Abstract
Background
Approximately 10% of patients with small cell lung cancer (SCLC) develop a paraneoplastic syndrome (PNS). Neurologic PNS are thought to improve prognosis, which we hypothesized is related to increased tumor infiltrating lymphocytes and immune recognition.
Methods
We queried 2,512,042 medical records from a single institution to identify SCLC patients with and without PNS and performed manual, retrospective chart review. We then performed multiplexed fluorescence immunohistochemistry and automated quantitative analysis (AQUA® Technology) on tumors to assess CD3, CD4, and CD8 T cell infiltrates and PD-1/PD-L1 interactions. T cell infiltrates and PD-1/PD-L1 interaction scores were compared among patients with neurologic PNS, endocrinologic PNS, and a control group without PNS. Clinical outcomes were analyzed using the Kaplan-Meier method and Cox proportional-hazards models.
Results
We evaluated 145 SCLC patients: 55 with PNS (25 neurologic and 30 endocrinologic) and 90 controls. Patients with neurologic PNS experienced improved overall survival (OS) compared to patients with endocrinologic PNS and controls (median OS 24mo vs. 12mo vs. 13mo, respectively). Of the 145 patients, we identified tumor tissue from 34 patients that was adequate for AQUA analysis. Among 37 specimens from these 34 patients, patients with neurologic PNS had increased T cell infiltrates (p=0.033) and PD-1/PD-L1 interaction (p=0.014) compared to tumors from patients with endocrinologic PNS or controls.
Conclusion
Tumor tissue from patients with SCLC with neurologic PNS demonstrated increased tumor infiltrating lymphocytes and PD-1/PD-L1 interaction consistent with an inflamed tumor microenvironment.
Keywords: Small cell lung cancer, paraneoplastic syndromes, tumor infiltrating lymphocytes, immunotherapy, tumor microenvironment
INTRODUCTION
Small cell lung cancer (SCLC) accounts for 15% of lung cancers and 30,000 deaths in the United States annually1. The median overall survival (OS) for SCLC patients is approximately 8–12 months for patients with extensive stage (ES-SCLC) disease and 12–20 months for patients with limited stage disease (LS-SCLC)1. The combination of elusive pathophysiology, poor prognosis, and minimal therapeutic improvement for several decades has led the National Cancer Institute to designate SCLC as a recalcitrant cancer2.
Among patients with SCLC, approximately 10% develop a paraneoplastic syndrome (PNS), such as Lambert-Eaton Myasthenic Syndrome (LEMS), syndrome of inappropriate antidiuretic hormone (SIADH), or Cushing’s Syndrome3–5. LEMS is a neurologic PNS, which is thought to be an immune-mediated phenomenon6. In contrast, endocrinologic PNS, such as SIADH and Cushing’s syndrome, reflect ectopic tumor hormonal secretion6,7.
Prognostic differences have been observed in small case series among patients with SCLC with endocrinologic PNS, neurologic PNS, or no PNS. Patients with SCLC with endocrinologic PNS, especially Cushing’s Syndrome, have demonstrated a worse prognosis than patients with SCLC with no PNS8. Similarly, patients with SCLC with neurologic PNS have improved OS compared to patients with SCLC with no PNS9–11. We sought to extend these observations in one of the largest series reported in patients with SCLC with neurologic PNS to date.
Furthermore, we evaluated tumor immune microenvironmental factors that contribute to unique outcomes in patients with SCLC with neurologic PNS by investigating both lymphocytic tumor infiltrates and PD-1/PD-L1 interactions. Blocking negative regulatory immune checkpoints, such as PD-1 and PD-L1, has revolutionized cancer care in many tumor subtypes12, and these treatments have shown promise in patients with SCLC13–15. However, in unselected populations of patients with SCLC, less than 20% have >1% tumor PD-L1 positivity, and better tumor immune recognition biomarkers are needed in this population16. We hypothesized that in tumors from patients with SCLC with neurologic PNS, a surrogate for adequate tumor immune recognition, tumor infiltrating lymphocytes are increased and PD-1 and PD-L1 are upregulated. To our knowledge, this is the first study to evaluate tumor PD-L1 staining in patients with SCLC with neurologic PNS, and it is the first to directly compare tumor tissue from patients with SCLC with neurologic PNS to tumor tissue from patients with SCLC with endocrinologic PNS.
METHODS
Study design and patients
An automated search algorithm of 2,512,042 patients in the Vanderbilt University Medical Center (VUMC) electronic medical record (EMR) was performed to identify cases with SCLC with neurologic or endocrinologic PNS, as well as control patients with SCLC without a PNS. There are no dedicated billing codes for SCLC or PNS. As a result, our search algorithm utilized free text search of all clinical notes in the EMR to flag potential patients, cross-referenced with cancer registry-designated SCLC cases.
Patient cohort validation
Through manual chart review we validated 145 patients with SCLC over a 16-year time span, from September 1998 to May 2014 under IRB approved protocol #141343. Case validation was based on the following definitions. The diagnosis SIADH was defined by serum sodium of less than 135 refractory to intravenous fluid administration with urine osmolarity greater than 100 and congruent clinical documentation noting SIADH. The diagnosis of Cushing’s syndrome was defined by an elevated adrenocorticotropic hormone (ACTH) with hypokalemia and congruent clinical documentation noting Cushing’s syndrome. The laboratory diagnosis of a neurologic PNS involved serologic, and rarely cerebrospinal fluid testing, for the presence of anti-neuronal nuclear antibodies type 1 (also known as anti-Hu antibody), 2, and 3, purkinje cell cytoplasmic antibodies types 1, 2, and Tr, striational antibody, N-type calcium channel binding antibody, P/Q type calcium channel antibody, voltage gated calcium channel antibody, voltage gated potassium channel antibody, CRMP-5 IgG antibody, acetylcholine receptor (muscle) antibody, acetylcholine receptor (ganglionic) neuronal antibody, and amphiphysin antibody. If patients had one positive serology for a paraneoplastic autoantibody in conjunction with a neurology consultation diagnosis or, in five cases, a neurologic consultation without a positive serology they were classified as having a neurologic PNS. Seven patients had both a neurologic PNS and SIADH, all seven had serum paraneoplastic autoantibodies assessed and 6 of 7 were positive; these 7 patients were included only in the neurologic PNS group as our primary hypothesis centered on the presence or absence of unique tumor immune recognition in the setting of any neurologic PNS. Any patients meeting the aforementioned criteria for a neurologic or endocrinologic PNS with histologic confirmation of SCLC were included. Control patients were defined as those patients with a histologically confirmed diagnosis of SCLC, no criteria for an endocrinologic nor neurologic PNS, and clinical annotation recording treatment modality, disease progression, and date of death. Patients were only excluded if they lacked sufficient histologic documentation of SCLC, diagnosis of a PNS as defined above, or, in control patients, sufficient clinical annotation as described above.
