Abstract
Epidemiologic studies link organophosphorus pesticides (OPs) to increased incidence of asthma. In guinea pigs, OP-induced airway hyperreactivity requires macrophages and TNF-α. Here, we determined whether OPs interact directly with macrophages to alter cytokine expression or release. Human THP1 cells were differentiated into macrophages and then exposed to parathion, chlorpyrifos, or diazinon, or their oxon, phosphate, or phosphorothioate metabolites for 24 hours in the absence or presence of reagents that block cholinergic receptors. TNF-α, IL-1β, platelet-derived growth factor, and transforming growth factor-β mRNA and protein were quantified by qPCR and ELISA, respectively. The effects of OPs on NF-κB, acetylcholinesterase, and intracellular calcium were also measured. Parent OPs and their oxon metabolites upregulated cytokine mRNA and stimulated cytokine release. TNF-α release, which was the most robust response, was triggered by parent, but not oxon, compounds. Cytokine expression was also increased by diethyl dithiophosphate but not diethyl thiophosphate or diethyl phosphate metabolites. Parent OPs, but not oxon metabolites, activated NF-κB. Parent and oxon metabolites decreased acetylcholinesterase activity, but comparable acetylcholinesterase inhibition by eserine did not mimic OP effects on cytokines. Consistent with the noncholinergic mechanisms of OP effects on macrophages, pharmacologic antagonism of muscarinic or nicotinic receptors did not prevent OP-induced cytokine expression or release. These data indicate that phosphorothioate OP compounds directly stimulate macrophages to release TNF-α, potentially via activation of NF-κB, and suggest that therapies that target NF-κB may prevent OP-induced airway hyperreactivity.
Keywords: chlorpyrifos, diazinon, macrophages, NF-κB, parathion
Organophosphorus pesticides (OPs) are extensively used to control insects in not only agricultural but also suburban and urban settings, and thus human exposure is widespread (1). Acute OP toxicity is mediated by inhibition of acetylcholinesterase (AChE), resulting in an overstimulation of nicotinic and muscarinic receptors that triggers a cholinergic crisis associated with peripheral and central respiratory paralysis. However, most human exposures involve considerably lower OP concentrations that do not cause a cholinergic crisis. Exposure occurs via inhalation, absorption through the skin and eyes, or ingestion (1). For example, in agricultural areas, OPs are tracked into homes, and concentrations in house dust have been shown to correlate with OP metabolites in the urine of children in those homes (2). Ingestion of OP-contaminated foods is another important source of exposure, as indicated by a study in Seattle in which OP metabolites were detected in the urine of 99% of the children examined in the study, but were no longer detectable when the children’s diets were switched to organic foods (3). In young children, urinary concentrations of OP metabolites were found to correlate with a significant decrease in pulmonary function and increase in other symptoms consistent with asthma (4). In adults, occupational exposure to OPs is associated with wheeze, respiratory dysfunction, and asthma (5), which can persist even after the exposure. Thus, there is widespread human exposure to OPs, and multiple studies have identified an association between OP exposure and chronic respiratory symptoms, including asthma.
In the lung, parasympathetic postganglionic nerves release acetylcholine to activate M3 muscarinic receptors on airway smooth muscle to cause bronchoconstriction. Acetylcholine also activates M2 muscarinic receptors on prejunctional parasympathetic nerves to inhibit further release of acetylcholine, thus limiting bronchoconstriction (6). Loss of neuronal M2 receptor function increases acetylcholine release, potentiating vagally induced bronchoconstriction, and this is associated with asthma (7). We have shown that chlorpyrifos, diazinon, and parathion each potentiate bronchoconstriction by inhibiting neuronal M2 receptor function (8–10) independently of AChE inhibition (8, 9). These observations suggest that environmental concentrations of OPs that do not cause significant AChE inhibition may, nonetheless, be sufficient to trigger airway hyperreactivity.
OP-induced loss of M2 function and potentiation of bronchoconstriction occur independently of direct effects of OPs on muscarinic receptors in airway nerves (11), suggesting that OPs alter neuronal M2 function downstream of direct effects on nonneuronal cells in the airways. There is increasing recognition that OPs are immunomodulators (5). For example, subacute doses of chlorpyrifos and diazinon increase TNF-α and IL-6 production in macrophages derived from multiple sources, including lungs (12). Macrophages release cytokines and growth factors that are known to modulate M2 muscarinic receptor function and/or expression (13–15). In a previous study, we demonstrated that inhibition of macrophages with clodronate or inhibition of TNF-α with etanercept each independently protected neuronal M2 receptors and prevented airway hyperreactivity in guinea pigs after exposure to parathion (10). In the same study, we showed that when guinea pig alveolar macrophages were isolated from parathion-treated animals, TNF-α and IL-1β mRNA expression was significantly increased, and when alveolar macrophages were isolated from naive guinea pigs and treated with parathion, IL-1β mRNA expression and TNF-α protein release were increased. These previously published data suggest a model in which OPs stimulate macrophages to increase cytokine expression, which consequently inhibits neuronal M2 receptor activity to cause airway hyperreactivity. Here, we further tested this hypothesis by determining whether these OPs and their metabolites directly stimulate macrophages to release cytokines and growth factors known to modulate M2 muscarinic receptor expression and/or function. We also investigated the mechanism(s) by which this may occur. Some of these results have been previously reported in the form of abstracts (16–19).
Methods
Materials
Parathion, chlorpyrifos, diazinon, paraoxon, chlorpyrifos oxon, and diazoxon were purchased from Chem Service. Diethyl phosphate (DEP) was obtained from Acros Organics. O,O-diethyl thiophosphate potassium salt (DETP), O,O-diethyl dithiophosphate (DEDTP), atropine, mecamylamine, eserine, and DMSO were obtained from Sigma-Aldrich.
THP1 Cells
THP1 cells (ATCC) were cultured in RPMI-1640 (Gibco) containing 100 I.U. penicillin and 100 μg/ml streptomycin, 10% FBS (Hyclone; GE Healthcare Life Sciences), and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich). THP1 cells were differentiated into macrophage-like cells using 25 ng/ml PMA (Sigma-Aldrich) for 48 hours (20).
qPCR
THP1 RNA was reverse transcribed with SuperScript III (Invitrogen). cDNA was amplified using QuantiTect SYBR Green (Qiagen) on a Veriti 96-well Thermal Cycler (Applied Biosystems). Specific primers were synthesized (Integrated DNA Technologies; Table 1), and PCR products were quantified on a 7500 Fast Real-Time PCR System (Applied Biosystems). The relative concentration of mRNA was calculated using a serially diluted sample (21) and normalized to 18S ribosomal RNA (rRNA).
Table 1.
