Significance
Climate change, habitat loss, and overharvesting are threatening coastal ecosystems worldwide. A less widely recognized threat is the decline in Indigenous mariculture practices. These practices, such as building of clam gardens, structured coastal ecosystems for millennia. Teasing out the dynamic and intertwined relationships between humans and culturally valued species, such as clams, requires long-term paleoecological and archaeological records. These records are requisite for creating meaningful management targets and for applying traditional mariculture practices, such as the tending of clam gardens, to increase the productivity and sustainability of our foods today. Documenting these interactions between humans and coastal ecosystems, such as we have done here, also counteracts the erasure of the long-term connections of Indigenous peoples to their lands and seas.
Keywords: historical ecology, clam gardens, traditional resource management, Northwest Coast, paleoecology
Abstract
Historical ecology can provide insights into the long-term and complex relationships between humans and culturally important species and ecosystems, thereby extending baselines for modern management. We bring together paleoecological, archaeological, and modern clam records to explore the relationship between humans and butter clams (Saxidomus gigantea) throughout the Holocene in the northern Salish Sea of British Columbia, Canada. We compare butter clam size and growth patterns from different temporal, environmental, and cultural contexts spanning 11,500 y to present. Butter clam size and growth were restricted in early postglacial times but increased over the next few millennia. During the early-Late Holocene, humans took increasing advantage of robust clam populations and after 3.5 ka, began constructing clam gardens (intertidal rock-walled terraces). Environmental and cultural variables, including coarse substrate, stabilized sea surface temperature, and the presence of a clam garden wall, increased clam growth throughout the Holocene. Measurements of clams collected in active clam gardens and deposited in middens suggest that clam gardens as well as other mariculture activities enhanced clam production despite increased harvesting pressure. Since European contact, decline of traditional management practices and increases in industrial activities are associated with reduced clam size and growth similar to those of the early postglacial clams. Deeper-time baselines that more accurately represent clam population variability and allow us to assess magnitudes of change throughout time as well as the complex interactions among humans and clams are useful for modern marine resource management.
Over the millennia, many peoples worldwide developed intimate knowledge of, and relationships with, particularly valued species of plants and animals (1). Tracking the development of these long-term human–species relationships requires temporally grounded records that provide insights into both the cultural and ecological sides of this equation. For instance, the archaeological faunal record can provide detailed information on the ecological and cultural effects of human–species interactions (2–5), whereas the paleoecological record can provide insights into species ecology in the absence of significant human intervention (6–8). Taken together, these 2 records can offer a powerful lens through which to assess coupled social–ecological systems over broad spatial and temporal scales and can help establish ecological baselines for modern management (8–12).
On the Northwest Coast of North America, clams are a valued cultural species (13, 14) with widespread importance that is reflected in origin stories, rituals, language, and in the kilometers of deep and ancient shell middens that line the coastline (15, 16). Detailed archaeological and ethnographic research indicates that clams, especially butter clams (Saxidomus gigantea) and littleneck clams (Leukoma staminea), were eaten in abundance both seasonally and year round (17) and both fresh and preserved. These species were a reliable, abundant, and easily harvested source of food (13, 18) that could be tended to increase abundance by applying various traditional cultivation techniques. One such technique, the building of rock-walled intertidal terraces called “clam gardens,” expanded and enhanced clam habitat and thus, clam production (14, 16, 19, 20).
Complimenting the archaeological and ethnographic records, studies of subfossil and fossil bivalves on the Northwest Coast have provided significant insights into the region’s paleoecology. Such data have been used for reconstructing both prehuman (21) and recent historical ecological conditions (22, 23). To our knowledge, no studies have combined both the archaeological and paleoecological marine bivalve records to fully explore the long-term relationships among humans and clams.
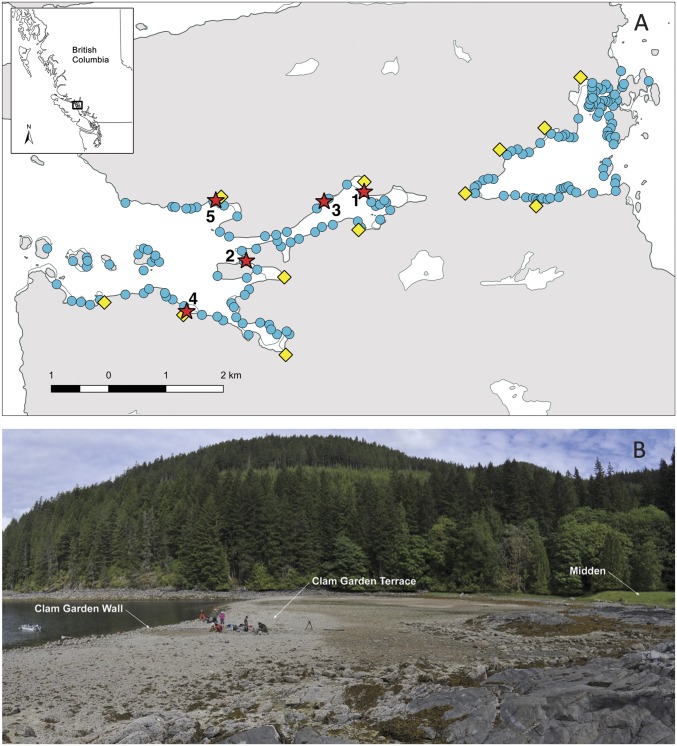
In this paper, we investigate the historical ecology of butter clams throughout the Holocene along the northern coast of Quadra Island, Salish Sea, British Columbia (Fig. 1) through analyses of the paleoecological, archaeological, and contemporary ecological records. Together, these records encompass 11,500 y of history—a period that spans the time before extensive human settlement to today. In our study sites in Kanish and Waiatt Bays, the coevolved history of humans and clams is reflected in the expansive archaeological shell middens with deposits dating to as old as 9 ka and the plethora of clam gardens dating from sometime after 3.5 ka (Fig. 1) (24).
Fig. 1.
(A) Study sites on northern Quadra Island, British Columbia, showing clam garden sites (blue dots), large midden settlement sites (yellow diamonds), and sampling sites (red stars; numbers 1 to 5 correspond to SI Appendix, Table S3). (B) Clam gardens, Quadra Island. Clam garden built on soft sediment showing wall, clam garden terrace, and ∼9,000-y-old midden. Living and dead (paleo) butter clams were collected from the clam garden terrace and the shell midden. Image courtesy of Mark Wunsch (Greencoast Media, British Columbia, Canada).
Based on our understanding of clam life history, coastal ecology, and local cultural attributes, we predicted that butter clam sizes and lifespan increased over the last 11,500 y as environmental conditions became more favorable and as humans altered the intensity and strategy with which they harvested and cultivated clams. We also predict that today’s butter clams are not as productive as those in past environments, likely due to a combination of less favorable ocean conditions, habitats modified by modern development, and the absence of traditional, Indigenous mariculture practices. To evaluate these hypotheses, we estimated butter clam sizes at various ages with sclerochronological analyses of clam shells from 5 beach sites and from 3 contexts at these sites: 1) paleobeaches below clam gardens that contain layers of clams that died in situ (“death assemblages”) before clam gardens were constructed, 2) terrestrial archaeological shell middens composed of clams originally harvested from active clam gardens, and 3) now largely defunct clam garden beaches containing clams that both are living and died relatively recently. Based on radiocarbon dating and stratigraphic context, we grouped these samples into 7 temporal periods, each characterized by particular cultural and environmental attributes that we predicted would differentially influence clam growth (SI Appendix, Table S1), and asked what variables best predict clam size across a spectrum of ages and age at death over the past 11,500 y (Table 1).
