Abstract
Background:
There are a number of surgical methods for undertaking anterior cruciate ligament (ACL) reconstruction (ACLR), although relatively high rates of ipsilateral retears and contralateral tears exist, with only 65% of patients returning to their preinjury level of sport. ACLR techniques adopting synthetic augmentation have been proposed in an attempt to improve clinical outcomes and reduce reinjury rates.
Purpose:
To determine the efficacy of ACLR using autologous hamstrings augmented with the Ligament Augmentation and Reconstruction System (LARS).
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 65 patients were prospectively treated with arthroscopically assisted single-bundle ACLR using hamstrings augmented with the LARS, of whom 50 were available for 1- and 2-year reviews. Patient-reported outcome measures (PROMs), KT-1000 arthrometer testing, knee range of motion, peak isokinetic knee strength testing, and a battery of 4 hop tests were employed. Limb symmetry indices (LSIs) were calculated. Analysis of variance was used to evaluate differences over time and between limbs. Data on return to the preinjury level of sport, retears, and reoperations were collected.
Results:
High PROM scores were demonstrated at 1 and 2 years. Before the injury, 47 patients (94%) were actively participating in level 1 or 2 sports, with 38 (76%) and 43 (86%) patients having returned at 1 and 2 years, respectively. Normal (<3 mm; 90%) or nearly normal (3-5 mm; 10%) KT-1000 arthrometer side-to-side differences were observed at 2 years. Apart from knee flexion (P < .0001), extension (P = .001), and the 6-m timed hop (P = .039), there were no between-limb differences at 1 year, and there were no differences on any objective measures at 2 years (all P > .05). Mean LSIs across all measures were ≥90%. At 2 years, 84% to 90% of patients were ≥90% on the hop tests, with 72% and 76% of patients having ≥90% for extension and flexion strength, respectively. Two reoperations were undertaken for meniscal tears (7 and 8.5 months), 1 patient (2%) suffered a retear at 7 months, and 2 patients (3%) suffered a contralateral tear (8 and 12 months).
Conclusion:
This augmented ACLR technique demonstrated good clinical scores, a high rate of return to sport, and low rates of secondary ruptures and contralateral ACL tears at 2 years. Some caution should be noted in interpreting these results, as 15 of 65 patients (23%) were not included in the 2-year follow-up.
Keywords: anterior cruciate ligament reconstruction, augmentation, clinical outcomes, retear, return to sport, knee function
Surgical reconstruction is considered the standard clinical treatment for anterior cruciate ligament (ACL) tears.50 Secondary reinjury rates after ACL reconstruction (ACLR) have been estimated at 7%, with 8% of patients also proceeding toward a contralateral ACL tear.62 ACLR aims to maximize knee stability and functional capacity while permitting a safe return to sport (RTS),5,7 although a systematic review by Ardern et al4 reported that only 65% of patients return to their preinjury level of sport after ACLR.
While a number of potential causes of graft failure have been reported,48 graft choice may be the only modifiable surgical factor for young, active patients wishing to RTS.11 A growing number of graft options exist for the orthopaedic surgeon,11 with autografts more commonly employed and appearing superior in terms of clinical outcomes and RTS capacity.11 During the ACLR graft revascularization phase of healing, reduced strength and stiffness occur,8,15,61 and successful incorporation (and subsequent maturation and function) of the graft is dependent on this process of graft ligamentization.1,25 Rehabilitation throughout this phase, therefore, needs to accommodate this process, and early and accelerated pathways can potentially lead to graft laxity, which may be associated with subsequent instability and/or reruptures.41 Synthetic prosthetic ligaments have been employed to bypass the aforementioned issues, remove donor site morbidity, and permit accelerated rehabilitation and RTS. While a recent 10-year longitudinal study9 demonstrated satisfactory clinical outcomes and failure rates in patients undergoing primary ACLR employing a synthetic ligament and remnant preservation, excessive synovitis and high failure rates have limited their ongoing use in earlier studies.26,27,29,36,43,45,63,64
In an attempt to improve clinical outcomes and reduce reinjury rates, particularly in patients with higher activity levels and those seeking an earlier RTS, concomitant extra-articular procedures such as anterolateral ligament reconstruction,10 as well as methods of ACLR employing an autograft or allograft augmented (or reinforced) with a synthetic device,14,19,20,49,51 have been proposed. As previously reported by Falconer et al,14 the proposed advantage of the combined intra-articular autograft/Ligament Augmentation and Reconstruction System (LARS; Corin Group) construct is to permit early ACL reinforcement and accelerated rehabilitation without the increased risk of graft stretching and/or failure. This study presents a remnant-sparing surgical technique for ACLR employing autologous hamstrings augmented with the LARS, together with clinical outcomes for 50 patients who underwent the technique. We sought to investigate the initial safety and efficacy of the surgical technique and to evaluate patient-reported and objectively measured clinical outcomes as well as satisfaction, rerupture, and RTS rates over the first 2 postoperative years.
Methods
Patients
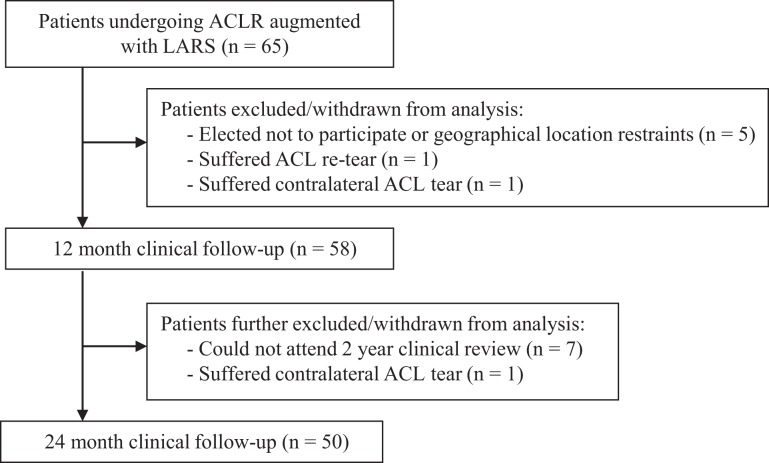
A total of 65 patients underwent ACLR with a hamstring tendon autograft, augmented with a synthetic LARS ligament, for a primary ACL tear between February 2015 and December 2016. All patients were consulted (and underwent surgery) by a single surgeon in a private orthopaedic clinic. The current study included clinical outcomes for 50 patients with a clinical review at both 1 and 2 years postoperatively (Figure 1). Details of the patient sample are provided in Table 1. Patients were invited to participate in the study if they were deemed candidates for surgery, including whether they were skeletally mature and required isolated primary ACLR, with or without concomitant meniscal surgery. Ethics approval was provided by the relevant hospital ethics committee, and the consent of all participants was obtained before the review.
