Abstract
Background
Precision medicine aims to identify those patients who will benefit the most from specific treatments. Recent work found large effects of naltrexone among “reward drinkers,” defined as individuals who drink primarily for the rewarding effects of alcohol. This study sought to replicate and extend these recent findings by examining whether the desire to drink mediated the effect of naltrexone among reward drinkers.
Methods
We conducted a secondary analysis of a 12‐week randomized clinical trial of daily or targeted naltrexone among problem drinkers (n = 163), with a focus on 86 individuals (n = 45 naltrexone and n = 41 placebo) who received daily medication. Interactive voice response technology was used to collect daily reports of drinking and desire to drink. Factor mixture models were used to derive reward and relief phenotypes. Moderation analyses were used to evaluate naltrexone effects, with phenotype as a moderator variable. Multilevel mediation tested average desire to drink as a mediator.
Results
Results indicated 4 phenotypes: low reward/low relief; low reward/high relief; high reward/low relief; and high reward/high relief. There was an interaction between the high reward/low relief subgroup (n = 10) and daily naltrexone versus placebo on drinks per drinking day (DPDD; p = 0.03), percent heavy drinking days (p = 0.004), and daily drinking (p = 0.02). As compared to placebo, individuals in the high reward/low relief phenotype who received daily naltrexone had significantly fewer DPDD (Cohen's d = 2.05) and had a lower proportion of heavy drinking days (Cohen's d = 1.75). As hypothesized, reductions in average desire to drink mediated the effect of naltrexone on average daily drinking among the high reward/low relief drinkers (moderated mediation effect: p = 0.029).
Conclusions
This theory‐driven study replicates the empirical finding that naltrexone is particularly efficacious among high reward/low relief drinkers. Our study brings the field a step closer to the potential of using a precision medicine approach to treating alcohol use disorder.
Keywords: Precision Medicine, Naltrexone, Reward Drinkers, Relief Drinkers, Alcohol Use Disorder
In a post‐hoc precision medicine analysis of a 12‐week clinical trial, the current study examined the efficacy of naltrexone versus placebo for reward drinkers, individuals who drink primarily for the rewarding effects of alcohol and not for the relieving effects. As compared to placebo, reward drinkers who received naltrexone (50 mg/d) had significantly fewer drinks per drinking day (see Figure, Cohen's d = 2.05). Reductions in average desire to drink mediated the effect of naltrexone on average daily drinking among reward drinkers.

Alcohol use disorder (AUD) is a common psychiatric disorder with greater than 10% of individuals in many countries worldwide meeting criteria for a current AUD (World Health Organization (WHO) 2014). Based on the 11 AUD criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM), fifth edition (American Psychiatric Association 2013), there are 2,048 combinations that could result in an AUD diagnosis (Lane and Sher, 2015). In view of such heterogeneity, attempts have been made to subtype affected individuals based on etiology, drinking patterns, course, personality traits, and comorbid psychopathology (Babor et al., 1992; Cloninger et al., 1993; Jellinek, 1960; Lesch and Walter, 1996; Moss et al., 2010).
Parsing the heterogeneity of AUD based on specific phenotypes holds promise for precision medicine approaches that aim to develop treatments tailored to specific dysfunction (Litten et al., 2012). Neuroimaging methods and genotyping have been used to detect subgroups of individuals with AUD for whom precision medicine approaches could be applied (Heilig and Leggio, 2016; Kranzler et al., 2014; Litten et al., 2015; Mann et al., 2009; Spanagel et al., 2013). For example, individuals with 1 or 2 Asp40 alleles of the rs179997 polymorphism in OPRM1 have been shown in some studies to respond better to naltrexone than Asn40 homozygotes (Chamorro et al., 2012; Oslin et al., 2003). The alcohol and addiction research domain criteria (AARDoC) (Litten et al., 2015) represent a combination of physiological, neuroimaging, genetic, self‐report, and clinical observation measures to guide precision medicine. Yet, usual treatment settings lack the capacity to obtain neuroimaging and genotyping measures to guide precision medicine. Thus, self‐report measures that guide precision medicine in real‐world AUD treatment settings could serve as invaluable tools for enhancing the treatment process by targeting specific treatments to specific AUD phenotypes. Such an approach could therefore decrease the public health burden of AUD.
In line with this goal, Verheul and colleagues (1999) hypothesized that reward drinkers, individuals whose drinking is primarily maintained through the positive reinforcing effects of alcohol, would respond better to treatment with naltrexone. This was based on the opioid antagonist effects of the medication, which have been shown to block the rewarding effects of alcohol, including stimulation, positive mood, craving, and enjoyment (Hendershot et al., 2017; Ray and Hutchison, 2007). Drawing on incentive‐sensitization theory, naltrexone may be particularly effective in reducing the desire to drink among individuals who have excessive wanting or liking of alcohol (Berridge and Robinson, 2016). Importantly, across species, naltrexone is more effective than placebo at reducing drinking in the context of alcohol availability and reward‐based learning paradigms (Hay et al., 2013; Kaminski et al., 2012; Ray et al., 2010b). On the contrary, relief drinkers, whose drinking is primarily maintained through consuming alcohol to relieve negative affective states (Koob et al., 2014), would potentially respond better to acamprosate, possibly as a consequence of effects on the glutamate system (Littleton, 2007; Mann et al., 2008). Our previous work tested these hypotheses using self‐report measures to examine heterogeneity in both the COMBINE study data (Roos et al., 2017) and the PREDICT study data (Mann et al., 2018). We found that individuals characterized by a high frequency of reward drinking and low frequency of relief drinking in the PREDICT study had a significantly better response to naltrexone than those who were low in reward and relief drinking (Mann et al., 2018). Individuals characterized by high relief drinking and moderate reward drinking in the COMBINE study had a significantly better response to acamprosate than those who were low in reward and relief drinking (Roos et al., 2017). We did not identify a subgroup of individuals who were high in reward drinking and low in relief drinking in the COMBINE study, and there was no interaction between reward drinking and naltrexone in predicting drinking outcomes in that study. Nonetheless, the findings from this prior work provide a basis for testing a precision medicine approach to AUD treatment, whereby individuals with specific phenotypes may be more likely to respond to specific medications.
Current Study
The current study was designed to replicate and extend our recent findings (Mann et al., 2018) by examining whether reward drinkers—defined as individuals who drink primarily for the rewarding effects of alcohol, relative to the relieving effects (i.e., high levels of reward drinking tendencies and low levels of relief drinking tendencies)—experienced a differential treatment response to naltrexone or placebo in a 12‐week clinical trial aimed at reducing heavy drinking (Kranzler et al., 2009). Further, we sought to examine whether a reduction in the desire to drink mediated naltrexone's efficacy among reward drinkers. Based on our prior work (Mann et al., 2018), we hypothesized that individuals identified as high reward/low relief drinkers at baseline would report significantly less drinking during the treatment trial when treated daily with naltrexone versus placebo. We also hypothesized that the average desire to drink would mediate the effect of naltrexone on drinking outcomes among high reward/low relief drinkers.
