Abstract
To evaluate the economic and operational effects of implementing a shorted diagnostic pathway during influenza epidemics. This retrospective study used emergency department (ED) data from the 2014/2015 influenza season. Alere i influenza A & B rapid molecular diagnostic test (RDT) was compared with the polymerase chain reaction (PCR) pathway. Differences in room occupancy time in the ED and inpatient ward and cost differences were calculated for the 14-week influenza season. The process flow was more streamlined with the RDT pathway, and the necessary isolation time in the ED was 9 h lower than for PCR. The difference in the ED examination room occupancy time was 2.9 h per patient on a weekday and 4 h per patient on a weekend day, and the difference in the inpatient room occupancy time was 2 h per patient on a weekday and 3 h per patient on a weekend day. Extrapolated time differences across the influenza season were projected to be 2733 h in the ED examination room occupancy and 1440 h in inpatient room occupancy. In patients with a negative diagnosis, the RDT was also estimated to reduce the total diagnostic costs by 41.52 € per patient compared with PCR. The total cost difference was projected to be 31,892 € across a 14-week influenza season. The improved process and earlier diagnosis with the RDT pathway compared with conventional PCR resulted in considerable savings in ED, inpatient room occupancy time and cost across the influenza season.
Keywords: Economic analysis, Emergency department, Influenza-like illness, Molecular test, Process optimization, Rapid diagnostic test
Introduction
Seasonal influenza epidemics are a huge global burden. They typically infect 5–15% of the population during an epidemic [1], cause 250,000–500,000 deaths annually worldwide [2], and are associated with a significant number of related hospitalizations [3]. A fast diagnosis enables infection control measures and patient treatment to be initiated in a timely manner and also helps to optimize the use of bed spaces [4].
The molecular techniques that are commonly used to diagnose influenza, such as reverse transcriptase polymerase chain reaction (PCR), require samples to be transported to a laboratory, where molecular testing may not be performed immediately. There is, therefore, a delay before the results are available, which is a particular issue during weekends and public holidays, when laboratory diagnostic facilities are frequently unavailable [5, 6], and during influenza epidemics, when diagnostic laboratories do not have the capacity to sustain demand [7]. This has implications for patient flow and infection control [8], and patients may need to remain in multi-bed rooms in the emergency department (ED) until a suitable room becomes available [9]. Furthermore, delays in the diagnosis of influenza have also been associated with inferior outcomes, including disease progression and mortality [8, 10].
Rapid diagnostic tests (RDTs) for influenza have previously been limited by the poor sensitivity of antigen detection tests [11]. Highly accurate molecular RDTs are now available and are likely to be of considerable use in the ED because this is where patients with influenza-like illness (ILI) most commonly present [12, 13]. The benefits of RDTs reported in previous studies include a reduction in the mean waiting time in the ED [14, 15] and the length of stay [14, 16, 17]. Rapid diagnostic testing for influenza would therefore be expected to ease capacity constraints, reduce the strain on EDs during epidemics, make it easier to comply with infection control requirements, and improve the patient experience. Rapid diagnostic testing also reduces the prescribing of antibiotics [16, 18, 19], which are often prescribed inappropriately to influenza patients, contributing to the development of antibiotic resistance [16, 18]. It also reduces the need for laboratory tests and chest X-rays, with a subsequent reduction in costs [17].
The Alere™ i Influenza A&B rapid test (Alere, Waltham, USA) was chosen for this study because it is an RDT that utilises the enzyme-mediated molecular amplification of nucleic acids to yield a qualitative diagnostic result within 15 min [20]. It does not require sample preparation and can be used near to the patient outside of the laboratory. Whilst the performance of this RDT has been established (94.8% positive percent agreement for Influenza A and 98.4% for Influenza B, 97.7% negative percent agreement for A and 99.4% for B measured against PCR [20, 21]), there is a paucity of data on the economic benefits of implementing an RDT into the diagnostic pathway in the ED during an influenza epidemic. This retrospective study was designed to evaluate the operational and economic benefits of a shortened diagnostic pathway using this RDT for managing suspected influenza cases in the ED of a hospital in Germany during an influenza epidemic.
Methods
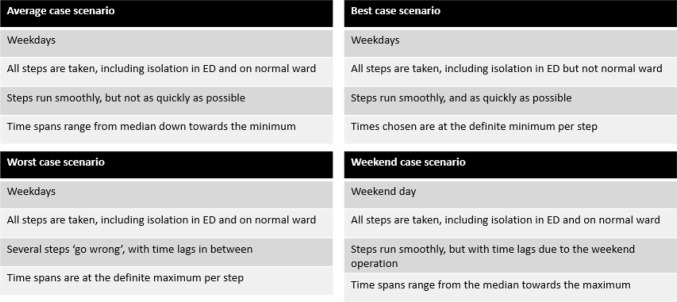
This retrospective analysis was based on data obtained from an ED that has approximately 56,000 emergency patients per year. The diagnostic pathways with PCR and the RDT in the management of patients with ILI, and their actual time requirements, were first mapped based on insight obtained from specialised hospital personnel (Head of ED, senior medical/nursing staff and a virologist). A conceptual model was created (Fig. 1) to enable process times to be compared between the two diagnostic pathways. The scenarios depicted represent those most commonly encountered in clinical practice and include a best, average and worst case scenario, each relating to a normal weekday. Additionally, a weekend average case was also constructed to reflect the diagnostic operation out of hours. The process times for the RDT and PCR pathways, the typical length of stay in the ED, and the time that ED and inpatient rooms were occupied or unusable pending decontamination was obtained for each possible case scenario from the ED and virology staff. Data on the number of patients and their respective test results were obtained from the RDT device.
