Abstract
Sustained locoregional control of disease is a significant issue in patients with inflammatory breast cancer (IBC), with local control rates of 80% or less at 5 years. Given the unsatisfactory outcomes for these patients, there is a clear need for intensification of local therapy, including radiation. Inhibition of the DNA repair protein poly adenosine diphosphate-ribose polymerase 1 (PARP1) has had little efficacy as a single agent in breast cancer outside of studies restricted to patients with BRCA mutations; however, PARP1 inhibition (PARPi) may lead to the radiosensitization of aggressive tumor types. Thus, this study investigates inhibition of PARP1 as a novel and promising radiosensitization strategy in IBC. In all existing IBC models (SUM-149, SUM-190, MDA-IBC-3), PARPi (AZD2281-olaparib and ABT-888-veliparib) had limited single agent efficacy (IC50 > 10 μM) in proliferation assays. Despite limited single agent efficacy, sub-micromolar concentrations of AZD2281 in combination with RT led to significant radiosensitization (rER 1.12–1.76). This effect was partially dependent on BRCA1 mutational status. Radiosensitization was due, at least in part, to delayed resolution of double strand DNA breaks as measured by multiple assays. Using a SUM-190 xenograft model in vivo, the combination of PARPi and RT significantly delays tumor doubling and tripling times compared to PARPi or RT alone with limited toxicity. This study demonstrates that PARPi improves the effectiveness of radiotherapy in IBC models and provides the preclinical rationale for the opening phase II randomized trial of RT +/− PARPi in women with IBC (SWOG 1706, ).
Keywords: inflammatory breast cancer, radiation sensitivity, PARP inhibitor
Introduction
Inflammatory breast cancer (IBC) diagnoses represent well under 5% of new breast cancer cases but account for a disproportionate share of breast cancer mortality(1). Despite aggressive, multimodal therapy, patients have high rates of locoregional recurrence and distant metastases(1). Treatment strategies for many breast cancer subtypes are largely directed against the protein drivers of each molecular subtype, including targeted therapies against the estrogen receptor (ER) or the human epidermal growth factor receptor 2 (HER2). IBC, however, represents a heterogeneous population that includes tumors across all of the molecular subtypes(2). Current treatment guidelines for IBC patients take into consideration the molecular subtype of the tumor and include anti-HER2 or anti-estrogen therapy when appropriate, but more effective and targeted therapeutic options for patients with IBC are extremely limited. Without more effective alternatives, IBC patients typically receive neoadjuvant chemotherapy followed by mastectomy and adjuvant radiation (RT) to the chest wall and regional lymphatics(1). The key molecular drivers of IBC are currently unknown, and this uncertainty manifests as ineffective clinical therapeutic strategies. In IBC, there is a critical need to identify more effective treatment strategies to decrease rates of locoregional recurrence.
In an attempt to understand the heterogeneity of IBC, a recent study of 53 IBC tumors demonstrated that over 90% of tumors studied contained actionable mutations in genes like PIK3CA and BRCA1/2 that could be targeted using therapies that are either FDA-approved or currently in clinical trial(3). In line with this finding, there are a number of phase I and phase II clinical trials seeking to repurpose other FDA-approved drugs for indication in IBC(1). Targeted therapies in these trials include agents against PD-1 (pembrolizumab), VEGF-A (bevacizumab)(4,5), JAK1/2 (ruxolitinib), and the viral agent T-VEC (talimogene laherparepvec)(1). Many different chemotherapy and radiation therapy regimens have been explored in IBC, but rates of recurrence and overall survival have not significantly improved(6). However, the ability to sensitize IBC tumors to current treatments like radiation represents a promising treatment strategy for patients with IBC.
Inhibition of poly adenosine diphosphate-ribose polymerase 1 (PARP1) has been explored in clinical trials for many cancer types. PARP1 inhibition (PARPi) does not demonstrate significant single agent efficacy in the treatment of most breast cancers(7),(8); however, PARPi is an effective targeted therapy in subsets of patients harboring BRCA1/2 mutations(9). In addition to the use of PARP1 inhibitors as monotherapy, our group has shown previously that PARPi can effectively radiosensitize a large range of breast cancer cell lines, including those with functional BRCA1 and BRCA2(10). PARP1, through the addition of poly-ADP ribose (PAR) moieties to sites of single strand DNA (ssDNA) damage, plays a critical role in recognition and recruitment of DNA repair machinery for a variety of different DNA repair processes. If ssDNA lesions go unrepaired, double strand DNA (dsDNA) breaks form. For cells with intact repair pathways, non-homologous end joining (NHEJ) or homologous recombination (HR) allows the cell to repair DNA. In the case of cancers with BRCA1/2 mutations, where BRCA-mediated homologous recombination is already deficient, the use of PARP1 inhibitors alone can promote the lethal accumulation of dsDNA breaks, leading to selective death of tumor cells – a concept referred to as synthetic lethality. In cells with wild type BRCA, other deficiencies in DNA repair pathways – and the addition of PARPi – may predispose tumor cells to higher levels of DNA damage caused by therapeutic radiation(10). To that end, the present study aimed to determine the effect and efficacy of combining PARP1 inhibition and radiation in multiple preclinical models of IBC.
Materials and Methods
Cell Culture
All IBC cell lines were grown in HAMS F12 media (Gibco 11765–054) in a 5% CO2 incubator. Media for SUM-149 cells was supplemented with 5% FBS (Atlanta Biologicals), 10mM HEPES (Thermo Fisher 15630080), 1x antibiotic-antimycotic (anti-anti, Thermo Fisher 15240062), 1μg/mL hydrocortisone (Sigma H4001), and 5μg/mL insulin (Sigma I9278). SUM-190 media was supplemented with 1% FBS, 1μg/mL hydrocortisone, 5μg/mL insulin (Sigma I0516), 50nM sodium selenite (Sigma S9133), 5μg/mL apo-Transferrin (Sigma T-8158), 10nM triiodo thyronine (T3, Sigma T5516), 10mM HEPES, and 0.03% ethanolamine (Sigma 411000). MDA-IBC-3 cells were grown with 10% FBS, 1μg/mL hydrocortisone, 1x anti-anti, and 5μg/mL insulin (Sigma I0516). SUM cell lines were obtained from Stephen Ethier at the Medical University of South Carolina, and MDA-IBC-3 cells were obtained directly from Wendy Woodward at the University of Texas MD Anderson Cancer Center. All cell lines were routinely tested for mycoplasma contamination (Lonza LT07–418) and were authenticated using fragment analysis at the University of Michigan DNA sequencing core. Olaparib (MedChem Express HY-10162) and veliparib (MedChem Express HY-10129) were reconstituted in 100% DMSO for cellular assays.
