In this review, we present a comprehensive discussion of matters related to the problem of blood culture contamination. Issues addressed include the scope and magnitude of the problem, the bacteria most often recognized as contaminants, the impact of blood culture contamination on clinical microbiology laboratory function, the economic and clinical ramifications of contamination, and, perhaps most importantly, a systematic discussion of solutions to the problem.
KEYWORDS: blood culture contamination
SUMMARY
In this review, we present a comprehensive discussion of matters related to the problem of blood culture contamination. Issues addressed include the scope and magnitude of the problem, the bacteria most often recognized as contaminants, the impact of blood culture contamination on clinical microbiology laboratory function, the economic and clinical ramifications of contamination, and, perhaps most importantly, a systematic discussion of solutions to the problem. We conclude by providing a series of unanswered questions that pertain to this important issue.
INTRODUCTION
Blood cultures have long been recognized as one of the most important tests performed in clinical microbiology laboratories. Unfortunately, blood cultures are frequently contaminated (1–5). There is a substantial cost associated with contaminated blood cultures, a defined impact on clinical microbiology laboratory practice, and, perhaps most importantly, the potential for negative outcomes among patients from whom blood cultures have been obtained (2, 6–13).
The intent of this review is to systematically address five issues relevant to the problem of contaminated blood cultures. These include the scope and magnitude of the problem, its impact on laboratory practice, the clinical implications of contaminated blood cultures, associated costs, and, finally and perhaps most importantly, potential solutions to the problem. Inherent in this discussion is the long-held notion that a certain percentage of blood cultures will be contaminated irrespective of what efforts are made to prevent contamination (14). Currently, health care institutions in the United States are held to a performance standard of <3% rates of blood culture contamination (15, 16). Clearly, as will be shown in this review, recent advances in practice can lead to much lower rates of contamination. If this is true, in view of the substantial negative consequences of contaminated blood cultures, the question arises, should this arbitrary 3% contamination rate threshold be reconsidered?
THE MICROBIOLOGY OF BLOOD CULTURE CONTAMINATION
Magnitude of the Problem
There are two metrics that can be used to quantify the extent of blood culture contamination: the percentage of all positive blood cultures that yield organisms judged to be contaminants (i.e., overall blood culture contamination rate) and the percentage of all blood cultures obtained that are contaminated. Using both of these approaches, the reported frequency with which blood culture contamination occurs has varied in published studies. In a series of large clinical studies examining blood cultures and bacteremia over 4 recent decades, Weinstein and colleagues found that one-third to one-half of all positive blood cultures were judged by infectious disease physicians to represent contamination (2, 3, 17). Other studies have reported lower rates. Story-Roller and Weinstein found that 26% of all positive blood cultures were judged to contain contaminants (4). The overall contamination rate at the university hospital where this study was done was 3.9% (4). Washer et al. found that 13% of all positive blood cultures represented contamination and that overall contamination rates were 0.8% when blood for culture was obtained peripherally by phlebotomists who performed venipuncture (18). Rupp et al. reported that 23% of all positive blood cultures represented contamination and that overall contamination rates were 1.8% during a defined study period (9). Interestingly, the institutional contamination rate in this study increased to 2.8% 6 months following conclusion of the study and reversion to standard practice (9). Other studies have noted that 20 to 56% of all positive blood cultures are found to be contaminated (19, 20), with overall contamination rates of 0.6 to 12.5% (21, 22). Clearly, a number of different variables impact the scope and magnitude of the problem of blood culture contamination.
Sources of Blood Culture Contamination
The potential sources of blood culture contamination are numerous and varied (23, 24). Poor technique by individuals obtaining blood cultures is a major factor (7, 25–27). So is insufficient disinfection of the skin, as the principal source of contaminants is commensal bacteria that colonize the skin (18, 20–22, 28). Collection of blood through indwelling vascular catheters is also problematic. Perhaps related to the difficulty in obtaining adequate antisepsis in the port (i.e., access) area of the intravenous device at the site where blood is obtained for culture, blood cultures that are obtained by peripheral venipuncture have generally been reported to be associated with lower contamination rates than those obtained from indwelling intravenous catheters (29, 30).
Yet another contributing factor in contamination is the type of broth medium used for blood cultures. Several decades ago, manufacturers of blood culture media and detection systems developed media that contained antibiotic-binding resins. In clinical studies comparing these so-called resin media to standard broth media without resins, the resin-containing media provided an increased yield (i.e., growth) of both pathogens and contaminants (31–36). This was particularly notable for both Staphylococcus aureus and coagulase-negative staphylococci (CoNS), the latter most often representing contamination.
Lastly, the standard of practice for obtaining blood cultures by venipuncture underwent a substantial change as the importance of blood-borne pathogens (e.g., HIV) and needlestick injuries to health care workers became widely recognized during the 1990s. Prior to the HIV era, blood cultures were often obtained by a 2-needle technique wherein a sterile needle was used for venipuncture and then, prior to inoculation of the blood culture vials, this needle was removed and a 2nd sterile needle was attached to the syringe for inoculation of blood into the culture vials. To minimize exposure to “sharps” that might result in injury, studies were conducted to determine whether a single-needle technique could be used. Three such studies were published and showed no statistically significant increase in contamination rates (37–39). However, a meta-analysis of these studies reported that single-needle blood cultures were associated with contamination rates of 3.7%, whereas contamination rates were only 2.0% when a two-needle technique was used (40).
Bacteria Associated with Contaminated Blood Cultures
Over many years, a number of clinical laboratory investigations have helped inform our current understanding of the microorganisms most likely to represent contamination when isolated from the blood (2, 3, 17, 41). These include most species of CoNS, most species of Corynebacterium and related genera, Bacillus spp. other than Bacillus anthracis, Micrococcus spp., and Cutibacterium acnes and related species. These organisms may be considered contaminants unless recovered from multiple blood cultures obtained in sequence, in which case, careful assessment of patients and additional laboratory information is required in defining significance (or lack thereof). Enterococci, viridans group streptococci, and Clostridium spp. are of variable clinical significance (i.e., may be either contaminants or true pathogens) when recovered from blood cultures. In contrast, microorganisms such as Staphylococcus aureus, Streptococcus pneumoniae, beta-hemolytic streptococci, Listeria monocytogenes, Escherichia coli and other members of the Enterobacteriaceae, Pseudomonas aeruginosa, Neisseria meningitidis, Haemophilus influenzae, anaerobic Gram-negative rods (e.g., Bacteroides spp. and Fusobacterium spp.), and Candida species rarely represent contamination.
THE LABORATORY IMPACT OF BLOOD CULTURE CONTAMINATION
According to the Clinical and Laboratory Improvements Act (CLIA), the clinical microbiology laboratory is responsible for the preanalytical phase of testing related to the diagnosis of infectious diseases (42). This includes the selection, collection, and transport of specimens. Therefore, the clinical laboratory plays a central role in providing instructions for preventing contamination during blood culture procurement. Table 1 lists key elements for optimization of blood cultures that may impact contamination. The last section of this review delineates in detail the various factors that must be considered at the time of blood culture procurement with the aim of minimizing contamination. Specific instructions on the proper collection of blood cultures should be provided in laboratory directories or specimen collection guidelines.
TABLE 1.
Essential elements for reduction of contamination and optimization of blood culturesa
| Element | Comment |
|---|---|
| Preanalyticalb | |
| Body site for specimen collection | Peripheral venipuncture preferred |
| Preparation of the venipuncture site | Use of alcoholic chlorhexidine gluconate and/or tincture of iodine with 70% alcohol; iodophors (povidone iodine) are not recommended |
| Specimen procurement | |
| Hand hygiene | Use of trained phlebotomy teams |
| Sterile gloves | Adherence to aseptic practices |
| Selective use of sterile drapes | Avoid cultures obtained through lines |
| No. of bottles | A minimum of two separate draws; usually one aerobic and anaerobic bottle per draw |
| Vol of blood (in adults) | 8–10 ml of blood per bottle; 20–30 ml per venipuncture |
| Transport to the laboratory | Transport time should be as rapid as possible at room temperature; follow manufacturers’ guidelines. |
| Analytical | |
| Use of continuously monitored blood culture systems | Reduces turnaround time |
| Timely Gram stain performance | 24/7 processing of positives |
| Rapid diagnostics | Provides faster identification of contaminants and true pathogens |
| Timely ASTc | Resistance marker detection direct from bottle AST |
| Postanalytical (result interpretation) | |
| Notify care providers when blood cultures are determined to be positive | Establish institutional critical action value policy for reporting |
| Define, minimize workup of contaminants | Clearly report as contaminants organisms that meet criteria as such; do not perform AST |
| Final report in electronic patient record | Concise, pertinent report highlighting pathogen and resistance |
Laboratories should have specimen collection guidelines available that address the preanalytical protocols for specimen procurement, training of phlebotomists or other staff, and feedback on individual contamination rates.
AST, antimicrobial susceptibility testing.
In addition, the clinical microbiology laboratory plays an essential role in providing information to care providers when blood culture isolates likely to represent contamination are recovered. Several algorithms have been described that provide criteria which can be used in the laboratory for determining whether isolates of bacteria frequently found to contaminate blood cultures, such as those mentioned above, are likely to be contaminants when recovered in blood cultures (1, 30, 43–45). In making this assessment, in addition to the specific bacterium recovered, the number of blood cultures obtained and the number positive are both important. (For purposes of this review, a blood culture is defined as a culture obtained by one venipuncture or line draw usually consisting of two bottles, one aerobic bottle and one anaerobic bottle.) Consultation with care providers may be required in determining the significance of a blood culture isolate when only a single blood culture set is submitted. When multiple cultures yield the same organism, the positive predictive value for true bacteremia increases (1, 41, 43, 46). Having made a determination that a blood culture isolate is likely to be a contaminant, it is incumbent upon the laboratory to provide this information to care providers both in written culture reports and, when warranted, in direct communications. For example, the report might state: “Coagulase-negative Staphylococcus, probable contaminant. No additional workup. Please call the laboratory within 2 days if susceptibility testing is needed.”
A related consideration is the length of time blood culture bottles are incubated. There exists at least anecdotal information which suggests that the length of time to positivity is of some value in discerning the likelihood of an individual blood culture isolate being a contaminant or of clinical significance (6, 23). The underlying premise is that growth of pathogens, which are likely to be present in higher concentrations in blood from patients with bacteremia, will be detected earlier than contaminants, in which case the magnitude of bacteremia is generally lower (41). This concept may have some validity, at least with blood culture systems that are characterized by prolonged incubation periods. However, today, with the widespread use of continuous monitoring blood culture systems and their attendant incubation periods of only 4 to 5 days, this notion may not be valid (47). In addition, given the extensive overlap in detection times between significant isolates and contaminants, length of time to detection cannot, by itself, be relied upon to accurately predict the clinical significance of individual blood culture isolates (48).
