Abstract
miR-132 and miR-212 are structurally-related microRNAs that are expressed from the same non-coding transcript. Accumulating evidence has shown that the dysregulation of these microRNAs contributes to aberrant neuronal plasticity and gene expression in the mammalian brain. Consistent with this, altered expression of miR-132 is associated with a number of affect-related psychiatric disorders. Here, we tested the functional contribution of the miR-132/212 locus to the development of stress-related and anxiety-like behaviors. Initially, we tested whether expression from the miR-132/212 locus is altered by stress-inducing paradigms. Using a 5-h acute-stress model, we show that both miR-132 and miR-212 are increased more than two-fold in the WT murine hippocampus and amygdala, whereas after a 15 day chronic-stress paradigm, expression of both miR-132 and miR-212 are upregulated more than two-fold within the amygdala but not in the hippocampus. Next, we used a tetracycline-inducible miR-132 overexpression mouse model and a miR-132/212 conditional knockout (cKO) mouse model to examine whether dysregulation of miR-132/212 expression alters basal anxiety-like behaviors. Interestingly, in both the miR-132 overexpression and cKO lines, significant increases in anxiety-like behaviors were detected. Importantly, suppression of transgenic miR-132 expression (via doxycycline administration) mitigated the anxiety-related behaviors. Further, expression of Sirt1 and Pten—two miR-132 target genes that have been implicated in the regulation of anxiety—were differentially regulated in the hippocampus and amygdala of miR-132/212 conditional knockout and miR-132 transgenic mice. Collectively, these data raise the prospect that miR-132 and miR-212 may play a key role in the modulation of stress responsivity and anxiety.
Keywords: miR-132, miR-212, Anxiety, Hippocampus, Amygdala, Pten, Sirt1
1. Introduction
Anxiety disorders are a class of neuropsychiatric ailments that often result from and/or are influenced by both genetic and epigenetic factors (Nugent et al., 2011; Poulton et al., 2008; Smoller, 2016). Along these lines, gene expression can alter anxiety onset/severity through mechanisms that include DNA and histone modifications and non-coding RNA regulation (for review, see Issler and Chen, 2015; McEwen et al., 2012; Nieto et al., 2016). As for the latter case, microRNAs are small, non-coding RNA molecules that modulate essential physiological processes, such as cellular homeostasis and immune/stress responsiveness (for review, see Leung and Sharp, 2010, 2007; Haramati et al., 2011; Mendell and Olson, 2012; Wiegand et al., 2017). Indeed, the ability of miRNAs to fine-tune gene expression positions them as excellent candidates for regulating stress and anxiety behaviors (Andolina et al., 2016; Cohen et al., 2017; Hollins and Cairns, 2016; Issler and Chen, 2015; Leung and Sharp, 2010; Malan-Müller et al., 2013; Mannironi et al., 2013, 2018; O’Connor et al., 2012; Volk et al., 2014).
Among these miRNAs is brain-enriched miR-132 (Vo et al., 2005). A large body of work has characterized the miR-132/212 locus as a synaptic activity-dependent regulator of gene expression and plasticity within the CNS (Cheng et al., 2007; Jimenez-Mateos et al., 2011; Mellios et al., 2011; Nudelman et al., 2010). With respect to its role in synaptic physiology, miR-132 has been shown to regulate hippocampal neuronal spine density, dendritic arborization, and long-term potentiation (Hansen et al., 2010; Nudelman et al., 2010; Tognini et al., 2011; Wayman et al., 2008).
Recently, miR-132 has been shown to function as a modulator of cognitive capacity. Along these lines, transgenic overexpression of miR-132 was found to reduce spatial and recognition memory (Hansen et al., 2010; Scott et al., 2012), while the targeted deletion of the miR-132/ 212 locus led to deficits in learning and memory (Hansen et al., 2016; Hernandez-Rapp et al., 2015). Interestingly, dysregulation of miR-132/ 212 expression has been reported in patients with schizophrenia and bipolar disorder (Kim et al., 2010; Perkins et al., 2007). Further, miR-132 is increased in patients with depression (Li et al., 2013), as well as in animal models that exhibit non-learned helplessness (Smalheiser et al., 2011). Several groups have also reported a correlation between stress and miR-132 expression. For example, Meerson et al. (2010) showed that miR-132 is induced in the hippocampal CA1 region following chronic immobilization stress, while Shaltiel et al. (2013) demonstrated that predator scent and footshock stress leads to long lasting increases in hippocampal miR-132 and concomitant decreases in miR-132 target genes. Likewise, miR-132 is significantly increased in the prefrontal cortex of maternally separated rat pups (Uchida et al., 2010) and has been shown to modulate stress-induced chemokine production (Strum et al., 2009).
These studies, coupled with our recent observation that miR-132/ 212 conditional KO mice displayed a heightened level of freezing behavior in the contextual fear conditioning paradigm (Aten et al., 2018a), led us to test the relationship between the dysregulation of miR-132/212 and the appearance of anxiety-like behavior. Here, using RT-qPCR, we show that miR-132 and miR-212 are induced after both acute and chronic stress paradigms. Further, utilizing forebrain miR-132/212 knockout and transgenic targeting approaches, we demonstrate that the dysregulation of miR-132/212 expression leads to anxiety-related behaviors. Collectively, these data indicate that the miR-132/212 locus contributes to the manifestation of anxiety-like behavioral states.
2. Materials and methods
2.1. miR-132 transgenic and miR-132/212 knockout mice
The CaMKII-Cre::miR-132/212f/f conditional forebrain neuron knockout (referred to as ‘miR-132/212 cKO’) mouse line was previously described by our lab (Hansen et al., 2016). The miR-132/212f/f animals were provided by Dr. J. Simon C. Arthur (University of Dundee, Scotland), and the CaMKII-Cre line (Tsien et al., 1996) was purchased from Jackson Labs (Stock number: 005359: Bar Harbor, ME, USA). Generation of the CaMKII-tTA::miR-132:CaMKII-Cre::miR-132/212f/f mouse line (referred to as ‘miR-132 transgenic’) was recently reported in Aten et al. (2018a). In brief, miR-132 transgenic animals were created by breeding homozygous CaMKII-Cre::miR-132/212f/f (miR-132/212 cKO) mice with a tetracycline-regulated bidirectional miR-132/cyan fluorescent protein (CFP) transgenic mouse line driven by CaMKII::tTA. Thus, miR-132 transgenic mice were homozygous for the miR-132/ 212f/f locus and positive for Cre, tTA, and miR-132 transgenes. This ‘Tet-off’ transgenic animal model allowed for selective deletion of endogenous miR-132/212 and transgenic over-expression of miR-132 within the same population of excitatory forebrain neurons. For all experiments with transgenic and knockout mice, control (referred to as ‘WT’) animals were littermates that were negative for either the driver and/or responder genes. Genotyping for both the miR-132/212 cKO and miR-132 transgenic lines was previously described by our lab (Aten et al., 2018a; Hansen et al., 2016, 2010).
All mice utilized for experiments were bred, housed, and maintained under standard 12 h/12 h Light/Dark (LD) conditions and had ad libitum access to food and water. All behavioral and molecular experiments took place from the mid-to late-day light period. Experimental animals were screened, as described in Hansen et al. (2013), to ensure that they did not have any vision deficits. Of note, for experiments examining miR-132 and miR-212 expression after stress paradigms (Fig. 1; Supplementary Fig. 1–2), only male WT mice (8–12 weeks of age) were utilized to eliminate potential sex-specific effects. However, given the limited number of miR-132/212 cKO and miR-132 transgenic mice available, both males and females were used for behavioral and molecular experiments (Figs. 3–6); a sex-parsed presentation of data for WT, miR-132/212 cKO, and miR-132 transgenic anxiety behavioral experiments (Figs. 3 and 4) are provided in Supplementary figure 3. The Ohio State University Institutional Animal Care and Use Committee approved all protocols and methods, and all experiments were in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
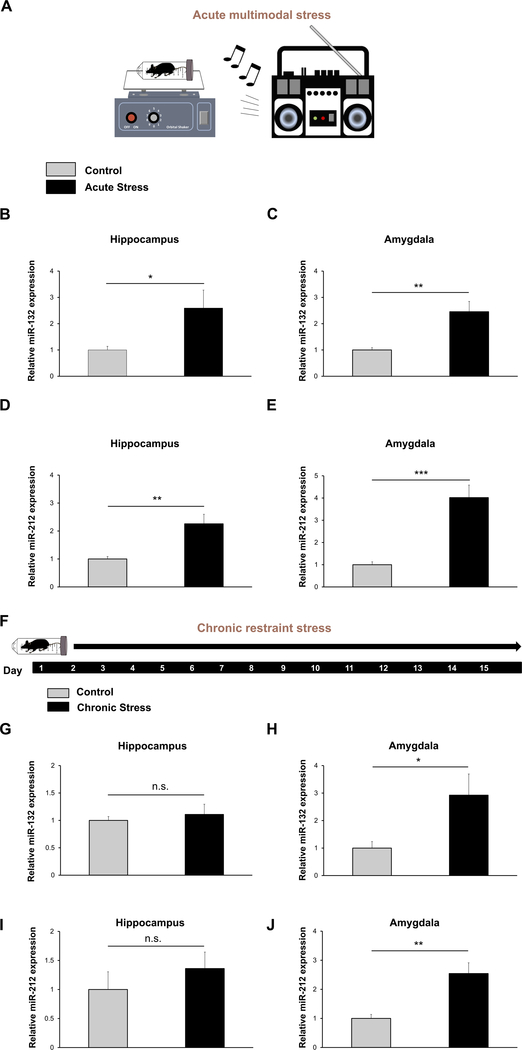
Fig. 1. miR-132 and miR-212 are upregulated in the hippocampus and amygdala after acute multimodal stress and in the amygdala after chronic restraint stress.
(A) Acute multimodal stress experimental design: WT animals were subjected to 5 h of acute multimodal stress, wherein mice were placed in restraint tubes which rocked on a laboratory shaker for 5 h. Loud music was also played in the room. (B) RT-qPCR for miR-132 was performed on RNA samples isolated from the hippocampus (B) and amygdala (C) of control and acutely stressed mice. Similarly, hippocampal miR-212 (D) and amygdalar miR-212 (E) expression was probed via RT-qPCR. (F) Chronic restraint stress experimental design: WT animals were subjected to 2 h of restraint each day, throughout the 15 day paradigm. On day 16, RNA was isolated from both chronically stressed and control mice and hippocampal (G) and amygdalar (H) miR-132 expression was examined using RT-qPCR. Similarly, hippocampal (I) and amygdalar miR-212 (J) expression was probed via RT-qPCR. For both acute and chronic paradigms, miR-132 and miR-212 expression in control animals was set equal to a value of one, and miR-132 and miR-212 expression in stressed mice was normalized to this value. Significance was examined with Student’s t-test for each brain region, and data are presented as the mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001; n.s: not significant (p > 0.05). N = 9–10 mice per group.