Figure 1.
Study flowchart demonstrating patient recruitment and clinical evaluation over the 24-month period. ACL, anterior cruciate ligament; ACLR, ACL reconstruction; LARS, Ligament Augmentation and Reconstruction System.
TABLE 1.
Characteristics of Patients (N = 50)a
| Male/female sex, n (% male) | 32/18 (64.0) |
| Age, mean ± SD (range), y | 26.3 ± 9.6 (16-49) |
| Body mass index, mean ± SD (range), kg/m2 | 24.8 ± 4.0 (18.1-31.9) |
| Time from injury to surgery, mean ± SD (range), wk | 12.1 ± 12.2 (2-72) |
| Noncontact/contact injuries, n | |
| Australian rules football | 12/2 |
| Soccer | 5/2 |
| Netball | 5/1 |
| Basketball | 6/0 |
| Hockey | 5/0 |
| Other | 6/6 |
| Concomitant procedures, n (%) | |
| Meniscal repair | 6 (12.0) |
| Meniscectomy | 19 (38.0) |
| Prior procedures, n (%) | |
| Meniscal repair | 1 (2.0) |
| Meniscectomy | 1 (2.0) |
| Contralateral ACLR | 1 (2.0) |
aACLR, anterior cruciate ligament reconstruction.
Surgical Technique
All surgical procedures were performed by the senior author (P.T.A.) via an arthroscopically assisted single-bundle surgical technique. Gracilis and semitendinosus hamstrings were harvested from the ipsilateral knee through a 2- to 3-cm transverse incision 1 cm above the pes anserinus. The doubled tendons and a doubled 3.5-mm prosthetic LARS ligament (product code 104.133: LARS anterior cruciate reinforcement) were combined for diameter sizing (Figure 2) in all patients. The LARS ligament added 1 mm of graft diameter, generally creating an overall cross-sectional area of approximately 9 mm2. A 20-mm closed-loop Endobutton (Smith & Nephew) was routinely used for femoral graft fixation, with a 25-mm Endobutton employed when the graft diameter exceeded 8 mm. The grafts and LARS ligament were individually looped through the Endobutton and whipstitched to themselves (Figure 3).
Figure 2.
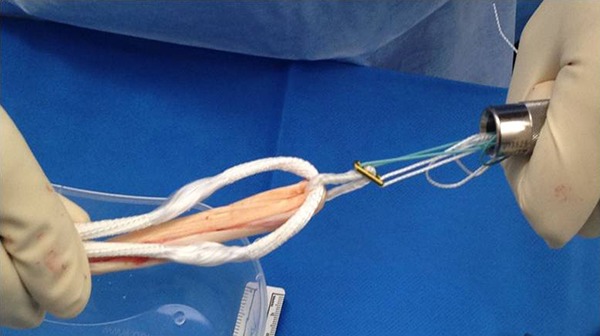
The doubled tendons and a doubled 3.5-mm prosthetic LARS (Ligament Augmentation and Reconstruction System) ligament combined in preparation for diameter sizing.
Figure 3.
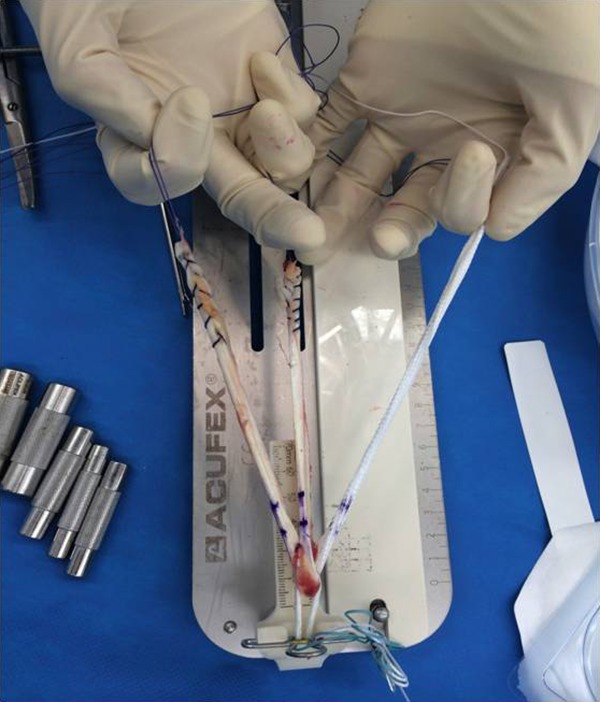
Individual preparation of the grafts and LARS (Ligament Augmentation and Reconstruction System) ligament, looped through the Endobutton and whipstitched to themselves.
Femoral tunnel preparation was performed through an anteromedial portal, allowing anatomic femoral tunnel positioning. A remnant-sparing technique was employed,2,16 which was recommended to minimize potential particulate wear, avoid synthetic impingement, and optimize autograft ligamentization. Therefore, only unstable remnant tissue was debrided, with all stable ACL remnants and the notch synovium spared, as was the fat pad and ligamentum mucosum. The graft was passaged within the retained remnant, with the LARS ligament lying in a posteromedial relationship to the autograft tendons and anterior to the posterior cruciate ligament. The LARS ligament therefore appeared “hidden” in the notch, minimizing impingement and exposure to the joint (Figures 4 and 5).
Figure 4.
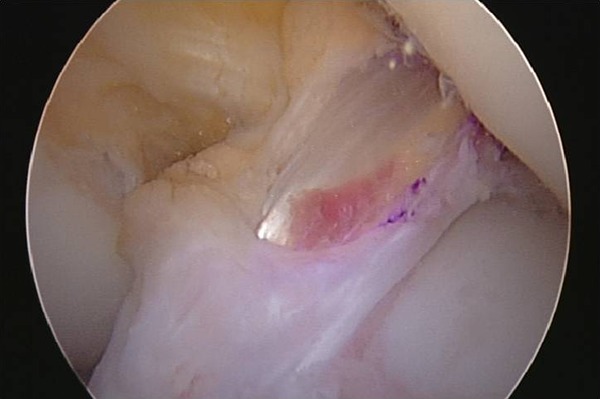
The graft passaged within the retained remnant, with the LARS (Ligament Augmentation and Reconstruction System) ligament lying in a posteromedial relationship to the autograft tendons and “hidden” in the notch, minimizing impingement and exposure to the joint.
