Abstract
Fungal cells colonize and proliferate in distinct niches, from soil and plants to diverse tissues in human hosts. Consequently, fungi are challenged with the goal of obtaining nutrients while simultaneously elaborating robust regulatory mechanisms to cope with a range of availability of nutrients, from scarcity to excess. Copper is essential for life but also potentially toxic. In this review we describe the sophisticated homeostatic mechanisms by which fungi acquire, utilize, and control this biochemically versatile trace element. Fungal pathogens, which can occupy distinct host tissues that have their own intrinsic requirements for copper homeostasis, have evolved mechanisms to acquire copper to successfully colonize the host, disseminate to other tissues, and combat host copper bombardment mechanisms that would otherwise mitigate virulence.
Keywords: metals, transporter, pathogenesis, transcription, virulence, compartmentalization
INTRODUCTION
Copper is an essential trace element for virtually all forms of eukaryotic life. The ability of ionic copper to cycle between an oxidized (Cu2+) and reduced (Cu+) state is exploited by many enzymes to catalyze biochemical processes including aerobic respiration, superoxide detoxification, and iron acquisition (119). However, labile copper ions inactivate other metalloenzymes by metal displacement, and inappropriately engage with intracellular metallophilic ligands provided by sulfur, oxygen, and nitrogen, and drive the generation of reactive oxygen species (ROS) through Fenton-like chemistry (56, 57, 109). The acquisition, distribution, storage, and efflux of copper must be tightly regulated to satisfy cellular demands under a range of environmental conditions while minimizing toxicity due to copper accumulation beyond homeostatic capacity. Indeed, like any eukaryotic cell, fungal cells must import, buffer, route, compartmentalize, utilize, detoxify, and sense different levels of copper that are critical for their fitness in distinct environmental niches where they thrive, or where they simply survive under conditions of deficiency (Figure 1). Although many of the fundamental mechanisms for copper homeostasis have been discovered in fungi, and are conserved in mammals, fungi have transcriptional responses to copper availability that are distinct from those occurring in metazoans. Here we review fundamental concepts and mechanisms in copper acquisition, utilization, and regulation in fungi and highlight recent advances in the burgeoning field of fungal copper biology as it relates to host-pathogen interactions and virulence.
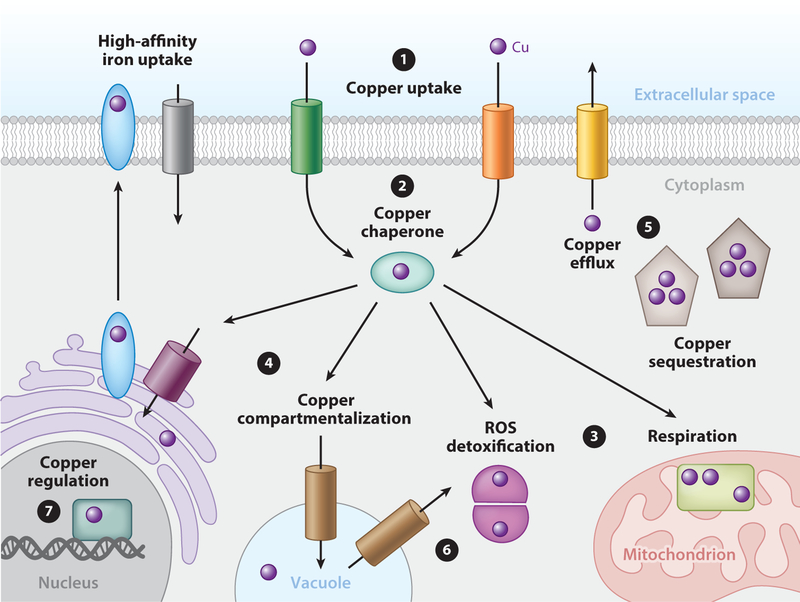
Figure 1.
Acquisition and distribution of copper in fungi. ❶. Dedicated high-affinity copper transporters at the plasma membrane facilitate the unidirectional import of copper (purple spheres) to the cytoplasm. ❷ Once internalized, copper is bound by copper chaperones or other buffering components that facilitate the delivery of copper to ❸ cytoplasmic and mitochondrial enzymes for copper-dependent activation or ❹ subcellular compartments for storage or loading into secretory copper-dependent enzymes. However, if copper accumulates beyond homeostatic capacities, fungi employ copper detoxification mechanisms such as ➎copper sequestration or efflux. ➏ Under conditions of extracellular copper deficiency cells mobilize vacuolar copper stores. ➐ In fungi, many of these processes are controlled at the level of transcription by copper-responsive transcription factors that regulate the expression of these components in response to intracellular copper status.
COPPER TRANSPORT ACROSS MEMBRANES
Fungi can assume many forms, such as hyphae, spores, and yeast cells, but in all forms they are surrounded by both a carbohydrate-rich cell wall and a plasma membrane. The fungal plasma membrane is largely impermeable to copper, creating the need for a copper uptake system that facilitates the import of copper across the lipid bilayer and into the cytosol. Moreover, copper transport must occur across a number of distinct membranes, such as that which demarcates the secretory compartments, the mitochondrial inner membrane, and specialized membranes in fungal spores (Figure 2). Low-affinity copper uptake systems such as the Fe2+ , Cu+ , and Zn2+ transporter Fet4 in the yeast Saccharomyces cerevisiae have been reported (75); however, an ideal copper transporter would have both high affinity and high specificity for copper, so as to acquire copper when it is scarce, respond to changing needs for copper, and ignore other charged ions. Given that copper can engage with multiple biological ligands, a copper transporter should outcompete other ligands in the local environment to coordinate to copper, yet release copper once it has been transported to the desired destination. Fungi express a family of dedicated, high-affinity copper transporters (Ctr) (39, 40, 122, 124, 175). Indeed, the Ctr proteins are not only specific for copper but also exquisitely selective, which is due to transporting the reduced form of copper, Cu+ , rather than Cu2+ (76). Ctr family transporters are characterized by the presence of three transmembrane domains per polypeptide, with a characteristic Met-X3-Met motif located in the second transmembrane domain that is absolutely necessary for Cu+ transport (131). Biochemical, genetic, and structural studies demonstrate that three Ctr protein monomers assemble as a homotrimer to form a functional transporter unit (3, 43, 131).
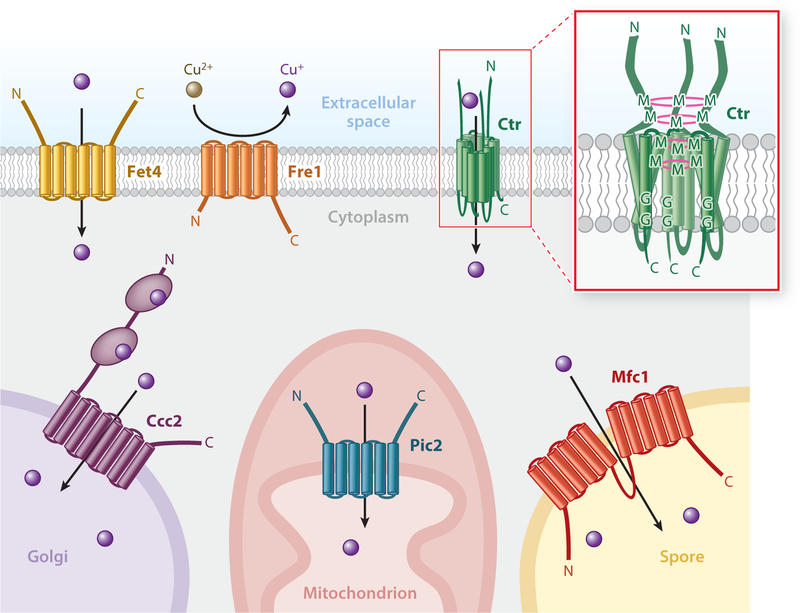
Figure 2.
Copper transport across membranes. The Fet4 iron and zinc transporter is capable of mediating low-affinity copper transport from the extracellular environment into the cytoplasm. The plasma membrane Ctr proteins, in combination with the cupric reductase Fre1, mediate high-affinity copper transport. (Inset) Ctr transporter organization as a homotrimer. Pink rings represent potential layers of copper coordination forming a selectivity filter at the mouth of the pore. Several additional transporters function to compartmentalize cytoplasmic copper. Mfc1 transports copper into the developing forespore during meiosis, Pic2 mediates copper transport into the mitochondrial matrix, and Ccc2 pumps copper into the Golgi network. Abbreviations: C, carboxyl terminus; G, glycine residue; M, methionine residue; N, amino terminus.
Ctr family members function both at the plasma membrane to import Cu+ and on the vacuolar membrane to mobilize luminal Cu+ stores. S. cerevisiae encodes two plasma membrane Ctr family members, Ctr1 and Ctr3, that possess an extracellular domain with methionine- and cysteine-rich motifs, respectively, that are proposed to concentrate copper at the transmembrane pore (Figure 2) (93, 131). Although the extreme amino termini of these metal-binding regions are dispensable for copper transport, a conserved Met/Cys-X-Met motif near the first transmembrane domain forming the pore is strictly required for function. These residues have been proposed to form a thiol-rich track, along with the Met-X3-Met motif of the second transmembrane domain, that funnels Cu+ into the pore formed by the homotrimeric transporter (82). The ability of Ag+ , a metal ion that is isoelectric to Cu+ , to compete for uptake and the requirement in S. cerevisiae for members of a ferric/cupric metalloreductase family of proteins located on the cell surface support the notion that Ctr proteins transport Cu+ (76). Other fungi also possess cupric reductases and two functionally redundant plasma membrane Ctr proteins, with Cryptococcus neoformans expressing Ctr1 and Ctr4 and Schizosaccharomyces pombe harboring Ctr4 and Ctr5 (18, 175). However, rather uniquely, S. pombe Ctr4 and Ctr5 form obligatory heteromultimers that constitute a functional transporter (175). Loss of either Ctr4 or Ctr5 renders the other subunit unable to drive high-affinity Cu+ uptake, likely owing to an inability to be properly trafficked to the plasma membrane. In contrast, S. cerevisiae and C. neoformans require that both plasma membrane transporters be absent for the cells to manifest a copper-deficiency growth phenotype due to loss of copper-dependent enzyme activity (45, 93).
In addition to the Ctr1/3 Cu+ transporters on the plasma membrane, S. cerevisiae Ctr2 and S. pombe Ctr6 mediate the transport of intracellular copper from the vacuolar lumen to the cytoplasm (21, 136). In keeping with a putative Cu+ substrate, Ctr2 requires the vacuolar-localized Fre6 reductase for the mobilization of copper stores to the cytosol, as a fre6Δ mutant phenocopies a ctr2Δ mutant (137). Further supporting Cu+ as substrate, a truncated Ctr2 variant also containing a W7R mutation mislocalizes to the plasma membrane and imports copper in a manner that is dependent on the cell surface Fre1 reductase (137). It is interesting that while expression of the homologous gene CTR6 in S. pombe is elevated in response to copper deficiency, S. cerevisiae CTR2 expression is induced in response to iron deficiency, perhaps reflecting its requirement largely under conditions where extracellular copper pools are not available to Ctr1/Ctr3 (21, 137).
A more recently characterized S. pombe copper transporter, Mfc1, is a member of the major facilitator superfamily (MFS) of transporters and possesses 12 helical transmembrane domains connected by short hydrophilic loops (13). An exception is loop 6, which is greatly enlarged and functions to separate the protein into two connected halves. This allows for interdomain movement between the amino and carboxyl halves required for substrate transport (172). Of note, amino acid sequence analysis of Mfc1 shows four pairs of methionine residues that could function to coordinate copper during transport. Interestingly, the MFC1 gene is highly expressed during meiosis, and Mfc1 localizes to forespore membranes that accrue the products of meiosis. Presumably this is to ensure that the developing spores have an adequate supply of copper, since the copper-dependent amine oxidase Cao1, which localizes primarily in the forespores, shows a dramatic decrease in activity in cells lacking MFC1 (128). Normal meiotic progression is also delayed in cells lacking MFC1 when copper is limited (13). Further study will be required to determine the nature of copper transported by Mfc1, whether it depends on a cupric reductase, and whether other fungal and mammalian species contain a homologous copper transporter.