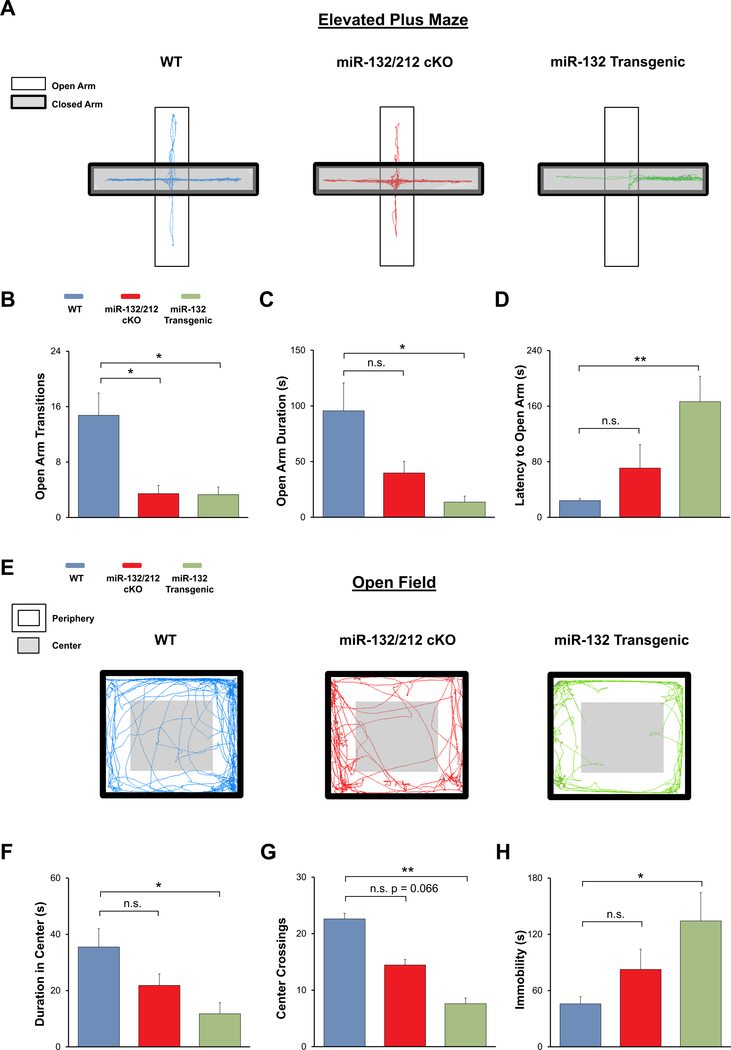
Fig. 3. Profiling anxiety phenotype in miR-132/212 cKO and miR-132 transgenic animals with elevated plus maze and open field assay.
(A) Representative elevated plus maze locomotor traces from WT (blue), miR-132/212 cKO (red), and miR-132 transgenic animals (green). (B) miR-132/212 cKO and miR-132 transgenic animals exhibited significantly fewer open arm transitions in the elevated plus maze compared to WT mice. Mean time spent in open arms (C) and mean latency to open arms (D) were significantly different between WT and miR-132 transgenic animals. (E) Representative open field test locomotor traces from WT, miR-132/212 cKO, and miR-132 transgenic animals. (F) Total duration in the center of the open field was significantly different between miR-132 transgenic and WT mice. (G) Number of crosses into the center was significantly reduced in miR-132 transgenic mice compared to WT mice. (H) Total immobility was significantly different between WT and miR-132 transgenic mice. Data were analyzed by one-way ANOVA with Bonferroni post-hoc tests. Data are presented as the mean ± SEM. *: p < 0.05; **: p < 0.01; n.s.: not significant (p > 0.05). N = 9–13 mice per group.
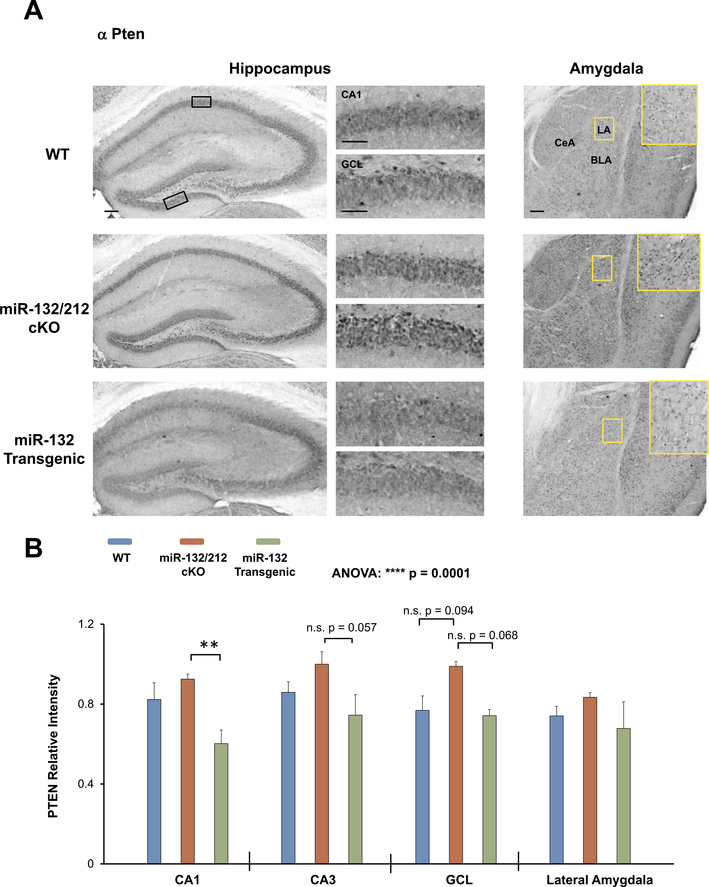
Fig. 6. Profiling PTEN expression in miR-132/212 cKO and miR-132 transgenic mice.
(A) Representative immunohistochemical labeling for PTEN in the hippocampus and amygdala of WT (blue), miR-132/212 cKO (red), and miR-132 transgenic animals (green). The boxed regions in the low-magnification images approximate the locations of the regions that are depicted in the high-magnification panels (to the right for the hippocampus, and inset for the amygdala). (B) Quantification of PTEN immunolabeling in the hippocampus and amygdala. Note the increased expression of PTEN within the miR-132/212 cKO mice. Scale bar = 50 μm for the low magnification images (i.e., whole hippocampus and amygdala) and 30 μm for the high magnification images (i.e., CA1 cell layer and GCL-lower blade). Data were analyzed by two-way ANOVA with Bonferroni post-hoc correction and are presented as the mean ± SEM. **: p < 0.01; n.s.: not significant (p > 0.05); N = 3–6 mice per genotype.
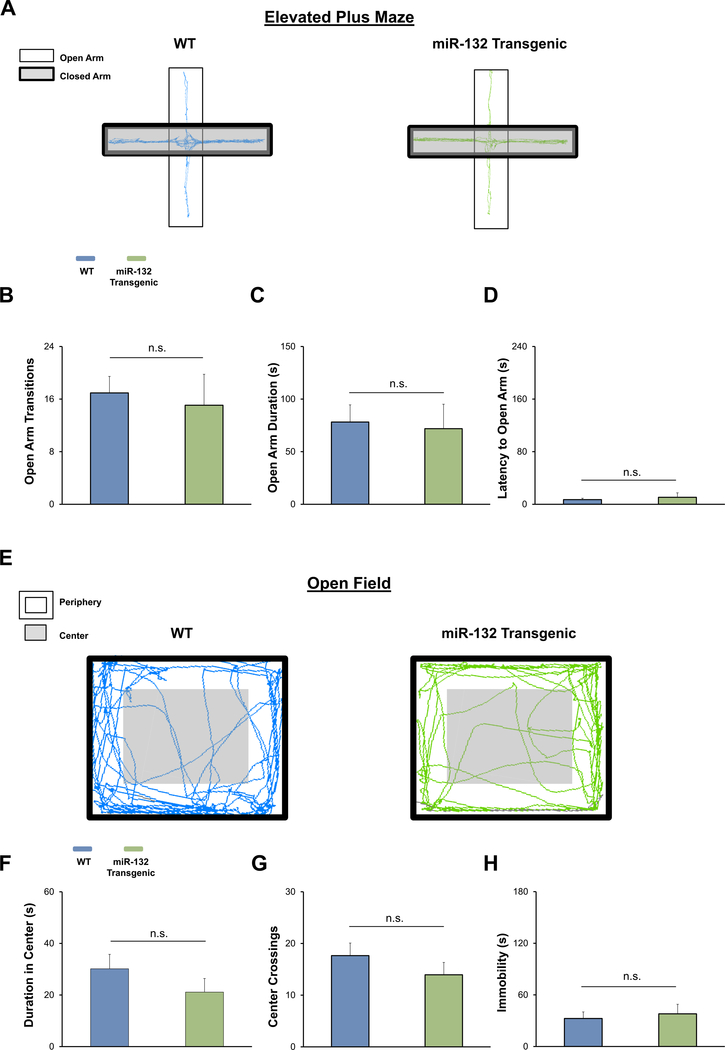
Fig. 4. Profiling anxiety phenotype in miR-132 transgenic animals treated with doxycycline.
(A) Representative elevated plus maze locomotor traces from WT (blue) and miR-132 transgenic animals (green). Note that both WT and miR-132 transgenic animals were given a 0.4 μg doxycycline dose in their drinking water for three weeks prior to testing (and throughout the testing period). No differences in open arm transitions (B), open arm cumulative duration (C), or latency to open arm (D) were observed between WT and miR-132 transgenic animals in the elevated plus maze. (E) Representative open field locomotor traces from WT and miR-132 transgenic animals. No significant differences in duration in the center of the arena (F), center crossings (G), or time spent immobile (H) were observed between WT and miR-132 transgenic animals in the open field test. Data were analyzed by Student’s t-tests and are presented as the mean ± SEM. n.s.: not significant (p > 0.05). N = 15–20 mice per group.