Continuously monitored blood culture systems and enhancements to culture media have improved the recovery and shortened the time to detection of both true pathogens and contaminants, thus shortening the time to provision of blood culture results to care providers (49, 50). Similarly, the availability of novel laboratory technologies to rapidly identify organisms and, in some cases, their resistance determinants directly from positive blood cultures also allows for more rapid detection of potential contaminants, in some cases by as much as 24 h (51–53). Methods include matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), nucleic acid detection, nucleic acid sequencing, and peptide nucleic acid fluorescence in situ hybridization (PNA-FISH). As stated above, the positive clinical implications of use of these methods are substantial (54–56). It should be noted, however, that MALDI-TOF MS, a rapid and relatively inexpensive identification method, is labor-intensive when applied directly to blood culture broth and, as a result, is most often applied to colonies after short incubation of subcultures from positive blood cultures (57). In contrast, nucleic acid-based methods can readily be applied directly to blood culture broth and thus do represent a practical rapid means for identifying causes of bacteremia. Nucleic acid-based methods are, however, typically costly ($70 to $250 per application). In determining whether such methods are cost justifiable in the workup of positive blood cultures, the accrued laboratory costs must be balanced against the potential cost savings in patient care.
Future technologies that measure host response to infection could be useful in determining whether an organism recovered in a blood culture is of clinical significance. This would be of particular value when combined with ultrasensitive detection/identification molecular methods.
Blood culture contamination directly affects analytical testing and laboratory efficiency. Workup of contaminated blood cultures increases technologists’ workloads at a time when many microbiology laboratories are experiencing staffing shortages. In addition, contaminated cultures divert technologist efforts away from other critical samples. There is also the issue of increased time spent in phoning false-positive blood cultures as critical action values to care providers. This is disruptive not only to the laboratory but also to recipients of phone calls, e.g., nurses, physicians, and other health care providers. Finally, high blood culture contamination rates may result in a negative impression of clinical laboratory services, as users of the laboratory often believe that contamination is the result of poor laboratory practices (6–8, 19, 22, 25).
Contaminated blood cultures also result in financial consequences to the laboratory, as they lead directly to unnecessary and costly additional laboratory testing. Examples include repeat blood cultures, cultures of ancillary sites, and nonmicrobiologic studies such as therapeutic drug monitoring for agents such as vancomycin, basic metabolic panels, and complete blood count (CBCs) (22). Microbiology laboratories may use more media, perform additional organism identification procedures, and conduct unnecessary antimicrobial susceptibility tests. Gander et al. reported a difference in median patient charges between negative and false-positive episodes of $8,720 per contaminant, a 47% increase (7). In this study, laboratory costs were not separated from the other expenses. Bates et al. were among the few investigators to specifically address additional microbiology costs between contaminated and negative cultures (6). Using charge analysis as their metric, they noted an 80% increase in total microbiology charges related to contaminated blood cultures, including a 30% increase in routine culture charges and a 40% increase in blood culture charges (6). In a small study of CoNS contaminants, Surdulescu et al. found a 150% increase in charges compared to negative blood cultures (25). Finally, Alahmadi and colleagues found a 4-fold increase in microbiology charges per contaminated culture compared to a negative culture (19). Clearly, contaminated blood cultures have a substantial negative financial impact on laboratory practice.
THE CLINICAL CONSEQUENCES OF BLOOD CULTURE CONTAMINATION
There are several untoward clinical consequences of contaminated blood cultures, the most obvious of which is increased antibiotic exposure. Bates et al. found that intravenous antibiotic charges were 39% higher for contaminant blood culture episodes than among culture-negative patients (6). Souvenir et al. demonstrated that 41% of blood culture contaminant episodes due to CoNS were treated with antibiotics (with 34% receiving vancomycin unnecessarily) (22). Similarly, Lee et al. showed that 41% of 178 patients with contaminants received unneeded intravenous antibiotics (58). Many patients who are started on antibiotics for contamination events receive prolonged therapy. van der Heijden and colleagues found that the median antibiotic course among patients receiving antibiotics after contamination events was 7 days (59), while Souvenir et al. found a mean duration of 6.5 days of vancomycin for CoNS contamination episodes (22).
This increased antibiotic exposure is associated with several potential adverse events, including allergic reactions, drug-drug interactions, antibiotic resistance emergence, and disruption of the host microbiome that can lead to Clostridioides difficile infection as well as other adverse consequences. Unfortunately, limited data exist to quantify the burden of the adverse events that are specifically associated with contaminated blood cultures. While one study revealed that patients with contaminated blood cultures who received antibiotics had higher 1- and 2-week crude mortality rates than those who did not, this finding was confounded by the fact that patients given antibiotics were sicker and had more comorbidities (e.g., malignancy), and their deaths were not felt to be directly related to the consequences of unnecessary antibiotic therapy (58).
Several other clinical consequences may follow blood culture contamination events. Venous access must be maintained in order to deliver parenteral antibiotics and can result in mechanical complications, thromboembolic disease, and infection (60). Unneeded consultation requests may be generated (e.g., infectious diseases consultations), along with additional laboratory or other diagnostic testing (e.g., repeat blood cultures and echocardiography). The search for a source of the bacteremia can lead to unwarranted concern over, or even removal of, indwelling devices such as pacemakers or implantable cardioverter defibrillators. Finally, the initial focus on the blood culture result as the etiology of the patient’s presenting clinical syndrome may result in “anchoring bias” (a form of cognitive bias in which one leans too heavily on an initial piece of information when making subsequent decisions). This can lead to a delay in obtaining the correct diagnosis and a delay in initiating appropriate therapy.
To quantify the frequency of some of these consequences, investigators at Vanderbilt University Medical Center selected a random sample of 100 consecutive patients with blood cultures positive for CoNS that were judged to be contaminants. Extrapolating from this cohort, they estimated an annual burden of almost 900 additional blood cultures obtained, over 350 additional antibiotic courses, and over 30 each of catheter removals, echocardiograms, and subspecialty consultations at their hospital (59). Moreover, an estimated 15 operative procedures would be postponed annually due to contamination events. All of the above may then lead to increased hospital lengths of stay.
Observational studies have described the magnitude of such increases in length of hospitalization. For example, blood culture contamination resulted in an increase of approximately 1 day of hospitalization (from 4 to 5 days) for patients with blood cultures obtained in the emergency department (ED) prior to admission (7). Among hospitalized patients, contaminated blood cultures were associated with a 5.4-day increase in hospital stay compared with that of hospitalized controls matched for age, comorbidity score, and admission month (19). Each additional hospital day due to blood culture contamination increases the chances for a hospital-acquired adverse event, including health care-associated infection, medication errors, falls, decubitus ulcers, and thromboembolic events. While the risks for these adverse events vary substantially by patient-level and facility-level variables, Hauck and Zhao estimated that each additional night in a hospital increases a patient’s risk for an adverse drug reaction by 0.5%, for hospital-acquired infections by 1.6%, and for pressure ulcers by 0.5% (61).
The negative clinical impact of blood culture contamination can be ameliorated in part by the use of rapid diagnostics combined with active antibiotic stewardship efforts. In a prospective, randomized controlled trial, Banerjee and colleagues at Mayo Clinic introduced a rapid multiplex PCR for detection of pathogens directly from positive blood cultures (62). They compared treatment with >24 h of antibiotic therapy of CoNS contamination episodes (1 positive culture out of ≥2 obtained) between the intervention (rapid PCR, with and without active stewardship) and control (standard care) arms. When combined with real-time audit and feedback from their antibiotic stewardship team, the rapid testing group had a significant reduction in treatment of presumed contaminants (from 25% in the control group to 8% [P = 0.015]). Rapid test results alone (with templated comments in the report but no active stewardship) also demonstrated a significant, though less pronounced, reduction in treatment of contaminants (11%). Nagel and colleagues likewise examined the impact of MALDI-TOF MS identification and an antibiotic stewardship intervention on the duration of antibiotic therapy for CoNS contamination episodes (63). Using a quasiexperimental, before-after study design, they demonstrated a reduction in days of unnecessary antibiotic therapy (from 3.89 to 1.31 days [P < 0.001]) in the postintervention cohort, as well as a 50% reduction in the number of vancomycin trough levels performed.
Therefore, the implementation of rapid diagnostic testing for positive blood cultures is likely to reduce, but not eliminate, the adverse clinical consequences of blood culture contamination events. These approaches are most effective when combined with an active antibiotic stewardship team. Such interventions are also justified based on many published studies that report an overall mortality reduction, summarized in a recent meta-analysis (64). However, these interventions (advanced rapid molecular diagnostics, stewardship-trained pharmacists, and infectious diseases specialists) may not be accessible to many hospitals. For example, the 2017 National Healthcare Safety Network (NHSN) patient safety annual survey revealed that only 30% of 4,792 acute-care hospitals used MALDI-TOF MS technology for bacterial organism identification, with those facilities using it more likely to be larger and affiliated with a medical school (65). Such hospitals may also be more likely to have dedicated phlebotomy services, also known to reduce blood culture contamination rates.
THE ECONOMIC CONSEQUENCES OF BLOOD CULTURE CONTAMINATION
Numerous investigations, using various study designs, have examined the economic impact of contaminated blood cultures. Studies dating back several decades have consistently identified an economic cost associated with blood culture contamination.
Two case series have described the added costs associated with blood culture contamination in pediatric patient populations (66, 67). In a retrospective, hospital-wide study conducted between 1993 and 1996 in Boston, a total of 9,465 blood culture specimens were obtained from febrile children; 87 blood cultures (0.9%) from 87 patients were considered to be contaminated (66). Among these 87 patients, 15 were treated in the hospital and 72 were managed on an outpatient basis. Hospital costs were not reported in this study, but additional charges attributable to the contaminated blood cultures were estimated to be $10,821 for patients treated in the outpatient setting and $16,200 for patients admitted to the hospital. A second retrospective case series study evaluated 8,306 children with blood cultures drawn in an ED in Washington, DC, between 1994 and 1996 (67). Of these cultures, 85 (1%) yielded contaminants. The average added cost for the care of patients with contaminated blood cultures in this study was $928 per patient. This cost was incurred as a result of hospital admissions (61% of total costs), reevaluation in the ED (31% of total costs), and antimicrobial susceptibility testing (8% of total costs).
Similar observations have been made for adult patients. A retrospective case series conducted in 1997 assessed the cost implications of contaminated blood cultures in adults (22). In this study, 3,276 blood cultures obtained from 1,433 adult patients hospitalized in Spokane, WA, were examined. The primary intent of this investigation was to assess the attributable hospital costs of blood culture contamination on antimicrobial therapy. Fifty-nine patients in this study were noted to have contaminated blood cultures; 52 (88.1%) of these cultures yielded CoNS. No species identification was performed. Among these 59 patients, 24 (41%) were treated with a systemic antimicrobial, most commonly vancomycin (n = 20 [34%]). No description of empirical versus directed therapy was reported, but the average length of vancomycin therapy was 6.5 days, suggesting that antimicrobial therapy was continued after identification of the organism. The per-patient average cost to perform pharmacokinetic monitoring and purchase of vancomycin in this study was $645.
Although retrospective case series such as those discussed above do not prove causality, they do provide insight into the potential for added costs associated with contaminated blood cultures. There have, however, been six published studies that have compared the economic differences between patients with contaminated blood cultures directly to those with negative blood cultures (6–8, 19, 68, 69). These comparative investigations, because of their design, provide more direct evidence of the cost implications of blood culture contamination.
