Abstract
The incidence of reported coccidioidomycosis in the past two decades has increased greatly; monitoring its changing epidemiology is essential for understanding its burden on patients and the healthcare system and for identifying opportunities for prevention and education. We provide an update on recent coccidioidomycosis trends and public health efforts nationally and in Arizona, California, and Washington State. In Arizona, enhanced surveillance shows that coccidioidomycosis continues to be associated with substantial morbidity. California reported its highest yearly number of cases ever in 2016 and has implemented interventions to reduce coccidioidomycosis in the prison population by excluding certain inmates from residing in prisons in high-risk areas. Coccidioidomycosis is emerging in Washington State, where phylogenetic analyses confirm the existence of a unique Coccidioides clade. Additional studies of the molecular epidemiology of Coccidioides will improve understanding its expanding endemic range. Ongoing public health collaborations and future research priorities are focused on characterizing geographic risk, particularly in the context of environmental change; identifying further risk reduction strategies for high-risk groups; and improving reporting of cases to public health agencies.
Keywords: Coccidioidomycosis, epidemiology, mycotic diseases, fungal diseases
Introduction
The epidemiology of coccidioidomycosis in the United States continues to evolve with increasing knowledge of the characteristics of at-risk populations, environmental conditions, changing climate and weather patterns, and other factors. It is essential to monitor the epidemiology of this disease to understand long- term trends and explore factors that contribute to environmental and human risk.
Here we describe the national epidemiology of the disease, highlighting findings of enhanced surveillance in Arizona and the increased incidence in California during 2016. We describe public health efforts undertaken to reduce the risk of coccidioidomycosis in vulnerable populations, such as prisoners incarcerated in California’s Central Valley. We highlighted the contribution of advancements in molecular and genomic methods and their role in source identification in outbreak investigations and understanding dispersal. These tools have been used to confirm the identification of newly endemic areas such as Washington State, which helps us learn about the environmental range of the fungus. This update of the epidemiology of coccidioidomycosis in the United States offers a foundation for researchers, clinicians, and public health professionals to better understand the disease and develop better treatment and prevention strategies.
Public health surveillance of coccidioidomycosis in the United States
Coccidioidomycosis is a disease of increasing public health concern because of challenges in its diagnosis and treatment, as well as the substantial morbidity and healthcare impact. Timely information about its national epidemiology is essential for understanding trends, overall burden, and geographic risk. Strong collaborations between state, local, and federal public health agencies are required to chart the epidemiology of the disease, and dissemination of this information to clinicians and researchers can enable them to advance approaches to treatment and prevention.
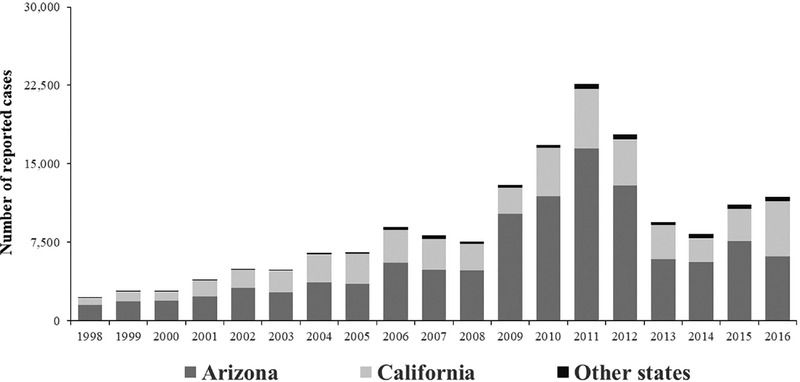
In the United States, coccidioidomycosis cases are reported to public health agencies based on a case definition established by the Council of State and Territorial Epidemiologists (CSTE).1 As of 2017, coccidioidomycosis was reportable in 22 states. Typically, cases are reported to a local or state health jurisdiction after a positive laboratory test result. The Centers for Disease Control and Prevention (CDC) receives case counts from states through the National Notifiable Disease Surveillance System (NNDSS). Approximately 10,000 cases are reported annually.2 The incidence of reported cases of coccidioidomycosis has notably increased since it became nationally notifiable in 1995, even after adjusting for changes in population size and characteristics.3 The number of cases peaked at 22,641 in 2011, declined during 2012–2014. and increased again in 2015; in 2016, California reported a record number of cases4 (Fig. 1). Although changes in reporting practices and laboratory testing likely explain some of the annual variation in case counts, much of the variation likely reflects true changes in disease incidence, probably related to environmental factors, including temperature and precipitation.5,6
Figure 1.
Reported coccidioidomycosis cases in the United States, 1998–2016.
Most cases (>95%) are reported from Arizona and California, and the highest incidence areas continue to be California’s Central Valley and southern Arizona. About 1–2% of cases are reported from Nevada, New Mexico, and Utah, where the disease is endemic, though with lower incidence than Arizona and California. The remaining cases occur in states with even lower levels of endemicity or in residents of nonendemic states who have traveled to endemic areas.
Cases reported to public health represent a small proportion of all infections. First, only about 40% of coccidioidomycosis infections are symptomatic.7 The true number of cases is substantially higher because among symptomatic infected persons, some do not seek medical care, others are misdiagnosed, and some identified cases are not reported. Preliminary estimates of the annual burden of symptomatic coccidioidomycosis suggest that the true number of cases is 6 to 14 times greater than that reported to public health.8
Understanding of geographical risk for coccidioidomycosis in the southwestern United States is largely based on studies of coccidioidin skin test reactors performed in the late 1940s and early 1950s.9 Since then, investigation of coccidioidomycosis outbreaks outside the traditionally defined range have expanded the recognized endemic areas. These include several outbreaks among archeologists in Northern California and at Dinosaur National Monument in Northern Utah.10–13 Additionally, a cluster of cases in south-central Washington State identified endemic areas much farther north than previously described.14 Efforts are underway to improve understanding of the ecological niche of Coccidioides species in the environment by modeling soil and climatic variables in collaboration with the United States Department of Agriculture.15
Efforts to enhance collaboration led to the establishment of a Coccidioidomycosis Public Health Working Group, which now provides a platform to share information, develop protocols, and assist in standardizing surveillance methods across the United States. This Working Group is composed of local, state, and federal public health agencies, working together to advance epidemiologic knowledge of the disease. Collaborations have also led to advancements in molecular tools to detect DNA of Coccidioides spp., which have allowed environmental surveillance to enhance epidemiologic investigations. Additionally, whole-genome sequencing has provided methods to investigate epidemiologically linked clusters and determine likely locations of exposure.16–18
Coccidioidomycosis causes substantial morbidity, with a burden that far exceeds the numbers of reported cases. Increasing clinical awareness is key to improving diagnosis and identification of coccidioidomycosis. Improving case reporting to public health will add to our knowledge geographic risk for this disease, which likely extend beyond the historically defined range. Because many cases occur in travelers to endemic areas who return to states, where the disease is uncommon, clinicians in non-endemic areas also need to be aware of the risk of coccidioidomycosis. Ensuring accurate coccidioidomycosis case reporting to public health improves our understanding of the disease and can strengthen prevention and treatment efforts.