2.2. Doxycycline treatment
In a subset of animals (Fig. 4), temporal modulation of tetracycline-inducible miR-132 transgene expression was accomplished via the administration of doxycycline (0.40 μg/mL) to the drinking water. Using this technique, as previously described by Hansen et al. (2013) and Aten et al. (2018a), we were able to reduce the expression of the miR-132 transgene. For the elevated plus and open field experiments, all animals (WT and miR-132 transgenic) were given this doxycycline dose for three weeks prior to the start of the experiment, and the animals remained on doxycycline throughout the duration of the experiment(s). Doxycycline water was changed every three days. Consistent with our previous data (Aten et al., 2018a; Hansen et al., 2013), no significant effect of doxycycline was observed in control, WT animals (i.e. animals that were not positive for both the tTA and miR-132 driver/responder transgenes; data not shown).
2.3. Acute multimodal stress paradigm
Our acute stress test was adapted from Chen et al. (2010) and Zimprich et al. (2014). Male WT mice (8–12 weeks of age) were divided into two groups (control and stress) and were given 30 min to acclimate to the testing room before the experiment began. For the acute stress paradigm, mice were placed in ventilated 50 mL conical tubes. Next, the tubes were secured on an orbital shaker in a brightly lit (~400 lux) room, with loud music. Stressed animals were maintained in ventilated conicals for 5 h. Control animals remained in their respective cages for the 5 h period. After cessation of the stress paradigm, control and stressed animals were divided into three cohorts: one cohort was used for RT-qPCR analysis (Fig. 1B–E), another for behavioral analysis (Supplementary Fig. 1A-D), and a final group for immunohistochemical analysis (Supplementary Fig. 1E and F). For RT-qPCR analysis of miR-132 and miR-212 expression, stressed and control mice were sacrificed 30 min after the stress session ended (approximately 5.5 h after stress onset). The timing of sacrifice was chosen as it reflects the kinetics for inducible miR-132 expression (Nudelman et al., 2010; Vo et al., 2005). For the behavioral analysis, stressed animals were given a 20 min resting period after the cessation of the multimodal stress paradigm before the open field test was conducted. This 20 min rest period was based on work by Zimprich et al. (2014), which demonstrated that this delay period between the acute stressor and examination of the stress response was required to eliminate the confounding effects of a transient bout of grooming behavior. Finally, animals (stressed and control) were killed immediately after the stress session ended for immunohistochemical profiling of cFos. The specific timecourse/protocol for each of the three mentioned experiments is described in separate sections below.
2.4. Chronic restraint stress paradigm
The chronic restraint paradigm was adapted from Jeong et al. (2013) and Meerson et al. (2010). In brief, stressed WT mice (males only) were restrained in ventilated 50 mL conical tubes for 2 h each day for a 15 day period. After each stress session, mice were placed back into their home cages. Control mice were handled daily while the stressed animals were restrained. Mice were also weighed every five days in order to assess body weight during the stress paradigm. Animals were sacrificed on day sixteen—24 h after the last restraint session—for RT-qPCR profiling of miR-132 and miR-212 expression (Fig. 1G–J). Post-mortem adrenal glands, thymus, and spleen were dissected from both control and stressed animals and were weighed to test the efficacy of the stress chronic restraint stress paradigm (Supplementary Fig. 2).
2.5. RT-qPCR quantification of miR-132 and miR-212 expression levels in the hippocampus and amygdala
WT animals were killed 30 min after the cessation of the acute multimodal stress paradigm and 24 h after the last chronic stress session; tissue processing and RNA isolation were performed using methods described previously for the hippocampus (Aten et al., 2018a), and the amygdala (Zapala et al., 2005). RNA was reverse transcribed using the miScript II Reverse Transcription kit (Qiagen). The miScript Primer System (Qiagen) was used to quantify miR-132 and miR-212 levels: miR-132 primer sequence: 5′ UAACAGUCUACAGCCAUGGUCG (Qiagen, Cat# MS00001561); miR-212 primer sequence: 5’UAACAGU CUCCAGUCACGGCCA (Qiagen, Cat# MS00024570). For RT-qPCR, QuantiFast SYBR Green thermocycling conditions were used, following methods previously described by Alemayehu et al. (2013) and Aten et al. (2018a). Data were normalized to RNU6B_2 levels, and fold change was calculated using Double Delta CT analysis.
2.6. Elevated plus maze
The elevated plus maze was run under dim white light (~50 lux). The maze dimensions and structure were previously described by our lab (Snider et al., 2016). During the experiment, mice were placed in the center of the plus-shaped maze, facing an open arm and were given 5 min to explore. 70% ethanol was used to clean the maze between each trial. The total number of open arm transitions, cumulative open arm duration, and latency to enter an open arm was scored using Noldus Ethovision XT version 11.5 (EthoVision XT).
For the behavioral experiments depicted in Figs. 3–4, all mice (WT, miR-132/212 cKO, and miR-132 transgenic) were first run through the elevated plus maze. Two days later, the same animals were subjected to the open field test.
2.7. Open field assay
For examination of anxiety-like behavior in the three noted mouse lines (WT, miR-132/212 cKO, and miR-132 transgenic), the open field test was conducted under dim white light (~50 lux). Mice were placed in the arena and were given 5 min to explore. The bottom surface and the walls of the arena were wiped with 70% ethanol between each trial. Total time spent in the center of the arena, number of crosses into the center of the arena, and cumulative freezing time was scored by Noldus EthoVision XT version 11.5 (EthoVision XT).
To confirm hypothalamic pituitary adrenal (HPA) axis activation in WT mice after the acute multimodal stress paradigm, the same open field protocol was used as described above. Three common readouts of acute stress-responsivity were measured: number of rears, distance traveled, and velocity (Zimprich et al., 2014). Note that number of rears was manually scored. A rear was defined as a mouse positioning itself on its hind legs, with its front legs off the ground.
2.8. Tissue processing for immunolabeling
Mice were sacrificed via rapid cervical dislocation, and brains were cut into 600 μm sections using a vibratome. Next, sections were fixed in 4% paraformaldehyde diluted in 1X phosphate-buffered saline (PBS) for 6 h (4 °C) and then cryoprotected via overnight incubation in 30% sucrose (in 1X PBS). In preparation for immunolabeling, sections were thin-cut to 40 μm on a freezing microtome.
2.9. Immunohistochemistry
Sections were incubated for 20 min in 0.3% hydrogen peroxide in 0.1% Triton X-100 in PBS (PBST). Next, tissue was blocked for 1 h in 10% normal goat serum, followed by incubation at 4 °C (for 8 h) with one of the following antibodies: rabbit anti-cFos (1:2000 dilution; Millipore, Cat# ABE457), rabbit anti-Sirt1/Sir2α (1:1000 dilution; Millipore, Cat# 09–845), or rabbit anti-Pten (1:100 dilution; Cell Signaling Technology, Cat # 9188P).
On the second day, sections were incubated in biotin-conjugated goat anti-rabbit IgG secondary antibody (1:1000 dilution; Vector Laboratories, Cat# BA-1000) for 2 h at room-temperature. Next, sections were processed using the ABC labeling method (Vector Laboratories Cat# PK-6100) and horseradish peroxidase enzymatic activity was visualized using nickel intensified diaminobenzidine labeling method (Vector Laboratories Cat# SK-4100). Of note, sections were washed three times (5 min/wash) in PBST between each labeling step. Finally, sections were mounted on gelatin-coated slides, washed in dH2O, and coverslipped with Permount Mounting Medium (Fisher Chemical). Bright field images were captured with a 16-bit digital camera (Micromax YHS 1300; Princeton Instruments) on a Leica DMIR microscope with Metamorph software (MetaMorph Microscopy Automation and Image Analysis Software). For the quantification of cFos labeling, digital images of the PVN, BNST, medial septum, central amygdala, and dorsal CA1 of the hippocampus were traced digitally from 1–3 sections per animal. Intensity levels for each section were background subtracted and quantitatively analyzed using ImageJ software. Mean intensity values for regions of interest were generated for each animal and the group average was displayed as the mean ± the standard error (SEM) for each noted brain region. For the quantification of Sirt1 and Pten labeling, digital images of the amygdala and the CA1, CA3, and GCL hippocampal subfields were acquired, and intensity values and quantitation methods were performed as described for c-Fos labeling.
2.10. Experimental design and statistical analysis
Statistics were performed using GraphPad Prism 7.0 (Graphpad Prism) software. All data are presented as the mean ± SEM. As denoted in the figure legends, significance for all experiments was set at *p < 0.05. Comparisons between two groups were performed using Student’s two-tailed t-tests, while comparisons between three or more groups were made using a one-way ANOVA. Bonferroni post-hoc tests were conducted to show an interaction obtained from significant ANOVA results. To determine if there were significant interactions between two independent variables on the dependent variable, a two-way ANOVA was performed. Additionally, Grubb’s test was conducted on data sets within each group, and animals that were found to be statistically significant outliers (p < 0.05) were removed from analysis. Grubb’s test was used to exclude two control animals from the acute stress RT-qPCR data set and one WT animal from the Pten immunolabeling analysis. Further, for behavioral experiments, one WT animal, two cKO, and one transgenic animal used in the no-doxycycline anxiety paradigms were also found to be significant outliers, while one WT and one transgenic animal in the doxycycline behavioral experiments were significant outliers.
3. Results
3.1. Hippocampal and amygdalar miR-132 and miR-212 are upregulated after acute stress
Extensive work in several brain regions has characterized miR-132 as an activity-inducible gene (Cheng et al., 2007; Hansen et al., 2013; Hernandez-Rapp et al., 2015; Mellios et al., 2011; Nudelman et al., 2010; Tognini et al., 2011). With these observations in mind, we aimed to test whether miR-132 is induced after an acute stress paradigm (Fig. 1A). RT-qPCR analysis revealed that a 5-h long acute multimodal stress paradigm led to a two-fold increase in hippocampal miR-132, relative to control animals (Fig. 1B, t(17) = 2.270, p = 0.0365; Student’s t-test); in the amygdala, stress also led to a two-fold relative increase in miR-132 expression (Fig. 1C, t(16) = 3.423; p = 0.0035; Student’s t-test). Interestingly, miR-212 hippocampal and amygdalar expression was also increased (> two-fold in the hippocampus and > four fold in the amygdala) in acutely stressed animals (Fig. 1D, t(17) = 3.583, p = 0.0023 for hippocampus and Fig. 1E, t(15) = 4.881, p = 0.00032 for amygdala; Student’s t-tests). Together, these results demonstrate the inducible nature of the miR-132/212 locus after an acute stress paradigm.