A retrospective case-control study conducted in Ireland (2007 to 2008) compared 142 hospitalized patients with contaminated blood cultures matched by age, comorbidities, and length of time in the hospital prior to collection of blood cultures to an equal number of hospitalized patients with negative blood cultures (19). The total length of hospital stay was 5.4 days longer for patients with contaminated blood cultures (95% confidence interval [CI], 2.8 to 8.1) and was associated with increased hospital costs of $7,502 (U.S. dollars) (95% CI, $4,925 to $10,078) per patient. Per-patient pharmacy costs increased by $95 and laboratory costs by $61. Another comparative study conducted in St. Louis, MO (1995 to 1996), examined cost differences between patients with contaminated versus negative blood cultures (68). This prospective cohort study of adult, hospitalized patients was conducted as part of a randomized controlled trial evaluating different disinfectant methods. All patients with contaminated blood cultures (n = 120) were compared to all other patients that participated in the study (n = 3,731). The average length of stay was 2 days longer for patients with contaminated blood cultures (11 ± 12 days) than for patients with negative blood cultures (9 ± 10 days), but these results were not statistically significant (P = 0.11). However, statistically significant increases in cost were noted in the total cost of hospitalization ($4,100 increase [95% CI, $740 to $7,400]), the total cost of antimicrobials ($700 increase [95% CI, $20 to $1,400]), and the total laboratory costs ($330 increase [95% CI, $140 to $300]).
Three other cohort studies assessed hospital charges as opposed to hospital costs, two involving hospitalized patients (6, 8) and the other involving patients in the ED (7). The first of these was a prospective study in Boston, MA, in 1989 in which 94 of 1,191 hospitalized patients (7.9%) who had blood cultured were found to have contaminated blood cultures (6). The length of stay was significantly longer (P = 0.003) for patients with contaminated blood cultures (median, 12.5; interquartile range [IQR], 7 to 19 days) compared to patients with negative blood cultures (median, 8; IQR, 5 to 17 days). Statistically significantly increased hospital charges were also noted, including total hospital charges ($4,385 increase [P = 0.004]), antimicrobial charges ($382 increase [P = 0.004]), and laboratory charges ($631 increase [P = 0.0003]). In multivariate analysis, total charges increased by 12% for patients with contaminated blood cultures compared to patients with negative blood cultures. In a retrospective cohort study of hospitalized patients conducted in 2002 in Denver, CO, Zwang and Albert compared 56 patients with contaminated blood cultures to 816 patients with negative blood cultures (8). An increase in total hospital stay of 3 additional days was noted for patients with contaminated blood cultures. This was associated with increased hospital charges of $8,756. Based on their overall 6% rate of blood culture contamination, they estimated that contamination of blood cultures would result in $1.8 million in excess annualized costs, most of that accounted for by an estimated 1,455 to 2,200 extra days of hospitalization per year. Gander et al. also examined the impact of blood culture contamination on total hospital charges in Dallas, TX, in 2006 to 2007 (7). This investigation was part of a randomized controlled study aimed at evaluating phlebotomist versus nonphlebotomist blood culture teams in the ED. All patients with contaminated blood cultures during the study period (n = 120) were compared to 960 randomly selected patients with negative blood cultures. Hospital charges were significantly higher for patients with contaminated blood cultures (median, $27,472; IQR, $21,063 to $37,841) than for patients with negative blood cultures (median: $18,752; IQR: $17,046 to $20,315).
And finally, in a recent retrospective comparative observational study predicated on matched survival data derived from two large teaching hospitals in Boston, Geisler et al. estimated a cost per blood culture contamination event of $6,463; 79% of the increased cost of care was the result of prolonged hospital stays and increased duration of antimicrobial therapy (69).
All six of the cohort studies presented above demonstrate consistently increased hospital costs or charges associated with blood culture contamination. Parenthetically, it is also interesting that over time the increased length of stay associated with contaminated blood cultures seems to have decreased. Possible explanations for this observation include the positive impact of rapid diagnostic procedures in the clinical microbiology laboratory, the increasing reliance on antimicrobial stewardship programs as a means for optimizing antimicrobial therapy, and/or the heightened general emphasis on shortening hospital stays.
Lastly, two investigations have assessed the economic benefits of routine use of interventions to decrease costs associated with blood culture contamination (70, 71). Self et al. compared routine use of sterile collection kits as well as phlebotomy teams for drawing blood cultures as means for reducing blood culture contamination rates (70). Using a decision analysis model, routine use of a sterile kit resulted in net annualized savings of $483,219 in comparison to the routine blood culture collection procedure that had been in place. An annual savings of $288,980, was observed when trained phlebotomists collected blood cultures. Skoglund et al. performed a similar decision analysis investigation in assessing the routine use of an initial specimen diversion device to reduce blood culture contamination. The Steripath diversion device (Magnolia Medical Technologies, Seattle, WA; discussed below) was compared to standard of care in patients with blood cultures drawn in the ED (71). In this study, it was estimated that the routine use of the diversion device to prevent contamination would result in overall hospital cost savings of $272 per blood culture obtained in the ED. Both of these cost-benefit analyses provide convincing evidence as to the cost effectiveness of interventions that reduce blood culture contamination.
PREVENTION OF BLOOD CULTURE CONTAMINATION
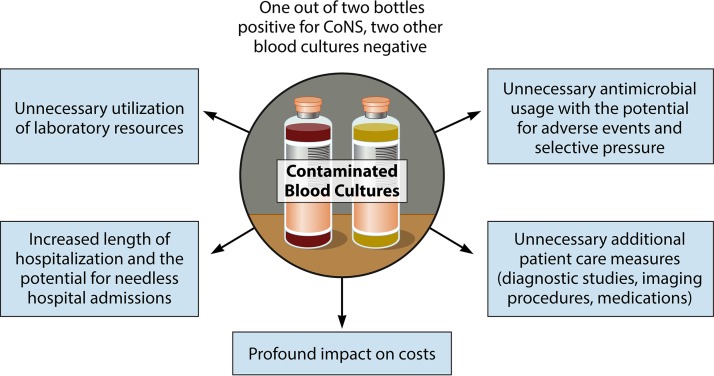
The clinical and cost implications of blood culture contamination together with the impact of contamination on clinical microbiology laboratory function are summarized in Fig. 1. The question then arises, what can be done to reduce the scope and magnitude of this problem?
FIG 1.
Consequences of blood culture contamination.
Patient Selection
Prevention of blood culture contamination starts with selecting appropriate patients for blood cultures. The pretest probability for bacteremia has a major impact on the positive predictive value of the blood culture result. Performing blood cultures on patients with a very low likelihood of bacteremia results in positive cultures more frequently representing false positives. Therefore, clinicians should be educated regarding clinical conditions more frequently associated with bacteremia and appropriate laboratory stewardship. For example, there is growing recognition of the waste associated with unrestrained blood culture use and the lack of utility of routinely repeating blood cultures for patients with Gram-negative bacteremia (72, 73). Bacteremia prediction models may eventually prove useful in guiding clinical decision making (74, 75). There is potential for decision support tools, associated with the electronic medical record, to positively affect the appropriateness of provider orders for blood cultures.
Skin Antisepsis
Extensive investigation has been conducted to discern the preferable disinfectant to be used for skin disinfection in blood culture procurement. Immediate antimicrobial activity at the venipuncture site is required, but prolonged residual effect is not needed. The results of a College of American Pathologists Q-Probe survey published in 1998 noted that the median blood culture contamination rate among adult inpatients was significantly lower in hospitals using tincture of iodine (2.1%) than in those institutions using an povidone iodine (2.9%) for blood culture skin disinfection (P = 0.036) (14). A year later, similar observations regarding the superiority of tincture of iodine versus povidone iodine as a skin disinfectant were made by Little et al. (68). Further reduction in reported contamination rates was observed when 0.5% chlorhexidine gluconate, used with alcohol, became available (76).
Subsequent reports, however, did not demonstrate a statistically significant difference with alcoholic chlorhexidine in comparison to aqueous povidone-iodine or tincture of iodine (4, 77–79). Maiwald and Chan performed a systematic review and meta-analysis on the efficacy of chlorhexidine in skin antisepsis in which 12 articles, including 2 previous systematic reviews, were included (80). Alcoholic chlorhexidine was noted to be superior to aqueous povidone iodine. However, there was no evidence to support alcoholic chlorhexidine over alcoholic povidone iodine or other comparative agents that contained alcohol. In fact, alcohol alone may be an adequate skin disinfectant (79, 81). Investigators at M. D. Anderson Cancer Center noted that the addition of 5% dimethyl sulfoxide (DMSO) to 70% isopropyl alcohol and 1% iodine resulted in a significant decrease in blood culture contamination (82). Guidelines generally recommend disinfection of the phlebotomy site with 2% alcoholic chlorhexidine or 70% isopropyl alcohol followed by 2% chlorhexidine, with at least 30 s allowed for drying (83, 84). We recommend careful disinfection of the skin prior to phlebotomy for blood cultures with an alcohol-containing disinfectant and allowance for drying. Data do not support preference for alcoholic chlorhexidine over an alcoholic iodine-containing preparation, alcohol followed by another disinfectant, or even alcohol alone.
Blood Culture Bottle Disinfection
Although they are covered with a lid, the rubber septa of blood culture vials are not sterile, and it is standard practice to disinfect the tops of culture bottles prior to inoculation (14, 15, 44, 83–85). Seventy percent isopropyl alcohol is most often used for this purpose. Iodine products should not be used alone to disinfect blood culture bottle tops, as the iodine may result in erosion of the rubber and introduction of contaminants (44).
Blood Culture Collection Site
It is preferable to obtain blood for culture via venipuncture rather than from intravascular catheters (21, 86). A meta-analysis of nine studies that met carefully chosen quality metrics (24) demonstrated that blood collected through an intravascular catheter had, on average, a 2.69-fold greater likelihood of being contaminated than blood collected by venipuncture (95% CI, 2.03 to 3.57) (21). When blood cultures are obtained through lines, organisms colonizing the catheter hubs may result in false-positive blood cultures with not only skin commensals but also recognized pathogens such as enterococci, S. aureus, and even Gram-negative bacilli (29, 30). This may falsely elevate an institution’s rate of central line-associated bloodstream infections (CLABSIs) as established by the NHSN (87). False-positive CLABSI rates may have negative patient and financial consequences if thresholds are exceeded and could affect an institution’s reputation as a quality organization (29, 30).
While blood cultures obtained via intravascular catheters are associated with decreased specificity and lower positive predictive value than for peripheral venipuncture blood cultures, they do have greater sensitivity and negative predictive value (86). Deriving data from 6 studies on this topic, Falagas et al. concluded that for every 1,000 patients with blood cultures obtained via intravascular catheters, an additional 8 patients with true bacteremia would be identified, but 59 false-positives would also result (86). Blood culture contamination rates may be decreased with newly inserted central venous catheters if the sample for culture is obtained via a lumen not exposed to the guidewire (88).
Furthermore, if a central venous catheter is thought to be a possible source of a patient’s bacteremia, paired blood samples may be obtained for culture from both the catheter and a peripheral site, and the length of time to positivity compared. In order for the time-to-positivity comparison to be valid, it is important that equal volumes of blood be drawn from both sites. Alternatively, both blood samples can be cultured quantitatively and the quantities of organisms recovered compared (89). Blood cultures obtained from a central venous catheter should be drawn via a freshly placed needleless connector, as connectors in clinical use are frequently colonized and result in blood culture contamination (90). In addition, if a catheter-related bloodstream infection is suspected, some data support obtaining blood for culture from each catheter lumen (91). However, in patients with multiple catheters and multiple lumens, this practice can result in a significant amount of blood sampled at a substantial cost as well as additional opportunities for blood culture contamination with each lumen sampled.