INTRACELLULAR COPPER BUFFERING
Once Cu+ enters the cytoplasm, cells are immediately faced with the challenge of buffering free Cu+ to preclude the inappropriate metalation of proteins with solvent-exposed copper-ligands and its participation in uncontrolled Fenton reactions. Moreover, the metal ion must be directed to its appropriate intracellular destinations with some specificity. In fact studies suggest that, in general, cells contain virtually no free copper, with the majority existing in a buffered pool (132). One component of this copper buffering pool is a family of small, cysteine-rich, metal-binding proteins known as metallothioneins (MTs) (101, 168).
The CUP1 gene from S. cerevisiae encodes, arguably, the best-characterized fungal MT and was initially identified by investigation into naturally occurring yeast strains resistant to high levels of copper (24). Copper-sensitive cells were not able to grow on plates containing 0.3 mM CuSO4, whereas isogenic copper-resistant cells were able to grow on plates containing 1.75 mM CuSO4. Subsequent stepwise selection yielded strains capable of growth in up to 12 mM CuSO4, and molecular analysis of these strains revealed that they had undergone tandem amplification of the CUP1 gene (53).
The Cup1 protein is capable of binding up to eight Cu+ ions, with each trigonally coordinated to å sulfur atom of cysteine residues at a distance of 2.23 Å, as determined by X-ray absorption analysis (66). Although largely unstructured in the apo form, the Cu+ - and Ag+ -bound three-dimensional solution structures of Cup1 have been determined by 1H two-dimensional NMR spectroscopy (23, 126). Although lacking any α-helix or β-sheet motifs, the peptide backbone folds around a single metal cluster in two large parallel loops. The Cu+ -bound structure of another MT, from the yeast Neurospora crassa, has also been investigated by 1H-NMR spectroscopy (29). The polypeptide backbone structure is markedly different from that of S. cerevisiae MT, presumably because spacing of the Cu+ -coordinating cysteine residues is different. This, and the lack of structure in the absence of Cu+, suggests that the overall tertiary structures of MTs are almost completely determined by metal coordination.
Aside from directly sequestering and preventing copper toxicity in S. cerevisiae, overexpression of CUP1 can partially rescue growth phenotypes of yeast lacking copper-zinc superoxide dismutase (SOD1) (149). These and other experiments suggest that MTs can play a role in cellular defense against oxidative stress, as well as copper detoxification (47, 74, 151). A second MT-like protein encoded by S. cerevisiae, Crs5 (33), is less effective than Cup1 in copper buffering but detoxifies excess copper (86). Other fungi such as Candida glabrata and C. neoformans also encode MTs that provide powerful cytosolic Cu+ -buffering capabilities (44, 113).
Sod1 is highly abundant in the cytosol of S. cerevisiae and in addition to its role in protection against oxidative stress due to disproportionation of oxygen radicals, it is also a copper-buffering agent (35). This function appears to be independent superoxide scavenging activity, since SOD1 is able to protect cells from copper toxicity in anaerobic environments, where there is little superoxide. Also, the PMR1, BSD, and ATX1 genes that suppress oxygen toxicity in cells lacking SOD1 fail to suppress copper sensitivity in the same cells.
A powerful source of intracellular copper buffering is also provided by the tripeptide glutathione (GSH), synthesized from cysteine, glutamic acid, and glycine in a two-step mechanism involving the enzymes glutamate cysteine ligase and glutathione synthetase (54, 55, 107). While serving as a general cellular antioxidant, GSH also binds metals including Cu+ through thiol ligands (32). Cellular fractionation of both N. crassa and S. cerevisiae demonstrates that a measurable portion of copper is GSH bound (67, 103). In vitro experiments suggest that GSH-Cu chelate complexes can donate bound Cu+ to known Cu+ -binding proteins such as apo-hemocyanin, MT, and the copper chaperone Atx1 (25, 51, 115), suggesting a potential copper-dissemination role. Free amino acids such as methionine, cysteine, and histidine have also been suggested to participate in intracellular copper buffering (123, 173).
COPPER TRANSPORT TO THE SECRETORY COMPARTMENT
Beyond intracellular copper buffering, it is critical to distribute copper to physiologically relevant proteins and compartments to drive copper-dependent biochemical reactions that take place inside fungal cells or in their local environment. Several fungal enzymes that traverse the secretory pathway—such as the multi-copper ferroxidase Fet3, critical for iron acquisition; laccase, required for melanin biogenesis; and secreted lytic polysaccharide monooxygenases (LPMOs) that degrade extracellular complex carbohydrates—require copper for proper folding, secretion, and activity (7, 85, 158). Fet3 activity, which requires Cu+, is a useful measure of the ability of copper to enter the secretory pathway, as S. cerevisiae lacking metalated Fet3 fails to grow under low-iron conditions. Interestingly, S. cerevisiae cells lacking the Tfp1 subunit of the vacuolar H+ -ATPase and defective in post-Golgi vesicle acidification, as well as cells lacking the post-Golgi localized CLC chloride (Cl− ) channel Gef1, are unable to grow on low-iron medium owing to inactive Fet3, but growth is rescued by exogenous copper (64). These observations prompted a model in which proper acidification of post-Golgi compartments is critical for Cu+ loading onto Fet3. Since acidification leads to an increase in membrane potential, Gef1 may be necessary to counterbalance this with an influx of Cl− . It has also been suggested that Cl− acts as an allosteric effector required for proper copper metalation of Fet3 (41).
An exciting new discovery relevant to the copper metalation of Fet3 suggests that compart-mentalization of K+ in the trans–Golgi network (TGN), by the K+ /H+ exchanger Kha1, plays a critical role in this process (169). S. cerevisiae cells lacking KHA1 display a growth defect in nonfermentable carbon sources that can be rescued by either iron or copper supplementation and show a decrease in iron accumulation, indicative of defective Fet3. Although Kha1 is a proposed K+/H+ exchanger, no difference in cytosolic or TGN pH was observed between cells possessing Kha1 and those lacking Kha1. These results, along with in vitro experiments showing that K+ promotes binding of copper to apo-Fet3 but not to the E487A or N49A mutants predicted to be deficient in K+ binding, support the notion that the requirement of Kha1 for proper Fet3 activity is due to a requirement for K+ . Consistent with these results and the requirement for Kha1 for copper-dependent Fe acquisition, KHA1 mRNA levels are increased by the iron-responsive transcription factors Aft1/2.
While Tfp1, Gef1, and Kha1 are required for Cu+ loading onto Fet3, they do not directly transport Cu+ into the secretory lumen. To accomplish this in a controlled manner, integral-membrane Cu+ -transporting ATPases, such as Ccc2 in S. cerevisiae, deliver Cu+ into the secretory compartment, where it is loaded onto luminal copper-requiring proteins (58). Deletion of the CCC2 gene results in a severe decrease in the activity of Fet3 and a concomitant iron deficiency (174). Ccc2 is, in general, conserved from bacteria to humans, and loss-of-function mutations in the corresponding human genes cause Menkes, or Wilson, disease (42).
Ccc2 operates in Cu+ delivery to the secretory compartment using two cytosolic metal-binding domains (MBDs) that adopt a ferredoxin-like βαββαβ fold capable of binding Cu+ via a conserved Cys-X2-Cys motif in which the two cysteine residues bind Cu+ in a solvent-exposed, bidentate fashion (9, 10). These domains are necessary for in vivo activity, in that a yeast strain expressing mutant Ccc2 lacking both MBDs fails to grow on low-iron media, presumably owing to an inability to metalate Fet3 and support iron import (116). The MBDs are thought to function by receiving Cu+ from an intracellular chaperone (see below) and passing Cu+ to the transmembrane exit tunnel. A homolog of Ccc2 (LpCopA) from Legionella pneumophila has been crystalized and provides a framework for modeling the mechanism by which this family of copper-ATPases function (72). Cu+ bound to the MBDs is first transferred to a Cu+ -binding site located on the cytoplasm-facing side of the transmembrane domains comprising glutamic acid, aspartic acid, and methionine residues. Notably, this pore entrance forms an α-helical platform that may serve as a docking station for the Cu+ -loaded MBD or a cytosolic Cu+ chaperone. Owing to the relative low affinity of Cu+ binding to carboxylate moieties of glutamic acid and aspartic acid residues, this binding event would be transient and result in Cu+ transfer to the high-affinity intramembrane Cu+ -binding site made up of sulfur ligands contributed by two cysteines and a methionine residue, where the Cu+ is bound in a stable trigonal planar coordination mediated by the three sulfur atoms at a distance of ~2.25Å, as determined by EXAFS analysis (111). Binding of Cu+ to this high-affinity site triggers ATP hydrolysis and subsequent Cu+ occlusion and transfer to a transient binding site composed of two methionines and a glutamic acid residue before dissociation and release into the secretory lumen (4). Catalytic activity requires the formation of an ATP-dependent acyl-phosphate intermediate at the essential aspartic acid residue in a conserved DKTGT motif to generate the force necessary to pump Cu+ against a gradient (106).
COPPER CHAPERONES
Since the cellular copper buffering pool sequesters free copper from the cell, copper needs to be directed to the desired compartments. For this purpose, fungi have evolved highly specific and dedicated chaperones to direct copper trafficking. Efficient Cu+ transfer to the secretory compartment, particularly under conditions of Cu+ limitation, requires a metallochaperone for Cu+ delivery to Ccc2. The metallochaperone for Ccc2 is Atx1, an ~8-kDa protein originally identified in a genetic screen as a multicopy suppressor of oxygen toxicity in cells lacking SOD1 (104). Cells lacking ATX1 show reduced Ccc2-mediated activation of Fet3, and a corresponding iron deficiency, a phenotype that can be rescued by the addition of exogenous copper (105). In keeping with the critical role for Cu+ loading onto Fet3 in the secretory compartment, expression of the ATX1 gene is induced under conditions of low iron, via the Aft1/2 iron-sensing transcription factors, rather than in response to low copper levels (105).
In keeping with the reactivity of copper ions when uncontrolled, Atx1 delivers Cu+ to Ccc2 by directly interacting with the cytosolic MBDs in a fashion that allows facile, yet controlled, copper transfer (Figure 3a) (130). Nature has cleverly reutilized the ferredoxin-like βαββαβ fold found in Ccc2, which is also adopted by Atx1, to drive protein-protein interactions along complementary surfaces (6, 140). As in the Ccc2 MBDs, Cu+ binds to Atx1 at an invariant Cys-X2-Cys motif, essential for this transfer, in which the two cysteine residues bind copper in a solvent-exposed, bidentate fashion. Additionally, Atx1 possesses a highly charged lysine-rich surface required for the Atx1 Cu+ transfer to Ccc2 (129). The Ccc2 MBDs possess a patch of negatively charged residues that form salt bridges with Atx1, orienting the proteins for the formation of metal-bridged coordination intermediates, which would both facilitate Cu+ transfer and preclude nonspecific interactions with Cu+ (5, 8, 22). The thermodynamic gradient for metal transfer is shallow (Kexchange ~1.4), allowing for bidirectional transfer between Atx1 and Ccc2, which could function in the regulation of copper loading into the secretory compartment (11, 83, 170). Outside of interactions with Ccc2, the Atx1 homolog in S. pombe has been shown to mediate Cu+ transfer to the amine oxidase Cao1, demonstrating that a single chaperone may metalate multiple proteins (125).