Multiplexed fluorescence immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) samples were obtained from the VUMC pathology archives for patients with a neurologic PNS, endocrinologic PNS, or no PNS under an IRB approved protocol (IRB# 160769). We performed multiplexed fluorescence immunohistochemistry (FIHC) combined with automated quantitative analysis (AQUA® Technology; Navigate BioPharma Services, Inc.) to assess CD3, CD4, CD8, PD-1, and PD-L1 expression as previously described17. Two slides were used, one to assess CD3, CD4, and CD8, and one to assess PD-1 and PD-L1. Staining for CD3, CD4, and CD8 was excluded for cytology specimens. The following primary antibodies were used: rabbit anti-CD3 (EP41, dilution 1:200, Biocare Medical), mouse anti-CD4 (4B12, dilution 1:50, DAKO), mouse anti-CD8 (C8/144B, 1:400, DAKO), 0.5 μg/mL mouse anti-PD1 (NAT105, Biocare), 3.6 μg/mL rabbit anti-PD-L1 (E1L3N, Cell Signaling Technology), mouse anti-TTF1 (8G7G31, 1:500, DAKO). The following secondary antibodies were used: anti-mouse Envision HRP (DAKO) and anti-rabbit Envision HRP (DAKO), plus 40,6-diamidino-2-phenylindole (DAPI). The following reagents were used to detect secondary antibodies: TSA-Cy3.5 (Perkin Elmer), TSA-Cy5 (Perkin Elmer), Opal™520 (Perkin Elmer), and TSA-Cy3 (Perkin Elmer).
FIHC image analysis
Forty high-power (20x) fluorescence images for each sample (one slide per sample/per test as described above) were acquired with the Vectra 2 Intelligent Slide Analysis System using Vectra software version 2.0.8 (Perkin Elmer) and quantified with AQUAnalysis™ software as previously described17. DAPI was used to identify all cells, and TTF1 expression, present in 70–90% of SCLC tumors18, was used to identify tumor cells. Each pixel in the image was identified as either positive or negative for the signal of interest to create a binary mask for each cell type. Similarly, the CD3, CD4, CD8, PD-1, and PD-L1, expression co-localized with DAPI positive or TTF1 positive cells was used to create binary masks of all cells expressing these biomarkers of interest, respectively. Percent positivity was calculated from the total area, measured in pixels, for the cell type of interest divided by the total area, measured in pixels, of the corresponding cells of interest (all cells, tumor cells, or T cells (CD3 positive)). Tumor scoring of T cell infiltrates and PD-1/PD-L1 was completed by pathologists blinded to patient clinical characteristics. T cell subtypes (CD4 or CD8 positive) were defined by double-positivity for CD3/CD4 or CD3/CD8. The PD-1/PD-L1 interaction score was calculated by measuring the total area of PD-1 positive cells within the proximity of PD-L1 positive cells. This area was then divided by the total area of all non-tumor nucleated cells in the image and multiplied by a factor of 10,000. The interaction score provides a numerical reflection of the overall proportion of PD-1 positive cells within an approximately one-cell distance to PD-L1 positive cells. The interaction score algorithm was designed based on the hypothesis that co-existence of two markers will be a better measure of immunosuppression than either PD1 or PD-L1 alone. This novel algorithm was demonstrated to predict response to anti-PD-L1 therapy in metastatic melanoma19 akin to the PD1/L1 proximity score reported by an independent study20. Similarly, the utility of interaction score to predict resistance to CAR-T therapy in a prospective DLBCL study21 and response to chemotherapy in a randomized NSCLC cohort have been reported22. Maximum PD-L1 score was defined as the PD-L1 score for the field of view with the highest percentage of tumor cells expressing PD-L1 out of all the 20x fields of view measured per sample.
Statistical analysis
Patient demographic and clinical characteristics were summarized using median with interquartile range (IQR) for continuous variables or frequency with percentages for categorical variables. The standardized CD3, CD4, CD8 cell counts (out of all cells) and PD-1/PD-L1 interaction scores were compared using a Kruskal-Wallis test among endocrinologic-, neurologic- and non- PNS groups and Mann-Whitney tests for pairwise comparisons. Progression free survival (PFS) was defined as radiographic disease growth on surveillance computed tomography or death and OS were graphically represented using the Kaplan-Meier method and compared using a Log-rank test. Multivariable Cox proportional-hazards models were used to assess the associations of PNS type on survival outcomes while adjusting for age and disease stage. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. Statistical significance was considered with a two-sided alpha < 0.05. Statistical analyses were performed using R (version 3.4.3).
RESULTS
Patient demographics
A total of 145 SCLC patients were identified, 55 with a PNS (25 neurologic and 30 endocrinologic) and 90 control patients without a PNS. The date range of SCLC diagnosis was similar among the groups, with each containing patients diagnosed from 1998 to 2014. The demographic and treatment information for the three patient cohorts is listed in Table 1. A greater proportion of patients with neurologic PNS (56%) had LS-SCLC compared to patients with endocrinologic PNS (37%), but this difference was not statistically significant (p=0.152). Patients were similar in age at diagnosis and extent of smoking history, though there was a notably higher proportion of patients in the neurologic PNS group (24%) that received no systemic therapy for SCLC compared to the endocrinologic PNS (7%) and control (5%) patient cohorts (p=0.007, Supplemental Table 1).