Table 1.
Proxy measures of clam growth: Expectations relative to poor* (11.5- to 11-ka) conditions and summary of results by time period
| Response variable | Expectations | How evaluated | Summary of results | Expectations met?/comments |
| Age at death | Improved growing conditions for clams will allow clams to live longer unless harvested | Count maximum number of annuli | Age at death increases through time up until harvested midden samples; age at death of all nonharvested samples are older than specimens from 11.5 to 11 ka | Yes. Clam dies youngest in Early Holocene and in Early Historic times when envirocultural conditions are poorest |
| Size at death | Improved growing conditions for clams will allow clams to grow larger unless harvested | Measure maximum growth-axis length | Size at death from all nonharvested samples larger than specimens from 11.5 to 11 ka | Yes. Clams are smallest in the Early Holocene and in Early Historic times, paralleling trends in age at death |
| Modeled maximum size | Healthier clams will be larger | Estimate Linf | Linf indicates increase in size from the Early Holocene to before Early Historic times. Most clams from middens and clam gardens are on average the same size as clams from unwalled beaches 4.2 to 2.9 ka; however, the largest clams in the Holocene are from the midden contexts. By the Early Historic, clams have a smaller maximum size. Linf of 11.5- to 11-ka specimens is smaller than all time periods, except 10 to 9.5 ka and living | Yes. Clams are smallest in the Early Holocene and in Early Historic and modern times. Linf indicates that midden samples from clam gardens are the same size or larger than nonmidden 4.2- to 2.9-ka clams, suggesting that the theoretical maximum size of clams in clam gardens is the same or larger than the “naturally” biggest clams in the Holocene; this, in turn, demonstrates the benefits of traditional clam management |
| Size at young age | Clams will be bigger in better growing conditions | Measure sizes (growth-axis length) at ages 1–5 | At ages 1 and 2, Early Historic clams are bigger than all others, but this evens out by age 3. By age 3, clams from all time periods, except living, are larger than those from 11.5 to 11 ka; by ages 4 and 5, clams from all time periods are larger than those 11.5 to 11 ka | Somewhat. While 11.5- to 11-ka clams are smaller than all other time periods, reflecting that period’s relatively poorer growing conditions, the Early Historic and modern clams are not distinguishable from this early period |
Poor conditions are those where abiotic factors (grain size, water temperature, slope) are outside the preferred range for butter clams and where there has been no human management. These conditions characterize the temporal category from 11.5 to 11 ka.
Results
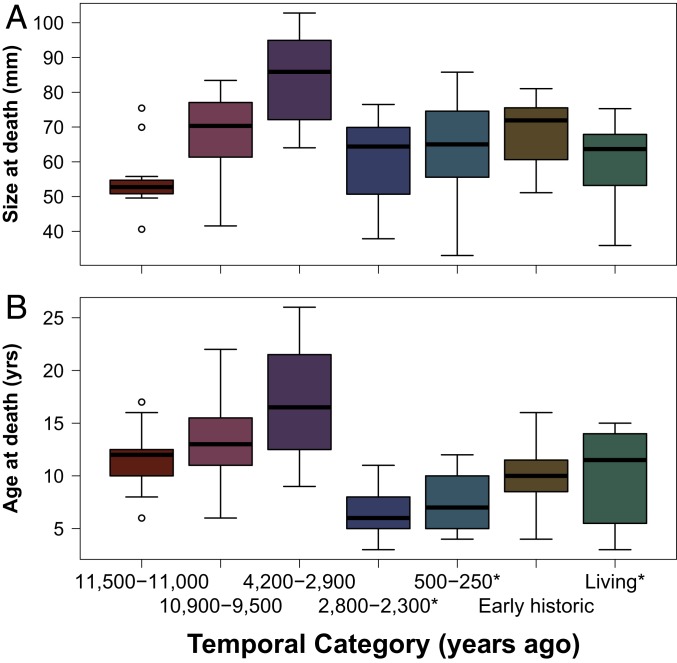
As predicted, nonharvested clams (i.e., clams that died in situ from natural mortality) show a steady increase in size at death and age at death from 11.5 to 11 ka until the early-Late Holocene (4.2 to 2.9 ka) (Figs. 2 and 3, Table 1, and SI Appendix, Table S2), corresponding to improving environmental conditions. In the midden (2.8 to 2.3 ka and 500 to 200 y ago) and living samples, clams were harvested from active and inactive clam gardens, respectively, and did not live out their full lifespans. Thus, the measurements do not evaluate natural mortality but rather, in the case of the middens, show the preferred size and age at which clams were harvested. However, in the Early Historic Period, where clams died of natural mortality in clam gardens, we can compare their age at death and size at death with the early-Late Holocene clams. We find that the median age at death and size at death of the Early Historic clams drop significantly and are 16 to 40% smaller than those in the early-Late Holocene samples (4.2 to 2.9 ka) (Fig. 2). As well, the Early Historic samples are statistically indistinguishable from the Early Holocene time periods (11.5 to 11 and 10.9 to 9.5 ka) when there was minimal human presence on the Pacific Northwest coastal landscapes. Notably, Early Historic Period clams grew in the years following 1782 CE when the Indigenous populations declined dramatically as a result of introduced diseases (25).
Fig. 2.
Size (millimeters) at death (A) and age (years) at death (B) of butter clams (S. gigantea) by temporal category. Sample size in each temporal period varies between 11 and 30 depending on temporal context (SI Appendix, Table S3). *Harvested clams collected from midden deposits and from modern (living) populations.
Fig. 3.
Butter clams from 11.5 to 11 ka (Left) and from 10.9 to 9.5 ka (Right), illustrating the differences in butter clam shell size in some of our samples.
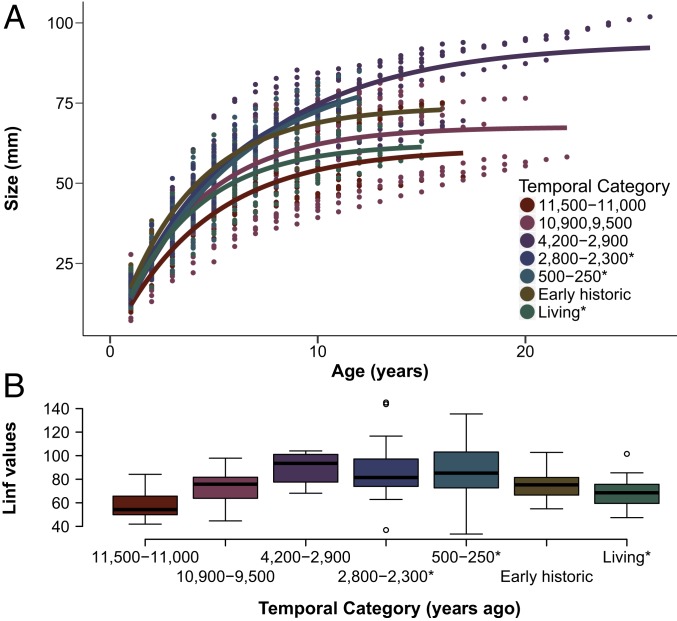
To further explore butter clam growth rates, we fit von Bertalanffy growth curves (26) to clam growth increments in each of the 7 time periods and compared estimated theoretical maximum clam sizes (Linf parameter) (Fig. 4). Visual inspection of the early life history growth in the von Bertalanffy growth curves suggests that the clam growth trajectories across time periods broadly fit our predictions that clams grow faster as environmental conditions become more favorable. Additionally, clams from 11.5 to 11 ka, clams from 10.9 to 9.5 ka, and those from today appear to follow the same growth pattern and grow relatively slowly in their early years. In contrast, clams living 4.2 to 2.9 ka, the midden clams harvested from clam gardens (2.8 to 2.3 ka and 500 to 200 y ago), and the Early Historic clams all have a relatively faster growth trajectory in their early life history.
