Abstract
Low socioeconomic status (SES) is associated with greater risk for symptoms of attention-deficit/hyperactivity disorder (ADHD). One mechanism through which SES may confer risk for ADHD is by influencing brain structure. Alterations to cortical thickness, surface area and subcortical volume have been associated with low SES and with the presence of ADHD across multiple studies. The current study examined whether cortical thickness, surface area or subcortical volume mediate the associations between SES and ADHD in youth 3–21 years old (N = 874) from the Pediatric Imaging, Neurocognition and Genetics Study. Freesurfer was used to estimate cortical thickness, surface area and subcortical volume from structural magnetic resonance imaging. Parents reported on demographics, family SES, ADHD diagnoses and the presence of child attention problems. Statistical mediation was assessed using a bootstrap resampling procedure. Controlling for parental ADHD, child age, gender, birth weight and scanner, children in low SES families were more likely to be in the ADHD group. Consistent with previous reports in this sample, low SES was associated with reduced surface area across the frontal lobe and reduced subcortical volume in the amygdala, cerebellum, hippocampus and basal ganglia. Of these regions, a significant indirect effect of SES on ADHD status through subcortical volume was observed for the left cerebellum (95% confidence interval: 0.004, 0.022), the right cerebellum (95% confidence interval: 0.006, 0.025), and the right caudate (95% confidence interval: 0.002, 0.022). Environmentally mediated changes in the cerebellum and the caudate may be neurodevelopmental mechanisms explaining elevated risk of ADHD in children in low SES families.
Keywords: attention-deficit/hyperactivity disorder (ADHD), brain development, cerebellum, cognitive neuroscience, developmental psychopathology, socioeconomic status
1 |. INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders, occurring in 7%–9% of children (Froehlich et al., 2007; Thomas, Sanders, Doust, Beller, & Glasziou, 2015). Children with ADHD exhibit a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with daily functioning (American Psychiatric Association & DSM-5 Task Force, 2013). Initial research demonstrates that adverse early environments—including maltreatment, neglect and low socioeconomic status (SES)—contribute to risk for ADHD diagnosis and persistence of ADHD over time (Law, Sideridis, Prock, & Sheridan, 2014; McLaughlin, Sheridan, Winter, et al., 2014; Ouyang, Fang, Mercy, Perou, & Grosse, 2008).
Relatedly, the impact of early adversity on brain structure and function has garnered increasing interest as a potential mechanism through which adversity could influence risk for psychopathology (Bick & Nelson, 2015; Noble et al., 2015). A study of children raised in institutional settings during early childhood showed that reductions in cortical thickness mediated the association between neglect and elevated rates of ADHD (McLaughlin, Sheridan, Winter, et al., 2014). These findings support that altered neural structure may be a key link between adversity and ADHD etiology. Low SES, while less severe than neglect, may influence brain development because children from low SES families are more likely to lack access to the same resources and experiences as high SES children (Bradley & Corwyn, 2002; Raviv, Kessenich, & Morrison, 2004; Tamis-LeMonda, Shannon, Cabrera, & Lamb, 2004). Prior work suggests that the effect of socioeconomic disparities on cognitive functioning and behaviour are mediated through differences in cognitive stimulation in a child’s home (Hackman & Farah, 2009; Noble, McCandliss, & Farah, 2007; Noble, Norman, & Farah, 2005; Sarsour et al., 2011).
Low parental SES in childhood is associated with reductions in grey matter volume in the amygdala, hippocampus, cerebellum, temporal, and prefrontal cortices (Hair, Hanson, Wolfe, & Pollak, 2015; Hanson et al., 2015; Holz, Laucht, & Meyer-Lindenberg, 2015; Jednoróg et al., 2012; Lawson, Duda, Avants, Wu, & Farah, 2013; Luby & et al., 2013; Noble, Houston, Kan, & Sowell, 2012). Cerebellar grey matter volume has been associated with family SES in early life (Cavanagh et al., 2013). With lower heritability than other brain structures, the cerebellum is particularly sensitive to the environment (Giedd, Schmitt, & Neale, 2007). In studies using the Pediatric Imaging Neurocognition and Genetics (PING) dataset, also reported on here, Noble and colleagues found that low parental SES was associated with lower total surface area (Noble et al., 2015), lower fractional anisotropy and volume (Ursache, Noble, & Pediatric Imaging, Neurocognition and Genetics Study, 2016) and smaller amygdala volume (Merz, Tottenham, & Noble, 2017). Additionally, family SES moderated the relationship between cortical thickness and cognition (language and executive function abilities) (Brito, Piccolo, & Noble, 2017). In sum, there is substantial evidence that low parental SES is associated with differences in brain structure across development.
Although alterations in neural structure and function are a plausible mechanism linking low childhood SES to risk for ADHD, current work has not linked SES, neural structure and risk for ADHD. Thus, while there is substantial evidence that SES disparities are associated with differences in neural structure, there is little evidence concerning if these differences in neural structure are linked with ADHD. Interestingly, the patterns of neural structure associated with low SES are highly similar to patterns observed in children with ADHD. There is evidence that children with ADHD have overall smaller brain volumes, most prominently in prefrontal regions compared to controls (Castellanos et al., 2002; Krain & Castellanos, 2006; Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002). Children with ADHD show reduced cortical thickness in frontal and temporal regions (Almeida et al., 2010; Batty et al., 2010; Narr et al., 2009; Schweren et al., 2015; Shaw et al., 2006) and altered subcortical volume, including reduced volume in the basal ganglia, amygdala, hippocampus and cerebellum (Brieber et al., 2007; Carmona et al., 2005; Castellanos et al., 2002; Durston et al., 2002; Hoogman et al., 2017; Qiu et al., 2009; Wyciszkiewicz, Pawlak, & Krawiec, 2017).