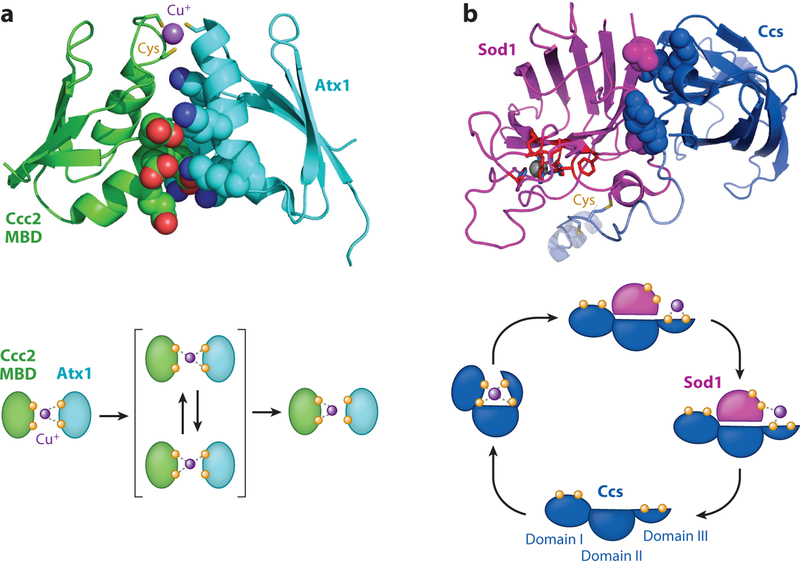
Figure 3.
Metallochaperone-mediated copper transfer. (a) Structure of Atx1 (cyan) bound to a metal-binding domain (MBD) of Ccc2 (green) reveals electrostatic interactions mediating protein-protein interactions (top). Atx1 contains several positively charged residues (blue space filling) that interact with several negatively charged residues (red space filling) of the MBD. This drives proper orientation of the copper (purple spheres)-coordinating cysteine residues (yellow sticks) to facilitate copper transfer (bottom). Modified from PDB 2GGP. (b) Structure of Ccs (blue) bound to Sod1 (magenta) reveals hydrophobic interactions (space filling) that mediate the docking interactions (top). Copper is transferred from the cysteine residues of Ccs to the active site pocket (red) of Sod1 (bottom). The three domains of Ccs are indicated. Modified from PDB 1JK9.
While Sod1 serves as a copper buffer to protect cells under elevated copper conditions, super-oxide disproportionation activity requires a dedicated chaperone to deliver the essential copper cofactor (36, 61, 80). The copper chaperone for Sod1 (Ccs) comprises three distinct domains (Figure 3b). Ccs domain 1 displays striking similarity to the Atx1 and Ccc2 MBD ferredoxin-like βαββαβ fold (100). The conserved Cys-X2-Cys motif within this domain binds Cu+ but is largely required for Cu+ loading of Sod1 under copper-limiting conditions. Domain 2 forms a Greek key β-barrel fold, similar to Sod1, and this domain is responsible for direct interactions with Sod1, forming a pseudodimer, and mutations abrogating this interaction prevent copper metalation and activation of the homodimeric Sod1 enzyme (98, 99). Domain 3, bearing a critical Cys-X-Cys motif, is responsible for Cu+ binding and essential for copper transfer to Sod1.
Ccs and Sod1 were initially thought to reside in the cytosol, but it is now recognized that a significant fraction of Ccs and Sod1 exists in the mitochondrial intermembrane space (IMS) (Figure 4) (146). In contrast to its noncritical role in loading of Sod1, Ccs domain 1 plays an indispensable role in targeting the protein to mitochondria. After import into the IMS through the TOM (translocase of the outer membrane) complex, Ccs domain 1 interacts with the mitochondrial disulfide relay system protein Mia40, which is anchored to the mitochondrial inner membrane (135). Mia40 contains six conserved cysteine residues that are maintained in an oxidized state by the flavin-linked sulfhydryl oxidase Erv1 (114). Upon interaction with oxidized Mia40, a disulfide bond is transferred to the two cysteine residues of the Cys-X2-Cys motif from Ccs domain 1 (92). This disulfide-containing form of Ccs is retained within the IMS, presumably owing to a stable folded conformation, as the TOM complex is only capable of transporting unfolded proteins. In the absence of Ccs, Sod1 fails to accumulate in the mitochondria, implicating the importance of Ccs in this compartment (146). The presence of Ccs in both the cytoplasm and the IMS suggests that it could serve as a mitochondrial metallochaperone providing copper for multiple mitochondrial functions. However, yeast lacking CCS retain wild-type mitochondrial copper levels and cytochrome c oxidase (COX) activity (30).
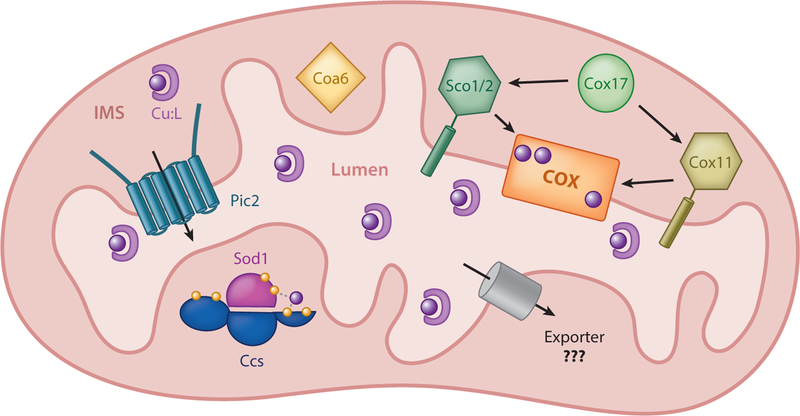
Figure 4.
Mitochondrial copper handling. Pic2 imports copper from the intermembrane space (IMS) to the lumen, where it is bound to a ligand (CuL). This copper is thought to be exported by an unidentified transporter from the matrix into the IMS, where it is acquired by Cox17 and transferred to Sco1/2 or Cox11 for insertion into COX subunits, or acquired by Ccs for insertion into Sod1. Coa6 plays a critical role in COX metalation and assembly through currently unknown mechanisms.
COPPER TRAFFICKING TO MITOCHONDRIA
COX is the terminal enzyme of the electron transport chain in mitochondria, playing a critical role in energy generation via oxidative phosphorylation. COX is composed of 12 subunits with 2, Cox1 and Cox2, requiring copper (Figure 4). The copper sites are buried within the structure, therefore requiring coordination between copper availability and the assembly of the complex. COX biogenesis requires >25 accessory proteins known as COX assembly factors. At least 9 of these factors (Cox11, Sco1, Sco2, Cox17, Coa6, Cmc1, Cmc2, Cox19, Cox23) facilitate insertion of the copper cofactors and trafficking of IMS copper.
Cox1 binds a single copper atom. The metal center is designated the CuB site and is located ~13 Å below the membrane surface (153). Experiments performed in Rhodobacter sphaeroides demonstrated that proper metalation of the CuB site requires the accessory factor Cox11 (78). The amino-terminal domain of Cox11 anchors the protein to the inner membrane, with the soluble carboxyl-terminal domain directly binding copper via three cysteine residues (27). S. cerevisiae cells lacking COX11 show reduced COX activity and have lower levels of Cox1 polypeptide, demonstrating the importance of copper for Cox1 stability within the fully assembled holoenzyme (155).
Cox2 harbors a binuclear copper site (CuA) and is buried in the enzyme complex ~8 Å above the membrane surface into the IMS. Proper metalation of this site requires a dedicated accessory factor, Sco1, as yeast sco1Δ mutants are devoid of COX activity and display dramatically decreased levels of Cox2 (71, 144). Similar to Cox11, the amino-terminal domain of Sco1 protrudes into the inner membrane, with the soluble carboxyl-terminal domain containing conserved cysteine residues that directly bind Cu+ in a solvent-exposed manner (120). Mutation of these cysteine residues results in nonfunctional COX in S. cerevisiae (138). A Sco1 paralog, Sco2, is also involved in copper loading of COX, although its function in fungi is at least partially redundant with that of Sco1.
Although Cox11 and Sco1 directly metalate COX subunits, they both require the presence of another accessory protein, Cox17, to obtain Cu+, and S. cerevisiae cox17Δ mutants lack detectable COX activity (70, 81). In contrast to Cox11 and Sco1, Cox17 is a soluble protein that exists in both the cytosol and the mitochondrial IMS (20). This observation was initially understood to support the hypothesis that Cox17 may be responsible for ferrying Cu+ into mitochondria; however, tethering of Cox17 to the inner membrane maintained normal COX activity and normal mitochondrial Cu+ levels (112), indicating that Cox17 critically functions in this regard in the IMS.
Another recently described factor involved in copper loading of COX is Coa6 (68). S. cerevisiae lacking COA6 displays decreased respiration and COX assembly. Addition of exogenous copper rescued these defects, suggesting a role for Coa6 in the Cu+ -delivery pathway. The presence of conserved Cys-X9-Cys-Xn-Cys-X10-Cys motif in Coa6 is reminiscent of previously described Cu+ -binding motifs, and mutation of any of the four cysteine residues to alanine results in a protein incapable of restoring COX activity to wild-type levels in S. cerevisiae lacking endogenous COA6. The Cys-X9-Cys-Xn-Cys-X10-Cys motif is also found in Cmc1, Cmc2, Cox19, and Cox23, and these proteins have been implicated in copper delivery in the IMS. Further studies will be required to uncover the molecular mechanisms by which these proteins are involved in COX metalation, both in yeast and in mammals.
A fascinating observation was that only a fraction of total mitochondrial copper was associated with Sod1 and COX (30, 62), with the majority found in a soluble, anionic copper ligand complex (CuL). The exact nature of the CuL complex awaits further investigation, but it is resistant to protease digestion, displays anionic characteristics, and has a fluorescence maximum at 360 nm, suggesting the ligand might be a metabolite or nucleotide. While CuL is found in the cytosol, the majority of Cu+ -bound CuL is in the mitochondrial matrix. Cleverly expressing SOD1 and CRS5 as Cu+ -chelating proteins that depleted the bioavailable matrix copper pool, Cobine et al. (31) suggested that matrix Cu+ pools, rather than those originating from the cytosol to IMS, are destined for loading onto COX and mitochondrial Sod1 in the IMS. As the inner membrane is impermeable to most ions, systems to import copper from the IMS to the matrix, as well as to mobilize matrix copper pools back out into the IMS, must be operational.
Pic2 is a mitochondrial carrier family (MCF) protein, previously implicated in phosphate transport, that was recently demonstrated to transport copper into the mitochondrial matrix (159). S. cerevisiae cells lacking PIC2 display growth inhibition and lower levels of Cox2 when Ag+ is present in the growth media, as well as decreased COX activity when grown on a nonfermentable carbon source. Also, deletion of PIC2 results in a decrease in total mitochondrial copper, and isolated mitochondria from pic2Δ cells show decreased rates of copper uptake. Lactococcus lactis bacterial cells expressing the S. cerevisiae Pic2 protein display enhanced copper uptake when the transporter is presented with either free copper or the CuL complex isolated from S. cerevisiae mitochondria. Further investigation will be required to determine the nature by which Pic2 trans-ports copper, or CuL and the identity of the transporter that mobilizes copper out of the matrix to the IMS.
In response to copper starvation, C. albicans induces a switch from Cu,Zn SOD1 to Mn SOD3 while maintaining COX activity (26). This switch in Sod enzymes eliminates Cu,Zn Sod1 from the IMS, potentially rendering mitochondria susceptible to superoxide. However, superoxide is maintained at low levels by inducing expression of an alternative iron-dependent oxidase, Aox2, which is able to accept electrons directly from coenzyme Q and bypass a major source of IMS superoxide, complex III of the electron transport chain. Thus, when copper is scarce, C. albicans is capable of prioritizing available copper for COX at the expense of Cu,Zn Sod1.
COPPER-RESPONSIVE TRANSCRIPTION FACTORS
Reciprocal and Separable Responses to Copper Toxicity and Copper Starvation
The regulation of copper homeostasis in fungi is primarily controlled at the level of transcription by copper-responsive transcription factors. These fungus-specific transcription factors sense changes in copper status and appropriately activate the copper acquisition or detoxification genes under conditions of copper limitation or excess, respectively. Characteristic of these transcription factors are cysteine-rich regions, generally arranged as Cys-X-Cys and/or Cys-X2-Cys repeats, which in some cases have been shown to bind Cu+ as a direct mechanism to survey and respond to copper status (37, 88). Many fungi contain two copper-responsive transcription factors that reciprocally activate genes in response to copper starvation or toxicity.