Fig. 1.
The conceptual model and its case scenarios (ED emergency department)
The number of patients tested was obtained from ED data relating to one week of the influenza epidemic in early 2015 (9th–15th March), during which time the rapid test had been performed by staff in the ED in accordance with the manufacturer’s instructions. PCR, which included confirmatory testing for negative rapid test results, was performed by a virologist on the same sample at an onsite diagnostic laboratory for all patients (PCR-Kit 1.0 Real Star®, Altona Diagnostics, analytic sensitivity for Influenza A: 0.45 copies/µl Influenza B: 2.42 copies/µl, analytic specificity: no cross-amplification with other respiratory pathogens detectable [22]). The proportion of patients that were tested and the ratio of weekday to weekend tests were calculated by comparing test data from the RDT device with patient statistics from the ED. The time and cost effects of both pathways were calculated per patient for the average weekday case scenario and the weekend case scenario (see conceptual model in Fig. 1). The effects per patient were then extrapolated to estimate the total effect across the influenza season.
The cost calculations were based on the cost of resources used for the management of patients with ILI and included expenditure related to the inpatient admission of patients who later received a negative diagnosis following PCR. The mean personnel and diagnostic unit costs assigned to every step of the two pathways, including the provision of technical personnel and diagnostic reagents, and the cost of performing the test were based on average rates which were either obtained from the ED or researched (see Table 1 for more detail). Incidental costs, such as the cost of protective clothing and its disposal, were researched. Inpatient reimbursement was based on the German base rate of 3231.20 € that was obtained from the National Association of Statutory Health Insurance Funds in Germany [23].
Table 1.
Costs and other economic assumptions
| Position | Value |
|---|---|
| Personnel costs ED nurse p.a.a | 60,000 € |
| Personnel costs consultant ED p.a.b | 111,000 € |
| Personnel costs cleaning staff p.a.a | 36,000 € |
| Personnel costs radiographer p.a.a | 50,000 € |
| Personnel costs transport service p.a.a | 24,000 € |
| Net annual working time in ha | 1650 |
| PCR testing costsa | 21.42 € |
| PCR on demand testing costsa | 42.84 € |
| Alere i testing costsc | 27.00 € |
All calculations that were based on data from the RDT device were adjusted to take account of false positive and negative results based on a specificity of 98.09% [24]. This was the lower of the two specificity values for influenza A and B respectively [24]. For calculations in which it was necessary to take account of the average hospital utilisation rate, a value of 77.4% was used, which is widely accepted as a representative figure in Germany [25]. All calculations were performed using Microsoft Excel.
Results
During the 14 weeks of the 2015 influenza epidemic, the ED treated 15,300 emergency patients overall. Data obtained from the RDT for the analysed week of the 2015 influenza epidemic indicated that 5.3% of patients were tested for influenza; therefore, approximately 812 patients would have been tested during the whole influenza epidemic. 31% of the tested patients were positive. The positivity rate underlines the necessity for the preventive but costly isolation. The percentage ratio of patients tested on a weekday to patients tested on a weekend day was considered to be 64:36, based on these data.
Process flow differences for the PCR pathway versus the RDT pathway
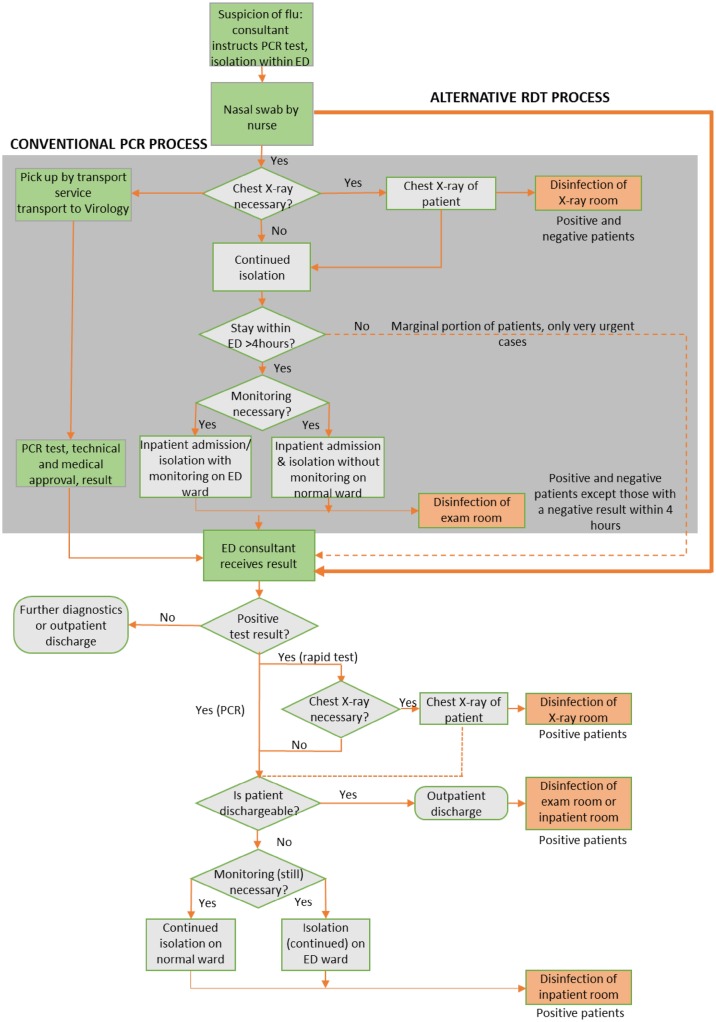
A conceptual flow diagram of the diagnostic process for influenza using either PCR or the RDT in the ED is presented in Fig. 2. This figure shows that the conventional pathway has more stages than the RDT pathway and also requires samples to be transported to a diagnostic laboratory. The uncertain diagnostic status of the patient prolongs the time during which patients must remain isolated in the ED and mandates the disinfection of multiple rooms (shown in orange in Fig. 2), including the X-ray suite and the ED examination room after inpatient admission. This is expensive and renders the room unusable, typically for 30 min. Patients diagnosed via this pathway often remain under isolation in the ED for over 4 h.