Primers
| 18S rRNA | 5′ | GTAACCCGTTGAACCCCATT |
| 3′ | CCATCCAATCGGTAGTAGCG | |
| Human TNF-α | 5′ | TCAGCCTCTTCTCCTTCCTG |
| 3′ | TCAGCTTGAGGGTTTGCTAC | |
| Human IL-1β | 5′ | AAGCTGATGGCCCTAAACAG |
| 3′ | CAGGTCATTCTCCTGGAAGG | |
| Human PDGF | 5′ | CAGTCAGATCCACAGCATCC |
| 3′ | TCTCGTAAATGACCGTCCTG | |
| Human TGF-β | 5′ | CAACAATTCCTGGCGATACC |
| 3′ | GTAGTGAACCCGTTGATGTCC |
Definition of abbreviations: PDGF = platelet-derived growth factor; rRNA = ribosomal RNA; TGF-β = transforming growth factor-β.
ELISA
TNF-α and IL-1β protein were measured in conditioned media on a VersaMax plate reader (450 nm; Molecular Devices). The detection limits were 15.6 pg/ml for TNF-α and 3.9 pg/ml for IL-1β (R&D Systems). The protein concentration was calculated from the slope of a standard curve.
NF-κB Activation
THP1-XBlue cells (InvivoGen) were maintained in THP1 media supplemented with 100 μg/ml normocin and 200 μg/ml zeocin (InvivoGen). Differentiated THP1-XBlue cells (25 ng/ml PMA for 48 h) were exposed to OPs for 24 hours, and secreted embryonic alkaline phosphatase activity was quantified using Quanti-Blue reagent (InvivoGen) on a SpectraMax spectrophotometer (630 nm; Molecular Devices) as a measure of NF-κB activation.
AChE Assay
AChE activity was determined using the standard Ellman assay (22) with 5,5′-dithio-bis(2-nitrobenzoic acid) and acetylthiocholine iodide as substrate, and 100 μM tetraisopropyl pyrophosphoramide to inhibit pseudocholinesterase. AChE activity was normalized to protein concentration (BCA assay; Pierce).
Data Analysis
All data were analyzed by Shapiro-Wilk and D’Agostino and Pearson normality tests. mRNA expression and protein concentration in exposed cultures were graphed as the fold change over controls in each experiment to demonstrate the magnitude of the effect and to account for changes in baseline expression. The data were then analyzed by Kruskal-Wallis (nonparametric one-way ANOVA) and corrected by Dunn’s multiple comparison test (Prism 7; GraphPad). NF-κB activity was analyzed by one-way ANOVA on log-transformed data for parathion, chlorpyrifos, chlorpyrifos oxon, and diazinon using Tukey’s multiple comparison test, and for paraoxon and diazoxon using the Kruskal-Wallis and post hoc Dunn’s multiple comparison test. AChE activity was analyzed by one-way ANOVA with post hoc Tukey’s multiple comparison test. A statistical probability of P ≤ 0.05 was considered significant. Data are represented as mean ± SEM.
Results
Parent OPs Increase TNF-α, IL-1β, Platelet-derived Growth Factor, and Transforming Growth Factor-β mRNA Expression
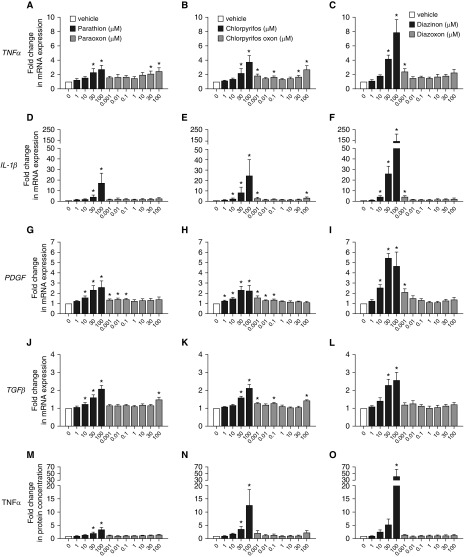
The parent OPs parathion (Figures 1A, 1D, 1G, and 1J; black bars), chlorpyrifos (Figures 1B, 1E, 1H, and 1K; black bars), and diazinon (Figures 1C, 1F, 1I, and 1L; black bars) concentration-dependently and significantly increased TNF-α (Figures 1A–1C), IL-1β (Figures 1D–1F), PDGF (platelet derived growth factor) (Figures 1G–1I), and TGF-β (transforming growth factor-β) (Figures 1J–1L) mRNA expression in differentiated THP1 cells after 24 hours of exposure. Diazinon induced the largest increase in cytokine and growth factor mRNA expression (for example, parathion induced a 17-fold increase in IL-1β, whereas diazinon caused a 143-fold increase in IL-1β).
Figure 1.
Organophosphorus pesticides (OPs) upregulate cytokine and growth factor mRNA expression and TNF-α release in THP1 cells. Differentiated THP1 cells were treated with (A, D, G, J, and M) parathion or paraoxon, (B, E, H, K, and N) chlorpyrifos or chlorpyrifos oxon, or (C, F, I, L, and O) diazinon or diazoxon for 24 hours. Cellular levels of mRNA specific for (A–C) TNF-α, (D–F) IL-1β, (G–I) PDGF (platelet-derived growth factor), and (J–L) TGF-β (transforming growth factor-β) were quantified by real-time PCR and normalized to 18S ribosomal RNA. Conditioned media was collected from THP1 cells and quantified by ELISA to quantify the amount of (M–O) TNF-α protein released by the cells into the media. The effect of OPs on mRNA expression and protein release was expressed as a fold change over mRNA expression or protein release, respectively, in vehicle-treated cells (0.1% DMSO) within each experiment. Data are presented as the mean ± SEM (each exposure was performed in triplicate wells; n = 4–10 separate experiments for each exposure). *Significantly different from vehicle control at P ≤0.05.
In contrast, although some concentrations of the oxon metabolites increased cytokine and growth factor mRNA expression (see gray bars for paraoxon in Figures 1A, 1D, 1G, and 1J; chlorpyrifos oxon in Figures 1B, 1E, 1H, and 1K; and diazoxon in Figures 1C, 1F, 1I, and 1L), with the exception of paraoxon’s effects on TNF-α mRNA expression (Figure 1A), the effects did not exhibit classic monotonic concentration–effect relationships. Moreover, the magnitude of the increase in mRNA levels observed in THP1 cells exposed to the oxon metabolites was less than that observed in THP1 cells exposed to the corresponding parent compound.
Parent OPs Increase TNF-α Protein Expression in Conditioned Media
Conditioned media was collected from THP1 cells 24 hours after exposure to parathion, chlorpyrifos, or diazinon, or their oxon metabolites. Among the proteins examined, only TNF-α protein was significantly increased in conditioned media by all three OPs (Figures 1M–1O and Figure E1 in the data supplement). Parathion, chlorpyrifos, and diazinon increased TNF-α protein at concentrations ≥30 μM (Figures 1M–1O, respectively, black bars). Similar to observations of OP effects on mRNA levels, the largest increase in TNF-α protein was seen in diazinon-exposed cells (42-fold increase; Figure 1O). None of the oxon metabolites significantly increased TNF-α protein in conditioned media (Figures 1M–1O, respectively, gray bars).
