Abstract
Introduction
Non-union after humeral shaft fractures are seen frequently in clinical practice. The incidence is 2–10% after conservative management and up to 30% after surgical treatment. The purpose of this study is to evaluate the outcomes of plate-and-bone-strut-allograft technique with bone chip augmentation for aseptic non-unions of the distal third of the humerus.
Materials and methods
26 consecutive cases were treated using a trans-triceps approach. The non-union was fixed with a 4.5 mm Locking Compression Plate combined with a strut bone allograft at the anterior part of the humerus and bone chips. All patients underwent the same rehabilitation protocol of 12 weeks. Clinical evaluation took place 12 months after surgery with the Mayo elbow score and Oxford elbow score.
Results
Complete bone healing without complications was achieved in all 26 patients. The average period of radiographic union was 106 days. The average range of flexion-extension was 108° (94°–180°) and pro-supination was 159° (102°–180°). Twelve months after surgery, average Mayo elbow score was 86 (68–100) and the Oxford elbow score was 83 (52–100).
Conclusion
The plate-and-bone-strut-allograft technique with bone chip augmentation in distal humeral shaft for aseptic non-unions resulted in union of all cases. No adverse events related to the surgery or the materials used were documented.
Keywords: Aseptic non-union, Bone strut allograft, Complications, Material failure, Distal third humeral shaft, Outcomes
1. Introduction
Non-unions of humeral shaft fractures are seen frequently in clinical practice at about 2–10% in conservative management and 30% in surgically treated patients1. Factors favouring non-union can be patient related or therapy-related. In every case, a precise technique and proper indication are essential for success2, 3, 4, 5, 6. Non-union, displacement, and fixation failure are complications, that can lead to impairment after humeral shaft fractures2, 3, 4, 5, 6. The aseptic non-union is a problem associated with unfavourable biology at the fracture site. Therefore, the biological response has to be optimized, with for example bone grafting and augmentation which have shown good results in metaphyseal aseptic non-unions2, 3, 4, 5, 6. Our plate-and-bone-strut-allograft technique consists of extramedullary fixation with a biological graft and augmentation of the defect. The purpose of this study is to evaluate the outcomes of our technique in the management of distal third shaft humeral aseptic non-unions.
2. Materials and methods
2.1. Patient selection and follow-up
All patients who visited our institution between 2006 and 2018 with a distal third humeral shaft non-union were screened for treatment using the plate-and-bone-strut-allograft technique. The exclusion criteria were hematological or oncological disease, acute or chronic systemic infections, ASAMI non-union classifications type A (aseptic non-union without bone defect) and C (infected non-union)7, age under 18 years, any bone metabolism disease, elbow osteoarthritis and rheumatoid disease. At the outpatient clinic, the patients were informed in a clear and comprehensive way of the type of treatment and other possible surgical and conservative alternatives. Patients were treated according to the ethical standards of the Helsinki Declaration, and were invited to read, understand, and sign the informed consent form. If the patients agreed and underwent our proposed treatment, they were enrolled in this study.
To diminish bias because of different rehabilitation, we let all patients undergo the same rehabilitation protocol (see appendix). The objective outcomes during follow-up were surgical data, adverse events during follow-up, range of motion, the Mayo Elbow Score (MES)8 and humeral alignment measured using plain radiographs. Subjective patient reported outcomes were measured by Oxford Elbow Score (OES)9.
Follow-up consisted of questionnaires at 3 weeks and 3, 6 and 12 months after surgery. The evaluation endpoint was set at 12 months after surgery, yet follow-up was prolonged to the last outpatient visit up to November 2018. Bone union was measured using the radiographic union score as described by Whelan et al. and the Non-Union Scoring System (NUSS)10,11.
2.2. Statistical analysis
Cohen's kappa coefficient (κ) is a statistic which measures inter-rater agreement for qualitative items. Through this parameter we calculated the concordance between bone healing (healing of the anterior, posterior, lateral and medial cortices) and the anatomical axis of the humerus measured on plain radiographs.
