Abstract
In this targeted review, we summarize current knowledge on substance-use disorder (SUD)-related cognitive deficits, the link between these deficits and clinical outcomes, and the cognitive training, remediation, and pharmacological approaches that have the potential to rescue cognition. We conclude that: (i) people with SUDs have moderate deficits in memory, attention, executive functions, and decision-making (including reward expectancy, valuation, and learning); (ii) deficits in higher-order executive functions and decision-making are significant predictors of relapse; (iii) cognitive training programs targeting reward-related appetitive biases, cognitive remediation strategies targeting goal-based decision-making, and pharmacotherapies targeting memory, attention, and impulsivity have potential to rescue SUD-related cognitive deficits. We suggest avenues for future research, including developing brief, clinically oriented harmonized cognitive testing suites to improve individualized prediction of treatment outcomes; computational modeling that can achieve deep phenotyping of cognitive subtypes likely to respond to different interventions; and phenotype-targeted cognitive, pharmacological, and combined interventions. We conclude with a tentative model of neuroscience-informed precision medicine.
Keywords: substance-use disorder, cognition, decision-making, treatment outcome, cognitive training, cognitive remediation, cognitive enhancer
Abstract
En este artículo de revisión, se resume el conocimiento actual acerca de los déficits cognitivos relacionados con el trastorno por uso de sustancias (TUS), el vínculo entre estos déficits y los resultados clínicos, y el entrenamiento cognitivo, la remediación y los enfoques farmacológicos potencialmente útiles para la recuperación cognitiva. Se concluye que: 1) las personas con TUS tienen déficits moderados en memoria, atención, funciones ejecutivas y en la toma de decisiones (incluidas la expectativa de recompensa, la valoración y el aprendizaje), 2) los déficits en las funciones ejecutivas superiores y en la toma de decisiones son importantes predictores de recaída, 3) los programas de entrenamiento cognitivo orientados a los sesgos en las apetencias relacionadas con la recompensa, las estrategias de remediación cognitiva dirigidas a la toma de decisiones basadas en objetivos y las farmacoterapias focalizadas en memoria, atención e impulsividad tienen el potencial de recuperar los déficits cognitivos relacionados con el TUS. Se sugieren alternativas para futuras investigaciones, incluyendo el desarrollo de secuencias de pruebas cognitivas breves, armónicas y con una orientación clínica para mejorar la predicción individual de los resultados del tratamiento; los modelos computacionales que pueden conseguir un fenotipado profundo de los subtipos cognitivos que respondan probablemente a diferentes intervenciones; y las intervenciones cognitivas y/o farmacológicas para un fenotipo específico. Se concluye con un modelo tentativo de medicina de precisión basada en la neurociencia.
Abstract
Nous résumons dans cet article ciblé les connaissances actuelles sur les troubles cognitifs liés à l’utilisation d’une substance (TUS), sur le lien entre ces troubles et les résultats cliniques, sur l’entraînement cognitif, la remédiation cognitive et les traitements pharmacologiques qui peuvent aider la cognition. Nos conclusions sont les suivantes : 1) les troubles des personnes souffrant de TUS sont modérés pour la mémoire, l’attention, les fonctions exécutives et la prise de décision (y compris l’attente de récompense, l’évaluation et l’apprentissage) ; 2) les troubles des fonctions exécutives et de prise de décision d’ordre supérieur sont des prédicteurs significatifs de rechute ; 3) les programmes d’entraînement cognitif ciblant les biais appétitifs liés à la récompense, les stratégies de remédiation cognitive ciblant la prise de décision basée sur objectif et les traitements médicamenteux ciblant la mémoire, l’attention et l’impulsivité peuvent aider les troubles cognitifs liés aux TLS. Nos propositions de recherche future sont : des séquences de tests cognitifs brèves, harmonisées et cliniquement orientées pour améliorer une prédiction individualisée des résultats thérapeutiques ; un modelage numérique pour un phénotypage profond des sous-types cognitifs susceptibles de répondre aux différentes interventions ; et des interventions cognitives ciblées sur le phénotype, pharmacologiques et associées. Nous proposons en conclusion un modèle de médecine de précision éclairé par les neurosciences.
Cognitive deficits in substance-use disorders
People with substance-use disorders (SUDs) including those related to alcohol, stimulants, and opioids, have cognitive deficits of moderate magnitude and longevity. 1 Meta-analytic research suggests that several cognitive processes are significantly impaired across users of different drugs, including selective attention and related attentional biases (automatic responses to drug-related stimuli), episodic memory, executive functions (working memory, inhibition, and shifting), and reward-based decision-making. 2 - 5 A systematic review of studies comparing different SUDs suggested that stimulant SUDs are particularly associated with inhibition and shifting deficits, whereas opioid SUDs are associated with reasoning deficits. 6 Alcohol-use disorder is associated with wide-ranging deficits in attention and executive functions. 6 Different SUDs have common deficits in reward-based decision-making. 7 Individual studies have shown that several factors moderate the severity of cognitive deficits, including principal drug of choice (greatest deficits in alcohol and stimulant SUDs), polysubstance use, drug-specific pharmacokinetics and pharmacodynamics, and psychiatric comorbidities. 6 , 8 - 10 However, there remain many gaps in our knowledge. For instance, although dose and duration of use are important, for none of the substances is there, as yet, evidence of a critical dosage, below which cognitive deficits can be excluded. In addition to these data-driven findings, theoretical models and expert consensus approaches have recently highlighted a limited number of domains that, according to experts, play a pivotal role in addiction. The Addiction Neuroclinical Assessment framework (ANA), leveraging on evidence from preclinical and human neuroimaging and neurocognitive studies, has categorized deficits into three key domains: incentive salience, negative emotionality, and executive functions. 11 , 12 In addition, a recent international Delphi consensus pinpointed key deficits in reward valuation, expectancy, action selection, reward learning, habit, and compulsivity. 13 Most of these constructs were deemed relevant for both addiction vulnerability and chronicity, although compulsivity was specifically associated with chronicity, in agreement with preclinical models of addiction. 14 , 15 Although these constructs were separately identified during the Delphi process, we and others have integrated several of these domains (eg, reward expectancy/valuation, action selection, reward learning) in the context of decision-making processes. 16 Therefore, both meta-analytic research, neuroscience models, and pragmatic-consensus approaches agree on the existence of SUD-related deficits in reward and salience valuation, executive functions, and decision-making.
