Abstract
Demand for edible oil from crops like groundnut, mustard and soybean, is increasing and likely to increase further. In this regard, Indian mustard seeds were treated with microwaves (MW), to investigate the possibility of enhancing oil yield and nutritional content. MW pre-treatment was given to the seeds of two Indian mustard varieties i.e. PM21 (V1) and PDZ1 (V2) for 0, 2, 4 and 6 min (H0, H2, H4 and H6 min respectively). MW treatment with increasing exposure time showed a linear reduction in the glucosinolate and erucic acid content with concomitant increase in oil yield and moisture loss in both the varieties, as evident from correlation and principal component analysis. Antioxidant capacity has increased with the reduction in phytic acid content (1.82) in V2 at 6 min exposure time with respect to untreated control. Free radical scavenging activity was improved with increasing MW treatment in both the varieties. Therefore, from the obtained results, it is advisable to treat mustard seeds with MW before extraction of oil, because it gives a relatively good oil yield, with enhanced nutritional factors. Moreover, microwaving was effective in reducing glucosinolates and erucic acid also.
Keywords: Indian mustard, Microwave irradiation, Glucosinolates, Erucic acid, Phytic acid, Antioxidants
Introduction
According to agricultural statistics (2017), Indian-mustard ranks third in terms of export after Canada and China with per cent share of 8.8 from the production of 7.98 million tonnes in the financial year 2016–2017 (http://agricoop.nic.in/sites/default/files/pocketbook_0.pdf). This crop has emerged as a nutritionally rich due to its superior edible oil with 2:1 ratio of omega-6 to omega-3 fatty acids and protein rich seed meal for animal feed (Jesch and Carr 2017). Nutritional assessment of Indian-mustard seed is acknowledged by its oil content, fatty acid profile, certain nutritional and anti-nutritional factors including glucosinolates and phytic acid. Stability of mustard oil largely depends on the presence of natural antioxidants which are mostly phenolics, tocopherols, phytosterols and β-carotene. These components essentially provide protection to polyunsaturated fats from oxidation (Sebei et al. 2007). Studies have shown that consumption of oils that are naturally rich in antioxidants is associated with a reduced risk of many cardiac diseases, obesity and cancer (Jan et al. 2018). Glucosinolates (plant thioglucosides) are found primarily among the members of Brassicaceae family. It is responsible for the characteristic pungency of Indian mustard oil. Cleavage products of glucosinolates hydrolysis are known to be detrimental to animal health but on the same hand it possesses antifungal, antibacterial properties and chemoprevention activity (Szydłowska-Czerniak et al. 2011). Phytic acid and erucic acid are another anti-nutritional compounds found in mustard oil and seed meal (Sharafi et al. 2015; Raboy 2001). Systematic research on nutritional and anti-nutritional compounds of Indian mustard may result in further popularization of this crop.
As oil content of the seed determines its commercial success, likewise market price of the Indian mustard is based on its oil content. It was reported that the moisture content affected the oil recovery. Low moisture caused brittleness and higher moisture content induces plasticity, which reduced the level of compression and given poor oil recovery. Due to less processing and simplicity of use, microwave (MW) radiation pre-treatment of seeds for oil extraction is drawing more attention (Azadmard-Damirchi et al. 2010; Gaikwad et al. 2017; Kittiphoom and Sutasinee 2015; Uquiche et al. 2008). MW treated seeds have shown higher oil yield due to the formation of permanent pores in the cell wall, enabling the oil to move through them rapidly (Abbey et al. 2017). The aim of the present study is to investigate the impact of pre-treatment by MW radiation on nutritional and anti-nutritional determinants in Indian mustard. In addition to it, extent of oil extraction through solvent extraction method and its quality was also analyzed.
Materials and methods
Seed material
Seeds rendered of dust of PM21 (V1) and PDZ1 (V2) varieties of Indian mustard seeds were obtained from ICAR-Directorate of Rapeseed-Mustard Research, Bharatpur, Rajasthan, India and were used in the analysis.
Microwave pre-treatment
For each MW pre-treatment of Indian mustard seeds, 50 g of seeds were placed in an even layer in Borosil petri dishes (26 cm diameter) inside the domestic microwave (Whirlpool, WM0951000430). Samples were microwave treated at a frequency of 2450 MHz, 900 W or 270 kJ (Abbey et al. 2017) for three exposure time of radiation 2 (H2), 4 (H4) and 6 (H6) minutes. Seed sample without radiation i.e. 0 s radiation time (H0) was used as control. The pre-treatment was repeated thrice with each set consisting of 50 g seeds. The treated seeds were pooled and mixed after each treatment to reduce the errors due to handling and microwave power.
Analytical methods
Phytate estimation
The method proposed by Haug and Lantzsch (1983) was used for the estimation of phytate content. Freshly ground seed samples (0.5 g) of all treatments were extracted with 25 ml of 0.2 N HCl in a shaking water bath at 70 rpm at 40 °C for 3 h. After extraction samples were filtered through Whattman No. 1 filter paper and an aliquot of 0.5 ml was used for assay. To the extract, 0.9 ml of distilled water and 1 ml of ferric ammonium sulphate (0.2 g of NH4Fe(SO4) × 12 H2O dissolved in 100 ml of 2 mol L−1 HCl and filled to mark with distilled water) was added and then placed in boiling water bath for 30 min. After cooling, 1 ml of the supernatant was transferred to a fresh test tube and 1.5 ml of bipyridine solution (10 g 2,2′-bipyridine dissolved in 10 ml thioglycolic acid and filled to mark with distilled water) was added. The absorbance of the reaction mixture was measured at 519 nm and results were expressed as mg g−1. Calibration curve was prepared by series of standard solutions of sodium salt of phytic acid (0–100 μg ml−1).