Materials and Methods
Participants and Procedure
The current study was a secondary analysis of a randomized clinical trial evaluating naltrexone for reducing problem drinking (Kranzler et al., 2009). Individuals (n = 163) who sought to reduce their drinking were recruited via the community through advertisements and clinician referrals and were randomly assigned in a 2 × 2 design to receive (i) active naltrexone (50 mg) or placebo naltrexone administered via (ii) daily dosing (50 mg/d) or targeted dosing in anticipation of high‐risk drinking situations. Those in the targeted dosing condition were encouraged to use at least 3 tablets (50 mg naltrexone or matching placebo) per week. All participants also received six 20‐minute coping skills training sessions every 2 weeks that focused on identifying and managing high‐risk situations for heavy drinking. The medication and coping skills training were delivered over the course of 12 weeks. The study was registered as NCT00369408, and detailed information on it can be found in Kranzler and colleagues (2009). Study participants were 18 to 65 years old, able to read in English (at least eighth‐grade reading level), not pregnant, and consuming 24 + standard drinks (men) or 18 + standard drinks (women) weekly on average during pretreatment. Exclusionary criteria were severe physical or psychiatric illnesses requiring medical treatment (including severe AUD), current drug dependence, lifetime opioid dependence, or regular use of opioids or psychotropic medications in the past month. Almost all participants (95.1%) met criteria for current alcohol dependence based on DSM‐IV (American Psychiatric Association 1994).
Analyses to establish baseline phenotypes were conducted with the full sample of 163 individuals. Demographic data among the full sample were as follows: male (58.3%), mean age = 49.1 (SD = 9.59), non‐Hispanic white (96.9%), black/African American (1.8%), Indian/South Asian (0.6%), and “other” not‐Hispanic race (0.6%). Average years of education was 15.4 (SD = 2.37).
To provide the closest replication with our prior study that examined daily dosing of 50 mg of naltrexone (Mann et al., 2018), the inferential analyses focused on individuals (n = 86) who received 50 mg of naltrexone (n = 45) or placebo equivalent (n = 41) daily. Demographic data among the sample included in the inferential analyses were as follows: male (61.6%), mean age = 48.3 (SD = 9.43), non‐Hispanic white (96.5%), black/African American (2.3%), and “other” not‐Hispanic race (1.2%). Average years of education was 15.3 (SD = 2.30).
Measures
Reward and Relief Drinking Phenotypes
Twenty‐seven items from the 42‐item short form of the Inventory of Drinking Situations (IDS; Annis et al., 1987) were used to assess reward and relief drinking phenotypes. Individuals responded to the frequency (0 = never, 3 = almost always) of heavy drinking in various situations over the past year. Items were selected based on overlap with the 30‐item version used in our prior work (Mann et al., 2018) and based on conceptual review of the items from the 42‐item scale. Twelve of the 42 items were selected for the current analyses to reflect drinking in social and rewarding situations (e.g., “with friends and want to relax and enjoy”), and 15 items were selected to reflect drinking to relieve negative emotional or physical states (e.g., “feeling upset”). Table 1 provides a summary of items and factor loadings from a confirmatory factor analysis. Internal consistency reliability for the 12 reward items was Cronbach's α = 0.901 and for the 15 relief items was Cronbach's α = 0.925 in the current sample.
Table 1.
Items From the IDS‐42 Representing Reward and Relief Drinking and Standardized Factor Loadings From a Confirmatory Factor Analysis
| Reward factor | Relief factor | |
|---|---|---|
| Want to celebrate with a friend | 0.91 | |
| Met a friend and s/he suggested we have a drink together | 0.89 | |
| Out with friends and wanted to increase my enjoyment | 0.88 | |
| Out with friends and they stopped by bar for a drink | 0.85 | |
| Enjoying myself at a party and wanted to feel even better | 0.83 | |
| At a party and others were drinking | 0.81 | |
| Something good happens and I feel like celebrating | 0.75 | |
| Relaxing with a good friend and wanted to have a good time | 0.73 | |
| I felt confident and relaxed | 0.63 | |
| To heighten my sexual enjoyment | 0.55 | |
| Everything was going well | 0.54 | |
| I remembered how good it tasted | 0.52 | |
| When I had problems with people at work | 0.91 | |
| When I was not getting along with others at work | 0.90 | |
| Was angry at the way things turned out | 0.84 | |
| When I felt I let myself down | 0.84 | |
| Others around me made me tense | 0.83 | |
| Someone criticized me | 0.83 | |
| Others treated me unfairly | 0.82 | |
| Others didn't seem to like me | 0.82 | |
| Afraid things were not working out | 0.81 | |
| When I had an argument with a friend | 0.74 | |
| When I felt pressure at work due to supervisor's demands | 0.70 | |
| Felt confused about what I should do | 0.70 | |
| Felt uneasy in the presence of someone | 0.68 | |
| When there were fights at home | 0.67 | |
| When I had trouble sleeping | 0.30 |
A 2‐factor confirmatory factor analysis, with reward and relief drinking factors, provided an adequate fit to the data based on Root Mean Square Error of Approximation (RMSEA) and Comparative Fit Index values at or below cutoffs (Kline, 2015) (χ2 (398) = 859.1, p < 0.001; RMSEA = 0.08 (90% CI: 0.07, 0.09); CFI = 0.94). The correlation between the reward and relief factor was significant (r = 0.371, p < 0.001).
Alcohol Use Outcomes
Alcohol use during treatment was assessed using the Timeline Follow‐back Interview (TLFB; Sobell and Sobell 1992). We examined 2 indices of alcohol consumption during treatment: drinks per drinking day (DPDD), calculated as the average number of drinks consumed on any drinking day, and the proportion of heavy drinking days (PHDD; 4 or more drinks for women, 5 or more drinks for men) during the 12‐week study.
Daily Drinking and Desire to Drink
Interactive voice response (IVR) technology was used to assess daily drinking and desire to drink over the course of the 12‐week study. As described in prior publications of these data (Kranzler et al., 2009, 2013), individuals completed daily IVR surveys between 5 pm and 9 pm for up to 84 days via a touch‐tone phone. Participants reported their desire to drink, number of standard drinks consumed the prior evening (after the last IVR survey), number of standard drinks consumed prior to the daily IVR survey, and whether they took the assigned medication since the prior call. Desire to drink was defined by the average of 3 items from the IVR survey (“I really don't feel like drinking” (reverse coded), “I feel like I could really use a drink,” and “The idea of drinking is appealing”) with 5 response options for each item ranging from “Definitely false” = 0 to “Definitely true” = 4. Internal consistency reliability of the 3 items was excellent (Cronbach's α = 0.86). Daily drinking was calculated based on the total number of drinks consumed per day (calculated as the sum of the prior evening standard drinks and the current day standard drinks).
Covariates
Consistent with prior analyses of these data (Kranzler et al., 2009, 2013), we controlled for sex, years of education, and the percentage of drinking days during the 90 days prior to the baseline assessment as covariates. In addition, because prior work showed that rs179997, a single nucleotide polymorphism (SNP) in the OPRM1 gene, a target of naltrexone, moderated the effects of desire to drink on subsequent drinking in these data (Kranzler et al., 2013), we included the OPRM1 genotype (Asn40‐allele homozygotes vs. Asp40‐allele carriers) as a covariate in the models.