To test the efficacy of the noted multimodal stress paradigm, a separate cohort of stressed and non-stressed animals was subjected to the open field test 20min after the cessation of the stress paradigm (Supplementary Fig. 1A). As expected, compared to control animals, mice that were exposed to the stress paradigm displayed behaviors associated with an elevated level of stress responsivity (Zimprich et al., 2014); hence, the stress paradigm led to a significant increase in the number of rears (Supplementary Fig. 1B, t(9) = 2.283, p = 0.0483; Student’s t-test), an increase in the total distance moved (Supplementary Fig. 1C, t(9) = 2.378, p = 0.0414; Student’s t-test), and an increase in the mean velocity (Supplementary Fig. 1D, t(9) = 2.376, p = 0.0415; Student’s t-test). An additional set of mice was sacrificed after termination of the stress paradigm, and tissue from these animals was processed for cFos induction—a readout of stress-induced neuronal activation (Hoffman et al., 1993; Senba et al., 1993; Cullinan et al., 1995) (Supplementary Fig. 1E and F). Similar to results obtained by Maras et al. (2014), the 5 h acute multimodal stress paradigm increased cFos counts in the paraventricular nucleus (PVN), bed nucleus of the stria terminalis (BNST), medial septum, and amygdala (Supplementary Fig. 1F, F(4, 36) = 16.5, p < 0.0001; two-way ANOVA). Specifically, cFos expression in the PVN, BNST, medial septum, and amygdala was significantly higher in stressed mice compared to control mice (Supplementary Fig. 1F t(8) = 7.389, p < 0.001 for PVN; t(8) = 6.105, p = 0.0015 for BNST, t(8) = 4.762, p = 0.0070 for medial septum, and t(5) = 8.721, p = 0.0015 for central amygdala; Bonferroni post-hoc tests). cFos expression in the dorsal CA1 of stressed mice also showed a trending relative increase, though significance was not reached (Supplementary Fig. 1F t(7) = 3.388, p = 0.0580; Bonferroni post-hoc test). Collectively, these data indicate that our multimodal stress paradigm elicits the expected behavioral and molecular responses.
3.2. Amygdalar miR-132 and miR-212 are upregulated after chronic restraint stress
Given our acute stress results, we aimed to determine whether miR-132/212 induction in the hippocampus and amygdala is specific to acute stress exposure, or if chronic stress also leads to its induction. To this end, we exposed WT mice to 2 h of restraint stress (or brief handling for control animals) each day, for a 15 day period, before sacrificing the animals on day 16 (Fig. 1F). Interestingly, no significant difference was observed in hippocampal miR-132 expression between control and chronically stressed mice (Fig. 1G t(16) = 0.5149, p = 0.6136; Student’s t-test), while amygdalar miR-132 was increased ~ three-fold (Fig. 1H t(16) = 2.254, p = 0.0386; Student’s t-test). The chronic stress paradigms also led to an increase in miR-212 in the amygdala, but not in the hippocampus (Fig. 1I t(16) = 1.036, p = 0.3156 for the hippocampus and Fig. 1J t(16) = 3.687, p = 0.0020 for the amygdala; Student’s t-test).
A hallmark of chronic stress in mice is a decrease in body weight gain (Krahn et al., 1990). To confirm the effectiveness of the chronic stress paradigm, mice were weighed every five days. Consistent with previous findings (Jeong et al., 2013; Voorhees et al., 2013; Yoon et al., 2014), after 15 days of chronic restraint, body weight gain was significantly reduced in stressed mice compared to control mice (Supplementary Fig. 2A F(3, 60) = 4.416, p = 0.0072; two-way ANOVA). Specifically, body weight was lower in stressed mice on day 16 (Supplementary Fig. 2A t(15) = 3.238, p = 0.0220; Bonferroni post-hoc test). Adrenal glands, thymus, and spleens were also weighed post-mortem, as a measure of stress reactivity (Blanchard et al., 1993; Engler et al., 2005). Again, consistent with previous findings (Stankiewicz et al., 2014; Voorhees et al., 2013), after 15 days of restraint, stressed mice had increased adrenal mass (Supplementary Fig. 2B, t(15) = 5.661, p < 0.0001; Student’s t-test), decreased thymic mass (Supplementary Fig. 2C, t(15) = 3.524, p = 0.0031; Student’s t-test), and a trend toward increased spleen weight (Supplementary Fig. 2D, t(14) = 1.835, p = 0.0879; Student’s t-test). Together, these support the effectiveness of the chronic restraint paradigm.
3.3. Basal anxiety is affected by the dysregulation of miR-132 in forebrain excitatory neurons
Given our finding that stress paradigms trigger the upregulation of miR-132/212 in brain regions that underlie stress and anxiety responsiveness, we examined whether anxiety levels are affected by the dysregulation of miR-132/212. To this end, we employed a combination of conditional miR132/212 gain- and loss-of-function mouse lines. As detailed in the Methods section, and in Aten et al. (2018a), the miR-132/212 loss-of-function mouse line (hereafter referred to as the ‘miR-132/212 cKO’ mouse line; Fig. 2A) uses a Cre/lox gene deletion strategy to eliminate the miR-132/212 locus from excitatory forebrain neurons—including neurons of the hippocampus, amygdala, and cortex. For the gain-of-function mouse line (hereafter referred to as the ‘miR-132 transgenic’ mouse line; Fig. 2B), a tetracycline/doxycycline-controlled CaMKII-tTA::TRE-miR-132 transgenic line (previously described by Hansen et al., 2010) was crossed with the miR-132/212 cKO mouse line. Transgenic expression of miR-132 was targeted to the same excitatory neuronal cell populations in which the miR-132/212 gene locus was deleted. Importantly, in the absence of doxycycline treatment, transgenic miR-132 is expressed at supraphysiological levels (Aten et al., 2018a), and thus by applying doxycycline to the drinking water, this ‘Tet-off’ mouse line can be used to examine the effects of titered miR-132 expression. Using the elevated plus maze paradigm (Fig. 3A), we found that the number of open arm transitions (a measure that is often used as a read-out of anxiety-like behavior; Walf and Frye (2007)), was significantly affected by the gain- and loss-of miR-132/ 212 expression (Fig. 3B; F(2,33) = 7.753; p = 0.0021; one-way ANOVA). In specific, both miR-132/212 cKO and miR-132 transgenic animals displayed significantly fewer transitions into the open arms of the elevated plus maze compared to control animals (Fig. 3B; t(19) = 2.793, p = 0.0348 for miR-132/212 cKO to WT comparison and t(20) = 2.984, p = 0.0219 for miR-132 transgenic to WT comparison; Bonferroni post-hoc tests). Moreover, the cumulative duration spent in the open arms of the maze was significantly different between genotypes (Fig. 3C; F(2,33) = 5.202, p = 0.0113; one-way ANOVA). While miR-132 transgenic animals spent significantly less time in the open arms compared to WT animals (Fig. 3C; t(21) = 2.704, p = 0.0399; Bonferroni post-hoc test), no significant differences were found between WT animals and miR-132/212 cKO animals (Fig. 3C; t(22) = 1.850, p = 0.2334; Bonferroni post-hoc test). Further, a significant genotypic difference in the latency to open arms was also observed (Fig. 3C; F(2,33) = 5.630, p = 0.0082; one-way ANOVA). miR-132 transgenic animals took significantly more time, on average, to enter the open arms of the maze compared to WT mice (Fig. 3D; t(21) = 3.636 p = 0.0045; Bonferroni post-hoc test) while the latency for miR-132/212 cKO animals to enter open arms did not vary significantly from WT animals (Fig. 3D; t(22) = 1.239 p = 0.6852; Bonferroni post-hoc test).
Fig. 2. miR-132/212 conditional knockout and miR-132 transgenic mouse models.
(A) Schematic depiction of the miR-132/212 conditional knockout mouse model (CaMKII-CremiR-132/212f/f (denoted as ‘miR-132/212 cKO’ in red lettering)). A Cre/lox strategy was used to delete the miR-132/212 locus from excitatory forebrain neurons in these animals. (B) Representation of the breeding strategy used to generate the CaMKII-tTA::miR-132:CamKII-Cre::miR-132/212f/f (‘miR-132 Transgenic’) mice, as depicted in green lettering. The miR-132/212f/f conditional knockout (cKO) line was crossed to a tetracycline-inducible transgenic miR-132 mouse line (denoted as ‘miR-132 Over-expression’ in black lettering). This tetracycline inducible (‘Tet-off’) transgenic mouse line was created by crossing a CaMKII-tTA driver mouse with a mouse expressing miR-132 under the control of the TRE promoter. Hence, crossing the miR-132/212 cKO line to the CaMKII-tTA::miR-132-CFP transgenic line, generated a quadruple transgenic mouse line: CaMKII-tTA::miR-132:CaMKII-CRE::miR-132/212f/f (‘miR-132 Transgenic’). This animal allowed for selective deletion of endogenous miR-132 and the transgenic expression of miR-132 within the same population of excitatory neurons of the forebrain. The schematic was adapted from Aten et al. (2018a).
To complement our elevated plus maze data, we also explored the anxiety phenotype of miR-132/212 cKO and miR-132 transgenic mice using the noted open field assay (Fig. 3E). We found significant genotype-dependent differences in the total time spent in the center of the field, (Fig. 3F; F(2,33) = 4.337, p = 0.0218; one-way ANOVA). Specifically, miR-132 transgenic animals spent less time in the center of the field compared to WT mice (Fig. 3F; t(20) = 2.616 p = 0.0498; Bonferroni post-hoc test), whereas mean time spent in the center by miR-132/212 cKO mice did not significantly differ from WT mice (Fig. 3F; t(23) = 1.676 p = 0.322; Bonferroni post-hoc test). Further, the number of crossings into the center of the open field arena also varied by genotype (Fig. 3G; F(2,33) = 10.038, p < 0.0001; one-way ANOVA). miR-132 transgenic animals crossed into the center of the arena fewer times than WT animals (Fig. 3G; t(21) = 4.103 p = 0.0015; Bonferroni post-hoc test), while the number of center crosses for miR-132/212 cKO mice did not significantly differ from WT animals (Fig. 3G; t(24) = 0.066; Bonferroni post-hoc test). Finally, a main effect of genotype on the overall immobility of animals in the open field test was observed (Fig. 3H; F(2, 33) = 4.043, p = 0.0269; one-way ANOVA). The total time spent freezing was significantly higher in miR-132 transgenic animals compared to WT animals (Fig. 3H; t(20) = 3.016, p = 0.0198; Bonferroni post-hoc test). The total immobility time was not significantly different between WT and miR-132/212 cKO mice (Fig. 3H; t(24) = 1.54 p = 0.4110). Together, the elevated plus maze and open field assay data indicate that the dysregulation of miR-132/212 led to anxiety-like behaviors.
