Abstract
Aim
The aim of the case-control study was to investigate if serum biomarkers indicative of vascular inflammation and endothelial dysfunction can predict the development of microalbuminuria in patients with diabetes mellitus type 2.
Methods
Among participants enrolled in the ROADMAP (Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention) and observational follow-up (OFU) studies, a panel of 15 serum biomarkers was quantified from samples obtained at initiation of the study and tested for associations with the development of new-onset microalbuminuria during follow-up. A case-control study was conducted with inclusion of 172 patients with microalbuminuria and 188 matched controls. Nonparametric inferential, nonlinear regression, mediation, and bootstrapping statistical methods were used for the analysis.
Results
The median follow-up time was 37 months. At baseline, mean concentrations of C-X-C motif chemokine ligand 16 (CXCL-16), transforming growth factor (TGF)–β1 and angiopoietin-2 were higher in patients with subsequent microalbuminuria. In the multivariate analysis, after adjustment for age, sex, body mass index, glycated hemoglobin, duration of diabetes, low-density lipoprotein (LDL), smoking status, blood pressure, baseline urine albumin-to-creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), time of follow-up and cardiovascular disease, CXCL-16 (odds ratio [OR] 2.60, 95% confidence interval [CI] 1.71–3.96), angiopoietin-2 (OR 1.50, 95% CI 1.14–1.98) and TGF-β1 (OR 1.03, 95% CI 1.01–1.04) remained significant predictors of new-onset microalbuminuria (P < 0.001). Inclusion of these biomarkers in conventional clinical risk models for prediction of microalbuminuria increased the area under the curve (AUC) from 0.638 to 0.760 (P < 0.001).
Conclusion
In patients with type 2 diabetes, elevated plasma levels of CXCL-16, angiopoietin-2, and TGF-β1 are independently predictive of microalbuminuria. Thus, these serum markers improve renal risk models beyond established clinical risk factors.
Keywords: albuminuria, atheromatosis, cardiovascular disease, diabetic kidney disease, inflammation
See Commentary on Page 1362
Although the incidence of diabetes-related complications has decreased considerably in the past 2 decades, a large disease burden still persists.1 The diagnosis of diabetes mellitus constitutes a major independent cardiovascular risk factor.2 The development of diabetic kidney disease, clinically diagnosed by new-onset albuminuria and/or mild impairment of glomerular filtration rate, has a considerable additional negative impact on outcome.3 Hence, there is an overwhelming need to optimize the care of the affected patients and foremost to detect early and treat appropriately those at the highest risk of diabetic kidney disease and cardiovascular complications.4
A growing number of molecules reflecting different stages in the inflammatory cascade of atheromatosis have been measured in patients with diabetes, to identify novel biomarkers for cardiovascular complications or cardiovascular death.5, 6, 7 On the other side, low-degree albuminuria is not merely a determinant of nephropathy in patients with diabetes but more an early and sensitive marker of widespread vascular damage with established cardiovascular prognostic value.8 Therefore, it is interesting to know if there is an association between pathways underlying atherosclerosis or atherosclerotic vascular disease and progression of albuminuria.
The aim of the present case-control study was to investigate whether early-on alterations in serum biomarkers representing distinct and potentially complementary pathways of ongoing vascular inflammation and endothelial dysfunction precede the development of microalbuminuria (defined as UACR >30 mg/g) in patients with diabetes mellitus type 2. For this purpose, a panel of 15 candidate biomarkers was assayed in biobanked serum samples from participants of the ROADMAP and OFU studies.9, 10 The goal was to examine their independent association with incident microalbuminuria and to explore their potential to improve renal risk prediction in patients with diabetes mellitus type 2 beyond and above classical clinical and biochemical risk factors.
Material and Methods
Study Population
A case-control study with inclusion of participants from ROADMAP and OFU (ROADMAP-OFU) was conducted. The ROADMAP study has been executed as a randomized, placebo-controlled, double-blinded, multinational trial assessing the effect of the angiotensin receptor blocker olmesartan on the onset of microalbuminuria in patients with diabetes mellitus type 2 diagnosis. A total of 4447 patients were assigned to receive olmesartan or placebo for a median of 3.2 years. The ROADMAP-OFU was a prespecified, multicenter, longitudinal observational follow-up of patients who formerly participated in the ROADMAP study. A total of 1758 patients were included with a mean follow-up of 3.3 ± 0.6 years. The design and outcomes of both studies have been described elsewhere.9, 10
In the ROADMAP study, new-onset microalbuminuria was detected in 210 patients randomized to the placebo group (9.8%) and in 178 randomized to the olmesartan group (8.2%). Of the initial 4447 participants, 2430 had serial serum samples taken and stored during the intervention period, permitting biomarkers to be assayed and were included in this study. Of these 2430 patients, 240 (9.8%) developed microalbuminuria during ROADMAP. A drawback of ROADMAP was that collection and storage of serum and urine probes was initiated in the second year of the study and therefore baseline serum samples at enrollment were not available for most of the study participants. Of the 240 patients with microalbuminuria, serum samples at any time before the development of microalbuminuria were available in only 65. A total of 107 patients who remained normoalbuminuric throughout ROADMAP and developed microalbuminuria exclusively during OFU, had serum samples available before the development of microalbuminuria. During OFU treatment, visits were not performed in agreement with a study protocol but according to local medical standards and at variable time points. Therefore, microalbuminuria was less stringently confirmed in OFU in comparison with ROADMAP. In summary, for the present study, a total of 172 patients with serum samples taken and stored before the occurrence of microalbuminuria were available for analysis. The average time span between serum collection and development of microalbuminuria overall was 36.9 ± 10.4 months. A schematic presentation of the collection of serum specimens of patients with subsequent microalbuminuria included in this study is shown in Supplementary Figure S1.
A case-control group consisting of 188 patients who remained normoalbuminuric during follow-up was generated. Cases and case-controls were matched for age, sex, body mass index, systolic and diastolic blood pressure, glycated hemoglobin, eGFR, and LDL. Due to the number of the matching criteria, only 188 from 2258 patients could be selected as controls. An increase in the number of controls in order to increase the power of the study would be possible only if less stringent matching criteria were applied.
Definition of Microalbuminuria as Outcome Parameter
In ROADMAP, microalbuminuria was defined as a UACR of more than 35 mg/g in women or more than 25 mg/g in men and had to be confirmed by at least 1 additional positive result from 2 separate samples taken within 2 weeks after the initial test. In the ROADMAP-OFU cohort, less stringent criteria were used, because microalbuminuria was detected according to local standards and was not centrally assessed. If the result was likely (counting rule 1) in the urine albumin dipstick, this value was counted as positive (approach 1) or was excluded (approach 2). If during a certain period more than 1 albumin measurement was obtained (counting rule 2), the highest value (approach 1) or the most frequent value (approach 2) was used. Therefore, 4 different criteria combinations for the diagnosis of microalbuminuria were possible. UACR values more than 30 mg/g were counted as positive.10 For the present analysis, patients with microalbuminuria in ROADMAP-OFU were chosen only if they had more than 1 positive urine value in consecutive visits, were normoalbuminuric during ROADMAP, and had available stored serum samples. Samples of patients with as many positive results as possible, gathered at different time points, were preferentially selected for the present analysis. In that case, time of new-onset microalbuminuria was chosen the time of the first positive sample.
Biomarker Measurements
The serum samples were prepared immediately following blood drawing and stored constantly at −80 °C until assays were performed. Commercially available, high-sensitivity enzyme-linked immunosorbent assays (Quantikine HS; R&D Systems, Minneapolis, MN) were used to quantify angiopoietin-1, angiopoietin-2, CCL2/monocyte chemotactic protein 1 (MCP-1), CXCL-16, endostatin, galectin-3, Receptor for Advanced Glycation Endproducts, soluble tumor necrosis factor receptor (RAGE), soluble Tumor Necrosis Factor Receptor 1 (sTNF-RI)/TNFRSF1A, sTNF-RII/TNFRSF1B, ST2/interleukin-1 (IL-1) R4, TGF-β1, thrombomodulin/BDCA-3, vascular adhesion protein 1 (VAP-1), vascular endothelial growth factor A (VEGF-A) and VEGF-R1/flt-1 at 450 nm using a microplate reader (TECAN, Männedorf, Switzerland) according to the manufacturer’s instructions. Enzyme-linked immunosorbent assay kits from CircuLex (Düsseldorf, Germany) and from Cloud-Clone Corp. (Houston, TX) were used for S100 calcium binding-protein A8/Myeloid Related Protein 8 (S100A8/MRP8) and for C1qR1, respectively.