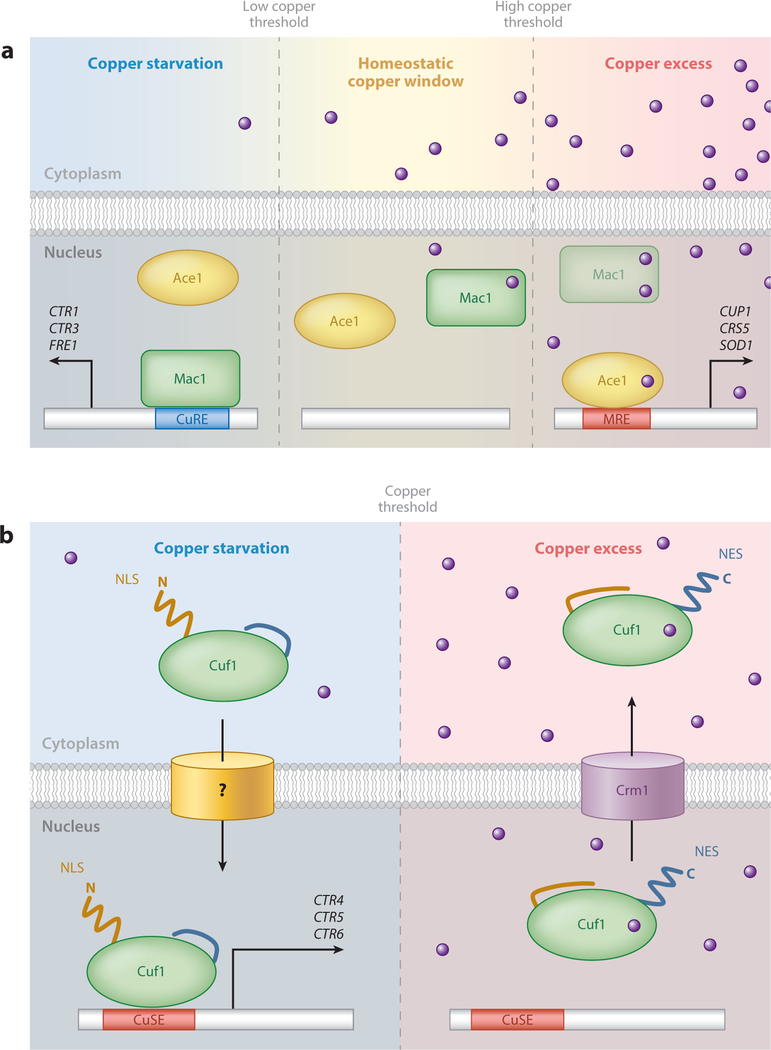
Initially discovered in S. cerevisiae, Ace1 activates expression of the MT genes CUP1 and CRS5, as well as SOD1, in response to excess copper (>1 μM) (Figure 5a) (33, 73, 150, 165). Ace1 contains a single cysteine-rich region directly downstream of its conserved minor groove DNA-binding domain (DBD) (60). This cysteine-rich region binds four Cu+ atoms to form a tetra-copper-thiolate cluster (37, 148). Cluster formation triggers intramolecular conformational changes within the amino-terminal DBD that activates monomeric Ace1 DNA binding (60). Metal ion–ligand activation is highly dependent upon and specific for Cu+ over other monovalent and divalent metal ions, with the exception of isoelectric Ag+ (60). Within the Ace1 DBD lies a Cys3-His zinc finger and basic KGRP amino acid motif required for establishing DNA minor groove contacts (46, 50, 154). Activated Ace1 recognizes specific metal response elements (MREs) in target gene promoters with the consensus sequence 5’-HTHNNGCTGD-3’(D = A, G, or T; H = A, C, or T), and MRE mutations result in reduced Ace1 occupancy and loss of copper-responsive gene expression (49, 60, 84). The importance of Ace1-mediated activation of copper detoxification genes is exemplified by the copper hypersensitivity phenotype of ace1Δ cells, which fail to induce MT gene expression. Studies using an amino-terminus-truncated variant of Ace1 (residues 1–122), which preserves the cysteine-rich and KGRP DBD, demonstrated this domain is competent for Cu+ cluster formation and MRE recognition both in vitro and in vivo (34, 59). However, target gene activation was abolished, indicating the amino-terminal domain confers DNA binding and specificity whereas the carboxyl-terminal domain is required for transcriptional activation (34).
Figure 5.
Copper-responsive transcription in fungi. (a) Two-component copper-sensing transcriptional regulation in Saccharomyces cerevisiae. Mac1 induces expression of the high-affinity copper acquisition machinery during copper limitation to facilitate copper uptake, through recognition of promoter CuREs. As intracellular copper equilibrates to homeostatic levels, copper cluster formation in Mac1 results in its inactivation, and Mac1 is degraded at higher copper concentrations. Under conditions of copper excess, activated Ace1 induces expression of the copper detoxification machinery through recognition of target gene promoter MREs. The stronger copper-binding affinity of Mac1 ensures no overlap in activation with the weaker copper-binding affinity of Ace1, effectively establishing a homeostatic copper window (163). (b) Single-component copper-sensing transcriptional regulation in Schizosaccharomyces pombe. During copper limitation, an exposed amino-terminal nuclear localization sequence (NLS) of Cuf1 directs its nuclear translocation, via an unknown importer, where Cuf1 induces expression of the high-affinity copper-acquisition machinery through recognition of target gene promoter CuSEs. As copper levels exceed a homeostatic threshold, copper cluster formation inactivates Cuf1 DNA binding, and the subsequent unmasking of a carboxyl-terminal nuclear export signal (NES) targets Cuf1 for nuclear export via the Crm1 exportin. Abbreviations: CuRE, copper-responsive element; CuSE, copper-signaling element; MRE, metal response element.
Additional Ace1 homologs have been discovered in several other fungal species, including AMT1 from the opportunistic pathogen C. glabrata, that function similarly to S. cerevisiae Ace1 (177). Expression of AMT1 in S. cerevisiae ace1Δ cells induces S. cerevisiae CUP1 expression in a copper-dependent manner, ameliorating the copper sensitivity phenotype (152). Similarly, S. cerevisiae Ace1 activates C. glabrata MTI in a copper-dependent manner, demonstrating these proteins are not just structural but also functional homologs that recognize heterologous MREs (152). In contrast to Ace1, which is basally expressed irrespective of copper status, Amt1 is under positive autoregulatory feedback due to the presence of an MRE within its own promoter and is critical for eliciting an adequate copper detoxification response whereby Amt1 levels are amplified to activate expression of MT genes in this organism (176).
S. cerevisiae has a second copper-regulated transcriptional activator that responds to copper limitation: Mac1 activation induces expression of the high-affinity Cu+ transporters encoded by CTR1 and CTR3 and the plasma membrane reductase encoded by FRE1 during copper limitation (Figure 5a) (65, 90, 96). Mac1 contains two functionally distinct cysteine-rich repeats located within the carboxyl-terminal region that collectively have the potential to coordinate up to eight Cu+ ions (87, 88). In contrast to Ace1, excess copper (>10 nM) results in Mac1 inactivation and potential degradation at even higher copper concentrations (≥10 μM) in vivo (178). This rapidly suppresses CTR1/3 transcription, effectively providing a secondary countermeasure to prevent copper toxicity. Under copper limitation, activated Mac1 binds to copper-responsive elements (CuREs) in the promoters of target genes with a defined consensus sequence 5 -TTTGCTC-3, strikingly similar to Ace1 MREs (96, 171). Consistent with the role of Mac1 in copper acquisition, mac1Δ cells display impaired iron acquisition, respiratory defects, and sensitivity to ROS due to reduced copper accumulation and defective metalation of Fet3, COX, and Sod1, respectively (90). Interestingly, a single mutation of a terminal histidine to glutamine in one of the cysteine-rich motifs results in constitutive Mac1 activation and induction of CTR1/3 and FRE1, regardless of copper status (90, 96). These cells display a copper hypersensitivity phenotype similar to that of ace1Δ cells owing to the chronic expression of the copper-acquisition machinery (178). Although copper-binding mutants have been useful tools to study Mac1, we currently have a very limited understanding of exactly how Mac1 senses copper limitation.
Single-Component Copper-Responsive Factors
Utilization of two distinct copper-sensing transcription factors such as Ace1 and Mac1 is not conserved throughout the fungal kingdom. Evolutionarily distant fungi have evolved to use a single copper-responsive transcription factor exemplified by Cuf1. Cuf1 has been thoroughly investigated in S. pombe, and while its overall sequence homology to Ace1 or Mac1 is low, hallmarks from both proteins are conserved (95). The amino-terminal DBD shares a high degree of identity with Ace1 (51%), and the carboxyl-terminal domain contains a cysteine-rich region with identity to Mac1 (48%), therefore appearing chimeric in nature (95). Cuf1 function is analogous to that of Mac1: It is activated during copper limitation and induces expression of the plasma membrane copper transporters CTR4 and CTR5 and the vacuolar copper transporter CTR6 by binding to cis-acting regulatory elements in their promoters (17, 21, 95, 141). Excess copper is sensed through the cysteine-rich region and results in Cuf1 inactivation (Figure 5b). Similar to S. cerevisiae mac1Δ cells, cuf1Δ cells display characteristic copper starvation phenotypes, which are reversed by cop-per supplementation. Interestingly, the S. pombe genome does not encode any known copper-detoxification MTs; therefore, there is no known role for Cuf1 in the copper detoxification response aside from transcriptional silencing of copper-uptake genes at high copper concentrations.
While Cuf1 is functionally analogous to Mac1, the two have key differences. Cuf1 binds DNA sequences with strong similarities to the Ace1/Amt1 MRE, termed copper-signaling elements (CuSEs), with the consensus sequence 5′-D(T/A)DDHGCTGD-3′ (D = A, G, or T; H = A, C, or T) (14). Studies of S. cerevisiae ACE1 transcomplementation into S. pombe cuf1Δ cells resulted in sustained activation of CTR transporter expression even at high copper concentrations (>100 μM) and a copper hypersensitivity phenotype. Additionally, a chimeric Cuf1 protein containing the amino-terminal 1–63 residues of S. cerevisiae Ace1 could rescue the copper starvation phenotype of cuf1Δ cells, suggesting that although Cuf1 is functionally homologous to Mac1, its mode of DNA binding and recognition is analogous to that of Ace1 (14). Therefore, Cuf1 represents a class of transcription factors composed of two separate functional domains from Ace1/Mac1 of S. cerevisiae.
Further structure-function analysis of Cuf1 revealed additional regulatory mechanisms controlling Cuf1 function. The amino-terminal 11–53 residues of Cuf1 contain a noncanonical nuclear localization sequence (NLS) rich in positively charged amino acids required for nuclear import during copper limitation (15). Cuf1 also contains a leucine-rich nuclear export signal (NES) down-stream of the cysteine-rich region and is exported from the nucleus during copper excess via the nuclear exportin Crm1. A functional NES and cysteine-rich domain are required for nuclear export, as mutations of either resulted in nuclear accumulation and unregulated expression of CTR4/5/6 (16). However, nuclear accumulation is not sufficient for unregulated gene expression, as a crm1+ mutant, which accumulates nuclear Cuf1, could still be inactivated by copper. From this data, a model has been proposed for Cuf1 regulation (Figure 5b).
The basidiomycete C. neoformans also encodes a protein with similarity to S. pombe Cuf1 (CnCuf1). CnCuf1 is unique in that it serves the dual regulatory functions of Mac1 and Ace1 for regulating both copper acquisition and copper detoxification (45). During copper limitation, CnCuf1 activates expression of the CTR1 and CTR4 copper transporters. As copper accumulates toward toxic levels, CnCuf1 switches its induction profile to express the copper-detoxifying MTs, MT1 and MT2. Surprisingly, one study reported cytoplasmic retention of CnCuf1 at the cell periphery under high-copper conditions, despite MT1/2 induction in a copper- and CnCuf1-dependent manner (89). Elucidation of how CnCuf1 senses and responds to both extremes in the copper spectrum requires further examination. Possibly, the presence of four cysteine-rich regions within the amino-terminal half of CnCuf1 increases binding capacity for copper compared to Ace1 or Mac1. Could each cysteine-rich region have a copper-binding affinity gradient per-mitting a fine-tuned response of CnCuf1 toward activating copper-acquisition or -detoxification genes? Additionally, a unique feature of CnCuf1 is a large (~500 amino acids) carboxyl-terminal domain with no known homology to other proteins. This large domain contains no cysteines; how-ever, it contains multiple methionine residues that could still participate in copper coordination. Perhaps the CnCuf1 amino-terminal and carboxyl-terminal domains separately regulate copper acquisition and detoxification, either by recognition of different cis-acting regulatory elements or by recruitment of different transcriptional accessory proteins required for target gene induction.