Fig. 2.
Comparison of the diagnostic process flow for PCR and the RDT (ED emergency department, PCR polymerase chain reaction, RDT rapid diagnostic test)
In the RDT pathway, a diagnosis is obtained directly from a nasal swab or nasopharyngeal swab in viral transport medium, and a result is typically available within 15 min. The continued isolation of patients and subsequent disinfection of the ED examination room and X-ray suite is only necessary when the rapid test returns a positive result. Patients who receive a negative result do not need to be kept under isolation, and hence spend less time in the ED examination room. Less time is also spent making provision for further isolation or inpatient bed space in the RDT pathway compared with the PCR pathway, which further improves patient flow and increases examination room availability in the ED. Discontinuing the isolation for negative results was possible since the described pathway was applied only in adult care and not the pediatric section with high prevalence of RSV and other highly contagious respiratory pathogens. The examination rooms were nonetheless cleaned with additional basis hygiene (wipe disinfection) but without the full disinfection program (residence time 30 min).
Influence of rapid diagnostic testing versus PCR on the ED resource management and inpatient room occupancy
Use of the RDT pathway in the ED was associated with a lower necessary isolation time (defined as the time elapsed between a medical practitioner considering flu as a potential diagnosis and confirmatory test results actually being available) in the ED for all scenarios depicted in the discrete model (Fig. 3). The necessary isolation time for the average case scenario was 10 h for the PCR pathway and 65 min for the rapid diagnostic pathway, representing a difference of almost 9 h. The difference was greatest for the weekend case scenario (Fig. 3).
Fig. 3.
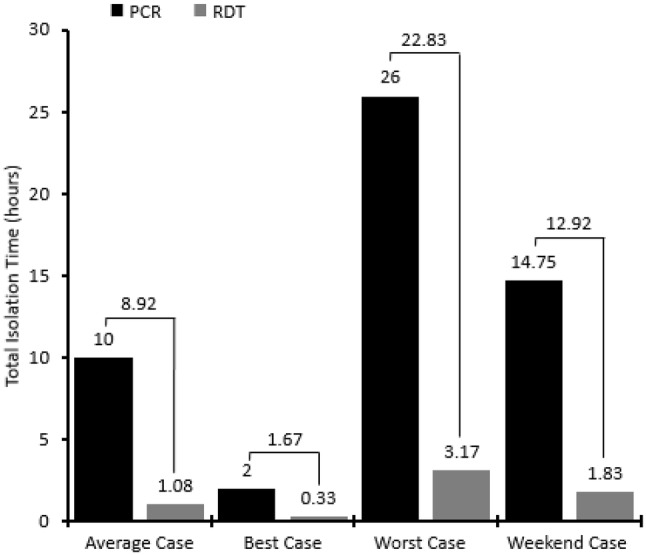
Total patient isolation times (PCR polymerase chain reaction, RDT rapiddiagnostic test)
The ED examination room occupancy time was shorter with the RDT pathway than the PCR pathway, with a difference of 2.9 h per patient on a weekday and 4 h per patient on a weekend day. Once extrapolated, this indicates a time saving of 2733 h across the 14-week influenza season (Table 2), which is the equivalent of 28 h of ED examination room time per day; hence, the RDT pathway could be expected to extricate a whole extra examination room in the ED per day. Similarly, the inpatient room occupancy was also lower with the RDT pathway, and there was a difference of 2 h per patient on a weekday and 4 h per patient on a weekend day, which is likely to be because the diagnosis is known in the ED so there is therefore less of a need for inpatient admission. This was extrapolated to indicate a reduction of 1440 h in inpatient room occupancy across the 14-week flu season (Table 2).
Table 2.
