisenberg RJ, Cohen GH, Friedman HM, Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in guinea pigs. PLoS Pathog 14, e1007095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cairns TM, Ditto NT, Lou H, Brooks BD, Atanasiu D, Eisenberg RJ, Cohen GH, Global sensing of the antigenic structure of herpes simplex virus gD using high-throughput array-based SPR imaging. PLoS Pathog 13, e1006430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardi N, Hogan MJ, Porter FW, Weissman D, mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kariko K, Buckstein M, Ni H, Weissman D, Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Midoux P, Pichon C, Lipid-based mRNA vaccine delivery systems. Expert Rev Vaccines 14, 221–234 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Kauffman KJ, Webber MJ, Anderson DG, Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release 240, 227–234 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu JS, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang YJ, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Kariko K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D, Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, Moody MA, Verkerke HP, Myles A, Willis E, LaBranche CC, Montefiori DC, Lobby JL, Saunders KO, Liao HX, Korber BT, Sutherland LL, Scearce RM, Hraber PT, Tombacz I, Muramatsu H, Ni H, Balikov DA, Li C, Mui BL, Tam YK, Krammer F, Kariko K, Polacino P, Eisenlohr LC, Madden TD, Hope MJ, Lewis MG, Lee KK, Hu SL, Hensley SE, Cancro MP, Haynes BF, Weissman D, Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, Tam YK, Kariko K, Barbosa CJ, Madden TD, Hope MJ, Krammer F, Hensley SE, Weissman D, Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nature communications 9, 3361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awasthi S, Hook LM, Shaw CE, Friedman HM, A trivalent subunit antigen glycoprotein vaccine as immunotherapy for genital herpes in the guinea pig genital infection model. Hum Vaccin Immunother, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosmann TR, Coffman RL, TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology 7, 145–173 (1989). [DOI] [PubMed] [Google Scholar]
- 34.Hook LM, Awasthi S, Dubin J, Flechtner J, Long D, Friedman HM, A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine 37, 664–669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Para MF, Goldstein L, Spear PG, Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. Journal of Virology 41, 137–144 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH, Herpes virus fusion and entry: a story with many characters. Viruses 4, 800–832 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH, Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol 72, 3595–3601 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns TM, Ditto NT, Atanasiu D, Lou H, Brooks BD, Saw WT, Eisenberg RJ, Cohen GH, Surface plasmon resonance (SPR) reveals direct binding of HSV glycoproteins gH/gL to gD and locates a gH/gL binding site on gD. J Virol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victora GD, Nussenzweig MC, Germinal centers. Annual review of immunology 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Crotty S, T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delagrave S, Hernandez H, Zhou C, Hamberger JF, Mundle ST, Catalan J, Baloglu S, Anderson SF, DiNapoli JM, Londono-Hayes P, Parrington M, Almond J, Kleanthous H, Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS One 7, e46714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halford WP, Geltz J, Messer RJ, Hasenkrug KJ, Antibodies Are Required for Complete Vaccine-Induced Protection against Herpes Simplex Virus 2. PLoS One 10, e0145228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernstein DI, Pullum DA, Cardin RD, Bravo FJ, Dixon DA, Kousoulas KG, The HSV-1 live attenuated VC2 vaccine provides protection against HSV-2 genital infection in the guinea pig model of genital herpes. Vaccine 37, 61–68 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D, Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release 217, 345–351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, Nakao C, Pauthner MG, Reiss S, Cottrell CA, Smith ML, Bastidas R, Gibson W, Wolabaugh AN, Melo MB, Cossette B, Kumar V, Patel NB, Tokatlian T, Menis S, Kulp DW, Burton DR, Murrell B, Schief WR, Bosinger SE, Ward AB, Watson CT, Silvestri G, Irvine DJ, Crotty S, Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 177, 1153–1171 e1128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR, Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. The Journal of infectious diseases 187, 542–549 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB, Impact of Immunization with Glycoprotein D2/AS04 on Herpes Simplex Virus Type 2 Shedding into the Genital Tract in Guinea Pigs That Become Infected. J Infect Dis 192, 2117–2123 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ, Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol 69, 4471–4483 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM, Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J Virol 80, 5283–5291 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awasthi S, Hook LM, Swaminathan G, Cairns TM, Brooks B, Smith JS, Ditto NT, Gindy ME, Bett AJ, Espeseth AS, Cohen GH, Friedman HM, Antibody responses to crucial functional epitopes as a novel approach to assess immunogenicity of vaccine adjuvants. Vaccine, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruhns P, Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119, 5640–5649 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA, Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13, 843–850 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA, HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107, 4781–4789 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, Cai M, Brown M, Smith JF, Kowalski R, Swoyer R, Galli J, Copeland V, Rios S, Davidson RC, Salnikova M, Kingsley S, Bryan J, Casimiro DR, Friedman HM, Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol 88, 2000–2010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pardi N, Muramatsu H, Weissman D, Kariko K, In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol 969, 29–42 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Weissman D, Pardi N, Muramatsu H, Kariko K, HPLC purification of in vitro transcribed long RNA. Methods Mol Biol 969, 43–54 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W, Tam YK, Ansell SM, Kumar V, Qin J, Zhang X, Wang Q, Panesar S, Hutabarat R, Carioto M, Hettinger J, Kandasamy P, Butler D, Rajeev KG, Pang B, Charisse K, Fitzgerald K, Mui BL, Du X, Cullis P, Madden TD, Hope MJ, Manoharan M, Akinc A, Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther 21, 1570–1578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, Butler D, Eltepu L, Matsuda S, Narayanannair JK, Rajeev KG, Hafez IM, Akinc A, Maier MA, Tracy MA, Cullis PR, Madden TD, Manoharan M, Hope MJ, Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 51, 8529–8533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH, Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol 63, 2325–2334 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.