Glucosinolate estimation
Spectrophotometric estimation was done (Kumar et al. 2010) using methanolic extract prepared from the same genotypes by homogenizing 0.2 g defatted seed meal in a 2 ml vial with 80% methanol. Defatted seed meal was prepared by incubating seed meal in n-Hexane. After an overnight incubation, the seed meal was air dried and resuspended in 80% methanol for overnight. The homogenate was then centrifuged at 3000 rpm for 4 min at room temperature. The supernatant was collected after centrifugation and made up to 2 ml with 80% methanol. 100 µl of this extract was used for estimation. 0.3 ml double distilled water and 3 ml of 2 mM sodium tetrachloropalladate (58.8 mg Sodium tetrachloropalladate, 170 µl concentrated HCl and 100 ml double distilled water) was added to it. After 1 h incubation at room temperature, absorbance was measured at 425 nm using a spectrophotometer (Labomed UV–Vis Double beam UVD-3500, Los Angeles, CA 90034 USA). A blank was set following the same procedure without the extract. Total glucosinolates were calculated by putting the OD of each sample at 425 nm into the predicted formula: y = 1.40 + 118.86 × OD425 (Mawlong et al. 2017).
Total antioxidant activity
Total antioxidant activity was estimated in defatted and methanolic extract of the samples using the method of Prieto et al. (1999). To 100 μl of the methanol extract of samples, 2.5 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added and the reaction mixture was incubated in boiling water bath for 90 min. After cooling, the absorbance was measured at 695 nm and results were expressed as μg g−1. Calibration curve was prepared by series of standard solutions of ascorbic acid (0–50 μg ml−1).
DPPH radical scavenging assay
The DPPH radical scavenging assay method is based on the reduction of 1,1-diphenyl-2-picrylhydrazyl (DPPH), a stable free radical (Mensor et al. 2001). All the samples were defatted and processed as done in case of glucosinolate estimation. Volumes of 500 µl of samples as well as standard compounds (Ascorbic acid, 1 mg/ml) were taken and the volume was made uniformly to 1 ml using 80% methanol. Each of the samples was then further diluted up to 5 ml with methanol and to each 5 ml DPPH (0.01 M) was added. Absorbance was taken after 30 min incubation in dark at 517 nm using methanol as blank on UV–visible spectrophotometer. The IC50 values for each compounds as well as standard preparation were calculated. The DPPH free radical scavenging activity was calculated using the formula: % Radical scavenging capacity = (Absorbance of control − Absorbance of test sample/Absorbance of control) × 100
β-Carotene estimation
The seeds of all treatments of both the varieties of Indian mustard were thoroughly grinded to make fine flour and 1 g of the flour from each sample was taken into screw capped glass vials to estimate the β carotene content (Sathya et al. 2014). Each vial was covered with aluminium foil in order to maintain dark condition. To each vial 5 ml of water saturated n-butanol (8:2) was added and mixed vigorously for 1 min. All the vials were kept for overnight in dark at room temperature for complete extraction of β carotene. Next day the content was thoroughly mixed and filtered through Whattman No. 40 filter paper. The absorbance of clear filtrate of all the samples were measured at 440 nm using pure water saturated n-butanol as blank. The β carotene content was calculated through calibration curve of standard β carotene and expressed in ppm.
Total tocopherol estimation
Tocopherol was extracted with slight modification from Backer et al. (1980) from seeds using acetone:methanol (7:3, v/v), based on ferrous-dipyridyl colour reagent. Briefly, 1 ml of the extracted sample was mixed with 1 ml of acetone:methanol, 1 ml of 2, 2′-dipyridyl reagent (0.125 g in 25 ml of absolute ethanol) and 0.2 ml of 0.06% ferric chloride reagent in absolute ethanol was added and the mixture was mixed well for 10 s. Slight modification in 0.2% ferric chloride reagent (0.4 ml) was done here from Backer et al. (1980). Another modification was the addition of 1 ml of absolute ethanol to all the tubes before measuring absorbance. The absorbance of the mixture was read within a minute at 522 nm using blank consisting of CHCl3, 2,2′-dipyridly reagent, and ferric chloride reagent. The standard curve was drawn (0–10 μg ml−1) and α-tocopherol contents in the extracts were calculated from the regression equation of the standard curve.
Total lipid extraction
Total lipid extraction was performed according to AOAC (2000); briefly, dried seeds (5 g) were ground to fine powder using a pestle and mortar prior to oil extraction. The oil from seed meal was extracted for 8–10 h by soxhlet method, using 200 ml of n-hexane as solvent. The solvent was then recovered to obtain the oil yield.
Moisture loss analysis
As the MW treatment affects the moisture content of the seeds, moisture loss or dehydration per unit weight (mg/g) of the fresh seed samples was determined from the difference between the initial and final weight after MW treatment.
Erucic acid
Fatty acid methyl esters (FAME) were prepared employing the method developed by Chauhan et al. (2002) for the estimation of erucic acid. Analysis of FAME was performed with gas chromatograph 5765 (Nucon gas chromatograph, New Delhi, India) equipped with a FID detector and using SP 2300 + 2310 SS columns. The programme was set at N2 flow rate: 30 ml min−1, H2 flow rate: 30 ml min−1, Zero air flow rate: 300 ml min−1, Injector temperature: 240 °C and Detector temperature: 250 °C. Erucic acid was compared to the retention time of standard FAME.