Proliferation Assays
SUM-190 and SUM-149 cells were plated in 96 well plates overnight and treated the next morning with either olaparib or veliparib using a dose range of 1pM to 10μM. After 72 hours, AlamarBlue (Thermo Fisher DAL1025) was added up to 10% of the final volume and read on a microplate reader after incubation at 37⁰C for 3 hours. MDA-IBC-3 cells were plated in 6-well plates and treated with a dose range of 1nM to 10μM of either olaparib or veliparib. After 72 hours, cells were trypsinized and counted with a hemocytometer.
Clonogenic Survival Assays
SUM-149 and SUM-190 cells were plated at various densities from single cell suspension in 6-well plates and radiated the following day after a one-hour pretreatment with olaparib. Cells were grown for up to three weeks, then fixed with methanol/acetic acid and stained with 1% crystal violet. Colonies with a minimum of 50 cells were counted for each treatment condition. Plating efficiency was determined and used to calculate toxicity. Cell survival curves were calculated as described previously(10). MDA-IBC-3 cells were grown in soft agar (Thermo Fisher 214050) with a base layer of 0.5% agar solution and a top layer of 0.4% agar containing the cell suspension. Drug treatments in supernatant media were added fresh each week. Colonies were grown for up to four weeks before staining with 0.005% crystal violet.
Immunofluorescence
Cells were plated on 18 mm coverslips in 12-well plates and allowed to adhere to coverslips overnight. The following day, cells were treated with media containing either olaparib or vehicle one hour before radiation (2 Gy), and coverslips were fixed at predetermined time points after radiation. γH2AX foci were detected using anti-phospho-histone H2AX (ser139) monoclonal antibody (Millipore 05–636), with a goat anti-mouse fluorescent secondary antibody (Invitrogen A11005). At least 100 cells were scored visually for γH2AX foci in three independent experiments. Cells containing ≥ 15 γH2AX foci were scored positive and were pooled for statistical analysis.
Immunoblotting
Cells were plated overnight and pre-treated the next morning with olaparib. Plates were irradiated one hour after pretreatment, and cells were harvested at 6 and 24 hours after radiation. Lysates were extracted using RIPA buffer (Thermo Fisher 89901) containing protease and phosphatase inhibitors (Sigma-Aldrich PHOSS-RO, CO-RO). Proteins were detected using the anti-PAR antibody (LS-B12794, 1:5000), the anti-PARP1 antibody (ab6079, 1:1000), and anti-β-Actin (8H10D10, Cell Signaling 12262S, 1:50,000).
Xenograft Models
Bilateral subcutaneous flank injections were performed on 4–6 week old CB17-SCID female mice with 1 × 106 SUM-190 cells resuspended in 100μL PBS with 50% Matrigel (Thermo Fisher CB-40234). Tumors were allowed to grow until reaching approximately 80mm3. Olaparib treatment was given by intraperitoneal injection 24 hours prior to the first radiation treatment. For long term studies, mice were treated with vehicle (10% 2-hydroxylpropyl-beta-cyclodextrin in phosphate buffered saline, Thermo Fisher 10010–023), olaparib (50mg/kg) alone, radiation alone (2 Gy × 8 fractions) or the combination of olaparib + RT, with 16–20 tumors per treatment group. Tumor growth was measured three times a week using digital calipers, and mice were weighed on the same days. Tumor volume was calculated using the equation V=(L*W2)*π/6. For short term studies, mice were treated with vehicle control, olaparib, or radiation for 48 hours before the tumors were harvested. Mice treated with both olaparib and radiation received olaparib treatment 24 hours before radiation treatment. The tumors were then harvested 48 hours after radiation. Immunohistochemical staining was performed on tumors for all four conditions. All procedures involving mice were approved by the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan and conform to their relevant regulatory standards.
Irradiation
Irradiation was carried out using a Philips RT250 (Kimtron Medical) at a dose rate of approximately 2 Gy/min in the University of Michigan Experimental Irradiation Core as previously described(10). Irradiation of mouse tumors was carried out as described previously(11).
Immunohistochemistry
Immunohistochemical staining was performed on the DAKO Autostainer (Agilent, Carpinteria, CA) using Envision+ or liquid streptavidin-biotin and diaminobenzadine (DAB) as the chromogen. De-paraffinized sections were labeled with the antibodies listed in Supplemental Table 1 for 30 minutes at ambient temperature. Microwave epitope retrieval, as specified in Supplemental Table 1, was used prior to staining for all antibodies. Appropriate negative (no primary antibody) and positive controls (as listed in Supplemental Table 1) were stained in parallel with each set of slides studied. Whole-slide digital images were generated using an Aperio AT2 scanner (Leica Biosystems Imaging, Vista, CA, USA) at 20X magnification, with a resolution of 0.5 μm per pixel. The scanner uses a 20x / 0.75 NA objective and an LED light source. The same instrument and settings were used throughout the study for all whole-slide images generated. The images were checked for quality before use, and scans were repeated as necessary. Digital slides were analyzed using the Visopharm image analysis software suite (DK-2970 Hoersholm, Denmark, v2019.2) to count stained and unstained nuclei.
Comet Assay
Cells were plated in 6 well plates and allowed to adhere overnight. Cells were pretreated with olaparib for one hour before radiation and collected at designated time points after radiation. Cells were mixed with low melting point agarose (Thermo Fisher 15–455–200) and spread on CometSlides (Trevigen 4250–050–03). The cells were lysed with lysis solution (Trevigen 4250–050–01), and DNA was separated by electrophoresis. Propidium iodide (Thermo Fisher P3566) was used to stain DNA. A fluorescent microscope was used to take images of at least 50 cells/treatment. Images were analyzed using Comet Assay IV Software Version 4.3 to calculate the Olive tail moment. Results were pooled for statistical analyses.
Statistical Analyses
GraphPad Prism 7.0 was used to perform statistical tests. In vitro statistical analyses were performed using the two-tailed student’s t-test or a one-way ANOVA in the case of multiple comparisons. For in vivo studies, a two way ANOVA was used to compare tumor growth, and the fractional tumor volume (FTV) method for assessing synergy in vivo was used as previously described(12,13).