Table 1.
Baseline demographics of small cell lung cancer patients with neurologic paraneoplastic syndromes (PNS), endocrinologic PNS, and control patients with no PNS.
| Neurologic PNS (n=25) | Endocrine PNS (n=30) | Control (n=90) | |
|---|---|---|---|
| Age at diagnosis (years) | 64 (57–67) | 64 (56–73) | 61 (54–69) |
| Male gender | 9 (36%) | 11 (37%) | 47 (52%) |
| Female gender | 16 (64%) | 19 (63%) | 43 (48%) |
| Race | |||
| Caucasian | 25 (100%) | 30 (100%) | 73 (81%) |
| African American | 0 (0%) | 0 (0%) | 10 (11%) |
| Asian | 0 (0%) | 0 (0%) | 1 (1%) |
| Unspecified | 0 (0%) | 0 (0%) | 6 (7%) |
| Smoking pack-years | 55 (48–78) | 50 (40–68) | 45 (30–70) |
| Stage at diagnosis | |||
| Limited stage (LS-SCLC) | 14 (56%) | 11 (37%) | 40 (44%) |
| Extensive stage (ES-SCLC) | 11 (44%) | 19 (63%) | 50 (56%) |
| Sites of metastasis | |||
| Hepatic | 3 (12%) | 11(37%) | 30 (33%) |
| Brain parenchymal | 3 (12%) | 1 (3%) | 12 (13%) |
| Osseous | 2 (8%) | 12 (40%) | 16 (18%) |
| Pleural | 6 (24%) | 9 (30%) | 16 (18%) |
| Adrenal | 2 (8%) | 6 (20%) | 10 (11%) |
| Mechanism of diagnosis of PNS | |||
| Lab with serum paraneoplastic autoantibody | 20 (80%) | 0 (0%) | 0 (0%) |
| Lab without paraneoplastic autoantibody | 0 (0%) | 30 (100%) | 0 (0%) |
| Clinical (neurology consultation) | 5 (20%) | 0 (0%) | 0 (0%) |
| First line cytotoxic chemotherapy | |||
| Platinum-based with etoposide | 18 (72%) | 22 (73%) | 83 (92%) |
| Platinum-based with irinotecan | 1 (4%) | 2 (7%) | 3 (3%) |
| Other | 0 (0%) | 4 (13%) | 0 (0%) |
| No systemic therapy | 6 (24%) | 2 (7%) | 4 (5%) |
| Prophylactic cranial irradiation | 14 (56%) | 15 (50%) | 49 (54%) |
| Concurrent chemoradiation (% of limited stage patients) | 10 (72%) | 8 (73%) | 34 (85%) |
Parenthetical ranges indicate the lower and upper quartiles for patient age and smoking history, and percent of total for each grouping for the remaining variables.
Description of Paraneoplastic Syndromes
Serological evaluation of SCLC patients who presented with a neurological disorder was completed using a clinical autoantibody panel. Among the 25 patients with a neurologic PNS, 20 (80%) had a detectable serum paraneoplastic autoantibody. Autoantibody tests from 3 patients were negative, but all 3 patients were clinically diagnosed with a neurologic PNS through consultation with the Neurology service. Two patients were not tested for serum paraneoplastic autoantibodies but were clinically documented as having a neurologic PNS upon consultation with a Neurologist, one case of autonomic neuropathy and the other with pseudoachalasia (Supplemental Table 2). The most commonly identified serum paraneoplastic autoantibodies were P/Q-type calcium channel antibodies (7 patients) and anti-Hu antibodies (6 patients). The most common clinical manifestations in patients with P/Q-type calcium channel antibodies and anti-Hu antibodies were LEMS (4 patients) and limbic encephalitis (3 patients), respectively. Patients with endocrinologic PNS manifested either SIADH (25 patients) or Cushing’s syndrome (5 patients) (Supplemental Table 3).
Notably, the manifestation of a PNS predated the diagnosis of SCLC in 9 patients (36%) with neurologic and 3 patients (10%) with endocrinologic PNS. The longest lag time between PNS development and identification of the underlying SCLC was 12 months in a patient with a neurologic PNS and 6 months in a patient with an endocrinologic PNS. The majority of patients with PNS that manifested before SCLC were diagnosed with SCLC within one month.