3.4. Doxycycline mitigates the anxiety phenotype in miR-132 transgenic mice
To further test the relationship between miR-132 and anxiety-like behaviors, we examined whether the anxiety phenotype in miR-132 transgenic animals could be suppressed via doxycycline administration. To this end, naïve miR-132 transgenic mice and WT mice (mice that were negative for either the driver and/or responder genes) were maintained on drinking water containing 0.4 μg/mL doxycycline for three weeks and then tested using the elevated plus maze (Fig. 4A). Of note, our prior work has shown that treatment with 0.4 μg/mL of doxycycline reduces transgenic miR-132 over-expression to physiological levels (Hansen et al., 2013). Remarkably, we found that doxycycline mitigated the anxiety phenotype of miR-132 transgenic mice. Thus, no significant difference in the number of open arm transitions was observed between WT and miR-132 transgenic mice (Fig. 4B t(30) = 0.3511, p = 0.7280; Student’s t-test). Further, the amount of time spent in the open arms and the latency to an open arm of the maze did not differ between WT and miR-132 transgenic mice (Fig. 4C t(30) = 0.2203, p = 0.8271 and Fig. 4D t(30) = 0.5122, p = 0.6122; Student’s tests). Similarly, in the open field test, we found that the anxiety phenotype was absent in the miR-132 transgenic mice upon administration of 0.40 μg/mL of doxycycline (Fig. 4E). No significant difference in the time spent in the center, the number of crosses, or the total seconds immobile was observed between WT and miR-132 transgenic mice (Fig. 4F t(33) = 1.131, p = 0.2661 for center time; Fig. 4G t(35) = 1.049, p = 0.30313 for center crosses and Fig. 4H t(33) = 0.3999, p = 0.6918 for immobility time Student’s t-test). Further, an analysis of elevated plus maze performance and open field activity between WT mice (Fig. 3) and WT mice maintained on doxycycline (Fig. 4) did not detect significant differences in any of the reported measures (data comparison not shown). Altogether, these data support the link between miR-132 and the induction of anxiety-like behavior.
Here, we note that both male and female WT, miR-132/212 cKO, and miR-132 transgenic animals were used in the above behavioral experiments. Hence, the results presented above (from Figs. 3–4) include both male and female pooled data sets. Given that sex differences in rodent anxiety paradigms have been reported (for review, see Donner and Lowry, 2013; Kokras and Dalla, 2014), the noted data sets were also analyzed as separate female and male cohorts (see Supplementary figure 3). In both the elevated plus maze experiments and in the open field experiments (both no-doxycycline and doxycycline conditions), we found no significant interaction between genotype and sex in any reported measure (Supplementary Fig. 3A-L; p > 0.05; two-way ANOVA). However, in the no-doxycycline open field experiment, we did observe a significant effect of sex in the total duration spent in the center and in the number of center crosses (Supplementary Fig. 3G F(1, 29) = 8.652; p = 0.0064 for center duration and Supplementary Fig. 3H F(1,30) = 8.703; p = 0.0061 for center crosses). Additionally, in the doxycycline open field experiment, we noted an effect of sex in the number of center crosses (Supplementary Fig. 3K F(1, 33) = 6.163; p = 0.0183). Further, paralleling our pooled results, in each parameter measured (in both the no-doxycycline elevated plus and open field experiments), we did find a significant effect of genotype (Supplementary Fig. 3A-C; 3G-I; p < 0.05 for each parameter tested; two-way ANOVA). This genotypic effect was not observed in animals treated with doxycycline (Supplementary Fig. 3D-F; 3J-L; p > 0.05 for each parameter tested; two-way ANOVA).
3.5. Altered expression of miR-132 target genes in miR-132/212 cKO and miR-132 transgenic mice
Given the significant alterations in baseline anxiety in miR-132 transgenic mice, and that miRNAs repress the translation of mRNA targets, we examined the expression of Sirt1 and Pten—two miR-132/ 212 targets that are expressed within the hippocampus and amygdala (Lachyankar et al., 2000; Ramadori et al., 2008; Zakhary et al., 2010) and that influence synaptic plasticity and anxiety (Gao et al., 2010; Kwon et al., 2006; Libert et al., 2011; Lugo et al., 2014; Michán et al., 2010).
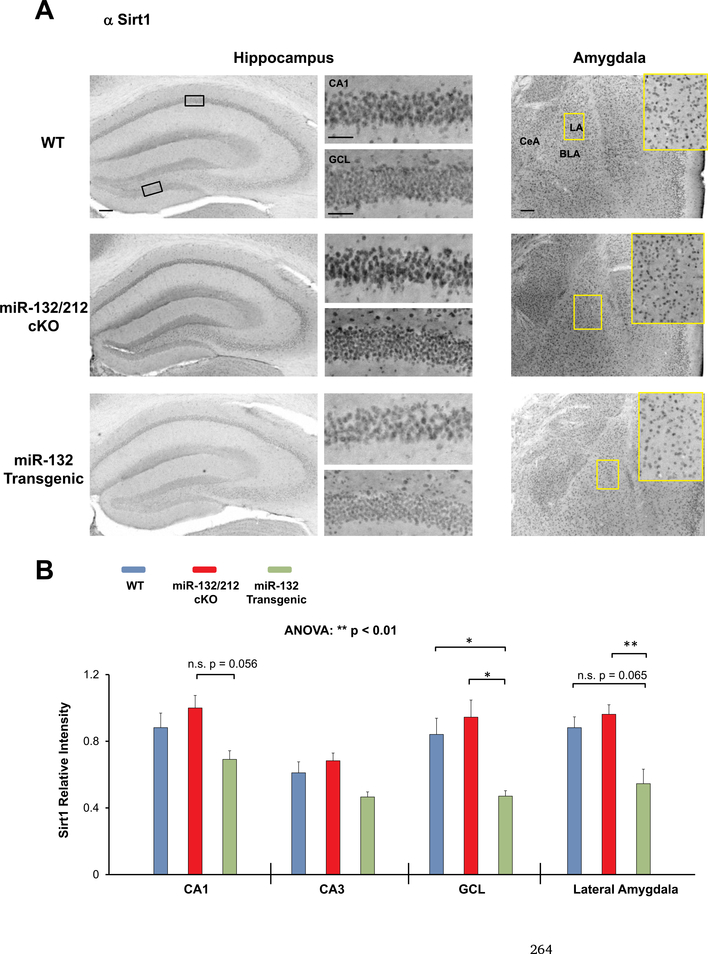
Marked Sirt1 immunohistochemical (IHC) labeling was observed throughout the hippocampus and amygdala of WT mice (Fig. 5A). Interestingly, quantitative densitometric analysis of the IHC labeling revealed a significant effect of genotype on the expression of Sirt1 (Fig. 5B F(2, 35) = 5.247, p = 0.0043; two-way ANOVA). Specifically, Sirt1 expression was significantly higher in the GCL of WT mice compared to miR-132 transgenic mice (Fig. 5B t(7) = 2.827, p = 0.023; Bonferroni post-hoc test), and in the GCL of miR-132/212 cKO mice compared to miR-132 transgenic mice (Fig. 5B t(8) = 3.785, p = 0.0017; Bonferroni post-hoc test). Additionally, expression of Sirt1 in the amygdala of miR-132/212 cKO mice was significantly higher relative to expression in miR-132 transgenic mice (Fig. 5B t(8) = 3.325, p = 0.0062; Bonferroni post-hoc test). Sirt1 expression within the CA1 and lateral amygdala showed a trending increase in miR-132/212 cKO mice relative to miR-132 transgenic mice, though significance was not reached after Bonferroni correction (Fig. 5B t(8) = 2.465, p = 0.0563 for hippocampus and Fig. 5B t(8) = 2.401, p = 0.0654 for lateral amygdala).
Fig. 5. Profiling Sirt1 expression in miR-132/212 cKO and miR-132 transgenic mice.
(A) Representative immunohistochemical labeling for Sirt1 in the hippocampus and amygdala of WT (blue), miR-132/212 cKO (red), and miR-132 transgenic mice (green). The boxed regions in the lowmagnification images approximate the locations of the regions that are depicted in the high-magnification panels (to the right for the hippocampus, and inset for the amygdala). (B) Quantification of Sirt1 immunolabeling in three hippocampal regions (the CA1, CA3, and GCL) and in the lateral amygdala. Note the increased expression of Sirt1 in the miR-132/212 cKO animals. Scale bar = 50 μm for the low magnification images (i.e., whole hippocampus and amygdala) and 30 μm for high magnification images (i.e., CA1 cell layer and GCL-lower blade). Data were analyzed by two-way ANOVA with Bonferroni post-hoc correction and are presented as the mean ± SEM. *: p < 0.05; **: p < 0.01; n.s.: not significant (p > 0.05). N = 3–5 mice per genotype.
Turning to Pten (Fig. 6), we found a significant effect of genotype on the expression of Pten within the forebrain (Fig. 6B F(2, 40) = 11.66, p = 0.0001; two-way ANOVA). In particular, Pten expression within the CA1 was significantly lower in miR-132 transgenic mice relative to miR-132/212 cKO mice (Fig. 6B t(8) = 3.318, p = 0.0058; Bonferroni post-hoc test). Further, in the CA3 and GCL subregions of the hippocampus, a trend was observed, wherein Pten expression was higher in miR-132/212 cKO animals relative to miR-132 transgenic animals, though significance was not reached (Fig. 6B t(8) = 2.448, p = 0.0566 for GCL and t(8) = 2.37, p = 0.0681 for CA3; Bonferroni post-hoc tests). Additionally, a trending increase in PTEN expression was also noted in the GCL of miR-132/212 cKO animals relative to WT animals, though the data did not pass Bonferroni correction (Fig. 6B t(9) = 2.23, p = 0.0944).