Cognitive deficits and treatment outcomes
Cognitive deficits in attention, memory, executive functions, reward/negative emotion valuation, and decision-making reflect performance differences between substance users and drug-naïve controls and/or consensus from experts. 2 , 11 , 13 , 17 , 18 A pertinent but different question is which of these cognitive deficits are relevant to clinical outcomes in the context of addiction treatment. Key treatment outcomes include treatment retention and adherence, reduction of drug use and abstinence, craving, and quality of life. 19 , 20 In a systematic review of prospective studies measuring retention and abstinence-related outcomes, we showed that a test battery of general cognitive functioning indexing speed/accuracy during attention and reasoning tasks (MicroCog) was the only consistent predictor of treatment retention, and tests of uncertainty and risk-based decision-making the only consistent and robust predictor of relapse. 21 The latter finding in line with recent neuroimaging data showing that quality of the neural networks integrating the executive control system (connectivity between frontoparietal and medial frontal networks) and the reward responsiveness system (connectivity between salience, motor/sensory, and subcortical networks) is associated with cocaine abstinence following treatment and at 6-month follow-up. 22
Some of the constructs identified by Delphi consensus (eg, reward expectancy and valuation) or ANA (eg, negative emotionality) have not been examined in prospective outcome studies and need more research. However, some other constructs pinpointed by these approaches (eg, habits/compulsivity and incentive salience) have been measured with well-validated paradigms such as shifting/perseveration and attentional bias tests and, although some studies showed significant predictive validity, there was not sufficient consistency across studies. 21 Tests of executive functions yielded mixed evidence although this may be due to test impurity (see section on cognitive training and rehabilitation) and significant heterogeneity in the selection of measures.
What is it about successful tests? General cognition tests predicting treatment retention may be capturing more complex aspects of the cognitive architecture. For example, speed and attention are needed to perform complex executive function tests including action selection and response inhibition tasks. 23 In addition, the performance measures of these tests (precise measures of reaction time and errors) can be more sensitive to individual differences relevant to prediction, compared with the traditional outcomes of neuropsychological tests, ie, gross measures such as the Stroop interference score or attentional lapses in the Wisconsin Card Sorting Test. In support of this view, we recently showed that the index of reaction time variability of the continuous performance test was a significant predictor of continuous treatment engagement, over and above a more specific measure of effort-related reward valuation. 24 Another possibility is that these tests are a proxy of IQ-related decline 25 and thus a global measure of the impact of SUDs on fluid intelligence and related outcomes. Decision-making tests predicting drug use and relapse suggest a much more straightforward story. The ability to make advantageous decisions in complex scenarios is essential to achieve long-term goals and life milestones. In the context of SUDs, greater alterations in the ability to make uncertain decisions (Iowa Gambling Task) and estimate risk (Cambridge Gambling Task) can compromise attempts to maintain abstinence. 26 , 27 At the same time, these tasks (especially the Iowa task) have been criticized for lack of reliability and construct validity. 28 Although our personal experience is that providing appropriate task instructions and performing detailed analyses (eg, block-by-block or trial-by-trial performance) partly control for these criticisms, decision-making tasks are inherently complex and open to several different response styles (see section on cognitive training and rehabilitation). The latter feature can be negative for reliability and construct validity but positive for predictive validity, by allowing individual differences and detection of specific cognitive phenotypes. 29 , 30
Several cognitive domains have clear face validity for predicting addiction treatment outcomes but may be suffering measurement problems. Response inhibition and action selection are integral aspects of decision-making processes, and clinicians consistently report anecdotal evidence of highly impulsive patients dropping out of treatment and restarting drug use. 31 However, available tests such as the Stroop or the Stop Signal task have not shown consistent predictive validity. 21 Therefore, the design of new tests that incorporate precise output metrics (to detect individual differences) and optimize both construct and predictive validity (to predict meaningful outcomes) will allow reassessment of the clinical significance of these cognitive domains. An important consideration for the design of new tests in the SUD space is the prioritization of features that can facilitate clinical usability, including brevity and automated instructions and feedback. 32 Complementarily, the outcomes of the tests should be suitable for computational modeling that can tease apart clinically significant subtypes to inform precision medicine approaches. These features (usability and suitability for modeling) will also contribute to the next frontier and most ambitious challenge in this context–the harmonization of a cognitive test battery for addiction (akin to the MATRICS battery for schizophrenia). Based on current knowledge, we propose that this battery incorporate indices of IQ, speed/accuracy-based attention and reasoning, decision-making, and novel measures of action selection/response inhibition and reward learning. Desired features include customized computerization, automated instructions and feedback reports, brevity (or potential to become briefer via psychometric modeling) and suitability to conduct cognitive neuroscience-informed computational modeling on output variables.
Computational modeling
An outstanding limitation of current methods of cognitive assessment, especially complex attention, executive functions and decision-making tasks frequently used in the context of SUDs, is the so-called test impurity. Complex cognitive tests simultaneously tap into several different cognitive abilities. Some of these abilities are prerequisite skills needed to engage more complex abilities. And some others are complex, higher-order cognitive skills, just not the ones that the test primarily intends to measure. For example, participants performing a decision-making test need to recruit basic attention and memory skills to process the task stimuli and remember instructions. When making decisions, some participants may use working memory strategies to “hold online” options with better reward values, whereas others may employ response inhibition skills to withhold responses driven by past immediate outcomes or predicted rewards. All of these influences permeate the final output test measures, which then reflects a combination of the performance of participants in the targeted cognitive skills + prerequisite skills + other complex strategies employed during test performance. This phenomenon may partly explaining why complex cognitive measures often suffer reliability and construct validity issues. 33 It may also contribute to explaining why some constructs with high face validity, such as response inhibition, have not consistently predicted clinical outcomes of SUD treatment.