Although the routine practice of drawing blood cultures via vascular catheters is discouraged, it remains a common practice in EDs when peripheral intravenous catheters are established and experience, particularly in pediatric patients, remains somewhat conflicting (37, 92–95). Interestingly, the use of antiseptic barrier caps or passive port protectors has been noted to decrease contamination of blood cultures obtained via central venous catheters (96).
Single Needle versus Double Needle
As discussed above, because the needle used for venipuncture can become contaminated, it used to be common practice to apply a new sterile needle to transfer blood from a syringe to the blood culture vial (double needle transfer technique), and indeed, as noted above, a meta-analysis of 8 studies demonstrated a decrease in contamination rate from 3.7% to 2.0% associated with the double-needle technique (40). However, due to concern regarding needlestick injury, the double-needle transfer technique has fallen into disfavor, and instead, it is recommended that equipment be utilized that allows for the direct collection of blood into culture vials (15, 83).
Sterile Gloves and Hand Hygiene
Gloves should be worn as personal protective equipment when obtaining blood cultures as part of standard infection prevention/blood-borne pathogen precautions. However, it should be noted that nonsterile gloves can become contaminated and have resulted in blood culture contamination (97). The use of sterile gloves was associated with a significant decrease in blood culture contamination in a single-center crossover trial (98). However, the baseline rate of contamination at the center was low (1.1%) and the overall decrease (0.5%) was small.Sterile glove use for blood culture procurement has been an element of successful multicomponent culture contamination reduction programs (99, 100). Clearly, sterile gloves should be employed if it is necessary to repalpate a venipuncture site after it has been disinfected (101, 102). Although the performance of hand hygiene is universally recommended prior to patient contact as part of standard infection prevention, and intuitively one would reason that hand hygiene could decrease blood culture contamination, there are few data to suggest that hand hygiene plays a role in blood culture contamination rates.
Blood Culture Kits and Standard Procedures
In some studies, the use of blood culture collection kits (with or without sterile gloves) and standardized operating procedures has been associated with a significant decrease in blood culture contamination (100, 103–106). However, in a meta-analysis of 7 studies, Snyder and colleagues noted that the utilization of prepackaged blood culture preparation kits did not result in a significant reduction in the rate of blood culture contamination (odds ratio [OR], 1.121; 95% CI, 0.94 to 1.35) (21). Also, it should be noted that in many studies the kits were one of several interventions, such as education, training, and data feedback, and thus, the relative contribution of the kit to the blood culture contamination rate is difficult to discern.
Sterile Drapes
The effect of sterile drapes on blood culture contamination has not been studied in isolation, but sterile drapes have been included in some blood culture kits as part of multicomponent improvement projects (100).
Blood Sampling and Volume
Sampling an appropriate amount of blood is essential in optimizing the performance characteristics of blood cultures. The detrimental effect on pathogen detection by culturing an inadequate amount of blood is well known (106). In this regard, whenever possible, two or three 20-ml volumes of blood should be obtained in the initial evaluation of adult patients suspected of having bacteremia. In pediatric patients in whom collection of high volumes of blood is not possible, two blood specimens should be obtained each consisting of as much blood as can safely be collected. Importantly, separate sticks should be employed in collecting each blood sample. The practice of obtaining multiple blood samples during a single phlebotomy as can happen, for example, at the time an intravenous (i.v.) catheter is placed, is discouraged. It is also essential that care be taken to inoculate appropriate volumes of blood into blood culture vials. Both underfilling and overfilling blood culture vials have been associated with contamination and/or false-positive results (107–109). As noted above, the clinical microbiology laboratory should be proactive in establishing policies and providing guidance to care providers with respect to optimizing the collection of blood for culture.
Phlebotomy Teams/Education
Aseptic collection of blood for culture is a complex skill that requires special knowledge and training. Unfortunately, gaps in knowledge and performance flaws are common (92, 110, 111). Therefore, it is not surprising that the use of trained phlebotomists to obtain blood cultures has been associated with decreased contamination of blood cultures (21, 112–114). In addition, a meta-analysis of five large studies conducted in several U.S. hospitals, four of which were designated good quality, showed excellent strength of evidence supporting reduced contamination rates by trained phlebotomists compared to nonphlebotomists (25). The mean odds ratio of all five studies of 2.58 (95% CI, 2.07 to 3.20) favors phlebotomy teams for decreasing blood culture contamination (21). Several authors have detailed educational programs associated with successful reduction in blood culture contamination (21, 112, 115, 116).
Multidisciplinary/Multimodal Performance Improvement
In many instances, interventions to minimize blood culture contamination have not been studied individually, and instead, multiple measures are introduced in multimodal performance improvement projects that often include education and training, kits, sterile gloves, phlebotomy teams, etc. (30, 102, 112, 117, 118). Translating evidence into sustained clinical practice via multidisciplinary improvement projects requires attention to both technical and adaptive work (119). Adaptive challenges to sustained performance improvement may include people’s beliefs, habits, and values.
Surveillance and Feedback
Surveillance for blood culture contamination, most commonly using laboratory parameters for the definition of contamination, is crucial in establishing baseline institutional performance metrics and measuring the effect of improvement efforts. Surveillance and feedback systems have been shown in multiple studies to result in improved blood culture contamination rates, particularly when contamination rates are reported in a timely manner and directed individually to those who perform phlebotomy (117, 120–123). In one study, education combined with feedback to individual phlebotomists proved more effective than education alone (124).
Initial Specimen Diversion
Hair follicles, sebaceous glands, and deeper layers of the epidermidis serve as sanctuary sites for skin flora. It is very difficult to eradicate microbes from these protected sites with topical antiseptics. Skin fragments that may be colonized with microbes can be dislodged as a result of venipuncture, potentially resulting in blood culture contamination (125, 126). The previously discussed methods to prevent blood culture contamination do not fully address this mode of blood culture contamination. As an alternative or at least as an adjunct to these methods, diversion of the first portion of blood, which presumably contains the contaminating bacteria, with culture of the remaining secondary aliquot of blood, has been proposed as a means to decrease contamination. Studies assessing the utility of this approach have reported overall blood culture contamination rates of 1.6 to 2.4% (127–129).
Recently, two commercially available, self-contained devices that do not require extra collection tubes but which achieve initial specimen diversion have been introduced to the market, the Steripath Gen2 system (Magnolia Medical Technologies, Seattle, WA) and the Kurin Lock device (Kurin, Inc., San Diego, CA). The question arises, can even lower blood culture contamination rates be achieved through use of these diversion devices?
The performance characteristics of the Steripath Gen2 device have been elucidated in several published reports, three of which are outlined here (9, 130, 131). In a prospective, controlled, matched-pair trial in an ED setting, an environment in which blood culture contamination is notoriously a major problem, Rupp and colleagues observed a nearly 90% decrease in blood culture contamination rates based on use of the Steripath device (9). Overall blood culture contamination rates decreased from a level of 1.78% to a level of 0.22% (P = 0.001) through use of this device during the 12-month intervention period involving 904 subjects and 1,808 blood cultures. Limitations of the study include a potential selection bias, lack of pediatric aged patients, and the fact that the study was conducted at a single center. In a multicenter controlled clinical study in four EDs, the historical blood culture contamination rate over a year-long period (35,392 blood cultures) was compared to contamination rates during a 7-month intervention period (6,293 blood cultures) (130). The cumulative average blood culture contamination rate decreased from a level of 3.52% to a level of 0.6% (P = 0.0001) when the Steripath device was used to process blood specimens obtained by both venipuncture and at the time that peripheral i.v. lines were placed, an 83% reduction in contamination rate. And finally, in a prospective study conducted on a single medical ward over a 6-month period involving 671 blood cultures obtained via peripheral phlebotomy or at the time of initial peripheral intravenous catheter insertion, use of the Steripath device (207 cultures) was associated with a 1.0% contamination rate, compared to a contamination rate of 5.2% in 464 cultures obtained via standard practice (P < 0.008) (131). There is one published study pertaining to the Kurin Lock device (132). In a small, single-center study, blood culture contamination rates obtained during a 3-month period in which the Kurin device was used to process blood cultures were reported as 0.44%. Contamination rates of 1.71% were noted during a 3-month comparison period. No information was provided regarding the numbers of blood cultures performed, the characteristics of the patient populations assessed, the criteria used to judge the clinical significance of blood culture isolates, or the identification of the microorganisms recovered from blood cultures. There are no head-to-head comparative trials with these two commercially available specimen diversion devices.
Table 2 summarizes those factors that are important in avoiding contamination of blood cultures.
TABLE 2.
Interventions to prevent blood culture contaminationa
| Intervention | Comment |
|---|---|
| Patient selection | Blood cultures should be performed for patients with a reasonable likelihood of bacteremia. |
| Skin disinfection | Use of an alcohol containing disinfectant is recommended. |
| Blood culture bottle cap disinfection | Tops of blood culture vials should be disinfected prior to inoculation of blood. |
| Phlebotomy site (intravascular catheter vs peripheral vein) | Blood cultures should not be obtained via intravascular catheters unless the catheter is thought to be the source of bacteremia. |
| Single-needle vs double-needle transfer | Although double-needle technique may be helpful, it is not recommended due to risk of needlestick injury. Use direct transfer technique. |
| Sterile gloves/hand hygiene | Limited data to support sterile glove use |
| Standardized kits | Standardized kits and procedures helpful in preventing blood culture contamination |
| Sterile drapes | Not studied as an isolated intervention; sometimes included in blood culture kits |
| Appropriate blood volume | Contamination and false-positive results associated with under- and overfilling blood culture bottles |
| Phlebotomy team/education | Proven useful in decreasing blood culture contamination in numerous studies |
| Multidisciplinary quality improvement | Requires both technical and adaptive work |
| Surveillance and feedback | A key part of any comprehensive program to decrease blood culture contamination |
| Initial specimen diversion | Commercially available device shows promise as a cost-effective means to decrease contamination |
Unanswered Questions
A number of unanswered questions remain related to the practice of obtaining blood cultures and their interpretation.
The first of these is, should there be a new universal standard which defines “acceptable” overall institutional blood culture contamination rates, and, if so, what should that threshold value be? Currently, rates of ≤3.0% are considered acceptable. It is our opinion, however, that overall institutional contamination rates of ≤1.0% are now achievable, and therefore, consideration should be given to the establishment of a new universal threshold value of ≤1.0%.
Second, in settings in which overall contamination rates rise above 1%, should objective, stepwise quality improvement programs designed to improve patient care and reduce unnecessary costs be implemented? The answer is clearly yes. In this regard, organizations concerned with patient safety and health care quality control such as The Joint Commission, the Centers for Disease Control and Prevention, and the Agency for Healthcare Research and Quality should assume a leadership role.
A third question is whether it is advisable for institutions to have a single, uniform standard operational policy for collecting blood cultures, one that reduces to the extent possible the problem of contamination. Again, the answer to this question is clearly yes, at least as it pertains to the routine evaluation of patients suspected of having bacteremia. However, in selected patients, e.g., patients with endocarditis or infected endovascular devices such as pacemakers or vascular grafts, patients with joint protheses, and profoundly immunocompromised patients, a routine policy might be insufficient. This is because positive blood cultures yielding organisms usually considered to be contaminants can be and often are of clinical significance for such patients. Further, the clinical consequences of a contaminated blood culture vary depending on the location from which a specimen is drawn (e.g., in an ED or on a ward caring for immunosuppressed patients). In addition, contaminated blood cultures are more likely to occur in patients with limited or difficult venous access such as neonates, patients with concurrent vascular lines, obese patients, or in patients in which blood cultures are drawn emergently. Thus, in these settings, even greater effort is warranted in attempting to avoid contamination of blood cultures. This may include collection of blood for culture by use of initial specimen diversion devices and/or use of specialized, highly trained personnel to draw blood cultures using sterile gloves or a double-needle method. A related question is whether special laboratory techniques to rapidly identify blood culture isolates and antimicrobial resistance determinants should be utilized in these situations. The answer to this secondary question is yes.
