Abstract
Coenzyme Q10 (CoQ10) is among the most widely used dietary and nutritional supplements on the market. CoQ10 has several fundamental properties that may be beneficial in several clinical situations. This article reviews the pertinent chemical, metabolic, and physiologic properties of CoQ10 and the scientific data and clinical trials that address its use in two common clinical settings: statin-associated myopathy syndrome (SAMS) and congestive heart failure (CHF).
Although clinical trials of CoQ10 in SAMS have conflicting conclusions, the weight of the evidence, as seen in meta-analyses, supports the use of CoQ10 in SAMS overall. In CHF, there is a lack of large-scale randomized clinical trial data regarding the use of statins in patients receiving contemporary treatment. However, one relatively recent randomized clinical trial, Q-SYMBIO, suggests an adjunctive role for CoQ10 in CHF. Recommendations regarding the use of CoQ10 in these clinical situations are presented.
Keywords: coenzyme Q10, CoQ10, ubiquinol, ubiquinone, statin myopathy, statin-associated muscle symptoms, congestive heart failure
INTRODUCTION
Frederick Crane and colleagues discovered coenzyme Q in 1957.1 The name coenzyme Q10 derives from its chemical structure, a benzoquinone ring with a side chain composed of 10 isoprene units. This naturally occurring compound is ubiquitous in nature; thus, it is also known as ubiquinone. Coenzyme Q10 exists in three oxidation states: the fully reduced ubiquinol form (CoQ10H2), the radical semiquinone intermediate (CoQ10H.), and the fully oxidized ubiquinone form (CoQ10; Figure 1). Although similar in structure to some vitamins (eg, vitamin K), CoQ10 is not a vitamin since it is synthesized in the body, whereas vitamins must be obtained from the diet.
Figure 1.
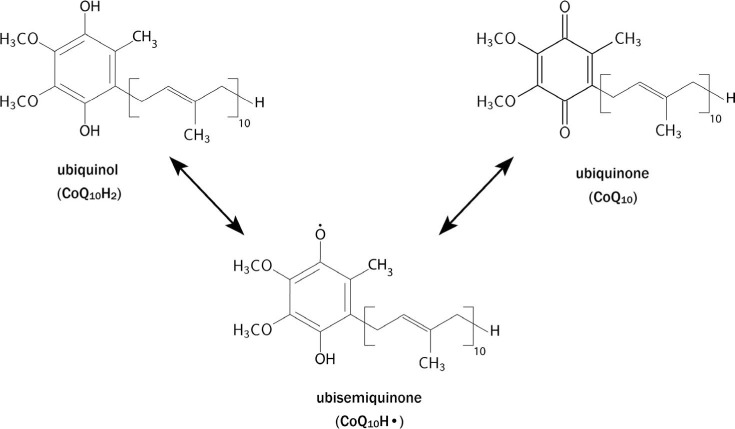
The three oxidative states of CoQ10: the fully reduced ubiquinol form (CoQ10H2), the radical semiquinone intermediate (CoQ10H.), and the fully oxidized ubiquinone form (CoQ10).
PROPERTIES
CoQ10 has fundamental properties that confer its potential benefit in a variety of clinical situations.2,3 First, CoQ10 is a cofactor for mitochondrial enzyme complexes involved in oxidative phosphorylation in the production of adenosine triphosphate (ATP). Hence, it has a fundamental role in cellular bioenergetics, which has led to its clinical application in problems involving tissues with high metabolic requirements, such as heart muscle. Second, beyond its role in generating ATP, CoQ10 serves as an antioxidant or free radical scavenger.