Saved ED and inpatient room occupancy time
| Weekday (average) case | Weekend case | Flu season (14 weeks) | |
|---|---|---|---|
| Patients tested for influenza (n)a | 522 | 290 | 812b |
| ED examination room occupancy (h per case type) | |||
| PCR pathway | 4.5 | 7.5 | |
| RDT pathway | 1.58 | 3.33 | |
| Difference | 2.92 | 4.17 | |
| Subtotal: Time saved (h) (patients*difference) | 1524 | 1209 | 2733 |
| Inpatient room occupancy (h per case type)c | |||
| PCR pathway | 2.08 | 3.28 | |
| RDT pathway | 0 | 0 | |
| Difference | 2.08 | 3.28 | |
| Subtotal: Time saved (h) (patients*difference) | 1086 | 951 | 1440 d |
ED emergency department, PCR polymerase chain reaction, RDT rapid diagnostic test
aAssumes a weekday: weekend day ratio of 64:36
bCalculated as 5.3% of the number of emergency patients in one flu season (15,300)
cInpatient ward and normal ward
dExcludes room capacity occupied as a result of false positives from the rapid test. Data from the rapid test device indicate that 31% of 812 patients (n = 252) received a positive rapid test result, so 560 patients would therefore have received a negative rapid test result. The specificity of the Alere™ i rapid test is 98.09% (manufacturers data), so the percentage of patients with a false positive result can be calculated as 1 − 0.981 × 560, equalling 10.7 patients. Assuming an average occupancy time is 3 days, and an average utilisation time of 77.4%, this would equal 596 h (10.7 pts × 3 days × 24 h × 0.774)
The average length of stay for inpatients in Germany is 7.4 days [22], which is the equivalent of 177.6 h. A total of 1440 h in saved ward capacity, which would therefore enable eight additional patients to be treated during the 14-week influenza season, with an anticipated reimbursement of 26,263 € (based on the German base rate for inpatient reimbursement of 3231.20 € per patient).
Integrating rapid testing into the management of ILI Patients had significant economic benefits
The shortened diagnostic pathway with the RDT compared with conventional PCR was associated with less expenditure on caring for isolated patients in the ED, disinfecting the X-ray suite, and ED specialists searching for inpatient bed space. The number of times that protective clothing (protective gloves, protective gown, FFP3 mask, protective goggles) typically needs to be used was lower for the RDT pathway than the conventional PCR pathway. The calculated cost difference in patients who received a negative diagnosis with the RDT instead of PCR was €41.52 (Table 3). The costs for both pathways were totalled, and the combined cost difference for the average case and the weekend day scenario across a 14-week influenza epidemic was estimated to be 31,892 € (Table 4).
Table 3.
Comparison of PCR and rapid test costs
| Process stage | Key costs (€) | PCR pathway | RDT pathway | Difference |
|---|---|---|---|---|
| Diagnostic test | Cost of the test | 21.42 | 27.00 | 5.58 |
| Cost of nursing staff to performing the rapid test | 12.12 | 12.12 | ||
| Transport of samples to the lab (weekend) | 0.61 | − 0.61 | ||
| Patient isolation in ED | Cost of labour to care for isolated patients | 45.45 | 13.26 | − 32.20 |
| Provision of protective clothing | 2.25 | 0.30 | − 1.95 | |
| Disposal of contaminated waste | 0.05 | 0.05 | N/A | |
| Possible X-raya | Disinfection of X-ray unit/lost radiographer time | 9.75 | N/A | − 9.75 |
| Provision of protective clothing | 0.25 | N/A | − 0.25 | |
| Disposal of contaminated waste | 0.03 | N/A | − 0.03 | |
| Inpatient admissiona | Cost of ED specialists searching for bed space | 13.05 | N/A | − 13.25 |
| Isolation on normal warda | Lost revenue from unnecessary bed occupancy | N/A | N/A | |
| Provision of protective clothing | 1.37 | N/A | − 1.37 | |
| Disposal of contaminated waste | 0.02 | N/A | − 0.02 | |
| Total costs | 94.25 | 52.73 | 41.52 |
ED emergency department, PCR polymerase chain reaction, RDT rapid diagnostic test
aUnnecessary costs for negative cases only
Table 4.
Total costs for both pathways
| Weekday (average) case | Weekend case | |
|---|---|---|
| Patients (n) | 522 | 290 |
| Total cost per patient (€) | ||
| PCR pathway | 82.97 | 105.52 |
| RDT pathway | 49.32 | 56.14 |
| Cost Differencea | 33.65 | 49.38 |
| Total cost per season (€)b | ||
| PCR pathway | 43,307.79 | 30,621.25 |
| RDT pathwaya | 25,744.68 | 16,292.72 |
| Cost Differencea | 17,563.11 | 14,328.53 |
| Total cost difference weekday and weekend(€) | 31,891.64 | |
PCR polymerase chain reaction, RDT rapid diagnostic test
aSubtotal of the PCR costs minus the RDT costs
bThe cost of the rapid test itself and the associated personnel cost, which were calculated to be 2.40 € and 1.33 € in total for the average case and the weekend case respectively, were deducted
Effects depending of the size of the ED
To estimate the cost and room occupancy reductions that could be expected with use of the RDT pathway instead of the PCR pathway for the detection of influenza in EDs of different sizes, the cost and room utilisation data were extrapolated to three representative examples of EDs with different patient numbers. The projected cost difference with the RDT instead of PCR during a typical flu season was estimated to range from 5633 € for a small ED with 10,000 patients annually to 33,649 € for a large ED with 60,000 patients annually (Table 5). Using the same extrapolation principles, the number of ED examination rooms extricated with use of the RDT instead of PCR was projected to range from 0.2 rooms per day for a representative small ED to 1.2 days per day for a representative large ED. The projected time difference in inpatient room occupancy on the ward enabled up to eight new inpatients to be admitted, with a projected remuneration of up to 30,000 € (Table 5).