Interassay variability is as follows: S100A8/MRP8: 3.6%–4.1%, C1qR1: <12%, angiopoietin-1: 5.5%–6.4%, angiopoietin-2: 7.4%–10.4%, CCL2/MCP-1: 4.6%–6.7%, CXCL-16: 9.1%–10.0%, endostatin: 5.7%–7.9%, galectin-3: 5.8%–6.3%, RAGE: 6.6%–8.3%, sTNF-RI/TNFRSF1A: 3.7%–8.8%, sTNF-RII/TNFRSF1B: 3.5%–5.1%, ST2/IL-1 R4: 5.4%–7.1%, TGF-β1: 5.7%–8.4%, thrombomodulin/BDCA-3: 5.7%–8.0%, VAP-1: 4.5%–4.8%, VEGF-A: 5.0%–8.5%, and VEGF-R1/flt-1: 5.5%–9.8%. Intra-assay variability is as follows: S100A8/MRP8: 3.4%–7.1%, C1qR1: <10%, angiopoietin-1: 2.4%–3.3%, angiopoietin-2: 4.2%–6.9%, CCL2/MCP-1: 4.7%–7.8%, CXCL16: 3.5%–4.9%, endostatin: 3.6%–6.9%, galectin-3: 3.5%–3.8%, RAGE: 4.8%–6.2%, sTNF-RI/TNFRSF1A: 3.6%–6.0%, sTNF-RII/TNFRSF1B: 2.6%–4.8%, ST2/IL-1 R4: 4.4%–5.6%, TGF-β1: 1.9%–2.9%, thrombomodulin/BDCA-3: 2.3%–3.6%, VAP-1: 1.5%–2.4%, VEGF-A: 3.5%–6.5%, and VEGF-R1/flt-1: 2.6%–3.8%.
Statistical Analyses
Comparisons of baseline characteristics between the microalbuminuria cases and case-controls were performed using the χ2 or Fisher’s exact tests for categorical variables. The Student’s t-test or Mann-Whitney test were applied when the assumptions of the t-test were not met (normality and homoscedasticity studied using the Fisher-Snedecor test) for quantitative variables. A Bonferroni-Holm correction was used to account for the increased possibility of a type 1 error due to multiple comparisons. The receiver operating characteristic (ROC)–AUC was calculated to evaluate diagnostic accuracy of each continuous marker in univariate analyses. Spearman correlation coefficients were calculated to characterize associations between biomarkers and clinical parameters. Coefficients of less than 0.4 were considered as indicating low, of 0.4 to 0.7 medium, and greater than 0.7 strong correlation.11
Mixed logistic regression models were used to test the association of each biomarker concentration at baseline and time until occurrence of microalbuminuria by backward and forward stepwise regression. Covariates for adjustment were selected a priori based on clinical relevance and previous literature as well as according to the results of the univariate analysis.12, 13 The Hosmer-Lemeshow goodness of fit test was used to assess calibration, and a nonsignificant test result was considered to indicate good calibration. Results were expressed as ORs with 95% CIs. The relationship between the concentration of the biomarker at baseline and new onset of microalbuminuria during follow-up was examined by time to event analysis. Because the time to onset of microalbuminuria was different within the cases, Kaplan-Meier curves were generated to examine the rates of new onset of microalbuminuria in general, and across quartiles of the biomarkers came to be significant in the multivariate analysis. Finally, to evaluate the utility of the biomarkers on risk stratification, we assessed improvement in predictive performance via DeLong’s test for differences in ROC-AUC and net reclassification index by comparing the clinical model with biomarkers and the clinical model.14
We performed bootstrap sampling to create replicate data sets that simulated the ratio of affected to unaffected individuals.15, 16 To create each replicate, a participant was randomly drawn one at a time from the pool of individuals. After each draw, the selected individual was replaced in the same pool and the draw was repeated until a designated sample size was reached.
To investigate whether baseline CXCL-16, TGF-β1, or angiopoietin-2 are more than baseline risk predictors and to assess the respective contributions of baseline CXCL-16, TGF-β1, and angiopoietin-2 for prognosis of microalbuminuria, we conducted a multilevel mediation analysis.17 When mediation analysis is applied, the goal is to determine whether a specific variable (the “mediator”) has an effect on outcome that explains, in whole or in part, the prognostic effects resulting from another independent variable.18, 19 A mediation proportion was estimated, indicating how much of the whole prognostic value provided by an independent variable can be explained by the indirect path in which changes in this independent variable drives a change in the mediator, and changes in the mediator then affect outcome. An average causal mediation effect was calculated, which expresses the independent hazard associated with this indirect path.18 The exposure-mediator interaction effect was tested. A P value less than 0.05 was considered significant in all comparisons. Statistical analysis was performed using SPSS software, v24 (IBM Corp, Armonk, NY) and IBM SPSS Statistics Essentials for R.
Results
Comparison of Cases With New-Onset Microalbuminuria and Case-Controls
The baseline demographics and clinical characteristics of the study participants are listed in Table 1. The case-controls did not differ regarding age, sex, details of medical history, relevant clinical examination findings, and appropriate biochemical measures. Patients who developed microalbuminuria had a higher prevalence of cardiovascular disease at baseline (P = 0.02). Baseline cardiovascular disease was generally low in microalbuminuria cases and case-controls of the present study (16.3% vs. 8.5%) and lower than that reported for the whole ROADMAP study population (approximately 30%).9
Table 1.
Clinical characteristics of the study cohorts at baseline, split by presence or absence of microalbuminuria
| Characteristics | Microalbuminuria (n = 172) | Controls (n = 188) | P |
|---|---|---|---|
| Demographic characteristics | |||
| Age, yr | |||
| Median (min–max) | 58 (39–75) | 57 (33–75) | 0.343 |
| <55, n (%) | 57 (33.1) | 61 (32.5) | |
| ≥55, n (%) | 115 (66.9) | 127 (67.5) | |
| Male sex, n (%) | 86 (50.0) | 90 (47.9) | 0.687 |
| Tobacco smoking, n (%) | |||
| Nonsmoker | 108 (62.8) | 118 (62.8) | 0.913 |
| Current smoker | 29 (16.9) | 30 (16.0) | |
| Former smoker | 35 (20.3) | 40 (21.3) | |
| Physical examination | |||
| Body mass indexa | 31.3 ± 4.9 | 31.3 ± 4.5 | 0.585 |
| Blood pressure, mm Hg | |||
| Systolic | 137.7 ± 15.9 | 136.5 ±15.0 | 0.665 |
| Diastolic | 80.4 ± 8.9 | 80.9 ± 8.6 | 0.412 |
| Mean arterial pressure | 99.4 ± 10.4 | 99.4 ± 9.7 | 0.769 |
| Laboratory values | |||
| eGFR, ml/min per 1.73 m2 | 85.9 ± 15.6 | 86.9 ± 16.9 | 0.656 |
| HbA1c, % | 7.9 ± 1.6 | 7.8 ± 1.5 | 0.811 |
| LDL cholesterol, mmol/l | 3.0 ± 1.0 | 3.1 ± 1.0 | 0.621 |
| Urine albumin-to-creatinine ratio, mg/g | 9.0 ± 7.9 | 7.5 ± 7.1 | 0.075 |
| Medical history | |||
| Cardiovascular disease, n (%) | 28 (16.3) | 16 (8.5) | 0.025 |
| Duration of diabetes, mo | 80.8 ± 72.6 | 72.6 ± 71.2 | 0.143 |
eGFR, estimated glomerular filtration rate (calculated with the use of the abbreviated Modification of Diet in Renal Disease formula); HbA1c, glycated hemoglobin; LDL, low-density lipoprotein.