Statistical Analyses
Reward and Relief Drinking Phenotypes
Factor mixture modeling, estimated in Mplus version 8.2 (Muthén and Muthén, 2017), was used to identify subgroups of individuals with similar item endorsement for reward and relief drinking factors. We simultaneously estimated reward and relief latent factors, which were used as indicators of a latent class variable. Items were treated as ordered categorical, and parameters were estimated with robust maximum likelihood estimation, which allowed us to use all available data in estimating the models (Witkiewitz et al., 2014). We used the full sample (n = 163) for the factor mixture model to maximize the sample size for phenotyping individuals based on responses to the IDS at baseline. Several model fit indices, as well as theoretical utility and parsimony, were used to select the factor mixture model (Nylund et al., 2007). In line with our prior work, we anticipated that a 4‐class solution would provide the best fit to the data. The most likely phenotype assignment from the final solution was saved using the estimated posterior probabilities of class membership.
Replication of Prior Findings Examining Aggregate Alcohol Use Outcomes
As a direct replication of our prior findings (Mann et al., 2018), we examined whether medication condition and reward and relief drinking phenotypes were associated with drinking as reported in the TLFB assessment over the course of the 12‐week treatment period. Moderation analyses were conducted in the context of moderated regression models with the relief and reward phenotypes predicting DPDD and PHDD, controlling for covariates. The treatment condition (0 = placebo naltrexone, 1 = active naltrexone) and dummy‐coded phenotype variables representing reward and relief drinking, with low reward/low relief drinking as the reference group, were entered in the regression models as main effects. Then, we created interaction terms by multiplying treatment by the dummy‐coded phenotype variables. Significant interaction effects from the regression models were probed by conducting simple slopes regression analyses to examine the associations between active naltrexone versus placebo and aggregate alcohol use outcomes in the reward and relief phenotype groups (Aiken and West, 1991).
Extension of Prior Findings Examining Daily Drinking and Desire to Drink
As an extension of the findings in Mann and colleagues (2018), multilevel modeling was used to examine the association between reward and relief drinking phenotypes and daily drinking, as well as the desire to drink obtained from the IVR assessment. Treatment condition, dummy‐coded phenotype variables representing reward and relief drinking, with low reward/low relief drinking as the reference group, and interaction terms were entered in the multilevel models predicting drinks per day. Next, we used multilevel mediation modeling to examine average daily desire to drink as the mechanism by which naltrexone and phenotypes predicted average drinks per day (Preacher et al., 2010).
For all models, we used a generalized linear multilevel model with a negative binomial distribution and log‐link function to predict total drinks per day. All between‐level variables were grand‐mean‐centered. Daily desire to drink was person‐mean‐centered on the within‐person level, and each individual's average desire to drink over the course of the 12 weeks was included on the between‐person level. Significant interaction effects were followed up by conducting multiple group models to examine the associations between active naltrexone versus placebo and daily drinking in the reward and relief phenotype groups (Aiken and West, 1991).
Sensitivity Analyses
We conducted several sensitivity analyses to further explore the phenotypes. First, we examined the same models with the high reward/low relief phenotype as the reference group. Second, we reestimated all models in the group that received targeted naltrexone (n = 38) and matching placebo (n = 39). Third, we conducted analyses of the daily naltrexone effects by sex to examine potential sex differences in the naltrexone response in the reward and relief phenotype groups by sex. Finally, given that the full 100‐item version of the IDS was administered in the PREDICT study sample (n = 426; Mann et al., 2009), we were able to replicate the analyses of the PREDICT study sample published by Mann and colleagues (2018) using the 27‐item version of the IDS tested in the current study. Factor mixture models to identify reward/relief phenotypes were conducted using the 27‐item version of the IDS (as described above). Logistic regression models and Cox proportional hazard models were used to examine associations between reward/relief phenotypes and any heavy drinking days and time to first heavy drinking day, respectively, as reported by Mann and colleagues (2018).
Clinical Application and Post Hoc Power Analysis
To increase the clinical utility of the subgroup analyses, we conducted supplementary analyses to identify reward and relief drinkers in the sample based on observed scores for the IDS reward and relief subscales (Supporting information). We also conducted post hoc power analyses to determine the sample size that would be required for power > 0.80 to detect a significant effect (2‐tailed, p < 0.05) of naltrexone versus placebo among high reward/low relief drinkers, given the effect sizes observed in the current study.
Results
Reward and Relief Drinking Phenotypes
Consistent with our prior work (Glöckner‐Rist et al., 2013; Mann et al., 2018), we found that a 4‐class factor mixture solution provided a better fit than the 3‐class model and a 5‐class model did not fit significantly better than a 4‐class model (p = 0.121). The 4‐class factor mixture model showed excellent classification precision (entropy = 0.95). The 4 classes could be defined by scores on the reward and relief factors: (i) low reward/low relief (n = 37; 22.8% of the sample); (ii) high relief/low reward (n = 59; 36.0% of the sample); (iii) high relief/high reward (n = 38; 23.6% of the sample); and (iv) high reward/low relief (n = 29; 17.6% of the sample). We then used the estimated posterior probabilities of class membership to recode the 4 reward/relief classes into dummy‐coded variables with the low reward/low relief class as the reference class.
Table 2 provides the descriptive statistics for demographics and aggregate alcohol use over the course of the 12‐week treatment trial for the naltrexone versus placebo groups, as well as effect sizes (Cohen's d) by the reward and relief drinking phenotypes for the total sample (n = 163). For participants likely classified in the high reward/low relief phenotype, there were medium‐to‐large effects of naltrexone versus placebo (DPDD: Cohen's d = 0.96; PHDD: Cohen's d = 0.72), whereas effect sizes for other phenotypes were small‐to‐medium (Cohen's d = 0.04 to 0.56). There were no significant treatment or phenotype interactions by age, gender, or genotype (all p > 0.10); however, there were differences for naltrexone versus placebo within phenotype groups. As compared to placebo, individuals in the low reward/low relief phenotype who received naltrexone were significantly older, F(1, 36) = 6.88, p = 0.01, individuals in the high relief/low reward phenotype who received naltrexone were significantly more likely to be Asp40‐allele carriers, χ 2(1) = 7.96, p = 0.01, and individuals in the high reward/low relief phenotype who received naltrexone were drinking significantly fewer DPDD, F(1, 26) = 9.43, p = 0.02. There were no differences between phenotype groups or treatment‐by‐phenotype differences in baseline drinking (all p > 0.05).
Table 2.