Statistical analysis
The experiment was conducted in CRD design with two replications for each parameter. Data was analyzed using two way ANOVA. Correlation analysis was also established using function cor and corrplot in R studio. The mean differences between the varieties, treatments and analytical parameters were compared by Tukey’s test using the R studio, Inc. (Boston; 2018; version-1.1.463). Values at p level < 0.05 were considered as statistically significant. PCA was performed and its biplot was prepared using the library FactoMineR and factoextra.
Results and discussion
Phytic acid
During ripening, phytic acid rapidly accumulates in seeds in the form of globoid crystal within the protein bodies. It binds to starch, proteins, and minerals leading to their decreased bioavailability in the digestive tract (Raboy 2001). In terms of MW treatment over the control, H2 showed the maximum mean statistical decrease in the phytic acid content (1.70). V2 has performed better than V1 with statistical mean of 1.82 over the control (Table 1). Reduction in phytic acid content was studied earlier in mung bean seeds (Mubarak 2005) and chickpea seeds (Alajaji and El-Adawy 2006). The reduction of phytic acid might be due to the chemical degradation of phytate to lower inositol phosphates and inositol or cleavage of the phytate ring itself (Chen and Betty 2003). This will in turn enhance the nutritive value of oilseeds.
Table 1.
Nutritional, anti-nutritional factors and oil yield of Indian mustard varieties obtained by microwave pre-treatment
| Analytical method/variety | Microwave pre-treatments (min) | ||||
|---|---|---|---|---|---|
| H0 | H2 | H4 | H6 | Varietal mean | |
| Phytic acid (mg g−1) | |||||
| V1 | 1.65 ± 0.007J | 1.69 ± 0.001KL | 1.71 ± 0.021KL | 1.74 ± 0.007JK | 1.70 ± 0.03B |
| V2 | 1.97 ± 0.014I | 1.71 ± 0.007KL | 1.77 ± 0.042JK | 1.82 ± 0.028J | 1.82 ± 0.10A |
| Treatment mean | 1.81 ± 0.18D | 1.70 ± 0.01F | 1.74 ± 0.04EF | 1.78 ± 0.05DE | |
| Glucosinolate content (μmoles g−1) | |||||
| V1 | 36.20 ± 0.86I | 28.04 ± 5.33IJ | 21.93 ± 0.98IJK | 15.00 ± 1.50JKL | 25.30 ± 8.61A |
| V2 | 20.46 ± 9.80IJK | 16.03 ± 0.52BDC | 11.11 ± 0.41KL | 3.57 ± 2.26L | 12.79 ± 7.70B |
| Treatment mean | 28.33 ± 10.71D | 22.03 ± 7.59DE | 16.52 ± 6.28EF | 9.88 ± 6.78F | |
| Total antioxidant activity (μg g−1) | |||||
| V1 | 25.60 ± 1.60L | 63.16 ± 16.32JKL | 101.69 ± 9.88IJK | 118.51 ± 10.23I | 77.24 ± 39.28A |
| V2 | 88.44 ± 20.80IJK | 113.39 ± 14.02IJ | 99.00 ± 15.29IJK | 53.48 ± 13.21KL | 88.58 ± 25.59A |
| Treatment mean | 57.02 ± 38.22E | 88.28 ± 31.55D | 100.35 ± 10.62D | 86.00 ± 38.76DE | |
| DPPH radical scavenging activity (%) | |||||
| V1 | 36.27 ± 8.85I | 36.41 ± 8.20I | 35.77 ± 7.54I | 35.04 ± 8.19I | 35.87 ± 6.23A |
| V2 | 36.84 ± 7.64I | 36.38 ± 8.26I | 35.95 ± 8.21I | 35.18 ± 7.71I | 36.09 ± 6.05A |
| Treatment Mean | 36.56 ± 6.76D | 36.40 ± 6.72D | 35.86 ± 6.44D | 35.11 ± 6.50D | |
| β-carotene content (ppm) | |||||
| V1 | 2.84 ± 0.10J | 3.85 ± 0.35IJ | 5.45 ± 1.18IJ | 6.46 ± 1.08IJ | 4.65 ± 1.62A |
| V2 | 3.31 ± 0.09IJ | 2.79 ± 0.26J | 3.60 ± 0.28IJ | 7.72 ± 3.02I | 4.35 ± 2.39A |
| Treatment mean | 3.07 ± 0.28E | 3.32 ± 0.66E | 4.52 ± 1.28DE | 7.09 ± 1.99D | |
| Tocopherol content (mg g−1) | |||||
| V1 | 68.81 ± 4.04K | 100.47 ± 5.14JK | 122.35 ± 17.42IJ | 132.02 ± 13.75IJ | 105.91 ± 27.39B |
| V2 | 112.41 ± 2.76IJK | 100.31 ± 16.73JK | 128.85 ± 12.71IJ | 160.94 ± 16.80I | 125.63 ± 26.40A |
| Treatment mean | 90.61 ± 25.33F | 100.39 ± 10.11EF | 125.60 ± 13.0DE | 146.48 ± 20.88D | |
| Oil yield (%) | |||||
| V1 | 24.50 ± 0.42M | 25.37 ± 0.24M | 29.75 ± 0.49L | 39.70 ± 0.42J | 29.83 ± 6.46B |
| V2 | 35.01 ± 0.30K | 43.90 ± 0.14I | 37.60 ± 0.57J | 43.92 ± 1.23I | 40.11 ± 4.21A |
| Treatment mean | 29.75 ± 6.07F | 34.63 ± 10.70E | 33.67 ± 4.55E | 41.81 ± 2.55D | |
| Erucic acid content (%) | |||||
| V1 | 11.69 ± 0.45I | 8.02b ± 0.12J | 2.94 ± 0.15K | 0.99 ± 0.02M | 5.91 ± 4.50A |
| V2 | 3.72 ± 0.23K | 3.49 ± 0.02K | 1.79 ± 0.10L | 0.99 ± 0.06M | 2.50 ± 1.22B |
| Treatment mean | 7.70 ± 4.60D | 5.76 ± 2.61E | 2.37 ± 0.67F | 0.99 ± 0.03G | |
Different letters within the same column indicate significant differences between parameters determined for two varieties (A–B) and MW treatment (D–G). Different letters (I–M) within the cells indicate significant differences between treatment combinations (two-way ANOVA and Tukey test, p < 0.05) A values are means ± standard deviations, n = 2. Where H0, H2, H4, H6 represents 0, 2, 4, 6 min microwave treatments on two varieties PM21 (V1) and PDZ1 (V2)