2.3. Surgical technique
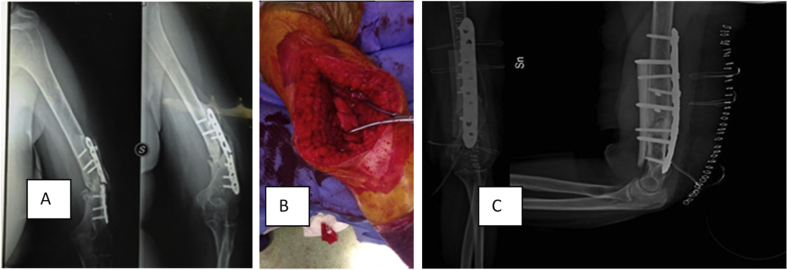
We used a standardised surgical technique. The patient was positioned prone with the arm on a radiolucent support or a padded post. Either option gives maximum freedom to approach the elbow. The approach was performed using a trans-triceps approach (TTA)12. The posterior midline skin incision was made, curving laterally around the tip of the olecranon. Full thickness skin flaps were elevated, and the ulnar nerve was identified, mobilized, and protected. The triceps tendon was split in its midline, and extending distally (Fig. 1), the incision was curved around the lateral aspect of the olecranon and then down along the lateral border of the proximal ulna. In extending the incision distally, we left a 3–5 mm cuff of triceps tendon and deep fascia on the lateral aspect of the proximal ulna, for subsequent side-to-side repair. After having exposed the non-union focus, we removed the previous implant and reduced the fracture. We reamed the intramedullary shaft and removed all non-vital tissue. The strut allograft (decellularized donor distal humeral shaft) was prepared on a separate table and a modelled 4.5 mm Locking Compression Plate® (LCP®, DepuySynthes™, Oberdorf, Switzerland) was temporarily fixed with a K-wire. Along the lateral side a window to the anterior side of the humerus was made, with regard to the radial nerve. Through this window the bone strut was placed anteriorly. Cortical lag screws were used to hold the plate-and-bone-strut-allograft, which was placed in such way to support the anterior humeral hinge (Fig. 1). Then we drilled the humeral shaft to implant a locking screw for angular stability for fixation of the allograft to the humeral shaft. Bone chips, either pre-chipped or manually chipped during surgery, were impacted as an augmentation inside the humeral shaft. We placed the bone chips mixed with Putty® Biocollagen Crunch bone paste (Biogen®, Bioteck™, Arcugnano, Vicenza, Italy) in the cortical gap. The triceps was reattached at its insertion using absorbable sutures. At the end of the surgery, the reduction result was inspected by fluoroscopy in three different views (Fig. 1) and by dynamic testing of the elbow. Following the muscle reattachment, we closed the subcutaneous layer with absorbable sutures and skin with metal staples. For the first three postoperative weeks, all patients were placed in a resin above-elbow cast with the elbow flexed in 90°.
Fig. 1.
A 37-year-old woman underwent surgery because of fixation failure and aseptic non-union (A). Trans-Triceps Approach (B). Removal of 3.5 mm Locking Compression Plate with two pre-existently broken screws, debridement of the non-union focus and reduction of the two bone segments. Fixation by 4.5 mm Locking Compression Plate® (LCP ®) (DepuySynthes ™, Oberdorf, Switzerland) and anterior bone strut allograft (C).
3. Results
From a total of 54 patients with a distal third humeral shaft fracture non-union, we included 26 patients with distal third humeral non-union after exclusion of 28 patients. The demographic data of the enrolled patients, including primary mode of fixation, are described in Table 1. The mean total follow-up was 38 months. All 26 patients demonstrated wound healing within 21 days. During the whole follow up we had no superficial of deep infections and no material failure.
Table 1.