Figure 5.
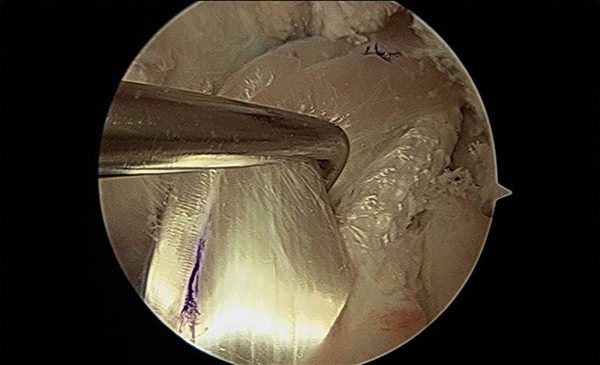
Intraoperative photograph demonstrating exposure of the LARS (Ligament Augmentation and Reconstruction System) ligament after retraction of the hamstring tendon autograft portion of the graft.
Femoral fixation occurred with the Endobutton seated and tensioned with maximal manual tension through 10 cycles of knee flexion. Tibial fixation was performed using Intrafix (DePuy Synthes) with maximal manual tension on the autograft and light tension on the LARS, with the LARS posteromedial to the autografts in full extension (Figure 6). Figure 7 shows final incorporation of the hybrid graft. Postoperative management included early splinting (for 2 weeks, only as a means of reducing movement-associated pain and swelling) and weightbearing as tolerated, with early range of motion (ROM) exercises commencing immediately, and progressive cycling and strengthening exercises being undertaken from 6 to 8 weeks.
Figure 6.
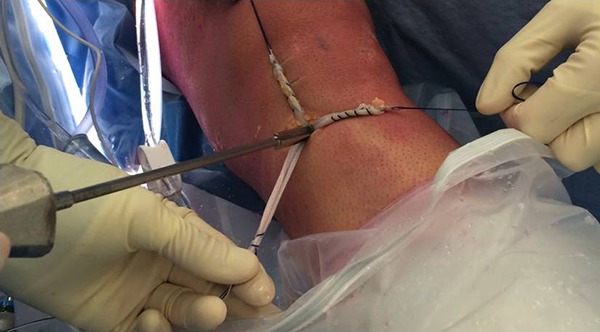
Tibial fixation using Intrafix with maximal manual tension on the autograft and light tension on the LARS (Ligament Augmentation and Reconstruction System), with the LARS posteromedial to the autograft in knee extension.
Figure 7.
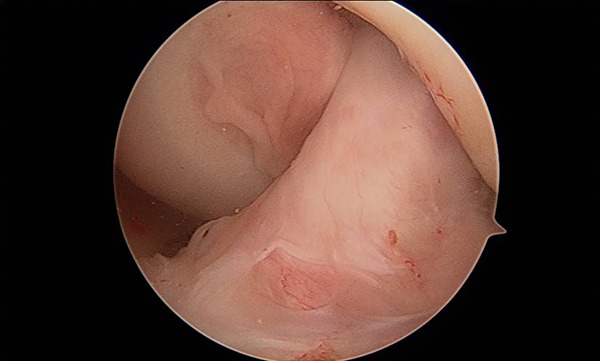
Incorporation of the hybrid graft.
Clinical Assessment
A number of patient-reported outcome measures (PROMs) were undertaken at 1 and 2 years postoperatively. These included the International Knee Documentation Committee (IKDC) subjective knee evaluation form,23 the Knee Outcome Survey (KOS) Activities of Daily Living subscale,24 the Knee injury and Osteoarthritis Outcome Score (KOOS),46 the Lysholm scale,34 the Tegner activity scale,52 the ACL–Return to Sport after Injury (ACL-RSI) scale,57 and the Noyes Sports Activity Rating Scale (NSARS).40 Furthermore, we used a global rating of change scale to evaluate the patient’s perceived status compared with that before surgery, with scores ranging from –5 (very much worse) to 0 (about the same) to 5 (completely recovered). Satisfaction with the surgical procedure overall was evaluated as well as satisfaction with the surgical procedure to relieve pain, improve the ability to perform normal daily and work activities, improve the ability to return to recreational activities (including walking, swimming, cycling, golf, and dancing), and improve the ability to participate in sport (including sports such as tennis, netball, soccer, and football). A Likert response scale was employed with the following descriptors: very satisfied, somewhat satisfied, somewhat dissatisfied, and very dissatisfied.
Objectively, maximal active knee flexion and extension ROM were initially measured. Graft stiffness was evaluated via the KT-1000 arthrometer (MEDmetric), employed to quantify anterior tibial translation42 during a maximal manual test; the difference between the operated and nonoperated knees was obtained. Patients underwent a previously validated battery of 4 hop tests in the following order: (1) the single hop for distance, (2) the 6-m timed hop, (3) the triple hop for distance, and (4) the triple crossover hop for distance.44 Finally, peak concentric knee extension (quadriceps) and flexion (hamstring) isokinetic strength were measured at a single isokinetic angular velocity of 90 deg/s using an isokinetic dynamometer (Isosport). To avoid fatigue, patients were given as much time as they wanted between hop and strength test trials; this time was not standardized and was based on the individual patient’s readiness to proceed.
Data and Statistical Analyses
First, the mean ± standard deviation (range) of all measures (operated and nonoperated limbs) were calculated at 1 and 2 years. The cohort (N = 50) included patients who underwent ACLR alone (n = 25) and those who underwent ACLR in conjunction with meniscal surgery (n = 25). The cohort was assessed collectively, given that independent t tests revealed no difference (P > .05) in characteristics or clinical outcomes between groups. Clinical differences over time (1 and 2 years), as well as between limbs, were assessed via analysis of variance. Limb symmetry indices (LSIs) were also calculated for all hop and strength tests, further categorized by the number and percentage of patients with LSIs <90% and ≥90%. For KT-1000 arthrometer laxity measures, these were further categorized based on the side-to-side difference as normal (<3 mm), nearly normal (3-5 mm), abnormal (6-10 mm), and severely abnormal (>10 mm).39 Only 49 of 50 patients were included in the KT-1000 arthrometer analysis, with the 1 patient who had undergone prior contralateral ACLR omitted. The NSARS was employed to present the number and percentage of patients participating in level 1 (4-7 d/wk) or level 2 (1-3 d/wk) activities that included jumping, hard pivoting, cutting, running, twisting, and/or turning sports. The number and type of surgical complications, postoperative adverse events, reoperations, and retears (ipsilateral and/or contralateral ACL retears) were presented. Statistical analysis was performed using SPSS software (version 23.0; IBM). Statistical significance was determined at P < .05.