Descriptive Statistics and Effect Sizes for Aggregate Alcohol Use Outcomes by Naltrexone and Reward and Relief Drinking Phenotypes in Total Sample (n = 163)
| Drinking phenotypes | Age | Sex | OPRM1 | DPDD | PHDD | ||
|---|---|---|---|---|---|---|---|
| Medication conditions | Mean (SD) | Male N (% within row) | Asp40 allele, N (% within row) | Mean (SD) | Cohen's d (placebo vs. naltrexone) | Mean (SD) | Cohen's d (placebo vs. naltrexone) |
| Low reward/low relief (n = 37) | |||||||
| Placebo (n = 14) | 47.21 (10.1)* | 11 (78.6%) | 3 (21.4%) | 4.11 (2.44) | 0.56 | 0.21 (0.24) | 0.04 |
| Naltrexone (n = 23) | 55.17 (8.2)* | 14 (60.9%) | 3 (13.6%) | 3.07 (0.95) | 0.20 (0.23) | ||
| High relief/low reward (n = 59) | |||||||
| Placebo (n = 32) | 51.09 (10.0) | 16 (50.0%) | 3 (9.4%)* | 3.42 (1.15) | 0.20 | 0.23 (0.24) | 0.29 |
| Naltrexone (n = 27) | 49.59 (8.7) | 11 (40.7%) | 11 (40.7%)* | 3.19 (1.17) | 0.17 (0.17) | ||
| High relief/high reward (n = 38) | |||||||
| Placebo (n = 18) | 44.17 (9.8) | 12 (66.7%) | 4 (26.7%) | 4.36 (1.64) | 0.27 | 0.36 (0.34) | 0.18 |
| Naltrexone (n = 20) | 45.45 (7.3) | 13 (65.0%) | 5 (26.3%) | 3.92 (1.60) | 0.30 (0.31) | ||
| High reward/low relief (n = 29) | |||||||
| Placebo (n = 16) | 46.87 (10.0) | 12 (75.0%) | 4 (25.0%) | 4.04 (1.45)* | 0.96 | 0.31 (0.27) | 0.72 |
| Naltrexone (n = 13) | 49.46 (9.4) | 6 (46.2%) | 3 (23.1%) | 2.85 (0.98)* | 0.15 (0.16) | ||
DPDD, drinks per drinking day; PHDD, proportion of heavy drinking days.
*p < 0.05 between naltrexone and placebo within the drinking phenotypes.
Table 3 provides the descriptives for the sample that received daily dosing of naltrexone or placebo (n = 86). As compared to placebo, individuals in the high reward/low relief phenotype who received naltrexone were drinking significantly fewer DPDD, F(1, 9) = 8.96, p = 0.02; Cohen's d = 2.05, and had a lower PHDD, F(1, 9) = 5.94, p = 0.04; Cohen's d = 1.75.
Table 3.
Descriptive Statistics and Effect Sizes for Aggregate Alcohol Use Outcomes by Naltrexone and Reward and Relief Drinking Phenotypes in Sample who Received Daily Doses (n = 86)
| Drinking phenotypes | Age | Sex | OPRM1 | DPDD | PHDD | ||
|---|---|---|---|---|---|---|---|
| Medication conditions | Mean (SD) | Male N (% within row) | Asp40 allele, N (% within row) | Mean (SD) | Cohen's d (placebo vs. naltrexone) | Mean (SD) | Cohen's d (placebo vs. naltrexone) |
| Low reward/low relief (n = 23) | |||||||
| Placebo (n = 10) | 49.20 (10.46) | 7 (70.0%) | 1 (10.0%) | 3.29 (1.68) | 0.26 | 0.10 (0.14) | 0.39 |
| Naltrexone (n = 13) | 56.07 (5.56) | 7 (53.8%) | 3 (23.1%) | 2.93 (1.07) | 0.18 (0.25) | ||
| High relief/low reward (n = 29) | |||||||
| Placebo (n = 16) | 49.25 (11.47) | 9 (56.3%) | 2 (12.5%) | 3.46 (1.13) | 0.09 | 0.22 (0.25) | 0.05 |
| Naltrexone (n = 13) | 48.00 (7.05) | 6 (46.2%) | 6 (46.2%) | 3.37 (0.93) | 0.21 (0.16) | ||
| High relief/high reward (n = 24) | |||||||
| Placebo (n = 9) | 40.44 (8.70) | 6 (66.7%) | 1 (16.7%) | 4.50 (1.59) | 0.10 | 0.39 (0.39) | 0.26 |
| Naltrexone (n = 15) | 44.13 (7.59) | 11 (73.3%) | 4 (28.6%) | 4.35 (1.53) | 0.30 (0.28) | ||
| High reward/low relief (n = 10) | |||||||
| Placebo (n = 6) | 49.17 (9.68) | 5 (83.3%) | 3 (50.0%) | 4.84 (1.48)* | 2.05 | 0.49 (0.33)* | 1.75 |
| Naltrexone (n = 4) | 50.00 (6.16) | 2 (50.0%) | 0 (0.0%) | 2.35 (0.86)* | 0.07 (0.08)* | ||
DPDD, drinks per drinking day; PHDD, proportion of heavy drinking days.
*p < 0.05 between naltrexone and placebo within the drinking phenotypes.
Replication of Prior Findings: Reward and Relief Drinking by Naltrexone Predicting Alcohol Outcomes
Consistent with our prior work (Mann et al., 2018), we also found a significant interaction between the high reward/low relief phenotype (with the low reward/low relief phenotype as the reference group) and naltrexone in predicting DPDD, B (SE) = −1.89 (0.89), p = 0.03, and PHDD, B (SE) = −0.51 (0.17), p = 0.004. As shown in Fig. 1, simple slopes analysis indicated that, among high reward/low relief drinkers, individuals treated with naltrexone reported significantly fewer DPDD and a lower proportion of heavy drinking occasions over the 12‐week treatment than those treated with placebo. In contrast, there were no effects of naltrexone treatment on DPDD or greater PHDD in the other phenotype groups (all p ≥ 0.206).
Figure 1.
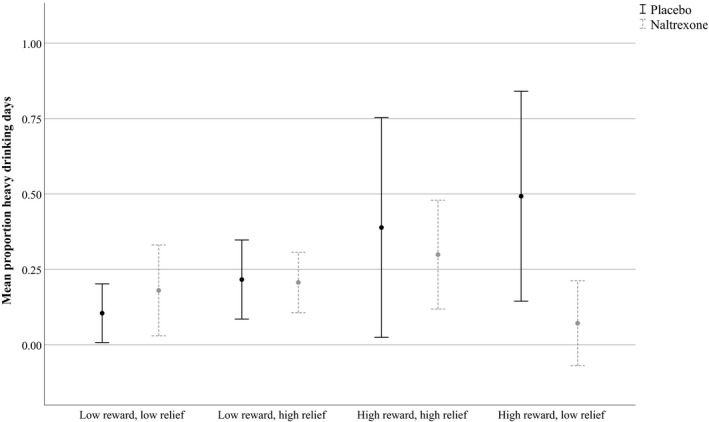
Reward and relief drinking by naltrexone predicting aggregate alcohol outcomes (Average alcohol use and 95% confidence intervals).
Although not a primary hypothesis in the current study, we included genotype based on the Asn40Asp SNP in OPRM1 as a covariate in the analyses based on prior evidence of its effects (Kranzler et al., 2013). OPRM1 genotype did not predict phenotype group, χ 2(3) = 1.09, p = 0.78, and there was no evidence of a pharmacogenetic effect involving that variant (all p ≥ 0.520). The small sample size and the absence of Asp40‐allele carriers in the high reward/low relief phenotype prevented our testing the OPRM1 × phenotype × treatment interaction.