Despite these similarities, only one study to date has investigated the relationship between early adversity, brain development and ADHD. As noted above, this study documented that children reared in institutions have widespread reductions in cortical thickness which mediated the association of institutionalization with ADHD symptoms (McLaughlin, Sheridan, Winter, et al., 2014). Additional work is warranted in broader populations of children given that deprivation present in institutionalization is less common than other adversities which may function through similar mechanisms, such as low parental SES.
The purpose of the current study is to examine if neural structure mediates the relationship between low parental SES and ADHD. We investigate these relationships in the PING dataset, a large cohort of youth from sites across the United States (Jernigan et al., 2016). This dataset is one of the largest and most diverse neuroimaging datasets available to identify associations between SES, neural structure and mental health. We assessed whether variation in neural structure accounts for elevations in ADHD in children raised in low SES families. Because previous studies examining parental SES in this sample have focused on surface area (Noble et al., 2015) but decreases in cortical thickness have been commonly linked with ADHD (Almeida et al., 2010; Shaw et al., 2006), we examine mediation in cortical surface area, cortical thickness and subcortical volume. We hypothesized that children with low SES will be more likely to have ADHD group membership and that alterations to brain structure would partially account for the association between SES and ADHD.
2 |. METHODS AND MATERIALS
2.1 |. Sample
Data were obtained from the PING Study, a data resource of standardized magnetic resonance imaging (MRI) data for a large cohort of children 3–21 years old (Jernigan et al., 2016). Participants were recruited at ten sites and were excluded due to major neurological disorders; diagnoses of autism spectrum disorder, bipolar disorder or schizophrenia; brain injury; prematurity; exposure to illicit drugs or alcohol prenatally; head trauma with loss of consciousness; or contraindications for MRI. All participants and parents gave their informed written consent/assent to all study procedures.
Each site administered a standardized structural MRI protocol. Pre-and post-processing techniques have been previously described but we review them succinctly here (Jernigan et al., 2016). The MRI protocol included a T1-weighted scan and a T2-weighted volume. Cortical reconstruction and volumetric segmentation were performed with FreeSurfer imaging analyses to obtain measures of cortical thickness and surface area (Destrieux, Fischl, Dale, & Halgren, 2010; Fischl & Dale, 2000). FreeSurfer procedures have demonstrated good test-retest reliability across scanner manufacturers and field strengths (Desikan et al., 2006; Han et al., 2006).
The PING study collected acceptable structural imaging data on 1,239 youth 3–21 years old. Information about SES, ADHD status and control variables were available for 874 participants; data were missing for age (n = 5), birth weight (n = 195) parental education (n = 28), parental diagnosis of ADHD (n = 8), parental report of attention problems (n = 26), household income (n = 31) and parental occupation (n = 72). SES was defined as a composite measure of parental education for the primary caregiver, parental occupation for the primary caregiver and family income (Akshoomoff et al., 2014) (see Data S1 for more information regarding SES coding). ADHD group membership was defined by a two-item questionnaire: a parent report of a previous child diagnosis of ADHD (n = 58; 6.7%) and/ or a parent report of significant child attention problems (n = 77; 8.8%) for a total sample of 91 (10.4%) children with ADHD group membership (Table 1). While this rate is consistent with prevalence rates of ADHD in population samples (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007), this manner of identifying ADHD does not comply with rigorous diagnostic criteria. Future work will need to investigate these associations in children with more carefully assessed ADHD diagnoses. Our approach of considering attention problems broadly is consistent with current approaches to understanding neurobiological mechanisms of psychopathology (Insel et al., 2010). However given the limited nature of diagnostic information, results should be considered preliminary vis-a-vis ADHD diagnostic status.
TABLE 1.
Distribution of key study variables, by ADHD Group (N = 874) ADHD, attention-deficit/hyperactivity disorder.