Data are presented as mean (SD) for normally distributed values, median (minimum–maximum) for skewed continuous values, and n (%) for categoric values. Cardiovascular disease was defined as history of coronary heart disease, myocardial infarction, stroke, or transient ischemic attack, or peripheral vascular disease. Statistically significant differences are in bold.
The body mass index is the weight in kilograms divided by the square of the height in meters.
Univariate Associations of Individual Biomarkers With New-Onset Microalbuminuria
The mean concentrations of all biomarkers determined in the specimens at baseline are shown in Table 2. Endostatin, CXCL-16, sTNF-RI, sST2, TGF-β1, angiopoietin-2, and MCP-1 were increased in cases with microalbuminuria; however, when P values were adjusted for multiple comparisons, only CXCL-16, TGF-β1, and angiopoieitin-2 remained significant. No significant differences between microalbuminuria cases and case-controls were observed for the following markers: S100A8, VAP-1, sTNF-RII, sThrombomodulin, VEGF-A, RAGE, VEGF-R1 (sFlt-1), and angiopoietin-1.
Table 2.
Mean concentrations of biomarkers at baseline determined in both groups of the study population
| Biomarkers | Microalbuminuria (n = 172) | Controls (n = 188) | P | Bonferroni-Holm correction |
|---|---|---|---|---|
| S100A8/MRP8, ng/ml | 94.77 ± 60.89 | 93.40 ± 65.79 | 0.650 | >0.999 |
| Endostatin, ng/ml | 121.2 ± 38.99 | 108.9 ± 31.91 | 0.005 | 0.075 |
| VAP-1, ng/ml | 624.6 ± 170.0 | 653.6 ± 169.6 | 0.090 | >0.999 |
| CXCL-16, ng/ml | 2.67 ± 0.61 | 2.16 ± 0.72 | <0.001 | <0.001 |
| sTNF-RI, ng/ml | 1.43 ± 0.46 | 1.39 ± 0.59 | 0.018 | 0.270 |
| sTNF-RII, ng/ml | 3.12 ± 1.02 | 2.97 ± 0.85 | 0.138 | >0.999 |
| sST2, ng/ml | 15.87 ± 8.24 | 13.74 ± 4.90 | 0.048 | 0.720 |
| sThrombomodulin, ng/ml | 4.59 ± 1.22 | 4.39 ± 0.92 | 0.257 | >0.999 |
| VEGF, pg/ml | 298.3 ± 170.6 | 276.9 ± 171.95 | 0.186 | >0.999 |
| RAGE, ng/ml | 1.10 ± 0.41 | 1.06 ± 0.47 | 0.272 | >0.999 |
| VEGF-R1, pg/ml | 90.72 ± 26.09 | 86.02 ± 38.87 | 0.059 | 0.885 |
| TGF-β1, ng/ml | 33.21 ± 13.14 | 23.57 ± 18.99 | <0.001 | <0.001 |
| Angiopoietin-2, ng/ml | 2.30 ± 1.30 | 1.85 ± 0.91 | <0.001 | <0.001 |
| Angiopoietin-1, ng/ml | 39.47 ± 12.84 | 39.94 ± 13.92 | 0.623 | >0.999 |
| MCP-1, pg/ml | 407.9 ± 120.3 | 378.3 ± 132.9 | 0.004 | 0.060 |
CXCL-16, C-X-C motif chemokine ligand 16; MCP-1, monocyte chemotactic protein 1; RAGE, receptor for advanced glycation endproducts; S100A8/MRP8, S100 calcium binding protein A8/myeloid related protein 8; sTNF-RI, soluble tumor necrosis factor receptor I; sTNF-RII, soluble tumor necrosis factor receptor II; sST2, soluble ST2; TGF-β1, transforming growth factor beta 1; VAP-1, vascular adhesion protein 1; VEGF-A, vascular endothelial growth factor-A; VEGF-R1, vascular endothelial growth factor-A receptor 1.
Data are presented as mean (SD) for normally distributed values or and n (%) for categoric values. Statistically significant differences are in bold.
The ROC-AUC of the clinical parameters and the 15 tested biomarkers were calculated (Supplementary Table S1). For 3 markers, the ROC-AUC was calculated above 0.6 (CXCL-16, TGF-β1, and angiopoietin-2), and 7 markers had a 95% CI lower limit above 0.5. After adjusting for multiple comparisons, only CXCL-16 (ROC-AUC = 0.696 [0.643–0.750]), TGF-β1 (0.669 [0.612–0.726]), and angiopoietin-2 (0.658 [0.602–0.714]) remained significant as predictors of microalbuminuria development.
Correlations of selected biomarkers found to be increased in cases with microalbuminuria, with each other, and with clinical parameters determined at baseline are given in Supplementary Tables S2A and S2B. Spearman correlation coefficient values were lower than 0.4 for all paired comparisons except for endostatin and sTNFR-I (Spearman ρ = 0.519, P < 0.001).
Data about the treatment arm were available for all patients of the roadmap primary study and only for few patients of the OFU cohort. For those patients (86 treated with olmesartan and 96 treated with placebo), no statistically significant differences were found in the clinical parameters, except for LDL cholesterol, or that marks came to be significantly different in the univariate analysis of all patients (Supplementary Table S3).
Multivariate Associations of Individual Biomarkers With New-Onset Microalbuminuria
Biomarkers that were significant in the univariate analysis and statistically not significant but clinically relevant variables as well were used to compute ORs for new-onset microalbuminuria using multivariate logistic regression analysis. Clinical covariates included in the models are age, sex, body mass index, smoking status, diabetes mellitus duration, glycated hemoglobin, mean arterial pressure, LDL, baseline UACR, baseline eGFR, follow-up duration, and prevalent cardiovascular disease.
Elevated baseline serum levels of CXCL-16, TGF-β1, and angiopoietin-2 were independently associated with higher risk of microalbuminuria development (OR for each 1-log increment in plasma CXCL-16, 2.603, 95% CI 1.705–3.957, OR for each 1-log increment in TGF-β1, 1.026, 95% CI 1.010–1.042, and OR for each 1-point increment in angiopoietin-2, 1.504, 95% CI 1.141–1.983, respectively, Table 3). The final model, including significant interactions, was validated using a bootstrapping method based on 10,000 replications (Supplementary Table S4).
Table 3.
Odds ratio for developing microalbuminuria before and after multivariate logistic regression
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.011 | 0.987–1.037 | 0.373 | 1.000 | 0.968–1.033 | 0.988 |
| Sex | 0.918 | 0.607–1.389 | 0.687 | 0.681 | 0.403–1.149 | 0.150 |
| BMI | 0.999 | 0.956–1.044 | 0.963 | 0.953 | 0.904–1.006 | 0.079 |
| Smoking status | 1.015 | 0.773–1.333 | 0.913 | 0.930 | 0.662–1.306 | 0.674 |
| Mean arterial pressure | 1.000 | 0.980–1.021 | 0.980 | 0.995 | 0.972–1.019 | 0.685 |
| eGFR | 0.997 | 0.984–1.009 | 0.594 | 0.997 | 0.981–1.013 | 0.691 |
| HbA1c | 1.024 | 0.893–1.174 | 0.737 | 0.938 | 0.797–1.103 | 0.436 |
| LDL cholesterol | 0.965 | 0.788–1.183 | 0.733 | 0.971 | 0.768–1.227 | 0.806 |
| Urine albumin-to-creatinine ratio | 1.026 | 0.997–1.055 | 0.074 | 0.996 | 0.963–1.030 | 0.817 |
| Cardiovascular disease | 2.090 | 1.088–4.016 | 0.027 | 1.516 | 0.703–3.271 | 0.288 |
| Duration of diabetes | 1.002 | 0.999–1.004 | 0.283 | 1.001 | 0.997–1.004 | 0.633 |
| Duration of follow-up | 0.981 | 0.969–0.993 | 0.003 | 0.992 | 0.977–1.006 | 0.248 |
| CXCL-16 | 3.168 | 2.217–4.525 | <0.001 | 2.603 | 1.705–3.957 | <0.001 |
| TGF-β1 | 1.036 | 1.022–1.049 | <0.001 | 1.026 | 1.010–1.042 | 0.001 |
| Angiopoietin-2 | 1.596 | 1.233–2.065 | <0.001 | 1.504 | 1.141–1.983 | 0.004 |
BMI, body mass index; CI, confidence interval; CXCL-16, C-X-C motif chemokine ligand 16; eGFR, estimated glomerular filtration rate (calculated with the use of the abbreviated Modification of Diet in Renal Disease formula); HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; OR, odds ratio; TGF-β1, transforming growth factor beta 1.