Extension of Prior Findings: Reward and Relief Drinking by Naltrexone Predicting Daily Drinking
Consistent with our hypothesis and previous findings (Mann et al., 2018), there was a significant interaction between the high reward/low relief phenotype (with the low reward/low relief phenotype as the reference group) and naltrexone in predicting daily drinking during treatment (p = 0.02; see Table 4). Simple slopes analysis indicated that, among high reward/low relief drinkers, those who were treated with naltrexone reported drinking significantly fewer drinks per day than those treated with placebo, B (SE) = −0.77 (0.25), p = 0.002; IRR = 0.46; Cohen's d = 0.62, whereas those with the low reward phenotypes reported consuming more drinks per day when treated with naltrexone than placebo (low reward/low relief: B (SE) = 0.46 (0.22), p = 0.04; low reward/high relief: B (SE) = 1.16 (0.49), p = 0.02. Naltrexone had no effect on drinks per day among those with the high reward and high relief phenotype, B (SE) = 0.17 (0.26), p = 0.50.
Table 4.
Estimates from Multilevel Model of Reward and Relief Drinking by Naltrexone Predicting Daily Drinking in Sample who Received Daily Doses (n = 86)
| B (SE) | IRR (95% CI) | |
|---|---|---|
| Time | 0.001 (0.00) | 1.00 (0.99, 1.002) |
| Years of education | −0.006 (0.03) | 0.99 (0.93, 1.06) |
| Sex (male coded 1) | 0.06 (0.17) | 1.06 (0.71, 1.42) |
| Baseline % drinking days | 0.02 (0.009)* | 1.02 (1.002, 1.04) |
| OPRM1 (Asn40‐allele homozygotes coded 1) | 0.03 (0.16) | 1.03 (0.70, 1.36) |
| Naltrexone (active naltrexone coded 1) | 0.39 (0.28) | 1.48 (0.66, 2.29) |
| High relief/low reward coded 1 | −0.14 (0.40) | 0.87 (0.19, 1.54) |
| High relief/high reward coded 1 | 0.42 (0.31) | 1.53 (0.59, 2.47) |
| High reward/low relief coded 1 | 0.90 (0.26)*** | 2.47 (1.23, 3.70) |
| Naltrexone by high relief/low reward | 0.26 (0.49) | 1.30 (0.04, 2.55) |
| Naltrexone by high relief/high reward | −0.13 (0.41) | 0.88 (0.17, 1.58) |
| Naltrexone by high reward/low relief | −1.14 (0.49)** | 0.32 (0.01, 0.63) |
*p < 0.05; **p < 0.01; ***p < 0.001.
B (SE) = unstandardized regression coefficient (standard error); IRR (95% CI) = incident rate ratio (95% confidence interval of the IRR). The IRR can be interpreted as the increase (above 1.0) or decrease (below 1.0) in number of drinks per day for a 1‐unit increase in the predictor (with other predictors in the model held constant).
Extension of Prior Findings: Desire to Drink Multilevel Mediation Models
Next, we examined whether average desire to drink mediated the moderating effect of reward drinking by naltrexone in predicting average daily drinking. Results from the multilevel mediation analysis (Table 5) indicated a significant interaction between the high reward/low relief phenotype (with the low reward/low relief phenotype as the reference group) and naltrexone in predicting average desire to drink, desire to drink predicting average daily drinking, and a significant mediation effect of desire to drink, indirect effect (95% CI) = −0.74 (−1.41, −0.07), p = 0.03. Simple slopes analysis, shown in Fig. 2, indicated that, among high reward/low relief drinkers, those treated with naltrexone (relative to placebo) reported significantly less desire to drink over time, B (SE) = −1.49 (0.45), p = 0.001, whereas there was no association between receiving naltrexone and desire to drink over time among the other phenotypes (all p ≥ 0.26).
Table 5.
Estimates from Multilevel Mediation Model of Reward and Relief Drinking by Naltrexone Predicting Average Daily Drinking Mediated by Desire to Drink
| Predicting average daily drinking | B (SE) | IRR (95% CI) |
|---|---|---|
| Time | 0.001 (0.00) | 1.00 (0.99, 1.00) |
| Desire to drink (mediator) | 0.44 (0.14)*** | 1.55 (1.15, 1.97) |
| Years of education | −0.03 (0.03) | 0.97 (0.91, 1.03) |
| Sex (male coded 1) | 0.08 (0.14) | 1.08 (0.78, 1.38) |
| Baseline % drinking days | 0.01 (0.007) | 1.01 (0.99, 1.03) |
| Naltrexone (active naltrexone coded 1) | −0.18 (0.26) | 0.75 (0.66, 2.29) |
| High relief/low reward coded 1 | −0.72 (0.26)** | 0.83 (0.19, 1.54) |
| High relief/high reward coded 1 | −0.14 (0.21) | 0.49 (0.59, 2.47) |
| High reward/low relief coded 1 | −0.14 (0.24) | 0.87 (1.23, 3.70) |
| Naltrexone by high relief/low reward | 0.72 (0.39) | 2.05 (0.48, 3.63) |
| Naltrexone by high relief/high reward | 0.24 (0.31) | 1.27 (0.49, 2.05) |
| Naltrexone by high reward/low relief | 0.16 (0.43) | 1.17 (0.19, 2.15) |
| Predicting average desire to drink | B (SE) | β |
|---|---|---|
| Time | −0.001 (0.00)** | −0.02 |
| Years of education | 0.06 (0.03)* | 0.20 |
| Sex (male coded 1) | −0.44 (0.14)** | −0.30 |
| Baseline % drinking days | 0.01 (0.004)** | 0.29 |
| OPRM1 (Asn40‐allele homozygotes coded 1) | 0.08 (0.14) | 0.04 |
| Naltrexone (active naltrexone coded 1) | 0.24 (0.25) | 0.16 |
| High relief/low reward coded 1 | 0.46 (0.28) | 0.31 |
| High relief/high reward coded 1 | 0.27 (0.29) | 0.16 |
| High reward/low relief coded 1 | 1.22 (0.41)** | 0.55 |
| Naltrexone by high relief/low reward | −0.40 (0.34) | −0.20 |
| Naltrexone by high relief/high reward | 0.20 (0.37) | 0.10 |
| Naltrexone by high reward/low relief | −1.68 (0.52)** | −0.50 |
*p < 0.05; **p < 0.01; ***p < 0.001.
B (SE) = unstandardized regression coefficient (standard error); IRR (95% CI) = incident rate ratio (95% confidence interval of the IRR); β = standardized regression coefficient.
Figure 2.