Table 5.
Extrapolation of differences in cost and room occupancy to ED departments of different sizes
| Small hospital | Medium hospital | Large hospital | |
|---|---|---|---|
| Patient statistics (n) | |||
| ED patients per year | 10,000 | 30,000 | 60,000 |
| Emergency patients tested for influenza per seasona | 143 | 428 | 856 |
| Patients tested on weekdays during the flu seasonb | 92 | 274 | 548 |
| Patients tested on weekend days during the flu season | 51 | 154 | 308 |
| Patients testing positive during the flu seasonc | 45 | 133 | 265 |
| Patients testing negative during the flu season | 99 | 295 | 591 |
| Cost data (€) | |||
| Difference per patient (cost of PCR − cost of RDT)d | |||
| Average case | 33.65 | 33.65 | 33.65 |
| Weekend case | 49.38 | 49.38 | 49.38 |
| Difference per influenza seasone | |||
| Average case | 3102 | 9220 | 18,440 |
| Weekend case | 2531 | 7605 | 15,209 |
| Cost savings with the RDT per flu season | 5633 | 16,825 | 33,649 |
| ED examination room occupancy (h) | |||
| Time difference per case (cost of PCR − cost of RDT)f | |||
| Average case | 2.92 | 2.92 | 2.92 |
| Weekend case | 4.17 | 4.17 | 4.17 |
| Time difference per influenza season | |||
| Average case | 268.64 | 800.08 | 1600.16 |
| Weekend case | 212.67 | 642.18 | 1284.36 |
| Total time difference | 481.34 | 1442.26 | 2884.52 |
| Hours per dayg | 4.91 | 14.72 | 29.43 |
| Rooms per dayh | 0.21 | 0.61 | 1.2 |
| Inpatient ward room occupancy (h) | |||
| Time difference per case | |||
| Average case | 2.1 | 2.1 | 2.1 |
| Weekend case | 3.28 | 3.28 | 3.28 |
| Time difference per influenza season | |||
| Average case | 193.2 | 575.4 | 1150.8 |
| Weekend case | 167.28 | 505.12 | 1010.24 |
| Total time differencei | 254.9 | 765 | 1529 |
| Capacity for new inpatients (n)j | 1.47 | 4.32 | 8.60 |
| Expected revenue (€)k | 4749.9 | 13,926.5 | 27,788.32 |
ED emergency department, PCR polymerase chain reaction, RDT rapid diagnostic test
aAssumes 5.3% of ED patients tested for influenza in a 14-week influenza season
bAssumes a weekday:weekend day ratio of 64:36
cData from the RDT indicated that 31% of patients tested received a positive diagnosis for influenza, so this was calculated as 0.031 multiplied by the number of emergency patients tested for influenza per season, and the remainder were assumed negative
dSee Table 4 for cost unit data
eCalculated as the difference per patient multiplied by the number of patients
fSee Table 2 for cost unit data calculations
gTotal time difference divided by 14 weeks multiplied by 7 days
hHours per day divided by 24
iExcludes room capacity occupied as a result of false positives from the rapid test calculated as [(1 − 0.981)*n for negatives]*3 days*24 h*0.774 occupancy rate
jCalculated as available capacity/(average length of stay in hours) = available capacity/(7.4*24)
kBased on a remuneration rate of 3231.20 €
Discussion
There is a paucity of evidence on the economic and functional benefits of using rapid molecular diagnostic tests for the detection of influenza during an influenza epidemic. This retrospective analysis used data obtained from an ED during an influenza epidemic to calculate differences in cost and room occupancy time with the simplified pathway using the molecular RDT compared with the conventional PCR pathway, and extrapolated the differences to estimate the actual savings across the 14-week flu season.
This study has demonstrated that patient flow in the ED is improved by implementation of a simplified patient pathway which used an RDT. Diagnosis in the ED with the rapid test eliminated the need for samples to be processed by a laboratory and reduced the time required to diagnose influenza, during which the patient must remain isolated, by 9 h in the average case.
Other similar studies investigating the benefits of early diagnosis have also reported that rapid diagnostic testing reduces the patient waiting time and isolation time in the ED [15, 16, 26] and a similar reduction in the ED waiting time of 9 h was reported in the prospective study of a rapid commercial PCR assay compared to an in-house PCR test by Soto et al. [15]. The most likely explanation is a reduction in the time spent awaiting a diagnosis. The same-day diagnosis with rapid testing enables infection control measures to be instigated in a timely manner [27], reducing the risk of nosocomial transmission, which prolongs hospital stays [17], warrants additional interventions and causes absenteeism among health workers [27]. Implementation of RDTs in the ED also enable an accurate diagnosis to be obtained in the ED [16]. Clinical diagnosis in the ED can otherwise be difficult because of the overcrowded environment and because ILI patients without complications are usually seen as less urgent when triaged [16]. Clinical diagnosis of influenza in the ED based on signs and symptoms alone has recently been reported to have sensitivity as low as 36% [28], leading to inappropriate isolation and treatment. In addition, rapid diagnostic testing in the ED and on the ward also reduces strain on diagnostic laboratories.