The epidemiology of coccidioidomycosis in Arizona
Surveillance
The first cases of coccidioidomycosis in Arizona were reported in the 1930s. In 1994, the Council of State and Territorial Epidemiologists (CSTE) established a national case definition for surveillance of coccidioidomycosis. Since then, cases reported from Arizona typically contribute approximately two thirds of the national case count annually. In 1997, the Arizona Department of Health Services made all positive laboratory test results for Coccidioides spp. reportable by administrators of clinical laboratories.19 Because of the large number of reported cases, confirmed cases are counted using only laboratory criteria. These were validated in 2007, and over 95% of patients with positive laboratory test results had compatible symptoms and met the CSTE case definition.20 Over the last two decades since instituting mandatory laboratory reporting of coccidioidomycosis, completeness of reporting in Arizona has increased and incidence has greatly increased.
Demographic characteristics of coccidioidomycosis patients in Arizona
Incidence of coccidioidomycosis increase steadily with age, with those over age 70 experiencing the highest rate at 209 cases per 100,000 population while the rate for those between 1 and 4 years of age is 7.7 per 100,000 population. From 1990 to 2008, the percent of cases in males fluctuated between 51% and 66%, but from 2009 to 2015 the gender of reported cases showed a slight female predominance.21–23 In 2016, males again predominated.24 Coccidioidomycosis cases are reported among residents of all 15 counties in Arizona, with 95% of cases reported from the most populous counties of Maricopa, Pima, and Pinal. Incidence is highest in central and southern counties, varying substantially by county.22 This pattern has been relatively consistent over time. In 2009, a major commercial laboratory began reporting all positive Coccidioides enzyme immunosorbant Assay (EIA) test results in addition to those confirmed by an immunodiffusion test. This resulted in a large increase in reported EIA-only positive coccidioidomycosis tests. During this time, the ratio of male to female cases changed to slightly more females than males.22 In 2012, the same commercial laboratory switched to a different Coccidioides EIA test kit. Reported EIA-only positive results dropped substantially.23 Although a portion of the 2009–2012 increase might have been attributable to EIA immunoglobulin M (IgM) only tests, this does not explain the entire increase.
The reason for the increase is unclear and is likely due to multiple causes. Arizona is a rapidly growing Sun Belt state, with a population increase of 75% from 1990 to 2010.25 The influx likely includes persons who are immune-naive to coccidioidomycosis. The Phoenix and Tucson metropolitan areas have experienced suburban expansion concurrent with population growth. Changes in land use from native desert to developed land may be associated with increased soil-borne dust exposure. Several studies have also correlated climate factors with coccidioidomycosis incidence.6,26,27 However, climate factors alone are unlikely to drive the nearly linear increase in incidence. In addition to the reporting changes mentioned earlier, public and clinician awareness of coccidioidomycosis in Arizona might have led to greater care seeking and testing. However, a retrospective cohort study among two large healthcare systems in metropolitan Phoenix published in 2008 reported that only 2–13% of patients presenting with community acquired pneumonia were tested for coccidioidomycosis,28 despite a public health recommendation for testing. Arizona’s population is, on average, older than the US population, and the proportion of the population aged over 65 has increased significantly over time.25 Since older populations have a higher prevalence of chronic disease, immunosuppressive therapy use, and health insurance coverage, they are at higher risk of severe or symptomatic infection and more likely to seek care and be diagnosed with the disease.29
Severity
Hospitalizations with a primary diagnosis of coccidioidomycosis based on ICD-9 and ICD-10 discharge diagnosis codes likely underestimates the true burden but is likely indicative of actual disease trends. The increase in coccidioidomycosis incidence during the laboratory reporting change was also seen in hospitalizations, though the increase was less dramatic. In 2015, hospitalized patients were mostly male (59%), and the median age was 51. There were $50 million in charges, and 56% of hospitalizations were paid for by Medicare or Medicaid.23 A review of coccidioidomycosis cases reported from 2009 to 2013 revealed that 1.5% of cases were hospitalized for disseminated coccidioidomycosis, compared with 8% and 4% in the 2007 and 2012 patient interviews from enhanced surveillance, respectively.20 Approximately 89 patients were admitted each year for coccidioidomycosis meningitis, with highest rates in males and African Americans. From 2009 and 2016, deaths from coccidioidomycosis ranged from 42 to 64 each year; however, a capture-recapture study of 2008–2013 coccidioidomycosis-associated deaths registered in the state vital record system compared to a database of hospital discharges estimated that deaths in Arizona are underestimated sevenfold by underlying cause of death and twofold by using any form of death certificate data (the current ADHS definition). Coccidioidomycosis was 2.4 times more likely to be listed as a cause of death if the patient was infected with human immunodeficiency virus (HIV) and 1.7 times more likely to be listed if they had disseminated coccidioidomycosis.30
To better understand the health impact of coccidioidomycosis in Arizona, we conducted population-based enhanced surveillance for reported coccidioidomycosis cases for 2007–2008.20 A systematic random sample of cases were interviewed with a standardized questionnaire about the diagnosis, course of illness, healthcare utilization, and the impact of the disease on their activities of daily living (ADL). In the 2007–2008 survey, 493 patients were symptomatic for a median of 120 days, 46% visited an emergency department, 41% were hospitalized for a median of 6 days, 26% saw a provider over 10 times, 75% were unable to perform their ADL for a median of 47 days, and of those working, 75% missed work for a median of 2 weeks. The median interval from first healthcare visit to diagnosis of coccidioidomycosis was 23 days. Patients who were aware of the disease were more likely to receive an earlier diagnosis and were more likely to ask to be tested for coccidioidomycosis than those who were not aware of the disease.20 In 2012, a second standardized questionnaire was used to interview 612 cases. Compared with the 2007–2008 results, 23% fewer respondents (18%) were hospitalized, and 18% fewer cases (28%) visited the emergency room. Of the 612 patients interviewed, 28% were diagnosed with pneumonia; 43% were prescribed antibiotics, with 30% having at least two courses; and 39% were prescribed an antifungal (K. Komatsu, unpublished data).