Glucosinolate content
Total glucosinolate content differed significantly among varieties and was also significantly influenced by the MW treatment. In the present investigation, glucosinolate content in V1 and V2 decreased with the increasing exposure time of MW. In V1, the glucosinolate content decreased from 36.20 μM g−1 (H0) to 15.01 μM g−1 (H6), whereas in V2, the decrease was from 20.46 μM g−1 (H0) to 3.57 μM g−1 (H6). It is evident from the statistical analysis that V1 has performed better (25.30) than V2 (12.79) variety. The results were in accordance with the results reported by Kumar et al. (2010) and Niu et al. (2015). Adverse effects of glucosinolate ingestion in animals are reduced palatability, digestion and growth. Glucosinolate reduction is due to inactivating myrosinase enzyme, glucosinolate degradation and removal of breakdown products such as isothiocyanates and oxazolidinethione. MW treatment of 6 min was more promising in lowering down the toxic glucosinolate and at the same time maintaining high oil yield.
Total antioxidant activity (TAA)
Antioxidants present in Indian mustard, have gained much attention due to their free radical scavenging abilities, which have been proved to be beneficial for human health (Szydłowska-Czerniak et al. 2011). In the present study, the TAA was found to be increasing with the MW treatments in V1, with H4 treatment performing better in both the varieties (Table 1) with treatment mean of 100.35. It may be noted that the variety in which the TAA was lowered, showed concomitant reduction in radical scavenging capacity (explained in the next section). Our results are in agreement with the results obtained in rapeseed after ultrasound–assisted extraction (Szydłowska-Czerniak and Tułodziecka 2014) and found to be better than many common antioxidant standards such as tert-butyl hydroquinone (TBHQ), butyl hydroxytoluene (BHT) etc. The high antioxidant capacity in turn provides high protection against oxidative stress, and thus increases potential uses of mustard seed oil in food, pharmaceutical and cosmetic industries (Szydłowska-Czerniak et al. 2011).
DPPH radical scavenging assay
The DPPH assay is a rapid, reliable, reproducible single electron transfer based method to assess general antioxidant activity of pure compounds as well as plant extracts. With rapid decrease in the colour intensity of DPPH with increase in the exposure time of MW treatment, suggests that both the varieties of Indian mustard have potent antioxidant activity due to its proton donating ability. Though significant differences were not observed in the varietal performance as far as DPPH radical scavenging activity is concerned with V1 showing mean of 35.87 and V2 as 36.09 (Table 1). However, MW pre-treatment mean was lowest at H6 (35.11) when compared to untreated control samples. The reducing capacity of compounds could serve as an indicator of potential antioxidant properties in rapeseed (Bandoniene et al. 2000; Szydłowska-Czerniak and Tułodziecka 2014).
β-Carotene
Phytochemical, β-carotene is the precursor of vitamin-A and is directly related to human health due to its antioxidant properties (Rao and Rao 2007). In the present study, the mean β-carotene was found to be increased in MW treated seeds of both the varieties. The content of β-carotene ranged from 2.79 to 7.72 ppm in V2 (7.72). Data shows that H6 treatment (7.09) can be considered best in both the varieties. Statistically, the varietal mean performance has been found to be at par i.e. 4.65 and 4.35 for V1 and V2 respectively (Table 1). Several attempts have been made to assess (Kumar et al. 2017) and enhance β-carotene content in Brassica sp. but MW treatment has come up as a rapid and reproducible method. As seen in the present study, antioxidant synergism between carotenoids and other bioactive compounds, such as tocopherols (Fig. 2), was also reported by Kamal-Eldin (2006). He stated that the oxidative stability of vegetable oils depends largely on their fatty acid composition and tocopherol content where tocopherols are most efficient in scavenging peroxyl radicals which is needed for the stabilization of vegetable oils.
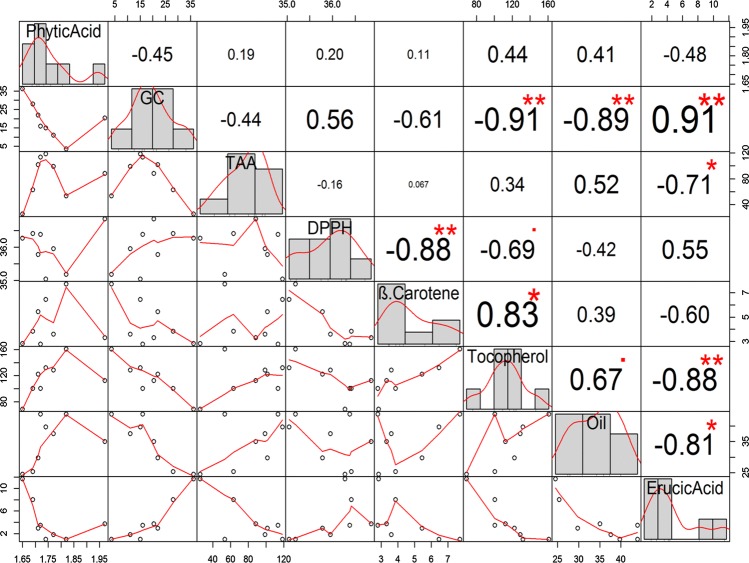
Fig. 2.