Clinical Outcomes
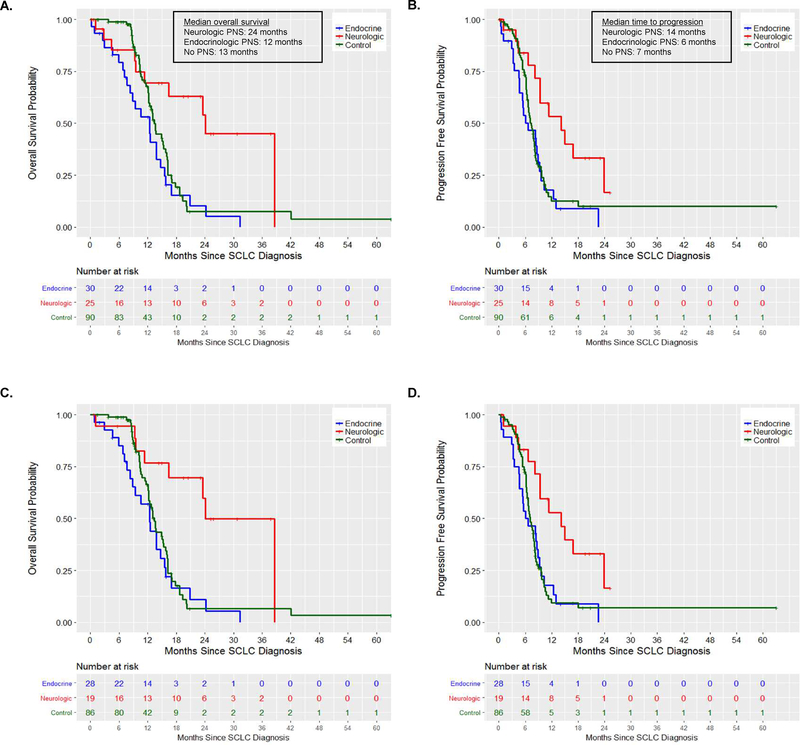
Most patients in all three groups were treated with first line platinum plus etoposide (Table 1), and no patients were treated with surgical resection. Six patients in the neurologic PNS cohort did not receive systemic therapy for SCLC: four were lost to follow-up, one died nearly 3 months after SCLC diagnosis, and one died 4.5 months after SCLC diagnosis (Supplemental Table 1). Including patients who did not receive systemic therapy for SCLC, patients with neurologic PNS experienced a doubling of their OS compared to patients with endocrinologic PNS and controls (median OS 24mo vs. 12mo vs. 13mo, respectively) (Figure 1A, Table 2). When evaluating PFS from the date of diagnosis, patients with neurologic PNS experienced a more than two-fold improvement in disease control compared to patients with endocrinologic PNS and controls (median PFS 14mo vs 6mo vs 7mo, respectively, Figure 1B, Table 2). When limited to only patients who received systemic therapy for their SCLC, the differences were further accentuated (OS: HR=0.24, 95% CI 0.10–0.55, p=0.007; PFS: HR=0.41, 95% CI 0.20–0.85, p=0.015; Figure 1C–D). Finally, when controlling for age and stage between patients with SCLC with neurologic PNS and SCLC with endocrinologic PNS, these findings remained statistically significant for both OS (HR=0.23, 95% CI 0.10–0.54, p=0.006 Supplemental Figure 1A–B) and PFS (HR=0.43, 95% CI 0.21–0.87, p=0.019, Supplemental Figure 1C–D). There was no statistically significant difference in OS or PFS when patients with endocrinologic PNS or no PNS were compared.
Figure 1. Progression free survival and overall survival according to the presence and type of paraneoplastic syndrome in patients with small cell lung cancer.
Kaplan-Meier estimates of (A) overall survival and (B) progression free survival in patients with small cell lung cancer according to the absence or presence of a paraneoplastic syndromes. Kaplan-Meier estimates of overall survival (C) and (D) progression free survival limited to only patients who received systemic therapy for small cell lung cancer.
Table 2.
Comparison of overall survival and progression free survival from diagnosis in patients with small cell lung cancer (SCLC) and neurologic paraneoplastic syndromes (PNS), SCLC and endocrinologic PNS, or SCLC and no PNS.
| SCLC and neurologic PNS | SCLC and endocrinologic PNS | SCLC and no PNS | |
|---|---|---|---|
| Overall Survival | 24 months, (16.4 - not reached) | 12 months, (8.3 – 15.5) | 13 months, (12.2 – 16.0) |
| Progression Free Survival | 14 months, (9.3 - not reached) | 6 months, (4.6 – 9.5) | 7 months, (6.6 – 8.1) |
Data displayed indicates median and 95% confidence interval in months.
Tumor Tissue CD3/CD4/CD8 and PD-1/PD-L1 Staining
The clinical data described above demonstrate that the presence of a neurologic PNS more than doubled median OS and PFS in our cohort. As improved tumor recognition by the host immune system is an increasingly appreciated mechanism of cancer control, we hypothesized that improved clinical outcomes in patients with SCLC with neurologic PNS are related to increased tumor infiltrating lymphocytes and PD-1/PD-L1 interactions in tumors from SCLC patients with neurologic PNS. We tested this hypothesis through examination of FFPE SCLC tumor samples using automated quantitative analysis (AQUA® technology) to assess infiltrating CD3, CD4, and CD8 positive lymphocytes as well as PD-1/PD-L1 interactions. T cell markers and PD-1/PD-L1 interaction scores were compared among patients with neurologic PNS, endocrinologic PNS, and a control group without PNS.
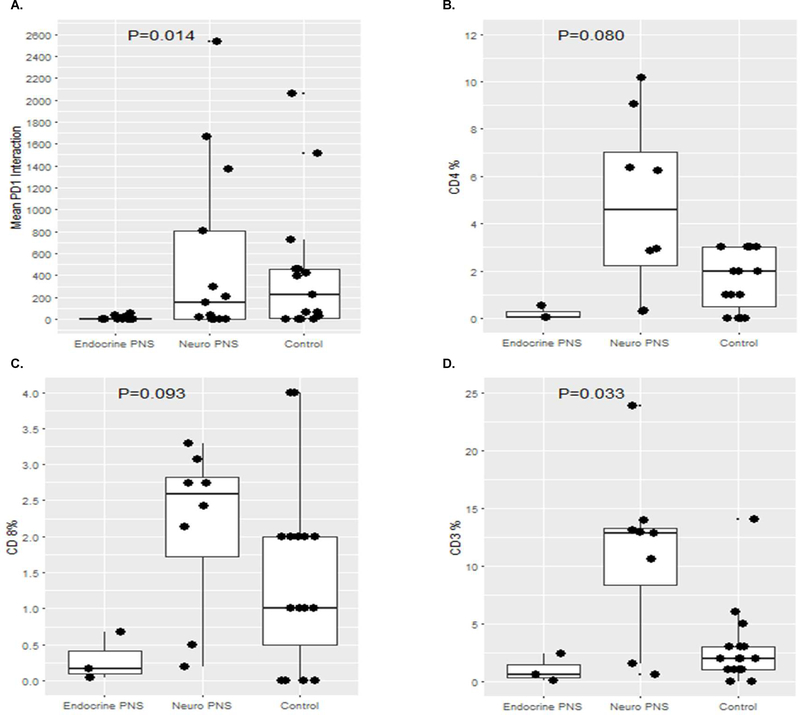