Awareness
In 2008, we assessed public awareness of coccidioidomycosis using the Behavioral Risk Factor Surveillance System, a telephone survey on health behavior of a representative sample of residents across Arizona. Twenty percent of respondents had never heard of Valley fever, and 33% did not know how it is transmitted. Twenty-five percent had lived in Arizona for less than 10 years, compared with 40% of the enhanced surveillance cases.20
In 2007, we conducted a survey of nurse practitioners’ and physicians’ knowledge, attitudes, and practices related to coccidioidomycosis. In 2007, reported confidence in the ability to treat coccidioidomycosis was 54%, and 60% had a working knowledge of different laboratory tests. However, those health-care providers who had received continuing medical education in the preceding 3 years were more likely to counsel patients about coccidioidomycosis, test patients with community-acquired pneumonia, offer treatment to immunosuppressed patients and test asymptomatic patients with underlying conditions.31
Update on epidemiology of coccidioidomycosis in California, 2016
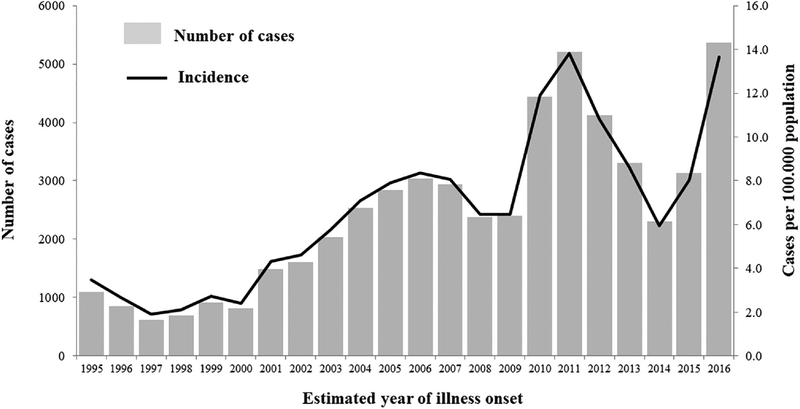
California’s San Joaquin Valley, from which “Valley Fever” was derived as a synonym of coccidioidomycosis, remains one of the locations with the highest incidence of the disease in the United States. In 2016, California reported 5372 cases statewide that involved estimated illness onset during that year, which was the highest yearly number of cases to date since California made coccidioidomycosis reportable in 1995 (Fig. 2).4 The incidence per 100,000 population was 13.7, a 71% increase from 2015, when it was 8.0. Cases affected predominantly males (3382 [63%]) with incidence of 17.3 per 100,000, compared to 1985 (37%) in females for a rate of 10.0. Incidence was highest, 18.8, among people 40–59 years old; rates by race and ethnicity were not calculated because of a substantial proportion missing this information (1759 [33%]).4 Seventy percent of cases were reported from seven endemic counties in the California Central Valley and Central Coast, with Kern County having the highest rate of 251.7 per 100,000 population.4
Figure 2.
Number of coccidioidomycosis cases and incidence, by estimated year of illness onset: California, 1995–2016 (1).
The increase in coccidioidomycosis cases observed in California was not seen elsewhere. Interestingly, the 2016 incidence Arizona was 89.3/100,000, lower than the 2015 incidence of 112.8; for other states where coccidioidomycosis is reportable, the combined 2016 incidence rate remained stable at 0.5.4
The reasons for the 2016 increase of coccidioidomycosis cases in California are not well understood but might include an increase in precipitation in January 2016 after several years of drought, other climatic and environmental factors favoring Coccidioides proliferation and airborne release, and dust generating activities. Some also attribute the rise to an increase in non-immune populations in endemic areas. Looking back to the 1991–1994 coccidioidomycosis epidemic in California, “abundant rain in March 1991 and February–March 1992” after several years of drought likely contributed to an increase in reported cases in late 1991 and an even larger increase in late 1992, mostly in Kern and other endemic counties.32 The winter of 2016–2017 turned out to be one of the rainiest winter seasons in California.33 At this time, the effect of this wet winter following a prolonged drought in California on the number of coccidioidomycosis cases in 2017 will not be fully known until after 2017 reported cases have been confirmed.
Efforts to reduce risk of coccidioidomycosis among inmates in the California State Correctional System
The incarcerated population in California (CA) increased from ~ 20,000 in 1980 to ~160,000 in 2000.34,35 This eightfold increase led to construction of new prisons, seven of which (for men) were built in the coccidioidomycosis endemic area of CA. Because 89% of inmates are convicted from nonendemic areas of CA, imprisonment in the coccidioidomycosis endemic area of CA places them at risk for a disease to which they may not have been previously exposed.
In 2005, an outbreak of coccidioidomycosis including 166 cases and four deaths occurred at one of the prisons built in the 1990s.36 As a result of this outbreak, and high rates of coccidioidomycosis among state prison inmates in CA, the California Correctional Health Care Services (CCHCS) initiated a series of interventions to prevent coccidioidomycosis among inmates from 2007 through 2017. The interventions included environmental modifications, as well as exclusion of individuals from prisons with expected highest rates of coccidioidomycosis based on: (1) inmate clinical and demographic risk factors, and (2) coccidioidomycosis skin test results of incoming inmates.
In 2007, all immunocompromised inmates were medically restricted from residing in any prisons in the coccidioidomycosis endemic area. Despite this restriction, from 2007 through 2012, high rates of coccidioidomycosis were identified at two of the prisons (5306 cases/100,000 population in 2011) and an average of six deaths per year due to coccidioidomycosis occurred among inmates.