COPPER AND FUNGAL PATHOGENESIS
Host Copper Mobilization and the Initial Copper Burst
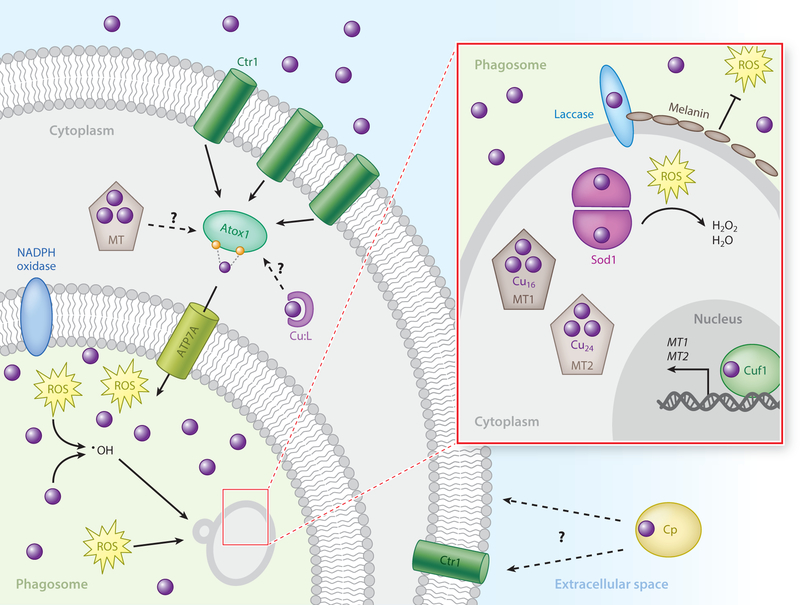
As frontline defenders in immunity, macrophages and neutrophils survey their environment for invading pathogens and activate to signal and recruit other immune cells in response to infection. Activated macrophages engulf foreign microbes, compartmentalizing them within the phagosome, which ultimately matures into a phagolysosome (160). The phagolysosome is a highly inhospitable and toxic environment, with low pH, high concentrations of ROS and reactive nitrogen species (RNS), and several proteases present (19, 143, 160). Whereas iron and zinc, two well-recognized nutritional factors required for virulence, are actively exported from the phagolysosome, copper is specifically delivered to this compartment to induce a state of copper toxicity (117). Accordingly, bacterial pathogens with mutations in copper detoxification P-type ATPase efflux pumps are more susceptible to macrophage killing within this compartment (1, 145, 166).
Macrophage activation by host-derived cytokines such as IFN-γ or bacteria-derived lipopolysaccharide induces expression of mammalian Ctr1 and stimulates copper uptake (166). This response is accompanied by increased expression and relocalization of the mammalian Cu+-transporting P-type ATPase ATP7A (yeast Ccc2 homolog) from the Golgi network to the phagolysosome membrane, effectively rerouting copper to pathogen-containing phagolysosomes (166). X-ray microprobe analyses have confirmed a significant increase in copper content in phagolysosomes of mycobacteria-infected macrophages (161). While evidence supports copper accumulation within the phagolysosome, the source of host-acquired copper is unknown. An immediate acute phase response to infection is increased serum circulation of the multi-copper oxidase ceruloplasmin (Cp), which is capable of binding six atoms of copper per Cp polypeptide and accounts for approximately 95% of total serum copper and thus could serve as a peripheral reservoir to supply copper to immune cells (28, 77). Alternatively, recruited immune cells may contain sufficient intracellular copper stores in endosomes or mitochondria, which could be mobilized to the phagolysosome during infection. While no direct link has been made for Cp supplying copper to immune cells, Cp has been shown to deliver copper to cells in both a Ctr1-dependent manner and a Ctr1-independent manner (91, 134). It is highly unlikely that free copper is a physiological source, as the toxic properties of unbound copper are just as detrimental to host cells.
Pathogen Response to Copper Bombardment
Successful pathogens have evolved effective mechanisms to circumvent host-imposed copper toxicity (Figure 6). Some fungal pathogens, such as C. neoformans, utilize copper-chelating MTs as a primary copper-resistance mechanism (44, 147). While mammalian and other metazoan MTs typically bind fewer than 10 Cu+ ions per polypeptide, the C. neoformans MT1 and MT2 proteins coordinate 16 and 24 Cu+ ions, respectively, providing an unprecedented efficient copper-scavenging mechanism (44). In vivo, live imaging of C. neoformans–infected mice has revealed an environment high in copper at the initial site of infection in the lungs, presumably within the phagosomes of resident alveolar macrophages (44). Copper-specific induction of MT1 and MT2 in the lungs is critical for C. neoformans survival in a pulmonary infection model, and data suggest these MTs serve redundant functions, as single deletions had no effect on virulence in a mouse model of infection. Virulence attenuation was dependent on deletion of both MTs, effectively removing a critical component of the cellular copper-buffering capacity and creating conditions permissive for copper toxicity (44). In addition, the virulence could not be returned if a MT in which all Cu+ -coordinating cysteine residues were mutated to alanine were expressed, confirming the role of copper chelation.
Figure 6.
Model of innate immune cell–directed copper bombardment of the fungal pathogen Cryptococcus neoformans. Activated macrophages induce the expression of the mammalian Ctr1 transporter at the plasma membrane to facilitate copper uptake. Additionally, ATP7A expression is elevated, and the protein localizes to the phagosomal membrane. The copper chaperone Atox1 directs the channeling of copper from Ctr1 at the plasma membrane to ATP7A. The source of extracellular antifungal copper is currently unknown and could involve the acute phase-responsive multi-copper oxidase Cp that has been shown to function in copper import in Ctr1-dependent and -independent mechanisms. Alternatively, intracellular mobilization of copper stores, such as from endosomal compartments, MTs, copper-glutathione, or other copper-ligand complexes in the cytoplasm could supply copper. In response to host-imposed copper toxicity, C. neoformans counters the copper bombardment strategy by inducing transcription of the MT genes MT1 and MT2 in a Cuf1-dependent manner (inset). Additionally, expression of Cu,Zn Sod1 neutralizes host-derived ROS in the cytoplasm. The expression of copper-requiring laccase produces a protective layer of melanin across the cell surface that has the ability to absorb and neutralize host-derived ROS extracellularly. Abbreviations: Cp, ceruloplasmin; MT, metallothionein; ROS, reactive oxygen species.
The fungal pathogen C. albicans is an oral-genito-urinary pathogen that commonly colonizes the kidney, and mass spectrometry imaging studies demonstrated increased copper accumulation in infected kidney tissue at the onset of infection (108). C. albicans also expresses two MTs, Cup1 and Crd2, as intracellular copper buffers (121, 139). However, these MTs are much smaller, with a lower copper-buffering capacity, and appear to be a secondary measure of preventing copper toxicity (139, 164). Instead, unique to Candida species is the Ace1- and copper-dependent expression of CRP1, which encodes a plasma membrane P-type ATPase (164). Crp1 is distinct from the P-type ATPase Ccc2, as it instead actively transports excess copper from the cytoplasm to the extracellular environment, whereas Ccc2 is not found localized at the plasma membrane under any conditions tested (174). While P-type ATPase pumps are a common mechanism for copper resistance in prokaryotes, this is the only known instance of a fungal pathogen with a dedicated copper efflux system. Accordingly, the MTs are dispensable when a functional Crp1 transporter is present, although C. albicans is extremely sensitive to low levels of copper in the absence of both detoxification mechanisms (139, 164). Recent reports have demonstrated Crp1 expression is required for full virulence and is highly induced during the initial stages of infection in the kidneys of mice (108).
Host Copper Sequestration During Late Stages of Infection
Nutritional immunity, the deliberate withholding of trace micronutrients by the host to the disad-vantage of invading pathogens, has been well described for iron, manganese, zinc, and calcium (38, 79, 118). Specific niches within the host are particularly limited in copper levels that are available to invading fungal pathogens, such as the brain and kidney for C. neoformans and C. albicans, respectively (102, 147). A copper-specific nutritional immunity response is evidenced during C. albicans infection of the kidney, where an initial rise in tissue copper concentrations subsequently diminishes as infection progresses (108). However, no bona fide host nutritional immunity mechanisms have yet been described for copper in host tissues.
Pathogen Response to Copper Deprivation
Given the requirement for several copper-dependent enzymes in fungal virulence, the ability to scavenge trace levels of copper from the host is paramount for establishing and maintaining an infection (52). In response to copper limitation in the host, C. albicans induces the expression of the CTR1/3 transporters in a manner that correlates with decreased host tissue copper concentrations as infection progresses in the kidney, and deletion of the CTR1 transporter results in a reduced capacity of C. albicans to colonize the kidneys in a mouse infection model (108). Interestingly, copper deprivation also impaired the ability of C. albicans to assimilate and utilize several carbon sources as well as altering core metabolic pathways (108). The ability to utilize alternative carbon sources during infection is a well-known virulence determinant, and Ctr1-mediated copper acquisition may play a role in promoting efficient carbon assimilation (12, 133).
In immune-compromised individuals, C. neoformans, which resists copper toxicity in alveolar macrophages, can disseminate from the lungs and colonize the brain, resulting in lethal cryptococcal meningoencephalitis (94). CTR1/4 transporters are highly induced in the brains of infected mice and humans, in contrast to their lungs, supporting the notion that C. neoformans encounters copper limitation within this niche (147, 162). Accordingly, inactivation of the CTR1/4 transporters resulted in reduced fungal burden and attenuation of virulence in mouse models of infection (147).
Copper-Dependent Enzymes Important for Pathogenesis
The pigment melanin, deposited on the fungal cell wall, is a phenolic polymer that has antioxidant and stress-protectant properties that may play a role in absorbing host-derived ROS/RNS (Figure 6). Melanin synthesis requires the copper-dependent enzyme laccase, and its expression in C. neoformans is modestly induced in response to elevated copper in vitro and in the cerebral spinal fluid of C. neoformans–infected rabbits (142, 167). Interestingly, L-DOPA, an abundant molecule in the human brain used for epinephrine synthesis, is an extracellular melanin precursor utilized by C. neoformans laccase (48). In contrast, Aspergillus fumigatus can synthesize melanin de novo via the polyketide pathway that involves noncanonical secretion of melanin precursor biosynthesis enzymes, which are targeted to endosomes through posttranslational palmitoylation (156, 157). Melanin precursors are then converted to melanin extracellularly by classically secreted laccases for final deposition onto the cell surface (157). Recently, an additional role for melanin was re-ported: during A. fumigatus infection, it inhibited macrophage LC3-associated phagocytosis by prohibiting formation of an active NADPH oxidase complex (2). It will be interesting to discern whether this is a common characteristic of all fungal pathogen melanin or specific to that of A. fumigatus.
The initial ROS/RNS burst generated within phagosomes, particularly in the presence of high copper levels, is an effective strategy for killing microbes, and an efficient ROS detoxification system is critical for survival of bacterial and fungal pathogens within this hostile environment (26, 63). Although ROS are primarily handled by Cu,Zn Sod1 in the cytoplasm and Mn Sod2 in mitochondria, several fungi have evolved alternative mechanisms to detoxify host-derived ROS. This is exemplified in C. albicans, which encodes at least six SOD enzymes. Sod4, 5, and 6 are all secreted to the extracellular environment and tethered to the cell wall via a glycosylphosphatidylinositol linkage, strategically placed to detoxify superoxide before it enters cells. Structural and biochemical analysis revealed these enzymes are unique in that they are copper-only enzymes and acquire copper extracellularly, without a known chaperone (69, 127). Perhaps through mass action, the elevated copper levels within the phagosomal compartment of macrophages facilitate copper loading of the copper-only Sod enzymes. Additionally, due to the copper-restricted conditions encountered within certain niches of the host, C. albicans Mac1 directly represses Cu,Zn Sod1 and induces an alternative cytosolic Sod3, which utilizes manganese as a catalytic cofactor (97, 102, 110). C. albicans has thus evolved a mechanism for swapping metal cofactors for cytosolic Sod during copper limitation, and this switch from Sod1 to Sod3 has been demonstrated to occur in vivo as a critical virulence determinant (102).