| ADHD group (n = 91) | Healthy control group (n = 783) | χ2 | p-value | |||
|---|---|---|---|---|---|---|
| % | (n) | % | (n) | |||
| Female | 29.7 | 27 | 49.8 | 390 | 13.25 | <0.001 |
| Race/Ethnicity | ||||||
| White | 72.5 | 66 | 70.0 | 548 | 0.45 | 0.798 |
| African American | 14.3 | 13 | 14.2 | 111 | 0.23 | 0.890 |
| Hispanic/Latino | 25.3 | 23 | 21.3 | 167 | 0.75 | 0.388 |
| Asian | 23.1 | 21 | 20.9 | 164 | 0.44 | 0.801 |
| American Indian | 5.5 | 5 | 4.6 | 36 | 0.38 | 0.829 |
| Pacific Islander | 18.7 | 17 | 10.2 | 80 | 6.12 | 0.047 |
| Scanner | 8.88 | 0.031 | ||||
| Achieva | 7.7 | 7 | 15.5 | 121 | ||
| Discovery | 13.2 | 12 | 13.3 | 104 | ||
| Signa | 14.3 | 13 | 7.0 | 55 | ||
| TrioTim | 64.8 | 59 | 64.2 | 503 | ||
| Parent ADHD diagnosis | 25.3 | 23 | 4.6 | 36 | 55.37 | <0.001 |
| Mean | (SD) | Mean | (SD) | t-value | p-value | |
| Age (years) | 11.21 | (3.20) | 11.19 | (4.68) | −0.06 | 0.953 |
| Birth weight | 7.62 | (1.09) | 7.54 | (1.15) | −0.57 | 0.570 |
| Socioeconomic status | 16.22 | (5.42) | 17.51 | (4.33) | 2.19 | 0.031 |
Parents reported demographic information, except for adult participants who provided self-report (Jernigan et al., 2016). There was no association between ADHD group membership and race or ethnicity, with the exception of Pacific Islander (Table 1). Participants who were identified by their caregiver as Hispanic (t = 7.65 p < 0.001), Pacific Islander (t = 7.44, p < 0.001), American Indian (t = 2.69, p < 0.01) or African American (t = 8.04, p < 0.001) had lower parental SES compared to all other participants. Gender was included as a covariate as children in the ADHD group were significantly more likely to be male, consistent with population estimates of ADHD (Polanczyk et al., 2007) (Table 1). There were no gender differences by SES (t = 0.41, p = 0.68). Because there were significant differences in scanner used to collect data by ADHD group and by SES (F(3,870) = 6.05, p < 0.001), scanner was included as a covariate. Parent diagnoses of ADHD obtained through self-report were included as a covariate (no parental history of ADHD or one or more parents with an ADHD diagnosis). There were no significant differences in parent ADHD by SES (t = 1.18, p = 0.24). However, children in the ADHD group were significantly more likely to have parents diagnosed with ADHD, consistent with high heritability estimates (Nikolas & Burt, 2010). There were significant differences in birth weight by SES; families with higher SES had children with higher birth weight on average (r = 0.07, p < 0.05). There were no significant differences in ADHD group membership by birth weight. There were no significant differences in age by SES (r = 0.04, p = 0.29) or ADHD group membership (Table 1) however, we included age as a covariate due to the wide age range.
2.2 |. Statistical analyses
We investigated whether the relationship between ADHD group membership and SES was at least partially accounted for by differences in neural structure using statistical mediation.
2.2.1 |. Data reduction
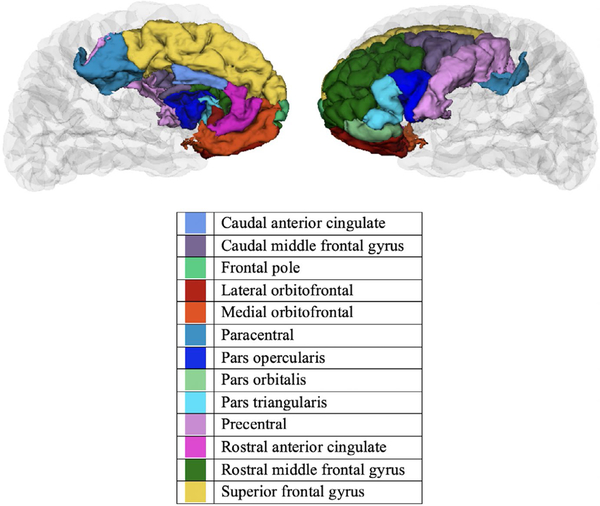
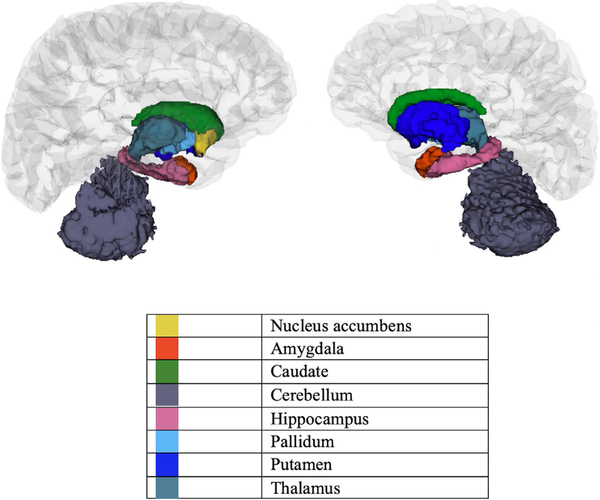
Using FreeSurfer, subcortical volume was segmented into eight regions and cortex was segmented into 32 regions in each hemisphere. We approached reducing the overall number of mediation paths tested using a priori theory coupled with a data-driven approach. First, given extensive prior evidence of differences in prefrontal cortical thickness and surface area in children with ADHD (Durston, 2003; Friedman & Rapoport, 2015; Krain & Castellanos, 2006; Vaidya, 2011), we selected thirteen prefrontal cortical regions a priori in each hemisphere identical to those used in a study examining differences in cortical thickness and grey matter volume for children with and without ADHD (Batty et al., 2010). These regions from the 2005 Desikan-Killiany FreeSurfer atlas are as follows: caudal anterior cingulate, caudal middle frontal, frontal pole, lateral orbitofrontal, medial orbitofrontal, paracentral, pars opercularis, pars orbitalis, pars triangularis, precentral, rostral anterior cingulate, rostral middle frontal and superior frontal (Desikan et al., 2006) (Figure 1). Differences in subcortical volume were investigated in: the nucleus accumbens, amygdala, caudate, cerebellum, hippocampus, pallidum, putamen and the thalamus (Figure 2). The analyses for cortical thickness, surface area and subcortical volume were conducted independently. Thus, we took this reduced group of regions and conducted analyses of the a, b and c paths using regression prior to testing the significance of the indirect effects for the full model. We only tested indirect effects (i.e. the full model) for regions with statistically significant a, b and c paths. For these analyses, a false discovery rate (FDR) correction, implemented in Statistical Analysis Software was applied to account for multiple comparisons (FDR corrected alpha of p < 0.05) for each group of analyses: cortical thickness, surface area and subcortical volume.