One way to address test impurity while simultaneously increasing measurement precision is by using cognitive modeling, or computational modeling of cognitive processes. Computational models enable researchers to deconstruct a cognitive task or activity on a limited number of subprocesses (parameters) and their predicted interactions, and to build models to precisely measure individual variation in each of those parameters (parameter estimates). Early applications of cognitive modeling to decision-making tasks in SUDs showed that the performance of substance users in the Iowa Gambling Task could be decomposed into several different parameters including sensitivity to reward and punishment, memory of recent versus distal decision outcomes, and choice consistency. 34 Using this modeling, they could establish that the decision-making deficits of cocaine users were mostly driven by hypersensitivity to reward and lack of choice consistency, 35 whereas the deficits of cannabis users were mostly driven by recency effects. Other simple forms of cognitive modeling, such as drift-diffusion modeling and hyperbolic curve modeling have been successfully applied to examine value-based and perceptual decision-making tasks (reviews in refs 36,37). More recently, model-based vs model-free modeling, which estimates the extent to which decision-making performance is driven by habitual versus goal-oriented responses, is gaining traction in the cognitive neuroscience literature and has been successfully applied to measure the decision-making deficits of methamphetamine users. 38 In addition to individual-based decision-making, novel computational models have started to deconstruct and estimate individual variation in complex social decision-making, incorporating complex abstract parameters such as guilt. 39 Altogether, growing evidence suggests that computational modeling could assist the design of novel cognitive tasks and test batteries that achieve more precise and predictive measures of latent cognitive processes.
Cognitive training and rehabilitation
There are two main approaches to restore cognitive deficits: (i) computerized cognitive training; (ii) cognitive rehabilitation. Computerized cognitive training uses software to retrain specific cognitive processes through repeated exercises aimed to build cognitive capacity. Cognitive rehabilitation or remediation focuses on meta-cognitive training and strategy learning, instructing participants to apply cognitive resources in a goal-driven and strategic way. 40 Unlike cognitive training, it is typically guided by therapists and focuses on real-life activities (instead of task-based exercises). A key assumption within addiction neuroscience is the existence of an imbalance between the bottom-up cognitive systems that are sensitized to the reward value of drug-related stimuli and the top-down executive and decision-making systems that fail to guide response selection according to long-term goals. 41 - 43 From a cognitive architectural and functional standpoint, it seems more feasible to retrain automatic bottom-up processes through cognitive training and repeated exercise. On the other hand, top-down goal-driven behavior requires greater complexity and entropy to adapt cognitive strategies to the current context and future goals, and thus is more suitable to be trained through cognitive rehabilitation approaches. In support of this notion, the two most successful cognitive remediation approaches for addiction use these principles. Cognitive bias modification (CBM) uses software-based cognitive exercises to retrain automatic attentional/approach biases towards drug stimuli. By training participants to avoid drug-related images and approach alternative reinforcers, CBM decreases the motivational appeal of drug stimuli. 44 The training has shown moderate efficacy to reduce alcohol use (and to increase approach to nonalcoholic drinks) in several independent trials, 45 although its application to other drugs of abuse is limited by lack of satisfactory alternative conditions (eg, there is no straightforward alternative to stimulant or opioid use). On the other hand, Goal Management Training (GMT) is a therapist-guided cognitive remediation training that instructs participants to implement a metacognitive strategy to decision-making, based on the sequence STOP-Mindfulness-Goal-Check. 46 This strategy enables participants to withhold impulsive behaviors, use mindfulness practice to align their attentional resources with goals (eg, abstinence), and select behaviors aligned with those goals. This training has been successfully applied to improve executive functions in alcohol and stimulant polysubstance users 47 , 48 and HIV+ participants with SUDs. 49
Strengthening executive functions via working memory (WM) training has also been proposed as a treatment strategy for SUDs. 50 WM training has been investigated in different SUD populations, with preliminary findings suggesting beneficial effects on working memory capacity, impulsive behavior, and reduction of alcohol/drug use among heavy drinkers, 51 stimulant-dependent individuals, 52 and methadone maintenance patients. 53 In more recent studies, WM training improved impulsivity in methamphetamine users 54 and among alcohol-dependent patients who were more impulsive at baseline. 55 In adolescents enrolled in cannabis use treatment, urinalysis results favored a WM-trained group compared with a control group. 56 In addition, WM training has shown feasibility within SUDs inpatient treatment settings. 57 Nevertheless, there are WM studies that have not reported transfer/generalization effects. For example, in Wanmaker et al, 58 WM training led to improvements on trained tasks but not on nontrained WM tasks or other cognitive measures. In another study with alcohol users, the WM-trained group demonstrated a significantly greater improvement in verbal WM compared with a control group; however, the results did not support an effect of WM training on other cognitive domains or drinking outcomes. 59 Taken together, these studies show promising results though further work is necessary to establish training effects on clinical outcomes.
An interesting emerging approach is to combine different neuroscience-informed interventions that synergistically tap into bottom-up versus top-down cognitive processes. Within this context, there are three potential approaches: (i) combining cognitive training with existing evidence-based interventions; (ii) combining cognitive training and exercise (regulates drug cues related salience and promotes neuroplasticity); and (iii) combining two different cognitive trainings. Initial evidence suggests that combining computerized cognitive training of general cognition and working memory with contingency management (financial incentives associated with completion of cognitive training sessions) improves the beneficial effects of training on top-down cognitive skills. 52 , 60 The next step is to explore the potential of this combination to improve clinical outcomes such as the reduction of drug use and abstinence. A promising approach in this context would be combining contingency management (CM) with Goal Management Training. 16 CM would facilitate the goal of maintaining abstinence in the short-term (ie, exchanging negative drug tests with financial incentives), while progressive training with GMT would enable participants to apply goal-based decision-making strategies in the long-term. The second approach leverages evidence showing that short- and mid-term regimens of aerobic exercise can significantly reduce drug cues related salience, 61 and increase the availability of dopamine D2-type receptors in the striatum, linked to reward valuation and impulsivity. 62 Thus, combining aerobic exercise and training of top-down impulse control via for example inhibitory control training may have synergistic benefits on cognitive control and craving. The third approach consisting of combining bottom-up and top-down cognitive training sounds immediately intuitive. However, its application is not without challenges. For example, we applied a combination of cognitive bias modification and working memory training among people with alcohol-use disorders, and found that the combination training did not improve cognitive or clinical outcomes. 63 We reasoned that one of the factors explaining the lack of success might be the risk of overwhelming cognitive abilities and generating frustration. Therefore, this approach should carefully consider the timing and the intensity of the “combination-training” for example, by alternating different trainings on different days and ensuring that difficulty is progressive, or by integrating both trainings in a single package.