IL-1β protein was significantly increased in conditioned media only by diazinon at 100 μM (Figure E1C in the data supplement). PDGF protein was significantly decreased by paraoxon at 0.001 μM and 0.1 μM and chlorpyrifos oxon at 100 μM (Figures E1D and E1E). TGF-β protein was undetectable in media from control or OP-exposed THP1 cells (data not shown).
Parent OPs Influence Cytokine Expression and Release at Concentrations that Do Not Cause Cellular Toxicity
A 24 hour exposure to parathion, chlorpyrifos, or diazinon (Figure E2, black bars), or their respective oxon metabolites (paraoxon, chlorpyrifos oxon, and diazoxon; Figure E2, gray bars) over the same concentration range used to assess OP effects on cytokine expression and release, had no effect on THP1 mitochondrial function as measured by an MTT assay (Figures E2A–E2C) or membrane integrity as measured by a lactate dehydrogenase cytotoxicity assay (Figures E2D–E2F). A live/dead cell assay indicated that some concentrations of the OPs and their oxons had a small but significant effect on THP1 cell viability (Figures E2G–E2J). The percentage of living cells observed after exposure to 30 μM parathion (99.2% ± 1.2%), 100 μM parathion (96.2% ± 2.2%), 1 μM paraoxon (96.9% ± 1.3%), 100 μM paraoxon (90.6% ± 2.8%), and 100 μM diazoxon (95.0% ± 1.0%) was significantly decreased compared with that observed in vehicle-treated cells (Figures E2G and E2I). None of the concentrations of chlorpyrifos, chlorpyrifos oxon, and diazinon decreased cellular viability (Figures E2H and E2I).
A Phosphorothioate OP Metabolite Similarly Increases Cytokine Expression in THP1 Cells
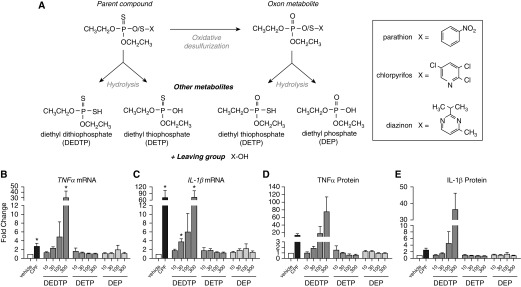
Parathion, chlorpyrifos, and diazinon are all classified as phosphorothioate OPs. The parent compounds have negligible AChE-inhibiting activity, and instead must be oxidized by exchanging a sulfur for an oxygen to form the oxon, which potently inhibits AChE (23). Oxidation can occur in the environment (24) or via cytochrome P450–mediated metabolism in biological organisms (25). OPs can also be hydrolyzed to form metabolites with no AChE activity, including DEP, DETP, and DEDTP (Figure 2A). These metabolites, which are not specific to any one OP, are often measured in urine as biomarkers of general OP exposure (26).
Figure 2.
Influence of phosphorothioate versus phosphate OP metabolites on TNF-α and IL-1β mRNA expression and protein release in THP1 cells. Differentiated THP1 cells were treated with 100 μM chlorpyrifos (CPF) or the OP metabolites O,O-diethyl dithiophosphate (DEDTP; 10–300 μM), O,O-diethyl thiophosphate potassium salt (DETP; 10–300 μM), or diethyl phosphate (DEP; 10–300 μM) for 24 hours. (A) Schematic of OP metabolism. X is the chemical structure that specifically identifies each OP. (B–E) Effects of CPF versus OP metabolites on (B and C) TNF-α and IL-1β mRNA and (D and E) protein levels in THP1 cells and conditioned media, respectively. The effect on cytokine expression was expressed as a fold change over expression in vehicle controls (0.1% DMSO) in each experiment. Data are presented as the mean ± SEM (each exposure was performed in triplicate wells; n = 4 separate experiments). *Significantly different from vehicle control at P ≤0.05.
A significant difference between the molecular structure of the parent OPs, which were observed to increase TNF-α release, and that of their oxon metabolites, which had no effect on TNF-α release, was a phosphorothioate linkage (P = S) in the parent compounds versus a phosphate group (P = O) in the oxon metabolites. Therefore, we tested the effect on cytokine expression and TNF-α release of phosphorothioate versus phosphate OP metabolites (Figure 2A). Neither DEP, a nonphosphorothioate, nor DETP, a phosphorothioate, increased TNF-α or IL-1β mRNA expression or protein release (Figures 2B–2E). These compounds also did not increase PDGF or TGF-β mRNA expression (Figures E3A and E3B). In contrast, DEDTP, a phosphorothioate with two sulfurs, induced a large, significant increase in TNF-α and IL-1β mRNA (Figures 2B and 2C) and a small but still significant increase in PDGF mRNA expression (Figure E3A). DEDTP did not increase TGF-β mRNA expression (Figure E3B). DEDTP increased TNF-α (Figure 2D) and IL-1β (Figure 2E) protein release, although not significantly.
Parent OPs Increase NF-κB Activity
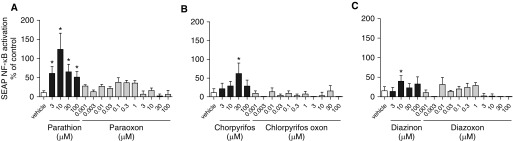
We next tested whether OPs activate NF-κB, using the NF-κB–reporter cell line THP1-XBlue. After a 24-hour exposure, the parent OPs parathion, chlorpyrifos, and diazinon each significantly increased NF-κB activation (Figures 3A–3C, respectively; black bars), whereas their oxon metabolites did not (Figures 3A–3C, respectively; gray bars). Parathion showed the largest increase, with peak activation of NF-κB observed at 10 μM (Figure 3A), and the effect of diazinon was minimal (Figure 3C).
Figure 3.
Parent OP compounds, but not oxon metabolites, activate NF-κB in THP1 cells. (A–C) Differentiated THP1-XBlue cells were exposed to parathion or paraoxon (A), chlorpyrifos or chlorpyrifos oxon (B), or diazinon or diazoxon (C) for 24 hours. Secreted embryonic alkaline phosphatase (SEAP) released into the culture medium was quantified as a measure of NF-κB activation. Values from OP-exposed cells were normalized to controls (0.1% DMSO) and expressed as a percent change from vehicle controls. Data are presented as the mean ± SEM (n = 8–20 wells per group in 4 different experiments). *Significantly different from vehicle control at P ≤0.05.