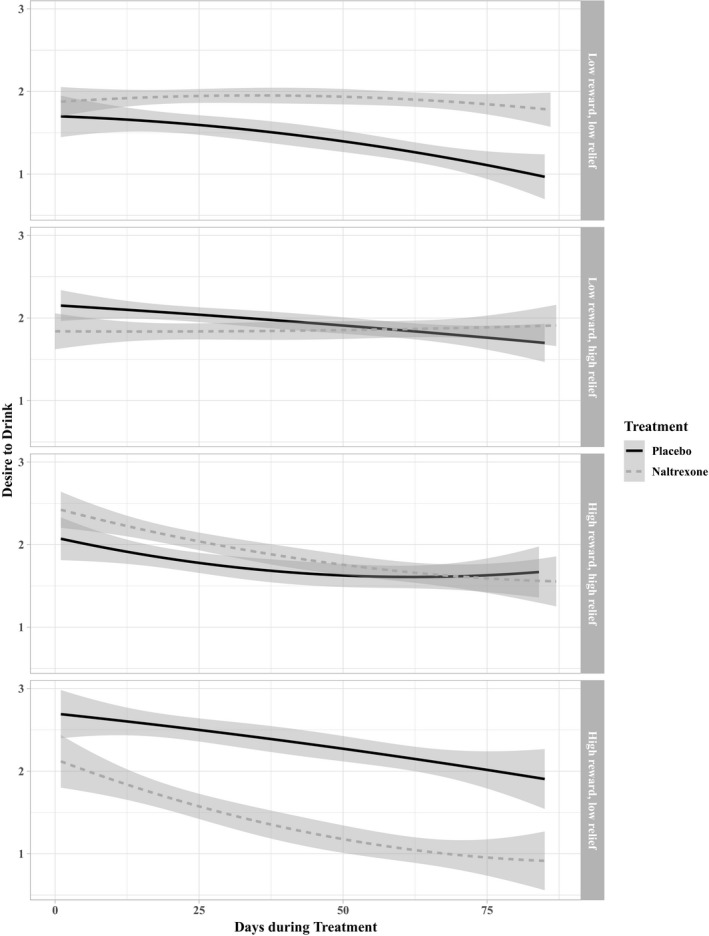
Simple slopes analysis of desire to drink over time by treatment and reward and relief phenotypes (average desire to drink and 95% confidence intervals).
Sensitivity Analyses
Several sensitivity analyses were conducted to further explore the moderation effects. First, we examined the same models with the high reward/low relief phenotype as the reference group. As seen in Table S1, we found a significant interaction between the low reward/high relief phenotype (with the high reward/low relief phenotype as the reference group) and naltrexone in predicting DPDD, PHDD, and daily drinks per day. We also found a significant interaction between the high reward/high relief phenotype (with the high reward/low relief phenotype as the reference group) in predicting daily drinks per day. Simple slopes analyses among high reward/low relief drinkers, not shown, indicated that those treated with naltrexone reported lower DPDD, PHDD, and drinks per day than those treated with placebo. Mediation analyses showed significant moderated mediation effects of the high reward/low relief versus all other phenotype groups by naltrexone in predicting average drinks per day via average desire to drink, low reward/low relief: indirect effect = 0.32 (95% CI: 0.10, 0.62); high relief/low reward: indirect effect = 0.37 (95% CI: 0.12, 0.70); high reward/high relief: indirect effect = 0.25 (95% CI: 0.05, 0.51).
Second, we conducted the same analyses with the targeted naltrexone group. Results of additional analyses indicated that targeted naltrexone was no more effective than placebo for high reward/low relief drinkers in reducing PHDD (interaction p = 0.08) or daily drinking (p = 0.23), and desire did not significantly mediate the effects of targeted naltrexone. However, there were significant interactions between targeted naltrexone versus placebo and drinking phenotypes in predicting DPDD (high reward/low relief vs. low reward/low relief B (SE) = 2.45 (1.10), p = 0.03; low reward/high relief vs. low reward/low relief B (SE) = 2.38 (1.05), p = 0.02. Simple slopes analyses indicated that individuals with the low reward/low relief phenotype reported consuming significantly fewer DPDD when treated with targeted naltrexone, B (SE) = −2.05 (0.77), p = 0.01; Cohen's d = 1.27, while targeted naltrexone versus placebo had no effect on DPDD among those with the other drinking phenotypes (all p > 0.71).
Next, we examined whether there were sex differences. There were no significant differences in the naltrexone response for high reward/low relief drinkers by sex in predicting DPDD (p = 0.58), PHDD (p = 0.48), or daily drinking (p = 0.19).
We also conducted a reanalysis of the PREDICT study data (n = 426; Mann et al., 2009), as published by Mann and colleagues (2018), using the 27‐item version of the IDS used in the current study. The 4‐class factor mixture solution provided a better fit than the 3‐class model, and a 5‐class model did not fit significantly better than a 4‐class model (p = 0.63). The 4‐class factor mixture model of the 27‐item IDS showed excellent classification precision (entropy = 0.93). The 4 classes could be defined by scores on the reward and relief factors: (i) low reward/low relief (n = 79; 18.8% of the sample); (ii) high relief/low reward (n = 135; 31.9% of the sample); (iii) high relief/high reward (n = 127; 29.8% of the sample); and (iv) high reward/low relief (n = 84; 19.8% of the sample). The outcomes reported by Mann and colleagues (2018) included any heavy drinking days and time to first heavy drinking day during treatment, which were examined using logistic regression and Cox proportional hazard regression models, respectively. Results from the 27‐item IDS indicated a significant phenotype‐by‐medication interaction in predicting any heavy drinking days in the PREDICT data, Wald χ 2 (6) = 14.68, p = 0.02, driven by the effect of high reward/low relief × naltrexone versus placebo: OR = 17.277, p = 0.01, and time to first heavy drinking day, Wald χ 2 (6) = 17.973, p = 0.006, driven by the effect of high reward/low relief × naltrexone versus placebo: OR = 5.659, p = 0.016. Individuals in the high reward/low relief phenotype (n = 85) in the PREDICT data who received naltrexone (n = 37) had a lower probability of heavy drinking days and longer time to the first heavy drinking day, as compared to those who received acamprosate or placebo (see Table S2 and Fig. S1).
Clinical Application and Post Hoc Power Analysis
As seen in the Tables S3 and S4 and Figs S2 and S3, observed scores on the reward and relief subscales of the IDS can be used to calculate the high reward/low relief subgroup by following a standard deviation rule. A high reward/low relief drinker is defined as an individual with a reward subscale score greater than 21 and a relief subscale score lower than 15. The naltrexone treatment effect for this subgroup in the current sample is large for PHDD (Cohen's d = 1.32) and DPDD (Cohen's d = 2.05). An alternative definition of high reward/low relief drinkers (reward greater than 22 and relief less than 14) provides an even larger naltrexone effect for PHDD (Cohen's d = 1.84) and similarly large effect for DPDD (Cohen's d = 1.86).
Using the effect sizes obtained from the current study, we had power > 0.80 to detect the largest treatment effect (i.e., the effect of naltrexone on DPDD, Cohen's d = 2.05) with only 10 individuals. For the smallest effect size of d = 1.32 for naltrexone predicting PHDD, post hoc power was only 0.44 and 22 subjects would be required for power > 0.80.
Discussion
Advancing precision medicine in treating AUD requires identifying homogeneous subgroups of individuals with AUD who respond better to particular treatment approaches (Kranzler and McKay, 2012; Mann et al., 2009). In prior secondary analyses of the PREDICT study, we found particularly large therapeutic effects of naltrexone (50 mg daily) among high reward/low relief drinkers, defined as individuals who drink primarily for the rewarding/pleasurable effects of alcohol, relative to the distress relieving effects (Mann et al., 2018). The current study replicates this finding in an independent sample of individuals who were treated with naltrexone (50 mg daily) or placebo for heavy drinking (Kranzler et al., 2009). The current study extends the initial findings by examining reductions in desire to drink as a purported mechanism of action of naltrexone (Mann et al., 2009; Ray et al., 2010a), which could explain this precision medicine hypothesis. In line with our hypothesis, the effect of daily naltrexone among high reward/low relief drinkers was mediated by reductions in average desire to drink, as measured by daily IVR.