Results
Subjective Assessment
Per the NSARS, 47 patients (94%) were actively participating in level 1 or 2 sports that included jumping, hard pivoting, cutting, running, twisting, and/or turning before the injury. These activities were being undertaken by 38 patients (76%) and 43 patients (86%) at 1 and 2 years postoperatively, respectively. All PROMs demonstrated high scores at 1 year postoperatively, with further significant improvement (P < .05) observed from 1 to 2 years on the IKDC; KOOS subscales of Pain, Symptoms, and Quality of Life; Lysholm scale; and global rating of change scale (Table 2). At 2 years postoperatively, 100% of patients were satisfied with the ability of their surgical procedure to relieve pain, improve activities of daily living, and improve recreational activities, with 49 of 50 patients (98%) satisfied with their ability to participate in sport (Table 3).
TABLE 2.
Patient-Reported Outcome Measure Scoresa
| Variable | 1 y | 2 y | P Value |
|---|---|---|---|
| IKDC (0 to 100) | 86.2 ± 10.6 (58.6 to 100.0) | 91.6 ± 8.3 (68.0 to 100.0) | .006 |
| KOS ADL (0 to 80) | 72.8 ± 7.5 (43.0 to 80.0) | 75.1 ± 6.3 (49.0 to 80.0) | .099 |
| KOOS Pain (0 to 100) | 90.7 ± 10.2 (58.3 to 100.0) | 95.1 ± 7.8 (58.3 to 100.0) | .017 |
| KOOS Symptoms (0 to 100) | 87.4 ± 12.3 (50.0 to 100.0) | 92.0 ± 9.9 (57.1 to 100.0) | .046 |
| KOOS ADL (0 to 100) | 96.2 ± 8.5 (50.0 to 100.0) | 96.9 ± 8.5 (50.0 to 100.0) | .679 |
| KOOS Sport/Recreation (0 to 100) | 86.9 ± 14.8 (50.0 to 100.0) | 90.8 ± 12.7 (50.0 to 100.0) | .161 |
| KOOS Quality of Life (0 to 100) | 73.8 ± 19.1 (31.3 to 100.0) | 82.4 ± 17.2 (37.5 to 100.0) | .020 |
| Lysholm (0 to 100) | 88.2 ± 11.8 (53.0 to 100.0) | 93.7 ± 8.1 (65.0 to 100.0) | .008 |
| Tegner (0 to 10) | 7.1 ± 1.9 (4 to 10) | 7.5 ± 1.6 (4 to 10) | .358 |
| ACL-RSI (0 to 100) | 67.1 ± 25.1 (19.2 to 100.0) | 74.8 ± 22.7 (18.3 to 100.0) | .218 |
| Global rating of change scale (–5 to 5) | 3.0 ± 1.8 (–2 to 5) | 4.0 ± 1.1 (–1 to 5) | .001 |
aData are shown as mean ± SD (range). Bolded P values indicate statistically significant differences between 1- and 2-year follow-up (P < .05). ACL-RSI, Anterior Cruciate Ligament–Return to Sport after Injury; ADL, Activities of Daily Living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; KOS, Knee Outcome Survey.
TABLE 3.
Patient-Reported Satisfaction Scores at 2 Years Postoperativelya
| Item | Very Satisfied | Somewhat Satisfied | Somewhat Dissatisfied | Very Dissatisfied |
|---|---|---|---|---|
| Pain relief | 40 (80.0) | 10 (20.0) | 0 (0.0) | 0 (0.0) |
| Improving activities of daily living | 45 (90.0) | 5 (10.0) | 0 (0.0) | 0 (0.0) |
| Improving recreational activities | 44 (88.0) | 6 (12.0) | 0 (0.0) | 0 (0.0) |
| Improving sport participation | 34 (68.0) | 15 (30.0) | 1 (2.0) | 0 (0.0) |
| Overall surgical outcome | 46 (92.0) | 4 (8.0) | 0 (0.0) | 0 (0.0) |
aData are shown as n (%).
Objective Assessment
KT-1000 arthrometer testing demonstrated normal (44/49; 90%) or nearly normal (5/49; 10%) side-to-side differences at 1 year postoperatively, which were unchanged at 2 years (Table 4). At 1 year postoperatively, active knee flexion (P < .0001) and extension (P = .001) ROM were significantly worse, while the 6-m timed hop was significantly slower (P = .039) in the operated compared with the nonoperated limb. There were no other side-to-side differences at 1 year (Table 5). By 2 years, there were no significant side-to-side differences in any of the objective measures (knee ROM, single-leg hop, or strength) (Table 5). Only active knee flexion ROM significantly improved from 1 to 2 years (P = .023) (Table 5).
TABLE 4.
KT-1000 Arthrometer Side-to-Side Differences (n = 49)a
| Variable | 1 y | 2 y | P (1 vs 2 y) |
|---|---|---|---|
| Side-to-side difference, mean ± SD (range), mm | 0.1 ± 1.5 (–2 to 4) | 0.9 ± 1.2 (–3 to 5) | .334 |
| Normal (<3 mm) | 44 (89.8) | 44 (89.8) | |
| Nearly normal (3-5 mm) | 5 (10.2) | 5 (10.2) | |
| Abnormal (6-10 mm) | 0 (0.0) | 0 (0.0) | |
| Severely abnormal (>10 mm) | 0 (0.0) | 0 (0.0) |
aData are shown as n (%) unless otherwise indicated.
TABLE 5.