Demographic and pre-operative measures of the included patients. SD: standard deviation. *: due to rounded percentages, totals now add up to 102%.
| Parameters | Descriptives |
|---|---|
| Number of patients | 26 |
| Distribution males: females | 14 : 12 |
| Average age, years (standard deviation) | 48 (±28) |
| Range of age, years | 18–69 |
| Occupation | Agricultural industry: 8 (31%) |
| Industrial sector: 12 (46%) | |
| Tertiary industry: 6 (23%) | |
| Injured upper limb side | Right: 6 (23%) |
| Left: 20 (77%) | |
| Injured dominant upper limb | Right: 6 (23%) |
| Left: 6 (23%) | |
| Type of primary accident | Fall from height: 6 (23%) |
| Traffic accident: 6 (23%) | |
| Work-related accident: 14 (54%) | |
| Primary type of distal humeral fracture according to the Arbeitsgemeinschaft für Osteosynthesefragen (AO)* | A1: 3 (12%) |
| A2: 2 (8%) | |
| A3: 2 (8%) | |
| B1: 6 (23%) | |
| B2: 4 (15%) | |
| B3: 3 (12%) | |
| C1: 2 (8%) | |
| C2: 2 (8%) | |
| C3: 2 (8%) | |
| Orthopedic devices used during primary surgery for proximal humeral fracture | Single plate: 5 (19%) |
| Two orthogonal plates: 9 (35%) | |
| Two parallel plates: 12 (46%) | |
| Average Non Union Scoring System (SD) | 45 (±19) |
| Range Non Union Scoring system | 23–65 |
After complete bone healing, the elbow alignment was normal in 20 patients (77%), valgus in 5 patients (19%), and varus in 1 patient (4%). At twelve months after surgery, the arcs of flexion-extension and pro-supination averaged 108° and 159°, respectively. Further surgical and follow-up details are described in Table 2.
Table 2.
Outcomes of the included patients. SD: standard deviation.
| Outcome measures | Outcomes |
|---|---|
| Duration of follow up (months, SD; range) | 38 (±14; 12–144). |
| Average surgical time (minutes, SD; range) | 96 (±13; 61–126) |
| Amount of red blood cell transfusions (SD; range) | 0.6 (±0.3; 0–4) |
| Wound closure within 21 days | 26/26 |
| Superficial wound infections | 0/26 |
| Deep infections | 0/26 |
| Material failure | 0/26 |
| Average time to bone healing registered by plain radiographs (days, SD; range) | 106 (±15; 74–123) |
| Post-operative axis of elbow alignment | Normal: 20 (77%) |
| Valgus: 5 (19%) | |
| Varus: 1 (4%) | |
| Arc of flexion-extension after 12 months (SD; range) | 108°(±23°; 94°–180°) |
| Arc of pro-supination after 12 months (SD; range) | 159°(±10°; 102°–180°) |
| Correlation between clinical-radiographic results and patient reported outcomes | Cohen's κ: 0.82 |
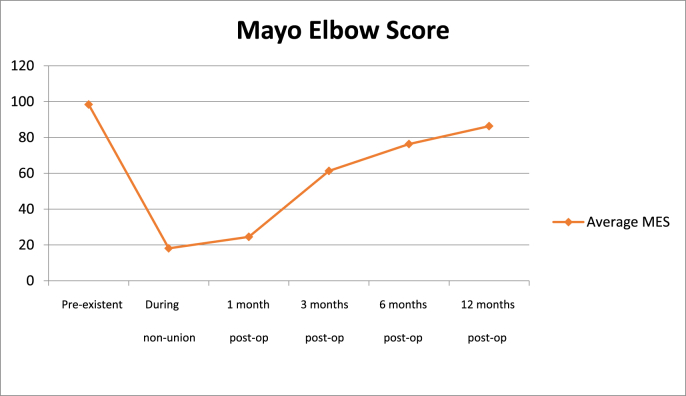
The objective quality of life and elbow function, measured by MES, before the trauma was on average 95 points (range 90–100). At the moment of non-union with eventual failure of orthopedic hardware, the MES averaged 18 (range 2–30). After 1, 3 and 6 months from the revision surgery the MES scores averaged 24 (range 18–56), 63 (range 46–80) and 79 (range 56–100) respectively. At final follow-up at twelve months the average MES score was 84 (range 68–100) (Fig. 2).
Fig. 2.
Trend of objective Mayo Elbow Score (MES) from pre-existent scores to 12 months after the revision surgery.