Results
Single agent PARPi does not significantly affect proliferation of IBC cell lines in vitro
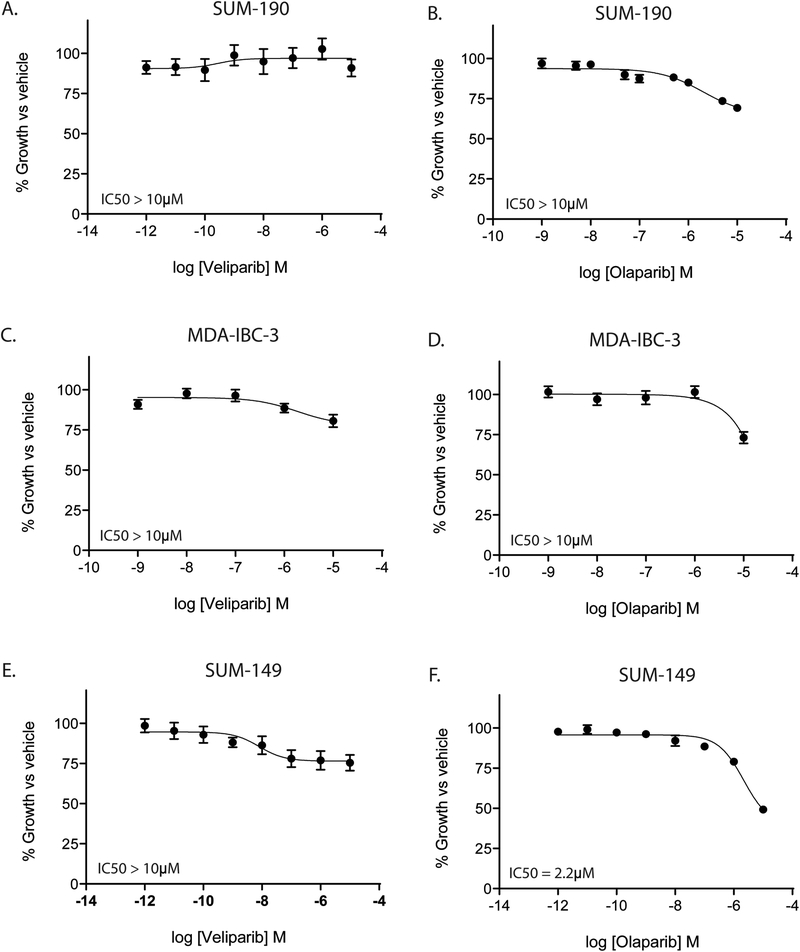
First, we sought to characterize the effect of two PARP1 inhibitors, olaparib (AZD2281) and veliparib (ABT-888) (14), on the proliferation of IBC cell lines. In SUM-190 and MDA-IBC-3 cells, single agent PARPi with olaparib or veliparb does not cause a significant decrease in proliferation at concentrations up to 10μM (Fig. 1A–D). While veliparib does not appear to impact proliferation of SUM-149 cells (IC50 > 10μM, Fig. 1E), olaparib does have a modest effect as a single agent in SUM-149 cells (IC50 = 2.2μM, Fig. 1F). All models tested were isolated from patients with IBC; however, SUM-149 cells are unique as they harbor a BRCA1 2288delT mutation as well as allelic loss of the wild type BRCA1 gene, rendering them BRCA1 deficient(15). Thus, SUM-149 cells may be especially sensitive to additional inhibition of DNA repair pathways(15).
Figure 1: PARP1 inhibition does not affect proliferation of IBC cell lines.
IBC cell lines were treated with either olaparib or veliparib and cell viability was measured 72 hours after treatment. In SUM-190 (A,B) and MDA-IBC-3 (C,D) cells, neither veliparib or olaparib showed significant effects on proliferation at doses up to 10μM. In SUM-149 cells (E,F), olaparib, but not veliparib, can inhibit proliferation at high doses (2.2μM). Graphs are shown as the average of three independent experiments ± SEM.
PARPi leads to radiosensitization of IBC cell lines in vitro
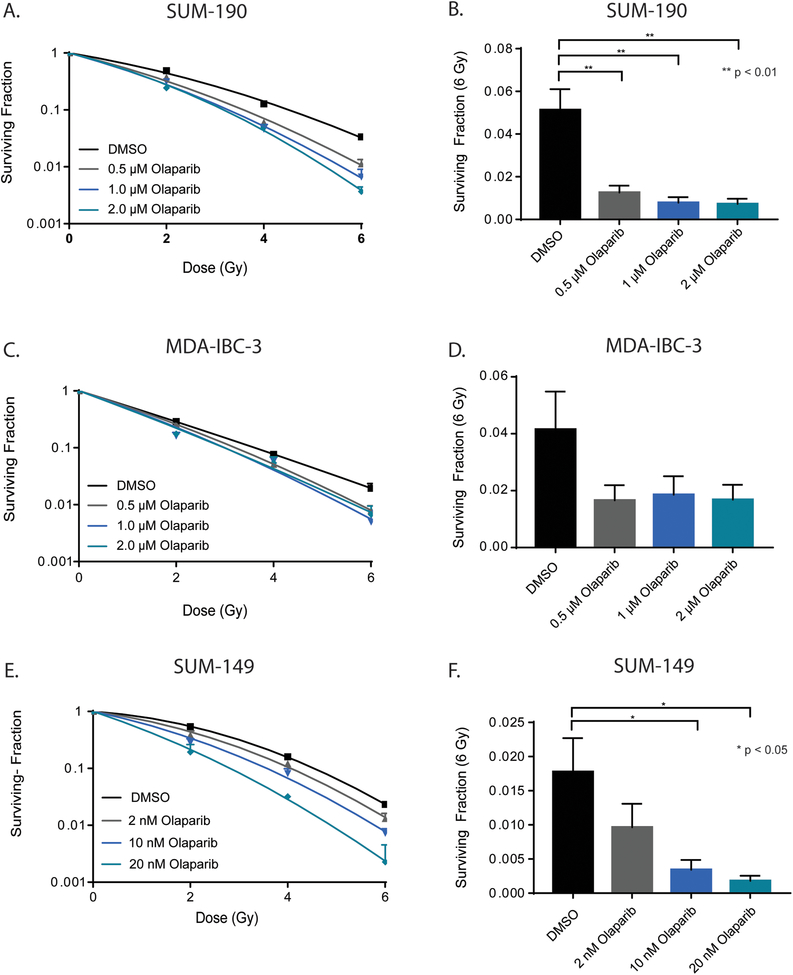
While single agent PARPi with either olaparib or veliparib did not inhibit cell proliferation, we sought to determine the effect of PARP1 inhibition on the radiosensitivity of IBC cell lines. Clonogenic survival assays were performed with olaparib in each of the three IBC cell lines, as olaparib is a more potent PARP1 inhibitor compared to veliparib, with both PARP1 enzymatic inhibition efficacy and PARP trapping function. All IBC cell lines displayed significant radiosensitization as a result of pretreatment with olaparib. In SUM-190 cells, a dose-dependent radiosensitization was observed, with average radiation enhancement ratios (rER) of 1.45 ± 0.03 and 1.64 ± 0.21 at concentrations of 1μM and 2μM olaparib, respectively (Fig. 2A, Supplemental Fig. 1A). A similar trend was observed in MDA-IBC-3 cells, with enhancement ratios of 1.12 ± 0.08 and 1.28 ± 0.06 under the same treatment conditions (Fig. 2C, Supplemental Fig. 1B). Because SUM-149 cells express a truncated form of the BRCA1 protein, treatment with olaparib leads to marked radiosensitization at much lower doses. At 10nM and 20nM, the average enhancement ratios for SUM-149 cells were approximately 1.42 ± 0.01 and 1.76 ± 0.11 (Fig. 2E, Supplemental Fig. 1C). The enhancement ratios observed here are similar to or greater than that of cisplatin (rER=1.2–1.3), a compound well-characterized for its ability to act as a radiosensitizing agent(16),(17). Furthermore, the surviving fraction of cells at 6 Gy (Fig. 2B, 2D, 2F) was significantly lower across all three inflammatory cell lines with the addition of olaparib. The radiation enhancement ratios demonstrated a marked dose-dependent increase, while toxicity from each treatment was minimal (Supplemental Fig. 1).