As a result, further environmental interventions were implemented to reduce exposure to Coccidiodes spp. in the two prisons with the highest coccidioidomycosis rates. Locally generated dust was reduced by placing either soil sealant or grass on bare areas of dirt. To reduce Coccidioides exposure indoors, high-grade filters were installed, as recommended by the National Institute of Occupational Health and Safety (NIOSH). An additional investigation of risk factors for coccidioidomycosis among male inmates in 2011 identified diabetes mellitus as a risk factor for severe coccidioidomycosis (with prolonged hospitalization), and African-American race was a risk factor for disseminated coccidioidomycosis.37 Subsequently, in 2013, African Americans, Filipinos, and those with diabetes mellitus were medically restricted from the two prisons with the highest coccidioidomycosis rates.
In 2014, CDC evaluated strategies using a newly formulated Coccidioides delayed type hypersensitivity (DTH) skin test, Spherusol ® for risk stratification of inmates. CDC estimated that a 61% reduction in coccidioidomycosis cases among inmates could be achieved if prisons were populated with inmates who test positive with the skin test (and therefore have a lower risk of coccidioidomycosis).38 In contrast, CDC estimated that CCHCS’s program of medical restrictions based on medical and demographic criteria would decrease coccidioidomycosis rates by only about 10%. Based on these recommendations, in 2015, CCHCS initiated a coccidioidomycosis prevention program with risk stratification using the coccidioidomycosis DTH skin test. We offered the coccidioidomycosis DTH skin test to all inmates eligible to reside in the two prisons with the highest rates of coccidioidomycosis. We defined a positive test as ≥5 mm of induration. Adverse reactions were defined as any localized or systemic signs or symptoms reported by the inmate or observed by the nurse at the time of the test reading. We assessed risk factors for skin test positivity using logistic regression.
Among the 96,987 inmates offered the test, 38% accepted. Of the 36,789 inmates tested, 1713 (4.7%) had adverse reactions of which nearly all were minor (including itching or rash at the injection site), and 3169 (8.6%) had a positive test. The mean induration of all positive tests was 12 mm. The positive skin test rate among inmates who ever resided at either of the two prisons with the highest coccidioidomycosis rates or other endemic prisons was 17%. For those inmates who resided at the time of testing at the two prisons with the highest rates, but not other endemic prisons, the positive skin test rate was 14%. For those inmates who resided in endemic prisons at the time of testing, excluding the two prisons with the highest rates, a positive skin test rate of 6% was identified. Finally, inmates of prisons in nonendemic areas had a positive skin test rate of 3%. Our results indicate the coccidioidomycosis skin test has a low rate of adverse reactions and performs as expected in identifying those who were infected with Coccidioides. The independent risk factors for a positive skin test identified are factors we would expect to find associated with past Coccidioides infections: residence at the two prisons with the highest rates of coccidioidomycosis, length of time at those two prisons, residence in an endemic county, and increasing age.39
Coccidioidomycosis skin tests have been used in past evaluations of Coccidioides exposure and in the medical management of coccidioidomycosis. This is the first time to our knowledge that results of coccidioidomycosis skin tests have been used for risk stratification in a coccidioidomycosis prevention program. We used the results of the coccidioidomycosis skin test to identify inmates at higher risk of coccidioidomycosis (those with negative coccidioidomycosis skin tests) and restricted them from residence in two prisons with higher rates of coccidioidomycosis.
The use of the coccidioidomycosis skin test in risk stratification might benefit other groups at risk of exposure to Coccidioides. For example, the coccidioidomycosis skin test could be offered to workers required to perform higher risk occupational activities (e.g., digging in dirt) in coccidioidomycosis endemic areas, such as archeologists, construction workers, solar farm workers, and wildland firefighters. Test results would permit risk stratification of these workers. Workers identified with a higher risk of coccidioidomycosis (those who test negative) could choose not to work in coccidioidomycosis-endemic areas or could be provided with personal protective equipment to reduce the risk of Coccidioides spp. exposure when performing high-risk activities in a coccidioidomycosis endemic area.
Coccidioidomycosis in Washington State
Coccidioidomycosis is an emerging infection in Washington State (WA). The suspicion of the first identification of locally acquired coccidioidomycosis in WA occurred in 2010 and was confirmed with genomic epidemiology (further discussed below in the genomic epidemiology section).14 Phylogenetic analysis of the initial locally acquired cases and an isolate grown from WA soil revealed that WA isolates form a distinct clade on the phylogenetic tree.18 The Washington State Department of Health (WA DOH) implemented surveillance for coccidioidomycosis statewide in April 2014. Prior to 2014, cases of coccidioidomycosis were reported sporadically, but no standard reporting procedure was in place. The historical epidemiology of coccidioidomycosis in WA is therefore poorly understood.
Since 2014, there has been a steady increase in cases reported to WA DOH, likely due to increasing awareness of the condition and improved compliance with reporting. The current WA DOH case reporting form consistently collects travel histories for coccidioidomycosis cases, as travel outside WA, especially to the American Southwest, remains the biggest risk factor for WA residents. As of August 2017, 12 confirmed cases with suspected or confirmed local exposures have been identified, all in four counties in south-central WA. Case-patients are considered to have acquired coccidioidomycosis locally if they are associated with a clinical isolate that matches the WA Coccidioides immitis clade or positive Coccidioides spp. serology results without travel to other endemic areas.
Among these 12 locally acquired cases, two (17%) deaths were reported, and 67% of patients were hospitalized. Most (75%) cases were in males. While most (75%) reported locally acquired case-patients presented with pneumonia, rare disease presentations have also been reported, including two cases of meningitis and one cutaneous wound infection. The majority of cases report significant soil or dust exposure. Locally acquired cases of coccidioidomycosis have higher rates of hospitalization and death compared with travel-associated cases and statistics reported from other endemic regions.40 As the majority of people infected with Coccidioides are asymptomatic or have only mild illness, and the majority of illness self-resolves, we believe only the most severe cases of coccidioidomycosis with exposure in WA are being identified and reported to public health authorities.
Environmental sampling efforts have identified the fungus in soil from Benton and Yakima counties; limited sampling has been done in other WA counties, so the geographic range of the organism is still undefined. Domestic and wild animals can be infected, and dogs and a horse without travel outside of WA have been diagnosed with coccidioido mycosis.