The current findings provide additional support for the efficacy of daily naltrexone for individuals who tend to drink primarily for the rewarding effects of alcohol, rather than drinking to relieve negative affect and discomfort. In addition to our prior work (Mann et al., 2018), the finding that daily naltrexone is more effective for high reward/low relief drinkers is consistent with findings that naltrexone is efficacious among individuals with more heavy drinkers in their social networks (Worley et al., 2015), among those who show greater reward‐related activation to alcohol cues (Bach et al., 2019; Mann et al., 2014), and among those with greater reductions in reward‐related activation to alcohol cues (Schacht et al., 2017). The significant moderated mediation effect, whereby naltrexone's effect among high reward/low relief drinkers was explained by greater reductions in desire to drink, extends this prior work and highlights a potential mechanism by which daily naltrexone treatment may be more effective among individuals who drink primarily for the rewarding effects of alcohol. These findings are consistent with incentive‐sensitization theory (Berridge and Robinson, 2016), provide further support of the importance of incentive salience, as proposed by the AARDoC (Kwako et al., 2018; Litten et al., 2015), and are consistent with human laboratory studies that have shown naltrexone to blunt the rewarding effects of alcohol (Hendershot et al., 2017; Ray and Hutchison, 2007).
The lack of naltrexone effects in the high reward/high relief phenotype is also consistent with our prior work, in which we found that individuals high on both reward and relief drinking responded no better to naltrexone than placebo (Mann et al., 2018; Roos et al., 2017). Thus, it may be that naltrexone is particularly effective for those individuals with high reward drinking tendencies in the context of low relief drinking tendencies, rather than individuals with similarly high reward and relief drinking tendencies (Mann et al., 2014; Mann et al., 2018; Roos et al., 2017). Importantly, it could be that individuals with both high reward and relief tendencies are more severely alcohol dependent (see Mann et al., 2018) and have more widespread neural dysfunction (Koob, 2014), including greater dysregulation of both reward and cognitive control systems (Lim et al., 2017). Research should aim to identify treatments that are most effective for this phenotype.
Interestingly, daily naltrexone was not effective in reducing desire to drink among the other phenotypes and targeted naltrexone was more effective than placebo when administered via targeted dosing among individuals in the low reward/low relief phenotype group. This finding was unexpected. It could be the case that targeting naltrexone to heavy drinking occasions for patients whose drinking is not primarily for reward or relief could be particularly beneficial. An alternative explanation is that individuals with strong reward or relief drinking tendencies are less likely to take naltrexone when they anticipate reward/relief drinking situations, although we did not find any significant differences in medication compliance by phenotype groups in the current study.
Limitations and Future Directions
The current study had several limitations. The very small sample size, particularly within phenotype groups, and the lack of stratification of medication conditions by reward and relief phenotypes are design limitations that prohibit stronger inferences. This point underscores the importance of replication to advance precision medicine (Litten et al., 2016, 2017). A second limitation is that the IDS mostly taps into social aspects of reward drinking and relief drinking mostly taps into problems and frustration, which are not entirely consistent with the definitions of reward and relief drinking used in the animal literature (Hay et al., 2013; Kaminski et al., 2012; Koob et al., 2014). Future work could use ecological momentary assessment to derive reward and relief drinking phenotypes based on actual drinking triggers. A prospective study that assigns individuals a priori to reward and relief drinking phenotypes and then randomizes them to treatment with naltrexone or placebo would provide a stronger test of the precision medicine aspect of our findings. Although it would require a large sample to ensure adequate statistical power, prospective evaluation of the Asn40Asp SNP as a moderator of naltrexone treatment response would also be of interest, particularly given the overlap of the OPRM1 genotype and the reward drinking phenotype across species (Bilbao et al., 2015; Korucuoglu et al., 2017).
Conflict of Interest
Dr. Kranzler is named as an inventor on PCT patent application #15/878,640 entitled: “Genotype‐guided dosing of opioid agonists,” filed January 24, 2018. Dr. Mann received honoraria for consultancies from Pfizer. Drs. Witkiewitz, Kranzler, and Mann are members of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative (ACTIVE Group), which over the time that this paper was developed was supported by AbbVie, Ethypharm, Indivior, Lilly, Lundbeck, Mitsubishi, Otsuka, and Pfizer. Dr. Roos has no disclosures.
Supporting information
Table S1. Main effects and interactions from regression and multilevel models of reward and relief drinking by naltrexone predicting daily drinking in subjects who received daily doses (n = 86) with high reward/low relief phenotype as reference group.
Table S2. Summary of medication effects in PREDICT study within latent classes defined by the 30‐item IDS (Mann et al., 2018) and the 27‐item IDS of the current study.
Table S3. Inventory of drinking situations ‐ 42 item (IDS‐42) – reward and relief drinking items.
Table S4. Summary of naltrexone response with reward drinkers identified by cutoff scores.
Fig. S1. Cox proportional hazards model of time to first heavy drinking day in the PREDICT data (Mann et al., 2009; n = 426).
Fig. S2. Observed reward subscale scores by reward and relief phenotypes.
Fig. S3. Observed relief subscale scores by reward and relief phenotypes.
ACKNOWLEDGMENTS
This research was supported by grants funded by the National Institutes of Health (R01 AA022328, R01 AA025539, P60 AA03510, K24 AA13736, M01 RR06192). The content is solely the responsibility of the authors and does not necessarily reflect the views of NIH.
Footnotes
The 30 IDS items used in the Mann and colleagues (2018) paper were derived from the 100‐item version of the IDS, whereas the current study administered the 42‐item short form. Only 13 items (7 for reward drinking and 6 for relief drinking) were overlapping. Initial confirmatory factor analysis of the 13 overlapping items indicated a poor fit to the data, and thus, we examined additional items from the 42‐item scale that were not included in the Mann and colleagues (2018) analyses.