Figure 2: Clonogenic survival of IBC cell lines decreases with olaparib treatment.
Olaparib treatment results in a dose-dependent reduction in survival fraction of SUM-190 (A), MDA-IBC-3 (C), and SUM-149 (E) cell lines. Representative data from single experiments are shown for each cell line. The surviving fraction of cells after 6 Gy (B, D, F) was calculated as the mean of three independent experiments and depicted ± SEM for each cell line. (p < 0.05 = *, p < 0.01 = **)
PARP1 inhibition and radiation leads to delayed repair of DNA double strand breaks compared to radiation alone
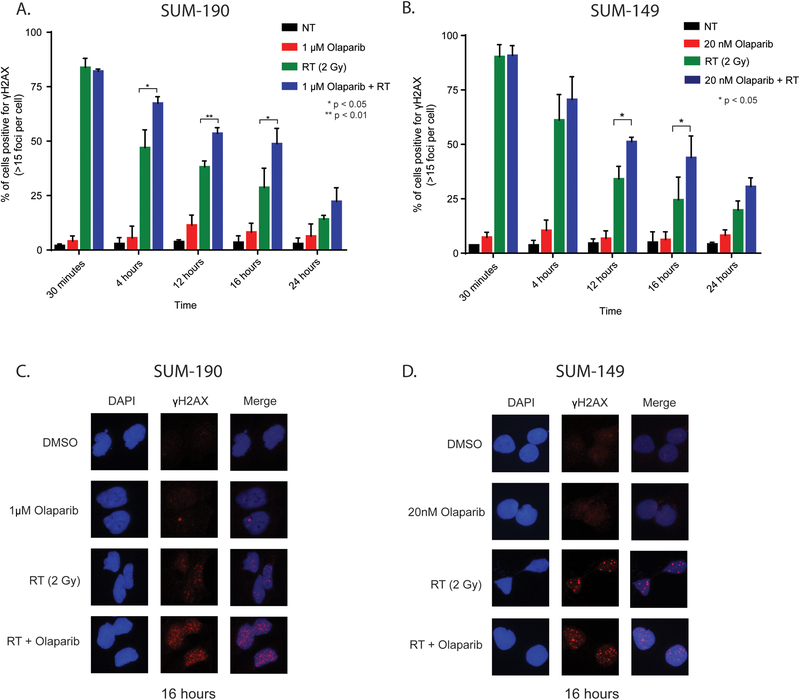
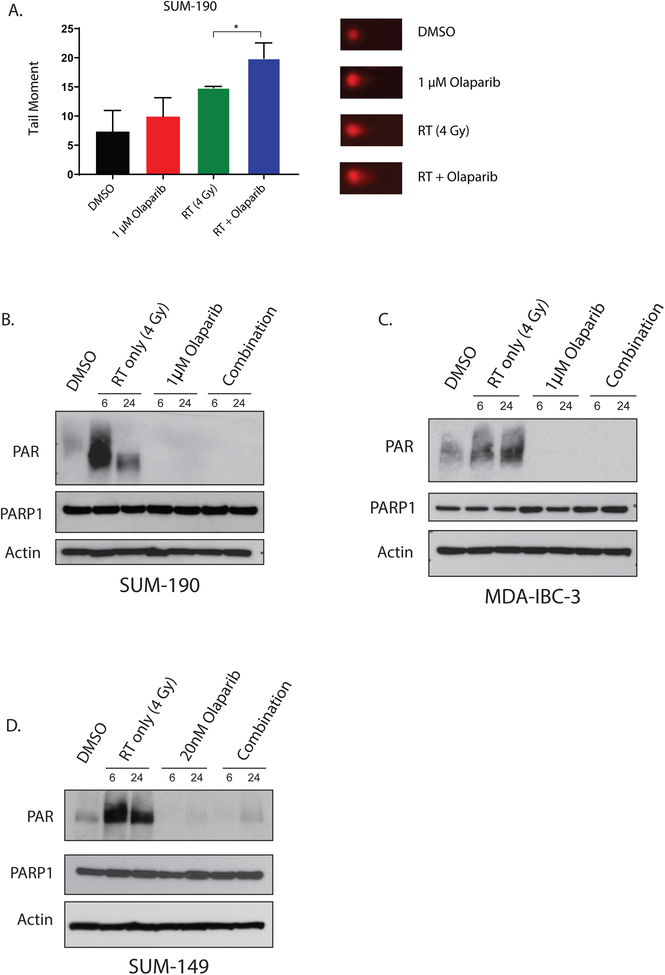
In cancer cells, ionizing radiation induces both single strand and double strand DNA breaks. In situations where DNA repair is inhibited and single strand breaks go unrepaired, the collapse of replication forks can propagate chromosomal damage and lead to the accumulation of lethal dsDNA breaks. Because PARP1 is involved in the recruitment of DNA repair proteins to DNA strand breaks, we sought to understand the effect of PARP1 inhibition and radiation on the accumulation of DNA damage in IBC cell lines. In SUM-190 and SUM-149 cells, radiation treatment alone (2 Gy) induces γH2AX foci in greater than 75% of cells (Fig. 3A,B). In both cell lines, dsDNA breaks are retained at significantly higher levels at 12 and 16 hours after treatment with olaparib and radiation compared to treatment with radiation alone (Fig. 3C,D). Furthermore, a similar difference in dsDNA breaks was observed between RT alone and combination treatment in SUM-190 cells at 4 hours after radiation. In short, the presence of olaparib leads to the accumulation and persistence of dsDNA breaks in the combination treatment compared to the radiation treatment alone in both SUM-190 and SUM-149 cells. In order to independently confirm these findings, we performed the neutral comet assay to assess for dsDNA breaks (Fig. 4A). In SUM-190 cells, the combination of PARPi and RT in SUM-190 cells lead to a significantly longer tail moment, indicating increased dsDNA breaks compared to treatment with RT alone (p = 0.029). The tail moment was also significantly higher compared to cells treated with vehicle or olaparib as a single agent (Fig. 4A). Representative images for each treatment condition are shown (Fig. 4A).