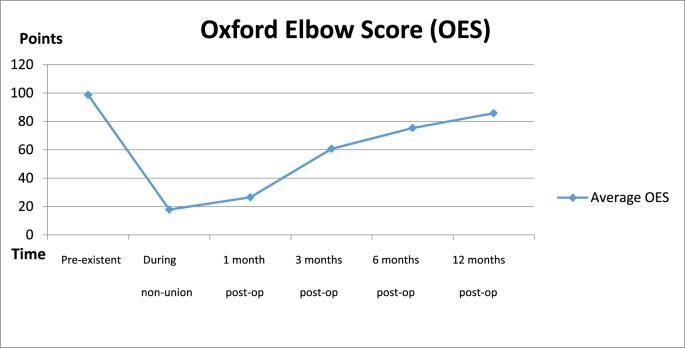
The subjective quality and elbow function measured by OES, was on average 99 points (range 90–100) before the first trauma. At the moment of non-union with eventual failure of orthopedic hardware, the OES averaged 20 (range 4–46). After 1, 3 and 6 months from the revision surgery the OES scores averaged 23 (range 22–48), 60 (range 30–84) and 78 (range 56–100) respectively. At final follow-up at twelve months the average OES score was 83 (range 52–100) (Fig. 3). The correlation of between neutral radiographic alignment, OES and MES was high according to Cohen's kappa (κ = 0,82). A complete follow-up course at the outpatient clinic is illustrated in Fig. 4.
Fig. 3.
Trend of mean subjective Oxford Elbow Score (OES) from pre-existing scores to 12 months after the revision surgery.
Fig. 4.
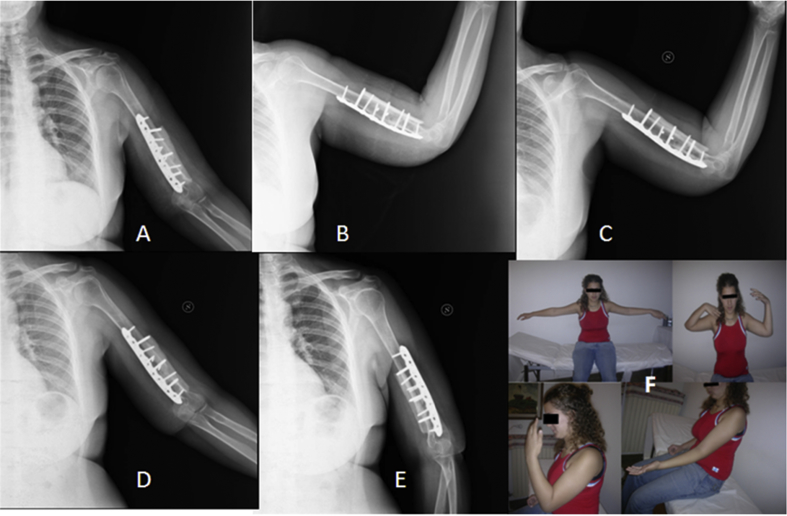
The images A and B present the 3-month X-rays control of the above described case (see Fig. 1). The images C, D and E show the healing of the non-union focus after 12 months. Image F shows the clinical results at 12 months of follow-up. In this case the patient had a deficit in flexion of the left elbow compared to the contralateral side.
4. Discussion
In our opinion besides patient-related factors, the main technical failure in primary osteosynthesis in our series was that the primary implant construct failed to adequately stabilize for fracture to heal (for example, Fig. 1). To successfully treat humeral shaft fractures with internal fixation, it is important to recognize that torsion is the predominant form of mechanical stress at the fracture site and because it is a non-weight bearing bone the lack of axial loading promotes fracture distraction2,13,15. Other common treatments of intra-articular or distal humeral atrophic non-union include elbow arthrodesis, arthroplasty16, allografts17, 18, 19, 20, open reduction and internal fixation21, prosthesis22 or the Ilizarov technique23. From these options, we have chosen the posterior plate to reduce the distracting forces and used the opposing anterior bone splint to further increase stability at the fracture site17, 18, 19,24. Besides stability, the osteo-inductive and osteo-formative abilities of the bone strut, bone chips and bone paste, provided a second mechanism to increase fracture healing and addition of mechanical load transfer2,5,6,20,25. This extramedullary fixation does not impede the intramedullary bone marrow, which was only reamed up to vital tissue.