We evaluated 105 potential tumor specimens from the 145 SCLC patients. Of these potential specimens, 37 specimens from 34 unique patients were adequate for AQUA analysis (Table 3). No surgically resected tumors were available for analysis, and most tumor specimens were obtained before chemotherapy administration (n=31, 84%). Of the 37 specimens, 11 (30%) were from nodal metastases, 19 (51%) were from the primary tumor, and 7 (19%) were from other metastatic sites (3 bone, 2 liver, 2 pleural). Details for all 37 specimens with accompanying patient-specific clinical descriptions, CD3, CD4, CD8, and PD-1/PD-L1 staining details are listed in Table 3. The maximum tumor PD-L1 positivity observed among 11 specimens from patients with SCLC with endocrinologic PNS was 8%, compared to a maximum tumor PD-L1 expression of 79% among 10 specimens from patients with SCLC with neurologic PNS. Furthermore, among the 10 specimens from SCLC patients with neurologic PNS, only 2 (20%) exhibited tumor PD-L1 of 0%, compared to 4 of 16 (25%) specimens from SCLC patients with no PNS and 6 of 11 (55%) specimens from SCLC patients with endocrinologic PNS. For the aggregate analysis, one specimen (sample ID #21) from a patient with SCLC with a neurologic PNS was excluded due to low TTF-1 staining and questionable tumor content. As a group, tumors from patients with SCLC with neurologic PNS (n=9) had an elevated PD-1/PD-L1 interaction score (p=0.014, Figure 2A) compared to tumors from patients with SCLC with endocrinologic PNS (n=11) or no PNS (n=16). In a pairwise comparison between the neurologic PNS and control group there was no statistically significant difference in PD-1/PD-L1 interaction score (p=0.963), however there was a statistically significant enrichment in PD-1/PD-L1 interaction in control samples compared to the endocrinologic PNS group (p=0.007). When comparing PD-1/PD-L1 interaction scores between the neurologic PNS and endocrinologic PNS, there was a significant increase in the neurologic PNS group (p=0.016). Limiting to only core biopsy specimens due to improved staining reproducibility, tumors from patients with SCLC with neurologic PNS (n=8) had a trend toward increased CD4 and CD8 positive T cell infiltrates (p=0.08 and p=0.09, respectively, Figures 2B–C), and a statistically significant increase in CD3 positive T cell infiltrates compared to tumors from patients with SCLC with endocrinologic PNS (n=3) or no PNS (n=17, p=0.033, Figure 2D). In a pairwise comparison, core biopsy samples from patients with neurologic PNS exhibited a statistically significant increase in CD3 positive T cells compared to controls (p=0.034) but not in CD4 or CD8 positive T cells (p=0.147 and p=0.184, respectively). There were no statistically significant differences in T cell infiltrates between core biopsy samples from patients with endocrinologic PNS and controls, but there were significant increases in both CD3 and CD4 positive T cell infiltrates comparing the neurologic PNS and endocrinologic PNS groups (p=0.048 and p=0.048, respectively) and a marginally significant difference in CD8 positive T cell infiltrate (p=0.052). Only 1 of 3 (33%) core biopsy specimens from patients with SCLC with endocrinologic PNS had >1% CD3+ T cell infiltration, compared to 7 of 8 (88%) core biopsy specimens from patients with SCLC with neurologic PNS and 10 of 16 (63%) core biopsy specimens from patients with SCLC with no PNS. Images from all PNS specimens evaluated that are not described in individual patient cases above are shown in Supplemental Figures 2–21.
Table 3.
Pathologic and clinical descriptions of 37 tumor samples assessed for PD-1/PD-L1 interaction scores (n=36) and CD4/CD8 T cell infiltrates (n=29).
| Sample ID | PNS Type | Specimen Type | Anatomic site | Treatment naïve specimen? |
PFS (months) | OS (months) | Maximum Tumor PD-L1 (%) | PD-1/PD-L1 interaction score |
% CD3 + | % CD4 + | % CD8 + |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Endocrinologic | Cytology | Primary tumor | Yes | 5 | Unknown | 0% | 0 | NA | NA | NA |
| 2 | Endocrinologic | Cytology | Primary tumor | Yes | 3 | 12 | 0% | 0 | NA | NA | NA |
| 3 | Endocrinologic | Cytology | Pleural fluid | Yes | 4 | 5 | 1% | 21 | NA | NA | NA |
| 4 | Endocrinologic | Cytology | Primary tumor | Yes | 1 | 8 | 0% | 0 | NA | NA | NA |
| 5 | Endocrinologic | Cytology | Primary tumor | Yes | 22 | 24a | 1% | 0 | NA | NA | NA |
| 6 | Endocrinologic | Cytology | Mediastinal LN | Yes | 1 | 9b | 2% | 52 | NA | NA | NA |
| 7 | Endocrinologic | Cytology | Mediastinal LN | Yes | 1 | 9b | 8% | 36 | NA | NA | NA |