Cardiovascular disease was defined as history of coronary heart disease, myocardial infarction, stroke or transient ischemic attack, or peripheral vascular disease. Statistically significant differences are in bold.
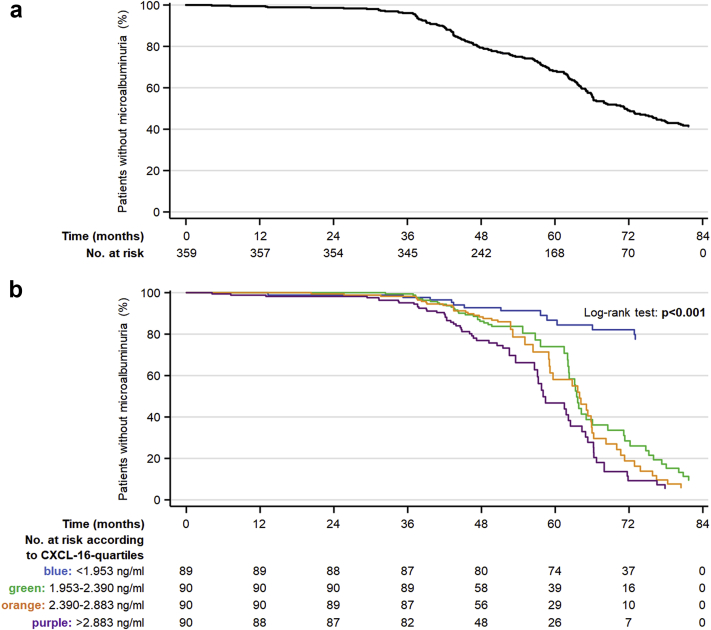
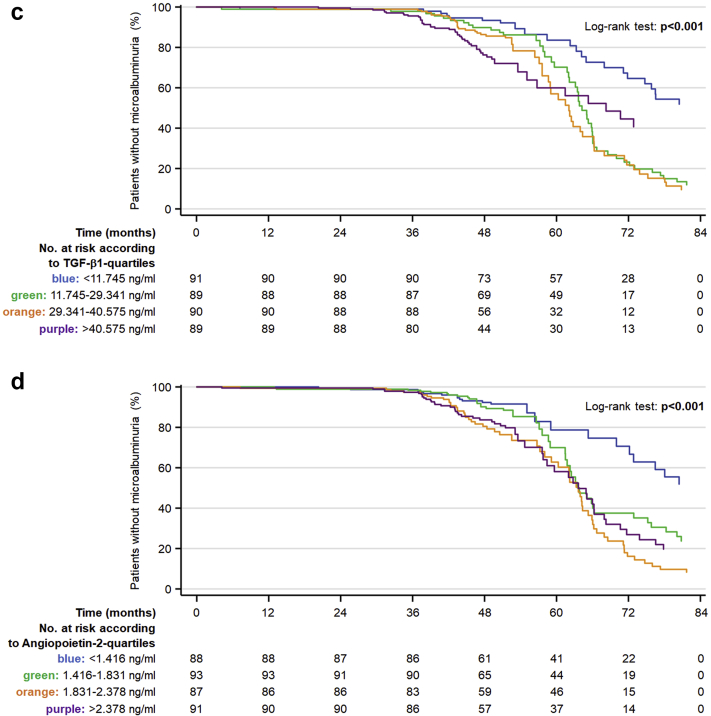
When dividing the patients into quartiles, subjects in the highest quartiles of CXCL-16, TGF-β1, and angiopoietin-2 had an increased risk of developing microalbuminuria compared with those in the lowest quartiles in fully adjusted models (Figure 1a–d, Supplementary Table S5).
Figure 1.
Kaplan-Meier curves of new-onset microalbuminuria in patients with type 2 diabetes mellitus at overall (a) and according to quartiles of C-X-C motif chemokine ligand 16 (CXCL-16) (b), transforming growth factor-β1 (TGF-β1) (c), and angiopoietin-2 (d) at baseline after adjusting for age, sex, body mass index, duration of diabetes, smoking status, mean blood pressure, glycated hemoglobin, estimated glomerular filtration rate, low-density lipoprotein, time of follow-up, urine albumin-to-creatinine ratio, and cardiac complications. The upper quartiles of the above 3 biomarkers are associated with an increased incidence of de novo microalbuminuria. The differences were statistically significant in comparison with the lower quartile.
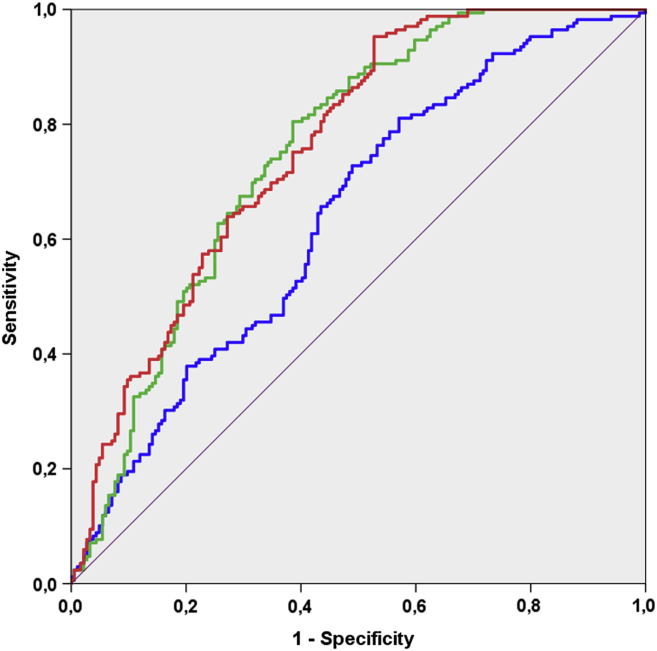
Next, risk prediction models for microalbuminuria were generated (Table 4). Fitting an entirely clinical model with all relevant clinical risk factors produced an adjusted C-index of 0.638 (95% CI 0.580–0.695). The second model encompassing biomarkers that were significant in the univariable analyses (CXCL-16, TGF-β1, and angiopoietin-2) produced a C-index of 0.752 (95% CI 0.702–0.803; ΔAUC P < 0.001 compared with clinical model alone). Inclusion of CXCL-16, TGF-β1 and angiopoietin-2 in the clinical model increased the AUC for predicting microalbuminuria development only from 0.752 to 0.760 (95% CI 0.711–0.809; ΔAUC P < 0.001 compared with clinical model alone). The ROC curves for the clinical model, the final biomarker model, and the combination of both as predictors of microalbuminuria development are shown in Figure 2. Prediction performance analysis, net reclassification improvement, Omnibus test, Nagelkerke R2, and Hosmer-Lemeshow goodness of fit tests are summarized in Supplementary Tables S6 and S7.
Table 4.
Risk prediction models with and without the inclusion of serum biomarkers
| Model | AUC | 95% CI | P | P value for AUC difference | P value for AUC difference | P value for AUC difference |
|---|---|---|---|---|---|---|
| Model 1 | 0.638 | 0.580–0.695 | <0.001 | Reference model | 0.003 | <0.001 |
| Model 2 | 0.752 | 0.702–0.803 | <0.001 | 0.003 | Reference model | 0.500 |
| Model 3 | 0.760 | 0.711–0.809 | <0.001 | <0.001 | 0.500 | Reference model |
AUC, area under the curve; CI, confidence interval.