Figure 3: Radiation in combination with the PARP1 inhibition leads to persistence of DNA damage in IBC cell lines.
Immunofluorescence microscopy was used to measure γH2AX foci in SUM-190 (A) and SUM-149 (B) cells. Cells were pretreated for one hour with olaparib and fixed at 0.5, 4, 12, 16, and 24 hours after radiation, then stained for DAPI and γH2AX. Cells containing ≥ 15 foci were scored as positive. In SUM-190 cells at 4, 12, and 16 hours, there were significantly higher levels of cells positive for γH2AX for those treated with the combination of 2 Gy radiation and 1μM olaparib compared to cells treated with 2 Gy radiation alone. In SUM-149 cells, 20nM olaparib and 2 Gy radiation results in a higher percentage of γH2AX positive cells compared to cells treated with radiation alone at both 12 and 16 hours. Representative images of γH2AX foci in SUM-190 (C) and SUM-149 (D) cells at 16 hours are shown for all treatment groups. Graphs represent the average of three independent experiments ± SD. (p < 0.05 = *)
Figure 4: PARP1 inhibition increases dsDNA breaks and significantly decreases PAR formation in IBC cell lines.
Neutral comet assay in SUM-190 cells (A) shows higher levels of dsDNA damage at 4 hours in cells treated with radiation and olaparib compared to untreated cells, or cells treated with RT or olaparib alone (p < 0.05 = *). Graphs represent the average of three independent experiments ± SD and representative images for each treatment are shown. In SUM-190 (B) and MDA-IBC-3 (C) cells, radiation induced DNA damage causes an increase in PAR formation at both 6 and 24 hours after 4 Gy radiation. In the combination group that receives a one-hour pretreatment of 1μM olaparib before radiation, PAR formation is significantly lower at 6 and 24 hours after RT. In SUM-149 (D) cells, this same trend can be observed at a much lower dose of olaparib (20nM). Though the enzymatic activity of PARP1 is efficiently inhibited at these doses, total levels of PARP are not significantly different across the treatment conditions.
Olaparib effectively inhibits PAR formation in IBC cell lines
In order to determine if inhibition of PARP1 enzymatic activity occurs at concentrations of olaparib that are sufficient to induce radiosensitization, we treated cells with olaparib ± 4 Gy radiation and measured the total PAR and PARP1 levels in IBC cell lines. In SUM-190 and MDA-IBC-3 cells, PAR formation is significantly inhibited with 1μM of olaparib (Fig. 4B,C). The same effect is seen in SUM-149 cells after treatment with 1μM olaparib (Supplemental Fig. 2). Inhibition of PAR formation, however, can also be achieved at the same level in SUM-149 cells with 20nM of olaparib (Fig. 4D). Therefore, inhibition of PAR formation with olaparib occurs at low concentrations (20nM) that are sufficient to confer radiosensitization in SUM-149 cells. Though olaparib effectively inhibits PARylation at these concentrations, the amount of PARP1 in the cell lines remains relatively constant in all models (Fig. 4B–D).
PARP1 inhibition significantly inhibits growth of SUM-190 xenografts in vivo
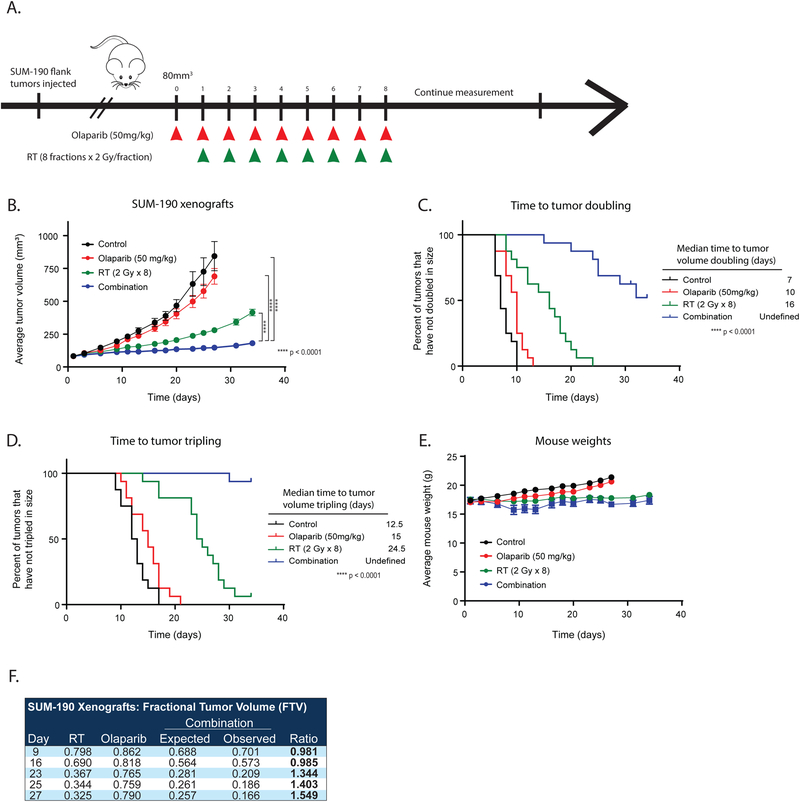
Having demonstrated that PARP1 inhibition can effectively radiosensitize IBC cell lines in vitro, we next sought to validate these findings in an in vivo xenograft model. For in vivo studies, subcutaneous tumors were allowed to reach ~80 mm3 in CB-17 SCID mice whereupon treatment was initiated with one of the following: vehicle, 50 mg/kg olaparib alone daily, radiation alone (8 fractions of 2 Gy), or the combination (olaparib 50 mg/kg + 2 Gy RT daily for 8 fractions) (Fig. 5A). To truly assess the radiosensitizing effects of PARP1 inhibition, olaparib treatment was started one day before initiation of radiation and discontinued after the last fraction of radiation. Consistent with the in vitro proliferation assays, treatment with olaparib alone did not significantly delay tumor growth or doubling time of xenograft tumors. As expected, radiation alone did lead to a decrease in tumor size initially, but tumors continued to grow after the completion of fractionated radiation (Fig. 5B). Mice receiving both radiation and olaparib treatment had significantly smaller tumors after completion of the study compared to those receiving radiation alone (p < 0.0001). There was a significant delay in the time to tumor doubling (p < 0.0001, Fig. 5C) and tripling (p < 0.0001, Fig. 5D) in the animals treated with combination olaparib and RT. In addition, time to tumor doubling and tripling was not reached in the combination treated group after 35 days. Weights of the mice (Fig. 5E) remained relatively constant throughout the experiment, indicating there was limited toxicity observed with combination treatment. Interestingly, the effects of the combination treatment with olaparib and radiation were found to be synergistic using the fractional tumor volume (FTV) method as previously described (Fig. 5F)(12). Immunohistochemistry studies in tumors harvested from the mice at the end of the experiment demonstrated that levels of Ki67, a marker of cell proliferation, were significantly decreased in all treatment groups compared to control mice, with the most significant decrease in the combination treated animals (p = 0.0004, Supplemental Fig. 3A,B). There was also a decrease in p16 staining levels in the mice treated with radiation alone (p = 0.0072) and the combination treated group (p = 0.0385) in the long-term experiments (Supplemental Fig. 3C,D), suggesting a decrease in cellular senescence in these tumors(18). The on-target effects of olaparib were confirmed in the short-term studies (48 hours of PARPi treatment alone or 24 hours of PARPi pretreatment before radiation). As expected, total levels of PARP1 were unaffected by treatment with olaparib, radiation, or the combination treatment (Supplemental Fig. 4A,B), while PAR levels were significantly lower in the PARPi treated animals (Supplemental Fig. 4C,D).