Fig. 4.
Modeled growth of butter clams from 7 temporal categories. (A) Average von Bertalanffy growth curve per time period. Curves have been forced through 0. (B) Boxplots showing L∞ by temporal category with model forced through 0 for each individual specimen (total sample = 124 clams; sample size in each temporal period varies between 11 and 30 depending on temporal context) (SI Appendix, Table S3). *Harvested clams collected from middens and from modern (living) populations.
Estimated Linf values from the von Bertalanffy growth curves (Fig. 4B) allow us to compare the theoretical maximum length for the midden and living samples as if they were not harvested, assuming that earlier rates of growth in an individual are predictive of later growth rates. The estimated Linf values of the 11.5- to 11-ka clams are significantly smaller (SI Appendix, Table S2) than most of the other time periods, except for the 10.9- to 9.5-ka, Early Historic, and living specimens (Fig. 4B). This again suggests that clams growing under modern conditions (in the last 200 y) reach sizes similar to clams that lived in the Earliest Holocene. The Linf estimates further suggest that clams from the middens, which were harvested from active clam garden beaches, would have reached roughly the same maximum length as the nonharvested clams in the early-Late Holocene (Fig. 4B). This suggests that clams under intense human management have the potential to grow as large as the largest clams living in environments with relatively few humans. Our estimates of Linf and our empirical data together indicate that, as environmental conditions improve and as traditional management intensifies, butter clams have the capacity to grow to older ages and larger sizes than before.
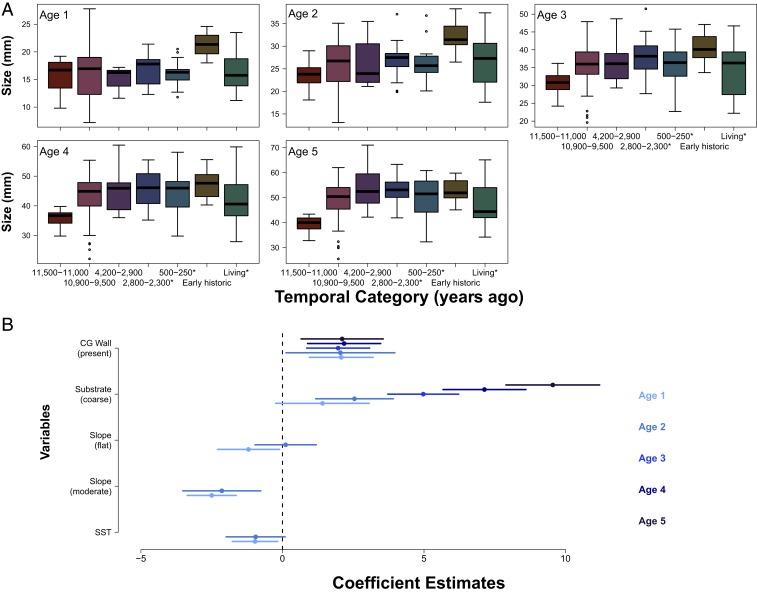
For each individual clam, we compared how size at age for the first 5 y changed across temporal category (Table 1). Each size at age follows a roughly similar pattern (Fig. 5) and mostly parallels that of age at death, size at death (Fig. 2), and Linf (Fig. 4). We find that, contrary to our predictions, age 1 clams in the Early Historic Period are bigger than all other times (SI Appendix, Table S2). By ages 4 and 5, clams in all time categories except living and those from 10.9 to 9.5 ka are larger than those in our oldest period (11.5 to 11 ka) (SI Appendix, Table S2).
Fig. 5.
Size (millimeters) at ages 1 to 5 (A) across temporal categories and model average coefficient estimates and SEs showing the factors affecting size at age (B), where age of clam is indicated from light to dark blue. Note different ranges on y axes in A and that slope and SST are not included in the model average for ages 3 to 5 clams. CG Wall indicates that a clam garden wall is present. *Clams collected from midden and living samples.
We compared the relative strength of evidence for several environmental and cultural attributes affecting clam growth with a multimodel inference approach, allowing us to explore alternative mechanisms driving variation in size at age. Depending on the age of the individual, different terms are included in the model average. For young clams (ages 1 to 2), we found evidence that beach slope and sea surface temperature (SST) inversely affect size at age but are less important for clams ages 3 to 5, suggesting that young clams are relatively more sensitive to changes in these factors. Coarse substrate has a strong positive influence on size at age for clams ages 1 to 5, with the strength of the effect being stronger as a clam gets older compared with fine substrate. Similar to fish, slight growth differences are more difficult to detect as clams age and growth slows (10), and therefore, the effects that we detect in older clams are actually conservative. Taken together, the inclusion of more terms in the model average (slope, SST, and substrate) for clams ages 1 to 2 indicates that relatively more environmental factors influence the growth of younger clams than older ones. As predicted, for clams at all ages (1 to 5), the presence or absence of a clam garden wall is an important factor affecting clam growth, and the presence of a clam garden results in a strong positive effect on size at age (Fig. 5B, Table 1, and SI Appendix, Table S1).
Similar factors affect both age at death and size at death, with clam garden wall, slope, and substrate having the most influence (SI Appendix, Fig. S1). Not surprisingly, the presence of a clam garden has a strong negative influence on both size at death and age at death, since most of the clams from clam gardens in our sample were harvested (midden samples from 2.8 to 2.3 ka and from 500 to 200 y ago and living), thus truncating their size and age at death. While substrate does not appear to affect age at death, size at death is affected by substrate, where coarse substrates result in larger size at death compared with fine substrates. This indicates that coarser substrates may be better for clam growth. Flat and moderate intertidal beach slopes, typical of clam gardens, result in older and larger age at death and size at death compared with steep beach slopes.
We also compared environmental and cultural factors affecting our von Bertalanffy estimated Linf values (SI Appendix, Fig. S2). Substrate is the most important variable governing Linf, where coarser substrates are associated with larger Linf compared with fine substrates. Flat and moderate beach slopes exert a positive influence on Linf as compared with steep slopes.
Taken together, we show that not only are there several factors that influence size of a clam throughout its life but also, that the effects of these factors vary throughout the life of the clam. For instance, SST appears to be negatively correlated with the size of clams at age 1, but we did not detect an effect of SST by age 2. The absence of effect in age 2 is at least in part due to the incongruity of comparing the millennia scale of SST data with yearly-scale growth patterns of clams that are individual snapshots of different years within that period of time. Similarly, flat and moderate beach slopes are negatively correlated with the size of clams at ages 1 and/or 2 but do not appear in the model average for individuals ages 3 to 5. Clam gardens, however, have a positive effect on the growth of young clams as suggested by previous experimental data (19). Finally, coarse substrate, characteristic of the unwalled early-Late Holocene beaches as well as cultivated clam gardens, appears to be beneficial to growth in all age categories, but the strength of this relationship increases with age.