OP Effects on Cytokine Expression and Release Are Not Mediated by Cholinesterase Inhibition
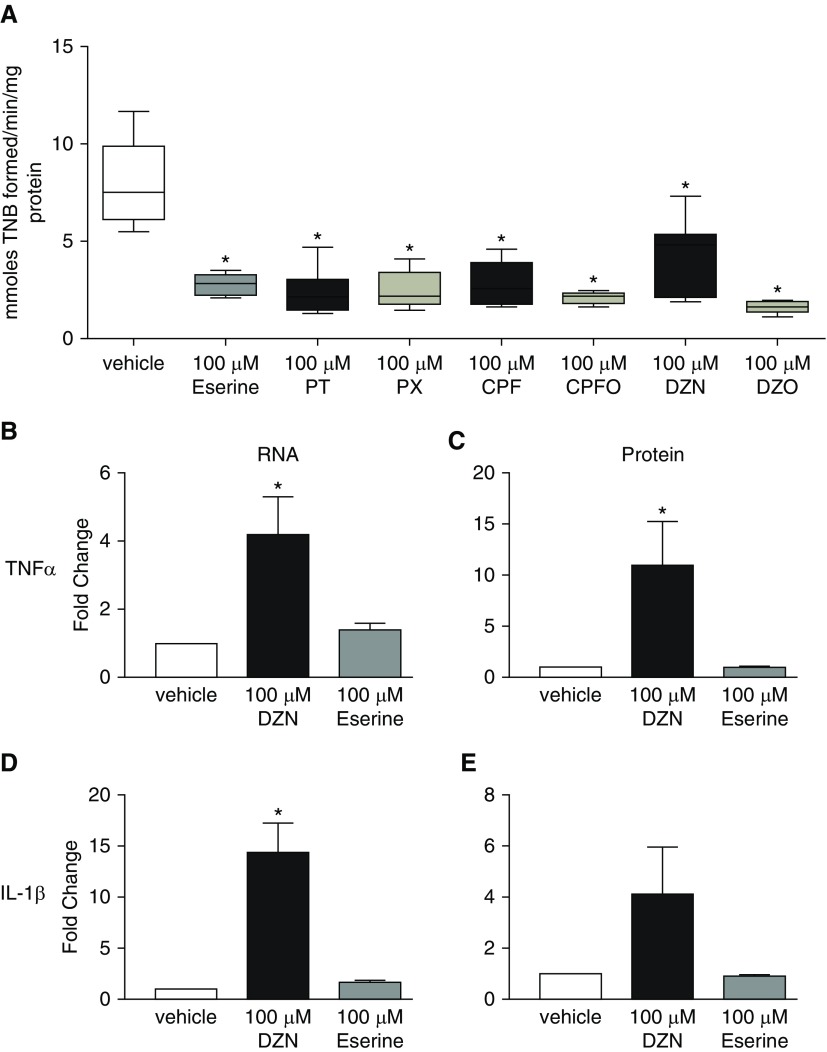
Many of the neurotoxic effects of OPs are mediated by AChE inhibition; therefore, AChE activity was measured at the highest concentrations of the OP parent compounds and oxon metabolites tested in the cytokine expression and release studies. At 100 μM, parathion, paraoxon, chlorpyrifos, chlorpyrifos oxon, diazinon, and diazoxon each significantly inhibited AChE activity by ∼75% compared with AChE activity in vehicle control THP1 cells (Figure 4A). To determine whether AChE inhibition mediated the effects of OPs on cytokine expression, we tested eserine at 100 μM, a concentration that inhibited AChE to a level comparable to that observed in the OP-exposed THP1 cells (Figure 4A). In contrast to diazinon at 100 μM, eserine at 100 μM did not increase cellular levels of TNF-α or IL-1β mRNA (Figures 4B and 4D), or media levels of TNF-α or IL-1β protein (Figures 4C and 4E).
Figure 4.
The effects of OPs on cytokines in THP1 cells are not mediated by acetylcholinesterase (AChE) inhibition. Differentiated THP1 cells were treated for 24 hours with either vehicle (0.2% DMSO), 100 μM of the cholinesterase inhibitor eserine, or 100 μM of the OPs parathion (PT), paraoxon (PX), chlorpyrifos (CPF), CPF oxon (CPFO), diazinon (DZN), and diazoxon (DZO). (A) AChE activity is expressed as activity per minute per milligram of protein. Data are presented as whisker box plots. The horizontal line in each box represents the mean; the lower and upper box limits represent the 25th and 75th percentiles, respectively; and whiskers represent the 1–99th percentile (n = 4 wells per group in 4 different experiments). (B–E) Concentrations of DZN and eserine that cause comparable inhibition of AChE activity differentially influence RNA (B and D) and protein (C and E) levels of TNF-α (B and C) and IL-1β (D and E) in THP1 cells. The effect of DZN and eserine on mRNA and protein expression is expressed as a fold change over expression in vehicle control cells (0.2% DMSO) in each experiment. Data are represented as mean ± SEM (each exposure was performed in triplicate wells; n = 5 separate experiments for each exposure). *Significantly different from vehicle control at P≤ 0.05. TNB = 2-nitro-5-thiobenzoic acid.
Pharmacologically Antagonizing Muscarinic or Nicotinic Receptors Did Not Prevent Effects of Diazinon on Cytokine Expression and Release
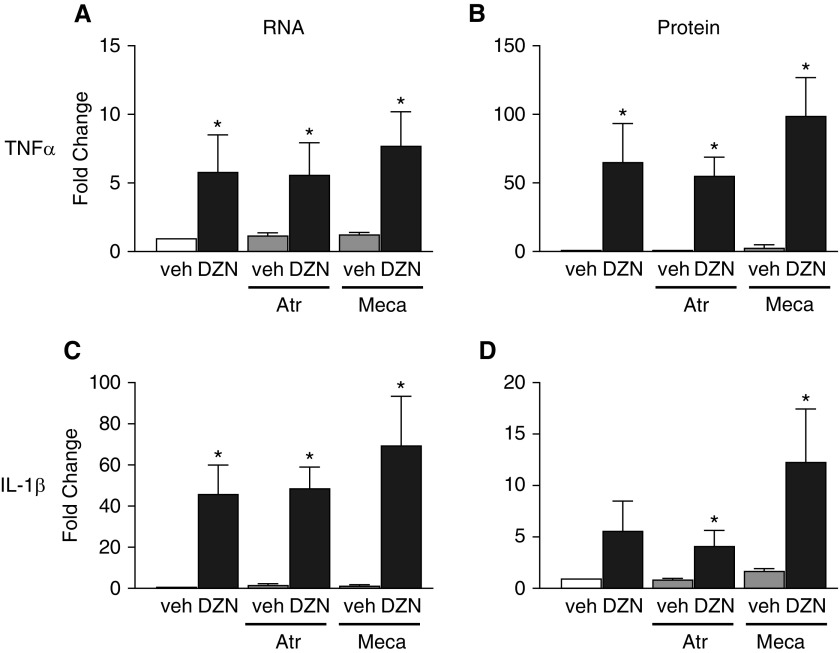
To determine whether muscarinic or nicotinic cholinergic receptors mediate OP-induced cytokine expression, we pretreated THP1 cells with either 100 μM atropine or 100 μM mecamylamine for 1 hour before adding 100 μM diazinon for 24 hours to block muscarinic or nicotinic receptors, respectively. The half-maximal inhibitory concentration for atropine is in the nanomolar range (27), whereas that for mecamylamine is in the low-micromolar range (28). Thus, at 100 μM, atropine and mecamylamine sufficiently blocks muscarinic and nicotinic receptors, respectively, on macrophages (29, 30). Neither atropine nor mecamylamine blocked upregulation of TNF-α or IL-1β mRNA, or increased the release of TNF-α or IL-1β protein into conditioned medium in THP1 cells exposed to diazinon (Figure 5). In the absence of diazinon, neither atropine nor mecamylamine had any effect on cytokine mRNA or protein levels.