In its reduced form, ubiquinol, it is itself a potent lipophilic antioxidant and can recycle and regenerate other antioxidants in the body. Numerous other functions of CoQ10 have been described, such as cell signaling, gene expression, and membrane stabilization.2
ABSORPTION AND FORMULATIONS
Since CoQ10 is lipophilic, its absorption is similar to that of lipids in the gastrointestinal tract and is increased if ingested with a fatty meal. It is absorbed in the small intestine, aided by secretions from the pancreas and bile. Following absorption, CoQ10 is reduced to ubiquinol and transported to the liver, where it is incorporated into very low-density lipoprotein (VLDL)/LDL particles and released into the circulation. After oral administration, the maximum plasma concentration of CoQ10 occurs at 6 to 8 hours and has an elimination half-life of over 30 hours.2
Notably, the efficiency of orally ingested CoQ10 is generally poor but varies depending on the form of preparation. CoQ10 supplements are available as tablets, powder-filled capsules, and oil suspensions in soft gel capsules. To improve absorption, Chopra et al. added the emulsifier polysorbate 80 to CoQ10 supplements.4 Langsjoen and Langsjoen showed that CoQ10 has superior bioavailability in its reduced form (ubiquinol) than in its oxidized form (ubiquinone),5 although both have been used in clinical studies.
DISTRIBUTION AND METABOLISM
CoQ10 is present in all tissues in varying amounts, with the highest concentrations seen in tissues with high energy requirements or metabolic activity, such as the heart, kidney, liver, and muscle. In these tissues, CoQ10 is primarily found as ubiquinol and is mostly concentrated in the mitochondria, reflecting its importance in mitochondrial function.
Since CoQ10 is synthesized in all tissues, the body is not normally dependent on exogenous supplies of CoQ10. However, endogenous biosynthesis tends to decline with age. Furthermore, tissue CoQ10 may be compromised in many pathophysiologic states. Under these circumstances, exogenous CoQ10 may be needed to maintain normal blood and tissue levels.
The metabolism of CoQ10 is not well studied in humans, but studies from animal models demonstrate that CoQ10 is metabolized in all tissues. The major routes of elimination are biliary and fecal excretion, with a small fraction excreted in the urine.2
SIDE EFFECTS AND INTERACTIONS
Although CoQ10 is present naturally in the human body and should therefore be well tolerated, a variety of adverse reactions have been reported, although infrequent and generally mild. These include decreased appetite, diarrhea, dizziness, dyspepsia, and nausea/vomiting. Despite one report suggesting a decrease in warfarin effect with supplemental CoQ10, a later controlled study showed no such interaction.6
DOSAGE
CoQ10 as a supplement is available in single capsule doses of 30, 60, 100, 200, 300, 400, and 600 mg. Although there is no established minimum or maximum effective dose, the average dose necessary to attain a therapeutic blood level of > 2.5 mcg/mL is 200 mg taken twice daily with a meal.5,7 In cardiac-related trials, daily doses of 100 to 400 mg have been used, while in neurodegenerative diseases (Huntington's disease, Parkinson's disease, and amyotrophic lateral sclerosis), doses of 600 to 3,000 mg have been used.
CoQ10 AND STATIN-ASSOCIATED MUSCLE SYMPTOMS
Atherosclerotic cardiovascular disease remains the leading cause of death in adults. Arguably, the most effective preventive therapies for atherosclerotic disease are the HMG-coenzyme A reductase inhibitors for lowering LDL and reducing cardiac events in patients with coronary artery disease and in previously healthy patients. Consequently, statins are one of the most prescribed drugs in the United States.
While generally well tolerated in most adults, statins are commonly associated with muscle complaints, termed statin-associated muscle symptoms (SAMS). Symptoms range from minor muscle aches to more severe muscle pains, severe cramps, muscle weakness, and, in rare instances, rhabdomyolysis. SAMS can occur in any gender and age group but appear to be more prevalent in women and older adults. The impact of SAMS is profound. Despite the proven life-saving benefits of statins, SAMS is by far the most common reason for noncompliance with statins and, more importantly, the discontinuation of these medications.8 Stopping statins has been associated with an increased risk of cardiovascular events.9
The mechanisms by which statins cause SAMS are unclear, but several observations are germane to understanding the pathophysiologic relationship. Statins reduce cholesterol production by inhibiting the mevalonate pathway, which also produces compounds needed for normal mitochondrial function, including ubiquinone or CoQ10.8 Studies in animals and humans have demonstrated statin-induced reduction of CoQ10 in blood and tissues such as the heart and liver.10 Since CoQ10 is fundamentally important to mitochondrial function and cellular energy production (ATP), the depletion of CoQ10 and resultant mitochondrial dysfunction is hypothesized as the primary pathophysiologic cause of SAMS. Therein lies the rational for using exogenous supplementation of CoQ10 to ameliorate SAMS.