| 8 | Endocrinologic | Cytology | Mediastinal LN | Yes | 1 | 9b | 1% | 1 | NA | NA | NA |
| 9 | Endocrinologic | Core Bx | Primary tumor | Yes | 5 | Unknown | 0% | 0 | 2.4 | 0.55 | 0.67 |
| 10 | Endocrinologic | Core Bx | Primary tumor | No | 22 | 24a | 0% | 0 | 0.62 | 0.03 | 0.16 |
| 11 | Endocrinologicc | Core Bx | Primary tumor | No | 9 | 14 | 0% | 0 | 0.12 | 0.05 | 0.04 |
| 12 | Neurologic | Core Bx | Mediastinal LN | Yes | Unknown | Unknown | 51% | 2535 | 13.9 | 2.85 | 2.43 |
| 13 | Neurologic | Core Bx | Supraclavicular LN | Yes | Unknown | Unknown | 23% | 1665 | 10.6 | 6.22 | 2.74 |
| 14 | Neurologic | Core Bx | Cervical LN | Yes | 2 | 3 | 16% | 16 | 0.64 | 0.31 | 0.19 |
| 15 | Neurologic | Cytology | Mediastinal LN | Yes | 4 | 5 | 0% | 0 | NA | NA | NA |
| 16 | Neurologic | Cytology | Mediastinal LN | Yes | >24 | >24 | 33% | 150 | NA | NA | NA |
| 17 | Neurologic | Core Bx | Mediastinal LN | Yes | 12 | >16 | 79% | 810 | 13.1 | 9.00 | 3.10 |
| 18 | Neurologic | Core Bx | Primary tumor | Yes | 12 | 16 | 6% | 293 | 12.8 | 2.92 | 2.74 |
| 19 | Neurologic | Core Bx | Mediastinal LN | Yes | 23 | 38 | 72% | 1367 | 23.8 | 10.10 | 2.10 |
| 20 | Neurologic | Core Bx | Primary tumor | Yes | 4 | 9 | 0% | 33 | 1.58 | 0.32 | 0.49 |
| 21d | Neurologic | Core Bx | Mediastinal LN | Yes | 18 | 24 | 34% | 506 | 12.9 | 6.40 | 3.30 |
| 22 | None | Core Bx | Primary tumor | Yes | 1.5 | 9 | 0% | 0 | 2.0 | 0 | 2.00 |
| 23 | None | Core Bx | Primary tumor | Yes | 9 | 10 | 0% | 0 | 2.0 | 3.00 | 4.00 |
| 24 | None | Core Bx | Primary tumor | Yes | 4 | 12 | 52% | 2062 | 14.0 | 20.00 | 6.00 |
| 25 | None | Core Bx | Primary tumor | Yes | 6 | 18 | 0% | 454 | 6.0 | 3.00 | 4.00 |
| 26 | None | Core Bx | Primary tumor | Yes | 10 | 20 | NA | NA | 1.0 | 0 | 0 |
| 27 | None | Core Bx | Primary tumor | Yes | 6 | 16 | 95% | 0 | 2.0 | 1.00 | 2.00 |
| 28 | None | Core Bx | Bone | No | 6 | 16 | 78% | 59 | 3.0 | 3.00 | 2.00 |
| 29 | None | Core Bx | Liver | No | 8 | 12 | 3% | 64 | 1.0 | 1.00 | 1.00 |
| 30 | None | Core Bx | Pleura | Yes | 3 | 14 | 9% | 423 | 5.0 | 3.00 | 2.00 |
| 31 | None | Core Bx | Bone | No | 11 | 17 | 1% | 0 | 0 | 0 | 0 |
| 32 | None | Core Bx | Primary tumor | Yes | 10 | 13 | 65% | 726 | 3.0 | 3.00 | 2.00 |
| 33 | None | Core Bx | Liver | Yes | 8 | 16 | 0% | 220 | 1.0 | 1.00 | 0 |
| 34 | None | Core Bx | Bone | No | 9 | 12 | 21% | 1510 | 1.0 | 2.00 | 1.00 |
| 35 | None | Core Bx | Primary tumor | Yes | 8 | 17 | 11% | 391 | 0 | 0 | 0 |
| 36 | None | Core Bx | Primary tumor | Yes | 9 | 14 | 3% | 457 | 3.0 | 2.00 | 1.00 |
| 37 | None | Core Bx | Primary tumor | Yes | 8 | 10 | 1% | 27 | 2.0 | 2.00 | 1.00 |
same patient
same patient
patient was treated with linsitinib at the time of diagnosis of endocrinologic paraneoplastic syndrome
dexcluded from cumulative analysis due to low TTF-1 staining.
Unknown: lost to follow-up; NA: not assessed; C: cytology; SP: surgical pathology; LN: lymph node; PFS: progression free survival; OS: overall survival.
Figure 2. Overall comparison of CD3, CD4, CD8, and PD-1/PD-L1 interaction scores.
Tumors from patients with small cell lung cancer with neurologic paraneoplastic syndromes had significantly increased PD-1/PD-L1 interaction scores (A) compared to tumors from patients with small cell lung cancer with endocrinologic paraneoplastic syndromes and tumors from patients with small cell lung cancer and no paraneoplastic syndrome (“control”). Tumors from patients with small cell lung cancer with neurologic paraneoplastic syndromes had a trend towards increased CD4 (B) and CD8 (C) infiltrates compared to tumors from patients with small cell lung cancer with endocrinologic paraneoplastic syndromes and tumors from patients with small cell lung cancer and no paraneoplastic syndrome (“control”). Tumors from patients with small cell lung cancer with neurologic paraneoplastic syndromes had significantly increased CD3 (D) infiltrates compared to tumors from patients with small cell lung cancer with endocrinologic paraneoplastic syndromes and tumors from patients with small cell lung cancer and no paraneoplastic syndrome (“control”).