Figure 5.
Pharmacologic antagonism of muscarinic or nicotinic acetylcholine receptors has no effect on diazinon-induced cytokine expression in THP1 cells. Differentiated THP1 cells were pretreated for 1 hour with 100 μM atropine (Atr) to block muscarinic receptors or 100 μM mecamylamine (Meca) to block nicotinic receptors before the addition of 100 μM DZN or vehicle (veh; 0.1% DMSO) for 24 hours. (A and C) Cellular levels of TNF-α (A) and IL-1β (C) mRNA were quantified by real-time PCR and normalized to 18S rRNA. (B and D) Levels of TNF-α (B) and IL-1β (D) protein in conditioned media were quantified by ELISA. The effect of DZN on mRNA and protein expression was expressed as a fold change over expression in vehicle control cells not exposed to atropine or mecamylamine (white bar) in each experiment. Data are presented as the mean ± SEM (each exposure was performed in triplicate wells; n = 4–5 separate experiments for each exposure). *Significantly different from vehicle control (white bar) at P ≤ 0.05.
OPs and Their Oxon Metabolites Do Not Increase Intracellular Calcium in THP1 Cells
To investigate whether Ca2+ mediates OP-induced cytokine expression in macrophages, we measured intracellular calcium levels in THP1 cells loaded with the Fluo4 Ca2+ indicator dye immediately before acute exposure to OPs (3–100 μM) or their oxon metabolites (0.1–100 μM). None of the three parent OPs or their oxon metabolites increased intracellular Ca2+ significantly above baseline levels within 10 minutes after administration (Figure E4). In contrast, ionomycin, added as a positive control after the 10-minute exposure to OPs, significantly increased intracellular calcium levels.
Discussion
Exposure to OPs is linked to an increased incidence of asthma and asthma exacerbations, as well as respiratory dysfunction (5). However, the mechanisms that mediate OP-induced asthma and airway hyperreactivity are not well understood. In guinea pigs, we have shown that OPs cause airway hyperreactivity (8–10) via neuronal M2 muscarinic receptor dysfunction. M2 receptors normally limit ACh release (8–10), and loss of their function leads to increased ACh release and increased bronchoconstriction. Loss of M2 receptor function in some asthma patients has been reported (7). We previously showed that OP-induced M2 dysfunction is mediated by macrophages and TNF-α, and that OPs increase TNF-α and IL-1β expression in guinea pig alveolar macrophages (10). Here, we extend those findings with the striking observation that the parent forms of parathion, chlorpyrifos, diazinon, and the diethyl dithiophosphate metabolite, but not the oxon metabolites, directly stimulate macrophages, potentially via NF-κB, to increase the release of TNF-α. This is significant because TNF-α has been reported to downregulate neuronal M2 receptors and increase airway reactivity (10). These data suggest that OPs cause airway hyperreactivity via noncholinergic mechanisms of immunomodulation, identifying a potential pathway to target for therapeutic interventions to prevent OP-induced airway dysfunction.
TNF-α, IL-1β, PDGF, and TGF-β have all been shown to modulate M2 muscarinic receptor expression and/or function (13–15), and therefore we focused on these cytokines and growth factors in this study. Here, we show that although mRNA was increased for all of these inflammatory cytokines by OPs, only TNF-α protein release into conditioned medium was increased in differentiated THP1 cells by all three OPs. These data confirm a prior study that showed enhanced TNF-α protein release from isolated guinea pig alveolar macrophages treated ex vivo with parathion (10). The link between macrophages and TNF-α is important because blocking TNF-α in vivo prevented OP-induced airway hyperreactivity and protected neuronal M2 muscarinic receptor function in guinea pigs 24 hours after OP exposure, whereas blocking IL-1β had no effect on acute OP-induced hyperreactivity (10). We previously demonstrated that OP-induced airway hyperreactivity and M2 dysfunction can persist for up to at least 7 days (9), so it may be possible that IL-1β, PDGF, and TGF-β have a role in the chronic effects of OP exposure; however, this possibility has not been tested. OPs may stimulate macrophages to release other cytokines and factors that were not investigated here, and future experiments using multiplex analysis may provide insights as to other factors released by OP-stimulated macrophages that may influence lung function.
OP-induced cytokine expression is not the result of macrophage cytotoxicity. THP1 mitochondrial function and plasma membrane integrity were unaffected by exposure to parent OPs or their oxon metabolites. High concentrations of parathion, paraoxon, and diazoxon caused a small but significant decrease in THP1 viability as determined by a live/dead cell assay. Similarly, 100 μM chlorpyrifos was reported to cause minimal cell death in the human monocyte cell line U937 (31), and neither 100 μM chlorpyrifos nor 100 μM diazinon caused significant cell death in human peripheral blood monocytes (32). Collectively, these data show that OPs at concentrations of <100 μM are not overtly cytotoxic to monocytic cells. Importantly, the small decrease in THP1 cell viability observed with high doses of parathion, paraoxon, and diazoxon in this study did not correlate with the increased cytokine expression observed in THP1 cells exposed to the parent OPs.
Little is known about the deposition of OPs in human lungs. We could find no published studies that measured OPs or their metabolites in induced sputum, BAL, or lung biopsies. Many OPs are lipophilic and can be stored in adipose tissue for days to weeks (33). For example, after oral administration of the OP malathion to rats, the initial highest concentrations were found in blood and muscle, but malathion was stored in adipose tissue (33). The OP fenthion is initially taken up by adipose tissue and does not cause cholinergic symptoms until 24–48 hours after exposure (34). Lung surfactant is 90% lipids and may be a reservoir for OPs in the lung. When parathion or paraoxon were infused directly into guinea pig lungs, both chemicals were nearly all retained (35). Although OPs are predominantly metabolized by liver cytochrome P450s, guinea pig lungs express some of the same hepatic cytochrome P450s (36) and can locally metabolize OPs (35). Thus, OPs can be retained and metabolized by the lung, where they would come into contact with lung macrophages.