Previous studies have already established that reduced waiting times in EDs improve patient satisfaction [29]. A previous large-scale survey concluded that increased time spent waiting for provider care was inversely related to patient-reported experience scores, particularly a patient’s perceived confidence in the care provider and perceived quality of care [30]. It is therefore reasonable to postulate that the reduction in waiting time and improved patient flow with use of an RDT would improve patient satisfaction levels, although the evaluation of this was unfortunately outside the scope of the present study. Patient satisfaction scores are important because they are increasingly considered in reimbursement decisions in some countries [31] and influence the reputation of the ED [32]. The reduced time to diagnosis with the RDT may also reduce inpatient admissions by eliminating the need to admit patients with ILI symptoms who later receive a negative diagnosis, although this was also not evaluated explicitly in this study. However, the improved process flow with the RDT will help to overcome constraints in waiting room capacity, which is an issue during influenza epidemics and pandemics [33].
In this study, implementation of the RDT pathway was projected to reduce room capacity requirements per patient in the ED. Indeed, the estimated 2733 ED examination room hours saved over the course of an entire flu season is the equivalent to having a whole extra room in the ED free for each day of the epidemic, which reduces the pressure on ED resources. The availability of rooms is the limiting factor in many hospitals, and is a particular issue during the influenza season [34]. An increased availability of rooms because of shorter occupancy times will therefore enable more patients to be seen and reduce elopement rates, which also increase during influenza pandemics [34]. In hospitals where room capacity is not the limiting factor, the extra capacity can also be used for alternative clinical services, such as an additional examination room for an outpatient clinic. The increased patient turnover also has financial benefits, and the average ED patient in Germany has a reimbursement rate of 1675 € [35].
Implementation of the RDT pathway also reduced the time that patients who later receive a negative diagnosis need to remain isolated for prior to receiving their diagnosis. The estimated reduction in inpatient room occupancy time with use of the rapid test was 1440 h across the flu season. This is particularly advantageous because hospital beds are one of the scarcest resources during influenza epidemics, and the demand for bed spaces in general medicine wards for influenza-related cases can increase by over 50% during influenza epidemics, compared with 25–32% for other specialties [33]. A reduction in the unnecessary isolation time can enable more inpatients to be treated, and this retrospective analysis indicated that eight additional inpatients could receive treatment based on an average length of stay of 7.4 days [25].
This study also demonstrated the financial benefits of rapid diagnostic testing. By eliminating unnecessary costs with the PCR pathway in patients who later receive a negative diagnosis, implementation of the RDT pathway reduced the total per patient costs by 41.52 €. This was extrapolated to a combined cost saving for the average weekday case and weekend day case across the influenza epidemic of 31,892 €. Other studies have also reported that use of RDTs reduce the cost per patient [15], indicating that rapid diagnostic testing has a lower cost per patient than traditional laboratory-based molecular diagnostic tests.
The lower cost with rapid diagnostic testing is likely to be due to the reduction in labour time required to care for isolated patients in the ED and the elimination of subsequent treatment stages that are unnecessary in patients who receive a negative rapid test result. This eliminates the cost of disinfecting the X-ray suite and of ED personnel searching for bed space, and prevents loss of revenue from unnecessary bed occupancy on the inpatient ward. The disinfection of the X-ray suite also renders the room unusable for a considerable amount of time, during which radiographers are unable to work. Unfortunately, thorough disinfection of examination and X-ray suites is necessary while awaiting diagnostic results from PCR because influenza particles on non-porous surfaces can remain infectious for 24–48 h [27]. Protective clothing is also only worn for a single use because influenza virus infectivity can remain on the surface of protective clothing for 8 h or more [36], so the cost of protective clothing and its disposal was also taken into consideration in this study. By reducing the number of hours spent searching for bed space and awaiting the completion of the disinfection process for treatment areas, RDTs liberate staff time, which can be used to improve the care of ED patients.
The limitations of this study include the retrospective study design, which make it difficult to exclude all confounding variables, and the fact that data were extrapolated to different scenarios. However, allowances were made in the data analysis for confounding variables that were identified, such as the possibility of false positives with the rapid test. The data extrapolations were also based on real process data obtained from the University of Marburg ED and the RDT, which is unlikely to vary significantly over a single flu season. The RDT was performed in the ED of the University Hospital of Marburg, which is a typical ED, during the 2014 influenza epidemic. The findings are therefore likely to be relevant to other similar hospital EDs during an influenza epidemic. A further limitation is the influenza focus of this study. There are other respiratory diseases which also require patient isolation with admission to the hospital (e.g. RSV). Some patients will have to remain isolated even with a negative influenza test when their presenting complaints suggest other infectious respiratory causes. But there are also molecular RDTs for RSV available.