CLINICAL STUDIES OF CoQ10 AND SAMS
Anecdotally, most cardiologists, internists, and other physicians who prescribe statins will cite instances in which CoQ10 treated or prevented SAMS in some of their patients. The concomitant use of a statin and CoQ10 has become a commonly prescribed strategy and an accepted medical practice. Consequently, it is important to examine the data that support or refute this common practice.
Multiple trials have investigated the use of CoQ10 in patients with SAMS. The published data is conflicting, with some trials showing benefit and others showing none. This discordance of results is illustrated by two of the larger randomized trials. Tóth et al. randomized 105 subjects with dyslipidemia into three groups: statin alone, statin plus omega-3 fatty acids, and statin plus omega-3-fatty acids plus CoQ10 200 mg/day. The group with CoQ10 had significantly less myalgia (P = .007), muscle weakness (P < .01), and muscle cramps (P < .001) than the other two groups without CoQ10. These authors concluded that CoQ10 “could attenuate the muscle adverse effects of statin therapy.”11
In contrast, Bookstaver et al. randomized 76 patients with SAMS to a lower dose of CoQ10, 60 mg twice daily, or matching placebo. In a 1-month follow-up using a visual analog scale for pain and the McGill Pain Questionnaire, the investigators found a reduction in muscle pain in both groups but with no statistically significant difference between the two.12
The diagnosis of true SAMS may be problematic. Taylor and colleagues randomized 120 patients with presumed statin myalgia in a double-blind crossover trial of simvastatin or placebo. Only 41 (approximately one-third) developed myalgia with simvastatin but not with placebo, illustrating the difficulty of attributing muscle pains to the statin drug in two-thirds of presumed statin myopathy patients. The 41 subjects with truly “confirmed” statin myopathy were then randomized to CoQ10 600 mg/day or placebo. Surprisingly, more subjects reported pain with CoQ10 (14 of 20, 70%) than placebo (7 of 18, 39%, P = .05). They concluded that CoQ10 supplementation does not reduce muscle pain in patients with statin myopathy.13
When there are multiple small trials with varied entry criteria, outcome measures, and drug dosages, a meta-analysis may be helpful. In 2015, Banach et al. reported such an analysis of five randomized controlled studies with 253 participants. According to the authors, their meta-analysis did not suggest a significant benefit of CoQ10 supplementation in improving statin-induced myopathy.14
A more recent and larger meta-analysis, however, reached a different conclusion. Qu et al. performed an elaborate meta-analysis of 12 randomized controlled trials with a total of 575 enrolled patients. Compared with placebo, CoQ10 supplementation significantly ameliorated statin-associated muscle symptoms, such as muscle pain (P < .001), muscle weakness (P = .006), muscle cramp (P < .001), and muscle fatigue (P < .001). This larger and more current meta-analysis (Figure 2) concluded that “CoQ10 supplementation may be a complementary approach to manage statin-induced myopathy.”15
Figure 2.