In addition to combination therapies primarily based on cognitive training, additional efforts have been made to develop modified “traditional” behavioral psychosocial treatments adapted to compensate the cognitive deficits of people with SUDs. Aharonovich et al 64 have integrated compensatory strategies for cognitive deficits used in brain injury patients in a Modified Cognitive Behavioral Therapy (M-CBT). They presented the therapeutic CBT activities in a less cognitively demanding way to improve learning, memory, and executive functions (eg, reduced session length and increased weekly frequency, short simplified communication, concrete and visual presentation of content of sessions, workbooks with visual illustrations and to-do lists, use of mnemonic and external memory aids, repetition until mastery of concept occurs). M-CBT was not superior to CBT in terms of treatment retention or drug use reduction, although participants enrolled in M-CBT reported higher treatment satisfaction, and those who completed at least 9 weeks of treatment showed a trend towards a greater reduction of cocaine use.
Finally, an important aspect to address in future studies of cognitive training and rehabilitation is what aspects of these interventions may work better for different patient subtypes. Research on moderators of cognitive training and rehabilitation effects is particularly useful in this context. For example, in the context of problematic alcohol use, Houben et al 51 found that participants with strong impulses to drink alcohol benefitted the most from WM training. This finding aligns with the view that WM training can be particularly useful to reduce impulsive behaviors given neurobiological overlap in lateral prefrontal cortex regions. 65 , 66 Furthermore, Eberl et al 67 demonstrated that older alcohol-dependent patients and patients with a strong pre-training approach-bias benefitted most from CBM.
Biological approaches
Pharmacotherapy
Cognitive enhancing pharmacotherapies are based on the premise that cognitive processes may be important targets for the treatment of SUD. 68 , 69 From this point of view pharmacotherapies that aim at improving cognition can be considered as potential transdiagnostic intervention, ie, enhancing cognitive processes underlying different types of addictions and associated psychiatric disorders. Broadly two categories of approaches can be identified: memory-enhancing drugs (acetylcholinesterase inhibitors) and stimulants.
Increasing synaptic concentrations of acetylcholine has shown potential to improve cognitive function in neuropsychiatric disorders, eg, dementia and schizophrenia. 70 , 71 In different, small-scale and short studies, galantamine and rivastigmine showed positive effects both on cognitive function (sustained attention, working memory) and clinical outcome in patients with stimulant SUDs (amphetamine, cocaine). 71 , 72 These results provide at least preliminary evidence meriting future research.
Modafinil is a cognitive enhancer with a complex pharmacological profile, ie, inhibitor of dopamine and norepinephrine transporters, and additional actions on GABA, glutamate, and Orexin. 73 Within cocaine-dependent patients modafinil has shown improvements of cognitive function (working memory) and clinical (reduction of cocaine use) effects. 74 - 77 Of interest, in a recent study baseline cognitive functioning, ie, impulsivity and attentional bias, predicted clinical outcomes in modafinil treated crack-cocaine dependent patients. 78 In alcohol-dependent patients modafinil improved impulsive decision-making, response inhibition, and working memory and had a positive effect on clinical outcome (time to relapse percentage of abstinent days). 79 - 83 However, both the positive clinical effect and the effect on working memory were limited to those patients with high impulsivity and low working memory. In reverse, patients with a normal-to-low baseline impulsivity had an adverse effect on their drinking outcomes when using modafinil. 80 , 82 These findings indicate the importance of baseline cognitive performance in differentiating the effect of modafinil and possibly other cognitive-enhancing medication.
Methylphenidate is another stimulant drug with a pharmacological action similar to amphetamines and cocaine, ie, increasing dopamine, norepinephrine, and serotonin. Different studies show its efficacy in improving decision-making, working memory, and set-shifting in ADHD patients. Recent studies show a positive effect of high dosages of methylphenidate on amphetamine and cocaine use in stimulant-dependent ADHD patients. 84 , 85 Interestingly, also other associated substance use in these patients, eg, alcohol and cannabis, diminished in these trials. This finding may indicate a substance “transdiagnostic” effect of high-dose methylphenidate. Overall, amphetamine-like drugs have been shown to have a positive effect on different cognitive functions. In healthy participants, D-amphetamine and lisdexamfetamine increase cognitive performance in processing speed, inhibition, and vigilance tasks. 86 However, the cognitive enhancing properties of these substances have been until recently hardly explored as a treatment for SUDs. The potential addictive properties of these substances play an important role here. In a recent study, high-dosed sustained-release dexamphetamine has shown positive clinical effects (fewer days of cocaine use) in cocaine-dependent heroin patients. 87
Taken together, treatment with cognitive enhancing drugs does seem to carry promise both in enhancing cognitive function and clinical outcome in SUD patients. However, the interrelation between these two outcome domains remains largely unexplored. Most studies focus on either cognition or SUD outcome and do not explore their intercorrelation or temporal (causal) interaction with cognitive outcomes. The complexity of this interrelation is highlighted in a recent study. In cocaine-dependent ADHD patients, the effect of extended-release mixed amphetamine salts (MAS-XR) came first on the ADHD symptoms (indicative of cognitive effect), with an effect on cocaine abstinence, only later on in treatment and limited to those patients who experienced a positive effect on ADHD. 88 Future studies should take into account the relationship between cognitive function improvement and clinical SUD improvement.