To improve surveillance for coccidioidomycosis, several projects are underway. These include analyzing commercial laboratory data to identify gaps in surveillance, establishing cross-reporting of occupational case notifications with the Department of Labor and Industries, and implementing a knowledge, attitudes, and practices (KAP) survey to assess the baseline knowledge of regional providers. Results from these projects will be used to evaluate and strengthen the surveillance system and prioritize areas for provider education.
Continuing efforts are needed to improve surveillance of coccidioidomycosis in WA to better understand the epidemiology of the disease in an emerging region, improve patient outcomes, and prevent disease.
Genomic epidemiology: Tracking Coccidioides around the hemisphere
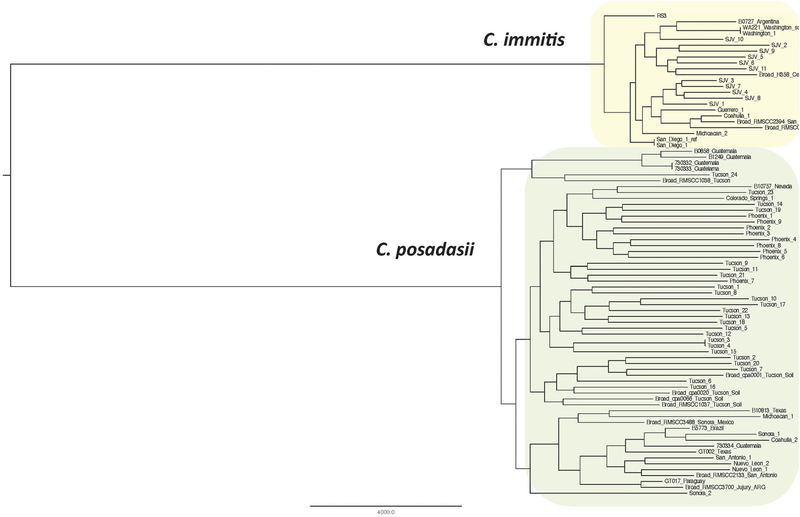
Coccidioides posadasii and C. immitis are geographically limited primarily to the American Southwest, with scattered foci in Mexico41,42 and in South America.43 Genomic epidemiology can help shed light on the distribution of these organisms and areas of increased disease risk. The organisms are primarily known to inhabit highly thermic soils in arid regions.44,45 While the two species are geographically and genomically distinct (Fig. 3), they appear to cause identical illness, with no detectable difference in virulence or clinical manifestation in mouse models or human disease,46 although in vitro differences in halotolerance and thermotolerance have been reported, possibly reflecting environmental adaptation in differing locales.47 Coccidioides reproduces asexually and, likely, sexually, providing challenges in understanding its population structure. This has led to several attempts to develop molecular typing methods that provide both large-scale phylogenetics and fine scale genotyping for molecular epidemiology.48–51
Figure 3.
Whole genome SNP phylogeny of Coccidioides genus including 59 C. posadasii and 22 C. immitis genomes. This Figure is reproduced in color in the online version of Medical Mycology.
Microsatellites allowed for the first true Coccidioides population genomics analyses, including the phylogenetic separation of the two species,52 and the identification of five sub-populations of Coccidioides: Central California, Southern California, Arizona, Texas, and Mexico, with South American isolates belonging to a subpopulation of the Texas group.53 However, microsatellites provided only limited ability to determine local population structures (e.g., within one of the five regional subpopulations). This, in turn, limited its utility for molecular epidemiology for studies of outbreaks and emergence in nonendemic regions.
Genomic epidemiology for Coccidioides
As with other eukaryotic and prokaryotic microbes, comprehensive whole genome analysis provides the greatest resolution for genotyping and phylogenetic analysis,54–57 allowing for the shift from “molecular epidemiology” to “genomic epidemiology.” Prior to the advent of next generation sequencing, limited C. immitis and C. posadasii genomes (n = 14) were sequenced using standard Sanger sequencing58 in an attempt to obtain geographic, clinical and environmental diversity.47 This work provided the initial backbone for developing a genomic epidemiology approach for Coccidioides. Genomic epidemiology was first proven useful as a tool for an investigation into the suspect Coccidioides outbreak associated with transplant of possibly infected solid organs.16 Three organ recipients each received solid organs from the same donor and all went on to develop serious C. immitis infections, two of them fatal. As no comparative samples were available from the donor, the isolates from the patient were analyzed by comparative genome analysis and compared to each other and to other genomes of C. immitis. The analysis showed that no more than two single-nucleotide polymorphisms (SNPs) occurred between patient strains, where at least 20,000 SNPs separated patient strains from background.16 This was the first use of genomic epidemiology for a fungal outbreak, proving its capability to link cases and place them within a population level context.
Genomic epidemiology was again employed in the analysis of a Guatemalan patient with HIV was infected with Coccidioides while traveling to obtain migrant farm work; these techniques determined that he was infected in Texas rather than in endemic zones in his home country.17
Coccidioides dispersal: From Pleistocene to the Pacific Northwest
The current biogeography of Coccidioides is well established, with the primary populations occurring in the California Central Valley for C. immitis and central and southern Arizona for C. posadasii.59 There has been apparent dispersal of both species, with additional endemic regions of C. immitis occurring in lower California and the Mexican state of Baja California, and endemic regions of C. posadasii occurring in northern Mexico, Texas, and several countries in South America.43,53 Although C. posadasii is more widespread and apparently more genomically variable, there is little agreement on whether one strain originated from the other. It is worthwhile to consider the possible paleo-epidemiology of the organism. Although the fungus is rarely found beyond the known current endemic region (see previous section regarding recent WA emergence), there is evidence of ancient fungal infections outside the current endemic zone, including the prehistoric burial midden from the Holocene period (8000 years ago) found at Dinosaur National Monument in northern Utah.45,60,61 This may represent a prehistoric North American endemic zone, as climate in this region during the Holocene period was more arid and warm than it is today. Conversely, that site may represent focal deposition of fungus by humans, as positive samples were only found in burial middens, where fungi may have seeded the environment during decomposition of human and/or companion animal bodies.