Correlation matrix showing correlation coefficients (r) of eight parameters of two varieties (V1 and V2) obtained after 4 MW pre-treatments (H0, H2, H4 and H6); where, *p < 0.05; **p < 0.01
Tocopherols
Tocopherol prevents lipid oxidation of fats and oils by modifying the radical chain autoxidation process. Though tocopherols are heat-sensitive, it was assumed that the MW treated seeds would have lower tocopherol content. However, as the MW exposure time was increased, the total tocopherol content of the seeds was increased in both the varieties. V2 had a higher content of total tocopherol (160.94) as compared with V1 (132.02) at H6 (Table 1). Both the varieties have performed significantly different with mean variation of 105.91 in V1 and 125.63 in V2. Present results also coincide with the results of oil extraction yield (Fig. 2). This increase is due to the fact that MW pre-treatment damages cell membrane, allowing rapid release of tocopherols which participate actively in the protection of cell membrane whose phospholipids consist majorly of polyunsaturated fatty acids (Ko et al. 2003; Sebei et al. 2007). Siger et al. (2017) explained the increase in the tocopherol and tocochromanol content in rapeseed oils by suggesting that, by increasing temperature, the matrix is disturbed, which results in better extraction of certain tocopherol and tocochromanols.
Oil yield
Effect of MW pre-treatment on oil extraction yield through n-Hexane was analyzed in treated and untreated samples. Results showed that MW exposure have increased oil recovery with respect to time in both the varieties with V2 performing better (40.11) than V1 (29.83). An increase of 3.4, 17.64 and 38.28% in V1, while 19.34, 6.88 and 19.39% increase in V2 was observed from H2 to H6 treatments (Table 1). Among all treatments, H6 have responded better (41.81). Obtained results concur with previously published results in hazelnuts (Uquiche et al. 2008), pomegranate seed (Gaikwad et al. 2017), mango seed (Kittiphoom and Sutasinee 2015) and rapeseed (Azadmard-Damirchi et al. 2010; Niu et al. 2015). Data of moisture loss analysis is in accordance with the results of oil yield, showing increased loss of moisture in V2 than V1 (Fig. 1). This could be due to the report stating that MW irradiation is characterized by polarization of water molecules with electromagnetic radiation at high frequencies (Pitchai 2011), causing molecular friction by dipole rotation. This might have increased the porosity of the cell wall, gas evolution and thermodynamic heat transfer through pore spaces (moisture loss). Thus, yielded better oil extraction in contrast with untreated samples in which intact cell wall itself imposes resistance to oil extraction (Uquiche et al. 2008).
Fig. 1.
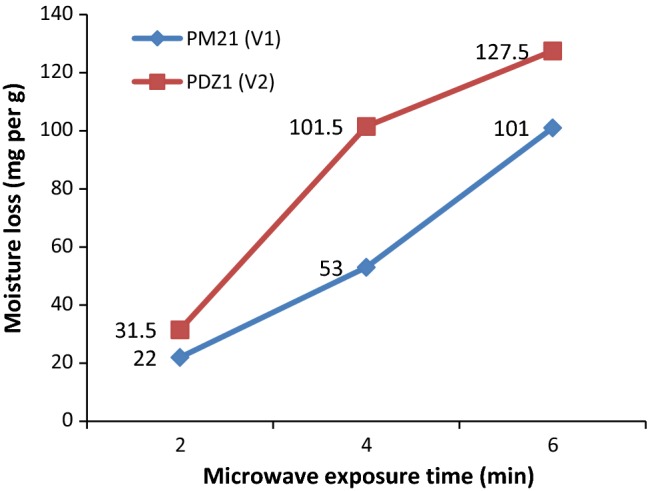
Effect of microwave exposure time (min) on loss of seed moisture (mg/g fresh weight)
Erucic acid
Erucic acid forms approximately 50% of the total fatty acid composition of Indian mustard oil and is known to be anti-nutritional (≤ 2%) in the edible oil, whereas higher amount of the same makes it industrially important (Sharafi et al. 2015). Both the varieties have shown a continuous decrease in erucic acid content with increasing MW exposure as found in the case of glucosinolate content. However, the extent of decrease was more pronounced in V1 (5.91) as compared to V2 (2.50) variety. The erucic acid content was lowest at H6 (0.99) in both the varieties (Table 1). Influence of microwave treatment on the quality of rapeseed oil was also studied earlier (Szydłowska-Czerniak et al. 2011; Valentová et al. 2002) and reported improved quality of oil through reduction in erucic acid.
Correlation analysis
Figure 2 shows the values of correlation analysis among the varieties, treatments and analytical parameters. This kind of analysis helps in establishing relationship between studied parameters for better understanding of the impact of treatments on varietal performance. In this study, phytic acid showed maximum positive correlation with tocopherol content (r = 0.44) and negative correlation with erucic acid (r = − 0.48). A significant, negative correlation of glucosinolates with tocopherol content and oil yield was found (r = − 0.91 and r = − 0.89 respectively) while a positive correlation was with erucic acid (r = 0.91) at p < 0.01 level. It is interesting to note here that erucic acid has shown a significant negative correlation with oil yield and TAA at p < 0.05 level (r = − 0.81, and r = − 0.71 respectively) and with tocopherol at p < 0.01 level (r = − 0.88) as reported by Khattab et al. (2010). It means that MW treatment has resulted in increase in oil yield and antioxidant activity with concomitant decrease in erucic acid. Surprisingly, the DPPH activity i.e. radical scavenging activity was found to be negatively correlated with TAA (r = − 0.16) and β-carotene (r = − 0.88). It might be due to the fact that the antioxidant property is governed mostly by antioxidant compounds other than radical scavengers (Kumar et al. 2017).