A fourth question is whether better criteria are needed to distinguish between contaminants and true pathogens when unusual organisms recovered from blood cultures are identified using non-culture-based molecular methods, e.g., MALDI-TOF MS, nucleic acid amplification, PNA-FISH, and/or nucleic acid sequencing. Notwithstanding situations where contamination is likely to have occurred, for example, multiple blood culture sets obtained with only a single bottle positive, blood culture isolates identified by these methods are often, a priori, considered to be of clinical significance. This can result in two unintended consequences. First, patients receive unnecessary antimicrobial therapy and additional diagnostic studies are performed. Second, the spurious diagnosis of certain conditions may result in unfair financial penalties being imposed on institutions based on the dictates of the Centers for Medicare Services. Expanding the list of known pathogens or otherwise changing the definition of what constitutes blood culture contamination may serve as solutions to this problem.
A fifth issue pertains to the increasingly important problem of central line-associated bloodstream infections (CLABSIs). CLABSIs are not only of clinical significance: CLABSI rates are publicly reported by the Center for Medicare and Medicaid Services (CMS) and can result in penalties via CMS pay-for-performance programs (value-based purchasing and hospital-acquired conditions programs) (133). However, as noted above, blood for culture when obtained through an indwelling vascular catheter is more likely to be contaminated than blood obtained by peripheral venipuncture. This underscores the importance of accurately assessing the significance of blood culture isolates in patients with central lines. The NHSN has provided criteria for defining the presence of a true CLABSI (134). Among these criteria, two or more blood cultures which yield organisms defined by the CDC as “common commensals” are to be considered significant. NHSN developed and maintains a list of organisms to be considered common commensals (135). Currently, there are over 700 hundred organisms on this list, the vast majority of which are unknown to all but a few clinical microbiology laboratories in the United States. Because of the nature of this list, it requires frequent updates, the most recent of which was in January of 2019 (136). Further, identification of most of the organisms on the list necessitates use of expensive molecular methods, e.g., MALDI-TOF and/or nucleic acid sequencing techniques, that may not be available in many clinical microbiology laboratories. As a consequence, there exists a tendency to overestimate the clinical significance of blood culture isolates from central lines, in turn leading to the potential for all of the negative downstream clinical consequences of false-positive blood cultures discussed above. Blood culture contamination events can also have a negative impact on hospital reimbursement if the positive blood culture result contributes to meeting the NHSN definition of central line-associated bloodstream infection (133). Clearly, better criteria are needed to more accurately define the presence of a true CLASBI, preferably, criteria that are predicated on technologies and approaches that are actually in place in clinical microbiology laboratories today.
Additional questions pertain to use of initial specimen diversion devices as a means for reducing blood culture contamination rates. To wit, should such devices be used routinely, or should they be applied selectively in circumstances where there exists a high likelihood of blood culture contamination? Also, can such devices be used to reduce contamination associated with blood drawn directly through indwelling venous catheters? The availability of more extensive data in peer-reviewed publications which assess the utility of the Kurin Lock device would be most instructive, as would published data describing the results of a head-to-head comparison of the Steripath and Kurin Lock devices. Additional data comparing contamination rates obtained with diversion devices versus simple initial specimen discard would be of value, as would data which assess the utility of diversion device over prolonged study periods, preferably with application to all patients rather than selected patient populations.
CONCLUSIONS
Blood cultures have been a critically important and potentially life-saving diagnostic test for over a century, yet the problem of blood culture contamination persists. There exists a universal consensus that contaminated blood cultures are costly and lead to important unintended consequences for patients. These unintended consequences are diverse and complex, but most are directly or indirectly related to unnecessary and prolonged antibiotic exposure, increased diagnostic testing, and prolonged periods of hospitalization. In general, in the past, overall institutional blood culture contamination rates of 3% have been considered acceptable.
However, we now have a comprehensive understanding of how blood culture contamination occurs and equally importantly, a clear understanding of how to diminish the scope and magnitude of the problem. The frequency of contamination and subsequent secondary harm and excess cost related to contamination can be reduced by careful selection of patients who really need blood cultures, meticulous and proper skin antisepsis, the use of dedicated phlebotomy teams to obtain blood samples, and/or adequate training of staff on how to properly obtain blood samples. Whenever possible, blood for culture should not be obtained from vascular catheters. Further, the use of initial specimen diversion devices as an alternative to standard blood specimen collection methods represents an exciting new advance that has the potential for reducing overall contamination rates to levels not previously considered to be attainable. In view of the foregoing, we believe that a new universal standard of ≤1% should be considered in defining allowable overall institutional blood culture contamination rates.
ACKNOWLEDGMENTS
We appreciate the critical evaluation of the manuscript and helpful suggestions for revision by Christopher D. Doern, Director, Clinical Microbiology Laboratories, Medical College of Virginia, Richmond, VA.
We disclose the following associations: G.V.D., consultant (Magnolia Medical Technologies) and honoraria (UpToDate); K.C.C., research support (GenMark, Singulex and Accelerate); D.J.D., research support (bioMérieux); K.W.G., research support (Magnolia Medical Technologies, Merck & Co, Summit Therapeutics, and Tetraphase); M.E.R., research support (Magnolia Medical Technologies, ContraFect, and XBioTech), consultant (3M, Citius, Telefex/Arrow, and Ariste), advisory panel (3M), and webinar participant (Magnolia Medical Technologies); M.P.W., research support (Beckman-Coulter, JMI Laboratories, and the NJ Department of Health), speaker’s honoraria (Beckman-Coulter), and consultant (SeLux Diagnostics and T2 Biosystems); D.J.S., medical advisory board (Magnolia Medical Technologies), stock ownership (Magnolia Medical Technologies), honoraria (UpToDate), research support (Centers for Disease Control and Prevention, the Duke-UNC Prevention Epicenter, and the National Football League), and consultant (Johnson & Johnson). Each of the authors was the recipient of an unrestricted educational grant from Magnolia Medical Technologies in support of this review.
Biographies

Gary V. Doern, Ph.D., is a professor emeritus of pathology and medicine at the University of Iowa Carver College of Medicine. He received his B.S. degree from Northwestern University and his Ph.D. degree in pathology from the Medical College of Wisconsin. Following 2 years of postdoctoral training in clinical microbiology at the University of Oregon Health Sciences Center in Portland, he served for 35 years as the Director of the Clinical Microbiology Laboratories, first at the University of Massachusetts Medical Center in Worcester, MA, and then at the University of Iowa Carver College of Medicine in Iowa City. He retired in 2008. He was Chairman of the Clinical Microbiology Division of the American Society for Microbiology, Head of the American Board of Medical Microbiology (ABMM), a member of the ICAAC program committee, and for 9 years a voting member of the Clinical and Laboratory Standards Institute (formerly the NCCLS). He served for 5 years as the editor in chief of the Journal of Clinical Microbiology as well as on the editorial boards of seven other journals in the disciplines of infectious diseases and clinical microbiology. He is an elected fellow of the American Academy of Microbiology and the Infectious Diseases Society of America and also a diplomat of the ABMM. He is the recipient of the ASM’s Becton-Dickinson Award and the ABMLI Award for contributions to the field of clinical microbiology.

Karen C. Carroll, M.D., is a professor of pathology at The Johns Hopkins University (JHU) School of Medicine. Dr. Carroll received her B.A. degree in biology from the College of Notre Dame of Maryland and her M.D. degree from the University of Maryland School of Medicine. She completed a residency in Internal Medicine in Rochester, NY, in the Associated Hospitals Program, University of Rochester School of Medicine. Following a clinical fellowship in Infectious Diseases at the University of Massachusetts Medical Center, Dr. Carroll did a second fellowship in medical microbiology at the University of Utah School of Medicine. She was granted a faculty appointment in the Department of Pathology and was the Director of the Infectious Diseases Laboratories at ARUP Inc. for 12 years. In 2002, Dr. Carroll joined the faculty at JHU as the Director of the Division of Medical Microbiology. She is a Fellow of the American Academy of Microbiology, the Infectious Diseases Society of America, and the College of American Pathologists. Dr. Carroll’s focused area of clinical research at JHU is in the diagnosis of health care-associated infections and bacteremia/sepsis.

Daniel J. Diekema, M.D., is a professor of internal medicine and pathology at the University of Iowa Carver College of Medicine. He is the director of the Division of Infectious Diseases, associate director of the Clinical Microbiology Laboratory, and associate hospital epidemiologist at the University of Iowa Healthcare. Dr. Diekema’s clinical and research interests include the role of the diagnostic laboratory in infection prevention and antimicrobial stewardship. He has served on national committees that establish standards in clinical microbiology and infection prevention, including the Clinical and Laboratory Standards Institute (CLSI) and the CDC’s Healthcare Infection Control Practices Advisory Committee (HICPAC), and he is a past president of the Society for Healthcare Epidemiology of America (SHEA).

Kevin W. Garey, Pharm.D., M.S., FASHP, is a professor at the University of Houston College of Pharmacy and Chair of the Department of Pharmacy Practice and Translational Research. He is an adjunct professor at the University of Texas School of Public Health and a clinical specialist and researcher at Baylor St. Luke’s Medical Center, Houston, TX. He received a bachelor of science in pharmacy degree from Dalhousie University in Halifax, Nova Scotia, Canada, a doctor of pharmacy degree from SUNY Buffalo in Buffalo, NY, and a master of science degree in biometry from the University of Texas School of Public Health. Postdoctoral training includes a pharmacy practice residency at Bassett Healthcare, Cooperstown, NY, and infectious disease specialty residency and fellowship training at the University of Illinois at Chicago, Chicago, IL.

Mark E. Rupp, M.D., is a professor and chief of the Division of Infectious Diseases at the University of Nebraska Medical Center. He is the medical director of The Nebraska Medical Center Department of Infection Control & Epidemiology. He serves as the chief of staff for Nebraska Medicine. Dr. Rupp received his medical degree from Baylor College of Medicine and holds a B.S. degree in chemical engineering from the University of Texas. He underwent training in internal medicine and infectious diseases at Virginia Commonwealth University. He is a fellow of the American College of Physicians, the Infectious Diseases Society of America, and the Society for Hospital Epidemiology of America (SHEA). He is a past president of SHEA. Dr. Rupp has published over 450 articles, chapters, and abstracts. He frequently serves as a guest lecturer and is an active teacher and researcher. Dr. Rupp’s research interests are in the areas of health care-associated infections and antimicrobial stewardship.