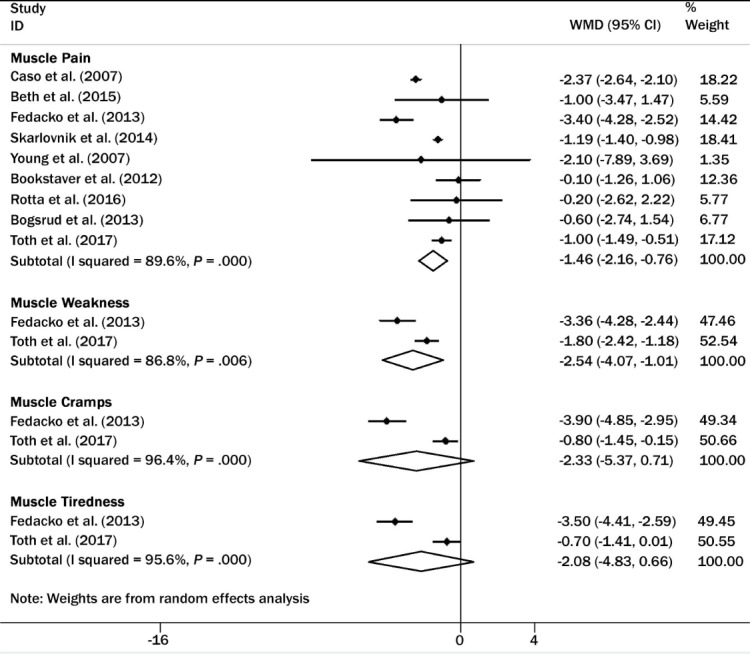
Forest plot for statin-associated muscle symptoms: Coenzyme Q10 versus placebo. CI: confidence interval; ID: study identification; WMD: weighted mean difference. Reprinted with permission.15
RECOMMENDATION FOR USE IN SAMS
Although individual clinical trials have arrived at conflicting conclusions, the largest and most recent meta-analysis by Qu et al. suggests a benefit of CoQ10 in preventing or treating statin myopathy.15 Additionally, clinicians and patients report anecdotally the effectiveness of CoQ10 in some patients with statin myopathy. Furthermore, because discontinuing statin treatment or being unable to take a statin may have important clinical implications for patients with hypercholesterolemia,9 a trial of CoQ10 to prevent or ameliorate the symptoms of statin myopathy seems to be a reasonable recommendation.
CoQ10 IN HEART FAILURE
Hypothetically, CoQ10 may also have a beneficial therapeutic effect in patients with heart failure. As mentioned previously, CoQ10 increases ATP production and cellular energy by mediating electron transfer in the electron transport chain. CoQ10 reduces oxidative stress, a marker of mortality in heart failure.16 Additionally, CoQ10 stabilizes calcium-dependent ion channels in the myocardium, thereby enhancing ATP synthesis.17
In patients with heart failure, there is a measurable deficiency of CoQ10 in both blood and myocardial tissue. Furthermore, the degree of CoQ10 deficiency directly correlates with the degree of impairment in left ventricular function. Exogenous CoQ10 increases blood and tissue levels of CoQ10 and incorporates into the mitochondria.3
The first randomized trial of CoQ10 was reported by Hashiba et al. in 1972 and suggested promising results.18 Langsjoen et al. reported significant improvement in ejection fraction (EF) with 100 mg of CoQ10 in the first controlled trial performed in the United States.19 Since then, a surprising number of randomized controlled clinical trials have been performed. Equally surprising is the observation that most of these trials have reported positive results but have been limited by small numbers and lack of contemporary therapies for heart failure.19
Fotino et al. performed a meta-analysis reported in 2013. Of 120 potentially relevant studies, 13 studies comprising 395 participants were included in the meta-analysis, 11 of which provided data on EF. Supplementation with CoQ10 resulted in a pooled mean net change in EF of 3.67% (95% CI, 1.60% to 5.74%). New York Heart Association (NYHA) Functional Class was also improved by −0.3 (95% CI, −0.66 to 0.06) by CoQ10. This study also suggested that the benefit may be limited to patients with less severe stages of congestive heart failure (CHF), such as those with EF ≥ 30% or those with NYHA Class II or III.20
Looking at 1,069 studies of CoQ10 in heart failure, Jafari et al. identified seven systematic reviews that reported data from 71 randomized clinical trials lasting 3 or more months and comprising 4,688 participants. Their “systematic review of systematic reviews” highlights the discordance of findings in the available literature. Nevertheless, despite the lack of consistency in individual trial results, their overview suggested that the current evidence and strong safety profile of CoQ10 warrant its use as an adjunctive therapy in CHF.21
Perhaps the strongest evidence supporting the use of CoQ10 for CHF comes from the Q-SYMBIO trial (Figure 3).22 This prospective, randomized, controlled, multicenter trial had an adequate sample size (420 patients enrolled), sufficient CoQ10 dosage (100 mg 3 times daily), and long enough duration of follow-up (2 years) to evaluate the efficacy of treatment on major adverse cardiac events and mortality in patients with moderate to severe CHF. Short-term (16 weeks) end points were changes in NYHA functional class, six-minute walk test, and serum N-terminal pro b-type natriuretic peptide (NT-proBNP). At 16 weeks, there were improvements in NYHA functional class and six-minute walk time in both the CoQ10 and placebo groups, but differences between groups were not statistically significant. Whereas differences in serum NT-proBNP were not significant, there was a mean 20% reduction in the CoQ10 subjects but a mean 12% increase in the placebo group.22
Figure 3.