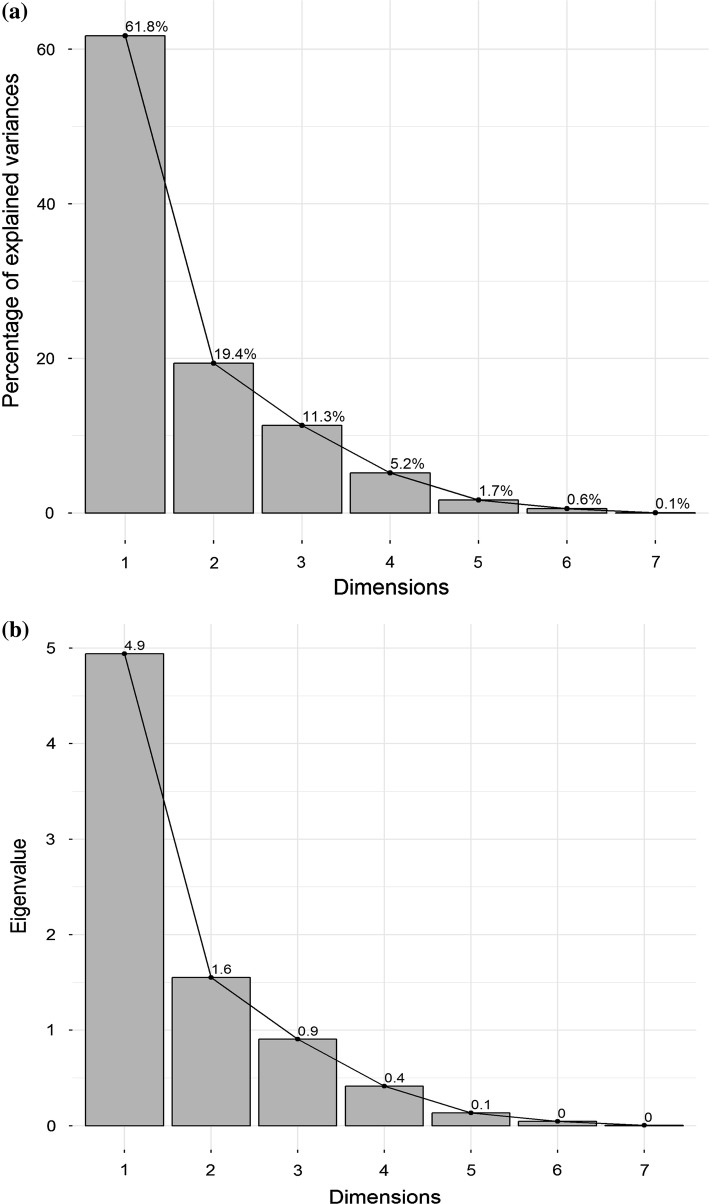
Principal component analysis
Principal component analysis (PCA) was applied to observe any possible clustering within the nutritional and anti-nutritional factors along with the MW treatments. The first two principal components account for 81.16% (PC1 = 61.8% and PC2 = 19.4%, respectively) of the total variation as seen in scree plot between dimensions on x-axis and percentage of explained variances on y-axis (Fig. 3a). These results are also evident from second scree plot between dimensions and Eigen values (Fig. 3b). Perhaps, scree plot is a graphic way to determine the number of principal components. An elbow (bend) in the scree plot determines the exact number of principal components. PC1 was inversely correlated with glucosinolate content (− 0.952), DPPH (− 0.690) and erucic acid (− 0.962), whereas PC2 was highly influenced by phytic acid (0.632), TAA (0.465), DPPH (0.680) and oil yield (0.268). PC1 component is showing values at higher side than PC2 component. This is due to the reason that the maximum variation is due to the PC1 (61.8%) component with higher positive value of 0.948, whereas it is 0.68 with PC2 component.
Fig. 3.