Discussion: The Historical Ecology of Human–Clam Relationships
With the retreat of the Cordilleran ice sheets ∼13.5 ka, coastal areas provided increased habitat for many species—including humans and bivalves. Our earliest butter clam samples consist of subfossil death assemblages dating to 11.5 to 11 ka. When alive, these clams burrowed into silts, fine sands, and poorly drained glacial-marine clays; on steep beach slopes; and in relatively cold SSTs (Fig. 6). These environmental conditions are not ideal habitats for this species of clam, and not surprisingly, this contributed to the small size, young age at death, and slow growth of these clams.
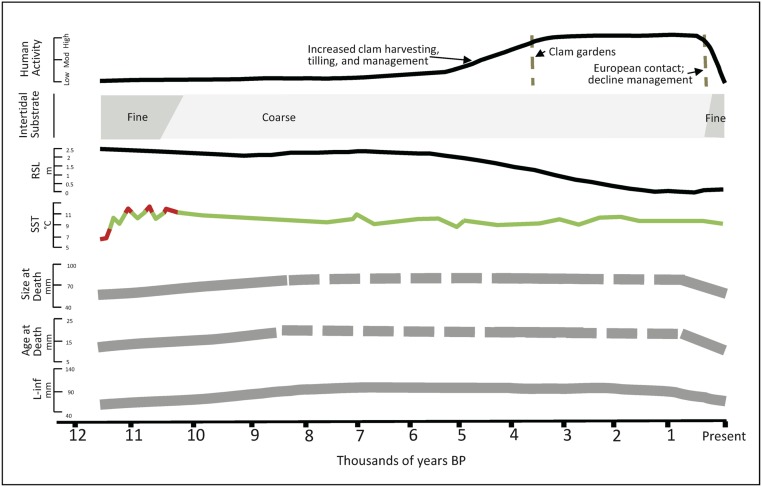
Fig. 6.
Summary of human–clam relationships through time. For age and size at death, the solid line represents actual data; the dashed line reflects inferred trends. RSL, relative sea level.
Butter clam environmental conditions began improving after ∼11 ka. These improvements include a transition to coarse (gravel–sand) substrate due to paraglacial deposits and hydrodynamic erosion (27), an increase in SSTs (28), and stabilizing sea levels (29, 30). These factors likely contributed to increased phytoplankton productivity (31) and more stable substrates for bivalve settlement (32). These improved conditions are reflected in our measured and modeled data by relatively larger size and older age at death of the 4.2- to 2.9-ka intertidal subfossils compared with those from 11.5 to 11 ka. However, the von Bertalanffy curves suggest that the growth trajectories for young clams are similar in these 2 time periods, which suggests similar ability to grow. Possibly, a decrease in mortality in the 4.2- to 2.9-ka period allowed for more larger and older individuals in that period’s death assemblage.
The abundance of Early Holocene subfossil shells within the intertidal sediments suggests that these early clam populations were not under significant predatory pressure by humans. Based on the Early Holocene archaeological record elsewhere on Quadra Island, we suspect that humans were visiting our study area at least by 10 ka, but there is no known record of sustained settlement. However, even if early campsites were found in the study area, it would be difficult to explore the role of clams in the human diet, because the region’s acidic soils limit preservation of clam shells in older archaeological deposits.
There is a temporal gap in our analysis for the period 9.5 to 4.2 ka due to the absence of both midden and intertidal clam samples. Although there are archaeological sites from this time, they have yet to be sampled for faunal analyses. Within the beach sediments, taphonomic factors are likely responsible for the lack of intertidal subfossil shells. In particular, the persistent wave action caused by relatively stable sea levels (Fig. 6) (29, 30) during this ∼5,000-y period coupled with reduced sedimentation (33) would have resulted in increased sediment erosion and the displacement and deterioration of intertidal subfossil shells. Before and after this time, the steady decline in sea level meant less wave action and thus, a regular influx of sediment that buried dead clams quickly, making them more likely to be preserved in the intertidal sediment column (34).
When we pick up the paleorecord again about 4 ka, we see that environmental conditions affecting butter clam growth continued to improve. These improvements, which differentially affect different age classes, include the further accumulation of coarse (gravel–sand) substrates and stabilized SSTs (Fig. 6). These beneficial environmental conditions are reflected in the trend of increased age at death, size at death, and maximum size of the 4.2- to 2.9-ka intertidal subfossil butter clams as compared with those from earlier in the Holocene. The large size of these clams must have been appealing to human harvesters, who began to harvest clams in earnest by ∼5 ka as indicated by the local preservation of shells in archaeological settlement sites. However, the prevalence of long-lived clams in our intertidal clam death assemblage, dating to 4.2 to 2.9 ka and originating from one particular beach (EbSh-77) 0.8 km from the nearest village, suggests that this location was not under heavy predatory pressure by humans. We suspect that this in turn reflects the fact that humans were focusing their harvesting on beaches immediately adjacent to their settlements.
After ∼3.5 ka, human–clam relationships in our study area intensify as indicated by the building of some of the first clam gardens (24). At this time, large settlements increase in number in Kanish and Waiatt Bays, filling all inhabitable coastal landforms and reflecting an increase in local human populations. By ∼2.7 ka, clam harvesting was so intensive that subfossil clams are virtually absent from intertidal deposits, and instead, there are large quantities of harvested clam shells in the middens. Clam garden construction was likely iteratively related to increasing human populations—both as an impetus for enhancing a reliable and productive food source and trade item and in turn, by allowing for the increasingly larger human population and complex social relations.
Despite intensive harvesting, several lines of evidence suggest that, as a result of traditional management practices, clam populations in our study area thrived throughout the Late Holocene. In general, building clam gardens increased the accessibility of clams to human harvesters by decreasing beach slope, increasing the amount of beach exposed during low tide, and increasing the proximity of clams to human settlements (14). Such increased accessibility of existing clam beaches could easily result in overharvesting. However, in our study area, this potential increase in harvesting pressure was offset by generations of Indigenous peoples building clam gardens, thereby increasing the viable clam habitat. We suggest that creation of this new clam habitat combined with other cultivation methods (e.g., tilling, removal of nonhuman predators, altering substrate, rock removal) (14, 16) and spatially explicit designated access rights (35, 36) ensured an ongoing, sustainable harvest of clams. Furthermore, the creation of the coarse sediment garden terrace and associated rock wall had the added benefit of increasing the abundance and availability of other edible marine foods (e.g., red rock crabs, sea cucumbers, snails, a variety of seaweeds) (37, 38).
In 2 different ways, the size of the clams in the middens in our study supports our inferences about the development of sustainable traditional harvesting practices. The first is the comparable estimated (Linf) maximum size of clams from the nonwalled early-Late Holocene beaches (4.2 to 2.9 ka) and those from the heavily harvested clam gardens in the middens (2.8 to 2.3 ka and 500 to 200 y ago) (Fig. 4B). Given the importance of substrate to the growth of young and old clams, we surmise that the similarity in clam size in these 2 time periods is in large part due to the coarse sediment substrate in both the naturally productive beaches and the created clam garden habitats.