Objective Knee ROM, Single-Leg Hop, and Isokinetic Strength Outcomesa
| Variable | 1 y | 2 y | P (Operated: 1 vs 2 y) | P (1 y: Operated vs Nonoperated) | P (2 y: Operated vs Nonoperated) | ||
|---|---|---|---|---|---|---|---|
| Operated Limb | Nonoperated Limb | Operated Limb | Nonoperated Limb | ||||
| Active knee flexion ROM, deg | 136.0 ± 7.4 (121 to 155) |
141.2 ± 6.4 (130 to 157) |
139.4 ± 7.2 (128 to 156) |
142.6 ± 6.4 (130 to 157) |
.023 | <.0001 | .088 |
| Active knee extension ROM, deg | –0.3 ± 2.6 (–5 to 9) |
–2.4 ± 3.4 (–10 to 6) |
–0.6 ± 2.4 (–10 to 6) |
–1.4 ± 2.5 (–10 to 5) |
.526 | .001 | .109 |
| Single hop for distance, m | 1.65 ± 0.42 (0.78 to 2.56) |
1.78 ± 0.31 (1.13 to 2.35) |
1.72 ± 0.39 (0.87 to 2.56) |
1.79 ± 0.31 (1.07 to 2.35) |
.360 | .089 | .334 |
| 6-m timed hop, s | 2.45 ± 0.84 (1.50 to 5.57) |
2.17 ± 0.46 (1.43 to 3.56) |
2.29 ± 0.97 (1.47 to 7.66) |
2.11 ± 0.47 (1.43 to 3.44) |
.385 | .039 | .248 |
| Triple hop for distance, m | 4.65 ± 1.26 (1.90 to 7.19) |
5.07 ± 1.01 (2.81 to 6.98) |
4.97 ± 1.21 (2.01 to 7.19) |
5.16 ± 1.02 (2.82 to 6.98) |
.201 | .073 | .399 |
| Triple crossover hop for distance, m | 4.20 ± 1.29 (1.59 to 6.68) |
4.57 ± 1.11 (2.42 to 6.65) |
4.56 ± 1.25 (1.42 to 6.68) |
4.68 ± 1.09 (2.25 to 6.40) |
.150 | .123 | .620 |
| Knee extension peak torque, N·m | 168.8 ± 74.8 (44.0 to 388.0) |
196.1 ± 72.6 (94.0 to 410.0) |
185.6 ± 74.9 (80.0 to 418.0) |
200.3 ± 75.6 (84.0 to 432.0) |
.264 | .067 | .332 |
| Knee flexion peak torque, N·m | 103.0 ± 37.7 (48.0 to 234.0) |
111.3 ± 34.8 (60.0 to 216.0) |
110.7 ± 36.0 (50.0 to 240.0) |
115.2 ± 35.7 (58.0 to 222.0) |
.349 | .303 | .537 |
aData are shown as mean ± SD (range). Bolded P values indicate statistical significance (P < .05). ROM, range of motion.
At 1 and 2 years postoperatively, the mean LSIs across all 4 single-leg hop tests and isokinetic strength measures were ≥90% (Table 6). At 1 year postoperatively, 66% to 82% of patients had LSIs ≥90% on the 4 hop tests, with 50% and 70% of patients demonstrating an LSI ≥90% for peak knee extension and flexion strength, respectively. At 2 years postoperatively, 84% to 90% of patients had LSIs ≥90% on the 4 hop tests, with 72% and 76% of patients demonstrating an LSI ≥90% for peak knee extension and flexion strength, respectively (Table 6).
TABLE 6.
Limb Symmetry Indices for 4 Hop Tests as Well as Peak Isokinetic Quadriceps and Hamstring Strengtha
| Variable | 1 y | 2 y | ||||
|---|---|---|---|---|---|---|
| Overall, Mean ± SD (Range) | <90%, n (%) | ≥90%, n (%) | Overall, Mean ± SD (Range) | <90%, n (%) | ≥90%, n (%) | |
| Single hop for distance, m | 92.0 ± 11.9 (56.3-113.8) | 9 (18.0) | 41 (82.0) | 95.6 ± 9.3 (58.1-113.8) | 7 (14.0) | 43 (86.0) |
| 6-m timed hop, s | 91.7 ± 11.6 (54.9-113.3) | 16 (32.0) | 34 (68.0) | 96.2 ± 11.5 (44.5-116.0) | 6 (12.0) | 44 (88.0) |
| Triple hop for distance, m | 90.9 ± 11.8 (46.5-105.1) | 16 (32.0) | 34 (68.0) | 95.5 ± 9.1 (62.3-107.7) | 8 (16.0) | 42 (84.0) |
| Triple crossover hop for distance, m | 90.7 ± 12.1 (54.1-105.9) | 17 (34.0) | 33 (66.0) | 96.7 ± 9.9 (60.4-111.3) | 5 (10.0) | 45 (90.0) |
| Knee extension peak torque, Nm | 84.7 ± 15.8 (37.3-122.0) | 25 (50.0) | 25 (50.0) | 92.4 ± 10.8 (62.3-111.2) | 14 (28.0) | 36 (72.0) |
| Knee flexion peak torque, Nm | 93.0 ± 14.7 (66.0-137.5) | 15 (30.0) | 35 (70.0) | 96.8 ± 15.4 (63.9-146.7) | 12 (24.0) | 38 (76.0) |
Complications, Reoperations, and Failures
Of all 65 patients who underwent surgery throughout the period (including the 50 patients retained in this analysis with complete 1- and 2-year data), 1 patient had an early wound infection that was treated accordingly without further issue. A further 2 patients underwent secondary surgical procedures, in both cases to debride a torn meniscus at 7 and 8.5 months postoperatively (1 of whom underwent meniscal repair concomitantly with ACLR) and both of whom were largely asymptomatic by 12 months and retained in the 50-patient cohort with complete follow-up data. To better evaluate complication, reoperation, and graft failure rates, all 65 patients who underwent surgery throughout the period were contacted at 1 and 2 years, irrespective of full participation in the follow-up clinical reviews. There were no further complications or subsequent surgical procedures besides those outlined above. However, 1 patient (2%) suffered an ACL retear at 7 months postoperatively because of an accelerated return to higher level activities, while 2 patients (3%) suffered a contralateral ACL tear. These occurred during sport (or sporting activities including training) at 8 and 12 months postoperatively.
Discussion
The use of synthetic ligaments in ACLR was proposed in the 1980s because of their abundant supply and strength, lack of harvest site morbidity, and potential of accelerated rehabilitation and RTS. While various synthetic materials have been employed,33 excessive synovitis and high failure rates limited their ongoing use.26,27,29,36,43,45,63,64 However, further work employing the LARS, made of polyethylene terephthalate, reported that under certain conditions, artificial ACLR could be successful.32,37 Moreover, in vivo and animal studies have suggested that the LARS may permit tissue in-growth properties,55,56 which, while not necessarily suitable as an ACL replacement,56 may provide benefit in augmentation as used in the current study. The most important findings from the current study, employing an ACLR technique using a hamstring tendon autograft augmented with the LARS, were the high clinical scores and satisfaction levels, together with comparative side-to-side limb laxity measurements and the high rate of return to preinjury sport levels (86%) by 2 years postoperatively. Furthermore, there were comparatively low ipsilateral retear (2%), contralateral tear (3%), and reoperation (3%) rates, with a relative absence of complications previously reported when employing synthetics such as loss of knee extension and clinical synovitis.