Melvin P. Weinstein, M.D., is board certified in internal medicine, infectious diseases, and medical microbiology. He is professor of medicine and pathology at Rutgers Robert Wood Johnson Medical School, where he was chief of the Division of Infectious Diseases from 2001 to 2019. A fellow of the American Academy of Microbiology and Infectious Diseases Society of America, Dr. Weinstein was a voting member of the CLSI Subcommittee on Antimicrobial Susceptibility Testing for 18 years and currently serves as its chair. He also serves on the CLSI Committee on Policies and Processes for Blood Cultures. He was previously a member of the FDA Anti-Infective Drug Advisory Committee and the FDA Microbiology Devices Panel and served as a Trustee of the ABMM. He directed the Microbiology Laboratory at RWJ University Hospital from 1983 to 2015 and currently is codirector. He has received the BD Award for Research in Clinical Microbiology, BioMerieux Sonnenwirth Award for Leadership in Clinical Microbiology, and ABMM/ABMLI Professional Recognition Award, all from ASM.

Daniel J. Sexton, M.D., is a professor of medicine in the Division of Infectious Diseases at Duke University Medical Center. He has been active in all aspects of hospital epidemiology since 1977 and was the hospital epidemiologist at Duke for 25 years. His research career has focused on tick-borne diseases, infective endocarditis, bloodstream infections, and hospital-acquired infections. In 1998 he founded the Duke Infection Control Outreach Network (DICON), which has since grown to include a total of 68 hospitals in 6 southeastern states. He has also held a number of leadership positions in the Duke Department of Medicine and the Infectious Diseases Society of America.
Footnotes
[This article was published on 30 October 2019 with the title “A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem.” The title was updated in the current version, posted on 20 July 2020.]
REFERENCES
- 1.Richter SS, Beekman SE, Croco JL, Diekema DJ, Koontz FP, Pfaller MA, Doern GV. 2002. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory-based algorithm. J Clin Microbiol 40:2437–2444. doi: 10.1128/jcm.40.7.2437-2444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 3.Pien BC, Sundaram P, Raoof N, Costa SF, Mirrett S, Woods CW, Reller LB, Weinstein MP. 2010. Evaluation of the microbiology, epidemiology, and outcome of bloodstream infections in hospitalized adults. Am J Med 123:819–828. doi: 10.1016/j.amjmed.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Story-Roller E, Weinstein MP. 2016. Chlorhexidine versus tincture of iodine for reduction of blood culture contamination rates: a prospective randomized crossover study. J Clin Microbiol 54:3007–3009. doi: 10.1128/JCM.01457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilligan P. 2013. Blood culture contamination: a clinical and financial burden. Infect Control Hosp Epidemiol 34:1–2. [DOI] [PubMed] [Google Scholar]
- 6.Bates DW, Goldman L, Lee TH. 1991. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA 265:365–369. doi: 10.1001/jama.1991.03460030071031. [DOI] [PubMed] [Google Scholar]
- 7.Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. 2009. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol 47:1021–1024. doi: 10.1128/JCM.02162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwang O, Albert RK. 2006. Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med 1:272–276. doi: 10.1002/jhm.115. [DOI] [PubMed] [Google Scholar]
- 9.Rupp M, Cavalieri S, Marolf C, Lyden E. 2017. Reduction in blood culture contamination through use of initial specimen diversion device. Clin Infect Dis 65:201–205. doi: 10.1093/cid/cix304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer G. 1985. Coagulase-negative staphylococci in blood cultures: the clinician’s dilemma. Infect Control 6:477–478. doi: 10.1017/S019594170006358X. [DOI] [PubMed] [Google Scholar]
- 11.Kim S-D, McDonald LC, Jarvis WR, McAllister SK, Jerris R, Carson LA, Miller JM. 2000. Determining the significance of coagulase-negative staphylococci isolated from blood cultures at a community hospital: a role for species and strain identification. Infect Control Hosp Epidemiol 21:213–217. doi: 10.1086/501747. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhoff LV, Sheagren JN. 1985. Epidemiology and clinical significance of blood cultures positive for coagulase-negative staphylococci. Infect Control 6:479–486. doi: 10.1017/S0195941700063591. [DOI] [PubMed] [Google Scholar]
- 13.Rupp ME, Archer GL. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis 19:231–243. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 14.Schifman RB, Strand CL, Meier FA, Howanitz PJ. 1998. Blood culture contamination. A College of American Pathologists Q-Probes study involving 640 institutions and 497134 specimens from adult patients. Arch Pathol Lab Med 122:216–221. [PubMed] [Google Scholar]
- 15.Wilson ML, Mitchell M, Morris A. 2007. Principles and procedures for blood cultures; approved guideline. CLSI document M47-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Baron EJ, Weinstein MP, Dunne WM Jr, Yagupsky P, Welch DF, Wilson DM. 2005. Cumitech 1C, blood cultures IV. ASM Press, Washington, DC. [Google Scholar]
- 17.Weinstein MP, Reller LB, Murphy JR, Lichtenstein KA. 1983. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis 5:35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Washer LL, Chenoweth C, Kim H-W, Rogers MAM, Malani AN, Riddell T IV, Kuhn L, Noeyack B Jr, Neusius H, Newton DW, Saint S, Flanders SA. 2013. Blood culture contamination: a randomized trial evaluating the comparative effectiveness of 3 skin antiseptic interventions. Infect Control Hosp Epidemiol 34:15–21. doi: 10.1086/668777. [DOI] [PubMed] [Google Scholar]
- 19.Alahmadi YM, Aldeyab MA, McElnay JC, Scott MG, Darwish Elhajji FW, Magee FA, Dowds M, Edwards C, Fullerton L, Tate A, Kearney MP. 2011. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect 77:233–236. doi: 10.1016/j.jhin.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Dargère S, Cormier H, Verdon R. 2018. Contaminants in blood cultures: importance, implications, interpretation and prevention. Clin Microbiol Infect 24:964–969. doi: 10.1016/j.cmi.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Snyder SR, Favoretto AM, Baetz RA, Derzon JH, Madison BM, Mass D, Shaw CS, Layfield CD, Christenson RH, Liebow EB. 2012. Effectiveness of practices to reduce blood culture contamination: a laboratory medicine best practices systematic review and meta-analysis. Clin Biochem 45:999–1011. doi: 10.1016/j.clinbiochem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souvenir D, Anderson DE, Palpant S, Mroch H, Askin S, Anderson J, Claridge J, Eiland J, Malone C, Garrison MW, Watson P, Campbell DM. 1998. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol 36:1923–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein MP. 2003. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol 41:2275–2278. doi: 10.1128/jcm.41.6.2275-2278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christenson RH, Snyder SR, Shaw CS, Derzon JH, Black RS, Mass D, Epner P, Favoretto AM, Liebow EB. 2011. Laboratory medicine best practices: systematic evidence review and evaluation methods for quality improvement. Clin Chem 57:816–825. doi: 10.1373/clinchem.2010.157131. [DOI] [PubMed] [Google Scholar]
- 25.Surdulescu S, Utamsingh D, Shekar R. 1998. Phlebotomy teams reduce blood-culture contamination rate and save money. Clin Perf Qual Health Care 6:60–62. [PubMed] [Google Scholar]
- 26.Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SS. 1997. Doing it right the first time: quality improvement and the contaminant blood culture. J Clin Microbiol 35:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norberg A, Christopher NC, Ramundo ML, Bower JR, Berman SA. 2003. Contamination rates of blood cultures obtained by dedicated phlebotomy vs intravenous catheter. JAMA 289:726–729. doi: 10.1001/jama.289.6.726. [DOI] [PubMed] [Google Scholar]
- 28.King TC, Price PB. 1963. An evaluation of iodophors as skin antiseptics. Surg Gynecol Obstet 116:361–365. [PubMed] [Google Scholar]
- 29.Boyce JM, Nadeau J, Dumigan D, Miller D, Dubowsky C, Reilly L, Hannon CV. 2013. Obtaining blood cultures by venipuncture versus from central lines: impact on blood culture contamination rates and potential effect on central line-associated bloodstream infection reporting. Infect Control Hosp Epidemiol 34:1042–1047. doi: 10.1086/673142. [DOI] [PubMed] [Google Scholar]
- 30.Garcia RA, Spitzer ED, Beaudry J, Beck C, Diblasi R, Gilleeny-Blabac M, Haugaard C, Heuschneider S, Kranz BP, McLean K, Morales KL, Owens S, Paciella ME, Torregrosa E. 2015. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am J Infect Control 43:1222–1237. doi: 10.1016/j.ajic.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen JH, Mirrett S, McDonald LC, Murray PR, Weinstein MP, Fune J, Trippy CW, Masterson M, Reller LB. 1997. Controlled clinical laboratory comparison of BACTEC Plus Aerobic/F resin medium with BacT/Alert Aerobic FAN medium for detection of bacteremia and fungemia. J Clin Microbiol 35:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald LC, Fune J, Gaido LB, Weinstein MP, Reimer LG, Flynn TM, Wilson ML, Mirrett S, Reller LB. 1996. Clinical importance of the increased sensitivity of BacT/Alert FAN aerobic and anaerobic blood culture bottles. J Clin Microbiol 34:2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JA, Bryce EA, Ngui-Yen JH, Roberts FJ. 1995. Comparison of BACTEC 9240 and BacT/Alert blood culture systems in an adult hospital. J Clin Microbiol 33:1905–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein MP, Mirrett S, Reimer LG, Wilson ML, Smith-Elekes S, Chuard CR, Joho KL, Reller LB. 1995. Controlled evaluation of BacT/Alert standard aerobic and FAN aerobic blood culture bottles for detection of bacteremia and fungemia. J Clin Microbiol 33:978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinstein MP, Mirrett S, Wilson ML, Harrell LJ, Stratton CW, Reller LB. 1991. Controlled evaluation of BACTEC Plus 26 and Roche Septi-Chek aerobic blood culture bottles. J Clin Microbiol 29:879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson ML, Weinstein MP, Mirrett S, Reimer LG, Feldman RJ, Chuard CR, Reller LB. 1995. Controlled evaluation of BacT/Alert standard anaerobic and FAN anaerobic blood culture bottles for the detection of bacteremia and fungemia. J Clin Microbiol 33:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaacman D, Karasic R. 1990. Lack of effect of changing needles on contamination of blood cultures. Pediatr Infect Dis J 9:274–278. doi: 10.1097/00006454-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Krumholz HM, Cummings S, York M. 1990. Blood culture phlebotomy: switching needles does not prevent contamination. Ann Intern Med 113:290–292. doi: 10.7326/0003-4819-113-4-290. [DOI] [PubMed] [Google Scholar]
- 39.Leisure MK, Moore DM, Schwartzman JD, Hayden GF, Donowitz LG. 1990. Changing the needle when inoculating blood cultures: a no-benefit and high-risk procedure. JAMA 264:2111–2112. doi: 10.1001/jama.1990.03450160081034. [DOI] [PubMed] [Google Scholar]
- 40.Spitalnic SJ, Wollard RH, Mermel LA. 1995. The significance of changing needles when inoculating blood cultures: a meta-analysis. Clin Infect Dis 21:1003–1006. [DOI] [PubMed] [Google Scholar]
- 41.MacGregor RR, Beaty HN. 1972. Evaluation of positive blood cultures. Guidelines for early differentiation of contaminated from valid positive cultures. Arch Intern Med 130:84–88. doi: 10.1001/archinte.1972.03650010072013. [DOI] [PubMed] [Google Scholar]
- 42.Anonymous. 1992. Clinical Laboratory Improvement Amendments of 1988. Register F, http://www.gpo.gov/fdsys/pkg/STATUTE-102/pdf/STATUTE-102-Pg2903.pdf.