In summary, this comparative study demonstrates the benefits of implementing a molecular RDT into the diagnostic pathway in EDs during influenza epidemics for improving patient flow, reducing the requirement for ED and inpatient room space for influenza patients, and reducing the total costs of managing such patients. Further research comparing patient satisfaction among influenza patients who were diagnosed using ward-based rapid testing in comparison with patients diagnosed using laboratory-based molecular testing would also help to demonstrate the benefits of rapid diagnostic testing on the patient experience.
Funding
The economic evaluation was supported by a grant to bcmed, Düsseldorf, Germany, from Alere International, Bedford, UK. Susanne Betz and Clemens Kill did not receive any form of honoraria for their participation in this study. Medical writing, editorial, and other assistance: medical writing support was provided by Ross Jarratt of integrated medhealth communication, London, UK. Support for this assistance was funded by Alere International.
Compliance with ethical standards
Conflict of interest
Matthias Brachmann is majority owner of bcmed GmbH which received a grant for an economic evaluation from Alere International, Galway, Ireland. Katja Kikull was a project manager at bcmed GmbH at time of writing which received a grant for an economic evaluation from Alere International, Galway, Ireland. Clemens Kill received speaker fees and travel costs from Alere International, Galway, Ireland. Susanne Betz has no conflict of interest.
Research involving human participants and/or animals
This article does not involve any new studies of human or animal subjects performed by any of the authors.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Change history
2/14/2019
The article Economic and operational impact of an improved pathway using rapid molecular diagnostic testing for patients with influenza-like illness in a German emergency department, written by Matthias Brachmann, Katja Kikull, Clemens Kill and Susanne Betz, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 04 January 2019 without open access.
References
- 1.Kovács G, Kaló Z, Jahnz-Rozyk K, Kyncl J, Csohan A, Pistol A, et al. Medical and economic burden of influenza in the elderly population in central and eastern European countries. Hum Vaccin Immunother. 2014;10(2):428–440. doi: 10.4161/hv.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Fact sheet: Influenza (seasonal). 2016. http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 14 Nov 2017.
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-Associated Hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Ginocchio CC, McAdam AJ. Current Best Practices for Respiratory Virus Testing. J Clin Microbiol. 2011;49(9 Suppl):44–48. doi: 10.1128/JCM.00698-11. [DOI] [Google Scholar]
- 5.Zheng W, Aitken R, Muscatello DJ, Churches T. Potential for early warning of viral influenza activity in the community by monitoring clinical diagnoses of influenza in hospital emergency departments. BMC Public Health. 2007;l7:250. doi: 10.1186/1471-2458-7-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaac-Renton JL, Chang Y, Prystajecky N, Petric M, Mak A, Abbott B, et al. Use of lean response to improve pandemic influenza surge in public health laboratories. Emerg Infect Dis. 2012;18(1):57–62. doi: 10.3201/eid1801.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi GP, Mitrache I, Pigal A, Freedman L. Public hospital-based laboratory experience during an outbreak of pandemic influenza A (H1N1) virus infections. J Clin Microbiol. 2010;48(4):1189–1194. doi: 10.1128/JCM.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber N, Khelili D, Martineau D, Dekimeche S, Szekely C, Lebon P, et al. Delay in diagnosis of influenza virus in an elderly hospitalized patient: a fatal outcome. Clin Med Insights Case Rep. 2012;5:5–8. doi: 10.4137/CCRep.S8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller MP, Junaid S, Matukas LM. Reduction in total patient isolation days with a change in influenza testing methodology. Am J Infect Control. 2016;44(11):1346–1349. doi: 10.1016/j.ajic.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Rorat M, Kurek T, Kuchar E, Szenborn L. The clinical couse of late diagnosed fatal cases of A (H1N1) influenza in Poland. Postepy Hig Med Dosw. 2013;67:595–600. doi: 10.5604/17322693.1053910. [DOI] [PubMed] [Google Scholar]
- 11.Brendish NJ, Schiff HF, Clark TW. Point-of-care testing for respiratory viruses in adults: the current landscape and future potential. J Infect. 2015;71(5):501–510. doi: 10.1016/j.jinf.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham MK, Perkins J, Vilke GM, Coyne CJ. Influenza in the emergency department: vaccination, diagnosis, and treatment: Clinical Practice Paper Approved by American Academy of Emergency Medicine Clinical Guidelines Committee. J Emerg Med. 2016;536–542. [DOI] [PubMed]
- 13.Afilalo M, Stern E, Oughton M. Evaluation and management of seasonal influenza in the emergency department. Emerg Med Clin North Am. 2012;30(2):271–305. doi: 10.1016/j.emc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagbuyi DB, Brown KM, Mathison DJ, Kingsnorth J, Morrison S, Saidinejad M, et al. A rapid medical screening process improves emergency department patient flow during surge associated with novel H1N1 influenza virus. Ann Emerg Med. 2011;57(1):52–59. doi: 10.1016/j.annemergmed.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Soto M, Sampietro-Colom L, Vilella A, Pantoja E, Asenjo M, Arjona R, et al. Economic impact of a new rapid PCR assay for detecting influenza virus in an emergency department and hospitalized patients. PLoS ONE. 2016;11(1):e0146620. doi: 10.1371/journal.pone.0146620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong HW, Heo JY, Park JS, Kim WJ. Effect of the influenza virus antigen test on a physician’s decision to prescribe antibiotics and on patient length of stay in the emergency department. PLoS ONE. 2014;9(11):e110978. doi: 10.1371/journal.pone.0110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112:363. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 18.Özkaya E, Cambaz N, Çoskun Y, Mete F, Geyik M, Samanc N. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr. 2009;98(10):1589–1592. doi: 10.1111/j.1651-2227.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 19.Tillekeratne LG, Bodinayake CK, Nagahawatte A, Vidanagama D, Devasiri V, Arachchi WK, et al. Use of rapid influenza testing to reduce antibiotic prescriptions among outpatients with influenza-like illness in Southern Sri Lanka. Am J Trop Med Hyg. 2015;93(5):1031–1037. doi: 10.4269/ajtmh.15-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen Van JC, Camelena F, Dahoun M, Pilmis P, Mizrahi A, Lourtet J, et al. Prospective evaluation of the Alere i Influenza nucleic acid amplification versus Xpert Flu/RSV. Diagn Microbiol Infect Dis. 2016;85(1):19–22. doi: 10.1016/j.diagmicrobio.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Manufacturer’s data. http://www.alere-i.com/en/index/alere-i-system/alere-i-tests/alere-i-influenza-a-b.html. Accessed 14 Nov 2017.