FIGURE 1.
Tested prefrontal regions from the 2005 Desikan–Killiany FreeSurfer atlas 127 × 94 mm (300 × 300 DPI)
FIGURE 2.
Tested subcortical regions from the 2005 Desikan– Killiany FreeSurfer atlas 126 × 107 mm (300 × 300 DPI)
2.2.2 |. Statistical mediation
First, we examined path c (if SES significantly predicted ADHD group status) using logistic regression while controlling for child age, gender, birth weight, scanner and parent ADHD. Age was mean-centered. Second, we investigated potential a paths (differences in cortical thickness, surface area and subcortical volume by SES) using linear regression analyses and the same controls. Third, we chose the regions which were significantly associated with SES following FDR correction and tested the b path (links between neural structure and ADHD group membership) using logistic regression and controlling for aforementioned covariates. Finally, for neural regions which met our strict criteria, we tested the significance of the indirect effects of parent SES on ADHD group membership through neural structure using a boot-strapping approach that provides bias-corrected confidence intervals (Preacher & Hayes, 2008). Confidence intervals that do not include zero indicate a significant indirect effect. These analyses were performed on the sample which had complete data (N = 874). Because the functional form of associations between age and subcortical volume differ across studies (Ostby et al., 2009; Wierenga et al., 2014), we additionally tested all significant mediation analyses including a non-linear age covariate (age2). We report when these results differ from our primary findings.
2.2.3 |. Multiple imputation
The sample which included complete data on all of our covariates and predictors was significantly smaller than the full dataset (N = 1,239). To ensure that data loss did not account for our observed associations, a supplementary analysis was conducted using multiple imputation. First, we determined that the pattern of data was not missing completely at random (Little’s MCARS test, χ2 = 140.39, p < 0.001). This lack of randomness may compromise the multiple imputation (Donders, Heijden, Stijnen, & Moons, 2006) however, violation of the missing completely at random assumption also affects listwise deletion procedures (Schafer & Graham, 2002). Thus, we report results from both listwise deletion (primary analysis) and multiple imputation for the full model. For multiple imputation, we use fully conditional specification (FCS), an iterative Markov chain Monte Carlo (MCMC) procedure, in SPSS 24. For every variable a model including all other variables is created to predict imputed values; this continued over 20 iterations, which were averaged to create the imputed dataset. We performed this procedure twice. Statistically significant mediation results observed in the primary analysis with complete data were reanalysed within these two datasets.
3 |. RESULTS
3.1 |. SES and ADHD group membership
Results of a logistic regression analysis indicated that ADHD group membership differed by composite SES (OR = 1.07, df = 1, p < 0.05). Children with lower SES were more likely to be in the ADHD group. In this and all subsequent analyses we controlled for child age, child gender, child birth weight, scanner and parent ADHD (see Table 1 and Sample section for more information on covariates).
3.2 |. SES and cortical thickness
Results of the linear regression analyses examining the relationship between SES and cortical thickness indicated that the right precentral region differed significantly by SES at p < 0.05 after FDR correction (see Table S2 for more information). Lower SES was associated with reduced thickness in the right precentral cortex.
3.3 |. SES and surface area
Results from the linear regression analyses identified 25 brain regions out of the 26 regions tested in which SES significantly predicts surface area at p < 0.05 after FDR correction (see Table S3 for more information). For all significant regions, lower SES was associated with reduced surface area.
3.4 |. SES and subcortical volume
Results from the linear regression analyses identified 10 subcortical regions out of the 16 regions tested in which SES was significantly associated with subcortical volume. SES significantly predicted subcortical volume in the left amygdala, caudate, cerebellum, hippocampus, putamen and the thalamus, as well as the right amygdala, caudate, cerebellum and the hippocampus (see Table S4 for more information). All analysed regions were significant at p < 0.05 after FDR correction. For all significant regions, lower SES was associated with less subcortical volume.
3.5 |. Cortical thickness and ADHD
Logistic regression analysis demonstrated that the right precentral cortex was not significantly associated with ADHD group membership.
3.6 |. Surface area and ADHD
Logistic regression analysis demonstrated that no cortical surface area regions associated with SES were significantly associated with ADHD group membership.
3.7 |. Subcortical volume and ADHD
Logistic regression analysis demonstrated that the left and right cerebellum were significantly associated with ADHD group membership at p < 0.05. Increases in subcortical volume in the left and right cerebellum were associated with higher risk for ADHD group membership. The right caudate was also significantly associated with ADHD group membership at p < 0.05. Increases in subcortical volume in the right caudate were associated with higher risk for ADHD group membership. All other regions associated with SES were not significant within the model. For all regions, increases in subcortical volume were associated with higher risk for ADHD group membership.