The ability of parent OPs to stimulate differentiated THP1 cells is independent of their ability to inhibit AChE, adding to a growing list of research showing that OPs target molecules other than AChE to cause toxicity. Inhibition of AChE is a property shared by both parent compounds and oxon metabolites; however, the oxon forms can be 100-fold more potent (23). Despite this, the oxon metabolites did not stimulate an increase in cytokine expression or release. Additionally, eserine, at a concentration that caused AChE inhibition comparable to that observed in THP1 cells exposed to OPs, did not mimic the effects of OP parent compounds on cytokine expression and release in differentiated THP1 cells. Macrophages express functional nicotinic and muscarinic receptors (37, 38), and OPs are capable of modulating both (39, 40). However, the ability of parent OPs to stimulate differentiated THP1 cells was unaffected by pharmacologic antagonism of nicotinic or muscarinic receptors. Using a calcium indicator dye, we additionally excluded increased calcium influx as a messenger contributing to OP-induced cytokine transcription and release. This is consistent with data obtained from guinea pig alveolar macrophages, which showed that parathion did not induce a calcium influx or potentiate a calcium influx in response to N-formylmethionine-leucyl-phenylalanine (B.J.P., unpublished results), a potent activator of macrophages. Collectively, these data show that the mechanism(s) underlying OP-induced increases in cytokine expression and TNF-α release in macrophages does not involve canonical mechanisms of OP toxicity involving AChE inhibition, modulation of cholinergic receptor activity, or changes in intracellular calcium levels.
A striking finding of this study is that the parent OPs, but not their oxon metabolites, stimulated differentiated THP1 cells to release TNF-α (Table 2). This confirms previous observations that parathion modestly increased IL-1β mRNA and significantly increased TNF-α protein release from cultured guinea pig alveolar macrophages, whereas paraoxon had no effect (10). Parent OPs are phosphorothioates and their oxon metabolites are phosphodiesters (see the chemical structures in Figure 2). We tested whether the presence of a sulfur group, which distinguishes the former from the latter, is critical for activation of THP1 cells. Neither DEP (a phosphodiester without a sulfur group) nor DETP (a phosphorothioate with one sulfur group) increased cytokine mRNA expression or protein release from THP1 cells. However, DEDTP (a phosphorothioate with two sulfur groups) significantly increased TNF-α, IL-1β, and PDGF mRNA, and increased TNF-α and IL-1β protein by 75-and 36-fold, respectively, although the differences were not significant. Thus, having a phosphorothioate bond and additional sulfur groups appears to increase the potential to stimulate macrophages, and may explain why parent OPs stimulate macrophages while oxon forms do not. DEDTP in urine is widely used as a biomarker of OP exposure but is believed to have little biological activity. Our data suggest that DEDTP directly affects macrophage function. In support of this possibility, Medina-Buelvas and colleagues reported an increase in alternatively activated (M2) macrophages in lymph nodes of mice treated with intraperitoneal DEDTP for 8 days (41). These data are important because many OP studies have focused on oxon metabolites in the context of neurotoxicity. Our data demonstrate that parent OPs and DEDTP may also have important biological effects that are unique from those associated with the neurotoxic oxon metabolites.
Table 2.
Summary of Data
| Increased | PTH (μM) | PX (μM) | CPF (μM) | CPFO (μM) | DZN (μM) | DZO (μM) | DEDTP (μM) | DETP (μM) | DEP (μM) | |
|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α | mRNA | 30, 100 | 30, 100 | 30, 100 | 0.001 and 0.1, 30, 100 | 30, 100 | 0.001 | 30 and 300 | ns | ns |
| protein | 30, 100 | ns | 30, 100 | ns | 100 | ns | 300* | ns | ns | |
| IL-1β | mRNA | 30, 100 | ns | 10, 30, 100 | 0.001 and 100 | 10, 30, 100 | 0.001 | 30 and 300 | ns | ns |
| protein | ns | ns | ns | ns | 100 | ns | 300* | ns | ns | |
| PDGF | mRNA | 10, 30, 100 | 0.001, 0.01, 0.1 | 1, 10, 30, 100 | 0.001, 0.01, 0.1 | 10, 30, 100 | 0.001 | 30 and 300 | ns | ns |
| protein | ns | ns | ns | ns | ns | ns | — | — | — | |
| TGF-β | mRNA | 10, 30, 100 | 100 | 30, 100 | 0.001, and 0.01, 100 | 30, 100 | ns | ns | ns | ns |
| protein | nd | nd | nd | nd | nd | nd | — | — | — | |
| NF-κB activation | 3, 10, 30, 100 | ns | 30 | ns | 10 | ns | — | — | — | |
Definition of abbreviations: “and” = indicates no dose response effect; CPF = chlorpyrifos; CPFO = CPF oxon; DEDTP = O,O-diethyl dithiophosphate; DEP = diethyl phosphate; DETP = O,O-diethyl thiophosphate potassium salt; DZN = diazinon; DZO = diazoxon; nd = not detected (i.e., below the standard curve); ns = no significant increase; PTH = parathion; PX = paraoxon.
This table summarizes the data for PTH, PX, CPF, CPFO, DZN, and DZO, as well as DEDTP, DETP, and DEP on cytokine mRNA and protein release in THP1 cells. All concentrations are in μM. Data for PTH/oxon, CPF/oxon and DZN/oxon are from Figures 1 and E1. Protein levels for TGF-β were all less than the lower limit of the standard curve (lowest concentration 31.3 ng/ml). Data for NF-κB activation are from Figure 3. Data for DEDTP, DEP, and DETP are from Figures 2 and E3.
DEDTP at 300 μM caused a large increase in TNF-α and IL-1β, but it was not statistically significant.
The mechanism by which the parent OPs and DEDTP alter cytokine synthesis and release in macrophages remains to be determined. Mac-1 (CD11b/CD18) is a heparin-binding integrin receptor that is expressed by macrophages and binds phosphorothioate oligonucleotides (42). A study by Hosoi and colleagues demonstrated that phosphorothioate oligonucleotides were 200 times more potent than phosphodiester oligonucleotides (of the same size) in inhibiting Mac-1–mediated DNA-dependent kinase activity in a fibroblast cell line (43). This may be one mechanism by which phosphorothioates, such as the parent OPs and DEDTP, interact with macrophages. In support of this possibility, only the parent OPs triggered NF-κB activation in a THP1 NF-κB reporter cell line. We did not determine whether parent OPs activated AP-1 to increase TGF-β and PDGF mRNA expression, or whether this increase in growth factors was the result of downstream signaling after NF-κB activation and/or TNF-α release. Further studies are needed to address these questions, as well as to identify the NF-ĸB and AP-1 binding sites in the promoter regions of cytokine genes upregulated by OPs. An extensive body of literature supports a critical role for NF-κB in transducing diverse environmental stimuli to upregulate cytokine expression in inflammatory cells (44). Although it remains to be determined whether blocking NF-κB activation prevents the effects of OPs on cytokine expression and release in macrophages, our data are consistent with that hypothesis.