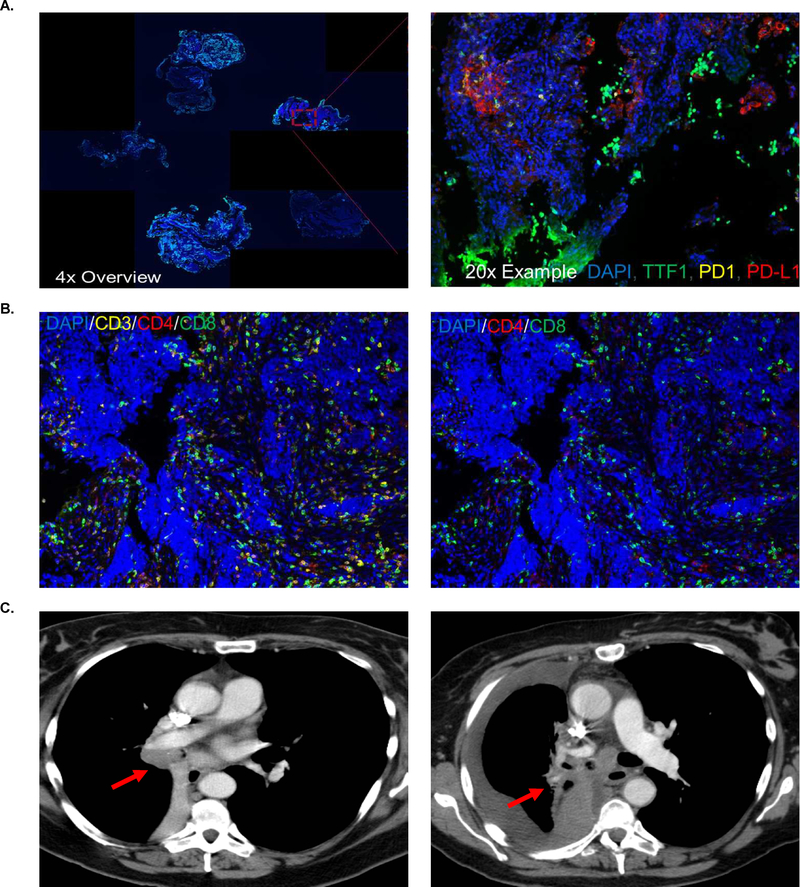
As an illustrative case, a 55 year-old woman (Table 3, sample ID #18) was diagnosed with LS-SCLC 6 months before a neurologic PNS (LEMS) became clinically apparent. Serum studies at the time of onset of significant lower extremity weakness revealed the presence of P/Q type calcium channel antibodies at 207 picomoles per liter. She was treated with concurrent chemoradiation (four cycles of cisplatin plus etoposide) with a partial response. She did not receive prophylactic cranial irradiation due to the occurrence of her neurologic PNS and management of her LEMS. She then had thoracic and hepatic progression 12 months after diagnosis of her SCLC and 6 months after onset of her neurologic PNS. She was treated with weekly topotecan, but had disease progression after 2 cycles and a declining performance status that did not permit further systemic therapy. She died 16 months after diagnosis. This patient’s pre-treatment primary tumor demonstrated 6% maximum PD-L1 positivity, and the PD-1/PD-L1 interaction score was 293 (Figure 3A). Of all cells in the diagnostic biopsy specimen, 12.8% were CD3 positive T cells, 2.9% were CD4 positive T helper cells, and 2.7% were CD8 positive cytotoxic T cells (Figure 3B). The patient’s right sided paratracheal tumor is denoted at diagnosis and at the time of second progression on topotecan (Figure 3C). The remaining tumor specimens from patients with neurologic PNS are shown in Supplemental Figures 1–9.
Figure 3. Immunologic correlates by automated quantitative analysis with accompanying computed tomography.
(A) 4x and 20x, DAPI in blue, TTF-1 in green, PD-1 in yellow, and PD-L1 in red. (B) 20x, DAPI in blue, CD3 in yellow, CD4 in red, and CD8 in green, first image with CD3 present, second image with CD3 removed. (C) Computed tomography demonstrates the primary tumor at diagnosis (left panel) followed by progression of disease (right panel)(red arrow indicates primary tumor in both panels).
DISCUSSION
To our knowledge, this is the most comprehensive report to interrogate both clinical outcomes and tumor immune microenvironment in patients with SCLC and paraneoplastic syndromes. Our study extends prior retrospective data that patients with SCLC with neurologic PNS have improved OS compared to patients with SCLC with endocrinologic PNS or SCLC and no PNS, despite nearly 25% of our patients with SCLC with neurologic PNS receiving no systemic therapy. Furthermore, our results demonstrate that increased tumor infiltrating lymphocytes, increased PD-L1 expression, and increased PD-1/PD-L1 interactions are apparent in tumors from patients with SCLC with neurologic PNS. These findings suggest that differences in tumor immune recognition and cellular immunity contribute to the improved tumor control observed in patients with SCLC with neurologic PNS.
These findings are especially relevant in patients with SCLC because initial clinical trials using checkpoint inhibitors have shown that 80% of SCLC tumors have <1% PD-L1 staining (vs. 30% in patients with SCLC with neurologic PNS in our cohort)13. This is inconsistent with the high tumor mutational burden in SCLC23, and it implies that SCLC tumors use immune evasion strategies independent of PD-1/PD-L1. Herein we have demonstrated that both tumor T cell infiltration and PD-1/PD-L1 interactions are enriched in patients with SCLC with neurologic PNS. This suggests that regardless of the outcome of checkpoint inhibitor clinical trials in large cohorts of patients with SCLC13–15,24, patients with SCLC with neurologic PNS may be an ideal target population for this treatment. However, as neurologic PNS can be debilitating independent of tumor progression6, caution must be used in balancing the anti-tumor effects of immunotherapy with exacerbation of the neurologic PNS. One patient with a neurologic PNS in our cohort received nivolumab, but response could not be assessed as the patient developed pneumonitis and disease progression after four doses.
The primary limitation of this study is the inclusion of both primary tumor and nodal metastases. This introduces potential confounding because it is difficult to accurately assess T cell infiltrates in nodal metastases. This may have biased our T cell infiltrate analysis, as more nodal tissue was evaluated in the neurologic PNS group compared to the endocrinologic PNS group and control group. Furthermore, it is possible that tumor immune infiltrates change over time and sampling the tumor at the time of onset of a neurologic PNS, which is not always simultaneous with SCLC diagnosis, may demonstrate the most accurate pathophysiologic link. Also, we included patients with no detectable serum paraneoplastic autoantibody (20% of patients with neurologic PNS), which may have biased the neurologic PNS cohort against an improved prognosis, as these patients may have had pathophysiology similar to controls. Finally, it is important to note that a higher proportion of patients with neurologic PNS had LS-SCLC compared to patients with endocrinologic PNS or controls.