Neuromodulation: transcranial stimulation
Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are two types of modulation with potential therapeutic utility in the treatment of a broad variety of psychiatric disorders, including SUDs. Studies have been published showing positive results (active versus sham condition), ie, reduction of craving and substance use, of TMS and tDCS in alcohol, nicotine, cocaine, obesity, and food addiction. 89 , 90 Of importance, for both techniques the produced effects are temporary, ie, long-term benefits would likely require chronic (repetitive) administration. The exact underlying working mechanisms of these interventions remain to be clarified, eg, whether these effects (craving, substance use) are direct or via the strengthening of cognitive functions. Indeed, studies using neuromodulation techniques such as transcranial direct current stimulation (tDCS) have demonstrated promising effects in modulating cognitive and motor functions. 91 In healthy individuals tDCS and rTMS induce alterations of cognitive functions, eg, reducing impulsivity and risk-taking. 92 Modification of these cognitive deficits that have been suggested to play a role in the pathogenesis of addictive behaviors may, at least from a theoretical stance, reduce these behaviors. In a broad variety of SUD patients, excitatory stimulation over the left DLPFC was associated with, improved inhibitory control, lower risk-taking, decreased delay-discounting, reduced attention towards alcohol cues, and improved executive functioning. 92 Right DLPFC stimulation was less studied, but also showed a reduction of risk-taking and improvements in memory and inhibitory control. However, findings were not consistent with some studies showing no or even negative effect. Of interest, studies suggest that SUD severity may differentiate the effects of neuromodulation, ie, a more significant effect on executive functioning in patients with more severe AUD. 93 Also, in different studies, baseline cognitive task performance proved to modulate the effectivity of neuromodulation. Baseline impulsivity is likely to be an essential determinant of neuromodulation effectivity. 94 Besides, earlier studies in SUDs populations showed rate-dependent effects for manipulations targeting delay discounting, suggesting that for future neuromodulation studies rate-dependent analysis should be considered. 55 , 95
Taken together, transcranial stimulation interventions seem to have both an effect on clinical outcome variables and cognitive functions within SUD patients. However, as yet no information is available on the question of whether these cognitive improvements are the drivers of the clinical effect.
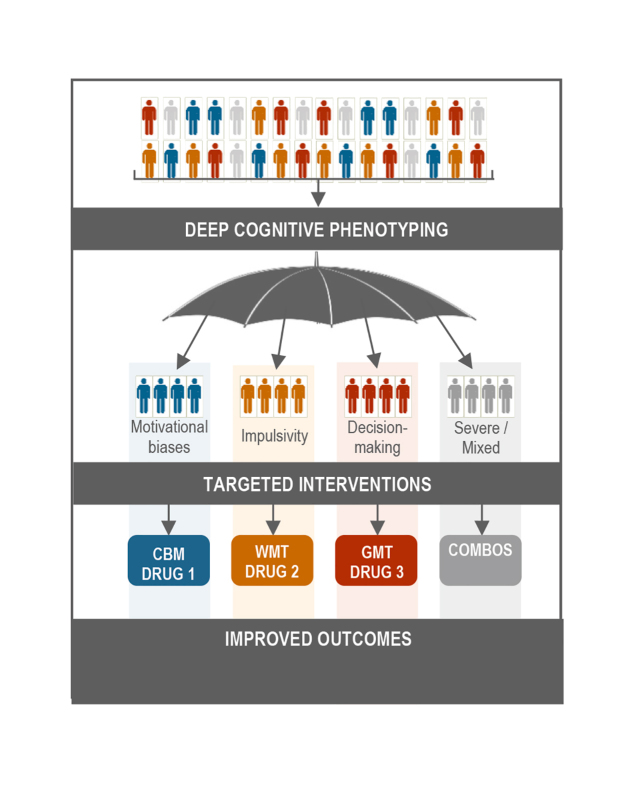
Figure 1. X Tentative model of deep cognitive phenotyping suggesting phenotype-type-matched cognitive and pharmacological approaches for substance use disorder treatment. CBM, Cognitive Bias Modification; WMT, Working Memory Training; GMT, Goal Management Training; COMBOS, combined interventions.
Conclusion
The reviewed evidence supports the central role of cognition in SUD symptomology, clinical prognosis, and potential therapeutic targets. Growing evidence about the relevance of attention, impulsivity, and decision-making for prediction and moderation of the outcomes of different cognitive and pharmacological approaches suggests that cognitive phenotyping and modulation will impregnate future treatment options. Future research is warranted to evaluate if this line of research can pave the way to precision medicine approaches. In the interim, we propose a tentative model (Figure 1) in which deep phenotyping of cognitive processes can lead to phenotype-matched cognitive and pharmacological approaches and putatively better SUD treatment outcomes. Current evidence suggests that cognitive approaches involving CBM, WM training, and Goal Management Training can be optimally suited for patients with strong automatic biases, high impulsivity levels, and deficient decision-making skills. Biological therapies, ie, pharmacotherapy and neuromodulation aiming at strengthening cognitive functions, are shown to be increasingly important, specifically for patients with high impulsivity and poor executive functioning. Meaningful combinations of cognitive and biological approaches can be particularly useful for patients with extreme presentations of identified phenotypes (eg, WM training and left dorsolateral prefrontal cortex stimulation for highly impulsive patients).
Acknowledgments
The authors report no conflict of interest. We acknowledge the support of Servier in the preparation of this manuscript.
Contributor Information
Antonio Verdejo-Garcia, Turner Institute for Brain and Mental Health, Monash University, Melbourne, Australia.
Gloria Garcia-Fernandez, Turner Institute for Brain and Mental Health, Monash University, Melbourne, Australia; Department of Psychology, University of Oviedo, Spain.
Geert Dom, Collaborative Antwerp Psychiatric Research Institute (CAPRI), Antwerp University (UA), Antwerp, Belgium.