4. Discussion
The over-arching goal of this project was to elucidate the role of the miR-132/212 locus in the regulation of stress responsiveness and anxiety. Here, we demonstrate that both miR-132 and miR-212 are induced in two stress-responsive regions—the hippocampus and amygdala—after an acute stress paradigm and in the amygdala after a chronic stress paradigm. Further, we show that dysregulation of forebrain miR-132/212 expression alters baseline anxiety levels. Taken together, these data suggest that the miR-132/212 locus modulates stress- and anxiety-like behavior.
4.1. Induction of the miR-132/212 locus after acute and chronic stress
With respect to the multimodal stress results, to our knowledge, this is the first study to show induction of miR-132 after an acute stress paradigm. The rather rapid (less than 6 h after stress onset) induction of miR-132 is not surprising given its highly inducible nature and classification as an early response miRNA (Lagos et al., 2010). Along these lines, Wibrand et al. (2010) noted an upregulation of both mature miR-132 and miR-212 just 2 h after the onset of LTP within the dentate gyrus of rats. Similarly, increased miR-212 expression has yet to be reported after an acute stressor, though its induction is not unexpected given that it is expressed from the same primary transcript as miR-132 (Vo et al., 2005).
Interestingly, our findings that the miR-132/212 locus is induced in the hippocampus and amygdala following acute stress and only in the amygdala following chronic stress—mirror the biphasic effects that are observed in many stress-related disorders. Indeed, acute stress often enhances synaptic transmission in forebrain regions, leading to increases in dendritic spine density within the hippocampus (Komatsuzaki et al., 2012; Shors et al., 2001), while repeated or chronic stress often reduces transmission and promotes dendritic retraction in the hippocampus (Magariños et al., 1996; Vyas et al., 2002; Watanabe et al., 1992), but not in the amygdala (Musazzi et al., 2015; Rosenkranz et al., 2010).
In line with the idea that acute stress facilitates synaptic transmission, the transgenic upregulation of miR-132 in hippocampal neurons increases dendritic spine density (Hansen et al., 2010) and enhances dendritic morphogenesis (Wayman et al., 2008). Given our observed induction of miR-132/212 within the hippocampus after acute stress, but not after chronic stress, it would be interesting to examine whether increases in dendritic spine density after acute stress are miR-132/212 dependent, and whether decreases in spine density after chronic stress are miR-132/212 independent. Consistent with the latter idea, Yi et al. (2014) reported a marked downregulation in miR-132 expression within the hippocampus following a chronic unpredictable stress paradigm, coinciding with the atrophy of dendrites and loss of synapses that is often observed during chronic stress paradigms as part of the biphasic action of stress (Musazzi et al., 2015).
Turning to the amygdala, both acute and chronic stress lead to increases in basolateral amygdalar neuron dendritic length/arborization (Cui et al., 2008; Hill et al., 2011; Kim et al., 2014; Mitra et al., 2005; Vyas et al., 2002). These neurons are also more hyperactive (Correll et al., 2005; Padival et al., 2013; Rosenkranz et al., 2010; Zhang and Rosenkranz, 2012) and have a greater number of NMDA receptors compared to non-stressed, control animals (Lei and Tejani-Butt, 2010). Consistent with this, imaging studies have shown that the amygdala and insula are hyperactive in patients with social anxiety and specific phobia disorder (Etkin, 2012; Etkin and Wager, 2007), and in patients who are ‘anxiety-prone’ (Stein et al., 2007). Given that bouts of both brief and prolonged stress increase the dendritic complexity of amygdalar neurons, and that we found miR-132/212 to be induced after both acute and chronic stress paradigms, one may postulate that miR-132 and miR-212 could be contributing to the observed increased physiological drive of amygdalar neurons after both transient and long-lasting stress. Hence, future studies aimed at examining if and how the miR-132/212 locus is involved in the molecular network that leads to changes in dendritic morphology and synaptic activity within the amygdala after stress, are highly merited.
4.2. Bidirectional miR-132 dysregulation increases anxiety-related behaviors
Our data showing an upregulation of the miR-132/212 locus after stress prompted us to turn to the question of whether this locus contributes to anxiety-like behavior. Interestingly, we found that miR-132 transgenic mice displayed a heightened anxiety phenotype in both the elevated plus maze and open field paradigms. To our surprise, miR-132/212 cKO mice also exhibited anxiety behaviors, albeit to a lesser degree than the miR-132 transgenic animals. Of note, this phenotype in the miR-132/212 cKO animals is in line with a prior study, which showed that miR-132/212 germline knockout mice spent slightly more time freezing in the open field test and less time in the open arms of the elevated plus maze, though significance was not reached (Hernandez-Rapp et al., 2015). On the surface, the observation that both miR-132/ 212 gain- and loss-of-function facilitates anxiety-like behaviors could be viewed as counter-intuitive. However, it should be noted that prior work from our lab has revealed that both miR-132 over-expressing mice and miR-132/212 cKO mice display cognitive deficits in several learning and memory paradigms (Hansen et al., 2016, 2013, 2010). Thus, in many respects, the anxiety phenotype that we observed after both knocking out and over-expressing miR-132, parallels the noted cognitive effects. Hence, these results suggest that tight regulation of miR-132 (and perhaps miR-212) is also necessary for maintenance of ‘normal’ basal anxiety levels. Consistent with this hypothesis, by titering transgenic miR-132 expression to physiological levels, we found that the anxiety phenotype was abolished in miR-132 transgenic animals. Further, given that the miR-132/212 locus is tightly regulated by the CREB/CRE transcriptional pathway (Vo et al., 2005), it is interesting to note that there also appears to be an optimal range of CREB activity that is required for the maintenance of ‘normal’ anxiety, as both knockdown and over-expression of CREB has been show to increase anxiogenic behaviors (Valverde et al., 2004; Vogt et al., 2014; Wallace et al., 2004).
The idea that there exists an optimal range of miR-132 (and perhaps miR-212) expression, below or above which anxiety-related behaviors are increased, is in line with several studies related to the role of forebrain excitatory/inhibitory (E/I) balance. Intriguingly, many groups have posited that anxiety disorders emerge and/or are potentiated by the dysregulation of E/I balance within the molecular network that comprises the limbic system (Ferraguti, 2018; Nuss, 2015; Tovote et al., 2015). In support of this idea, synaptic transmission is reduced in the hippocampus and cortex of miR-132/212 knockout animals (Luikart et al., 2011; Remenyi et al., 2013), while mEPSC amplitude and frequency are increased and synaptic depression is decreased after miR-132 over-expression (Edbauer et al., 2010; Lambert et al., 2010). Whether miR-132/212 regulates E/I balance in limbic brain regions remains an intriguing avenue for future study.
Here it should be noted that in the CNS, miR-132 is expressed at a much higher level than miR-212 (Remenyi et al., 2016; our unpublished data). This observation combined with the fact that miR-132 and miR-212 share a common seed sequences (and thus, are predicted to have a good degree of functional redundancy), led us to focus our transgenic over-expression approach solely on miR-132. The combination of findings showing that 1) transgenic miR-132 elicits anxiety-like behavior, and 2) that this phenotype is reversed with doxycycline treatment provides solid support for the idea that elevated levels of miR-132 (and possibly miR-212) couple stress to the manifestation of anxiety-like behavior. Here, one caveat regarding the transgenic miR-132 mouse line should be noted: tonically high levels of transgenic miR-132 are expressed via the CaMKII-tTA driver through late stage development and into maturity. As such, there may be long-term compensatory processes that could contribute to the anxiety-like phenotype. Approaches in which transient induction of transgenic miR-132 expression is initiated (via withdrawal of doxycycline from the drinking water after maturation) would be needed to obviate these issues. Similarly, deletion of the miR-132 locus (via the same CaMKII promotor construct), could also result in compensatory processes; additional experiments that employ inducible gene deletion approaches in mature animals (e.g., via the use of a tamoxifen-inducible CRE) would provide an excellent, further, test of the data reported here.
4.3. Altered expression of miR-132/212 target genes
The anxiety-like phenotype of miR-132 transgenic and miR-132/ 212 cKO mice reported here likely results from alterations in the expression patterns of both direct and indirect targets of miR-132/212. Notably, prior gene profiling methods have found that the deletion or over-expression of miR-132/212 leads to changes in the expression of hundreds of genes (Hansen et al., 2016; Mazziotti et al., 2017). Given the complexity of the miR-132/212-gated transcriptome, we chose to examine the expression levels of two miR-132/212 targets that have been shown to affect anxiety-like behavior: Sirt1 and Pten. Sirt1 is a NAD-deacetylase that has been characterized as a direct target of miR-132 (Gong et al., 2016; Miyazaki et al., 2014; Strum et al., 2009; Zhang et al., 2014). Though not yet validated, Sirt1 and miR-212 also show strong hybridization capacity (microRNA.org). Of relevance to this study, Sirt1 has been shown to mediate anxiety behavior and exploratory drive (Libert et al., 2011). Indeed, knocking down or over-expressing Sirt1 leads to anxiogenic behaviors (Libert et al., 2011; Kim et al., 2016). Though miR-132-mediated actuation of anxiety-like behavior likely involves multiple gene targets, it is interesting to note that Sirt1 expression was reduced in the hippocampus and amygdala of miR-132 transgenic mice. Further, consistent with data obtained from our chronic stress qPCR results (Aten et al., 2018b; Fig. 1G and J), Sirt1 protein was decreased in the amygdala of chronically stressed WT mice, whereas it remained unchanged within the hippocampus (Fig. 2, Data in Brief; submitted). Taken together, these data raise the prospect that miR-132 regulation of Sirt1 may play a role in stress-induced anxiety.
We next examined the expression of Pten, a key regulator of the PI3K/AKT/mTOR signaling pathway. With respect to its post-transcriptional regulation, Pten has been validated as a target of miR-132 by multiple groups (Hanin et al., 2017; Wong et al., 2013; Zhang et al., 2017). Additionally, miR-212 and Pten also show a robust predicted hybridization capacity (microRNA.org). Our interest in Pten was heightened by data showing that Pten conditional knockout mice display social deficits and anxiety-like behavior (Kwon et al., 2006; Zhou et al., 2009) and that knockdown of Pten also decreases amygdalar spine density and increases mEPSC frequency and amplitude (Haws et al., 2014), suggestive of a role for its regulation of synaptic activity in limbic regions. Intriguingly, our immunohistochemical data revealed a decrease in Pten in the CA1 of miR-132 transgenic animals. Given these results, a deeper understanding of the cellular signaling events that regulate Pten expression at baseline and after stress, may be of merit.