Model 1: Age, sex, body mass index, duration of diabetes, smoking status, mean blood pressure, glycated hemoglobin, estimated glomerular filtration rate, low-density lipoprotein, time of follow-up, urine albumin-to-creatinine ratio, cardiac complications.
Model 2: C-X-C motif chemokine ligand 16, transforming growth factor-β1, angiopoietin-2.
Model 3: Model 1 + 2.
Figure 2.
Receiver operating characteristic curves for risk prediction models of new-onset microalbuminuria in patients with type 2 diabetes mellitus. Blue line: Model 1 (age, sex, body mass index, duration of diabetes, smoking status, mean blood pressure, glycated hemoglobin, estimated glomerular filtration rate, low-density lipoprotein, time of follow-up, urine albumin-to-creatinine ratio, cardiac complications); green line: Model 2 (C-X-C motif chemokine ligand 16, transforming growth factor-1, angiopoietin-2); red line: Model 3 (Model 1 + 2); area under the curve and 95% confidence interval are given in Table 4.
Mediation Analysis
Elevated CXCL-16, TGF-β1 and angiopoietin-2 serum levels at baseline are significantly associated with risk for microalbuminuria development in the analyzed cases (step 1, 2, 3 of mediation analysis, Supplementary Figure S2) (OR: 3.21; 95% CI: 2.18–4.74 and OR: 1.04; 95% CI: 1.02–1.05 and OR: 1.59; 95% CI: 1.20–2.10, respectively). When TGF-β1 and angiopoietin-2 were tested as separate mediators of the effects of CXCL16 on the risk of microalbuminuria development, the direct association between CXCL16 and microalbuminuria remained significant. Baseline TGF-β1 serum levels mediated 20% (average causal mediation effect) and angiopoietin-2 mediated 9% (average causal mediation effect) of the effects of CXCL-16 on microalbuminuria (Supplementary Figure S2A–C). When CXCL-16 and angiopoietin-2 are tested as separate mediators of TGF-β1, the effect of TGF-β1 is mediated only by CXCL-16 and was 1% (Supplementary Figure S2D–F). When CXCL-16 and TGF-β1 are tested as separate mediators of angiopoietin-2, the effect of angiopoietin-2 is mediated (10%) by serum CXCL-16 only (Supplementary Figure S2G–I). Results from a parallel mediation analysis of the combination of the 3 variables altogether are presented in Supplementary Figure S3A–F.
Discussion
In the present study, we sought to clarify the relevance of an inflammatory and/or fibrosis-related systemic milieu at an early time point for the development of functional impairment and kidney disease at later time points. To this extent, serum specimens collected within the ROADMAP and ROADMAP-OFU studies were ideally suited to test for our hypothesis that serum markers of inflammation and fibrosis predict the new onset of microalbuminuria in patients with diabetes mellitus type 2. The analyses with 15 cytokines identified a combination of 3 biomarkers that predicts new-onset microalbuminuria and may discriminate patients with diabetes mellitus type 2 at risk for development of diabetic kidney disease. The associations remained generally robust after adjustment for established clinical risk and confounding factors, such as baseline eGFR, baseline UACR, and prevalent cardiovascular disease.
Diabetic kidney disease presents in a more heterogeneous manner as previously thought, with an increasing minority of patients showing primarily a more or less progressive decline in renal function in the absence of albuminuria and a decreasing majority developing albuminuria before or concomitantly with renal function decline.20, 21, 22, 23 Both phenotypes reflect complex, partially overlapping but also completely distinct, underlying disease processes,24 and correlate with renal histopathologic changes, which are generated and progress long before the occurrence of clinical abnormalities.25, 26, 27 However, given that the performance of kidney biopsies is generally not advocated for patients with suspected diabetic nephropathy, such relevant information often is lacking.
The ability to diagnose diabetic kidney disease at an early, not clinically apparent stage may have major clinical implications.28, 29 Accordingly, there has been a substantial interest in discovering molecular biomarkers that may more specifically reflect pathophysiology involved in diabetic kidney disease progression.30 This is particularly important in the case of microalbuminuria, due to its high physiological fluctuation and the concerns regarding its predictive value as marker of established kidney disease.31, 32 Nevertheless, the detection of microalbuminuria constitutes the gold standard and earliest marker for overt diabetic kidney disease when a kidney biopsy is lacking.
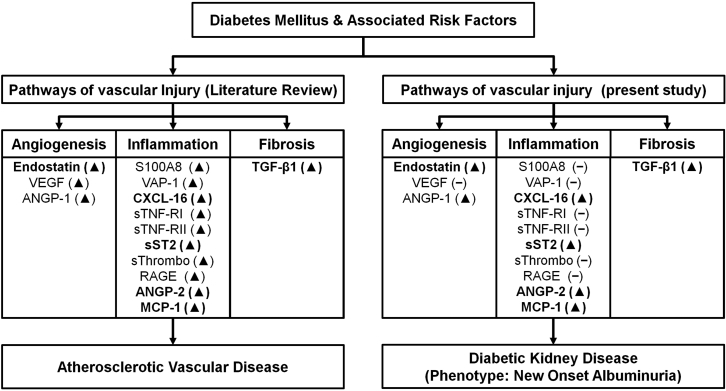
With the assumption that immune system activation underlies the development and progression of diabetic nephropathy and atherosclerosis, and that systemic atherosclerotic vascular and local renal disease share common and interdependent pathophysiologic pathways,33, 34, 35, 36, 37, 38 we focused on molecules relevant for cardiovascular disease pathology (Supplementary Table S8) and investigated if they were independently associated with future microalbuminuria. Case-control samples were available that matched for most of the clinical characteristics. Cytokines, chemokines, adhesion molecules, and growth factors, supposed to capture distinct aspects of the pathophysiology of atherosclerosis-related inflammatory responses, were found to be differentially regulated before the development of microalbuminuria, whereas some others remained unaffected (Figure 3). The changes in most of the biomarkers are plausible and in accordance with published evidence, but in some others difficult to interpret on the basis of current knowledge (Supplementary References in Supplementary Table S8); for example, it is unknown whether the elevated concentration of the soluble form of a receptor protein within the serum plays a direct pathogenetic role, or serves to protect cells from the deleterious effects of the respective ligand, or merely reflects long-term exposure of the receptor to the respective ligand. Nevertheless, our results suggest distinct and interconnected operating pathways of angiogenesis, fibrosis, and inflammation in the early stages of diabetic kidney disease (Figure 3) and implicate that microalbuminuria is present once renal injury has already occurred.
Figure 3.
Putative pathophysiologic mechanisms in the development of atherosclerotic vascular and albuminuric diabetic kidney disease. The investigated markers have been associated with development, progression or complications of atherosclerotic vascular disease. The changes in their levels presented in the left box are based on published study data summarized in the reference list of Supplementary Table S8. The results of our study are shown in the right box. ▲ denotes increases and ▼ decreases. For many of the markers, the changes in patients with future microalbuminuria (right box), were concordant with the changes observed in patients with future major cardiovascular events or adverse outcomes after a cardiovascular event (left box). The concordant changes in the markers included in the analysis are in bold. ANGP-1, angiopoietin-1; ANGP-2, angiopoietin-2; CXCL16, C-X-C motif chemokine ligand 16; MCP-1, monocyte chemotactic protein 1; RAGE, receptor for advanced glycation endproducts; S100A8, S100 calcium binding protein A8/myeloid related protein 8; sTNF-RI, soluble tumor necrosis factor receptor I; sTNF-RII, soluble tumor necrosis factor receptor II; sST2, soluble ST2; sThrombo, sThrombomodulin; TGFβ-1, transforming growth factor beta 1; VAP-1, vascular adhesion protein 1; VEGF, vascular endothelial growth factor.