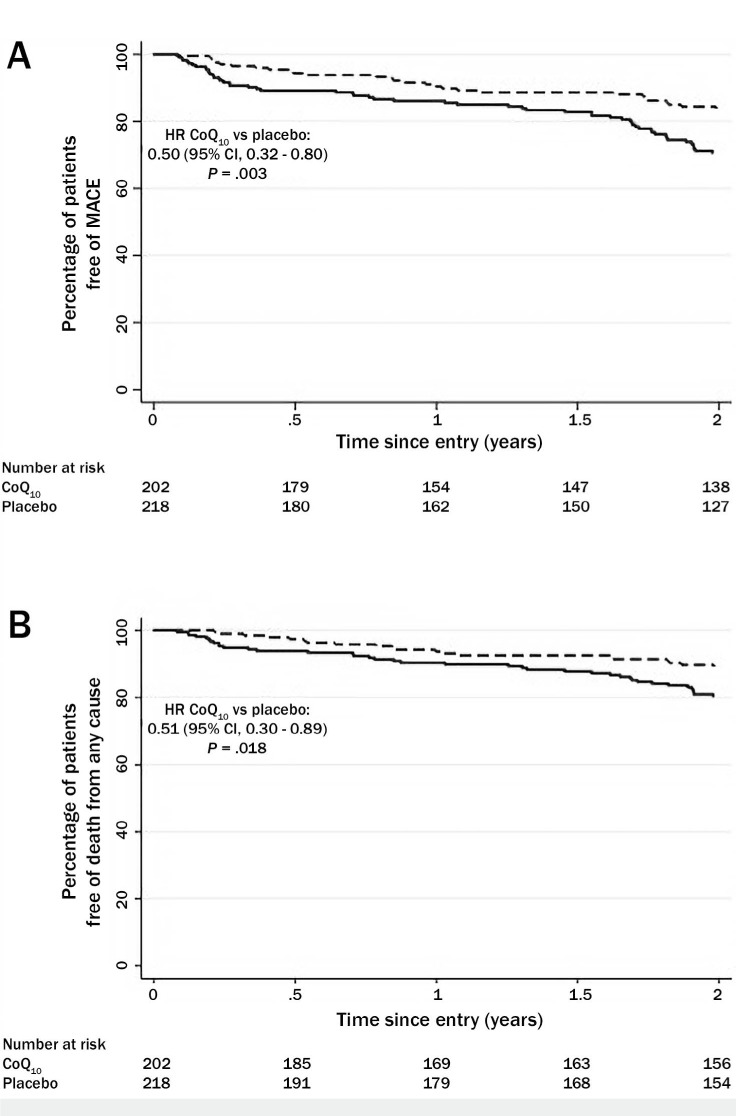
Kaplan-Meyer estimates of (A) the time to primary end point (major adverse cardiac events, or MACE), and (B) secondary end point (death) in the Q-SYMBIO trial. MACE included cardiovascular death, mechanical support, hospital stay for worsening heart failure, and urgent cardiac transplantation. A specified secondary outcome was death from any cause. Reprinted with permission.22
At 2 years, however, the differences between groups were impressive. There were significantly fewer major adverse cardiac events (death, hospitalization, need for left ventricular assist device) in the CoQ10 group (n = 30, 15%) than in the placebo group (n = 57, 26%), a 43% relative reduction (P = .005). There were fewer cardiovascular deaths in the CoQ10 group (18.9%) compared to placebo (16%), a 43% reduction (P = .039). The number of hospitalizations for CHF was lower in the CoQ10 group (17.8%) versus placebo (14%) (P = .033). In addition, a significant improvement in NYHA class was found in the CoQ10 group after 2 years (P = .028). Interestingly, improvement in left ventricular ejection fraction was seen in CoQ10 patients with baseline EF ≥ 30% but not in those with EF < 30%.22
Ultimately, Q-SYMBIO investigators concluded that long-term CoQ10 treatment of patients with chronic HF is safe, improves symptoms, and reduces major adverse clinical events.21
SUMMARY AND RECOMMENDATIONS
CoQ10 is among the most popular dietary and nutritional supplements on the market. It has been studied extensively in both animal and human scientific investigations. This review focused on its application in two clinical cardiac situations: SAMS and CHF. On purely hypothetical grounds, there is rationale for the use of CoQ10 in both of these settings. Yet, the data for or against its use in SAMS or CHF have been conflicting. Large-scale, multicenter, randomized, controlled trials would be required to provide irrefutable evidence supporting the use of CoQ10 in these entities. However, given the economics of clinical trials and the ubiquitous commercial availability of CoQ10, it is unlikely that such a trial would ever be conducted. It should be noted that CoQ10 is not approved by the US Food and Drug Administration for the treatment of any medical condition; instead, it is sold as a dietary supplement and its manufacturing is not regulated the same as drugs.