Figure 5: PARP1 inhibition with radiation is more effective than radiation alone in a SUM-190 xenograft model.
SUM-190 cells were subcutaneously injected into CB17-SCID mice, and treatment was started when tumors reached approximately 80 mm3 (A). Olaparib treatment began one day before the initiation of radiation treatment and ended on the same day as the last fraction of radiation. With this paradigm, the combination treatment leads to delayed growth of tumors (B) and an increased time to tumor doubling (C) and tumor tripling (D) (p < 0.0001 = ****). The treatment did not display significant toxicities, and animal weights were not significantly different between the treatment groups (E). Using the FTV method, there was a synergistic effect with olaparib and RT treatment to antagonize tumor growth (ratios >1 indicate synergism) (F). A two-way ANOVA was performed to compare tumor volume between experimental groups.
Discussion
In this study, we demonstrate that PARP1 inhibition alone is insufficient in delaying IBC cell line growth and proliferation (Fig. 1). Combination treatment with PARP1 inhibition and ionizing radiation, however, results in significant radiosensitization of IBC models in vitro (Fig. 2), and the combination treatment results in delayed tumor growth in vivo (Fig. 5). Additionally, we demonstrate that PARP1 inhibition in combination with radiation significantly delays resolution of dsDNA breaks using in vitro models of IBC (Fig. 3, Fig. 4). Taken together, these results suggest that PARP1 inhibition with radiation therapy may be a promising strategy for the treatment of inflammatory breast cancer. Although these studies suggest that PARP1 inhibition may be an effective radiosensitization strategy for the treatment of IBC, other potential targets for treatment have also been identified. Several groups have identified molecular alterations in IBC tumors and in vitro models that may help to describe the aggressive phenotype associated with IBC(1). Owing to the inflammatory nature of these cancers, the use of lipid lowering agents like statins has been met with some success(19,20). Preclinical data using statins in IBC show statin treatment can lead to increased apoptosis and radiosensitivity, inhibition of proliferation and invasion, and decreased metastatic dissemination of tumors(21). In a population-based cohort study in patients with IBC, statin use was associated with improved progression-free survival in IBC patients(21). Recent studies have sought to better define this inflammatory microenvironment, and many have noted that macrophages may be important in mediating the radiosensitivity and metastatic potential of IBC tumors(22–25). Immune regulating agents have also been implicated in the aggressiveness of IBC. In addition to the role that cytokines like INFα and TNFα may play in pathogenesis(26), many studies have reported that PD-L1 is consistently overexpressed in IBC tumors(27,28). Upregulation of downstream signaling proteins like mTOR and JAK2/STAT3 have also been observed(28,29). The role of RhoC in IBC has also been reported, but recent evidence suggests that downstream signaling may lead to unique metabolic regulation(30) and changes in lipid raft formation(31). Transcriptional reprogramming of IBC cells is also common, including C/EBPδ-mediated upregulation of VEGF-A(32) and upregulation of the redox-sensitive transcription factor NFκB and the E3 ubiquitin ligase XIAP(33–36). These promising studies suggest that more effective treatment strategies are on the horizon. Although we have demonstrated the radiosensitizing effects of olaparib in our models, these studies highlight the challenges of studying IBC. This study uses most of the available preclinical models of IBC but also highlights that there are a limited number of available models in which to study IBC. Thus, the need for additional model systems is critical to gaining a better understanding of the heterogeneity and pathogenesis of inflammatory breast cancers. While our studies were conducted in IBC cell lines, an important future direction of this work will involve the use of patient-derived xenograft (PDX) models of IBC. In addition, this study primarily utilized the more potent PARPi olaparib, though our previous studies also evaluated the efficacy of radiosensitization using veliparib(10). Olaparib may be more potent given its dual functionality as a PARP enzymatic inhibitor and PARP trapper, whereas veliparib only has functions as an enzymatic inhibitor of PARP1 at the doses used for these studies(37). Although more potent, toxicity in clinical trials to date does not appear worse with olaparib and clinical data suggests that olaparib is well tolerated in vivo(8). The dual functionality of some PARP inhibitors (like olaparib) to both inhibit enzymatic activity of the PARP1 protein as well as induce PARP trapping has been well documented(37–40). Recent literature also suggests that PARPi may cause an increase in replication fork acceleration, resulting in replicative stress that ultimately leads to cell death(41). Though the study reported here does not directly address the relative contributions of enzymatic PARP1 inhibition verses PARP trapping on radiosensitization, studies are underway to determine how these functions may differentially contribute to the compounds’ radiosensitizing effects. In addition to olaparib, PARP inhibitors such as talazoparib and rucaparib are used to treat other types of breast cancer(42,43). These inhibitors may also be valuable in the treatment of IBC in combination with radiation and are currently being investigated.