Of the 15 biomarkers evaluated, CXCL-16, angiopoietin-2, and TGF-β1 remained significant predictors of subsequent microalbuminuria in the multivariate analyses. Their combination resulted in the largest improvement in predictive performance beyond traditional risk factors. The only small, insignificant increase in the AUC (from 0.752 to 0.760, P = 0.50), with the inclusion of the biomarker panel into the clinical risk model, is attributed to the fact that the baseline samples were matched for most of the clinical risk variables used in the model. Other relevant factors (UACR) or parameters came to be significant in the univariate analysis (duration of follow-up and prevalent cardiovascular disease) for which the final clinical model was additionally adjusted did not affect the results. Olmesartan did not affect the levels of the measured biomarkers in the cohort derived from the ROADMAP primary study.
CXCL-16 belongs to the CXC chemokine family and exists in a transmembrane and a soluble form. The transmembrane form functions as a receptor for CXCR-6–expressing cells and as a scavenger receptor for oxidized LDL. The soluble form of CXCL-16 results from cleavage at the cell surface and mirrors increased activity of upstream inflammatory pathways but also has the ability to directly promote downstream inflammatory responses.39 For example, the release of the soluble form of CXCL-16 is induced by inflammatory cytokines, such as TNF-α, interleukin-1β, and interferon-γ,40 whereas CXCL-16 itself enhances platelet adhesion to the endothelium under normal and pathologic conditions.41, 42 Furthermore, CXCL-16 is expressed in podocytes and renal tubular cells and contributes to renal fibrosis by recruiting bone marrow–derived fibroblast precursors.43, 44, 45 In other experimental models, CXCL-16 has been implicated in the pathogenesis of primary37 and secondary forms of glomerulonephritis,43, 46 and was found to be a key mediator of angiotensin II–induced renal inflammation and fibrosis in hypertensive renal disease.47, 48 In humans, CXCL-16 has been associated with the progression of diabetic and nondiabetic chronic kidney disease in prior cross-sectional studies.49, 50, 51 Our study is the first to show a significant and independent association of CXCL-16 serum levels with future kidney disease in a prospective study of patients with type 2 diabetes with normoalbuminuria and normal renal function at baseline.
The angiopoietin/Tie-2 ligand receptor system is an important regulator of vascular homeostasis in physiologic conditions and in various disease states. Disruption of the angiopoietin-2/angiopoietin-1 balance in favor of angiopoietin-2 impairs vascular permeability and increases systemic vascular inflammation,52, 53 whereas podocyte-specific overexpression of angiopoietin-2 leads to glomerular endothelial cell apoptosis and proteinuria.54 Accordingly, elevated circulating angiopoietin-2 levels are found in hypertensive, diabetic, and vascular disease patients.55, 56 In the case of established chronic kidney disease, angiopoietin-2 has been clearly associated with further progression of kidney disease, with subclinical and overt clinical cardiovascular disease and with all-cause mortality.57, 58, 59 In cross-sectional studies, serum and urinary angiopoietin-2 levels positively correlate with albuminuria.60, 61 Our study is the first to show an independent association of angiopoietin-2 with incident microalbuminuria, and supports from a clinical point of view the ample experimental evidence linking angiopoietin-2 with atherosclerotic plaque development and progression of diabetic kidney disease.62, 63
TGF-β1 is a multifunctional cytokine that stimulates production of extracellular matrix in multiple kidney cell types but has also anti-inflammatory effects, direct, by acting on renal resident cells, and indirect, through interactions with cells of the immune system.64 A plethora of experimental and clinical data support the role of TGF-β1 as a key pathogenetic factor in the progression of diabetic kidney disease. For example, TGF-β1 affects the integrity of the glomerular filtration barrier through induction of VEGF-A and disrupts the uptake of filtered albumin by the proximal tubular epithelial cells through downregulation of the megalin receptor complex,65, 66 whereas abolishment of TGF-β1 signaling in proximal tubular epithelial cells enhances interstitial fibrosis.67 Thus, either too much or too little TGF-β1 seems to be equally deleterious. In the clinical setting, TGF-β1 has been associated with unfavorable renal outcomes or progression of albuminuria but pharmacological blockade of TGF-β1 provided no benefit in patients with diabetic nephropathy.68, 69, 70, 71 Our findings imply that circulating TGF-β1 levels are indicative of subsequent kidney damage. Whether the elevated TGF-β1 levels are a physiological counterregulation within a proinflammatory systemic milieu or the early sign of fibrosis-prone kidneys remains to be clarified.
Strengths and limitations of the study should be acknowledged. The major strength is that it is part of an international multicenter randomized controlled study focusing exclusively on new onset of microalbuminuria as a primary endpoint and trying to avoid confounding factors as far as possible. A major limitation is that no standardized baseline samples were available for all patients before randomization and that the samples used for analysis were collected at different time points after enrollment and randomization. Therefore, we may not exploit the complete follow-up time of the primary studies. Another limitation is that the present study was a combined post hoc analysis of a randomized controlled trial, and its OFU trial with all its inherent drawbacks. The diagnosis of microalbuminuria differed between the ROADMAP and OFU and because different criteria were applied, the definition of microalbuminuria in OFU was less stringent in comparison with the primary ROADMAP study. A selection of patients with microalbuminuria in a manner similar to that of the primary ROADMAP primary study was not feasible in OFU. Nevertheless, because in OFU, patients with repeatedly positive results were selected as cases, the occurrence of microalbuminuria in the cases of the OFU cohort can be regarded as confirmed.
Furthermore, the ability of the investigated markers to predict a decline of eGFR or a 2- to 4-fold increase in albuminuria levels (within the microalbuminuric range), was not analyzed. Preexisting cardiovascular disease was more prevalent in patients with subsequent new-onset microalbuminuria; however, was not by itself a significant predictor for microalbuminuria development. This finding is in accord with our previous publications of the ROADMAP cohort and, admittedly, difficult to interpret, because a rather bidirectional association between microalbuminuria and cardiovascular disease would be anticipated.72, 73
Finally, the proportion of cases with microalbuminuria and case-controls of the OFU study population included in the present analysis being treated with olmesartan remained unknown. Thus, a possible impact of the intervention by renin-angiotensin system blockade on the serum markers may not be entirely excluded, however unlikely due to the randomization of the medication. Besides that, olmesartan had no impact on the serum markers in the study population derived from the ROADMAP primary study.
Conclusion
Our study with a panel of serum markers reflecting systemic inflammation, extracellular matrix remodeling, and angiogenesis provide a basis for risk prediction of kidney injury (microalbuminuria development) in patients with type 2 diabetes. Furthermore, our findings provide mechanistic insights on the complex interplay of these biologic pathways on the developmental process of diabetic nephropathy and underline the clinical significance of albuminuria as a marker of cardiovascular and renal disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The work of CC and JM is supported by the European Union (HEALTH-2011-278249-EU-MASCARA). FGS, PRM, AB, SB, and CC are supported by the Deutsche Forschungsgemeinschaft grants SFB 854, project A01 (PRM); GRK 2408, project 8 (PRM); and grants ME-1365/7-2 and ME-1365/9-1 (PRM).
Author Contributions
FGS researched data, performed statistical analysis, and wrote, reviewed, and edited the manuscript; JM designed the study, researched data, and reviewed the manuscript; AF and SB researched and interpreted data; PRM reviewed the manuscript; HH is principal investigator of ROADMAP and ROADMAP-OFU and reviewed the manuscript; and CC designed the study, researched and interpreted data, and wrote the manuscript.
Footnotes
Figure S1. Flow diagram of the Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention (ROADMAP) and observation follow-up (OFU) studies and illustration of participant inclusion in the current study.