REFERENCES
- 1.Verdejo-Garcia A. The neuropsychologist working in addiction: What to know? Ten questions and answers. Revista Iberoamericana de Neuropsicologia. 2018;1(2):170–179. [Google Scholar]
- 2.Baldacchino A, Balfour DJ, Passetti F, Humphris G, Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev. 2012;36(9):2056–2068. doi: 10.1016/j.neubiorev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Biernacki K, McLennan SN, Terrett G, Labuschagne I, Rendell PG. Decision-making ability in current and past users of opiates: A meta-analysis. Neurosci Biobehav Rev. 2016;71:342–351. doi: 10.1016/j.neubiorev.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Leung D, Staiger PK, Hayden M, et al Meta-analysis of the relationship between impulsivity and substance-related cognitive biases. Drug Alcohol Depend. 2017;172:21–33. doi: 10.1016/j.drugalcdep.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8(5):368–376. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. 2011;35(3):377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Verdejo-Garcia A, Chong TT, Stout JC, Yucel M, London ED. Stages of dysfunctional decision-making in addiction. Pharmacol Biochem Behav. 2018;164:99–105. doi: 10.1016/j.pbb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Albein-Urios N, Martinez-Gonzalez JM, Lozano-Rojas O, Verdejo-Garcia A. Executive functions in cocaine-dependent patients with cluster B and cluster C personality disorders. Neuropsychology. 2014;28(1):84–90. doi: 10.1037/neu0000007. [DOI] [PubMed] [Google Scholar]
- 9.Cuyas E, Verdejo-Garcia A, Fagundo AB, et al The influence of genetic and environmental factors among MDMA users in cognitive performance. PLoS One. 2011;6(11):e27206. doi: 10.1371/journal.pone.0027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdejo-Garcia A, Fagundo AB, Cuenca A, et al COMT val158met and 5-HTTLPR genetic polymorphisms moderate executive control in cannabis users. Neuropsychopharmacology. 2013;38(8):1598–1606. doi: 10.1038/npp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwako LE, Schwandt ML, Ramchandani VA, et al Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. Am J Psychiatry. 2019 doi: 10.1176/appi.ajp.2018.18030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80(3):179–189. doi: 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yucel M, Oldenhof E, Ahmed SH, et al A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114(6):1095–1109. doi: 10.1111/add.14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ, Robbins TW, Fiske ST. Drug addiction: updating actions to habits to compulsions ten years on 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 16.Verdejo-Garcia A, Alcazar-Corcoles MA, Albein-Urios N. Neuropsychological interventions for decision-making in addiction: a systematic review. Neuropsychol Rev. 2019;29(1):79–92. doi: 10.1007/s11065-018-9384-6. [DOI] [PubMed] [Google Scholar]
- 17.Potvin S, Pelletier J, Grot S, Hebert C, Barr A, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addict Behav. 2018;80:154–160. doi: 10.1016/j.addbeh.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addiction Biology. 2013;18(2):203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- 19.Donovan DM, Bigelow GE, Brigham GS. et al Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107(4):694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction. 2012;107(4):709–718. doi: 10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez-Salas S, Diaz-Batanero C, Lozano-Rojas OM, Verdejo-Garcia A. Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neurosci Biobehav Rev. 2016;71:772–801. doi: 10.1016/j.neubiorev.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Yip SW, Scheinost D, Potenza MN, Carroll KM. Connectome-based prediction of cocaine abstinence. Am J Psychiatry. 2019;176(2):156–164. doi: 10.1176/appi.ajp.2018.17101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenis AJ, Fitzpatrick RE, Lubman DI, Verdejo-Garcia A. Sustained attention but not effort-based decision-making predicts treatment motivation change in people with methamphetamine dependence. J Subst Abuse Treat. 2018;95:48–54. doi: 10.1016/j.jsat.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Dean AC, Morales AM, Hellemann G, London ED. Cognitive deficit in methamphetamine users relative to childhood academic performance: link to cortical thickness. Neuropsychopharmacology. 2018;43(8):1745–1752. doi: 10.1038/s41386-018-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passetti F, Clark L, Davis P, et al Risky decision-making predicts short-term outcome of community but not residential treatment for opiate addiction. Implications for case management. Drug Alcohol Depend. 2011;118(1):12–18. doi: 10.1016/j.drugalcdep.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Verdejo-Garcia A, Albein-Urios N, Martinez-Gonzalez JM, Civit E, de la Torre R, Lozano O. Decision-making impairment predicts 3-month hair-indexed cocaine relapse. Psychopharmacology. 2014;231(21):4179–4187. doi: 10.1007/s00213-014-3563-9. [DOI] [PubMed] [Google Scholar]
- 28.Buelow MT, Suhr JA. Construct validity of the Iowa Gambling Task. Neuropsychol Rev. 2009;19(1):102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- 29.Dai JY, Kerestes R, Upton DJ, Busemeyer JR, Stout JC. An improved cognitive model of the Iowa and Soochow Gambling Tasks with regard to model fitting performance and tests of parameter consistency. Front Psychol. 2015;6:229. doi: 10.3389/fpsyg.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005;16(12):973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 31.Stevens L, Verdejo-Garcia A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: A review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat. 2014;47(1):58–72. doi: 10.1016/j.jsat.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Verdejo-Garcia A. Neuroclinical assessment of addiction needs to incorporate decision-making measures and ecological validity. Biol Psychiatry. 2017;81(7):E53–E54. doi: 10.1016/j.biopsych.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Enkavi AZ, Eisenberg IW, Bissett PG, et al Large-scale analysis of test-retest reliabilities of self-regulation measures. Proc Natl Acad Sci U S A. 2019;116(12):5472–5477. doi: 10.1073/pnas.1818430116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: Decomposing performance on the bechara gambling task. Psychol Assess. 