CONCLUDING REMARKS
The baker’s yeast S. cerevisiae has proven to be a powerful model organism for deciphering the copper biology of fungi. Many of the described functions in this model translate directly to other fungi and to higher eukaryotic organisms, underscoring a high level of conservation for the acquisition and distribution of copper. Given the diversity of the fungal kingdom, it is fascinating that different fungi have evolved distinct mechanisms for responding to copper excess or deficiency, likely specialized for their unique environments. However, gaps in our understanding of fungal copper biology remain, particularly mechanistic details of transport across cellular membranes in via the Ctr family of transporters that will ultimately require high-resolution structures and in vitro copper-transport reconstitution assays. Also, the nature and function of small-molecule copper ligands for copper delivery and compartmentalization await further investigation to discover their biogenesis and role in cellular copper homeostasis. A new and exciting area of investigation is the role of copper at the host-pathogen axis. Historically, iron and manganese have taken the spotlight in this field; however, copper is now taking center stage. While certain copper-regulated genes in S. cerevisiae are also conserved in pathogenic fungi, it appears pathogens have expanded their copper homeostasis and utilization repertoire to enhance survival and virulence within the host. Understanding variations in copper-responsive mechanisms between fungal pathogens that infect distinct host niches with drastic differences in copper availability is likely to shed light on host copper bombardment and withholding mechanisms.
ACKNOWLEDGMENTS
We thank Paul Cobine and Sarela Garcia-Santamarina for critical comments on this manuscript, and we gratefully acknowledge many who have contributed to understanding copper homeostasis in fungi over the last several decades. A.D.S. was supported by training grant 5T32AI052080, and research in the Thiele laboratory on copper in fungi is supported by grant GM041840-28 from the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Achard ME, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, et al. 2012. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem. J 444:51–57 [DOI] [PubMed] [Google Scholar]
- 2.Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, et al. 2016. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 19:79–90 [DOI] [PubMed] [Google Scholar]
- 3.Aller SG, Eng ET, De Feo CJ, Unger VM. 2004. Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J. Biol. Chem 279:53435–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson M, Mattle D, Sitsel O, Klymchuk T, Nielsen AM, et al. 2014. Copper-transporting P-type ATPases use a unique ion-release pathway. Nat. Struct. Mol. Biol 21:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnesano F, Banci L, Bertini I, Cantini F, Ciofi-Baffoni S, et al. 2001. Characterization of the binding interface between the copper chaperone Atx1 and the first cytosolic domain of Ccc2 ATPase. J. Biol. Chem 276:41365–76 [DOI] [PubMed] [Google Scholar]
- 6.Arnesano F, Banci L, Bertini I, Huffman DL, O’Halloran TV. 2001. Solution structure of the Cu(I) and apo forms of the yeast metallochaperone, Atx1. Biochemistry 40:1528–39 [DOI] [PubMed] [Google Scholar]
- 7.Askwith C, Eide D, Van Ho A, Bernard PS, Li L, et al. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403–10 [DOI] [PubMed] [Google Scholar]
- 8.Banci L, Bertini I, Cantini F, Felli IC, Gonnelli L, et al. 2006. The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat. Chem. Biol 2:367–68 [DOI] [PubMed] [Google Scholar]
- 9.Banci L, Bertini I, Chasapis CT, Rosato A, Tenori L. 2007. Interaction of the two soluble metal-binding domains of yeast Ccc2 with copper(I)-Atx1. Biochem. Biophys. Res. Commun 364:645–49 [DOI] [PubMed] [Google Scholar]
- 10.Banci L, Bertini I, Ciofi-Baffoni S, Huffman DL, O’Halloran TV. 2001. Solution structure of the yeast copper transporter domain Ccc2a in the apo and Cu(I)-loaded states. J. Biol. Chem 276:8415–26 [DOI] [PubMed] [Google Scholar]
- 11.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. 2010. Affinity gradients drive copper to cellular destinations. Nature 465:645–48 [DOI] [PubMed] [Google Scholar]
- 12.Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. 2006. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol 8:961–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudoin J, Ioannoni R, Mailloux S, Plante S, Labbé S. 2013. Transcriptional regulation of the copper transporter Mfc1 in meiotic cells. Eukaryot. Cell 12:575–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaudoin J, Labbé S. 2001. The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J. Biol. Chem 276:15472–80 [DOI] [PubMed] [Google Scholar]
- 15.Beaudoin J, Labbé S. 2006. Copper induces cytoplasmic retention of fission yeast transcription factor Cuf1. Eukaryot. Cell 5:277–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaudoin J, Labbé S. 2007. Crm1-mediated nuclear export of the Schizosaccharomyces pombe transcription factor Cuf1 during a shift from low to high copper concentrations. Eukaryot. Cell 6:764–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaudoin J, Mercier A, Langlois R, Labbé S. 2003. The Schizosaccharomyces pombe Cuf1 is composed of functional modules from two distinct classes of copper metalloregulatory transcription factors. J. Biol. Chem 278:14565–77 [DOI] [PubMed] [Google Scholar]
- 18.Beaudoin J, Thiele DJ, Labbé S, Puig S. 2011. Dissection of the relative contribution of the Schizosaccharomyces pombe Ctr4 and Ctr5 proteins to the copper transport and cell surface delivery functions. Microbiology 157:1021–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- 20.Beers J, Glerum DM, Tzagoloff A. 1997. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J. Biol. Chem 272:33191–96 [DOI] [PubMed] [Google Scholar]
- 21.Bellemare DR, Shaner L, Morano KA, Beaudoin J, Langlois R, Labbé S. 2002. Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J. Biol. Chem 277:46676–86 [DOI] [PubMed] [Google Scholar]
- 22.Bertini I, Felli IC, Gonnelli L, Pierattelli R, Spyranti Z, Spyroulias GA. 2006. Mapping protein-protein interaction by 13C′-detected heteronuclear NMR spectroscopy. J. Biomol. NMR 36:111–22 [DOI] [PubMed] [Google Scholar]
- 23.Bertini I, Hartmann HJ, Klein T, Liu G, Luchinat C, Weser U. 2000. High resolution solution structure of the protein part of Cu7 metallothionein. Eur. J. Biochem 267:1008–18 [DOI] [PubMed] [Google Scholar]
- 24.Brenes-Pomales A, Lindegren G, Lindegren CC. 1955. Gene control of copper-sensitivity in Saccharomyces. Nature 176:841–42 [DOI] [PubMed] [Google Scholar]
- 25.Brouwer M, Brouwer-Hoexum T. 1992. Glutathione-mediated transfer of copper(I) into American lobster apohemocyanin. Biochemistry 31:4096–102 [DOI] [PubMed] [Google Scholar]
- 26.Broxton CN, Culotta VC. 2016. SOD enzymes and microbial pathogens: surviving the oxidative storm of infection. PLOS Pathog 12:e1005295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr HS, George GN, Winge DR. 2002. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J. Biol. Chem 277:31237–42 [DOI] [PubMed] [Google Scholar]
- 28.Chiarla C, Giovannini I, Siegel JH. 2008. Patterns of correlation of plasma ceruloplasmin in sepsis. J. Surg. Res 144:107–10 [DOI] [PubMed] [Google Scholar]
- 29.Cobine PA, McKay RT, Zangger K, Dameron CT, Armitage IM. 2004. Solution structure of Cu6 metallothionein from the fungus Neurospora crassa. Eur. J. Biochem 271:4213–21 [DOI] [PubMed] [Google Scholar]
- 30.Cobine PA, Ojeda LD, Rigby KM, Winge DR. 2004. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem 279:14447–55 [DOI] [PubMed] [Google Scholar]
- 31.Cobine PA, Pierrel F, Bestwick ML, Winge DR. 2006. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem 281:36552–59 [DOI] [PubMed] [Google Scholar]
- 32.Corazza A, Harvey I, Sadler PJ. 1996. 1H, 13C-NMR and X-ray absorption studies of copper(I) glutathione complexes. Eur. J. Biochem 236:697–705 [DOI] [PubMed] [Google Scholar]
- 33.Culotta VC, Howard WR, Liu XF. 1994. CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J. Biol. Chem 269:25295–302 [PubMed] [Google Scholar]
- 34.Culotta VC, Hsu T, Hu S, Fürst P, Hamer D. 1989. Copper and the ACE1 regulatory protein reversibly induce yeast metallothionein gene transcription in a mouse extract. PNAS 86:8377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Culotta VC, Joh HD, Lin SJ, Slekar KH, Strain J. 1995. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J. Biol. Chem 270:29991–97 [DOI] [PubMed] [Google Scholar]
- 36.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. 1997. The copper chaperone for superoxide dismutase. J. Biol. Chem 272:23469–72 [DOI] [PubMed] [Google Scholar]
- 37.Dameron CT, Winge DR, George GN, Sansone M, Hu S, Hamer D. 1991. A copper-thiolate polynuclear cluster in the ACE1 transcription factor. PNAS 88:6127–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, et al. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. PNAS 110:3841–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dancis A, Haile D, Yuan DS, Klausner RD. 1994. The Saccharomyces cerevisiae copper transport protein (Ctr1p): biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Biol. Chem 269:25660–67 [PubMed] [Google Scholar]
- 40.Dancis A, Yuan DS, Haile D, Askwith C, Eide D, et al. 1994. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76:393–402 [DOI] [PubMed] [Google Scholar]
- 41.Davis-Kaplan SR, Askwith CC, Bengtzen AC, Radisky D, Kaplan J. 1998. Chloride is an allosteric effector of copper assembly for the yeast multicopper oxidase Fet3p: an unexpected role for intracellular chloride channels. PNAS 95:13641–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bie P, Muller P, Wijmenga C, Klomp LW. 2007. Molecular pathogenesis of Wilson and Menkes disease: Correlation of mutations with molecular defects and disease phenotypes. J. Med. Genet 44:673–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. 2009. Three-dimensional structure of the human copper transporter hCTR1. PNAS 106:4237–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding C, Festa RA, Chen YL, Espart A, Palacios Ò, et al. 2013. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 13:265–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, Thiele DJ. 2011. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol. Microbiol 81:1560–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobi A, Dameron CT, Hu S, Hamer D, Winge DR. 1995. Distinct regions of Cu(I) ·ACE1 contact two spatially resolved DNA major groove sites. J. Biol. Chem 270:10171–78 [DOI] [PubMed] [Google Scholar]
- 47.Ecker DJ, Butt TR, Sternberg EJ, Neeper MP, Debouck C, et al. 1986. Yeast metallothionein function in metal ion detoxification. J. Biol. Chem 261:16895–900 [PubMed] [Google Scholar]
- 48.Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, et al. 2007. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 153:3954–62 [DOI] [PubMed] [Google Scholar]
- 49.Evans CF, Engelke DR, Thiele DJ. 1990. ACE1 transcription factor produced in Escherichia coli binds multiple regions within yeast metallothionein upstream activation sequences. Mol. Cell Biol 10:426–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrell RA, Thorvaldsen JL, Winge DR. 1996. Identification of the Zn(II) site in the copper-responsive yeast transcription factor, AMT1: a conserved Zn module. Biochemistry 35:1571–80 [DOI] [PubMed] [Google Scholar]