Figure S2. Mediation analysis of risk for microalbuminuria development with inclusion of C-X-C motif chemokine ligand (CXCL)-16, transforming growth factor (TGF)-β1 and angiopoietin-2. (A–C) Tested mediators: changes in baseline serum TGF-β1 and angiopoietin-2. Independent variable: changes in CXCL-16. (A) The first step was the demonstration that higher CXCL-16 levels had a measurable impact on microalbuminuria development after accounting for baseline risk covariates. (B1 and B2) Second, we checked if mediator changes (TGF-β1, angiopoietin-2) correlated with higher risk for microalbuminuria development, after accounting for baseline risk covariates. (C1 and C2) Subsequently, we calculated the influence of higher CXCL-16 levels on the tested mediators (TGF-β1, angiopoietin-2). Finally, we jointly calculated the influence of the mediator on microalbuminuria development, after accounting for baseline risk covariates, and the direct effects of the independent variable (CXCL-16). This last step shows that higher serum TGF-β1 partially mediates (20%, P = 0.003 for the average causal mediation effect [ACME]) and angiopoietin-2 partially mediates (9%, P = 0.007 for the ACME) the original effect of CXCL-16 on microalbuminuria development and, consequently, CXCL-16 remains directly associated with microalbuminuria development in an independent manner (characterizing incomplete mediation). (D–F) Tested mediators: changes in baseline serum CXCL-16 and angiopoietin-2. Independent variable: changes in TGF-β1. (D) The first step was the demonstration that higher TGF-β1 levels had a measurable impact on microalbuminuria development after accounting for baseline risk covariates. (E1 and E2) Second, we checked if mediator changes (CXCL-16 and angiopoietin-2) correlated with microalbuminuria development, after accounting for baseline risk covariates. (F1 and F2) Third, we calculated the influence of higher CXCL-16 on the tested mediators (TGF-β1, angiopoietin-2). Finally, we jointly calculated the influence of the mediator on microalbuminuria development, after accounting for baseline risk covariates, and the direct effects of the independent variable (TGF-β1). This last step shows that higher serum CXCL-16 and angiopoietin-2 levels do not mediate the original effect of TGF-β1 on microalbuminuria development and, consequently, TGF-β1 remains directly associated with microalbuminuria development in an independent manner. (G–I) Tested mediators: changes in baseline serum CXCL-16 and TGF-β1. Independent variable: changes in angiopoietin-2. (G) The first step was the demonstration that higher angiopoietin-2 levels had a measurable impact on microalbuminuria development after accounting for baseline risk covariates. (H1 and H2) Second, we checked if mediator changes (CXCL-16 and TGF-β1) correlated with microalbuminuria development, after accounting for baseline risk covariates. (I1 and I2) Third, we calculated the influence of higher angiopoietin-2 on the tested mediators (CXCL-16 and TGF-β1). Subsequently, we jointly calculated the influence of the mediator on microalbuminuria development, after accounting for baseline risk covariates, and the direct effects of the independent variable (angiopoietin-2). This last step shows that higher serum CXCL-16 partially mediates (10%, P < 0.001 for the ACME) and TGF-β1 does not mediate (0%, P < 0.001 for the ACME) the original effect of angiopoietin-2 on microalbuminuria development and, consequently, angiopoietin-2 remains directly associated with microalbuminuria development in an independent manner (characterizing incomplete mediation). CI, confidence interval; OR, odds ratio.
Figure S3. Multilevel mediation analysis of risk for microalbuminuria development with inclusion of the combination of the 3 markers (C-X-C motif chemokine ligand [CXCL-16], transforming growth factor (TGF)-β1, and angiopoietin-2).
Table S1. Univariable association of individual clinical risk factors (top) and measured biomarkers (bottom) with new-onset microalbuminuria.
Table S2A. Spearman correlation coefficients between selected serum biomarker levels.
Table S2B. Spearman correlation coefficients between selected serum biomarker concentrations and clinical risk factors.
Table S3. Clinical characteristics of the available datasets at baseline, split by presence or absence of olmesartan in the Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention (ROADMAP) trial.
Table S4. Log-rank test of comparing the different quartiles of all 3 biomarkers associated with an increasing incidence of the composite outcome.
Table S5. Odds ratio (OR) for developing microalbuminuria before and after bootstrapped multivariate regression analysis.
Table S6. Calibration and sensitivity analysis of the risk prediction models.
Table S7. Prediction performance analysis for individual and combined biomarkers.
Table S8. Biomarkers of interest. Relevant clinical studies in patients with early stage cardiovascular disease or cardiovascular risk factors. Supposed pathways and underlying molecular mechanisms.
Modified STROBE Statement.
Contributor Information
Christos Chatzikyrkou, Email: christos.chatzikyrkou@med.ovgu.de.
ROADMAP Steering Committee:
Sadayoshi Ito, Josphe L. Izzo, Andrzeij Januszewicz, Shigerhiro Katayama, Jan Menne, Albert Mimram, Ton J. Rabelink, Eberhard Ritz, Luis M. Ruilope, Lars C. Rump, Giancarlo Viberti, and Herrman Haller
Supplementary Material
References
- 1.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Fox C.S., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen C.P., Chang C.H., Tsai M.K. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017;92:388–396. doi: 10.1016/j.kint.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V., Agarwal R., Fioretto P. Management of patients with diabetes and CKD: conclusions from a "Kidney Disease:Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2016;90:1175–1183. doi: 10.1016/j.kint.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Gerstein H.C., Pare G., McQueen M.J. Identifying novel biomarkers for cardiovascular events or death in people with dysglycemia. Circulation. 2015;132:2297–2304. doi: 10.1161/CIRCULATIONAHA.115.015744. [DOI] [PubMed] [Google Scholar]
- 6.Dweck M.R., Aikawa E., Newby D.E. Noninvasive molecular imaging of disease activity in atherosclerosis. Circ Res. 2016;119:330–340. doi: 10.1161/CIRCRESAHA.116.307971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schanstra J.P., Zurbig P., Alkhalaf A. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26:1999–2010. doi: 10.1681/ASN.2014050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menne J., Chatzikyrkou C., Haller H. Microalbuminuria as a risk factor: the influence of renin-angiotensin system blockade. J Hypertens. 2010;28:1983–1994. doi: 10.1097/HJH.0b013e32833c206d. [DOI] [PubMed] [Google Scholar]
- 9.Haller H., Ito S., Izzo J.L., Jr. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 10.Menne J., Ritz E., Ruilope L.M. The Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) observational follow-up study: benefits of RAS blockade with olmesartan treatment are sustained after study discontinuation. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukaka M.M. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 12.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Malek M.H., Berger D.E., Coburn J.W. On the inappropriateness of stepwise regression analysis for model building and testing. Eur J Appl Physiol. 2007;101:263–264. doi: 10.1007/s00421-007-0485-9. ; author reply 265–266. [DOI] [PubMed] [Google Scholar]
- 14.Pencina M.J., D'Agostino R.B., Sr., D'Agostino R.B., Jr. Evaluating the added predictive ability of a new marker:from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. ; discussion. 207–112. [DOI] [PubMed] [Google Scholar]
- 15.Henderson A.R. The bootstrap: a technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta. 2005;359:1–26. doi: 10.1016/j.cccn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Efron B., Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986:54–75. [Google Scholar]
- 17.Krull J.L., MacKinnon D.P. Multilevel modeling of individual and group level mediated effects. Multivariate Behav Res. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- 18.Imai K., Keele L., Tingley D. Unpacking the black box of causality: learning about causal mechanisms from experimental and observational studies. Am Polit Sci Rev. 2011;105:765–789. [Google Scholar]