The second way in which our midden data support inferences of sustainable harvest practices is that there is no indication of fishing pressure selection, such as a gradual decline in harvested clam size over time (i.e., compare 2.8 to 2.3 ka and 500 to 200 y ago) (Fig. 2A) (compare with refs. 2 and 39). Furthermore, the majority (62%) of clams within the midden samples are larger than today’s fisheries size limit of 63 mm (40), suggesting the possibility of a culturally prescribed set of harvesting size restrictions as well as preferences for particular clam sizes.
The larger clams that characterized Kanish and Waiatt Bays beaches throughout much of the Late Holocene declined in numbers sometime in the Early Historic Period, which was instead characterized by slower-growing, smaller clams. We attribute this decline in clam productivity to the disease-related decimation of Indigenous human populations beginning 1782 CE and the consequent reduction in management of clam gardens. Since fewer clams were being harvested at this time, more nonharvested clams died in situ of natural mortality and formed intertidal death assemblages. While there must have initially been some positive legacy effect on clam growth from the engineered intertidal slope and years of cultivation, we suggest that eventually clam habitats degraded as a result of the breakdown in the traditional clam management practices that had been part and parcel of daily human–clam interactions for millennia.
Our analyses of the clams currently living in the now-defunct clam garden beach suggest that growing conditions for clams continued to worsen in the last century. It is striking that the growth patterns of clams living in the beach today are most similar to the clams that lived and died in the unstable and relatively unproductive habitats of the Early Holocene. As in the Early Historic Period, we propose that the current low productivity is due to the decline in traditional management, including ongoing tilling through harvesting. However, given the importance of substrate on clam growth, we also attribute this recent pattern to the logging-induced deposition of silts on the clam beaches, possibly compounded by recent changes in ocean temperatures and productivity. These fine sediments created a substrate less conducive to clam growth than the coarse-grained substrates of the clam gardens and early-Late Holocene nonwalled beaches. Ironically, the shallow slope of the clam garden beaches and the lip of the clam garden wall itself—initially created to enhance clam production—now act as a sediment trap for logging silts in some clam gardens.
Conclusions
Our understanding of the historical ecology of humans and butter clams on Quadra Island not only illustrates the long-term and intertwined relationships of these 2 species but also, serves as a model for studying the intricacies of other human–species relationships. In the case of butter clams, a culturally valued species, there was a myriad of ecological and cultural factors that influenced population viability throughout the Holocene. We expect similarly complex interactions among humans and other species through time—whether through direct and deliberate interaction or through more indirect processes. Such complex human–clam interactions highlight the value of deeper-time baselines for informing modern fisheries management.
On the Northwest Coast of North America, as in coastal communities worldwide, the human–clam relationship is age old and continues today. Tracing that history and situating these relationships in the context of modern management decisions take bringing together data from multiple sources and using diverse types of analyses. They also require recognizing the sometimes-active role of humans in modifying coastal ecosystems of the past as well as the present (9, 41) and that not all long-term human–ecological interactions have negative ecological consequences on biological diversity (42–45).
In our study area, our analyses of shells from intertidal death assemblages, archaeological shell middens, and modern clams provide insights into how clams, clam habitats, and human–clam relationships changed through time in a specific place. More specifically, the analyses reveal how clam life histories have responded to shifts in harvesting, habitat alterations, climate and environmental factors, and management practices. Taken together, the temporal and spatial variability that we document is another reminder of the need to gather site- and time-specific baselines for modern management. We have demonstrated that ocean temperatures and substrate play a role in butter clam life history. Thus, it is no surprise that there is considerable variation in estimates of butter clam size in the literature (46–49), just as there are in our modern data and paleodata. Management plans based on local, modern, and paleoecological data are likely to be more robust than those based on more general spatiotemporal data from the literature. However, under future climate change scenarios, environmental variables are likely to resort in different combinations than those of recent history and perhaps, with few analogs in the past.
Previous research on clam gardens in our study area demonstrated that clam gardens today are at least twice as productive as nonwalled beaches (19, 20). This has implications for the numbers of people who can be locally supported by this ancient innovation in mariculture. Our data, however, show that clams in clam gardens today are far less productive than they were before European contact and industrial logging—that is, when traditional management systems were active and shell–sand–gravel vs. silt-rich beaches dominated clam habitats. This highlights the possibility that, if traditional mariculture methods were applied to clam beaches today, they could produce even greater yields than those estimated based on current ecological conditions—assuming similar pelagic production and oceanic conditions. In fact, many Indigenous communities along the Pacific Northwest Coast are exercising their rights to access and collective choice by restoring clam gardens and the traditional protocols associated with them (50).
Many coastal First Nations with whom we work observe that today’s clam beaches are less productive, because they are no longer managed in traditional ways. In recognition of widespread marine environment degradation and the loss of coastal resources, local communities worldwide have spearheaded efforts to manage, restore, and conserve coastal resources and improve biodiversity and food security (51). When such efforts incorporate local and traditional ecological knowledge (50) as well as archaeological and paleoecological data (11, 12), they are more likely to promote ecological sustainability. Examining the deep and specific history of human–species relationships, such as that between people and clams, is requisite for understanding and better managing our resources and ecosystems today.
Materials and Methods
Kanish and Waiatt Bays, Quadra Island.
Kanish and Waiatt Bays (Fig. 1) on northern Quadra Island are within the traditional territories of the southern Kwakwaka’wakw (Laich-kwil-tach) and northern Coast Salish First Nations. These bays were ideal locations for long-term human settlement throughout most of the Holocene, offering numerous sheltered coves, protection from the elements, access to freshwater, and easy access to neighboring settlements. The bays offer an abundance and variety of terrestrial and marine plants and animals, including shellfish. SSTs fluctuated somewhat throughout the Holocene, but since ∼10 ka, SSTs are within the temperature range favored by butter clams (28).
The archaeological record indicates long-term human settlement of the bays. Small settlements date to at least 9 ka. These occupations probably had low to moderate impact on the landscape in the form of settlement creation and predation on local plants and animals. Between 5 and 3 ka, the human population in Kanish and Waiatt Bays seems to have expanded considerably. Many large villages established at that time continued to be occupied until the late 1700s CE, at which time European diseases severely decreased Indigenous populations (25). Clam gardens were first constructed at some time around 3.5 ka, and they continued to be built and maintained, although in a much reduced way, into the mid-1900s (24). The number and extent of shell midden sites and the density of clam garden features (Fig. 1) reflect the central importance of clams in the diet. Similar to much of the rest of the Salish Sea (52), zooarchaeological analyses of middens dating to after ∼4 ka indicate that littleneck and butter clams specifically along with herring were the preferred marine foods throughout this time.
European settlement and logging of the bays were extensive in the early 20th century (53). Based on archival photographs and our excavations of beach deposits, we surmise that logging had a considerable impact on the forests and some of the foreshore both as a result of dragging logs and from slope wash of fine sediments after the removal of trees from the hillsides. Today, some areas are being logged again, but the beaches themselves are relatively undisturbed by industrial activity. In our experience, there is excellent preservation of subfossil shells in death assemblages in these beaches.
Butter Clams (S. gigantea).
Butter clams are found along the Pacific Northwest Coast of North America from Alaska to California in a mixed intertidal substrate of gravel, sand, and shell (46). This species lives up to 30 cm below the beach surface and normally, lives in the middle to lower intertidal (46). Today, butter clams are reported to live upward of 20 y (49) and mature at ∼35 mm in size at ∼4 y of age (46). Butter clams have robust shells that appear to preserve well in the intertidal paleo- and archaeological records, which make them strong candidates for historical ecological analyses.