2002;14(3):253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- 35.Stout JC, Busemeyer JR, Lin AL, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychonom Bull Rev. 2004;11(4):742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen ML, Frank MJ, Biele G. The drift diffusion model as the choice rule in reinforcement learning. Psychonom Bull Rev. 2017;24(4):1234–1251. doi: 10.3758/s13423-016-1199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdejo-Garcia A, Chong TTJ, Stout JC, Yucel M, London ED. Stages of dysfunctional decision-making in addiction. Pharmacol Biochem Behav. 2018;164:99–105. doi: 10.1016/j.pbb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Voon V, Derbyshire K, Ruck C, et al Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry. 2015;20(3):345–352. doi: 10.1038/mp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hula A, Vilares I, Lohrenz T, Dayan P, Montague PR. A model of risk and mental state shifts during social interaction. Plos Computational Biology. 2018;14(2) doi: 10.1371/journal.pcbi.1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey PD, McGurk SR, Mahncke H, Wykes T. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907–915. doi: 10.1016/j.bpsc.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98(5):886–903. doi: 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gladwin TE, Wiers CE, Wiers RW. Cognitive neuroscience of cognitive retraining for addiction medicine: From mediating mechanisms to questions of efficacy. Prog Brain Res. 2016;224:323–344. doi: 10.1016/bs.pbr.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 45.Wiers RW, Boffo M, Field M. What's in a Trial? On the importance of distinguishing between experimental lab studies and randomized controlled trials: the case of cognitive bias modification and alcohol use disorders. J Stud Alcohol Drugs. 2018;79(3):333–343. [PubMed] [Google Scholar]
- 46.Levine B, Schweizer TA, O'Connor C, et al Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfonso JP, Caracuel A, Delgado-Pastor LC, Verdejo-Garcia A. Combined Goal Management Training and Mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011;117(1):78–81. doi: 10.1016/j.drugalcdep.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Valls-Serrano C, Caracuel A, Verdejo-Garcia A. Goal Management Training and Mindfulness Meditation improve executive functions and transfer to ecological tasks of daily life in polysubstance users enrolled in therapeutic community treatment. Drug Alcohol Depend. 2016;165:9–14. doi: 10.1016/j.drugalcdep.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 49.Casaletto KB, Moore DJ, Woods SP, Umlauf A, Scott JC, Heaton RK. Abbreviated goal management training shows preliminary evidence as a neurorehabilitation tool for hiv-associated neurocognitive disorders among substance users. Clin Neuropsychol. 2016;30(1):107–130. doi: 10.1080/13854046.2015.1129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bickel WK, Moody L, Quisenberry A. Computerized working-memory training as a candidate adjunctive treatment for addiction. Alcohol Res-Curr Rev. 2014;36(1):123–126. [PMC free article] [PubMed] [Google Scholar]
- 51.Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychol Sci. 2011;22(7):968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- 52.Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rass O, Schacht RL, Buckheit K, Johnson MW, Strain EC, Mintzer MZ. A randomized controlled trial of the effects of working memory training in methadone maintenance patients. Drug Alcohol Depend. 2015;156:38–46. doi: 10.1016/j.drugalcdep.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks SJ, Wiemerslage L, Burch KH, et al The impact of cognitive training in substance use disorder: the effect of working memory training on impulse control in methamphetamine users. Psychopharmacology. 2017;234(12):1911–1921. doi: 10.1007/s00213-017-4597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snider SE, Deshpande HU, Lisinski JM, Koffarnus MN, LaConte SM, Bickel WK. Working memory training improves alcohol users' episodic future thinking: a rate-dependent analysis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(2):160–167. doi: 10.1016/j.bpsc.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sweeney MM, Rass O, DiClemente C, et al Working memory training for adolescents with cannabis use disorders: a randomized controlled trial. J Child Adolesc Subst Abuse. 2018;27(4):211–226. doi: 10.1080/1067828X.2018.1451793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendershot CS, Wardell JD, Vandervoort J, McPhee MD, Keough MT, Quilty LC. Randomized trial of working memory training as an adjunct to inpatient substance use disorder treatment. Psychol Addict Behav. 2018;32(8):861–872. doi: 10.1037/adb0000415. [DOI] [PubMed] [Google Scholar]
- 58.Wanmaker S, Leijdesdorff SMJ, Geraerts E, van de Wetering BJM, Renkema PJ, Franken IHA. The efficacy of a working memory training in substance use patients: A randomized double-blind placebo-controlled clinical trial. J Clin Exp Neuropsychol. 2018;40(5):473–486. doi: 10.1080/13803395.2017.1372367. [DOI] [PubMed] [Google Scholar]
- 59.Khemiri L, Brynte C, Stunkel A, Klingberg T, Jayaram-Lindstrom N. Working memory training in alcohol use disorder: a randomized controlled trial. Alcohol Clin Exp Res. 2019;43(1):135 146. doi: 10.1111/acer.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiluk BD, Buck MB, Devore KA, Babuscio TA, Nich C, Carroll KM. Performance-based contingency management in cognitive remediation training: a pilot study. J Subst Abuse Treat. 2017;72:80–88. doi: 10.1016/j.jsat.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conklin CA, Soreca I, Kupfer DJ, et al Exercise Attenuates negative effects of abstinence during 72 hours of smoking deprivation. Exp Clin Psychopharmacol. 2017;25(4):265–272. doi: 10.1037/pha0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson CL, Ishibashi K, Chudzynski J, et al Effect of Exercise Training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacology. 2016;41(6):1629–1636. doi: 10.1038/npp.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manning V, Mroz K, Garfield JB, et al Combining approach bias modification with working memory training during inpatient alcohol withdrawal: An open-label pilot trial of feasibility and acceptability. Subst Abuse Treat Prev Policy. 2019;14(1):24. doi: 10.1186/s13011-019-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aharonovich E, Hasin DS, Nunes EV, et al Modified Cognitive Behavioral Therapy (M-CBT) for cocaine dependence: development of treatment for cognitively impaired users and results from a stage 1 trial. Psychol Addict Behav. 