- 19.Shrout P.E., Bolger N. Mediation in experimental and nonexperimental studies:new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 20.Afkarian M., Zelnick L.R., Hall Y.N. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krolewski A.S., Skupien J., Rossing P. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int. 2017;91:1300–1311. doi: 10.1016/j.kint.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonneijck L., Muskiet M.H., Smits M.M. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28:1023–1039. doi: 10.1681/ASN.2016060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders H.J., Huber T.B., Isermann B. CKD in diabetes:diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 24.Van J.A., Scholey J.W., Konvalinka A. Insights into diabetic kidney disease using urinary proteomics and bioinformatics. J Am Soc Nephrol. 2017;28:1050–1061. doi: 10.1681/ASN.2016091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauer M., Zinman B., Gardiner R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekinci E.I., Jerums G., Skene A. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes care. 2013;36:3620–3626. doi: 10.2337/dc12-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klessens C.Q., Woutman T.D., Veraar K.A. An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 2016;90:149–156. doi: 10.1016/j.kint.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Doshi S.M., Friedman A.N. Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12:1366–1373. doi: 10.2215/CJN.11111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanouchi M., Skupien J., Niewczas M.A. Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int. 2017;92:258–266. doi: 10.1016/j.kint.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campion C.G., Sanchez-Ferras O., Batchu S.N. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117705371. 2054358117705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witte E.C., Lambers Heerspink H.J., de Zeeuw D. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–443. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakris G.L., Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care. 2014;37:867–875. doi: 10.2337/dc13-1870. [DOI] [PubMed] [Google Scholar]
- 33.Navarro-Gonzalez J.F., Mora-Fernandez C., Muros de Fuentes M. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 34.Ait-Oufella H., Taleb S., Mallat Z. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thomb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 35.Nair V., Komorowsky C.V., Weil E.J. A molecular morphometric approach to diabetic kidney disease can link structure to function and outcome. Kidney Int. 2018;93:439–449. doi: 10.1016/j.kint.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson A.C., Ingelsson E., Sundstrom J. Use of proteomics to investigate kidney function decline over 5 years. Clin J Am Soc Nephrol. 2017;12:1226–1235. doi: 10.2215/CJN.08780816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mise K., Hoshino J., Ubara Y. Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transplant. 2014;29:109–118. doi: 10.1093/ndt/gft349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez E.J., Lolis E. Structure, function, and inhibition of chemokines. Ann Rev Pharmacol Toxicol. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 40.Abel S., Hundhausen C., Mentlein R. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 41.Linke B., Meyer Dos Santos S., Picard-Willems B. CXCL16/CXCR6-mediated adhesion of human peripheral blood mononuclear cells to inflamed endothelium. Cytokine. 2019;122:154081. doi: 10.1016/j.cyto.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Borst O., Munzer P., Gatidis S. The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circ Res. 2012;111:1297–1307. doi: 10.1161/CIRCRESAHA.112.276444. [DOI] [PubMed] [Google Scholar]
- 43.Gutwein P., Abdel-Bakky M.S., Doberstein K. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. J Cell Mol Med. 2009;13:3809–3825. doi: 10.1111/j.1582-4934.2009.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutwein P., Abdel-Bakky M.S., Schramme A. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am J Pathol. 2009;174:2061–2072. doi: 10.2353/ajpath.2009.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G., Lin S.C., Chen J. CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J Am Soc Nephrol. 2011;22:1876–1886. doi: 10.1681/ASN.2010080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia G.E., Truong L.D., Li P. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol. 2007;170:1485–1496. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Z., Jin X., He L. CXCL16 regulates renal injury and fibrosis in experimental renal artery stenosis. Am J Physiol Heart Circ Physiol. 2016;311:H815–H821. doi: 10.1152/ajpheart.00948.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y., Entman M.L., Wang Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension. 2013;62:1129–1137. doi: 10.1161/HYPERTENSIONAHA.113.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z., Gong Q., Zhou Z. Increased plasma CXCL16 levels in patients with chronic kidney diseases. Eur J Clin Invest. 2011;41:836–845. doi: 10.1111/j.1365-2362.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 50.Elewa U., Sanchez-Nino M.D., Mahillo-Fernandez I. Circulating CXCL16 in diabetic kidney disease. Kidney Blood Press Res. 2016;41:663–671. doi: 10.1159/000447935. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L., Wu F., Jin L. Serum CXCL16 as a novel marker of renal injury in type 2 diabetes mellitus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiedler U., Reiss Y., Scharpfenecker M. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 53.Parikh S.M. The angiopoietin-Tie2 signaling axis in systemic inflammation. J Am Soc Nephrol. 2017;28:1973–1982. doi: 10.1681/ASN.2017010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis B., Dei Cas A., Long D.A. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol. 2007;18:2320–2329. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- 55.David S., Kumpers P., Lukasz A. Circulating angiopoietin-2 in essential hypertension: relation to atherosclerosis, vascular inflammation, and treatment with olmesartan/pravastatin. J Hypertens. 2009;27:1641–1647. doi: 10.1097/HJH.0b013e32832be575. [DOI] [PubMed] [Google Scholar]
- 56.Lim H.S., Blann A.D., Chong A.Y. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes:implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918–2924. doi: 10.2337/diacare.27.12.2918. [DOI] [PubMed] [Google Scholar]
- 57.Tsai Y.C., Lee C.S., Chiu Y.W. Angiopoietin-2, angiopoietin-1 and subclinical cardiovascular disease in chronic kidney disease. Sci Rep. 2016;6:39400. doi: 10.1038/srep39400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.David S., John S.G., Jefferies H.J. Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transplant. 2012;27:1867–1872. doi: 10.1093/ndt/gfr551. [DOI] [PubMed] [Google Scholar]
- 59.Tsai Y.C., Chiu Y.W., Tsai J.C. Association of angiopoietin-2 with renal outcome in chronic kidney disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang F.C., Lai T.S., Chiang C.K. Angiopoietin-2 is associated with albuminuria and microinflammation in chronic kidney disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S., Li H., Zhang C. Urinary angiopoietin-2 is associated with albuminuria in patients with type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:163120. doi: 10.1155/2015/163120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Theelen T.L., Lappalainen J.P., Sluimer J.C. Angiopoietin-2 blocking antibodies reduce early atherosclerotic plaque development in mice. Atherosclerosis. 2015;241:297–304. doi: 10.1016/j.atherosclerosis.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gnudi L. Angiopoietins and diabetic nephropathy. Diabetologia. 2016;59:1616–1620. doi: 10.1007/s00125-016-3995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sureshbabu A., Muhsin S.A., Choi M.E. TGF-beta signaling in the kidney:profibrotic and protective effects. Am J Physiol Renal Physiol. 2016;310:F596–F606. doi: 10.1152/ajprenal.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Hu J., Zhang Q. Urocortin 1 improves renal function in rats with streptozotocin-induced diabetes by inhibiting overproduction of TGF-beta 1 and VEGF. Br J Pharmacol. 2009;157:994–1003. doi: 10.1111/j.1476-5381.2009.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo L.M., del Re E., Brown D. Evidence for a role of transforming growth factor (TGF)-beta1 in the induction of postglomerular albuminuria in diabetic nephropathy:amelioration by soluble TGF-beta type II receptor. Diabetes. 2007;56:380–388. doi: 10.2337/db06-1018. [DOI] [PubMed] [Google Scholar]
- 67.Nlandu-Khodo S., Neelisetty S., Phillips M. Blocking TGF-beta and beta-catenin epithelial crosstalk exacerbates CKD. J Am Soc Nephrol. 2017;28:3490–3503. doi: 10.1681/ASN.2016121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong M.G., Perkovic V., Woodward M. Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 2013;83:278–284. doi: 10.1038/ki.2012.383. [DOI] [PubMed] [Google Scholar]
- 69.Mehta T., Buzkova P., Kizer J.R. Higher plasma transforming growth factor (TGF)-beta is associated with kidney disease in older community dwelling adults. BMC Nephrol. 2017;18:98. doi: 10.1186/s12882-017-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao Y.C., Shen J., Hong X.Z. Changes of regulatory T cells, transforming growth factor-beta and interleukin-10 in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Clin Immunol. 2016;170:61–69. doi: 10.1016/j.clim.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Voelker J., Berg P.H., Sheetz M. Anti-TGF-beta1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 2017;28:953–962. doi: 10.1681/ASN.2015111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chatzikyrkou C., Menne J., Izzo J. Predictors for the development of microalbuminuria and interaction with renal function. J Hypertens. 2017;35:2501–2509. doi: 10.1097/HJH.0000000000001491. [DOI] [PubMed] [Google Scholar]
- 73.Garg J.P., Bakris G.L. Microalbuminuria:marker of vascular dysfunction, risk factor for cardiovascular disease. Vasc Med. 2002;7:35–43. doi: 10.1191/1358863x02vm412ra. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.