C. posadasii dispersal
Based on data collected by microsatellites, Fisher et al. argued that the South American populations belong to a distinct subpopulation typified by the Texas regional population. Using molecular clock calculations, they estimated that the South American population arrived as little as 8900 years before present (ybp). This roughly coincides with the expansion of humans from North America to South America. Conversely, they also suggest that arrival of Coccidioides in South America may have occurred during the previous 100,000 years along with southward movement of several rodent and small mammal reservoir species.53
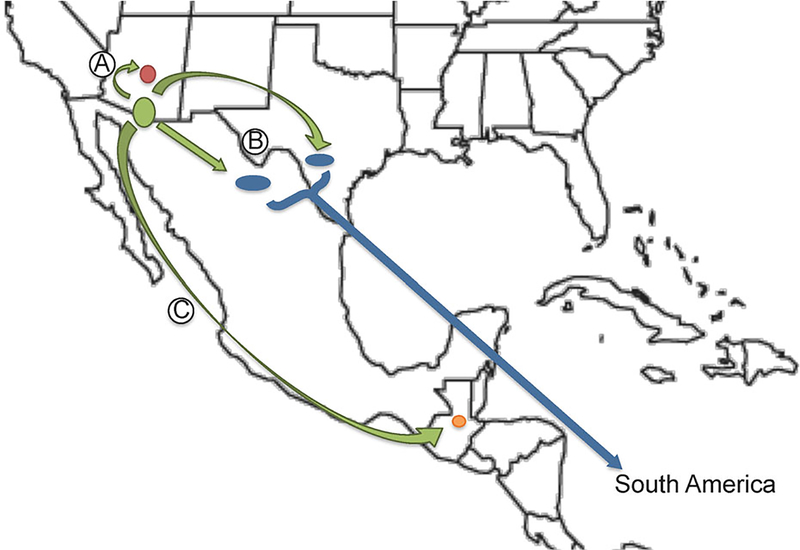
By employing Baeysian analysis of over 80 genomes, we have recalculated the initial expansion of C. posadasii out of southern Arizona as occurring roughly 800,000 years ago, with populations expanding into Texas and Mexico about 675,000 years ago and into South America no later than 500,000 years ago17 (Fig. 4). This timing would coincide with, or be subsequent to, the large-scale movements of mammals during the Great American Biotic Interchange events between North and South America, following the Panamanian connection between the continents during the Pleistocene. These data suggest that Coccidioides likely dispersed from its original endemic loci via animal transport, that is, infected mammals likely moved from endemic regions and died in previously nonendemic locales, thereby contaminating soils and establishing new endemic loci, rather than from anthropogenic causes.17
Figure 4.
Dispersal model of C. posadasii out of southern AZ, with (A) northward spread to central AZ 700 KYA (thousand years ago); (B) southeastern spread to Texas and Mexico 675 KYA and then to South America 520KYA; and (C) southern spread to Guatemala 190 KYA (adapted from (21)). This Figure is reproduced in color in the online version of Medical Mycology.
C. immitis dispersal
It is estimated that C. immitis as a species diverged about 370,000 years ago in the San Joaquin Valley region of California, perhaps due to other paleogeographic effects.17 During the shift from the Miocene to the Pleistocene, the Baja peninsula began to form and much of the western coastline of California surfaced, with the Central (San Joaquin) Valley still a large inland body of water, which was replaced by fresh water from the glacial melt of the Sierra Nevada Mountains.62 The ultimate draining of this body occurred 700,000 years ago, forming the Central Valley, and subsequently becoming a fertile ground for C. immitis growth and expansion.17 The Sierra Nevadas therefore may have acted as both a glacial refuge and as a reproductive barrier to the most western portion of the paleo-populations of Coccidioides, allowing for divergence into the two species to occur at this time.
It is now understood that modern dispersal is also occurring with Coccidioides. In 2010, three separate cases of coccidioidomycosis were identified in southeastern Washington state, well outside any known endemic zone.63 All three patients had travel to endemic regions (i.e., California and Arizona) several years prior to onset. In one particular instance, however, a patient had a knee-wound infection following a crash on an all-terrain vehicle at an off-road park in southeastern WA. Soils collected from the crash site during the initial investigation were recently analyzed and found positive by polymerase chain reaction, and subsequent cultures provided isolates for genomic analysis to compare to the patient’s isolate and other genomes. The patient and soil isolates were nearly genomically identical (i.e., no more than 3 SNPs separated clinical and soil isolates), providing a clear link to autochthonous exposure.18 The WA genomes were clearly C. immitis and fell within a clade most similar to isolates collected from San Joaquin Valley.18 A follow-up study of the soil samples from the same region as the original ATV park 4 years later has demonstrated that C. immitis remains present in the park and the surrounding regions, and additional cases have since been found providing evidence that Coccidioides is now endemic in this region of eastern WA. Studies are underway to better explore the habitat niches being exploited by both new and old populations of Coccidioides to produce models for areas at risk for additional expansion of the endemic range.
The epidemiology—both traditional and genomic—of coccidioidomycosis is crucially important to improve understanding of the disease as it continues to evolve over time. Accurate and standardized reporting of cases to public health is needed to determine the burden of morbidity on public health and healthcare systems. The overall trend of reported cases of coccidioidomycosis had been one of dramatic increase over the past two decades. We discussed some of the potential reasons for these changes, such as changes in the susceptible populations and effects of climate, precipitation, and drought. Future directions for epidemiologic work related to coccidioidomycosis include efforts to improve understanding of how these factors influence disease incidence.
Enhanced surveillance efforts have identified delays in seeking healthcare, delayed diagnoses, and extensive treatment with ineffective antibiotics. Efforts to collect enhanced case investigation of surveillance data beyond capturing case counts, basic demographics and seasonality have been important to describe disease severity, the burden on the healthcare system, and the economy. Future improvements in surveillance should include standardized collection of key data elements systematically to gain insight into the impact of the disease across different regions.
Better definition of populations at risk for coccidioidomycosis and those at highest risk for severe disease are important for targeting prevention efforts. In areas where the disease is endemic, it is difficult for people to avoid exposure to Coccidioides. One strategy to reduce risk is to implement population risk stratification as described for inmates in the California Corrections system. This or other strategies to reduce exposure risk may be used for groups at increased risk for severe or disseminated disease, or those at increased risk for exposure, such as workers participating in soil-disturbing activities. Future directions will include the development of methods to evaluate the implementation and effectiveness of various prevention strategies.