References
- Aiken LS, West SG (1991) Multiple Regression: Testing and Interpreting Interactions Sage Publications, Thousand Oaks, CA. [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM‐IV). American Psychiatric Association, Washington, DC. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn American Psychiatric Association, Washington, DC. [Google Scholar]
- Annis HM, Graham JM, Davis CS (1987) Inventory of Drinking Situations: User's Guide Addiction Research Foundation, Toronto, ON. [Google Scholar]
- Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H (1992) Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict 87:1415–1431. [DOI] [PubMed] [Google Scholar]
- Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstädt‐Klein S, Mann K, Perez‐Ramirez U, Moratal D, Canals S, Dursun SM, Greenshaw AJ, Kirsch P, Kiefer F, Sommer WH (2019) Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict Biol. 10.1111/adb.12717. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (2016) Liking, wanting, and the incentive‐sensitization theory of addiction. Am Psychol 71:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, Thorsell AA (2015) Pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol Psychiatry 77:850–858. 10.1016/j.biopsych.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Chamorro AJ, Marcos M, Miron‐Canelo JA, Pastor I, Gonzalez‐Sarmient R, Laso FJ (2012) Association of μ‐opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta‐analysis. Addict Biol 17:502–512. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR (1993) A psychobiological model of temperament and character. Arch Gen Psychiatry 50:975–990. [DOI] [PubMed] [Google Scholar]
- Glöckner‐Rist A, Lémenager T, Mann K, PREDICT Study Research Group (2013) Reward and relief craving tendencies in patients with alcohol use disorders: results from the PREDICT study. Addict Behav 38:1532–1540. [DOI] [PubMed] [Google Scholar]
- Hay RA, Jennings JH, Zitzman DL, Hodge CW, Robinson DL (2013) Specific and nonspecific effects of naltrexone on goal‐directed and habitual models of alcohol seeking and drinking. Alcohol Clin Exp Res 37:1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Leggio L (2016) What the alcohol doctor ordered from the neuroscientist. Prog Brain Res 224:401–418. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Samokhvalov AV, Rehm J (2017) Effects of naltrexone on alcohol self‐administration and craving: meta analysis of human laboratory studies. Addict Biol 22:1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinek EM (1960) The Disease Concept of Alcoholism Hillhouse Press, New Haven, CT. [Google Scholar]
- Kaminski BJ, Duke AN, Weerts EM (2012) Effects of naltrexone on alcohol drinking patterns and extinction of alcohol seeking in baboons. Psychopharmacology 223:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB (2015) Principles and Practice of Structural Equation Modeling Guilford Press, New York, NY. [Google Scholar]
- Koob GF (2014) Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol 125:33–54. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O (2014) Addiction as a stress surfeit disorder. Neuropharmacology 76(Pt B): 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korucuoglu O, Gladwin TE, Baas F, Mocking RJT, Ruhé HG, Groot PFC, Wiers RW (2017) Neural responses to alcohol taste cues in youth: effects of the OPRM1 gene. Addict Biol 22:1562–1575. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Covault J, Tennen H (2013) Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol 18:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM (2014) Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry 171:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, McKay JR (2012) Personalized treatment of alcohol dependence. Curr Psychiatry Rep 14:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Tennen H, Armeli S, Chan G, Covault J, Arias A, Oncken C (2009) Targeted naltrexone for problem drinkers. J Clin Psychopharmacol 29:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Blanco C, Goldman D (2018) Addictions neuroclinical assessment: initial validation and next steps. Alcohol Clin Exp Res 42:276A. [Google Scholar]
- Lane SP, Sher KJ (2015) Limits of current approaches to diagnosis severity based on criterion counts: an example with DSM‐5 alcohol use disorder. Clin Psychol Sci 3:819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch OM, Walter H (1996) Subtypes of alcoholism and their role in therapy. Alcohol Alcohol 31(Suppl. 1):63–67. [PubMed] [Google Scholar]
- Lim AC, Cservenka A, Ray LA (2017) Effects of alcohol dependence severity on neural correlates of delay discounting. Alcohol Alcohol 52:506–515. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A (2012) Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17:513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB (2016) Discovery, development, and adoption of medications to treat alcohol use disorder: goals for the phases of medications development. Alcohol Clin Exp Res 40:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig J, Leggio L (2017) Advances in pharmacotherapy development: human clinical studies, in Handbook of Experimental Pharmacology, pp. 579–613. Springer Nature, Cham, Switzerland. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF (2015) Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res 39:579–584. [DOI] [PubMed] [Google Scholar]
- Littleton JM (2007) Acamprosate in alcohol dependence: implications of a unique mechanism of action. J Addict Med 1:115–125. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A, PREDICT Study Research Team (2009) Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the PREDICT study. Alcohol Clin Exp Res 33:674–683. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J (2008) Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res 32:1105–1110. [DOI] [PubMed] [Google Scholar]
- Mann K, Roos CR, Hoffmann S, Nakovics H, Leménager T, Heinz A, Witkiewitz K (2018) Precision medicine in alcohol dependence: a controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharmacology 43:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Vollstädt‐Klein S, Reinhard I, Leménager T, Fauth‐Bühler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN (2014) Predicting naltrexone response in alcohol‐dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res 38:2754–2762. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi H‐Y (2010) Prospective follow‐up of empirically derived alcohol dependence subtypes in wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC): recovery status, alcohol use disorders and diagnostic criteria, alcohol consumption behavior. Alcohol Clin Exp Res 34:1073–1083. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (2017) Mplus Users Guide (Version 8) Muthén & Muthén, Los Angeles, CA. [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model 14:535–569. [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP (2003) A functional polymorphism of the mu‐opioid receptor gene is associated with naltrexone response in alcohol‐dependent patients. Neuropsychopharmacology 28:1546–1552. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z (2010) A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods 15:209–33. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin P, Miotto K (2010a) Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets 9:13–22. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2007) Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double‐blind placebo‐controlled study. Arch Gen Psychiatry 64:1069–1077. [DOI] [PubMed] [Google Scholar]
- Ray LA, Krull JL, Leggio L (2010b) The effects of Naltrexone among alcohol non‐abstainers: results from the COMBINE Study. Front Psychiatry 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Mann KF, Witkiewitz K (2017) Reward and relief dimensions of temptation to drink: construct validity and role in predicting differential benefit from acamprosate and naltrexone. Addict Biol 22:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF (2017) Predictors of naltrexone response in a randomized trial: reward‐related brain activation, OPRM1 genotype, and smoking status. Neuropsychopharmacology 42:2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline Follow-back: A technique for assessing self-reported ethanol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods (Allen J, Litten RZ. eds), pp 41–72. Humana Press, Totowa, NJ. [Google Scholar]
- Spanagel R, Durstewitz D, Hansson A, Heinz A, Kiefer F, Köhr G, Matthäus F, Nöthen MM, Noori HR, Obermayer K, Rietschel M, Schloss P, Scholz H, Schumann G, Smolka M, Sommer W, Vengeliene V, Walter H, Wurst W, Zimmermann US, Stringer S, Smits Y, Derks EM, Derks EM (2013) A systems medicine research approach for studying alcohol addiction. Addict Biol 18:883–896. [DOI] [PubMed] [Google Scholar]
- Verheul R, van den Brink W, Geerlings P (1999) A three‐pathway psychobiological model of craving for alcohol. Alcohol Alcohol 34:197–222. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Falk DE, Kranzler HR, Litten RZ, Hallgren KA, O'Malley SS, Anton RF (2014) Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials. Alcohol Clin Exp Res 38:2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2014) Global Status Report on Alcohol and Health World Health Organization, Geneva, Switzerland. [Google Scholar]
- Worley MJ, Witkiewitz K, Brown SA, Kivlahan DR, Longabaugh R (2015) Social network moderators of naltrexone and behavioral treatment effects on heavy drinking in the COMBINE study. Alcohol Clin Exp Res 39:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Main effects and interactions from regression and multilevel models of reward and relief drinking by naltrexone predicting daily drinking in subjects who received daily doses (n = 86) with high reward/low relief phenotype as reference group.
Table S2. Summary of medication effects in PREDICT study within latent classes defined by the 30‐item IDS (Mann et al., 2018) and the 27‐item IDS of the current study.
Table S3. Inventory of drinking situations ‐ 42 item (IDS‐42) – reward and relief drinking items.
Table S4. Summary of naltrexone response with reward drinkers identified by cutoff scores.
Fig. S1. Cox proportional hazards model of time to first heavy drinking day in the PREDICT data (Mann et al., 2009; n = 426).
Fig. S2. Observed reward subscale scores by reward and relief phenotypes.
Fig. S3. Observed relief subscale scores by reward and relief phenotypes.