A limitation to this research is that we did not confirm whether OPs increase cytokine expression in human primary alveolar macrophages. We have previously shown that OPs increased TNF-α and IL-1β expression in guinea pig alveolar macrophages isolated from BAL (10). Although useful information might be obtained with the use of alveolar macrophages collected from healthy humans, BAL is not routinely performed on healthy individuals. Human alveolar macrophages obtained from individuals with pulmonary disease would likely be more activated and heterogeneous than THP1 cells. In a previous study, we showed that when guinea pigs were sensitized to ovalbumin, parathion-induced airway hyperreactivity was significantly enhanced compared with what was observed in nonsensitized animals (45). Although we did not investigate macrophages in that study, we would infer that human alveolar macrophages isolated from individuals with asthma or atopic individuals may have an enhanced response to OP exposure, which may potentiate airway reactivity. Macrophages differentiated from human peripheral blood monocytes could also be used to investigate OP-induced increases in cytokine expression. THP1 cells are spontaneously immortalized monocytes derived from the blood of a child with acute monocytic leukemia. THP1 cells, differentiated THP1 cells, human monocytes, and human macrophages derived from peripheral monocytes have some differences and some similarities in gene expression profiles in response to stimulants (46, 47).
In conclusion, our data suggest a novel mechanism by which OPs induce airway hyperreactivity through stimulation of alveolar macrophages by parent OPs to increase NF-κB activation, resulting in TNF-α protein release. Although the oxon forms of these pesticides have been long considered to be mediators of OP toxicity, our data convincingly demonstrate that parent OP compounds more potently and consistently stimulate macrophages. We further postulate that the phosphorothioate linkage in parent OPs may be important in determining how parent OPs interact with macrophages and perhaps other cells.
Specialized macrophages are located throughout the body and may be similarly affected by OPs. Parent OPs increase IL-6 and TNF-α mRNA in cultured microglia (48), a macrophage-like glia cell in the brain, and exposure to OPs has been linked to an increased incidence of Parkinson’s disease (49) and autism (50). Collectively, these data add to a growing body of research indicating that parent OPs have biological effects, especially on cells in the macrophage family, and that these effects are independent of AChE inhibition. Future experiments to determine the mechanism by which parent OPs and other phosphorothioates interact with macrophages to induce cytokine expression will provide promising targets for the development of therapeutics to prevent the deleterious effects of OP exposure on airway function.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Thomas Scanlan for his consultation on chemical structures, Susan Hulsizer for her assistance with calcium experiments, and Jasmine Garg and Meredith Datena for their assistance with culturing the THP1 cells and processing cell samples.
Footnotes
Supported by National Institutes of Health grants U54 HD079125 (A.C.G.G. and P.J.L.), P30 ES023513 (A.C.G.G.), ES017592 (P.J.L. and A.D.F.), HL131525 (A.D.F.), and HL124165 (D.B.J.), and Health Effects Institute grant 4905-RFPA10-3 (A.D.F.). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Contributions: B.J.P. and A.C.G.G. performed the research. B.J.P., A.C.G.G., D.B.J., P.J.L., and A.D.F. designed the research study. B.J.P. and A.C.G.G. analyzed the data. B.J.P., A.C.G.G., P.J.L., and A.D.F. wrote the paper.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0257OC on April 12, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Voorhees JR, Rohlman DS, Lein PJ, Pieper AA. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Front Neurosci. 2017;10:590. doi: 10.3389/fnins.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamaro CM, Smith MN, Workman T, Griffith WC, Thompson B, Faustman EM. Characterization of organophosphate pesticides in urine and home environment dust in an agricultural community. Biomarkers. 2018;23:174–187. doi: 10.1080/1354750X.2017.1395080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C, Knutson DE, Fisker-Andersen J, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among pre-school children in the Seattle metropolitan area. Environ Health Perspect. 2001;109:299–303. doi: 10.1289/ehp.01109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raanan R, Balmes JR, Harley KG, Gunier RB, Magzamen S, Bradman A, et al. Decreased lung function in 7-year-old children with early-life organophosphate exposure. Thorax. 2016;71:148–153. doi: 10.1136/thoraxjnl-2014-206622. [DOI] [PubMed] [Google Scholar]
- 5.Shaffo FC, Grodzki AC, Fryer AD, Lein PJ. Mechanisms of organophosphorus pesticide toxicity in the context of airway hyperreactivity and asthma. Am J Physiol Lung Cell Mol Physiol. 2018;315:L485–L501. doi: 10.1152/ajplung.00211.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984;83:973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol (1985) 1989;67:2461–2465. doi: 10.1152/jappl.1989.67.6.2461. [DOI] [PubMed] [Google Scholar]
- 8.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci. 2005;83:166–176. doi: 10.1093/toxsci/kfi001. [DOI] [PubMed] [Google Scholar]
- 9.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol. 2004;286:L963–L969. doi: 10.1152/ajplung.00343.2003. [DOI] [PubMed] [Google Scholar]
- 10.Proskocil BJ, Bruun DA, Jacoby DB, van Rooijen N, Lein PJ, Fryer AD. Macrophage TNF-α mediates parathion-induced airway hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2013;304:L519–L529. doi: 10.1152/ajplung.00381.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proskocil BJ, Bruun DA, Thompson CM, Fryer AD, Lein PJ. Organophosphorus pesticides decrease M2 muscarinic receptor function in guinea pig airway nerves via indirect mechanisms. PLoS One. 2010;5:e10562. doi: 10.1371/journal.pone.0010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogasawara N, Matsushima M, Kawamura N, Atsumi K, Yamaguchi T, Ochi H, et al. Modulation of immunological activity on macrophages induced by diazinon. Toxicology. 2017;379:22–30. doi: 10.1016/j.tox.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Haddad EB, Rousell J, Lindsay MA, Barnes PJ. Synergy between tumor necrosis factor alpha and interleukin 1beta in inducing transcriptional down-regulation of muscarinic M2 receptor gene expression: involvement of protein kinase A and ceramide pathways. J Biol Chem. 1996;271:32586–32592. doi: 10.1074/jbc.271.51.32586. [DOI] [PubMed] [Google Scholar]