The current study provides a key link between decades-old clinical observations in patients with SCLC with neurologic PNS and the current immunomodulation paradigm in oncology focusing on tumor-infiltrating lymphocytes and PD-1/PD-L1 interactions. These hypothesis generating data are expected to lead to studies evaluating the full spectrum of circulating anti-tumor antibodies in patients with SCLC, with the hope of eventually identifying new disease specific drug targets. Further study should also investigate the application of checkpoint inhibitors in patients with SCLC with neurologic PNS and the full spectrum of immune evasion strategies used in SCLC tumors.
Supplementary Material
Acknowledgements
Foremost, we would like to acknowledge all of the patients who made this study possible and all of the health care providers who cared for these patients. We are grateful to Yingjun Yan and Sherry Smith for their help with project coordination. We would like to acknowledge the Vanderbilt Redcap project funded by NCATS/NIH grant UL1 TR000445 and the Clinical and Translational Science Award at Vanderbilt.
Financial support: This study was supported in part by a Vanderbilt Ingram Cancer Center Young Ambassadors Award and a Lung Cancer Foundation of America / International Association for the Study of Lung Cancer Lori Monroe Scholarship. CML was also supported by a Damon Runyon Clinical Investigator Award, a LUNGevity Career Development Award, a V Foundation Scholar-in-Training Award, P30-CA086485, U10CA180864, R01CA121210, U54CA217450-01, and U01CA224276-01. WTI was supported by the National Institutes of Health (NIH) and National Cancer Institute (NCI) Vanderbilt Clinical Oncology Research Career Development Award (VCORCDP) 2K12CA090625-17 and an American Society of Clinical Oncology / Conquer Cancer Foundation Young Investigator Award. ZZ, LLW, JTS, and JLW were supported in part by CCSG NCI/NIH P30CA068485-19. ES was supported by NIH (T32GM0734) and NCI (F30 CA216891-01) grants. CBM was supported by NIH (T32GM0734). All data storage for this research project utilized Vanderbilt’s Redcap data storage infrastructure, funded by NCATS/NIH grant UL1TR000445.
Abbreviations
- SCLC
small cell lung cancer
- PNS
paraneoplastic syndrome
- OS
overall survival
- PFS
progression free survival
- ES-SCLC
extensive stage small cell lung cancer
- LS-SCLC
limited stage small cell lung cancer
- NSCLC
non-small cell lung cancer
- LEMS
Lambert-Eaton myasthenic syndrome
- SIADH
syndrome of inappropriate antidiuretic hormone
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- FFPE
formalin fixed, paraffin embedded
- FIHC
fluorescence immunohistochemistry
Footnotes
Conflicts of interest: WTI has collaborated with EMD Serono on clinical trial implementation. CML has served on Advisory Boards for Pfizer, Novartis, Astra Zeneca, Genoptix, Sequenom, Ariad, and Takeda, Foundation Medicine, Blueprints Medicine, and Cepheid and has received research funds from Astra Zeneca, Novartis and Xcovery. JB, IS, and CV are employees of Navigate BioPharma Services, Inc., a Novartis subsidiary. ES, CBM, MR, ZZ, LLW, JTS, and JW report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernhardt EB, Jalal SI. Small Cell Lung Cancer. Cancer Treat Res 2016;170:301–22. [DOI] [PubMed] [Google Scholar]
- 2.Gazdar AF, Minna JD. Developing New, Rational Therapies for Recalcitrant Small Cell Lung Cancer. J Natl Cancer Inst 2016;108. [DOI] [PubMed] [Google Scholar]
- 3.Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest 2003;123:97s–104s. [DOI] [PubMed] [Google Scholar]
- 4.Kanaji N, Watanabe N, Kita N, et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 2014;5:197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol 1986;4:1191–8. [DOI] [PubMed] [Google Scholar]
- 6.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543–54. [DOI] [PubMed] [Google Scholar]
- 7.Chesler L Paraneoplasia, cancer development and immunity: what are the connections? Nat Rev Cancer 2014;14:447–8. [DOI] [PubMed] [Google Scholar]
- 8.Nagy-Mignotte H, Shestaeva O, Vignoud L, et al. Prognostic impact of paraneoplastic cushing’s syndrome in small-cell lung cancer. J Thorac Oncol 2014;9:497–505. [DOI] [PubMed] [Google Scholar]
- 9.Maddison P, Newsom-Davis J, Mills KR, Souhami RL. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet. England1999:117–8. [DOI] [PubMed] [Google Scholar]
- 10.Altman AJ, Baehner RL. Favorable prognosis for survival in children with coincident opsomyoclonus and neuroblastoma. Cancer 1976;37:846–52. [DOI] [PubMed] [Google Scholar]
- 11.Maddison P, Gozzard P, Grainge MJ, Lang B. Long-term survival in paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 2017;88:1334–9. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 13.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. [DOI] [PubMed] [Google Scholar]
- 14.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;35:3823–9. [DOI] [PubMed] [Google Scholar]
- 15.Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
- 16.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. [DOI] [PubMed] [Google Scholar]
- 17.Siska PJ, Johnpulle RAN, Zhou A, et al. Deep exploration of the immune infiltrate and outcome prediction in testicular cancer by quantitative multiplexed immunohistochemistry and gene expression profiling. Oncoimmunology 2017;6:e1305535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol 2012;25 Suppl 1:S18–30. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DB, Bordeaux J, Kim JY, et al. Quantitative Spatial Profiling of PD-1/PD-L1 Interaction and HLA-DR/IDO-1 Predicts Improved Outcomes of Anti-PD-1 Therapies in Metastatic Melanoma. Clin Cancer Res 2018;24:5250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 22.Bourdeaux JDN, Pennell NA, Stevenson J, et al. PD-1/PD-L1 interaction and CD25/FOXP3+ T cells to predict survival benefit from adjuvant chemotherapy in early stage non-small cell lung cancer (ES-NSCLC). J Clin Oncol 36 (suppl; abstr 12059): ASCO annual meeting; 2018. [Google Scholar]
- 23.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.