- 51.Ferreira AM, Ciriolo MR, Marcocci L, Rotilio G. 1993. Copper(I) transfer into metallothionein mediated by glutathione. Biochem. J 292 (Part 3):673–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Festa RA, Thiele DJ. 2011. Copper: an essential metal in biology. Curr. Biol 21:R877–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fogel S, Welch JW. 1982. Tandem gene amplification mediates copper resistance in yeast. PNAS 79:5342–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedman JH, Ciriolo MR, Peisach J. 1989. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem 264:5598–605 [PubMed] [Google Scholar]
- 55.Freedman JH, Peisach J. 1989. Intracellular copper transport in cultured hepatoma cells. Biochem. Biophys. Res. Commun 164:134–40 [DOI] [PubMed] [Google Scholar]
- 56.Fridovich I 1978. The biology of oxygen radicals. Science 201:875–80 [DOI] [PubMed] [Google Scholar]
- 57.Fridovich I 1983. Superoxide radical: an endogenous toxicant. Annu. Rev. Pharmacol. Toxicol 23:239–57 [DOI] [PubMed] [Google Scholar]
- 58.Fu D, Beeler TJ, Dunn TM. 1995. Sequence, mapping and disruption of CCC2, a gene that cross-complements the Ca2+ -sensitive phenotype of csg1 mutants and encodes a P-type ATPase belonging to the Cu2+ -ATPase subfamily. Yeast 11:283–92 [DOI] [PubMed] [Google Scholar]
- 59.Fürst P, Hamer D. 1989. Cooperative activation of a eukaryotic transcription factor: interaction between Cu(I) and yeast ACE1 protein. PNAS 86:5267–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fürst P, Hu S, Hackett R, Hamer D. 1988. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell 55:705–17 [DOI] [PubMed] [Google Scholar]
- 61.Gamonet F, Lauquin GJ. 1998. The Saccharomyces cerevisiae LYS7 gene is involved in oxidative stress protection. Eur. J. Biochem 251:716–23 [DOI] [PubMed] [Google Scholar]
- 62.Garber Morales J, Holmes-Hampton GP, Miao R, Guo Y, Munck E, Lindahl PA. 2010. Biophysical characterization of iron in mitochondria isolated from respiring and fermenting yeast. Biochemistry 49:5436–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.García-Santamarina S, Thiele DJ. 2015. Copper at the fungal pathogen-host axis. J. Biol. Chem 290:18945–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaxiola RA, Yuan DS, Klausner RD, Fink GR. 1998. The yeast CLC chloride channel functions in cation homeostasis. PNAS 95:4046–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D. 1997. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J. Biol. Chem 272:13786–92 [DOI] [PubMed] [Google Scholar]
- 66.George GN, Byrd J, Winge DR. 1988. X-ray absorption studies of yeast copper metallothionein. J. Biol. Chem 263:8199–203 [PubMed] [Google Scholar]
- 67.Germann UA, Lerch K. 1987. Copper accumulation in the cell-wall-deficient slime variant of Neurospora crassa: comparison with a wild-type strain. Biochem. J 245:479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghosh A, Trivedi PP, Timbalia SA, Griffin AT, Rahn JJ, et al. 2014. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum. Mol. Genet 23:3596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gleason JE, Galaleldeen A, Peterson RL, Taylor AB, Holloway SP, et al. 2014. Candida albicans SOD5 represents the prototype of an unprecedented class of Cu-only superoxide dismutases required for pathogen defense. PNAS 111:5866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glerum DM, Shtanko A, Tzagoloff A. 1996. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem 271:14504–9 [DOI] [PubMed] [Google Scholar]
- 71.Glerum DM, Shtanko A, Tzagoloff A. 1996. SCO1 and SCO2 act as high copy suppressors of a mito-chondrial copper recruitment defect in Saccharomyces cerevisiae. J. Biol. Chem 271:20531–35 [DOI] [PubMed] [Google Scholar]
- 72.Gourdon P, Liu XY, Skjorringe T, Morth JP, Moller LB, et al. 2011. Crystal structure of a copper-transporting PIB-type ATPase. Nature 475:59–64 [DOI] [PubMed] [Google Scholar]
- 73.Gralla EB, Thiele DJ, Silar P, Valentine JS. 1991. ACE1, a copper-dependent transcription factor, activates expression of the yeast copper, zinc superoxide dismutase gene. PNAS 88:8558–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamer DH, Thiele DJ, Lemontt JE. 1985. Function and autoregulation of yeast copperthionein. Science 228:685–90 [DOI] [PubMed] [Google Scholar]
- 75.Hassett R, Dix DR, Eide DJ, Kosman DJ. 2000. The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem. J 351(Part 2):477–84 [PMC free article] [PubMed] [Google Scholar]
- 76.Hassett R, Kosman DJ. 1995. Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J. Biol. Chem 270:128–34 [DOI] [PubMed] [Google Scholar]
- 77.Hellman NE, Gitlin JD. 2002. Ceruloplasmin metabolism and function. Annu. Rev. Nutr 22:439–58 [DOI] [PubMed] [Google Scholar]
- 78.Hiser L, Di Valentin M, Hamer AG, Hosler JP. 2000. Cox11p is required for stable formation of the CuB and magnesium centers of cytochrome c oxidase. J. Biol. Chem 275:619–23 [DOI] [PubMed] [Google Scholar]
- 79.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol 10:525–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horecka J, Kinsey PT, Sprague GF Jr. 1995. Cloning and characterization of the Saccharomyces cerevisiae LYS7 gene: evidence for function outside of lysine biosynthesis. Gene 162:87–92 [DOI] [PubMed] [Google Scholar]
- 81.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. 2004. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome c oxidase. J. Biol. Chem 279:35334–40 [DOI] [PubMed] [Google Scholar]
- 82.Howell SB, Safaei R, Larson CA, Sailor MJ. 2010. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol 77:887–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huffman DL, O’Halloran TV. 2000. Energetics of copper trafficking between the Atx1 metallochaperone and the intracellular copper transporter, Ccc2. J. Biol. Chem 275:18611–14 [DOI] [PubMed] [Google Scholar]
- 84.Huibregtse JM, Engelke DR, Thiele DJ. 1989. Copper-induced binding of cellular factors to yeast metallothionein upstream activation sequences. PNAS 86:65–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iwasaki H, Matsubara T, Mori T. 1967. A fungal laccase, its properties and reconstitution from its protein and copper. J. Biochem 61:814–16 [DOI] [PubMed] [Google Scholar]
- 86.Jensen LT, Howard WR, Strain JJ, Winge DR, Culotta VC. 1996. Enhanced effectiveness of copper ion buffering by CUP1 metallothionein compared with CRS5 metallothionein in Saccharomyces cerevisiae. J. Biol. Chem 271:18514–19 [DOI] [PubMed] [Google Scholar]
- 87.Jensen LT, Posewitz MC, Srinivasan C, Winge DR. 1998. Mapping of the DNA binding domain of the copper-responsive transcription factor Mac1 from Saccharomyces cerevisiae. J. Biol. Chem 273:23805–11 [DOI] [PubMed] [Google Scholar]
- 88.Jensen LT, Winge DR. 1998. Identification of a copper-induced intramolecular interaction in the transcription factor Mac1 from Saccharomyces cerevisiae. EMBO J 17:5400–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang N, Liu X, Yang J, Li Z, Pan J, Zhu X. 2011. Regulation of copper homeostasis by Cuf1 associates with its subcellular localization in the pathogenic yeast Cryptococcus neoformans H99. FEMS Yeast Res 11:440–48 [DOI] [PubMed] [Google Scholar]
- 90.Jungmann J, Reins HA, Lee J, Romeo A, Hassett R, et al. 1993. MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J 12:5051–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kidane TZ, Farhad R, Lee KJ, Santos A, Russo E, Linder MC. 2012. Uptake of copper from plasma proteins in cells where expression of CTR1 has been modulated. Biometals 25:697–709 [DOI] [PubMed] [Google Scholar]
- 92.Kloppel C, Suzuki Y, Kojer K, Petrungaro C, Longen S, et al. 2011. Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol. Mol. Biol. Cell 22:3749–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knight SA, Labbé S, Kwon LF, Kosman DJ, Thiele DJ. 1996. A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev 10:1917–29 [DOI] [PubMed] [Google Scholar]
- 94.Kronstad JW, Attarian R, Cadieux B, Choi J, D’Souza CA, et al. 2011. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol 9:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Labbé S, Peña MM, Fernandes AR, Thiele DJ. 1999. A copper-sensing transcription factor regulates iron uptake genes in Schizosaccharomyces pombe. J. Biol. Chem 274:36252–60 [DOI] [PubMed] [Google Scholar]
- 96.Labbé S, Zhu Z, Thiele DJ. 1997. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J. Biol. Chem 272:15951–58 [DOI] [PubMed] [Google Scholar]
- 97.Lamarre C, LeMay JD, Deslauriers N, Bourbonnais Y. 2001. Candida albicans expresses an unusual cytoplasmic manganese-containing superoxide dismutase (SOD3 gene product) upon the entry and during the stationary phase. J. Biol. Chem 276:43784–91 [DOI] [PubMed] [Google Scholar]
- 98.Lamb AL, Torres AS, O’Halloran TV, Rosenzweig AC. 2000. Heterodimer formation between superoxide dismutase and its copper chaperone. Biochemistry 39:14720–7 [DOI] [PubMed] [Google Scholar]
- 99.Lamb AL, Torres AS, O’Halloran TV, Rosenzweig AC. 2001. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat. Struct. Biol 8:751–55 [DOI] [PubMed] [Google Scholar]
- 100.Lamb AL, Wernimont AK, Pufahl RA, Culotta VC, O’Halloran TV, Rosenzweig AC. 1999. Crystal structure of the copper chaperone for superoxide dismutase. Nat. Struct. Biol 6:724–29 [DOI] [PubMed] [Google Scholar]
- 101.Lerch K 1980. Copper metallothionein, a copper-binding protein from Neurospora crassa. Nature 284:368–70 [DOI] [PubMed] [Google Scholar]
- 102.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. 2015. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. PNAS 112:E5336–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin CM, Crawford BF, Kosman DJ. 1993. Distribution of 64Cu in Saccharomyces cerevisiae: cellular locale and metabolism. J. Gen. Microbiol 139:1605–15 [DOI] [PubMed] [Google Scholar]
- 104.Lin SJ, Culotta VC. 1995. The ATX1 gene of Saccharomyces cerevisiae encodes a small metal homeostasis factor that protects cells against reactive oxygen toxicity. PNAS 92:3784–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin SJ, Pufahl RA, Dancis A, O’Halloran TV, Culotta VC. 1997. A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J. Biol. Chem 272:9215–20 [PubMed] [Google Scholar]
- 106.Lowe J, Vieyra A, Catty P, Guillain F, Mintz E, Cuillel M. 2004. A mutational study in the transmembrane domain of Ccc2p, the yeast Cu(I)-ATPase, shows different roles for each Cys-Pro-Cys cysteine. J. Biol. Chem 279:25986–94 [DOI] [PubMed] [Google Scholar]
- 107.Lu SC. 2013. Glutathione synthesis. Biochim. Biophys. Acta 1830:3143–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mackie J, Szabo EK, Urgast DS, Ballou ER, Childers DS, et al. 2016. Host-imposed copper poisoning impacts fungal micronutrient acquisition during systemic Candida albicans infections. PLOS ONE 11:e0158683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. PNAS 106:8344–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marvin ME, Mason RP, Cashmore AM. 2004. The CaCTR1 gene is required for high-affinity iron uptake and is transcriptionally controlled by a copper-sensing transactivator encoded by CaMAC1. Microbiology 150:2197–208 [DOI] [PubMed] [Google Scholar]
- 111.Mattle D, Zhang L, Sitsel O, Pedersen LT, Moncelli MR, et al. 2015. A sulfur-based transport pathway in Cu+ -ATPases. EMBO Rep 16:728–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maxfield AB, Heaton DN, Winge DR. 2004. Cox17 is functional when tethered to the mitochondrial inner membrane. J. Biol. Chem 279:5072–80 [DOI] [PubMed] [Google Scholar]
- 113.Mehra RK, Tarbet EB, Gray WR, Winge DR. 1988. Metal-specific synthesis of two metallothioneins and gamma-glutamyl peptides in Candida glabrata. PNAS 85:8815–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, et al. 2005. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121:1059–69 [DOI] [PubMed] [Google Scholar]
- 115.Miras R, Morin I, Jacquin O, Cuillel M, Guillain F, Mintz E. 2008. Interplay between glutathione, Atx1 and copper: 1. Copper(I) glutathionate induced dimerization of Atx1. J. Biol. Inorg. Chem 13:195–205 [DOI] [PubMed] [Google Scholar]