Field and Laboratory Sample Selection and Analyses.
We collected butter clams from 3 ecocultural contexts in 5 sites within Kanish Bay: 1) paleobeach deposits below clam garden beach deposits (n = 3 sites), 2) now defunct clam garden beaches (including specimens that were living and those that died in the Early Historic Period; n = 2 sites), and 3) archaeological shell midden deposits from clams harvested in active clam gardens (n = 2 sites) (Fig. 1 and SI Appendix, Table S3). The paleobeach and clam garden sampling sites were selected, because they have a soft sediment substrate that allowed us to collect specimens from both pre- (paleobeach) and post-clam garden contexts. The position and articulation of clam shells from the paleobeaches and clam garden beaches indicate that these specimens died of natural causes in situ and remained relatively undisturbed within the sediment column.
To retrieve these samples, excavation units were placed in clam garden terraces, in beach sediments seaward of the clam garden walls, and under clam garden rock walls themselves. When possible, we aimed to reach the basal clay deposited after the retreat of the Cordilleran glaciers 13.5 ka (54, 55). This was not always possible due to the constraints of the tide and groundwater flooding of the excavation units. In each excavation unit, shells in good condition (full, articulated valves, limited weathering) were collected for laboratory analysis. This may have resulted in some sampling bias toward more robust shells and against the smaller, more fragile shells that are more susceptible to taphonomic processes. Keeping the selection criteria biased toward larger clams across all sampling contexts (middens and in situ beach assemblages) should maintain consistency and reduce skewed results.
Clams that did not die in situ include those that we collected live and the specimens collected by ancient harvesters from clam garden beaches and deposited in shell middens. Living butter clams are relatively rare in Kanish Bay beaches, likely in part due to the recent influx of logging sediments; therefore, we collected every living specimen that was encountered from within 5 shallow shovel tests along a transect (7, 16, 25, 28, and 32 m perpendicular from the clam garden wall) within the clam garden at EbSh-13 (Fig. 1B). This introduced considerable variability in substrate across our sampling universe. Clam specimens from the 2 midden locations (EbSh-14, EbSh-13) come from excavated and dated column samples. Since midden specimens represent clams that were harvested selectively by Indigenous people, they do not represent the full natural range of variation in clam sizes and ages. Our midden samples date to 2.8 to 2.3 ka and 500 to 200 y ago. Older midden samples within the study area do exist but were not sampled for this study.
The temporal context of all clam specimens was determined in the field by analyzing the depositional context and in the laboratory by radiocarbon dating select samples from each depositional context. Dated samples were processed at the W. M. Keck Carbon Cycle AMS Laboratory at the University of California, Irvine using accelerator mass spectrometry. Based on these analyses, we divided our sample into 7 temporal categories spanning the time from the Early Holocene (11.5 ka) to modern. These temporal periods are characterized by a distinct set of environmental and cultural attributes (SI Appendix, Table S3). In general, the first 5 categories reflect preindustrial environments, with varying degree of human influence.
The Early Historic category encompasses the time just before and the decades immediately following direct European contact (1792 CE) (56). During this time, introduced diseases had a severe impact on local Indigenous populations and their traditional resource management systems. Although the wide radiocarbon date ranges for the clams from this period extend into the precontact era, we reason that these deposits are postcontact in age, because the clams died in the beaches rather than being harvested and deposited in middens as they were prior to European contact, when human population numbers were high. We assume that these beaches retained some legacies of the previous management system (coarse sediment substrates) but were not subject to the full range of traditional management strategies (tilling, selective harvest, beach clearing, predator removal). The living category includes clams growing today in the absence of traditional management and often in silty substrates as a result of 20th century logging.
Using a number randomizer, a total of 124 butter clam shells, representing the 7 temporal categories, were selected for metric and sclerochronological analyses (SI Appendix, Table S3). We measured the growth-axis length (millimeters) for each whole clam and then, measured the number and width of annual growth increments (millimeters) using thin sections cut along the axis of maximum growth of each butter clam. The preparation and processing of shell thin sections followed standard laboratory methods (57). Collectively, these measurements allow us to analyze age and size at death, and size at ages 1 to 5, which are, in turn, proxies for how well the clam grew in its cultural and ecological conditions (Table 1).
There are several reasons why we focus on age and size of individuals aged 1 to 5 as well as age at death and size at death. A practical reason for examining younger individuals is, unlike the comparisons at death, that we can compare the size at age of the harvested (midden and live-collected) clams with the other temporal periods. Another practical reason for focusing on comparative growth of this age class is the relative sensitivity that we have in detecting differences in growth between the clams of different time periods. Because annual growth increments are largest in the first several years of a clam’s life, we have higher resolution in our measurements, which in turn, reduces our measurement error. In addition, we are interested in young clams, because they are the most vulnerable to predation and disease, and thus, the faster they mature and grow to a larger size, the more chance they will contribute to the population. Previous research (19) showed that juvenile clam growth is relatively greater in clam gardens than nonwalled beaches today; our data allow us to explore whether this pattern also holds in the past.
Environmental and Cultural Attributes Affecting Butter Clam Growth.
To test which abiotic and cultural conditions influence clam growth, we included 6 environmental and cultural factors in our growth rate analyses: site (n = 5), substrate (coarse, fine), beach slope (steep, moderate, flat), SST, presence/absence of a modified clam garden, and the degree of human interaction (high, medium, low) (SI Appendix, Tables S1, S3, and S4). Substrate was evaluated during intertidal excavations, which exposed intertidal stratigraphy; pre-clam garden beach slope was assessed qualitatively by considering the geomorphology of the beach and surrounding landform. SST data were provided by Kienast and McKay (28). To determine degree of human interaction, we reasoned that beaches that were temporally and spatially distant from archaeological settlements experienced relatively less human interaction. Clam specimens recovered from beaches with large clam death assemblages and from presettlement times accumulated under conditions of less intensive harvesting pressure. Clam specimens from beaches with no death assemblages and associated with large archaeological shell middens represent clams that grew during human occupation and harvest. We assume that clams from middens directly associated with clam gardens were harvested from those clam gardens. Assessment of all of these attributes allowed us to make predictions about butter clam growth in the different environmental and cultural contexts of each temporal category (SI Appendix, Table S1).
Statistical Analyses.
For evaluating age and size at death, and size at ages 1 to 5, we conducted 2 quantitative analyses. We fit an ordinary least squares ANOVA to compare age at death (years), size at death (millimeters), and size at ages 1 to 5 (millimeters) across time period (within-group sample sizes ranging from 11 to 31 clams) and using the 11.5- to 11-ka time period as the baseline. To compare differences between groups, we used package emmeans (58) to estimate marginal means between time periods.
We then used multimodel inference (59, 60) to model the relationship between age and size at death, and size at ages 1 to 5 against environmental and cultural factors (SI Appendix, Table S5). We checked for collinearity between all factors using a variance inflation factor (VIF; package car) and excluded site and human interaction from the model due to their VIF ≥ 5. We standardized continuous variables by centering and dividing by 2 SDs (package “arm”). We used the dredge function in the MuMIn package (61) to evaluate all possible combinations of models from a specified global model containing slope, substrate, clam garden, and standardized SST (62). We used Akaike information criterion corrected for small sample sizes (AICc) to compare models, and if there was no clear top model, we model averaged to within 2 ΔAICc values (SI Appendix, Table S5) using the full averaging method (59, 60). Using the model averages, we calculated relative variable importance and variable effect sizes using the model average coefficients (Fig. 5B and SI Appendix, Fig. S1 and Table S4).