Finally, we should reiterate that while altered expression of either Sirt1 or Pten could significantly affect molecular/cellular signaling pathways that modulate anxiety states, we are not attributing the anxiety phenotype of the miR-132/212 cKO or miR-132 transgenic mice to the singular dysregulation of either target gene. However, in line with the idea of miRNA-target signaling regulation, the results that we present here suggest that dysregulation of the miR-132/212 locus leads to profound changes in gene expression that underlie stress and anxiety behavior.
5. Conclusions
Our data reveal a novel role for the miR-132/212 locus in the regulation of stress response and anxiety. Given that dysregulation of this locus is associated with a number of neurological diseases (see Wanet et al., 2012 for review), these findings raise compelling questions about the molecular underpinnings of miR-132/212 signaling and the importance of this locus in the etiology and regulation of other psychiatric and mood related disorders. Indeed, new genome editing technologies, novel small-molecule delivery techniques, and recent advances in exosomal non-coding RNA, may be utilized to characterize miRNAs as both biomarkers and/or therapeutic targets for several psychiatric conditions. To this end, additional work investigating the miR-132/212 locus and its molecular interactions in other psychopathologies is highly merited.
Supplementary Material
HIGHLIGHTS.
Hippocampal and amygdalar miR-132 and miR-212 are induced after acute stress.
Amygdalar miR-132 and miR-212 are induced after chronic stress.
miR-132/212 cKO mice and miR-132 transgenic mice display anxiety-like behaviors.
Moderate doxycycline dose abolishes anxiety phenotype in miR-132 transgenic mice.
Sirt1 and PTEN expression are altered in miR-132/212 cKO and miR-132 transgenic mice.
Acknowledgements:
The authors thank Dr. Kate Karelina for her input on experimental design.
Funding
This work was supported by National Institutes of Health; Grant code: MH103361, NS066345, NS091302 and National Science Foundation, United States; Grant code: 1354612.
Footnotes
Declarations of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2018.10.020.
References
- Alemayehu S, Feghali KC, Cowden J, Komisar J, Ockenhouse CF, Kamau E, 2013. Comparative evaluation of published real-time PCR assays for the detection of malaria following MIQE guidelines. Malar. J 12, 277 10.1186/1475-2875-12-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina D, Di Segni M, Bisicchia E, D’Alessandro F, Cestari V, Ventura A, Concepcion C, Puglisi-Allegra S, Ventura R, 2016. Effects of lack of microRNA-34 on the neural circuitry underlying the stress response and anxiety. Neuropharmacology 107, 305–316. 10.1016/j.neuropharm.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aten S, Hansen KF, Snider K, Wheaton K, Kalidindi A, Garcia A, Alzate-Correa D, Hoyt KR, Obrietan K, 2018a. miR-132 couples the circadian clock to daily rhythms of neuronal plasticity and cognition. Learn. Mem 25, 214–229. 10.1101/lm.047191.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aten S, Hansen KF, Snider K, Wheaton K, Kalidindi A, Garcia A, Alzate-Correa D, Hoyt KR, Obrietan K, 2018b. Data Highlighting the Expression of Two MiR-132/ 212 Target Genes—Sirt1 and Pten—after Chronic Stress. Data Brief (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ, 1993. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav. Brain Res 58, 113–121. 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ, 2010. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl. Acad. Sci. U.S.A 107, 13123–13128. 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-YM, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K, 2007. microRNA modulation of circadian clock period and entrainment. Neuron 54, 813–829. 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Jackson NL, Ballestas ME, Webb WM, Lubin FD, Clinton SM, 2017. Amygdalar expression of the microRNA miR-101a and its target Ezh2 contribute to rodent anxiety-like behaviour. Eur. J. Neurosci 46, 2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA, 2005. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol. Psychiatry 58, 382–391. 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Cui H, Sakamoto H, Higashi S, Kawata M, 2008. Effects of single-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience 152, 703–712. 10.1016/j.neuroscience.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JB, Battaglea DF, Akil H, Watson SJ, 1995. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA, 2013. Sex differences in anxiety and emotional behavior. Pflügers Archiv 465, 601–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang C-F, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M, 2010. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65, 373–384. 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF, 2005. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J. Neuroimmunol 163, 110–119. 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Etkin A, 2012. Neurobiology of anxiety: from neural circuits to novel solutions? Depress. Anxiety 29, 355–358. 10.1002/da.21957. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, 2018. Metabotropic glutamate receptors as targets for novel anxiolytics. Curr. Opin. Pharmacol 38, 37–42. 10.1016/j.coph.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang W-Y, Mao Y-W, Gräff J, Guan J-S, Pan L, Mak G, Kim D, Su SC, Tsai L-H, 2010. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109. 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong K, Qu B, Liao D, Liu D, Wang C, Zhou J, Pan X, 2016. MiR-132 regulates osteogenic differentiation via downregulating Sirtuin1 in a peroxisome proliferator-activated receptor β/δ-dependent manner. Biochem. Biophys. Res. Commun 478, 260–267. 10.1016/j.bbrc.2016.07.057. [DOI] [PubMed] [Google Scholar]
- Hanin G, Yayon N, Tzur Y, Haviv R, Bennett ER, Udi S, Krishnamoorthy YR, Kotsiliti E, Zangen R, Efron B, Tam J, Pappo O, Shteyer E, Pikarsky E, Heikenwalder M, Greenberg DS, Soreq H, 2017. miRNA-132 induces hepatic steatosis and hyperlipidaemia by synergistic multitarget suppression. Gut. 10.1136/gutjnl-2016-312869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Wayman GA, Impey S, Obrietan K, 2010. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS One 5 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Karelina K, Sakamoto K, Wayman GA, Impey S, Obrietan K, 2013. miRNA-132: a dynamic regulator of cognitive capacity. Brain Struct. Funct 218, 817–831. 10.1007/s00429-012-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Aten S, Snider KH, Loeser J, Hesse AM, Page CE, Pelz C, Arthur JSC, Impey S, Obrietan K, 2016. Targeted deletion of miR-132/−212 impairs memory and alters the hippocampal transcriptome. Learn. Mem 23, 61–71. 10.1101/lm.039578.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A, 2011. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J. Neurosci 31, 14191–14203. 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws ME, Jaramillo TC, Espinosa F, Widman AJ, Stuber GD, Sparta DR, Tye KM, Russo SJ, Parada LF, Stavarache M, Kaplitt M, Bonci A, Powell CM, 2014. PTEN knockdown alters dendritic spine/protrusion morphology, not density. J. Comp. Neurol 522, 1171–1190. 10.1002/cne.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rapp J, Smith PY, Filali M, Goupil C, Planel E, Magill ST, Goodman RH, Hébert SS, 2015. Memory formation and retention are affected in adult miR-132/212 knockout mice. Behav. Brain Res 287, 15–26. 10.1016/j.bbr.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS, 2011. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor–deficient mice parallel the effects of chronic stress. Cerebr. Cortex 21, 2056–2064. 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG, 1993. cFos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol 14, 173–213. [DOI] [PubMed] [Google Scholar]
- Hollins SL, Cairns MJ, 2016. MicroRNA: small RNA mediators of the brains genomic response to environmental stress. Prog. Neurobiol 143, 61–81. 10.1016/j.pneurobio.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Issler O, Chen A, 2015. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci 16, 201–212. 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- Jeong JY, Lee DH, Kang SS, 2013. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol. Metabol. (Seoul) 28, 288–296. 10.3803/EnM.2013.28.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Bray I, Sanz-Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T, Saugstad JA, Simon RP, Stallings RL, Henshall DC, 2011. miRNA expression profile after status epilepticus and hippocampal neuroprotection by targeting mir-132. Am. J. Pathol 179, 2519–2532. 10.1016/j.ajpath.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, van den Oord EJCG, Riley BP, Kendler KS, Vladimirov VI, 2010. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res 124, 183–191. 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-D, Hesterman J, Call T, Magazu S, Keeley E, Armenta K, Kronman H, Neve RL, Nestler EJ, Ferguson D, 2016. SIRT1 mediates depression-like behaviors in the nucleus accumbens. J. Neurosci 36, 8441–8452. 10.1523/JNEUROSCI.0212-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Yi JH, Choi K, Hong S, Shin KS, Kang SJ, 2014. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neurosci. 15, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N, Dalla C, 2014. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol 171, 1–25. 10.1111/bph.12710. Epub 2014 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki Y, Hatanaka Y, Murakami G, Mukai H, Hojo Y, Saito M, Kimoto T, Kawato S, 2012. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS One 7, e34124 10.1371/journal.pone.0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ, 1990. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol. Psychiatry 27, 1094–1102. [DOI] [PubMed] [Google Scholar]
- Kwon C-H, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF, 2006. Pten regulates neuronal arborization and social interaction in mice. Neuron 50, 377–388. 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, Miller SJ, Ohta S, Neel BG, Ross AH, 2000. A role for nuclear PTEN in neuronal differentiation. J. Neurosci 20, 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RSB, Gotch F, Boshoff C, 2010. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat. Cell Biol 12, 513–519. 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Storm DR, Sullivan JM, 2010. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS One 5 10.1371/journal.pone.0015182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Tejani-Butt SM, 2010. NMDA receptors are altered by stress and alcohol in wistar-kyoto rat brain. Neuroscience 169, 125–131. 10.1016/j.neuroscience.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKL, Sharp PA, 2007. microRNAs: a safeguard against turmoil? Cell 130, 581–585. 10.1016/j.cell.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Leung AKL, Sharp PA, 2010. MicroRNA functions in stress responses. Mol. Cell 40, 205–215. 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-J, Xu M, Gao Z-H, Wang Y-Q, Yue Z, Zhang Y-X, Li X-X, Zhang C, Xie S-Y, Wang P-Y, 2013. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS One 8, e63648 10.1371/journal.pone.0063648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, Kendler KS, Chen X, Hettema JM, van den Oord EJ, Rubio JP, Guarente L, 2011. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 147, 1459–1472. 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Smith GD, Arbuckle EP, White J, Holley AJ, Floruta CM, Ahmed N, Gomez MC, Okonkwo O, 2014. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front. Mol. Neurosci 7 10.3389/fnmol.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Bensen AL, Washburn EK, Perederiy JV, Su KG, Li Y, Kernie SG, Parada LF, Westbrook GL, 2011. miR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLoS One 6, e19077 10.1371/journal.pone.0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E, 1996. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci 16, 3534–3540. 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan-Müller S, Hemmings SMJ, Seedat S, 2013. Big effects of small RNAs: a review of MicroRNAs in anxiety. Mol. Neurobiol 47, 726–739. 10.1007/s12035-012-8374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannironi C, Camon J, De Vito F, Biundo A, De Stefano ME, Persiconi I, Bozzoni I, Fragapane P, Mele A, Presutti C, 2013. Acute stress alters amygdala microRNA miR-135a and miR-124 expression: inferences for corticosteroid dependent stress response. PLoS One 8, e73385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannironi C, Biundo A, Rajendran S, De Vito F, Saba L, Caioli S, Zona C, Ciotti T, Caristi S, Perlas E, Del Vecchio G, Bozzoni I, Rinaldi A, Mele A, Presutti C, 2018. miR-135a regulates synaptic transmission and anxiety-like behavior in amygdala. Mol. Neurobiol 55, 3301–3315. 10.1007/s12035-017-0564-9. [DOI] [PubMed] [Google Scholar]
- Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ, 2014. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol. Psychiatr 19, 811–822. 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotti R, Baroncelli L, Ceglia N, Chelini G, Sala GD, Magnan C, Napoli D, Putignano E, Silingardi D, Tola J, Tognini P, Arthur JSC, Baldi P, Pizzorusso T, 2017. Mir-132/212 is required for maturation of binocular matching of orientation preference and depth perception. Nat. Commun 8, 15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM, 2012. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62, 3–12. 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D, 2010. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J. Mol. Neurosci 40, 47–55. 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Sugihara H, Castro J, Banerjee A, Le C, Kumar A, Crawford B, Strathmann J, Tropea D, Levine SS, Edbauer D, Sur M, 2011. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat. Neurosci 14, 1240–1242. 10.1038/nn.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JT, Olson EN, 2012. MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187. 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michán S, Li Y, Chou MM-H, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LEH, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD, 2010. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci 30, 9695–9707. 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S, 2005. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A 102, 9371–9376. 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Li R, Rezk A, Misirliyan H, Moore C, Farooqi N, Solis M, Goiry LG, de Faria O Junior, Dang VD, Colman D, Dhaunchak AS, Antel J, Gommerman J, Prat A, Fillatreau S, Bar-Or A, 2014. A novel MicroRNA-132-surtuin-1 Axis underlies aberrant B-cell cytokine regulation in patients with relapsing-remitting multiple sclerosis. PLoS One 9 10.1371/journal.pone.0105421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Popoli M, 2015. Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Front. Psychiatr 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto SJ, Patriquin MA, Nielsen DA, Kosten TA, 2016. Don’t worry; be informed about the epigenetics of anxiety. Pharmacol. Biochem. Behav 146–147, 60–72. 10.1016/j.pbb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR, 2010. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20, 492–498. 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH, 2011. Gene–environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berlin) 214, 175–196. 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss P, 2015. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatric Dis. Treat 11, 165–175. 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Dinan TG, Cryan JF, 2012. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol. Psychiatr 17, 359–376. 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- Padival M, Quinette D, Rosenkranz JA, 2013. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology 38, 1748–1762. 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM, 2007. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 8, R27 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Andrews G, Millichamp J, 2008. Gene-environment interaction and the anxiety disorders. Eur. Arch. Psychiatr. Clin. Neurosci 258, 65–68. 10.1007/s00406-007-0784-5. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R, 2008. Brain SIRT1: anatomical distribution and regulation by energy availability. J. Neurosci 28, 9989–9996. 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi J, Bajan S, Fuller-Pace FV, Arthur JS, Hutvagner G, 2016. The loop structure and the RNA helicase p72/DDX17 influence the processing efficiency of the mice miR-132. Sci. Rep, 22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi J, van den Bosch MW, Palygin O, Mistry RB, McKenzie C, Macdonald A, Hutvagner G, Arthur JS, Frenguelli BG, Pankratov Y, 2013. miR-132/212 knockout mice reveal roles for these miRNAs in regulating cortical synaptic transmission and plasticity. PLoS One 8, e62509 10.1371/journal.pone.0062509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M, 2010. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 67, 1128–1136. 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HL, Tamagnini F, Narduzzo KE, Howarth JL, Lee Y-B, Wong L-F, Brown MW, Warburton EC, Bashir ZI, Uney JB, 2012. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur. J. Neurosci 36, 2941–2948. 10.1111/j.1460-9568.2012.08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senba E, Matsunaga K, Tohyama M, Noguchi K, 1993. Stress-induced c-fos expression in the rat brain: activation mechanism of sympathetic pathway. Brain Res. Bull 31, 329–344. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Hanan M, Wolf Y, Barbash S, Kovalev E, Shoham S, Soreq H, 2013. Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct. Funct 218, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J, 2001. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci 21, 6292–6297. 10.1523/jneurosci.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Rizavi HS, Zhang H, Torvik VI, Pandey GN, Davis JM, Dwivedi Y, 2011. MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. Int. J. Neuropsychopharmacol 14, 1315–1325. 10.1017/S1461145710001628. [DOI] [PubMed] [Google Scholar]
- Smoller JW, 2016. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology 41, 297–319. 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider KH, Dziema H, Aten S, Loeser J, Norona FE, Hoyt K, Obrietan K, 2016. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav. Brain Res. 308, 222–235. 10.1016/j.bbr.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz AM, Goscik J, Swiergiel AH, Majewska A, Wieczorek M, Juszczak GR, Lisowski P, 2014. Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neurosci. 15, 130 10.1186/s12868-014-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP, 2007. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J.Psychiatry 164, 318–327. 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM, 2009. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol 23, 1876–1884. 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognini P, Putignano E, Coatti A, Pizzorusso T, 2011. Experience-dependent expression of miR132 regulates ocular dominance plasticity. Nat. Neurosci 14, 1237–1239. 10.1038/nn.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A, 2015. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16, 317–331. 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S, 1996. Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y, 2010. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J. Neurosci 30, 15007–15018. 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Mantamadiotis T, Torrecilla M, Ugedo L, Pineda J, Bleckmann S, Gass P, Kretz O, Mitchell JM, Schütz G, Maldonado R, 2004. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology 29, 122–1133. [DOI] [PubMed] [Google Scholar]
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S, 2005. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. U.S.A 102, 16426–16431. 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt MA, Inta D, Luoni A, Elkin H, Pfeiffer N, Riva MA, Gass P, 2014. Inducible forebrain-specific ablation of the transcription factor creb during adulthood induces anxiety but no spatial/contextual learning deficits. Front. Behav. Neurosci 8, 407 10.3389/fnbeh.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk N, Paul ED, Harmati S, Eitan C, Fields BK, Zwang R, Gil S, Lowry CA, Chen A, 2014. microRNA-19b associates with Ago2 in the amygdala following chronic stress and regulates the adrenergic receptor beta 1. J. Neurosci 34, 15070–15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB, 2013. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One 8, e58488 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S, 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci 22, 6810–6818. https://doi.org/20026655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc 3, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Stellitano KE, Neve RL, Duman RS, 2004. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol. Psychiatry 56, 151–160. [DOI] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P, 2012. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 40, 4742–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS, 1992. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 588, 341–345. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng H-YM, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S, 2008. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc. Natl. Acad. Sci. U. S. A 105, 9093–9098. 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibrand K, Panja D, Tiron A, Ofte ML, Skaftnesmo K-O, Lee CS, Pena JTG, Tuschl T, Bramham CR, 2010. Differential regulation of mature and precursor microRNA expression by NMDA and metabotropic glutamate receptor activation during LTP in the adult dentate gyrus in vivo. Eur. J. Neurosci 31, 636–645. 10.1111/j.1460-9568.2010.07112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand C, Savelsbergh A, Heusser P, 2017. MicroRNAs in psychological stress reactions and their use as stress-associated biomarkers, especially in human saliva. BMH 2 4–4. 10.1159/000481126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H-KA, Veremeyko T, Patel N, Lemere CA, Walsh DM, Esau C, Vanderburg C, Krichevsky AM, 2013. De-repression of FOXO3a death axis by microRNA-132 and −212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet 22, 3077–3092. 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]
- Yi L-T, Li J, Liu B-B, Luo L, Liu Q, Geng D, 2014. BDNF–ERK–CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J. Psychiatry Neurosci. 39, 348–359. 10.1503/jpn.130169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Kim B-H, Ye S-K, Kim M-H, 2014. Chronic non-social stress affects depressive behaviors but not anxiety in mice. KOREAN J. PHYSIOL. PHARMACOL 18, 263–268. 10.4196/kjpp.2014.18.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary SM, Ayubcha D, Dileo JN, Jose R, Leheste JR, Horowitz JM, Torres G, 2010. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat Rec (Hoboken) 293, 1024–1032. 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapala MA, Hovatta I, Ellison JA, Wodicka L, Rio JAD, Tennant R, Tynan W, Broide RS, Helton R, Stoveken BS, Winrow C, Lockhart Daniel J., Reilly JF, Young WG, Bloom FE, Lockhart David J., Barlow C, 2005. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc. Natl. Acad. Sci. Unit. States Am 102, 10357–10362. 10.1073/pnas.0503357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA, 2012. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience 226, 459–474. 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang D, Wang Q, Shen D, Wang Y, Chen B, Zhang J, Gai L, 2014. MiR-132 inhibits expression of SIRT1 and induces pro-inflammatory processes of vascular endothelial inflammation through blockade of the SREBP-1c metabolic pathway. Cardiovasc. Drugs Ther. 28, 303–311. 10.1007/s10557-014-6533-x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu A, Feng X, Tian L, Bo W, Wang H, Hu Y, 2017. MiR-132 promotes the proliferation, invasion and migration of human pancreatic carcinoma by inhibition of the tumor suppressor gene PTEN. Prog. Biophys. Mol. Biol 10.1016/j.pbiomolbio.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon C-H, Zhang W, Sinton C, Powell CM, Parada LF, 2009. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci 29, 1773–1783. 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Garrett L, Deussing JM, Wotjak CT, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Hölter SM, 2014. A robust and reliable non-invasive test for stress responsivity in mice. Front. Behav. Neurosci 8, 125 10.3389/fnbeh.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.