Genomic epidemiology is rapidly becoming an important tool for next generation public health science, as a technological approach that provides empirical evidence for outbreak investigations and new areas of endemicity. This epidemiological approach provides multi-layered population structure for understanding historic distributions and dispersal, ongoing dispersal in Washington, and will provide an important tool for determining any future dispersal events. As these tools are used to better define new areas of coccidioidomycosis endemicity, it will be critical to focus on standardizing surveillance for coccidioidomycosis, even in states outside of the historically described endemic areas. The disease is reportable in 22 states in 2017, but expansion to reporting in all states is key to understanding the epidemiology and impacts of the disease nationwide.
Acknowledgement
We gratefully acknowledge all county health departments, reporting laboratories and providers.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1.Council of State and Territorial Epidemiologist Coccidioidomycosis Case Definition. 2011. https://wwwn.cdc.gov/nndss/conditions/coccidioidomycosis/case-definition/2011/. Accessed 9/28/17, 2017.
- 2.Centers for Disease Control and Prevention Valley Fever (Coccidioidomycosis) Statistics. https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html. Accessed January 13, 2018.
- 3.CDC Increase in Reported Coccidioidomycosis — United States, 1998–2011. MMWR. 2013; 62: 217–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Cooksey GS, Nguyen A, Knutson K et al. Notes from the field: increase in coccidioidomycosis—California, 2016. MMWR Morb Mortal Wkly Rep. 2017; 66: 833–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talamantes J, Behseta S, Zender CS. Fluctuations in climate and incidence of coccidioidomycosis in Kern County, California: a review. Ann N Y Acad Sci. 2007; 1111: 73–82. [DOI] [PubMed] [Google Scholar]
- 6.Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect. 2005; 113: 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CE, Whiting EG et al. The use of coccidioidin. Am Rev Tuberc. 1948; 57: 330–360. [DOI] [PubMed] [Google Scholar]
- 8.Freedman MAS, Benedict K, McCotter O et al. Preliminary estimates of annual burden of coccidioidomycosis in the United States, 2010–2014. Paper presented at The 7th International Coccidioidomycosis Symposium; 2017; Standford, CA. [Google Scholar]
- 9.Edwards PQ, Palmer CE. PRevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Chest. 1957; 31: 35–60. [DOI] [PubMed] [Google Scholar]
- 10.Loofbourow JC, Pappagianis D, Cooper TY. Endemic coccidioidomycosis in Northern California: an outbreak in the Capay Valley of Yolo County. Calif Med. 1969; 111: 5–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Werner SB, Pappagianis D, Heindl I, Mickel A. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med. 1972; 286: 507–512. [DOI] [PubMed] [Google Scholar]
- 12.Werner SB, Pappagianis D. Coccidioidomycosis in Northern California: an outbreak among archeology students near Red Bluff. Calif Med. 1973; 119: 16–20. [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. Coccidioidomycosis in workers at an archeologic site: Dinosaur National Monument, Utah, June–July 2001. MMWR Morb Mortal Wkly Rep. 2001; 50: 1005–1008. [PubMed] [Google Scholar]
- 14.Marsden-Haug N, Goldoft M, Ralston C et al. Coccidioidomycosis acquired in Washington State. Clin Infect Dis. 2013; 56: 847–850. [DOI] [PubMed] [Google Scholar]
- 15.Dobos R, McCotter O. Modeling and mapping of coccidioides soil habitat. Paper presented at The 7th International Coccidioidomycosis Symposium; 2017; Stanford, CA. [Google Scholar]
- 16.Engelthaler DM, Chiller T, Schupp JA et al. Next-generation sequencing of Coccidioides immitis isolated during cluster investigation. Emerg Infect Dis. 2011; 17: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelthaler DM, Roe CC, Hepp CM et al. Local population structure and patterns of western hemisphere dispersal for Coccidioides spp., the fungal cause of Valley Fever. MBio. 2016; 7: e00550–00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvintseva AP, Marsden-Haug N, Hurst S et al. Valley Fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin Infect Dis. 2015; 60: e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arizona Administrative Register 1997. 3: 1187 http://apps.azsos.gov/public_services/register/1997/18/contents.shtm. Accessed March 13, 2018. [Google Scholar]
- 20.Tsang CA, Anderson SM, Imholte SB et al. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis. 2010; 16:1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunenshine RH, Anderson S, Erhart L et al. Public health surveillance for coccidioidomycosis in Arizona. Ann N Y Acad Sci. 2007; 1111: 96–102. [DOI] [PubMed] [Google Scholar]
- 22.Hector RF, Rutherford GW, Tsang CA et al. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Res Public Health. 2011; 8: 1150–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arizona Department of Health Services. Valley Fever 2015 Annual Report. http://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/valley-fever/reports/valley-fever-2015.pdf. Accessed March 13, 2018.
- 24.Arizona Department of Health Services. Valley Fever 2016 Annual Report. http://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/valley-fever/reports/valley-fever-2016.pdf. Accessed March 13, 2018.
- 25.United States Census https://www.census.gov/quickfacts/fact/table/US/PST045217. Accessed January 15, 2018.
- 26.Kolivras KN, Comrie AC. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int J Biometeorol. 2003; 47: 87–101. [DOI] [PubMed] [Google Scholar]
- 27.Tamerius JD, Comrie AC. Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PLoS One. 2011; 6: e21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang DC, Anderson S, Wannemuehler K et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008; 14: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leake JA, Mosley DG, England B et al. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996–1997. J Infect Dis. 2000; 181: 1435–1440. [DOI] [PubMed] [Google Scholar]
- 30.Jones JM, Koski L, Khan M, Brady S, Sunenshine R, Komatsu KK. Coccidioidomycosis: an underreported cause of death-Arizona, 2008–2013. Med Mycol. 2018; 56: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S, Erhart LM, Anderson S et al. Coccidioidomycosis: knowledge, attitudes, and practices among healthcare providers: Arizona, 2007. Med Mycol. 2011; 49: 649–656. [DOI] [PubMed] [Google Scholar]
- 32.Pappagianis D. Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin Infect Dis. 1994; 19: S14–18. [DOI] [PubMed] [Google Scholar]
- 33.Weather Nation California. http://www.weathernationtv.com/news/wet-2017-water-year-ends-california/. Accessed November 15, 2017.