We used the trans-triceps approach (TTA), which has additional advantages over the triceps-sparing approach (TSA) in our view. TTA offers adequate exposure to the lateral column and limits the formation of intramuscular scar formation, reduces the chances of elbow contracture because of triceps contracture and shows good post-operative triceps function12,15. Besides, the TTA exposure can be extended proximally and distally. Proximal extension is possible by elevating the triceps off the humerus and mobilizing the radial nerve. Distal extension to provide an intra-articular view can be accomplished by converting the approach into an olecranon osteotomy approach, TRAP approach26 or Bryan and Morrey approach27 in case of an intra-articular extension of the shaft fracture that needs open reduction. The increased traction at the supracondylar level using TSA could lead to excessive scar formation and inflammation around the ulnar nerve what might lead to ulnaropathy14. The extra-articular distal humeral locking plate is based on a similar concept of single column plating. Owing to a greater screw hole density distally, it allows the placement of an adequate number of screws in the distal fragment, and the locking construct increases stability. Since only the lateral column was exposed in our series, it decreases both the soft tissue dissection and the surgical time15.
Union rates of conservatively treated humeral shaft fractures have been reported to be 67–98%13. Despite these high rates, some patients are unable or unwilling to undergo non-operative management. Especially healthy, young and active patients could benefit from earlier start of motion and return-to-work in our opinion. In our rehabilitation protocol, we used a hinged elbow brace to gradually increase the range of motion after three weeks and had the brace removed after 6 weeks if possible to regain motor control and psychological improvement gradually. Clinical union and brace removal takes an average of 12 weeks (range of 4–22 weeks) with functional bracing, compared with 6–10 weeks for intramedullary nailing and 9–10 weeks for compression plating13. In addition to functional limitations, functional bracing carries a 1–10% risk of malunion and skin and soft tissue complications13. However, the humerus can heal with deformities without leading to major impairment of function (<20° anterior angulation, < 30° varus/valgus angulation, and <3 cm shortening)5. Return to weight-bearing activities remains an interaction of bone quality and surgical fixation. Weight-bearing restrictions may be devastating to the elderly, who often require their arm to make a transfer or to mobilize with crutches or rollators. We allowed patients light weight bearing activities after 12 weeks and full weight baring activities after complete bone healing was observed on plain radiographs, on average after 15 weeks.
Open reduction and internal or thin wire fixation results in a flexion-extension motion arc between 71° and 102° (average 91°)14. In our series, the average arc of flexion-extension was 108°, which provides ample range of motion to regain activities of daily living. This is reflected in a high quality of life measured with the Mayo Elbow Performance Score, and Oxford Elbow Score as they were all clinically significantly better after a follow-up period of one year since the surgery, almost at a pre-existent level.
The limitations of the current study are the limited number of patients at a single Level 1 trauma center to provide more generalizable results. It also reflects a single-center, which could lead to bias as the experience and familiarity with this treatment is relatively high, and selection bias for the patients who are referred to our center. Another limitation is the retrospective nature of the study and possible recall bias in the pre-trauma questionnaires. In general, as it has been a retrospective non-comparative trial, confounders have not been investigated or corrected for.
5. Conclusion
We suggest the use our plate-and-bone-strut-allograft technique with bone chip augmentation technique in distal humeral shaft aseptic non-unions to restore the biomechanics, support the humeral and elbow axis and to maintain reduction until complete fracture healing. This technique has shown it can produce a high percentage of unions without adverse events related to the surgery itself or the materials used.
Conflict of interest statement
All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Disclosures
None, for all authors.
Rehabilitation protocol after plate-and-bone-strut-allograft repair of distal third humeral fractures
The purpose of our protocol is to provide the clinician with an orientation of the postoperative course of rehabilitation, in order to support a standardised physiotherapy program for the entire patient population.
Week 1–3
During the first three postoperative weeks, the patients wore a resin cast from the humerus to the metacarpals, with the elbow flexed at 90°.
Week 4–6
After the first three weeks, the patients received a hinged elbow brace.
At Week 4 the brace was placed in full elbow flexion, with up to 30° of extension deficit.