- 22.Manufacturer’s data. https://www.altona-diagnostics.com/files/public/Content%20Homepage/-%2002%20RealStar/MAN%20-%20CE%20-%20EN/RealStar%20Influenza%20S&T%20RT%20PCR%20Kit%201.0_WEB_CE_EN-S01.pdf. Accessed 14 Nov 2017.
- 23.The National Association of Statutory Health Insurance Funds. https://www.gkv-spitzenverband.de/startseite/startseite.jsp. Accessed 14 Nov 2017.
- 24.Bell J, Bonner A, Cohen DM, Birkhahn R, Yogev R, Triner W, et al. Multicenter clinical evaluation of the novel Alere™ i Influenza A&B isothermal nucleic acid amplification test. J Clin Virol. 2014;61(1):81–86. doi: 10.1016/j.jcv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Federal Statistics Office (Destatis). https://www.destatis.de/EN/FactsFigures/FactsFigures.html. Accessed 14 Nov 2017.
- 26.Li-Kim-Moy J, Dastouri F, Rashid H, Khandaker G, Kesson A, McCaskill M, et al. Utility of early influenza diagnosis through point-of-care testing in children presenting to an emergency department. J Paediatr Child Health. 2016;52(4):422–429. doi: 10.1111/jpc.13092. [DOI] [PubMed] [Google Scholar]
- 27.Salgada CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2(3):145–155. doi: 10.1016/S1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 28.Dugas A, Valsamakis A, Atreya MR, Thind K, Mancheqo A, Faisal A, et al. Clinical diagnosis of influenza in the ED. Am J Emerg Med. 2015;33(6):770–775. doi: 10.1016/j.ajem.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker BT, Marco MD. Emergency department length of stay: accuracy of patient estimates. West J Emerg Med. 2014;15(2):170–175. doi: 10.5811/westjem.2013.9.15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleustein C, Rothschild DB, Valen A, Valaitis E, Schweitzer L, Jones R. Wait times, patient satisfaction scores, and the perception of care. Am J Manag Care. 2014;20(5):393–400. [PubMed] [Google Scholar]
- 31.Tevis SE, Kennedy GD, Kent C. is there a relationship between patient satisfaction and favorable surgical outcomes? Adv Surg. 2015;49(1):221–233. doi: 10.1016/j.yasu.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worthington K. Customer satisfaction in the emergency department. Emerg Med Clin North Am. 2004;22(1):87–102. doi: 10.1016/S0733-8627(03)00121-4. [DOI] [PubMed] [Google Scholar]
- 33.Fusco F, Schilling S, De Iaco G, Brodt H, Brouqui P, Maltezou HC, et al. Infection control management of patients with suspected highly infectious diseases in emergency departments: data from a survey in 41 facilities in 14 European countries. BMC Infect Dis. 2012;12:27. doi: 10.1186/1471-2334-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugerman D, Nadeau KH, Lafond K, Cameron W, Soetebier K, Jhung M, et al. A survey of emergency department 2009 Pandemic Influenza A (H1N1) Surge Preparedness––Atlanta, Georgia, July–October 2009. Clin Infect Dis. 2011;52(Suppl1):177–182. doi: 10.1093/cid/ciq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brachmann M, et al. Management der Notaufnahme. 2. Berlin: Kohlhammer; 2016. Prozessoptimierung durch Point-of-Care-Testung; pp. 321–326. [Google Scholar]
- 36.Sakaguchi H, Wada K, Kajioka J, Watanabe M, Nakano R, Hirose T, et al. Maintenance of influenza virus infectivity on the surfaces of personal protective equipment and clothing used in healthcare settings. Environ Health Prev Med. 2010;15:344–349. doi: 10.1007/s12199-010-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]