In comparison with other proposed augmented methods, the relative lack of published data makes comparison with the current results employing ACLR with LARS augmentation difficult. Furthermore, the current surgical technique employed remnant preservation, although systematic reviews and meta-analyses have suggested similar clinical outcomes between remnant preservation and debridement methods.22,35,54 Falconer et al14 reported on the clinical outcomes of a double-bundle ACLR technique with the anteromedial bundle augmented with the LARS. At a minimum 2-year follow-up, no increase in laxity and a low failure rate were reported, although lower clinical scores and a higher reoperation rate (15.4%) compared with the current cohort were reported. Hamido et al19,20 reported on the outcomes of augmenting a short and/or undersized hamstring tendon autograft with the LARS. They initially reported the technique to be safe and satisfactory, especially in the presence of a required earlier RTS,20 and a comparison of outcomes with a 4-strand hamstring tendon autograft revealed comparable PROM scores (albeit better IKDC scores in the augmented LARS group) but a more stable knee with the LARS augmented procedure.19 There have been 2 other surgical techniques proposed in which FiberTape suture (Arthrex) was employed to reinforce a quadriceps tendon autograft49 or allograft,51 although no patient outcomes were reported.
The study patients reported high mean PROM and satisfaction scores. While 100% of patients were satisfied overall, 98% were satisfied with their ability to participate in sport. In the current study, 76% and 86% of patients actually returned to their preinjury level of sport by 1 and 2 years, compared with 94% of patients who were participating in level 1 or 2 sports before the injury. Ardern et al4 reported that only 65% of patients return to their preinjury level of sport, with 55% returning to competitive sport. A number of factors may be associated with a return to preinjury sport. Physical function may be important, by which patients with perceived “normal” knees (vs nearly normal, abnormal, or severely abnormal) and/or more symmetrical hop performance are more likely to return to their preinjury level of sport.4 This may be reflected in the current cohort given the high rate of patients with ≥90% LSIs for the hop tests. Psychological readiness to RTS and a lower fear of reinjuries have also been associated with returning to preinjury sport levels,3 and mean ACL-RSI scores of 67 at 1 year and 75 at 2 years were reported in the current cohort. Interestingly, it has been reported that while patellar tendon autografts may increase the chance of returning to preinjury sport, hamstring tendon autografts may increase the chance of returning to competitive sport. In the current study, it is unknown whether some patients underwent an augmented ACLR procedure with a preconceived idea that it permits an accelerated early recovery and RTS.
The high RTS rates in the current study did not appear to adversely affect anterior laxity or reinjury rates. KT-1000 arthrometer differences were graded “normal” in 90% of patients, with the remaining 10% being “nearly normal.” It has been shown that an elevated retear risk may extend well after the patient’s RTS; Grindem et al18 reported an increased retear rate up until 9 months postoperatively, after which no further increase in the retear risk was observed. While graft failure may occur because of a number of reasons, including delayed and/or inadequate revascularization of new tissue, earlier RTS may increase the risk of secondary retears,31 as may returning to higher level sports that involve side-stepping, pivoting, and/or jumping.18,47,58 Given the majority of patients in this study have returned to their preinjury level of sport, the low incidence of ipsilateral retears and contralateral tears are encouraging. However, it remains important that these patients continue to be followed clinically.
Mean LSIs ≥90% were observed for all hop and strength measures at both 1 year (apart from knee extensor strength) and 2 years postoperatively. This may be an associated factor in the comparably higher RTS rates observed. Single-leg hop tests21,44 and maximal muscle strength assessments6,28,38 are often employed to determine physical capacity, commonly reported via LSIs.53 At 1 year, 66% to 82% of patients demonstrated an LSI ≥90% for each of the 4 hop tests, a finding that had increased to 84% to 90% of patients at 2 years. However, the percentage of patients demonstrating an LSI ≥90% for peak knee extension strength was lower (50% at 1 year and 72% at 2 years). Lower LSIs during peak isokinetic testing (vs functional hop testing) have been previously reported12,59 and may be more sensitive in detecting side-to-side physical asymmetries.
While existing research has suggested an increased reinjury risk if patients do not meet strength and hop test LSIs of ≥90%,18,30 the current findings suggest that many patients returned to sport at 1 and/or 2 years without adequately meeting currently recommended strength criteria (quadriceps symmetry ≥90%). Despite the low reinjury rates observed, the majority of patients in this study did not undergo a formal test battery before their RTS. Interestingly, another study investigating outcomes in a community-level cohort of patients who underwent ACLR with a hamstring tendon autograft reported significant side-to-side differences on all hop and strength measures at 10 to 14 months, together with mean LSIs that were generally <90%.12 In that cohort, only 57 of 111 (51%) returned to level 1 or 2 sports, although the mean LSIs were generally ≥90% in patients specifically participating in these sports. This further highlights the aforementioned association between physical capacity and the incidence of postoperative RTS.
A number of limitations exist within the present study. First, it was a prospective study with no comparative group, given the nature and preliminary use of this augmented surgical technique. Nonetheless, despite the encouraging pilot outcomes and the justification that this provides for a randomized controlled trial, the large body of ACLR research that currently exists permitted us to compare our findings with historical outcomes. Second, this pilot case series was performed exclusively with a clinical review, and no magnetic resonance imaging or second-look arthroscopic surgery was undertaken. Synthetic ligaments have previously been shown to cause graft tunnel widening and joint synovitis. While this could have been assessed in the current series, there was no clinical evidence of synovitis. Falconer et al14 found no evidence of tunnel widening or synovitis on postoperative magnetic resonance imaging at a mean 2.42-year follow-up in patients with combined LARS/hamstring tendon grafts for ACLR. Furthermore, we could not evaluate the incidence of partial retears, although clinical KT-1000 arthrometer measures at 1 and 2 years postoperatively suggested satisfactory graft stability (90% normal and 10% nearly normal).