Principal component analysis where a Scree plot showing % of variance vs dimensions accounted for variations; b Scree plot constructed from eigen values versus dimensions accounted for variations
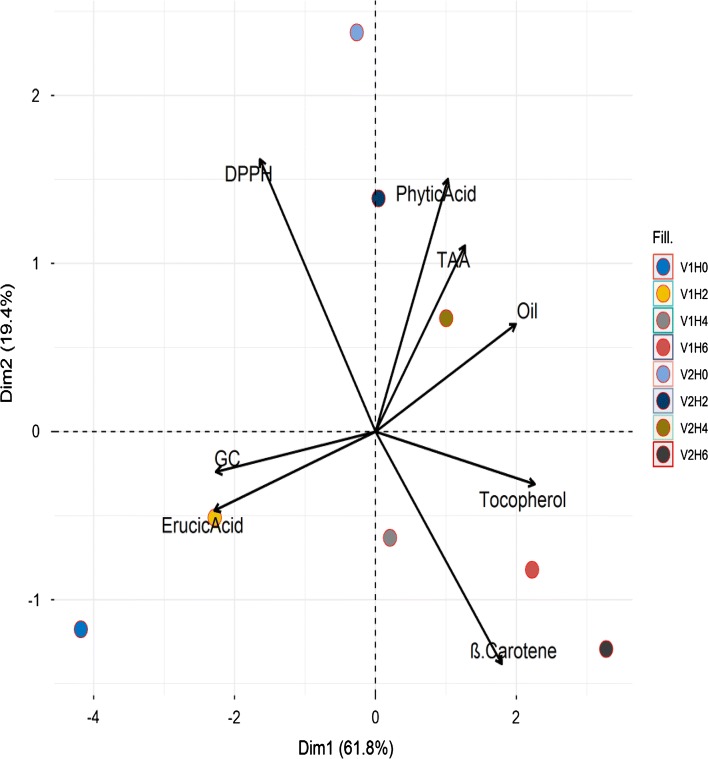
This analysis clearly shows the differences among the eight samples of two varieties and four treatments. Although there are 16 different samples (eight samples and eight parameters) in the graph, the graph can be divided into four sections (Fig. 4). It is noteworthy that the glucosinolate content (GC) and erucic acid, present at lower left section of the PCA biplot, were the variables with negative loadings on PC1 and PC2. Contrastingly, tocopherol and β-carotene content showed (lower right section) positive loadings on PC1 and PC2. This is important to note here that nutritional and anti-nutritional factors are clearly localized in lower right and left sections of the plot, respectively. DPPH activity, located in the upper left section, is the variable with negative loadings on PC1 and positive loadings on PC2 component. Lastly, phytic acid, TAA and oil yield variables (upper right section) showed positive loadings on PC1 and negative loadings on PC2. Furthermore, all the treatment variables of V1 (H0–H6), including V2H6 of V2 with higher GC, erucic acid, β-carotene and tocopherol content were located under the abscissa axis. On the contrary, rest of the three treatments (V2H0, V2H2 and V2H4) with higher DPPH, phytic acid, TAA and oil yield were located on the upper side of abscissa axis. The results demonstrate that the MW pre-treatment had a great influence on oil yield and nutritional factors. Through PCA analysis, Zhou et al. (2013) has shown the reduction in glucosinolate derivatives by pre-treatment with MW and dehulling in rapeseed oil. Furthermore, the increasing length of the arrows in the PCA biplot represents increasing variability which is the case found with DPPH and β–carotene content in the upper left and lowers right sections, respectively.
Fig. 4.
PCA biplot of scores and loadings of data obtained from DPPH, phytic acid, TAA, oil yield, tocopherol, β-carotene, erucic acid and glucosinolate (GC) analysis for two varieties (V1 and V2) prepared by the 4 MW pre-treatments (H0, H2, H4 and H6)
Conclusion
Low moisture content in the MW treated samples had made them more brittle and therefore resulted in easy rupture of tissue and showed increased oil extraction from the seeds (Uquiche et al. 2008). In untreated samples, oil extraction yield is low because of their intact cell wall itself poses resistance to oil extraction. Therefore, in the present study, it has been demonstrated that MW treatment has caused a modification in the cellular wall with greater porosity (Uquiche et al. 2008). Light microscopic studies by Gaikwad et al. (2017) have showed the rupture of cells and loosening of oil bodies in pomegranate seed. MW treated seeds have showed enhanced antioxidant potential which would be helpful in treating diseases related to free radicals. The MW treatment was successful in reducing anti-nutritional factors such as glucosinolates and phytic acid content (especially H6) in both the varieties, thereby improving oil content. Thus, treating mustard seeds with MW before oil extraction is suggested to be as an added advantage to the conventional extraction methods of oils to provide higher oil recovery, oil stability and higher amount of nutraceuticals, and in turn longer shelf life of the produced oil.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbey L, Udenigwe C, Mohan A, Anom E. Microwave irradiation effects on vermicasts potency, and plant growth and antioxidant activity in seedlings of Chinese cabbage (Brassica rapa subsp. pekinensis) J Radiat Res Appl Sci. 2017;10:110–116. doi: 10.1016/j.jrras.2017.01.002. [DOI] [Google Scholar]
- Alajaji SA, El-Adawy TA. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J Food Compos Anal. 2006;19:806–812. doi: 10.1016/j.jfca.2006.03.015. [DOI] [Google Scholar]
- AOAC (2000) Official methods of analysis of AOAC International (17th edn), Association of Official Analytical Chemists, USA
- Azadmard-Damirchi S, Habibi-Nodeh F, Hesari J, Nemati M, Fathi Achachlouei B. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010;121:1211–1215. doi: 10.1016/j.foodchem.2010.02.006. [DOI] [Google Scholar]
- Backer H, Frank O, De Angells B, Feingold S. Plasma tocopherol in man at various times after ingesting free or ocetylaned tocopherol. Nutr Rep Int. 1980;21:531–536. [Google Scholar]
- Bandoniene D, Pukalskas A, Venskutonis PR, Gruzdiene D. Preliminary screening of antioxidant activity of some plant extracts in rapeseed oil. Food Res Int. 2000;33:785–791. doi: 10.1016/S0963-9969(00)00084-3. [DOI] [Google Scholar]
- Chauhan JS, Tyagi P, Tyagi MK. Inheritance of erucic acid content in two crosses of Indian mustard. SABRAO J Breed Genet. 2002;34:19–26. [Google Scholar]
- Chen QC, Betty WL. Separation of phytic acid and other related inositol phosphates by high-performance ion chromatography and its applications. J Chromatogr A. 2003;1018:41–52. doi: 10.1016/j.chroma.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Gaikwad NN, Yedle VH, Yenge G, Suryavanshi S, Babu KD, Pal RK, Sarkar S. Effect of microwave pretreatment on extraction yield of pomegranate seed (cv. Bhagwa) oil. Int J Chem Stud. 2017;5:1291–1294. [Google Scholar]