3.8 |. Mediation analysis
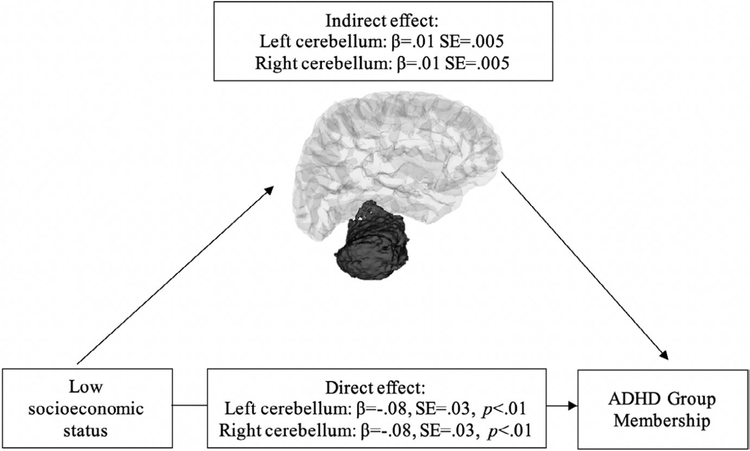
No cortical regions met our stringent criteria for conducting the mediation analysis examining either thickness or surface area. A significant indirect effect of SES on ADHD group membership through subcortical volume was observed for the left cerebellum (95% confidence interval: 0.004, 0.022) and the right cerebellum (95% confidence interval: 0.006, 0.025) (Figure 3). When including age2 as an additional covariate, the significant indirect effect of SES on ADHD group membership through the left and right cerebellum remains significant.
FIGURE 3.
Left and right cerebellum mediates family socioeconomic status and child attention-deficit/hyperactivity disorder group membership (N = 874) 101 × 60 mm (300 × 300 DPI)
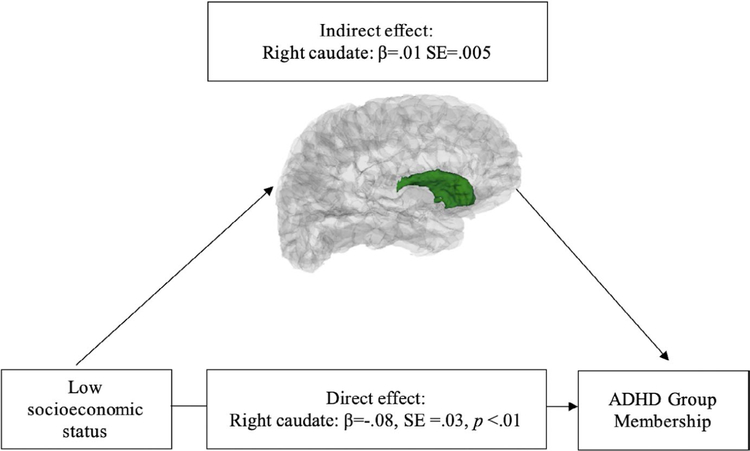
A significant indirect effect of SES on ADHD group membership through subcortical volume was additionally observed for the right caudate (95% confidence interval: 0.002, 0.022) (Figure 4). When including age2 as an additional covariate, the significant indirect effect of SES on ADHD group membership through the right caudate is no longer significant. In both imputed datasets, the indirect effect of the left cerebellum, the right cerebellum and the right caudate between SES and ADHD group were significant at the 95% confidence interval.
FIGURE 4.
Right caudate mediates family socioeconomic status and child attention-deficit/hyperactivity disorder group membership (N = 874) 101 × 60 mm (300 × 300 DPI)
Given the unexpected direction of effects, we additionally analysed cerebellar white matter volume to probe the indirect effect of SES on ADHD group membership and explore if the same pattern observed in cerebellar grey matter occurs in cerebellar white matter, which are often included together in an overall measure of subcortical volume. A significant indirect effect of SES on ADHD group membership through subcortical volume was observed for the left cerebellar white matter (95% confidence interval: 0.0003, 0.013) and the right cerebellar white matter (95% confidence interval: 0.0003, 0.014).
4 |. DISCUSSION
Low SES is one form of adverse early experience that has been previously linked to greater risk for ADHD (e.g. Law et al., 2014). In a large, diverse cohort of youth, we investigated whether differences in brain structure constituted a mechanism linking low SES and ADHD group membership. Subcortical volume in the left and right cerebellum partially mediated the relationship between low SES and ADHD group membership.
Consistent with the present findings, prior work indicates that low parental SES has been associated with decreases in cerebellar volume (Cavanagh et al., 2013; De Bellis & Kuchibhatla, 2006; Edmiston et al., 2011). Surprisingly, we also found that ADHD group membership was associated with increases in cerebellar grey matter and white matter. This could have been due to our inclusion of numerous covariates (e.g. due to collinearity) however, this is unlikely as we observe this direction of effects in the bivariate associations as well (see Table S1). Numerous studies have documented smaller cerebellar volume in children with ADHD (Berquin et al., 1998; Castellanos et al., 2002; Durston et al., 2004; Hill et al., 2003). The discrepancy between our findings and others may be accounted for by differences in methodology in measuring cerebellar volume, such as slice-by-slice hand-tracing or use of other automated image analysis techniques (Berquin et al., 1998; Bledsoe, Semrud-Clikeman, & Pliszka, 2011; Castellanos et al., 2002).
The cerebellum is important for coordination of motor movements and higher-order cognitive functions, including attentional shifting (Gottwald, Mihajlovic, Wilde, & Mehdorn, 2003). Relatedly, numerous theories have proposed that psychological deficits in ADHD are linked with alterations to frontal-cerebellar circuits in addition to frontal-striatal circuits (Castellanos & Proal, 2012; Durston, van Belle, & de Zeeuw, 2011; Nigg & Casey, 2005). Thus, the current study provides evidence that the environmental sensitivity of the cerebellum (Giedd et al., 2007) may be one mechanism through which the experience of low SES increases risk for ADHD.