Nevertheless, clinicians every day are faced with patients' problems and no definitive clinical trial evidence to guide their treatment decisions. Based on the review presented herein, the following recommendations can be offered.
Statin-Associated Muscle Symptoms
The diagnosis of SAMS should be made with reasonable clinical certainty. Patients on a statin drug who develop muscle pains, muscle weakness, or severe cramps should discontinue the statin for 1 month. Resolution of symptoms supports the diagnosis of SAMS. Starting another statin at low dose should then be tried. Return of symptoms is strong evidence of SAMS.
Initiate a trial of CoQ10. There is enough clinical trial evidence and anecdotal experience to initiate treatment with 200 to 400 mg CoQ10, preferably ubiquinol in water-soluble softgel capsules. The safety profile of CoQ10 suggests there is little risk. It is reasonable to temporarily discontinue the statin to allow the SAMS symptoms to resolve before restarting the statin and, concomitantly, CoQ10.
If symptoms of SAMS recur but are milder, continued use of combined therapy is justified. If symptoms of equal severity recur, CoQ10 and statin should be discontinued. Alternative treatment of hypercholesterolemia should be considered.
Applying the American College of Cardiology/American Heart Association (ACC/AHA) Recommendation System, CoQ10 could be considered Class of Recommendation IIa, with Level of Evidence B-R.
Congestive Heart Failure
There is reasonable evidence, most notably the Q-SYMBIO study, to support the adjunctive use of CoQ10 in some patients with CHF, particularly those with less severe CHF (eg, EF > 30%).22 CoQ10 should be considered as add-on therapy to proven medications, such as afterload-reducing drugs, beta blockers, and spironolactone.
It should be noted that cardiologists specializing in the management of patients with advanced HF do not advocate the routine use of CoQ10 as adjunctive treatment.
Applying the ACC/AHA Recommendation System, CoQ10 could be considered Class of Recommendation IIb, with Level of Evidence B-R.
KEY POINTS
Coenzyme Q10 (CoQ10) is an essential cofactor in oxidative phosphorylation in mitochondria and is fundamentally important to cellular energy (ATP) production. Additionally, CoQ10 has direct antioxidant effects.
Cellular depletion of CoQ10 is hypothesized as one pathophysiologic cause of statin-associated myopathy syndrome (SAMS) and may be a contributing factor to myocardial dysfunction in patients with congestive heart failure (CHF).
Although clinical trials of CoQ10 in SAMS have produced conflicting conclusions, meta-analysis supports the use of CoQ10 in patients with SAMS.
In patients with chronic CHF, one randomized clinical trial, Q-SYMBIO, suggests a role for CoQ10 in improving symptoms and reducing major adverse clinical events, particularly in patients with milder left ventricular dysfunction.
At this time, CoQ10 is not approved by the US Food and Drug Administration for the treatment of any medical condition.