To compare differences in growth rate across the life of a clam, we modeled growth using von Bertalanffy (26) growth curves for each specimen (n = 124). We estimated von Bertalanffy parameters for curves forced through 0. Using the estimated Linf from the von Bertalanffy curves, we used an ordinary least squares ANOVA to compare how growth changes across temporal category with the 11.5 to 11 ka time period and compared estimated marginal means between groups. We used a multimodel inference approach (see above) to evaluate the importance of factors affecting Linf. We excluded site and human influence due to high colinearity; constructed a global model including substrate, clam garden wall, slope, and SST; and used AICc to compare models from all possible combinations of the global model. As there was no top model, we model averaged to within 2 ΔAICc values (SI Appendix, Table S5) and compared relative variable importance and effect sizes using model average coefficients (SI Appendix, Fig. S2). All analyses were performed in R, version 3.3.2 (63).
Data Sharing Statement.
All data are available as supplementary material (Dataset S1).
Supplementary Material
Acknowledgments
This work was conducted on the traditional territories of the We Wai Kai, We Wai Kum, K’omoks, Xwemalhkwu, and Klahoose Nations. We are grateful to Eric Peterson and Christina Munck for the financial and logistical support provided by Tula Foundation and Hakai Institute. Thank you to Nicole Smith, Louie Wilson, Christine Roberts, Susan Kidwell, and others in the Clam Garden Network for conversations that sparked some of the ideas woven throughout this paper. Thank you to Louise Williams for the production of Figure 1. Portions of the work were derived from coauthor G.T.’s Master’s thesis “11,000 Years of Human-Clam Relationships on Quadra Island, Salish Sea, British Columbia.”
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905921116/-/DCSupplemental.
References
- 1.Garibaldi A., Turner N. J., Cultural keystone species: Implications for ecological conservation and restoration. Ecol. Soc. 9, 1 (2004). [Google Scholar]
- 2.Erlandson J. M., Rick T. C., Braje T. J., Steinberg A., Vellanoweth R. L., Human impacts on ancient shellfish: A 10,000 year record from San Miguel Island, California. J. Archaeol. Sci. 35, 2144–2152 (2008). [Google Scholar]
- 3.Erlandson J. M., Rick T. C., Braje T. J., Fishing up the food web?: 12,000 years of maritime subsistence and adaptive adjustments on California’s channel Islands. Pac. Sci. 63, 711–724 (2009). [Google Scholar]
- 4.Cannon A., Burchell M., Clam growth-stage profiles as a measure of harvest intensity and resource management on the Central Coast of British Columbia. J. Archaeol. Sci. 36, 1050–1060 (2009). [Google Scholar]
- 5.Braje T. J., Rick T. C., Erlandson J. M., A trans-holocene historical ecological record of shellfish harvesting on California’s Northern channel Islands. Quat. Int. 264, 109–120 (2012). [Google Scholar]
- 6.Kvenvolden K. A., Blunt D. B., Clifton H. E., Amino-acid racemizarion in quaternary shell deposits at Willapa Bay, Washington. Geochim. Cosmochim. Acta 43, 1505–1520 (1979). [Google Scholar]
- 7.Kidwell S. M., Time-averaged molluscan death assemblages: Palimpsests of richness, snapshots of abundance. Geology 30, 803–806 (2002). [Google Scholar]
- 8.Kidwell S. M., Biology in the Anthropocene: Challenges and insights from young fossil records. Proc. Natl. Acad. Sci. U.S.A. 112, 4922–4929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson J. B. C., et al. , Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Rowell K., et al. , Diverting the Colorado River leads to a dramatic life history shift in an endangered marine fish. Biol. Conserv. 141, 1138–1148 (2008). [Google Scholar]
- 11.Rick T. C., et al. , Millennial-scale sustainability of the Chesapeake Bay Native American oyster fishery. Proc. Natl. Acad. Sci. U.S.A. 113, 6568–6573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grier C., Alessa L., Kliskey A., Looking to the past to shape the future: Addressing social-ecological change and adaptive trade-offs. Reg. Environ. Change 17, 1205–1215 (2017). [Google Scholar]
- 13.Moss M. L., Shellfish, gender, and status on the Northwest Coast of North America: Reconciling archaeological, ethnographic, and ethnohistorical records of the Tlingit. Am. Anthropol. 93, 631–652 (1993). [Google Scholar]
- 14.Deur D., Dick A., Recalma-Clutesi K., Turner N. J., Kwakwaka’wakw “clam gardens”: Motive and agency in traditional Northwest Coast mariculture. Hum. Ecol. 43, 201–212 (2015). [Google Scholar]
- 15.Cannon A., Burchell M., Bathurst R., “Trends and strategies in shellfish gathering on the Pacific Northwest Coast of North America” in Early Human Impact on Megamolluscs, Antezak A., Cipriani R., Eds. (British Archaeological Reports International Series 1865, Archaeopress, Oxford, UK, 2008), pp. 7–22. [Google Scholar]
- 16.Lepofsky D., et al. , Ancient shellfish mariculture on the Northwest Coast of North America. Am. Antiq. 80, 236–259 (2015). [Google Scholar]
- 17.Cannon A., Burchell M., Reconciling oxygen isotope sclerochronology with interpretations of millennia of seasonal shellfish collection on the Pacific Northwest Coast. Quat. Int. 427, 184–191 (2016). [Google Scholar]
- 18.Burchell M., Cannon A., Hallmann N., Schwarcz H. P., Schöne B. R., Inter-site variability in the season of shellfish collection on the Central Coast of British Columbia. J. Archaeol. Sci. 40, 626–636 (2013). [Google Scholar]
- 19.Groesbeck A. S., Rowell K., Lepofsky D., Salomon A. K., Ancient clam gardens increased shellfish production: Adaptive strategies from the past can inform food security today. PLoS One 9, e91235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackley J., Gardner L., Djunaedi A. F., Salomon A. K., Ancient clam gardens, traditional management portfolios, and the resilience of couple human-ocean systems. Ecol. Soc. 21, 20 (2016). [Google Scholar]
- 21.Klein R. T., Lohmann K. C., Kennedy G. L., Elemental and isotopic proxies of paleotemperature and paleosalinity: Climate reconstruction of the marginal Northeast Pacific ca. 80 ka. Geology 25, 363–366 (1997). [Google Scholar]
- 22.Strom A., Francis R. C., Mantua N. J., Miles E. L., Peterson D. L., North Pacific climate recorded in growth rings of geoduck clams: A new tool for paleoenvironmental reconstruction. Geophys. Res. Lett. 31, L06206 (2004). [Google Scholar]
- 23.Black B. A., Copenheaver C. A., Frank D. C., Stuckey M. J., Kormanyos R. E., Multi-proxy reconstructions of northeastern Pacific sea surface temperature data from trees and Pacific geoduck. Palaeogeogr. Palaeoclimatol. Palaeoecol. 278, 40–47 (2009). [Google Scholar]
- 24.Smith N. F., et al. , 3500 years of shellfish mariculture on the Northwest Coast of North America. PLoS One 14, e0211194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris C., Voices of disaster: Smallpox around the Strait of Georgia in 1782. Ethnohistory 41, 591–626 (1994). [Google Scholar]
- 26.von Bertalanffy L., A quantitative theory of organic growth (Inquiries on growth laws. II). Hum. Biol. 10, 181–213 (1938). [Google Scholar]
- 27.Orford J. D., Forbes D. L., Jennings S. C., Organisational controls, typologies and time scales of paraglacial gravel-dominated coastal systems. Geomorphology 48, 51–85 (2002). [Google Scholar]
- 28.Kienast S. S., McKay J. L., Sea surface temperatures in the subarctic Northeast Pacific reflect millennial-scale climate oscillations during the last 16kyrs. Geophys. Res. Lett. 28, 1563–1566 (2001). [Google Scholar]
- 29.Crowell T., “Settlement and sea-level histories: Refining holocene sea-levels in Kanish and Waiatt Bays, Quadra Island,” Master’s thesis, Department of Archaeology, Simon Fraser University, Burnaby, BC, Canada (2017).