- 14.Jackson DA, Nathanson NM. Regulation of expression and function of m2 and m4 muscarinic receptors in cultured embryonic chick heart cells by transforming growth factor-beta 1. Biochem Pharmacol. 1997;54:525–527. doi: 10.1016/s0006-2952(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 15.Rousell J, Haddad el-B, Lindsay MA, Barnes PJ. Regulation of m2 muscarinic receptor gene expression by platelet-derived growth factor: involvement of extracellular signal-regulated protein kinases in the down-regulation process. Mol Pharmacol. 1997;52:966–973. doi: 10.1124/mol.52.6.966. [DOI] [PubMed] [Google Scholar]
- 16.Proskocil B, Bruun D, Lein P, Jacoby D, Fryer A. Late-breaking abstract: organophosphorus pesticides induce airway hyperreactivity via stimulation of alveolar macrophage Mac-1. Eur Respir J. 2016;48:OA3540. [Google Scholar]
- 17.Proskocil B, Lein P, Jacoby D, Fyer A. Organophosphorus pesticides directly simulate macrophages to increase expression of growth factors and cytokines. Eur Respir J. 2014;44:P4779. [Google Scholar]
- 18.Proskocil B, Lein P, Jacoby D, Fryer A. Organophosphorus pesticides increase inflammatory cytokines by activating macrophage Mac-1. Eur Respir J. 2017;50:PA4926. [Google Scholar]
- 19.Grodzki C, Fryer A, Lein P. Organophosphorus pesticides promote cytokine production in human macrophages. FASEB J. 2016;30:1195.10. [Google Scholar]
- 20.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- 21.Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006;Chapter 15:Unit 15.8. doi: 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Colovic MKD, Uscumlic G, Vasic V. Single and simultaneous exposure of acetylcholinesterase to diazinon, chlorpyrifos and their photodegradation products. Pestic Biochem Physiol. 2011;100:16–22. [Google Scholar]
- 24.Pehkonen SO, Zhang Q. The degradation of organophosphorus pesticides in natural waters: a critical review. Crit Rev Environ Sci Technol. 2002;32:17–72. [Google Scholar]
- 25.Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos. 2007;35:189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–e1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander SP, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al. CGTP Collaborators. The concise guide to pharmacology 2017/18: G protein-coupled receptors. Br J Pharmacol. 2017;174:S17–S129. doi: 10.1111/bph.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD, et al. CGTP Collaborators. The concise guide to pharmacology 2017/18: ligand-gated ion channels. Br J Pharmacol. 2017;174:S130–S159. doi: 10.1111/bph.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Torre E, Genaro AM, Ribeiro ML, Pagotto R, Pignataro OP, Sales ME. Proliferative actions of muscarinic receptors expressed in macrophages derived from normal and tumor bearing mice. Biochim Biophys Acta. 2008;1782:82–89. doi: 10.1016/j.bbadis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Bai X, Stitzel JA, Bai A, Zambrano CA, Phillips M, Marrack P, et al. Nicotine impairs macrophage control of Mycobacterium tuberculosis. Am J Respir Cell Mol Biol. 2017;57:324–333. doi: 10.1165/rcmb.2016-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakadai A, Li Q, Kawada T. Chlorpyrifos induces apoptosis in human monocyte cell line U937. Toxicology. 2006;224:202–209. doi: 10.1016/j.tox.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 32.Oostingh GJ, Wichmann G, Schmittner M, Lehmann I, Duschl A. The cytotoxic effects of the organophosphates chlorpyrifos and diazinon differ from their immunomodulating effects. J Immunotoxicol. 2009;6:136–145. doi: 10.1080/15476910902977407. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Repetto R, Martinez D, Repetto M. Malathion and dichlorvos toxicokinetics after the oral administration of malathion and trichlorfon. Vet Hum Toxicol. 1995;37:306–309. [PubMed] [Google Scholar]
- 34.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 35.Lessire F, Gustin P, Delaunois A, Bloden S, Nemmar A, Vargas M, et al. Relationship between parathion and paraoxon toxicokinetics, lung metabolic activity, and cholinesterase inhibition in guinea pig and rabbit lungs. Toxicol Appl Pharmacol. 1996;138:201–210. doi: 10.1006/taap.1996.0118. [DOI] [PubMed] [Google Scholar]
- 36.Castell JV, Donato MT, Gómez-Lechón MJ. Metabolism and bioactivation of toxicants in the lung: the in vitro cellular approach. Exp Toxicol Pathol. 2005;57:189–204. doi: 10.1016/j.etp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 38.Koarai A, Traves SL, Fenwick PS, Brown SM, Chana KK, Russell RE, et al. Expression of muscarinic receptors by human macrophages. Eur Respir J. 2012;39:698–704. doi: 10.1183/09031936.00136710. [DOI] [PubMed] [Google Scholar]
- 39.Jett DA, Abdallah EA, El Fakahany EE, Eldefrawi ME, Eldefrawi AT. High-affinity activation by paraoxon of a muscarinic receptor subtype in rat brain striatum. Pestic Biochem Physiol. 1991;39:149–157. [Google Scholar]
- 40.Katz EJ, Cortes VI, Eldefrawi ME, Eldefrawi AT. Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol. 1997;146:227–236. doi: 10.1006/taap.1997.8201. [DOI] [PubMed] [Google Scholar]
- 41.Medina-Buelvas D, Estrada-Muñiz E, Flores-Valadez M, Vega L. Genotoxic and immunotoxic effects of the organophosphate metabolite diethyldithiophosphate (DEDTP) in vivo. Toxicol Appl Pharmacol. 2019;366:96–103. doi: 10.1016/j.taap.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Benimetskaya L, Loike JD, Khaled Z, Loike G, Silverstein SC, Cao L, et al. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997;3:414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 43.Hosoi Y, Matsumoto Y, Tomita M, Enomoto A, Morita A, Sakai K, et al. Phosphorothioate oligonucleotides, suramin and heparin inhibit DNA-dependent protein kinase activity. Br J Cancer. 2002;86:1143–1149. doi: 10.1038/sj.bjc.6600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh S, Dass JF. Study of pathway cross-talk interactions with NF-κB leading to its activation via ubiquitination or phosphorylation: a brief review. Gene. 2016;584:97–109. doi: 10.1016/j.gene.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Proskocil BJ, Bruun DA, Lorton JK, Blensly KC, Jacoby DB, Lein PJ, et al. Antigen sensitization influences organophosphorus pesticide-induced airway hyperreactivity. Environ Health Perspect. 2008;116:381–388. doi: 10.1289/ehp.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohro T, Tanaka T, Murakami T, Wada Y, Aburatani H, Hamakubo T, et al. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J Atheroscler Thromb. 2004;11:88–97. doi: 10.5551/jat.11.88. [DOI] [PubMed] [Google Scholar]
- 47.Bosshart H, Heinzelmann M. THP-1 cells as a model for human monocytes. Ann Transl Med. 2016;4:438. doi: 10.21037/atm.2016.08.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian J, Dai H, Deng Y, Zhang J, Li Y, Zhou J, et al. The effect of HMGB1 on sub-toxic chlorpyrifos exposure-induced neuroinflammation in amygdala of neonatal rats. Toxicology. 2015;338:95–103. doi: 10.1016/j.tox.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122:1103–1109. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.