- 43.Beekmann SR, Diekema DJ, Doern GV. 2005. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol 26:559. doi: 10.1086/502584. [DOI] [PubMed] [Google Scholar]
- 44.Hall KK, Lyman JA. 2006. Updated review of blood culture contamination. Clin Microbiol Rev 19:788–802. doi: 10.1128/CMR.00062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riedel S, Carroll KC. 2010. Blood cultures: key elements for best practices and future directions. J Infect Chemother 16:301–316. doi: 10.1007/s10156-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 46.Mirrett S, Weinstein MP, Reimer LG, Wilson ML, Reller LB. 2001. Relevance of the number of positive bottles in determining clinical significance of coagulase-negative staphylococci in blood cultures. J Clin Microbiol 39:3279–3281. doi: 10.1128/jcm.39.9.3279-3281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron EJ, Scott JD, Tompkins LS. 2005. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures. Clin Infect Dis 41:1677–1680. doi: 10.1086/497595. [DOI] [PubMed] [Google Scholar]
- 48.Ilstrup D. 1978. Sepsis: organisms from blood cultures at the Mayo Clinic: 1968–1975, p 23–25. In Washington J., II (ed), The detection of septicemia. CRC Press, West Palm Beach, FL. [Google Scholar]
- 49.Krisher KK, Whyburn DR, Koepnick FE. 1993. Comparison of the BacT/Alert pediatric blood culture system, Pedi-BacT, with conventional culture using the 20-millimeter Becton-Dickinson supplemented peptone broth bottle. J Clin Microbiol 31:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy GT, Barr JG, Goldsmith C. 1995. Detection of bacteraemia by the continuously monitoring BacT/Alert system. J Clin Pathol 48:912–914. doi: 10.1136/jcp.48.10.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banerjee R, Ozenci V, Patel R. 2016. Individualized approaches are needed for optimized blood cultures. Clin Infect Dis 63:1332–1339. doi: 10.1093/cid/ciw573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riedel S, Carroll KC. 2016. Early identification and treatment of pathogens in sepsis: molecular diagnostics and antibiotic choice. Clin Chest Med 37:191–207. doi: 10.1016/j.ccm.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Messacar K, Hurst AL, Child J, Campbell K, Palmer C, Hamilton S, Dowell E, Robinson CC, Parker SK, Dominguez SR. 2017. Clinical impact and provider acceptability of real-time antimicrobial stewardship decision support for rapid diagnostics in children with positive blood culture results. J Pediatric Infect Dis Soc 6:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Box MJ, Sullivan EL, Ortwine KN, Parmenter MA, Quigley MM, Aguilar-Higgins LM, MacIntosh CL, Goerke KF, Lim RA. 2015. Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 35:269–276. doi: 10.1002/phar.1557. [DOI] [PubMed] [Google Scholar]
- 55.Pliakos EE, Andreatos N, Shehadeh F, Ziakas PD, Mylonakis E. 2018. The cost effectiveness of rapid diagnostic testing for the diagnosis of bloodstream infections with or without antimicrobial stewardship. Clin Microbiol Rev 31:e00095-17. doi: 10.1128/CMR.00095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamy B, Dargère Arendrup MC, Parienti JJ, Tattevin P. 2016. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol 7:697. doi: 10.3389/fmicb.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verhoeven PO, Haddar CH, Rigaill J, Fonsale N, Carricajo A, Grattard F, Pozzetto B. 2018. Comparison of the fully automated FilmArray BCID assay to a 4-hour culture test coupled to mass spectrometry for day 0 identification of microorganisms in positive blood cultures. Biomed Res Int 21:7013470. doi: 10.1155/2018/7013470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CC, Lin WJ, Shih HI, Wu CH, Chen PL, Lee HC, Lee NY, Chang CM, Wang LR, Ko WC. 2007. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J Microbiol Immunol Infect 40:438–444. [PubMed] [Google Scholar]
- 59.van der Heijden YF, Miller G, Wright PW, Shepherd BE, Daniels TL, Talbot TR. 2011. Clinical impact of blood cultures contaminated with coagulase-negative staphylococci at an academic medical center. Infect Control Hosp Epidemiol 32:623–625. doi: 10.1086/660096. [DOI] [PubMed] [Google Scholar]
- 60.Bell J, Goyal M, Long S, Kumar A, Friedrich J, Garfinkel J, Chung S, Fitzgibbons S. 2018. Anatomic site-specific complication rates for central venous catheter insertions. J Intensive Care Med 19:885066618795126. doi: 10.1177/0885066618795126. [DOI] [PubMed] [Google Scholar]
- 61.Hauck K, Zhao X. 2011. How dangerous is a day in the hospital? A model of adverse events and length of stay for medical inpatients. Med Care 49:1068–1075. doi: 10.1097/MLR.0b013e31822efb09. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagel JL, Huang AM, Kunapuli A, Gandhi TN, Washer LL, Lassiter J, Patel T, Newton DW. 2014. Impact of antimicrobial stewardship intervention on coagulase-negative Staphylococcus blood 368 cultures in conjunction with rapid diagnostic testing. J Clin Microbiol 52:2849–2854. doi: 10.1128/JCM.00682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timbrook TT, Morton JB, McConeghy WK, Caffrey AR, Mylonakis E, LaPlante KL. 2017. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. December 2018. National Healthcare Safety Network newsletter. https://www.cdc.gov/nhsn/newsletters/index.html.
- 66.Waltzman ML, Harper M. 2001. Financial and clinical impact of false-positive blood culture results. Clin Infect Dis 33:296–299. doi: 10.1086/321881. [DOI] [PubMed] [Google Scholar]
- 67.Segal GS, Chamberlain JM. 2000. Resource utilization and contaminated blood cultures in children at risk for occult bacteremia. Arch Pediatr Adolesc Med 154:469–473. doi: 10.1001/archpedi.154.5.469. [DOI] [PubMed] [Google Scholar]
- 68.Little JR, Murray PR, Traynor PS, Spitznagel E. 1999. A randomized trial of povidone-iodine compared with iodine tincture for venipuncture site disinfection: effects on rates of blood culture contamination. Am J Med 107:119–125. doi: 10.1016/s0002-9343(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 69.Geisler BP, Jilq N, Patton RG, Pietzsch JB. 27 March 2019. A model to evaluate the impact of hospital-based interventions targeting false-positive blood cultures on economic and clinical outcomes. J Hosp Infect. doi: 10.1016/j.jhin.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Self WH, Talbot TR, Paul BR, Collins SP, Ward MJ. 2014. Cost analysis of strategies to reduce blood culture contamination in the emergency department: sterile collection kits and phlebotomy teams. Infect Control Hosp Epidemiol 35:1021–1028. doi: 10.1086/677161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skoglund E, Dempsey C, Chen H, Garey KW. 2019. Estimated clinical and economic impact through use of a novel blood collection device to reduce blood culture contamination in the emergency department: a cost-benefit analysis. J Clin Microbiol 57:e01015-18. doi: 10.1128/JCM.01015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiggers JB, Xiong W, Daneman N. 2016. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 16:286. doi: 10.1186/s12879-016-1622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. 2017. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 65:1776–1779. doi: 10.1093/cid/cix648. [DOI] [PubMed] [Google Scholar]
- 74.Eliakim-Raz N, Bates DW, Leibovici L. 2015. Predicting bacteraemia in validated models—a systematic review. Clin Microbiol Infect 21:295–301. doi: 10.1016/j.cmi.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Woods-Hill CZ, Fackler J, McMillan KN, Ascenzi J, Martinez DA, Toerper MF, Voskertchian A, Colantuoni E, Klaus SA, Levin S, Milstone AM. 2017. Association of a clinical practice guideline with blood culture use in critically ill children. JAMA Pediatr 17:157–164. doi: 10.1001/jamapediatrics.2016.3153. [DOI] [PubMed] [Google Scholar]
- 76.Mimoz O, Karim A, Mercat A, Cosseron M, Falissard B, Parker F, Richard C, Samii K, Nordmann P. 1999. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. A randomized, controlled trial. Ann Intern Med 131:834–837. doi: 10.7326/0003-4819-131-11-199912070-00006. [DOI] [PubMed] [Google Scholar]
- 77.Barenfanger J, Drake C, Lawhorn J, Verhulst SJ. 2004. Comparison of chlorhexidine and tincture of iodine for skin antisepsis in preparation for blood sample collection. J Clin Microbiol 42:2216–2217. doi: 10.1128/JCM.42.5.2216-2217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trautner BW, Clarridge JE, Darouiche RO. 2002. Skin antisepsis kits containing alcohol and chlorhexidine gluconate or tincture of iodine are associated with lower rates of blood culture contamination. Infect Control Hosp Epidemiol 23:397–401. doi: 10.1086/502073. [DOI] [PubMed] [Google Scholar]
- 79.Caldeira D, David C, Sampaio C. 2011. Skin antiseptics in venous puncture-site disinfection for prevention of blood culture contamination: systematic review with meta-analysis. J Hosp Infection 77:223–232. doi: 10.1016/j.jhin.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 80.Maiwald M, Chan ES. 2012. The forgotten role of alcohol: a systematic review and meta analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS One 7:e44277. doi: 10.1371/journal.pone.0044277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez J, Macias JH, Arreguin V, Alvarez JA, Macias AE, Mosqueda-Gomez JL. 2017. Isopropyl alcohol is as efficient as chlorhexidine to prevent contamination of blood cultures. Am J Infect Control 45:350–353. doi: 10.1016/j.ajic.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 82.Tarrand JJ, LaSala PR, Han XY, Rolston KV, Kontoyiannis DP. 2012. Dimethyl sulfoxide enhances effectiveness of skin antiseptics and reduces contamination rates of blood cultures. J Clin Microbiol 50:1552–1557. doi: 10.1128/JCM.05106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emergency Nurses Association. 2016. Clinical practice guideline: prevention of blood culture contamination. https://www.ena.org/docs/default-source/resource-library/practice-resources/cpg/bcccpg2c37f1815b664d2fa8d7e9fd0f475a41.pdf?sfvrsn=6d1899fb_12. [DOI] [PubMed]
- 84.Department of Health. 2007. Saving lives: taking blood cultures-a summary of best practice. https://webarchive.nationalarchives.gov.uk/20120118171812/http://hcai.dh.gov.uk/files/2011/03/Document_Blood_culture_FINAL_100826.pdf.
- 85.Septimus E. 2015. Clinician guide for collecting cultures. https://www.cdc.gov/antibiotic-use/healthcare/implementation/clinicianguide.html.