Footnotes
Conflict of Interest Disclosure:
The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Crane FL, Hatefi Y, Lester RL, Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta. 1957 Jul;25(1):220–1. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 2.Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006 May;40(5):445–53. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 3.Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9(2–4):273–84. doi: 10.1002/biof.5520090224. [DOI] [PubMed] [Google Scholar]
- 4.Chopra RK, Goldman R, Sinatra ST, Bhagavan HN. Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vitam Nutr Res. 1998;68(2):109–13. [PubMed] [Google Scholar]
- 5.Langsjoen PH, Langsjoen AM. Comparison study of plasma Coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014 Jan;3(1):13–7. doi: 10.1002/cpdd.73. [DOI] [PubMed] [Google Scholar]
- 6.Engelsen J, Nielsen JD, Wither K. Effect of coenzyme Q10 and Ginkgo biloba on warfarin dosage in stable, long-term warfarin treated outpatients. Thromb Haemost. 2002 Jun;87(6):1075–6. [PubMed] [Google Scholar]
- 7.Langsjoen PH, Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors. 2008;32(1–4):119–28. doi: 10.1002/biof.5520320114. [DOI] [PubMed] [Google Scholar]
- 8.Taylor BA. Does Coenzyme Q10 Supplementation Mitigate Statin-Associated Muscle Symptoms? Pharmacological and Methodological Considerations. Am J Cardiovasc Drugs. 2018 Apr;18(2):75–82. doi: 10.1007/s40256-017-0251-2. [DOI] [PubMed] [Google Scholar]
- 9.Serban MC, Colantonio LD, Manthripragada AD et al. Statin Intolerance and Risk of Coronary Heart Events and All-Cause Mortality Following Myocardial Infarction. J Am Coll Cardiol. 2017 Mar 21;69(11):1386–1395. doi: 10.1016/j.jacc.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Langsjoen PH, Langsjoen AM. The clinical use of HMG CoA-reductase inhibitors and the associated depletion of coenzyme Q10. A review of animal and human publications. Biofactors. 2003;18(1–4):101–11. doi: 10.1002/biof.5520180212. [DOI] [PubMed] [Google Scholar]
- 11.Tóth Š, Šajty M, Pekárová T et al. Addition of omega-3 fatty acid and coenzyme Q10 to statin therapy in patients with combined dyslipidemia. J Basic Clin Physiol Pharmacol. 2017 Jul 26;28(4):327–336. doi: 10.1515/jbcpp-2016-0149. [DOI] [PubMed] [Google Scholar]
- 12.Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of Coenzyme Q10 Supplementation on Statin-Induced Myalgias. Am J Cardiol. 2012 Aug 15;110(4):526–9. doi: 10.1016/j.amjcard.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Taylor BA, Lorson L, White CM et al. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis. 2015 Feb;238(2):329–35. doi: 10.1016/j.atherosclerosis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banach M, Serban C, Saheblar A et al. Effects of Coenzyme Q10 on Statin-Induced Myopathy. Mayo Clin Proc. 2015 Jan;90(1):24–34. doi: 10.1016/j.mayocp.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Qu H, Guo M, Chai H et al. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Clinical Trials. J Am Heart Assoc. 2018 Oct 2;(19) doi: 10.1161/JAHA.118.009835. e009835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsutsui T, Tsutamoto T, Wada A et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002 Mar 20;39(6):957–62. doi: 10.1016/s0735-1097(02)01721-7. [DOI] [PubMed] [Google Scholar]
- 17.Rauchová H, Drahota Z, Lenaz G. Function of coenzyme Q in the cell: some biochemical and physiological properties. Physiol Res. 1995;44(4):209–16. [PubMed] [Google Scholar]
- 18.Hashiba K, Kuramoto K, Ishimi Z et al. Coenzyme-Q10 (in Japanese) Heart (Japanese) 1972;4:1579–1589. [Google Scholar]
- 19.Langsjoen PH, Vadhanavikit S, Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4240–4. doi: 10.1073/pnas.82.12.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q10 supplementation on heart failure: a meta-analysis. Am J Clin Nutr. 2013 Feb;97(2):268–75. doi: 10.3945/ajcn.112.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafari M, Mousavi SM, Asgharzadeh A, Yazdani N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. 2018 Jul;70(Suppl 1):S111–S117. doi: 10.1016/j.ihj.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen SA, Rosenfeldt F, Kumar A et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014 Dec;2(6):641–9. doi: 10.1016/j.jchf.2014.06.008. ; Q-SYMBIO Study Investigators. [DOI] [PubMed] [Google Scholar]