- 34.Offender Information Services Branch, California Department of Corrections and Rehabilitation. California Prisoners Report 1980: summary statistics of felon prisoners and parolees Youth and Adult Correctional Agency, Department of Corrections, Administrative Services Division. Sacramento: 1981. https://www.cdcr.ca.gov/Reports_Research/Offender_Information_Services_Branch/Annual/CalPris/CALPRISd1980.pdf. [Google Scholar]
- 35.Offender Information Services Branch, California Department of Corrections and Rehabilitation. California Prisoners Report 2001: summary statistics of felon prisoners and parolees Youth and Adult Correctional Agency, Department of Corrections, Administrative Services Division. Sacramento: 2001. https://www.cdcr.ca.gov/Reports_Research/Offender_Information_Services_Branch/Annual/CalPris/CALPRISd2001.pdf#page33. [Google Scholar]
- 36.Lee LA, Yuan J, Vugia D, Wheeler C, Chapnick R, Mohle-Boetani J. Increased coccidioidomycosis among inmates at a California Prison: initial investigation in 2005 to 2006. J Correct Health Care. 2017; 23: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015; 21: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purfield A, Derado G, Mohle-Boetani J, Wheeler C, Park B. Preventing coccidioidomycosis (Valley Fever) at highly endemic prisons in California: estimating the effect of a screening skin test to identify immune inmates. Open Forum Infect Dis. 2014; 1: S464–S464. [Google Scholar]
- 39.Wheeler C, Lucas K., Derado G, McCotter O et al. Risk stratification with coccidioidal skin test to prevent Valley Fever among inmates, California, 2015. J Correct Health Care. 2018; 24: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Washington State Department of Health. Washington State Annual Report on Fungal Disease, 2014. Vol DOH 420–14. http://www.doh.wa.gov/Portals/1/Documents/5100/420-146-FungalDiseaseAnnualReport.pdf. Accessed December 15, 2017.
- 41.Catalan-Dibene J, Johnson SM, Eaton R et al. Detection of coccidioidal antibodies in serum of a small rodent community in Baja California, Mexico. Fungal Biol. 2014; 118: 330–339. [DOI] [PubMed] [Google Scholar]
- 42.Luna-Isaac JA, Muniz-Salazar R, Baptista-Rosas RC et al. Genetic analysis of the endemic fungal pathogens Coccidioides posadasii and Coccidioides immitis in Mexico. Med Mycol. 2014; 52: 156–166. [DOI] [PubMed] [Google Scholar]
- 43.Hector RF, Laniado-Laborin R. Coccidioidomycosis: a fungal disease of the Americas. PLoS Med. 2005; 2: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown J, Benedict K, Park BJ, Thompson GR 3rd. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013; 5: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher FS, Bultman MW, Johnson SM, Pappagianis D, Zaborsky E. Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Ann N Y Acad Sci. 2007; 1111: 47–72. [DOI] [PubMed] [Google Scholar]
- 46.Pappagianis D. Epidemiology of coccidioidomycosis. Curr Topics Med Mycol. 1988; 2: 199–238. [DOI] [PubMed] [Google Scholar]
- 47.Barker BM, Jewell KA, Kroken S, Orbach MJ. The population biology of coccidioides: epidemiologic implications for disease outbreaks. Ann N Y Acad Sci. 2007; 1111: 147–163. [DOI] [PubMed] [Google Scholar]
- 48.Bialek R, Kern J, Herrmann T et al. PCR assays for identification of Coccidioides posadasii based on the nucleotide sequence of the antigen 2/proline-rich antigen. J Clin Microbiol. 2004; 42: 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burt A, Carter DA, Koenig GL, White TJ, Taylor JW. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc Natl Acad Sci U S A. 1996; 93: 770–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher MC, White TJ, Taylor JW. Primers for genotyping single nucleotide polymorphisms and microsatellites in the pathogenic fungus Coccidioides immitis. Mol Ecol. 1999; 8: 1082–1084. [DOI] [PubMed] [Google Scholar]
- 51.Koufopanou V, Burt A, Taylor JW. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc Natl Acad Sci U S A. 1997; 94: 5478–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher MC, Koenig G, White TJ, Taylor JW. A test for concordance between the multilocus genealogies of genes and microsatellites in the pathogenic fungus Coccidioides immitis. Mol Biol Evol. 2000; 17: 1164–1174. [DOI] [PubMed] [Google Scholar]
- 53.Fisher MC, Koenig GL, White TJ et al. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc Natl Acad Sci U S A. 2001; 98: 4558–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etienne KA, Gillece J, Hilsabeck R et al. Whole genome sequence typing to investigate the Apophysomyces outbreak following a tornado in Joplin, Missouri, 2011. PLoS One. 2012; 7: e49989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Litvintseva AP, Hurst S, Gade L et al. Whole-genome analysis of Exserohilum rostratum from an outbreak of fungal meningitis and other infections. J Clin Microbiol. 2014; 52: 3216–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engelthaler DM, Hicks ND, Gillece JD et al. Cryptococcus gattii in North American Pacific Northwest: whole-population genome analysis provides insights into species evolution and dispersal. MBio. 2014; 5: e01464–01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendriksen RS, Price LB, Schupp JM et al. Population genetics of Vibrio cholerae from Nepal in 2010: evidence on the origin of the Haitian outbreak. MBio. 2011; 2: e00157–00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.BroadInstitute. Coccidioides Group Database. https://www.broadinstitute.org/scientific-community/science/projects/fungal-genome-initiative/coccidioidesgenomes. Accessed December 15, 2009.
- 59.Pappagianis D. Epidemiology of coccidioidomycosis. Curr Topics Med Mycol. 1988; 2: 199–238. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease C, Prevention. Coccidioidomycosis in workers at an archeologic site: Dinosaur National Monument, Utah, June-July 2001. MMWR Morb Mortal Wkly Rep. 2001; 50: 1005–1008. [PubMed] [Google Scholar]
- 61.Petersen LR, Marshall SL, Barton-Dickson C et al. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg Infect Dis. 2004; 10: 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blakely R. Paleogeography and Geologic Evolution of North America. https://www2.nau.edu/rcb7/nam.html. Accessed January 15, 2018.
- 63.Marsden-Haug N, Hill H, Litvintseva AP et al. Coccidioides immitis identified in soil outside of its known range - Washington, 2013. MMWR Morb Mortal Wkly Rep. 2014; 63: 450. [PMC free article] [PubMed] [Google Scholar]