Third, as mentioned above, we did not assess the actual time to RTS but rather whether patients returned by 1 or 2 years. Actual RTS timing is difficult given that there are other factors that may determine timing out of the patient’s control, such as the fact that RTS (and competitive sport) may be dictated by the playing season. Fourth, it is acknowledged that the level of rehabilitation can affect strength and function after ACLR13,17 and subsequently RTS ability. While a general rehabilitation plan was provided, this was a community-level cohort of patients (none of whom were elite athletes) that were provided guidance and rehabilitation from an array of physical therapists. Therefore, despite the encouraging strength and functional LSIs observed at 1 and 2 years, together with the comparably higher RTS rates, the rehabilitation among patients differed in content, frequency, and duration and was not closely assessed or strictly standardized. Furthermore, the inherent limitations with using LSIs to report postoperative strength and functional outcomes are acknowledged,60 although these still remain the most common way of reporting objective measures and provide an effective means of comparing outcomes with that of other studies. Finally, caution should be noted in interpreting these results, as 15 of the original 65 patients (23%) were not included in the 2-year follow-up.
Conclusion
This augmented ACLR technique demonstrated good clinical outcomes, high levels of satisfaction including the ability to participate in sport, and a high rate of return to preinjury sport. In addition to the high rate of RTS compared with that reported in the literature, comparative side-to-side limb laxity measurements were observed, with a low rate of secondary ipsilateral ACL ruptures and/or contralateral ACL ruptures, again compared with the existing literature. Ongoing and close follow-up of this ACLR technique (and other novel and varied surgical techniques) is required to ascertain any longer term benefit to patient outcomes, reinjury rates, and participation in activity, often a primary outcome of the surgical procedure.
Footnotes
One or more of the authors declared the following potential conflict of interest or source of funding: Independent funding in the form of a research grant was provided by Corin Group to assist this research. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethics approval was obtained from the Hollywood Private Hospital Research Ethics Committee (HPH382).
References
- 1. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162–172. [DOI] [PubMed] [Google Scholar]
- 2. Annear PT, Rohr EJ, Hille DM, Gohil S, Ebert JR. No clinical difference in 10-year outcomes between standard and minimal graft debridement techniques in patients undergoing anterior cruciate ligament reconstruction using autologous hamstrings: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):516–523. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Osterberg A, Tagesson S, Gauffin H, Webster KE, Kvist J. The impact of psychological readiness to return to sport and recreational activities after anterior cruciate ligament reconstruction. Br J Sports Med. 2014;48(22):1613–1619. [DOI] [PubMed] [Google Scholar]
- 4. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. [DOI] [PubMed] [Google Scholar]
- 5. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. [DOI] [PubMed] [Google Scholar]
- 6. Augustsson J, Thomee R, Karlsson J. Ability of a new hop test to determine functional deficits after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):350–356. [DOI] [PubMed] [Google Scholar]
- 7. Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(12):1697–1705. [DOI] [PubMed] [Google Scholar]
- 8. Beynnon BD, Johnson RJ. Anterior cruciate ligament injury rehabilitation in athletes: biomechanical considerations. Sports Med. 1996;22(1):54–64. [DOI] [PubMed] [Google Scholar]
- 9. Chen T, Zhang P, Chen J, Hua Y, Chen S. Long-term outcomes of anterior cruciate ligament reconstruction using either synthetics with remnant preservation or hamstring autografts: A 10-year longitudinal study. Am J Sports Med. 2017;45(12):2739–2750. [DOI] [PubMed] [Google Scholar]
- 10. DePhillipo NN, Cinque ME, Chahla J, Geeslin AG, LaPrade RF. Anterolateral ligament reconstruction techniques, biomechanics, and clinical outcomes: a systematic review. Arthroscopy. 2017;33(8):1575–1583. [DOI] [PubMed] [Google Scholar]
- 11. Duchman KR, Lynch TS, Spindler KP. Graft selection in anterior cruciate ligament surgery: who gets what and why? Clin Sports Med. 2017;36(1):25–33. [DOI] [PubMed] [Google Scholar]
- 12. Ebert JR, Edwards P, Yi L, et al. Strength and functional symmetry is associated with post-operative rehabilitation in patients following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(8):2353–2361. [DOI] [PubMed] [Google Scholar]
- 13. Failla MJ, Logerstedt DS, Grindem H, et al. Does extended preoperative rehabilitation influence outcomes 2 years after ACL reconstruction? A comparative effectiveness study between the MOON and Delaware-Oslo ACL cohorts. Am J Sports Med. 2016;44(10):2608–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falconer TM, Tusak L, Breidahl WH, Annear PT. The LARS augmented 4-tunnel hamstring ‘hybrid’ ACLR graft construction allows accelerated rehabilitation without knee laxity: case series of 111 patients after 2 years. J Musculoskelet Res. 2015;14(4):1550020. [Google Scholar]
- 15. Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14(2):197–205. [DOI] [PubMed] [Google Scholar]
- 16. Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br. 2007;89(9):1165–1171. [DOI] [PubMed] [Google Scholar]
- 17. Grindem H, Granan LP, Risberg MA, Engebretsen L, Snyder-Mackler L, Eitzen I. How does a combined preoperative and postoperative rehabilitation programme influence the outcome of ACL reconstruction 2 years after surgery? A comparison between patients in the Delaware-Oslo ACL cohort and the Norwegian National Knee Ligament Registry. Br J Sports Med. 2015;49(6):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamido F, Al Harran H, Al Misfer AR, et al. Augmented short undersized hamstring tendon graft with LARS(R) artificial ligament versus four-strand hamstring tendon in anterior cruciate ligament reconstruction: preliminary results. Orthop Traumatol Surg Res. 2015;101(5):535–538. [DOI] [PubMed] [Google Scholar]
- 20. Hamido F, Misfer AK, Al Harran H, et al. The use of the LARS artificial ligament to augment a short or undersized ACL hamstrings tendon graft. Knee. 2011;18(6):373–378. [DOI] [PubMed] [Google Scholar]
- 21. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642–648. [DOI] [PubMed] [Google Scholar]
- 22. Hu J, Qu J, Xu D, Zhang T, Zhou J, Lu H. Clinical outcomes of remnant preserving augmentation in anterior cruciate ligament reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1976–1985. [DOI] [PubMed] [Google Scholar]
- 23. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 24. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132–1145. [DOI] [PubMed] [Google Scholar]