- 116.Morin I, Gudin S, Mintz E, Cuillel M. 2009. Dissecting the role of the N-terminal metal-binding domains in activating the yeast copper ATPase in vivo. FEBS J 276:4483–95 [DOI] [PubMed] [Google Scholar]
- 117.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. 2008. Interferon-γ limits the availability of iron for intramacrophage Salmonella typhimurium. Eur. J. Immunol 38:1923–36 [DOI] [PubMed] [Google Scholar]
- 118.Nakashige TG, Zhang B, Krebs C, Nolan EM. 2015. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol 11:765–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nevitt T, Ohrvik H, Thiele DJ. 2012. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta 1823:1580–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nittis T, George GN, Winge DR. 2001. Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J. Biol. Chem 276:42520–26 [DOI] [PubMed] [Google Scholar]
- 121.Oh KB, Watanabe T, Matsuoka H. 1999. A novel copper-binding protein with characteristics of a metallothionein from a clinical isolate of Candida albicans. Microbiology 145 (Part 9):2423–29 [DOI] [PubMed] [Google Scholar]
- 122.Park YS, Lian H, Chang M, Kang CM, Yun CW. 2014. Identification of high-affinity copper transporters in Aspergillus fumigatus. Fungal Genet. Biol 73:29–38 [DOI] [PubMed] [Google Scholar]
- 123.Pearce DA, Sherman F. 1999. Toxicity of copper, cobalt, and nickel salts is dependent on histidine metabolism in the yeast Saccharomyces cerevisiae. J. Bacteriol 181:4774–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pena MM, Puig S, Thiele DJ. 2000. Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J. Biol. Chem 275:33244–51 [DOI] [PubMed] [Google Scholar]
- 125.Peter C, Laliberté J, Beaudoin J, Labbe S. 2008. Copper distributed by Atx1 is available to copper amine oxidase 1 in Schizosaccharomyces pombe. Eukaryot. Cell 7:1781–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peterson CW, Narula SS, Armitage IM. 1996. 3D solution structure of copper and silver-substituted yeast metallothioneins. FEBS Lett 379:85–93 [DOI] [PubMed] [Google Scholar]
- 127.Peterson RL, Galaleldeen A, Villarreal J, Taylor AB, Cabelli DE, et al. 2016. The phylogeny and active site design of eukaryotic copper-only superoxide dismutases. J. Biol. Chem 291:20911–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Plante S, Ioannoni R, Beaudoin J, Labbé S. 2014. Characterization of Schizosaccharomyces pombe copper transporter proteins in meiotic and sporulating cells. J. Biol. Chem 289:10168–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Portnoy ME, Rosenzweig AC, Rae T, Huffman DL, O’Halloran TV, Culotta VC. 1999. Structure-function analyses of the ATX1 metallochaperone. J. Biol. Chem 274:15041–45 [DOI] [PubMed] [Google Scholar]
- 130.Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, et al. 1997. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278:853–56 [DOI] [PubMed] [Google Scholar]
- 131.Puig S, Lee J, Lau M, Thiele DJ. 2002. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem 277:26021–30 [DOI] [PubMed] [Google Scholar]
- 132.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. 1999. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284:805–8 [DOI] [PubMed] [Google Scholar]
- 133.Ramírez MA, Lorenz MC. 2007. Mutations in alternative carbon utilization pathways in Candida albicans attenuate virulence and confer pleiotropic phenotypes. Eukaryot. Cell 6:280–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramos D, Mar D, Ishida M, Vargas R, Gaite M, et al. 2016. Mechanism of copper uptake from blood plasma ceruloplasmin by mammalian cells. PLOS ONE 11:e0149516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reddehase S, Grumbt B, Neupert W, Hell K. 2009. The disulfide relay system of mitochondria is required for the biogenesis of mitochondrial Ccs1 and Sod1. J. Mol. Biol 385:331–38 [DOI] [PubMed] [Google Scholar]
- 136.Rees EM, Lee J, Thiele DJ. 2004. Mobilization of intracellular copper stores by the Ctr2 vacuolar copper transporter. J. Biol. Chem 279:54221–29 [DOI] [PubMed] [Google Scholar]
- 137.Rees EM, Thiele DJ. 2007. Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J. Biol. Chem 282:21629–38 [DOI] [PubMed] [Google Scholar]
- 138.Rentzsch A, Krummeck-Weiss G, Hofer A, Bartuschka A, Ostermann K, Rodel G. 1999. Mitochondrial copper metabolism in yeast: mutational analysis of Sco1p involved in the biogenesis of cytochrome c oxidase. Curr. Genet 35:103–8 [DOI] [PubMed] [Google Scholar]
- 139.Riggle PJ, Kumamoto CA. 2000. Role of a Candida albicans P1-type ATPase in resistance to copper and silver ion toxicity. J. Bacteriol 182:4899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosenzweig AC, Huffman DL, Hou MY, Wernimont AK, Pufahl RA, O’Halloran TV. 1999. Crystal structure of the Atx1 metallochaperone protein at 1.02 Å resolution. Structure 7:605–17 [DOI] [PubMed] [Google Scholar]
- 141.Rustici G, van Bakel H, Lackner DH, Holstege FC, Wijmenga C, et al. 2007. Global transcriptional responses of fission and budding yeast to changes in copper and iron levels: a comparative study. Genome Biol 8:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med 184:377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. 1998. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol 160:1290–96 [PubMed] [Google Scholar]
- 144.Schulze M, Rodel G. 1988. SCO1, a yeast nuclear gene essential for accumulation of mitochondrial cytochrome c oxidase subunit II. Mol. Gen. Genet 211:492–98 [DOI] [PubMed] [Google Scholar]
- 145.Schwan WR, Warrener P, Keunz E, Stover CK, Folger KR. 2005. Mutations in the cueA gene encoding a copper homeostasis P-type ATPase reduce the pathogenicity of Pseudomonas aeruginosa in mice. Int. J. Med. Microbiol 295:237–42 [DOI] [PubMed] [Google Scholar]
- 146.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. 2001. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria: a physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem 276:38084–89 [DOI] [PubMed] [Google Scholar]
- 147.Sun TS, Ju X, Gao HL, Wang T, Thiele DJ, et al. 2014. Reciprocal functions of Cryptococcus neoformans copper homeostasis machinery during pulmonary infection and meningoencephalitis. Nat. Commun 5:5550. [DOI] [PubMed] [Google Scholar]
- 148.Szczypka MS, Thiele DJ. 1989. A cysteine-rich nuclear protein activates yeast metallothionein gene transcription. Mol. Cell Biol 9:421–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tamai KT, Gralla EB, Ellerby LM, Valentine JS, Thiele DJ. 1993. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. PNAS 90:8013–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thiele DJ. 1988. ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol. Cell Biol 8:2745–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thiele DJ, Walling MJ, Hamer DH. 1986. Mammalian metallothionein is functional in yeast. Science 231:854–56 [DOI] [PubMed] [Google Scholar]
- 152.Thorvaldsen JL, Sewell AK, McCowen CL, Winge DR. 1993. Regulation of metallothionein genes by the ACE1 and AMT1 transcription factors. J. Biol. Chem 268:12512–18 [PubMed] [Google Scholar]
- 153.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, et al. 1995. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science 269:1069–74 [DOI] [PubMed] [Google Scholar]
- 154.Turner RB, Smith DL, Zawrotny ME, Summers MF, Posewitz MC, Winge DR. 1998. Solution structure of a zinc domain conserved in yeast copper-regulated transcription factors. Nat. Struct. Biol 5:551–55 [DOI] [PubMed] [Google Scholar]
- 155.Tzagoloff A, Capitanio N, Nobrega MP, Gatti D. 1990. Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. EMBO J 9:2759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Upadhyay S, Xu X, Lin X. 2016. Interactions between melanin enzymes and their atypical recruitment to the secretory pathway by palmitoylation. mBio 7:e01925–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Upadhyay S, Xu X, Lowry D, Jackson JC, Roberson RW, Lin X. 2016. Subcellular compartmentalization and trafficking of the biosynthetic machinery for fungal melanin. Cell Rep 14:2511–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, et al. 2010. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330:219–22 [DOI] [PubMed] [Google Scholar]
- 159.Vest KE, Leary SC, Winge DR, Cobine PA. 2013. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J. Biol. Chem 288:23884–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci 111 (Part 7):897–905 [DOI] [PubMed] [Google Scholar]
- 161.Wagner D, Maser J, Moric I, Vogt S, Kern WV, Bermudez LE. 2006. Elemental analysis of the Mycobacterium avium phagosome in Balb/c mouse macrophages. Biochem. Biophys. Res. Commun 344:1346–51 [DOI] [PubMed] [Google Scholar]
- 162.Waterman SR, Hacham M, Hu G, Zhu X, Park YD, et al. 2007. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J. Clin. Investig 117:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wegner SV, Sun F, Hernandez N, He C. 2011. The tightly regulated copper window in yeast. Chem. Commun 47:2571–73 [DOI] [PubMed] [Google Scholar]
- 164.Weissman Z, Berdicevsky I, Cavari BZ, Kornitzer D. 2000. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. PNAS 97:3520–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Welch J, Fogel S, Buchman C, Karin M. 1989. The CUP2 gene product regulates the expression of the CUP1 gene, coding for yeast metallothionein. EMBO J 8:255–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem 284:33949–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Williamson PR. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J. Bacteriol 176:656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Winge DR, Nielson KB, Gray WR, Hamer DH. 1985. Yeast metallothionein: sequence and metal-binding properties. J. Biol. Chem 260:14464–70 [PubMed] [Google Scholar]
- 169.Wu X, Kim H, Seravalli J, Barycki JJ, Hart PJ, et al. 2016. Potassium and the K+/H+ exchanger Kha1p promote binding of copper to ApoFet3p multi-copper ferroxidase. J. Biol. Chem 291:9796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiao Z, Brose J, Schimo S, Ackland SM, La Fontaine S, Wedd AG. 2011. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J. Biol. Chem 286:11047–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamaguchi-Iwai Y, Serpe M, Haile D, Yang W, Kosman DJ, et al. 1997. Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J. Biol. Chem 272:17711–18 [DOI] [PubMed] [Google Scholar]
- 172.Yan N 2015. Structural biology of the major facilitator superfamily transporters. Annu. Rev. Biophys 44:257–83 [DOI] [PubMed] [Google Scholar]
- 173.Yasokawa D, Murata S, Kitagawa E, Iwahashi Y, Nakagawa R, et al. 2008. Mechanisms of copper toxicity in Saccharomyces cerevisiae determined by microarray analysis. Environ. Toxicol 23:599–606 [DOI] [PubMed] [Google Scholar]
- 174.Yuan DS, Dancis A, Klausner RD. 1997. Restriction of copper export in Saccharomyces cerevisiae to a late Golgi or post-Golgi compartment in the secretory pathway. J. Biol. Chem 272:25787–93 [DOI] [PubMed] [Google Scholar]
- 175.Zhou H, Thiele DJ. 2001. Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem 276:20529–35 [DOI] [PubMed] [Google Scholar]
- 176.Zhou P, Thiele DJ. 1993. Rapid transcriptional autoregulation of a yeast metalloregulatory transcription factor is essential for high-level copper detoxification. Genes Dev 7:1824–35 [DOI] [PubMed] [Google Scholar]
- 177.Zhou PB, Thiele DJ. 1991. Isolation of a metal-activated transcription factor gene from Candida glabrata by complementation in Saccharomyces cerevisiae. PNAS 88:6112–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu Z, Labbé S, Peña MM, Thiele DJ. 1998. Copper differentially regulates the activity and degradation of yeast Mac1 transcription factor. J. Biol. Chem 273:1277–80 [DOI] [PubMed] [Google Scholar]