At Week 5 the brace was placed in full elbow flexion, with up to 20° of extension deficit, and at week 6 the brace was adjusted to full elbow flexion, with up to 10° of extension deficit.
Strengthening program
The postoperative strengthening program included single plane active ROM elbow flexion, extension, supination, and protonation.
Week 7–11
The patients were allowed full range of motion of the elbow, and could discontinue the brace if they demonstrated adequate motor control.
The patients could begin composite motions (i.e. extension with protonation).
If at 8 weeks postoperatively the patient had significant range of motion deficits, the therapist could consider more aggressive management after consultation with the referring surgeon.
Strengthening program
A progressive active-resistance exercise program was initiated for elbow flexion, extension, supination, and protonation.
Week 12
At week 12 the Hinged Elbow Brace was removed with standard procedure. At this time, the patients were able to initiate light upper extremity weight training.
Strengthening program
12 weeks marked the initiation of an endurance program that simulated desired work activities/requirements. The program focused on stimulation of the elbow and shoulder range of motion, strength and coordination.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2019.05.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kashayi-Chowdojirao S., Vallurupalli A., Chilakamarri V.K. Role of autologous non-vascularised intramedullary fibular strut graft in humeral shaft nonunions following failed plating. J Clin Orthop Trauma. 2017;8:21–30. doi: 10.1016/j.jcot.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J., Cho J., Cho W. Aseptic humeral Nonunion : what went wrong? What to do? A retrospective analysis of 20 cases. J Korean Soc Traumatol. 2016;29(4):129–138. [Google Scholar]
- 3.Micic D.I., Mitkovic B.M., Mladenovic S.D., Golubovic Z.V., Ho J.I. Treatment of the humeral shaft aseptic nonunion using plate or unilateral external fixator. J Trauma Inj Infect Crit Care. 2008;64(5):1290–1296. doi: 10.1097/TA.0b013e3180582471. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Z.K., Yuan L.M., Jiang P.P., Huang F. Treatment of 10 year humeral shaft nonunion with segment bony defect: a case report. Int J Clin Exp Med. 2016;9(10):20242–20246. [Google Scholar]
- 5.Marinelli A., Antonioli D., Guerra E., Bettelli G., Zaccarelli L., Rotini R. Humeral shaft aseptic nonunion: treatment with opposite cortical allograft struts. Musculoskelet Surg. 2009;93:821–828. doi: 10.1007/s12306-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 6.Murena L., Canton G., Vulcano E., Surace M.F., Cherubino P. Treatment of humeral shaft aseptic nonunions in elderly patients with opposite structural allograft, BMP-7, and mesenchymal stem cells. Orthopedics. 2014;37(2):201–206. doi: 10.3928/01477447-20140124-26. [DOI] [PubMed] [Google Scholar]
- 7.Yin P., Zhang L., Li T. Infected nonunion of tibia and femur treated by bone transport. J Orthop Surg Res. 2015;10:49. doi: 10.1186/s13018-015-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gundes H., Selek Ö., Gok U., Gumuslu B., Buluc L. The relation between elbow range of motion and patient satisfaction after open release of stiff elbow. Acta Orthop Traumatol Turcica. 2017 doi: 10.1016/j.aott.2017.05.005. 30329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beirer M., Friese H., Lenich A. The Elbow Self-Assessment Score (ESAS): development and validation of a new patient-reported outcome measurement tool for elbow disorders. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2230–2236. doi: 10.1007/s00167-015-3647-z. [DOI] [PubMed] [Google Scholar]
- 10.Calori G.M., Colombo M., Mazza E.L. Validation of the non-union scoring System in 300 long bone non-unions. Injury. 2014;45(6):93–97. doi: 10.1016/j.injury.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Whelan D.B., Bhandari M., Stephen D. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma Inj Infect Crit Care. 2010;68:629–632. doi: 10.1097/TA.0b013e3181a7c16d. [DOI] [PubMed] [Google Scholar]