- 25. Janssen RP, Scheffler SU. Intra-articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jenkins DH. The repair of cruciate ligaments with flexible carbon fibre: a longer term study of the induction of new ligaments and of the fate of the implanted carbon. J Bone Joint Surg Br. 1978;60(4):520–522. [DOI] [PubMed] [Google Scholar]
- 27. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H., Jr Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1–10. [DOI] [PubMed] [Google Scholar]
- 28. Kockum B, Heijne AI. Hop performance and leg muscle power in athletes: reliability of a test battery. Phys Ther Sport. 2015;16(3):222–227. [DOI] [PubMed] [Google Scholar]
- 29. Kumar K, Maffulli N. The ligament augmentation device: an historical perspective. Arthroscopy. 1999;15(4):422–432. [DOI] [PubMed] [Google Scholar]
- 30. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50(15):946–951. [DOI] [PubMed] [Google Scholar]
- 31. Laboute E, Savalli L, Puig P, et al. Analysis of return to competition and repeat rupture for 298 anterior cruciate ligament reconstructions with patellar or hamstring tendon autograft in sportspeople. Ann Phys Rehabil Med. 2010;53(10):598–614. [DOI] [PubMed] [Google Scholar]
- 32. Lavoie P, Fletcher J, Duval N. Patient satisfaction needs as related to knee stability and objective findings after ACL reconstruction using the LARS artificial ligament. Knee. 2000;7(3):157–163. [DOI] [PubMed] [Google Scholar]
- 33. Legnani C, Ventura A, Terzaghi C, Borgo E, Albisetti W. Anterior cruciate ligament reconstruction with synthetic grafts: a review of literature. Int Orthop. 2010;34(4):465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–154. [DOI] [PubMed] [Google Scholar]
- 35. Ma T, Zeng C, Pan J, Zhao C, Fang H, Cai D. Remnant preservation in anterior cruciate ligament reconstruction versus standard techniques: a meta-analysis of randomized controlled trials. J Sports Med Phys Fitness. 2017;57(7-8):1014–1022. [DOI] [PubMed] [Google Scholar]
- 36. Makisalo SE, Visuri T, Viljanen A, Jokio P. Reconstruction of the anterior cruciate ligament with carbon fibres: unsatisfactory results after 8 years. Knee Surg Sports Traumatol Arthrosc. 1996;4(3):132–136. [DOI] [PubMed] [Google Scholar]
- 37. Nau T, Lavoie P, Duval N. A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament: two-year follow-up of a randomised trial. J Bone Joint Surg Br. 2002;84(3):356–360. [DOI] [PubMed] [Google Scholar]
- 38. Neeter C, Gustavsson A, Thomee P, Augustsson J, Thomee R, Karlsson J. Development of a strength test battery for evaluating leg muscle power after anterior cruciate ligament injury and reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):571–580. [DOI] [PubMed] [Google Scholar]
- 39. Nicholas SJ, D’Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP. A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(8):1881–1886. [DOI] [PubMed] [Google Scholar]
- 40. Noyes FR, Barber SD, Mooar LA. A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res. 1989;246:238–249. [PubMed] [Google Scholar]
- 41. Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. [DOI] [PubMed] [Google Scholar]
- 42. Pugh L, Mascarenhas R, Arneja S, Chin PY, Leith JM. Current concepts in instrumented knee-laxity testing. Am J Sports Med. 2009;37(1):199–210. [DOI] [PubMed] [Google Scholar]
- 43. Rading J, Peterson L. Clinical experience with the Leeds-Keio artificial ligament in anterior cruciate ligament reconstruction: a prospective two-year follow-up study. Am J Sports Med. 1995;23(3):316–319. [DOI] [PubMed] [Google Scholar]
- 44. Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther. 2007;87(3):337–349. [DOI] [PubMed] [Google Scholar]
- 45. Richmond JC, Manseau CJ, Patz R, McConville O. Anterior cruciate reconstruction using a Dacron ligament prosthesis: a long-term study. Am J Sports Med. 1992;20(1):24–28. [DOI] [PubMed] [Google Scholar]
- 46. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 47. Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–957. [DOI] [PubMed] [Google Scholar]
- 48. Samitier G, Marcano AI, Alentorn-Geli E, Cugat R, Farmer KW, Moser MW. Failure of anterior cruciate ligament reconstruction. Arch Bone Joint Surg. 2015;3(4):220–240. [PMC free article] [PubMed] [Google Scholar]
- 49. Saper MG. Quadriceps tendon autograft anterior cruciate ligament reconstruction with independent suture tape reinforcement. Arthrosc Tech. 2018;7(11):e1221–e1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shea KG, Carey JL, Richmond J, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on management of anterior cruciate ligament injuries. J Bone Joint Surg Am. 2015;97(8):672–674. [DOI] [PubMed] [Google Scholar]
- 51. Smith PA, Bley JA. Allograft anterior cruciate ligament reconstruction utilizing internal brace augmentation. Arthrosc Tech. 2016;5(5):e1143–e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985. 198:43–49. [PubMed] [Google Scholar]
- 53. Thomee R, Neeter C, Gustavsson A, et al. Variability in leg muscle power and hop performance after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1143–1151. [DOI] [PubMed] [Google Scholar]
- 54. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: a meta-analysis. Knee. 2016;23(4):566–574. [DOI] [PubMed] [Google Scholar]
- 55. Vaquette C, Viateau V, Guerard S, et al. The effect of polystyrene sodium sulfonate grafting on polyethylene terephthalate artificial ligaments on in vitro mineralisation and in vivo bone tissue integration. Biomaterials. 2013;34(29):7048–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Viateau V, Manassero M, Anagnostou F, Guerard S, Mitton D, Migonney V. Biological and biomechanical evaluation of the ligament advanced reinforcement system (LARS AC) in a sheep model of anterior cruciate ligament replacement: a 3-month and 12-month study. Arthroscopy. 2013;29(6):1079–1088. [DOI] [PubMed] [Google Scholar]
- 57. Webster KE, Feller JA, Lambros C. Development and preliminary validation of a scale to measure the psychological impact of returning to sport following anterior cruciate ligament reconstruction surgery. Phys Ther Sport. 2008;9(1):9–15. [DOI] [PubMed] [Google Scholar]
- 58. Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. [DOI] [PubMed] [Google Scholar]
- 59. Welling W, Benjaminse A, Seil R, Lemmink K, Zaffagnini S, Gokeler A. Low rates of patients meeting return to sport criteria 9 months after anterior cruciate ligament reconstruction: a prospective longitudinal study. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3636–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017;47(5):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207. [DOI] [PubMed] [Google Scholar]
- 62. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Woods GW. Synthetics in anterior cruciate ligament reconstruction: a review. Orthop Clin North Am. 1985;16(2):227–235. [PubMed] [Google Scholar]
- 64. Zoltan DJ, Reinecke C, Indelicato PA. Synthetic and allograft anterior cruciate ligament reconstruction. Clin Sports Med. 1988;7(4):773–784. [PubMed] [Google Scholar]