- 86.Falagas ME, Kazantzi MS, Bliziotis IA. 2008. Comparison of utility of blood cultures from intravascular catheters and peripheral veins: a systematic review and decision analysis. J Med Microbiol 57:1–8. doi: 10.1099/jmm.0.47432-0. [DOI] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention. 2013. The National Healthcare Safety Network (NHSN) manual: patient safety component protocol. Division of Healthcare Quality Promotion, National Center for Infectious Diseases, Atlanta, GA. [Google Scholar]
- 88.Levin PD, Moss J, Stohl S, Fried E, Cohen MJ, Sprung CL, Benenson S. 2013. Use of the non-wire central line hub to reduce blood culture contamination. Chest 143:640–645. doi: 10.1378/chest.12-0863. [DOI] [PubMed] [Google Scholar]
- 89.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society for America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sherertz RJ, Karchmer TB, Palavecino E, Bischoff W. 2011. Blood drawn through valved catheter hub connectors carries a significant risk of contamination. Eur J Clin Microbiol Infect Dis 30:1571–1577. doi: 10.1007/s10096-011-1262-6. [DOI] [PubMed] [Google Scholar]
- 91.Guembe M, Rodríguez-Créixems M, Sánchez-Carrillo C, Pérez-Parra A, Martín-Rabadán P, Bouza E. 2010. How many lumens should be cultured in the conservative diagnosis of catheter-related bloodstream infections? Clin Infect Dis 50:1575–1579. doi: 10.1086/652766. [DOI] [PubMed] [Google Scholar]
- 92.Moeller D. 2017. Eliminating blood culture false positives: harnessing the power of nursing shared governance. J Emerg Nurs 43:126–132. doi: 10.1016/j.jen.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin LM, Inglis GD, Hoellering AB, Davies MW. 2013. Relationship between blood culture collection method and proportion of contaminated cultures in neonates. J Paediatr Child Health 49:105–108. doi: 10.1111/jpc.12088. [DOI] [PubMed] [Google Scholar]
- 94.Pourcyrous M, Korones SB, Bada HS, Patterson T, Baselski V. 1988. Indwelling umbilical arterial catheter: a preferred sampling site for blood culture. Pediatrics 81:821–825. [PubMed] [Google Scholar]
- 95.Hall RT, Domenico HJ, Self WH, Hain PD. 2013. Reducing the blood culture contamination rate in a pediatric emergency department and subsequent cost savings. Pediatrics 31:e292–e297. doi: 10.1542/peds.2012-1030. [DOI] [PubMed] [Google Scholar]
- 96.Mermel LA. 6 March 2019. Drawing blood cultures through intravascular catheters: controversy and update. Infect Control Hosp Epidemiol. doi: 10.1017/ice.2019.37. [DOI] [PubMed] [Google Scholar]
- 97.York MK. 1990. Bacillus species pseudobacteremia traced to contaminated gloves used in collection of blood from patients with acquired immunodeficiency syndrome. J Clin Microbiol 28:2114–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim N-H, Kim M, Lee S, Yun NR, Kim K-H, Park SW, Kim HB, Kim N-J, Kim E-C, Park WB, Oh M-D. 2011. Effect of routine sterile gloving on contamination rates in blood culture: a cluster randomized trial. Ann Intern Med 154:145–151. doi: 10.7326/0003-4819-154-3-201102010-00003. [DOI] [PubMed] [Google Scholar]
- 99.Park WB, Myung SJ, Oh M-D, Lee J, Kim N-J, Kim E-C, Park JS. 2015. Educational intervention as an effective step for reducing blood culture contamination: a prospective cohort study. J Hosp Infect 91:111–116. doi: 10.1016/j.jhin.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 100.Self WH, Mickanin J, Grijalva CG, Grant FH, Henderson MC, Corley G, Blaschke DG II, McNaughton CD, Barrett TW, Talbot TR, Paul BR. 2014. Reducing blood culture contamination in community hospital emergency departments: a multicenter evaluation of a quality improvement intervention. Acad Emerg Med 21:274–282. doi: 10.1111/acem.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bowen CM, Coleman T, Cunningham D. 2016. Reducing blood culture contaminations in the emergency department: it takes a team. J Emerg Nurs 42:306–311. doi: 10.1016/j.jen.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Denno J, Gannon M. 2013. Practical steps to lower blood culture contamination rates in the emergency department. J Emerg Nurs 39:459–464. doi: 10.1016/j.jen.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 103.Thomas S, Cheesbrough J, Plumb S, Bolton L, Wilkinson P, Walmsley J, Diggle P. 2011. Impact of a blood culture collection kit on the quality of blood culture sampling: fear and the law of unintended consequences. J Hosp Infect 78:256–259. doi: 10.1016/j.jhin.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 104.Madeo M, Jackson T, Williams C. 2005. Simple measures to reduce the rate of contamination of blood cultures in accident and emergency. Emerg Med J 22:810–811. doi: 10.1136/emj.2005.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dhillon RH, Clark J, Azadian BS. 2009. Reducing blood culture contamination. J Hosp Infect 73:97–99. doi: 10.1016/j.jhin.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Mermel LA, Maki DG. 1993. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 119:270–272. doi: 10.7326/0003-4819-119-4-199308150-00003. [DOI] [PubMed] [Google Scholar]
- 107.Gonsalves WI, Cornish N, Moore M, Chen A, Varman M. 2009. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol 47:3482–3485. doi: 10.1128/JCM.02107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alfa M, Sanche S, Roman S, Fiola Y, Lenton P, Harding G. 1995. Continuous quality improvement for introduction of automated blood culture instrument. J Clin Microbiol 33:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meessen NEL, Jacobs JA. 1998. Blood volume in BACTEC(PLUS)/F culture bottles sampled using the direct-draw technique. Clin Microbiol Infect 4:471–472. doi: 10.1111/j.1469-0691.1998.tb00399.x. [DOI] [Google Scholar]
- 110.Nair A, Elliott SP, Al Mohajer M. 2017. Knowledge, attitude, and practice of blood culture contamination: a multicenter study. Am J Infect Control 45:547–548. doi: 10.1016/j.ajic.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Rowley S. 2012. Blood culture collection: quality assurance for ANTT. Br J Nurs 21:158. doi: 10.12968/bjon.2012.21.3.158. [DOI] [PubMed] [Google Scholar]
- 112.Weightman NC, Kerr KG. 2012. Blood culture contamination: having your cake and eating it. J Hosp Infect 80:101–102. doi: 10.1016/j.jhin.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 113.Bekeris LG, Tworek JA, Walsh MK, Valenstein PN. 2005. Trends in blood culture contamination: a College of American Pathologists Q-tracks study of 356 institutions. Arch Pathol Lab Med 129:1222–1225. doi: 10.1043/1543-2165(2005)129[1222:TIBCCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 114.Mtunthama N, Gordon SB, Kusimbwe T, Zijlstra EE, Molyneux ME, French N. 2008. Blood culture collection technique and pneumococcal surveillance in Malawi during the four-year period 2003–2006: an observational study. BMC Infect Dis 8:137–138. doi: 10.1186/1471-2334-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larkin S, Baker N, Anderson R, Ward S, Forde S. 2010. An interactive approach to reducing blood culture contamination. J Hosp Infect 76:273–275. doi: 10.1016/j.jhin.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Ramirez P, Gordón M, Cortes C, Villarreal E, Perez-Belles C, Robles C, de Hevia L, Marti JV, Botella J, Bonastre J. 2015. Blood culture contamination rate in an intensive care setting: effectiveness of an education-based intervention. Am J Infect Control 43:844–847. doi: 10.1016/j.ajic.2015.04.183. [DOI] [PubMed] [Google Scholar]
- 117.Hopkins K, Huynh S, McNary C, Walker A, Nixon R, Craighead JE. 2013. Reducing blood culture contamination rates: a systematic approach to improving quality of care. Am J Infect Control 41:1272–1274. doi: 10.1016/j.ajic.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 118.Self WH, Speroff T, Grijalva CG, McNaughton CD, Ashburn J, Liu D, Arbogast PG, Russ S, Storrow AB, Talbot TR. 2013. Reducing blood culture contamination in the emergency department: an interrupted time series quality improvement study. Acad Emerg Med 20:89–97. doi: 10.1111/acem.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pronovost PJ. 2011. Navigating adaptive challenges in quality improvement. BMJ Qual Saf 20:560–563. doi: 10.1136/bmjqs-2011-000026. [DOI] [PubMed] [Google Scholar]
- 120.Zimmerman FS, Assous MV, Yinnon AM, Wiener-Well Y. 2018. Reducing blood culture contamination using a departmental report card. J Hosp Infect 99:236–237. doi: 10.1016/j.jhin.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 121.Youssef D, Shams W, Bailey B, O'Neil TJ, Al-Abbadi MA. 2012. Effective strategy for decreasing blood culture contamination rates: the experience of a Veterans Affairs medical center. J Hosp Infect 81:288–291. doi: 10.1016/j.jhin.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 122.Harding AD, Bollinger S. 2013. Reducing blood culture contamination rates in the emergency department. J Emerg Nurs 39:e1–e6. doi: 10.1016/j.jen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 123.Gibb AP, Hill B, Chorel B, Brant R. 1997. Reduction in blood culture contamination rate by feedback to phlebotomists. Arch Pathol Lab Med 121:503–507. [PubMed] [Google Scholar]
- 124.Lin C-M, Lee W-S, Lin F-Y, Yu F-L, Ou T-Y, Teng S-O. 2012. Reducing blood culture contamination rates by educational intervention and one-on-one feedback in the emergency department. J Exp Clin Med 4:154–156. doi: 10.1016/j.jecm.2012.04.005. [DOI] [Google Scholar]
- 125.Gibson T, Norris W. 1958. Skin fragments removed by injection needles. Lancet ii:983–985. doi: 10.1016/s0140-6736(58)90475-6. [DOI] [PubMed] [Google Scholar]
- 126.Buchta C, Nedorost N, Regele H, Egerbacher M, Kormoczi G, Hocker P, Dettke M. 2005. Skin plugs in phlebotomy puncture for blood donation. Wien Klin Wochenschr 117:141–144. doi: 10.1007/s00508-005-0310-6. [DOI] [PubMed] [Google Scholar]
- 127.Patton RG, Schmitt T. 2010. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol 48:4501–4503. doi: 10.1128/JCM.00910-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Binkhamis K, Forward K. 2014. Effect of the initial specimen diversion technique on blood culture contamination rates. J Clin Microbiol 52:980–981. doi: 10.1128/JCM.02773-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lalezari A, Cohen NJ, Svinik O, Tel-Zur O, Sinvani S, Block C, Moses AE, Oster Y, Strahilevitz J. A simplified blood culture sampling protocol for reducing contamination and costs: a randomized controlled trial. Clin Microbiol Infect, in press. [DOI] [PubMed] [Google Scholar]
- 130.Bell M, Bogar C, Plante J, Rasmussen K, Winters S. 2018. Effectiveness of a novel specimen collection system in reducing blood culture contamination rates. J Emerg Nurs 44:570–575. doi: 10.1016/j.jen.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 131.Zimmereman FS, Assous MV, Zevin S, Wiener-Well Y. 10 January 2019. Reducing blood culture contamination using an initial specimen diversion device. Am J Infect Control. doi: 10.1016/j.ajic.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 132.O’Sullivan DM, Steer l. 2019. Reducing false-positive blood cultures: using a blood diversion device. Conn Med 83:54–55. [Google Scholar]
- 133.Diekema DJ. 2017. Rising stakes for healthcare associated infection prevention: implications for the clinical microbiology laboratory. J Clin Microbiol 55:996–1001. doi: 10.1128/JCM.02544-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Centers for Disease Control and Prevention. 2019. Surveillance for bloodstream infections. https://www.cdc.gov/nhsn/acute-care-hospital/clabsi/index.html.
- 135.Centers for Disease Control and Prevention. 2019. National Healthcare Safety Network Master Organism List. https://www.cdc.gov/nhsn/xls/master-organism-com-commensals-lists.xlsx.
- 136.Centers for Disease Control and Prevention. 2019. Bloodstream infection event definitions. https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.