While we have shown that PARP1 inhibition can be used for the radiosensitization of inflammatory breast cancer, olaparib and other PARP1 inhibitors are currently being investigated as radiosensitization agents for the treatment of triple negative breast cancer (RadioPARP/), head and neck cancer(44), pancreatic cancer(45), prostate cancer(46), and ovarian cancer(47). More recent trials are testing whether PARP1 inhibition is effective in combination with radiation in squamous cell carcinoma(48) (), locally advanced rectal cancer ( and), high grade gliomas (), non-small cell lung cancer ( and ), and soft tissue sarcoma(49) (). Thus, while this study is the first to report that PARP inhibition may be an effective strategy in patients with IBC, the concept of PARP inhibitor-mediated radiosensitization is being explored in many other cancer contexts. IBC is a subset of breast cancer with limited treatment options and the lowest 5-year survival rates of any breast cancer type(1). Despite the limitations of the model systems, these data have provided the preclinical rationale for further clinical investigation. In a phase I trial, our group previously demonstrated that PARP1 inhibition in combination with radiation may be a safe and effective strategy for women with IBC (and in women with locoregionally recurrent breast cancer)(50). To that end, a randomized phase II trial (SWOG 1706, ) comparing the effects of olaparib and radiation therapy to radiation therapy alone in patients with IBC is now underway. Patients in the combination arm begin treatment with olaparib one day prior to the initiation of radiation therapy, and olaparib is administered until the final day of radiation treatment. Invasive disease-free survival of women receiving treatment with olaparib and radiation will be compared to that of the group receiving radiation alone. Secondary endpoints, like local disease control, distant relapse-free survival and overall survival will also be assessed. In addition, correlative studies from this trial will be used to see if biomarkers of treatment response and efficacy can be identified. These correlative studies will also define the genomic and transcriptomic landscape of IBC in a large patient population and will assess how circulating tumor DNA (ctDNA) levels are affected by combination and single agent treatment. Though it is evident from our study that PARP1 inhibition with olaparib leads to radiosensitization of IBC cell lines, further studies are needed to determine the exact mechanism of olaparib-induced radiosensitization in IBC. Future transcriptomic and proteomic analysis of current model systems across multiple platforms may provide some insight as to the mechanism of this radiosensitization, and such studies are currently underway. Finally, correlative studies from SWOG 1706 will help inform future mechanistic studies and will provide a platform in which to evaluate potential predictive or prognostic biomarkers that may be able to help more effectively guide selection of IBC patients for this approach to treatment intensification.
Supplementary Material
Acknowledgments:
The Breast Cancer Research Foundation (N026000 to L. Pierce), The Komen for the Cure Foundation (N053349 to R. Jagsi), the University of Michigan Rogel Cancer Center (P30CA046592 to C. Speers). A. Michmerhuizen, A. Pesch and B. Chandler are all supported by training grants through the National Institute of Health. A. Michmerhuizen is supported by T32-GM007315 (NIGMS), A. Pesch is supported by T32-GM007767 (NIGMS), and B. Chandler is supported by T32-CA140044 (NCI). Wendy Woodward at MD Anderson Cancer Center for providing MDA-IBC-3 cells, and Stephen Ethier at the Medical University of South Carolina for providing SUM-149 and SUM-190 cells.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no relevant conflicts of interest.
References
- 1.Menta A, Fouad TM, Lucci A, Le-Petross H, Stauder MC, Woodward WA, et al. Inflammatory Breast Cancer: What to Know About This Unique, Aggressive Breast Cancer. Surg Clin North Am 2018;98:787–800. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Xia Y, Wu Q, Zhu S, Chen C, Yang W, et al. Outcomes of patients with inflammatory breast cancer by hormone receptor-and HER2-defined molecular subtypes: A population-based study from the SEER program. Oncotarget. 2017;8:49370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross JS, Ali SM, Wang K, Khaira D, Palma NA, Chmielecki J, et al. Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. Breast Cancer Res Treat 2015;154:155–62. [DOI] [PubMed] [Google Scholar]
- 4.Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 2006;24:769–77. [DOI] [PubMed] [Google Scholar]
- 5.Yang SX, Steinberg SM, Nguyen D, Wu TD, Modrusan Z, Swain SM. Gene expression profile and angiogenic marker correlates with response to neoadjuvant bevacizumab followed by bevacizumab plus chemotherapy in breast cancer. Clin Cancer Res 2008;14:5893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgier C, Pessoa EL, Dunant A, Heymann S, Spielmann M, Uzan C, et al. Exclusive alternating chemotherapy and radiotherapy in nonmetastatic inflammatory breast cancer: 20 years of follow-up. Int J Radiat Oncol Biol Phys 2012;82:690–5. [DOI] [PubMed] [Google Scholar]
- 7.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852–61. [DOI] [PubMed] [Google Scholar]
- 8.Dent RA, Lindeman GJ, Clemons M, Wildiers H, Chan A, McCarthy NJ, et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res 2013;15;R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comen EA, Robson M. Poly(ADP-Ribose) Polymerase Inhibitors in Triple-Negative Breast Cancer. Cancer J 2010;16:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng FY, Speers C, Liu M, Jackson WC, Moon D, Rinkinen J, et al. Targeted radiosensitization with PARP1 inhibition: optimization of therapy and identification of biomarkers of response in breast cancer. Breast Cancer Res Treat 2014;147:81–94. [DOI] [PubMed] [Google Scholar]
- 11.Speers C, Zhao SG, Kothari V, Santola A, Liu M, Wilder-Romans K, et al. Maternal Embryonic Leucine Zipper Kinase (MELK) as a Novel Mediator and Biomarker of Radioresistance in Human Breast Cancer. Clin Cancer Res 2016;22:5864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res 2004;10:6487–501. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res 2000;60:2190–6. [PubMed] [Google Scholar]
- 14.Donawho CK, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007;13:2728–37. [DOI] [PubMed] [Google Scholar]
- 15.Elstrodt F, Hollestelle A, Nagel JH, Gorin M, Wasielewski M, van den Ouweland A, et al. BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res 2006;66:41–5. [DOI] [PubMed] [Google Scholar]
- 16.Skov K, Macphail S. Interaction of platinum drugs with clinically relevant x-ray doses in mammalian cells: a comparison of cisplatin, carboplatin, iproplatin, and tetraplatin. Int J Radiat Oncol 1991;20:221–5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Yang H, Gu K, Chen J, Rui M, Jiang G-L. In vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizer. Int J Nanomedicine 2011;6:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Althubiti M, Lezina L, Carrera S, Jukes-Jones R, Giblett SM, Antonov A, et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis 2014;5:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe AR, Debeb BG, Lacerda L, Larson R, Bambhroliya A, Huang X, et al. Simvastatin prevents triple-negative breast cancer metastasis in pre-clinical models through regulation of FOXO3a. Breast Cancer Res Treat 2015;154:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Wyhe RD, Rahal OM, Woodward WA. Effect of statins on breast cancer recurrence and mortality: a review. Breast Cancer 2017;9:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewer TM, Masuda H, Liu DD, Shen Y, Liu P, Iwamoto T, et al. Statin use in primary inflammatory breast cancer: a cohort study. Brit J Cancer 2013;109:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy JP, Atkinson RL, Larson R, Burks JK, Smith D, Debeb BG, et al. Mammary stem cell and macrophage markers are enriched in normal tissue adjacent to inflammatory breast cancer. Breast Cancer Res Treat 2018;171:283–93. [DOI] [PubMed] [Google Scholar]