Subcortical volume in the right caudate partially mediated the relationship between low SES and ADHD group membership. There is evidence that children with ADHD show reductions in caudate volume (Castellanos et al., 2002; Nakao, Radua, Rubia, & Mataix-Cols, 2011; Qiu et al., 2009; Valera, Faraone, Murray, & Seidman, 2007), although some studies have not found a significant effect (Bussing, Grudnik, Mason, Wasiak, & Leonard, 2002; Pineda et al., 1999). Interestingly, little prior research has linked parental SES to caudate volume, although links between childhood adversity and striatal structures have been observed (Edmiston et al., 2011). When we examined quadratic age effects, caudate volume no longer mediated the association between ADHD and SES. However, it is unlikely that non-linearity in age accounts for our findings because there were no age differences between children with and without ADHD. Overall this observation warrants follow-up and further investigation.
Consistent with previous work in this sample and elsewhere, we additionally observed bilateral reductions in subcortical volume associated with SES in the amygdala and hippocampus (Hanson, Chandra, Wolfe, & Pollak, 2011; Jednoróg et al., 2012; Luby et al., 2013; Merz et al., 2017; Noble et al., 2015, 2012). The amygdala and hippocampus are critical to social emotional functioning and are closely linked with hypothalamic-pituitary-adrenal axis stress response, one mechanism through which low parental SES could influence brain structure and function (Frodl & O’Keane, 2013; Lupien, McEwen, Gunnar, & Heim, 2009). Interestingly, volume of the hippocampus and amygdala did not mediate the association between SES and ADHD.
Findings in the present study build on prior research using the PING dataset (e.g. Noble et al., 2015) by linking low parental SES to ADHD through brain structure. The indirect effect of subcortical volume on the relationship between low parental SES and ADHD group membership constitutes an ‘inconsistent mediation’ indicating that the direct and indirect effects occur in opposite directions (MacKinnon & Fairchild, 2009; MacKinnon, Krull, & Lockwood, 2000). An inconsistent mediation indicates that there are multiple pathways through which SES confers higher risk for ADHD, not only the positive indirect effect through subcortical volume.
Low SES puts children at risk for environments characterized by low cognitive stimulation, exposure to complex language and careful supervision by adults (McLaughlin, Sheridan, & Lambert, 2014; Noble et al., 2007; Windsor, Moraru, Nelson, Fox, & Zeanah, 2013). Differences in cognitive stimulation and exposure to language may impact brain structure through typical experience-dependent neurodevelopmental processes (Brito & Noble, 2014; Sheridan & McLaughlin, 2014, 2016). Thus, the differences in subcortical volume that, in part, statistically account for the relationship between children with low family SES and ADHD symptoms may stem from differences in the developmental environment.
The present study presents novel findings linking socioeconomic disparities, brain structure and ADHD group membership observed in a large dataset comprised of a socioeconomically and racially diverse group of participants across a wide age range. However, several limitations should be noted. First, validated research diagnoses of ADHD were not obtained for the PING study. The PING study relied on parental report of ADHD diagnosis and/or parent-reported significant attention problems meaning that results are best interpreted as mediating the association between SES and inattention problems or community diagnosis. In light of this limited diagnostic information, these results should be considered preliminary observations. Future work should confirm that these associations are consistent when ADHD diagnosis is confirmed. Second, the PING study did not evaluate the presence of other psychological disorders in the full sample. Future work should explore if these associations are consistent when controlling for other psychological conditions. Third, the present study did not measure other forms of early adversity, such as abuse, exposure to domestic violence or exposure to community violence. Future studies should investigate depriving experiences and threatening experiences. Fourth, the present study was not a longitudinal analysis, compromising the directional interpretation of the mediation analysis.
In summary, this study documents that the link between low SES, a common early adverse experience and ADHD group membership is explained, in part, through alterations in bilateral cerebellar volume and right caudate volume. We provide this evidence in a large, diverse sample and across a wide range of ages, controlling for many potential confounders. The findings suggest that alterations to the cerebellum and caudate are potential neurodevelopmental mechanisms linking low SES with ADHD.
Supplementary Material
Research highlights.
The current study examined the relationship between family socioeconomic status (SES), alterations in brain structure and attention-deficit/hyperactivity disorder (ADHD) status in youth.
Low SES was associated with reduced surface area across the frontal lobe and reduced subcortical volume in the amygdala, cerebellum, hippocampus and basal ganglia.
Subcortical volume in the left cerebellum, right cerebellum and the right caudate statistically mediated the relationship between low SES and ADHD status in children.
These findings were for ADHD status as reported by parents and thus should be considered preliminary.
Funding information
National Science Foundation, Grant/Award Number: DGE-1144081
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available from the Pediatric Imaging, Neurocognition and Genetics Study at http://pingstudy.ucsd.edu.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, … Jernigan TL (2014). The NIH Toolbox Cognition Battery: Results from a large normative developmental sample (PING). Neuropsychology, 28(1), 1–10. 10.1037/neu0000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Ávila D, & Martínez RB (2010). Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross-sectional study. Journal of Psychiatric Research, 44(16), 1214–1223. 10.1016/j.jpsychires.2010.04.026 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association & DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Retrieved from http://dsm.psychiatryonline.org/book.aspx?bookxml:id=556.