- Haug W, Lantzsch HJ. Sensitive method for the rapid determination of phytate in cereals and cereal products. J Sci Food Agric. 1983;34:1423–1426. doi: 10.1002/jsfa.2740341217. [DOI] [Google Scholar]
- Jan SA, Shinwari ZK, Malik M, Ilyas M. Antioxidant and anticancer activities of Brassica rapa: a review. MOJ Biol Med. 2018;3:175–178. [Google Scholar]
- Jesch ED, Carr TP. Food ingredients that inhibit cholesterol absorption. Prev Nutr Food Sci. 2017;22:67–80. doi: 10.3746/pnf.2017.22.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal-Eldin A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur J Lipid Sci Technol. 2006;58:1051–1061. doi: 10.1002/ejlt.200600090. [DOI] [Google Scholar]
- Khattab R, Goldberg E, Lin L, Thiyam U. Quantitative analysis and free radical scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem. 2010;122:1266–1272. doi: 10.1016/j.foodchem.2010.03.081. [DOI] [Google Scholar]
- Kittiphoom S, Sutasinee S. Effect of microwaves pretreatments on extraction yield and quality of mango seed kernel oil. Int Food Res J. 2015;22:960–964. [Google Scholar]
- Ko SN, Kim CJ, Kim CT, Kim H, Chung SH, Lee SM, Yoon HH, Kim IH. Changes of vitamin E content in rice bran with different heat treatment. Eur J Lipid Sci Technol. 2003;105:225–228. doi: 10.1002/ejlt.200390045. [DOI] [Google Scholar]
- Kumar S, Chauhan JS, Andy A, Meena ML. Pattern of glucosinolate changes in Indian mustard (Brassica juncea L.) during different developmental stages. Indian J Plant Physiol. 2010;15:69–72. [Google Scholar]
- Kumar MSS, Mawlong I, Nanjundan J, Aravind J, Singh D. Variation in β-carotene and other antioxidants among different species of oilseed Brassica. Indian J Agric Biochem. 2017;30:129–134. doi: 10.5958/0974-4479.2017.00021.1. [DOI] [Google Scholar]
- Mawlong I, Kumar MSS, Gurung B, Singh KH, Singh D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int J Food Prop. 2017;20:3274–3281. doi: 10.1080/10942912.2017.1286353. [DOI] [Google Scholar]
- Mensor LL, Menezes FS, Leitao GG, Reis AS, Santos TCD, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mubarak AE. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005;89:489–495. doi: 10.1016/j.foodchem.2004.01.007. [DOI] [Google Scholar]
- Niu Y, Rogiewicz A, Wan C, Guo M, Huang F, Slominski BA. Effect of microwave treatment on the efficacy of expeller pressing of Brassica napus rapeseed and Brassica juncea mustard seeds. J Agric Food Chem. 2015;63:3078–3084. doi: 10.1021/jf504872x. [DOI] [PubMed] [Google Scholar]
- Pitchai K (2011) Electromagnetic and heat transfer modelling of microwave heating in domestic ovens. Dissertation and Theses in Food Science and Technology, Paper 38. University of Nebraska-Lincoln. http://digitalcommons.unl.do/foodscidiss/38/. Accessed on 10 Dec 18
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Raboy V. Seeds for a better future: ‘low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001;10:458–462. doi: 10.1016/S1360-1385(01)02104-5. [DOI] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Sathya M, Sumathi P, John Joel A. A simple and rapid screening technique for grain β-carotene content in pearl millet through spectrophotometric method. Afr J Agric Res. 2014;9:572–576. doi: 10.5897/AJAR2013.8296. [DOI] [Google Scholar]
- Sebei K, Boukhchina S, Kallel H. Evolution of tocopherols in relation of unsaturated fatty acids during maturation of seeds of rapeseed (Brassica napus L.) C R Biol. 2007;330:55–61. doi: 10.1016/j.crvi.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sharafi Y, Majidi MM, Goli SAH, Rashidi F. Oil content and fatty acids composition in Brassica species. Int J Food Prop. 2015;18:2145–2154. doi: 10.1080/10942912.2014.968284. [DOI] [Google Scholar]
- Siger A, Witulska MG, Broda IB. Antioxidant (tocopherol and canolol) content in rapeseed oil obtained from roasted yellow seeded Brassica napus. J Am Oil Chem Soc. 2017;94:37–46. doi: 10.1007/s11746-016-2921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydłowska-Czerniak A, Tułodziecka A. Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J Am Oil Chem Soc. 2014;91:2011–2019. doi: 10.1007/s11746-014-2557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydłowska-Czerniak A, Bartkowiak-Broda I, Karlović I, Karlovits G, Szłyk E. Antioxidant capacity, total phenolics, glucosinolates and colour parameters of rapeseed cultivars. Food Chem. 2011;127:556–563. doi: 10.1016/j.foodchem.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Uquiche E, Jeréz M, Ortíz J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol) Innov Food Sci Emerg Technol. 2008;9:495–500. doi: 10.1016/j.ifset.2008.05.004. [DOI] [Google Scholar]
- Valentová O, Novotná Z, Svoboda Z, Pejchar P, Káš J. Influence of microwave treatment on the quality of rapeseed oil. J Am Oil Chem Soc. 2002;79:1271. doi: 10.1007/s11746-002-0639-7. [DOI] [Google Scholar]
- Zhou Q, Yang M, Huang F, Zheng C, Deng Q. Effect of pretreatment with dehulling and microwaving on the flavour characteristics of cold-pressed rapeseed oil by GC–MS–PCA and electronic nose discrimination. J Food Sci. 2013;78:961. doi: 10.1111/1750-3841.12161. [DOI] [PubMed] [Google Scholar]