- 12.Amir S., Jannis S., Daniel R. Distal humerus fractures: a review of current therapy concepts. Curr Rev Musculoskelet Med. 2016:199–206. doi: 10.1007/s12178-016-9341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoch B.S., Padegimas E.M., Maltenfort M., Krieg J., Namdari S. Humeral shaft fractures: national trends in management. J Orthop Traumatol. 2017;18(3):259–263. doi: 10.1007/s10195-017-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donders J.C.E., Lorich D.G., Helfet D.L., Kloen P. Surgical technique: treatment of distal humerus nonunions. HSS J. 2017;13(3):282–291. doi: 10.1007/s11420-017-9551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharbanda Y., Tanwar Y.S., Srivastava V., Birla V., Rajput A., Pandit R. Retrospective analysis of extra-articular distal humerus shaft fractures treated with the use of pre-contoured lateral column metaphyseal LCP by triceps-sparing posterolateral approach. Strateg Trauma Limb Reconstr. 2017;12(1):1–9. doi: 10.1007/s11751-016-0270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollo G., Rotini R., Eygendaal D. The waterfall fascia lata interposition arthroplasty “grika technique” as treatment of posttraumatic osteoarthritis of the elbow in a high-demand adult patient: validity and reliability. Case Rep Orthop. 2018 doi: 10.1155/2018/8253732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollo G., Rotini R., Pichierri P. Grafting and fixation of proximal humeral aseptic non union: a prospective case series. Clin Cases Miner Bone Metab. 2017;14(3):298–304. doi: 10.11138/ccmbm/2017.14.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rollo G., Pichierri P., Marsilio A., Filipponi M., Bisaccia M., Meccariello L. The challenge of nonunion after osteosynthesis of the clavicle: is it a biomechanical or infection problem? Clin Cases Miner Bone Metab. 2017;14(3):372–378. doi: 10.11138/ccmbm/2017.14.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollo G., Tartaglia N., Falzarano G. The challenge of non-union in subtrochanteric fractures with breakage of intramedullary nail: evaluation of outcomes in surgery revision with angled blade plate and allograft bone strut. Eur J Trauma Emerg Surg. 2017;43(6):853–861. doi: 10.1007/s00068-016-0755-5. [DOI] [PubMed] [Google Scholar]
- 20.Rollo G., Vicenti G., Rotini R. Clavicle aseptic nonunion: is there a place for cortical allogenic strut graft? Injury. 2017;48:60–65. doi: 10.1016/S0020-1383(17)30660-5. [DOI] [PubMed] [Google Scholar]
- 21.Feng D., Zhang J., Zhu Y. Plate fixation with autogenous bone grafting for longstanding humeral shaft nonunion: a retrospective study of 6 cases. Medicine (Baltim) 2018;97(35) doi: 10.1097/MD.0000000000011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prkić A., Van Bergen C., The B., Eygendaal D. Total elbow arthroplasty is moving forward: review on past, present and future. World J Orthoped. 2016;18(7):44–49. doi: 10.5312/wjo.v7.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinker M.R., O'Connor D.P., Crouch C.C., Mehlhoff T.L., Bennett J.B. Ilizarov treatment of infected nonunions of the distal humerus after failure of internal fixation: an outcomes study. J Orthop Trauma. 2007;21:178–184. doi: 10.1097/BOT.0b013e318032c4d8. [DOI] [PubMed] [Google Scholar]
- 24.Hettrich C., Paul O., Neviaser A., Borsting E., Lorich D. The anterolateral approach to the proximal humerus for nonunions and delayed unions. Int J Shoulder Surg. 2011;5(1):21–25. doi: 10.4103/0973-6042.80466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters C.L., Bachus K.N., Davitt J.S. Fixation of periprosthetic femur fractures: a biomechanical analysis comparing cortical strut allograft plates and conventional metal plates. Orthopedics. 2003;26(7):695–699. doi: 10.3928/0147-7447-20030701-13. [DOI] [PubMed] [Google Scholar]
- 26.O'Driscoll S.W. The triceps-reflecting anconeus pedicle (TRAP) approach for distal humeral fractures and nonunions. Orthop Clin N Am. 2000:91–101. doi: 10.1016/s0030-5898(05)70130-9. [DOI] [PubMed] [Google Scholar]
- 27.Bryan R.S., Morrey B.F. Extensive posterior exposure of the elbow. A triceps-sparing approach. Clin Orthop Relat Res. 1982;166:188–192. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.