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, … Hollis C (2010). Cortical gray matter in attention-deficit/hyperactivity disorder: A structural magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 229–238. 10.1016/j.jaac.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, & Castellanos FX (1998). Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurology, 50(4), 1087–1093. 10.1212/WNL.50.4.1087 [DOI] [PubMed] [Google Scholar]
- Bick J, & Nelson CA (2015). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177–196. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe JC, Semrud-Clikeman M, & Pliszka SR (2011). Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder – combined type. Journal of the American Academy of Child and Adolescent Psychiatry, 50(6), 593–601. 10.1016/j.jaac.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, & Corwyn RF (2002). Socioeconomic status and child development. Annual Review of Psychology, 53(1), 371–399. 10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, … Konrad K (2007). Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry, 48(12), 1251–1258. 10.1111/j.1469-7610.2007.01799.x [DOI] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Piccolo LR, & Noble KG (2017). Associations between cortical thickness and neurocognitive skills during childhood vary by family socioeconomic factors. Brain and Cognition, 116, 54–62. 10.1016/j.bandc.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing R, Grudnik J, Mason D, Wasiak M, & Leonard C (2002). ADHD and conduct disorder: An MRI study in a community sample. The World Journal of Biological Psychiatry, 3(4), 216–220. 10.3109/15622970209150624 [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Trèmols V, Soliva JC, Rovira M, … Bulbena A (2005). Global and regional gray matter reductions in ADHD: A voxel-based morphometric study. Neuroscience Letters, 389(2), 88–93. 10.1016/j.neulet.2005.07.020 [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, … Rapoport JL (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA, 288(14), 1740–1748. 10.1001/jama.288.14.1740 [DOI] [PubMed] [Google Scholar]
- Castellanos FX, & Proal E (2012). Large-scale brain systems in ADHD: Beyond the prefrontal–striatal model. Trends in Cognitive Sciences, 16(1), 17–26. 10.1016/j.tics.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J, Krishnadas R, Batty GD, Burns H, Deans KA, Ford I, … McLean J (2013). Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterised population sample. The Cerebellum, 12(6), 882–891. 10.1007/s12311-013-0497-4 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, & Kuchibhatla M (2006). Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry, 60(7), 697–703. 10.1016/j.biopsych.2006.04.035 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, & Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders ART, van der Heijden GJMG, Stijnen T, & Moons KGM (2006). Review: A gentle introduction to imputation of missing values. Journal of Clinical Epidemiology, 59(10), 1087–1091. 10.1016/j.jclinepi.2006.01.014 [DOI] [PubMed] [Google Scholar]
- Durston S (2003). A review of the biological bases of ADHD: What have we learned from imaging studies? Mental Retardation and Developmental Disabilities Research Reviews, 9(3), 184–195. 10.1002/mrdd.10079 [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, … van engeland H (2004). Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry, 43(3), 332–340. 10.1097/00004583-200403000-00016 [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, Casey BJ (2002). A neural basis for the development of inhibitory control. Developmental Science, 5(4), F9–F16. 10.1111/1467-7687.00235 [DOI] [Google Scholar]
- Durston S, van Belle J, & de Zeeuw P (2011). Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biological Psychiatry, 69(12), 1178–1184. 10.1016/j.biopsych.2010.07.037 [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, & Blumberg HP (2011). Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics &Adolescent Medicine, 165(12), 1069–1077. 10.1001/archpediatrics.2011.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LA, & Rapoport JL (2015). Brain development in ADHD. Current Opinion in Neurobiology, 30, 106–111. 10.1016/j.conb.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Frodl T, & O’Keane V (2013). How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of Disease, 52, 24–37. 10.1016/j.nbd.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, & Kahn RS (2007). Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Archives of Pediatrics & Adolescent Medicine, 161(9), 857–864. 10.1001/archpedi.161.9.857 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Schmitt JE, & Neale MC (2007). Structural brain magnetic resonance imaging of pediatric twins. Human Brain Mapping, 28(6), 474–481. 10.1002/hbm.20403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald B, Mihajlovic Z, Wilde B, & Mehdorn HM (2003). Does the cerebellum contribute to specific aspects of attention? Neuropsychologia, 41(11), 1452–1460. 10.1016/S0028-3932(03)00090-3 [DOI] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, & Pollak SD (2015). Association of child poverty, brain development, and academic achievement. JAMA Pediatrics, 169(9), 822–829. 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, … Fischl B (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between Income and the Hippocampus. PLoS ONE, 6(5), e18712 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, … Davidson RJ (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, & Brooks W (2003). Magnetic resonance imaging correlates of attention-deficit/ hyperactivity disorder in children. Neuropsychology, 17(3), 496–506. 10.1037/0894-4105.17.3.496 [DOI] [PubMed] [Google Scholar]
- Holz NE, Laucht M, & Meyer-Lindenberg A (2015). Recent advances in understanding the neurobiology of childhood socioeconomic disadvantage. Current Opinion in Psychiatry, 28(5), 365–370. 10.1097/YCO.0000000000000178 [DOI] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, … Franke B (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. The Lancet. Psychiatry, 4(4), 310–319. 10.1016/S2215-0366(17)30049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, … Ramus F (2012). The influence of socioeconomic status ON CHILDREN’S BRAIN STRUCTURE. PLoS ONE, 7(8), e42486 10.1371/journal.pone.0042486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ, Akshoomoff N, Bartsch H, & Newman E, … Pediatric Imaging, Neurocognition and Genetics Study (2016). The pediatric imaging, neurocognition, and genetics (PING) data repository. NeuroImage, 124(Pt B), 1149–1154. 10.1016/j.neuroimage.2015.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, & Castellanos FX (2006). Brain development and ADHD. Clinical Psychology Review, 26(4), 433–444. 10.1016/j.cpr.