- 23.Allen SG, Chen Y-C, Madden JM, Fournier CL, Altemus MA, Hiziroglu AB, et al. Macrophages enhance migration in inflammatory breast cancer cells via RhoC GTPase signaling. Sci Rep 2016;6:39190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking Interleukin (IL)4- and IL13-Mediated Phosphorylation of STAT6 (Tyr641) Decreases M2 Polarization of Macrophages and Protects Against Macrophage-Mediated Radioresistance of Inflammatory Breast Cancer. Int J Radiat Oncol Biol Phys 2018;100:1034–43. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe AR, Trenton NJ, Debeb BG, Larson R, Ruffell B, Chu K, et al. Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models. Oncotarget 2016;7:82482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertucci F, Finetti P, Vermeulen P, Van Dam P, Dirix L, Birnbaum D, et al. Genomic Profiling of Inflammatory Breast Cancer: A Review. Breast 2014;23:538–45. [DOI] [PubMed] [Google Scholar]
- 27.Bertucci F, Finetti P, Colpaert C, Mamessier E, Parizel M, Dirix L, et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015;6:13506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamm CA, Moran D, Rao K, Trusk PB, Pry K, Sausen M, et al. Genomic and Immunological Tumor Profiling Identifies Targetable Pathways and Extensive CD8+/PDL1+ Immune Infiltration in Inflammatory Breast Cancer Tumors. Mol Cancer Ther 2016;15:1746–56. [DOI] [PubMed] [Google Scholar]
- 29.Jhaveri K, Teplinsky E, Silvera D, Valeta-Magara A, Arju R, Giashuddin S, et al. Hyperactivated mTOR and JAK2/STAT3 pathways: molecular drivers and potential therapeutic targets of inflammatory and invasive ductal breast cancers after neoadjuvant chemotherapy. Clin Breast Cancer 2016;16:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynn ML, Yates JA, Evans CR, Van Wassenhove LD, Wu ZF, Bridges S, et al. RhoC GTPase is a potent regulator of glutamine metabolism and N-acetylaspartate production in inflammatory breast cancer cells. J Biol Chem 2016;291:13715–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joglekar M, Elbezanti WO, Weitzman MD, Lehman HL, van Golen KL. Caveolin-1 mediates inflammatory breast cancer cell invasion via the Akt1 pathway and RhoC GTPase. J Cell Biochem 2015;116:923–33. [DOI] [PubMed] [Google Scholar]
- 32.Balamurugan K, Sterneck E. The Many Faces of C/EBPδ and their Relevance for Inflammation and Cancer. Int J Biol Sci. 2013;9:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allensworth JL, Evans MK, Bertucci F, Aldrich AJ, Festa RA, Finetti P, et al. Disulfiram (DSF) acts as a copper ionophore to induce copper‐dependent oxidative stress and mediate anti‐tumor efficacy in inflammatory breast cancer. Mol Oncol. 2015;9:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora J, Sauer SJ, Tarpley M, Vermeulen P, Rypens C, Van Laere S, Williams KP, Devi GR, Dewhirst MW. Inflammatory breast cancer tumor emboli express high levels of anti-apoptotic proteins: use of a quantitative high content and high-throughput 3D IBC spheroid assay to identify targeting strategies. Oncotarget. 2017; 8:25848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, et al. NF-kappa B genes have a major role in Inflammatory Breast Cancer. BMC Cancer 2008;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Laere SJ, Van der Auwera I, Van den Eynden GG, van Dam P, Van Marck EA, Vermeulen PB, et al. NF-κB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Brit J Cancer 2007;97:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murai J, Huang Sy N, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Differential trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012;72:5588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, et al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin- 1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem 2008;51:6581–91. [DOI] [PubMed] [Google Scholar]
- 39.Parsels LA, Karnak D, Parsels JD, Zhang Q, Velez-Padilla J, Reichert ZR, et al. PARP1 Trapping and DNA Replication Stress Enhance Radiosensitization with Combined WEE1 and PARP Inhibitors. Mol Cancer Res 2018;16:222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murai J, Zhang Y, Morris J, Ji J, Takeda S, Doroshow JH, et al. Rationale for Poly(ADP-ribose) Polymerase (PARP) Inhibitors in Combination Therapy with Camptothecins or Temozolomide Based on PARP Trapping versus Catalytic Inhibition. J Pharmacol Exp Ther 2014;349:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maya-Mendoza A, Moudry P, Merchut-Maya JM, Lee M, Strauss R, Bartek J. High speed of fork progression induces DNA replication stress and genomic instability. Nature 2018;559:279–84. [DOI] [PubMed] [Google Scholar]
- 42.Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Brit J Cancer 2016;114:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karam SD, Reddy K, Blatchford PJ, Waxweiler T, DeLouize AM, Oweida A, et al. Final Report of a Phase I Trial of Olaparib with Cetuximab and Radiation for Heavy Smoker Patients with Locally Advanced Head and Neck Cancer. Clin Cancer Res 2018;24:4949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vance S, Liu E, Zhao L, Parsels JD, Parsels LA, Brown JL, et al. Selective radiosensitization of p53 mutant pancreatic cancer cells by combined inhibition of Chk1 and PARP1. Cell Cycle 2011;10:4321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han S, Brenner JC, Sabolch A, Jackson W, Speers C, Wilder-Romans K, et al. Targeted radiosensitization of ETS fusion-positive prostate cancer through PARP1 inhibition. Neoplasia 2013;15:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi Y, Verginadis II, Dey S, Lin L, Guo L, Zheng Y, et al. Radiosensitization by the PARP inhibitor olaparib in BRCA1-proficient and deficient high-grade serous ovarian carcinomas. Gynecol Oncol 2018;150:534–44. [DOI] [PubMed] [Google Scholar]
- 48.Wurster S, Hennes F, Parplys AC, Seelbach JI, Mansour WY, Zielinski A, et al. PARP1 inhibition radiosensitizes HNSCC cells deficient in homologous recombination by disabling the DNA replication fork elongation response. Oncotarget 2016;7:9732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangoni M, Sottili M, Salvatore G, Meattini I, Desideri I, Greto D, et al. Enhancement of Soft Tissue Sarcoma Cell Radiosensitivity by Poly(ADP-ribose) Polymerase-1 Inhibitors . Radiat Res 2018;190:464–72. [DOI] [PubMed] [Google Scholar]
- 50.Jagsi R, Griffith K, Bellon J, Woodward W, Horton J, Ho A, et al. TBCRC 024 initial results: A multicenter phase 1 study of veliparib administered concurrently with chest wall and nodal radiation therapy in patients with inflammatory or locoregionally recurrent breast cancer. Int J Radiat Onc 2015;93:S137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.