2018;32(7):800–811. doi: 10.1037/adb0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snider SE, DeHart WB, Epstein LH, Bickel WK. Does delay discounting predict maladaptive health and financial behaviors in smokers? Health Psychol. 2019;38(1):21–28. doi: 10.1037/hea0000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J. Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci. 2013;4:38–51. doi: 10.1016/j.dcn.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacol Biochem Behav. 2011;99(2):285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deutsch SI, Schwartz BL, Schooler NR, Brown CH, Rosse RB, Rosse SM. Targeting alpha-7 nicotinic neurotransmission in schizophrenia: a novel agonist strategy. Schizophr Res. 2013;148(1-3):138–144. doi: 10.1016/j.schres.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive function as a transdiagnostic treatment target in stimulant use disorders. J Dual Diagn. 2016;12(1):90–106. doi: 10.1080/15504263.2016.1146383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carroll KM, Nich C, DeVito EE, Shi JM, Sofuoglu M. Galantamine and computerized cognitive behavioral therapy for cocaine dependence: a randomized clinical trial. J Clin Psychiatry. 2018;79(1) doi: 10.4088/JCP.17m11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology. 2013;229(3):415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalechstein AD, Mahoney JJ, Yoon JH, Bennett R, De la Garza R. Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology. 2013;64:472–478. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 75.Kampman KM, Lynch KG, Pettinati HM, et al A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug Alcohol Depend. 2015;155:105–110. doi: 10.1016/j.drugalcdep.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dackis CA, Kampman KM, Lynch KG, et al A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43(3):303–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson AL, Reid MS, Li SH, et al Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104(1-2):133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nuijten M, Blanken P, Van den Brink W, Goudriaan AE, Hendriks VM. Impulsivity and attentional bias as predictors of modafinil treatment outcome for retention and drug use in crack-cocaine dependent patients: Results of a randomised controlled trial. J Psychopharmacol. 2016;30(7):616–626. doi: 10.1177/0269881116645268. [DOI] [PubMed] [Google Scholar]
- 79.Schmaal L, Goudriaan AE, Joos L, et al Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychol Med. 2014;44(13):2787–2798. doi: 10.1017/S0033291714000312. [DOI] [PubMed] [Google Scholar]
- 80.Joos L, Goudriaan AE, Schmaal L, van den Brink W, Sabbe BG, Dom G. Effect of modafinil on cognitive functions in alcohol dependent patients: a randomized, placebo-controlled trial. J Psychopharmacol. 2013;27(11):998–1006. doi: 10.1177/0269881113503505. [DOI] [PubMed] [Google Scholar]
- 81.Schmaal L, Goudriaan AE, Joos L, et al Modafinil modulates resting-state functional network connectivity and cognitive control in alcohol-dependent patients. Biol Psychiatry. 2013;73(8):789–795. doi: 10.1016/j.biopsych.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 82.Joos L, Goudriaan AE, Schmaal L, et al Effect of modafinil on impulsivity and relapse in alcohol dependent patients: a randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2013;23(8):948–955. doi: 10.1016/j.euroneuro.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE. Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry. 2013;73(3):211–218. doi: 10.1016/j.biopsych.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 84.Konstenius M, Jayaram-Lindstrom N, Guterstam J, Beck O, Philips B, Franck J. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addiction. 2014;109(3):440–449. doi: 10.1111/add.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skoglund C, Brandt L, D'Onofrio B, Larsson H, Franck J. Methylphenidate doses in Attention Deficit/Hyperactivity Disorder and comorbid substance use disorders. Eur Neuropsychopharmacol. 2017;27(11):1144–1152. doi: 10.1016/j.euroneuro.2017.08.435. [DOI] [PubMed] [Google Scholar]
- 86.Dolder PC, Strajhar P, Vizeli P, Odermatt A, Liechti ME. Acute effects of lisdexamfetamine and D-amphetamine on social cognition and cognitive performance in a placebo-controlled study in healthy subjects. Psychopharmacology. 2018;235(5):1389–1402. doi: 10.1007/s00213-018-4849-0. [DOI] [PubMed] [Google Scholar]
- 87.Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10034):2226–2234. doi: 10.1016/S0140-6736(16)00205-1. [DOI] [PubMed] [Google Scholar]
- 88.Levin FR, Choi CJ, Pavlicova M, et al How treatment improvement in ADHD and cocaine dependence are related to one another: A secondary analysis. Drug Alcohol Depend. 2018;188:135–140. doi: 10.1016/j.drugalcdep.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bari A, DiCesare J, Babayan D, Runcie M, Sparks H, Wilson B. Neuromodulation for substance addiction in human subjects: A review. Neurosci Biobehav Rev. 2018;95:33–43. doi: 10.1016/j.neubiorev.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forcano L, Mata F, de la Torre R, Verdejo-Garcia A. Cognitive and neuromodulation strategies for unhealthy eating and obesity: Systematic review and discussion of neurocognitive mechanisms. Neurosci Biobehav Rev. 2018;87:161–191. doi: 10.1016/j.neubiorev.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Lapenta OM, Marques LM, Rego GG, Comfort WE, Boggio PS. tDCS in addiction and impulse control disorders. J ECT. 2018;34(3):182–192. doi: 10.1097/YCT.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 92.Naish KR, Vedelago L, MacKillop J, Amlung M. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug Alcohol Depend. 2018;192:338–351. doi: 10.1016/j.drugalcdep.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakamura-Palacios EM, de Almeida Benevides MC, da Penha Zago-Gomes M, et al Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int J Neuropsychopharmacol. 2012;15(5):601–616. doi: 10.1017/S1461145711001040. [DOI] [PubMed] [Google Scholar]
- 94.Cheng GL, Lee TM. Altering risky decision-making: Influence of impulsivity on the neuromodulation of prefrontal cortex. Soc Neurosci. 2016;11(4):353–364. doi: 10.1080/17470919.2015.1085895. [DOI] [PubMed] [Google Scholar]
- 95.Bickel WK, Quisenberry AJ, Snider SE. Does impulsivity change rate dependently following stimulant administration? A translational selective review and re-analysis. Psychopharmacology. 2016;233(1):1–18. doi: 10.1007/s00213-015-4148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]