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Law EC, Sideridis GD, Prock LA, & Sheridan MA (2014). Attention-deficit/hyperactivity disorder in young children: Predictors of diagnostic stability. Pediatrics, 133(4), 659–667. 10.1542/peds.2013-3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science, 16(5), 641–652. 10.1111/desc.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167(12), 1135–1142. 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, & Fairchild AJ (2009). Current directions in mediation analysis. Current Directions in Psychological Science, 18(1), 16–20. 10.1111/j.1467-8721.2009.01598.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, & Lockwood CM (2000). Equivalence of the mediation, confounding and suppression effect. Prevention Science: The Official Journal of the Society for Prevention Research, 1(4), 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry, 76(8), 629–638. 10.1016/j.biopsych.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, & Noble KG (2017). Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 47(2), 312–323. 10.1080/15374416.2017.1326122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, & Kaufmann WE (2002). Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry, 52(8), 785–794. 10.1016/S0006-3223(02)01412-9 [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, & Mataix-Cols D (2011). Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry, 168(11), 1154–1163. 10.1176/appi.ajp.2011.11020281 [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del’Homme M, … Levitt JG (2009). Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 48(10), 1014–1022. 10.1097/CHI.0b013e3181b395c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, & Casey BJ (2005). An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology, 17(3), 785–806. 10.1017/S0954579405050376 [DOI] [PubMed] [Google Scholar]
- Nikolas MA, & Burt SA (2010). Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: A meta-analysis. Journal of Abnormal Psychology, 119(1), 1 10.1037/a0018010 [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, … Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–527. 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, & Farah MJ (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10(4), 464–480. 10.1111/j.1467-7687.2007.00600.x [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, & Farah MJ (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science, 8(1), 74–87. 10.1111/j.1467-7687.2005.00394.x [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, & Walhovd KB (2009). Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of Neuroscience, 29(38), 11772–11782. 10.1523/JNEUROSCI.1242-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang L, Fang X, Mercy J, Perou R, & Grosse SD (2008). Attentiondeficit/hyperactivity disorder symptoms and child maltreatment: A population-based study. The Journal of Pediatrics, 153(6), 851–856. 10.1016/j.jpeds.2008.06.002 [DOI] [PubMed] [Google Scholar]
- Pineda D, Ardila A, Rosselli M, Arias BE, Henao GC, Gomez LF, … Miranda ML (1999). Prevalence of attention-deficit/hyperactivity disorder symptoms in 4-to 17-year-old children in the general population. Journal of Abnormal Child Psychology, 27(6), 455–462. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, & Rohde LA (2007). The worldwide prevalence of ADHD: A systematic review and metaregression analysis. The American Journal of Psychiatry, 164(6), 942–948. 10.1176/appi.ajp.164.6.942 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, & Mostofsky SH (2009). Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. The American Journal of Psychiatry, 166(1), 74–82. 10.1176/appi.ajp.2008.08030426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv T, Kessenich M, & Morrison FJ (2004). A mediational model of the association between socioeconomic status and three-year-old language abilities: The role of parenting factors. Early Childhood Research Quarterly, 19(4), 528–547. 10.1016/j.ecresq.2004.10.007 [DOI] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, & Boyce WT (2011). Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society, 17(01), 120–132. 10.1017/S1355617710001335 [DOI] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. 10.1037//1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Schweren LJS, Hartman CA, Heslenfeld DJ, van der Meer D, Franke B, Oosterlaan J, … Hoekstra PJ (2015). Thinner medial temporal cortex in adolescents with attention-deficit/hyperactivity disorder and the effects of stimulants. Journal of the American Academy of Child & Adolescent Psychiatry, 54(8), 660–667. 10.1016/j.jaac.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, … Rapoport J (2006). Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/ hyperactivity disorder. Archives of General Psychiatry, 63(5), 540–549. 10.1001/archpsyc.63.5.540 [DOI] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–585. 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2016). Neurobiological models of the impact of adversity on education. Current Opinion in Behavioral Sciences, 10, 108–113. 10.1016/j.cobeha.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Shannon JD, Cabrera NJ, & Lamb ME (2004). Fathers and mothers at play with their 2- and 3-year-olds: Contributions to language and cognitive development. Child Development, 75(6), 1806–1820. 10.1111/j.1467-8624.2004.00818.x [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, & Glasziou P (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135(4), e994–e1001. 10.1542/peds.2014-3482 [DOI] [PubMed] [Google Scholar]
- Ursache A, Noble KG, &Pediatric Imaging, Neurocognition and Genetics Study. (2016). Socioeconomic status, white matter, and executive function in children. Brain and Behavior, 6(10), e00531 10.1002/brb3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ (2011). Neurodevelopmental abnormalities in ADHD In Stanford C & Tannock R (Eds.), Behavioral neuroscience of attention deficit hyperactivity disorder and its treatment (pp. 49–66). Heidelberg, Berlin: Springer. [Google Scholar]
- Valera EM, Faraone SV, Murray KE, & Seidman LJ (2007). Metaanalysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry, 61(12), 1361–1369. 10.1016/j.biopsych.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, & Durston S (2014). Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage, 96, 67–72. 10.1016/j.neuroimage.2014.03.072 [DOI] [PubMed] [Google Scholar]
- Windsor J, Moraru A, Nelson CA, Fox NA, & Zeanah CH (2013). Effect of foster care on language learning at eight years: Findings from the Bucharest Early Intervention Project. Journal of Child Language, 40(03), 605–627. 10.1017/S0305000912000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyciszkiewicz A, Pawlak MA, & Krawiec K (2017). Cerebellar volume in children with attention-deficit hyperactivity disorder (ADHD): Replication study. Journal of Child Neurology, 32(2), 215–221. 10.1177/0883073816678550 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.