- 30.Fedje D., et al. , A revised sea level history for the northern Strait of Georgia, British Columbia, Canada. Quat. Sci. Rev. 192, 300–316 (2018). [Google Scholar]
- 31.Eppley R. W., Temperature and phytoplankton growth in the sea. Fish Bull. 70, 1063–1085 (1972). [Google Scholar]
- 32.Roegner G. C., Transport of molluscan larvae through a shallow estuary. J. Plankton Res. 22, 1779–1800 (2000). [Google Scholar]
- 33.Aitken A. E., Bell T. J., Holocene glacimarine sedimentation and macrofossil palaeoecology in the Canadian high arctic: Environmental controls. Mar. Geol. 145, 151–171 (1998). [Google Scholar]
- 34.Claassen A., Shells (Cambridge University Press, Cambridge, UK, 1998). [Google Scholar]
- 35.Lepofsky D., Caldwell M., Indigenous marine resource management on the Northwest Coast of North America. Ecol. Process. 2, 12 (2013). [Google Scholar]
- 36.Mathews D. L., Turner N. J., “Ocean cultures: Northwest Coast ecosystems and indigenous management systems” in Conservation for the Anthropocene Ocean, Levin P., Poe M., Eds. (Elsevier Academic Press, London, UK, 2017), pp. 169–206. [Google Scholar]
- 37.Cox K. D., et al. , Infaunal community responses to ancient clam gardens. ICES J. Mar. Sci., 10.1093/icesjms/fsz153 (2019). [DOI] [Google Scholar]
- 38.Caldwell M. E., et al. , A bird’s eye view of northern Coast Salish intertidal resource management features, southern British Columbia, Canada. J. Island Coast. Archaeol. 7, 219–233 (2012). [Google Scholar]
- 39.Broughton J. M., Prey spatial structure and behavior affect archaeological tests of optimal foraging models: Examples from the Emeryville shellmound vertebrate fauna. World Archaeol. 34, 60–83 (2002). [Google Scholar]
- 40.FOC Fisheries and Oceans Canada, Intertidal clam http://www.pac.dfo-mpo.gc.ca/fm-gp/commercial/shellfish-mollusques/clam-palourde/index-eng.html. Accessed 27 April 2019.
- 41.Pauly D., Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Anderson E. N., Ecologies of the Heart: Emotion, Belief, and the Environment (Oxford University Press, Oxford, UK, 1996). [Google Scholar]
- 43.Turner N. J., The Earth’s Blanket: Traditional Teachings for Sustainable Living (D & M Publishers Inc., Vancouver, BC, Canada, 2005). [Google Scholar]
- 44.Balée W., Cultural Forests of the Amazon: A Historical Ecology of People and Their Landscapes (University of Alabama Press, Tuscaloosa, AL, 2013). [Google Scholar]
- 45.Harris M., Weisler M., Intertidal foraging on atolls: Prehistoric forager decision-making at Ebon Atoll, Marshall Islands. J. Island Coast. Archaeol. 12, 200–223 (2017). [Google Scholar]
- 46.Quayle D. B., Bourne N., The Clam Fisheries of British Columbia (Fisheries Research Board of Canada, Ottawa, ON, Canada, 1972). [Google Scholar]
- 47.Paul A. J., Paul J. M., Feder H. M., Age, growth, and recruitment of the butter clam, Saxidomus gigantea, on Porpoise Island, Southeast Alaska. Proc. Natl. Shellfish. Assoc. 66, 26–28 (1976). [Google Scholar]
- 48.Kozloff E., Seashore Life of the Northern Pacific Coast (University of Washington Press, Seattle, WA, 1993). [Google Scholar]
- 49.Harbo R. M., Shells & Shellfish of the Pacific Northwest: A Field Guide (Harbour Publishing Co. Ltd., Madiera Park, BC, Canada, 1996). [Google Scholar]
- 50.Augustine S., Dearden P., Changing paradigms in marine and coastal conservation: A case study of clam gardens in the Southern Gulf Islands, Canada. Can. Geogr. 58, 305–314 (2014). [Google Scholar]
- 51.Berkes F., Coasts for People: Interdisciplinary Approaches to Coastal and Marine Resource Management (Routledge, New York, NY, 2015). [Google Scholar]
- 52.McKechnie I., et al. , Archaeological data provide alternative hypotheses on Pacific herring (Clupea pallasii) distribution, abundance, and variability. Proc. Natl. Acad. Sci. U.S.A. 111, E807–E816 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor J., The Quadra Story: A History of Quadra Island (Harbour Publishing Co. Ltd., Madeira Park, BC, Canada, 2009). [Google Scholar]
- 54.Ryder J., Fulton R., Clague J., The Cordilleran ice sheet and the glacial geomorphology of Southern and Central British Colombia. Geogr. Phys. Quat. 45, 365–377 (1991). [Google Scholar]
- 55.Blais-Stevens A., Bornhold B. D., Kemp A. E. S., Dean J. M., Vaan A. A., Overview of late quaternary stratigraphy in Saanich Inlet, British Columbia: Results of ocean drilling program leg 169S. Mar. Geol. 174, 3–26 (2001). [Google Scholar]
- 56.Kendrick J., The Voyage of Sutil and Mexicana 1792: The Last Spanish Exploration of the Northwest Coast of North America. (The Arthur H. Clark Company, Spokane, WA, 1991). [Google Scholar]
- 57.Schöne B. R., Dunca E., Fiebig J., Pfeiffer M., Mutvei’s solution: An ideal agent for resolving microgrowth structures of biogenic carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 228, 149–166 (2005). [Google Scholar]
- 58.Lenth R. V., Emmeans: Estimated Marginal Means, aka Least-Squares Means (R Package Version 1.3.3). https://cran.r-project.org/web/packages/emmeans/index.html.
- 59.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer Science & Business Media, Berlin, Germany, 2003). [Google Scholar]
- 60.Grueber C. E., Nakagawa S., Laws R. J., Jamieson I. G., Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 24, 699–711 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Bartoń K., MuMIn: Multi-Model Inference (R Package Version 1.15.6). https://cran.r-project.org/web/packages/MuMIn/index.html.
- 62.Gelman A., et al. , Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. 2015 (R Package Version 1–9). https://cran.r-project.org/web/packages/arm/index.html.
- 63.R Core Team , R: A Language and Environment for Statistical Computing (Version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.