Abstract
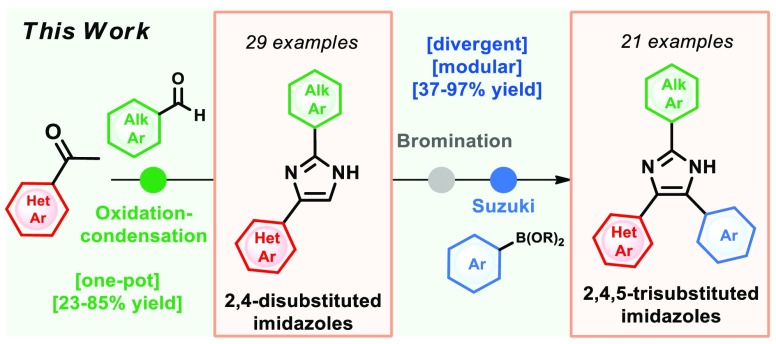
A one-pot and modular approach to the synthesis of 2,4(5)-disubstituted imidazoles was developed based on ketone oxidation, employing catalytic HBr and DMSO, followed by imidazole condensation with aldehydes. This methodology afforded twenty-nine disubstituted NH-imidazoles (23%–85% yield). A three-step synthesis of 20 kinase inhibitors was achieved by employing this oxidation–condensation protocol, followed by bromination and Suzuki coupling in the imidazole ring to yield trisubstituted NH-imidazoles (23%–69%, three steps). This approach was also employed in the synthesis of known inhibitor GSK3037619A.
Accessibility and availability of small organic molecules remains one of the major challenges in the drug discovery process.1 Efficient and rapid approaches to access these molecules are highly desirable in order to provide medicinal chemists and chemical biologists the right tools in their scientific endeavors.2 In this context, synthetic organic chemistry plays a pivotal role in creating pathways to access these molecules in a short, economic and efficient way from commercially and widely available building blocks. Moreover, the synthetic approach must offer versatility by allowing modular changes in a divergent fashion in order to generate several different molecules from a single precursor.
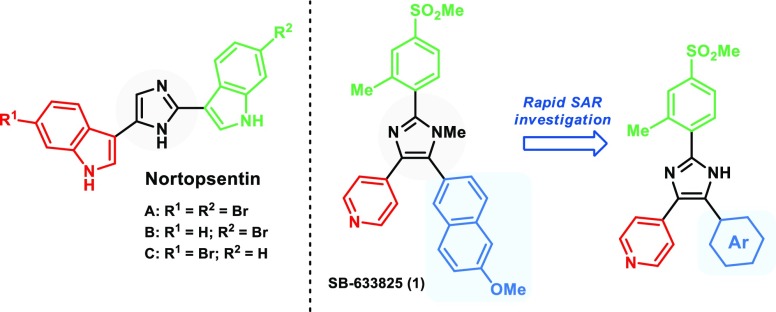
Substituted imidazoles are one class of such small organic molecules with broad interest, ranging from applications in materials and polymer science3,4 to their use as ionic liquids,5 and as therapeutic agents6 and bioactive molecules such as the marine alkaloids Nortopsentins A–C7 (Figure 1). Methods to access these scaffolds have been intensely explored and can be roughly divided into two approaches. The first approach involves the formation of the imidazole ring from suitable precursors,8 while the second involves the functionalization of a preformed imidazole ring.9 Combinations of both approaches can also be employed to efficiently assemble substituted imidazoles.10−14
Figure 1.
Bioactive Nortopsentins A–C and pyridyl–imidazole kinase inhibitor SB-633825 (1).
In our search for selective and potent inhibitors of the kinase STK10,15 which is a serine–threonine kinase important due to its role in lymphocyte migration,16−19 we were challenged with the task of providing an efficient, modular and divergent synthetic route for rapid evaluation of the structure–activity relationship (SAR) of trisubstituted pyridyl-imidazole 1 (Figure 1), focusing on changes in the naphthyl moiety since the previous synthetic approach introduced the naphthalene in the first step of a six-step route.20
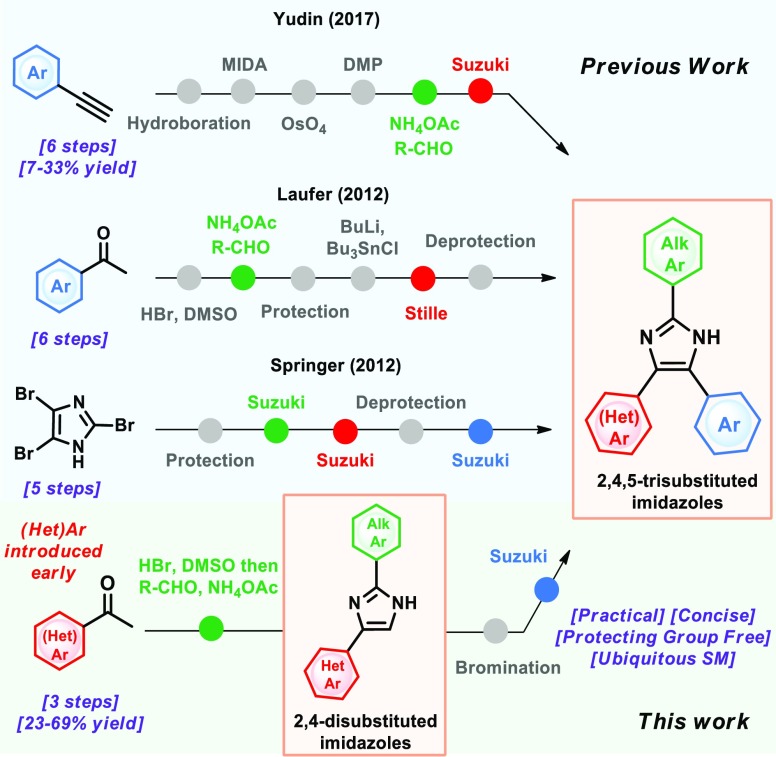
Previous work from Laufer12 and Springer21 already provided access to trisubstituted pyridyl imidazoles in a divergent and modular fashion, although with the use of protecting groups, thus increasing the step count by two (Figure 2). The use of oxalyl boronates by Yudin offers a regioselective, protecting group-free and modular approach to imidazoles. However, the key intermediate is accessed in five steps, and the cross-coupled product is obtained in moderate yields (Figure 2).11
Figure 2.
Modular access to 2,4,5-trisubstituted imidazoles.
We sought to address both challenges by implementing an efficient step- and redox-economical approach to disubstituted 2,4(5)-NH imidazoles, followed by the Suzuki reaction to introduce the aromatic substituent in a protecting group-free fashion. Herein, we report an improved one-pot approach to disubstituted 2,4(5)-NH imidazoles consisting of a sequential Kornblum oxidation,22 followed by Radziszewski23 imidazole condensation, which allowed the synthesis of twenty-nine 2,4(5)-disubstituted imidazoles in yields ranging from 23% to 85%. Moreover, representative imidazoles 32 and 36 were further functionalized to rapidly access a small kinase inhibitor library of 20 trisubstituted 2,4,5-NH imidazoles in yields ranging from 23% to 69% for three steps.
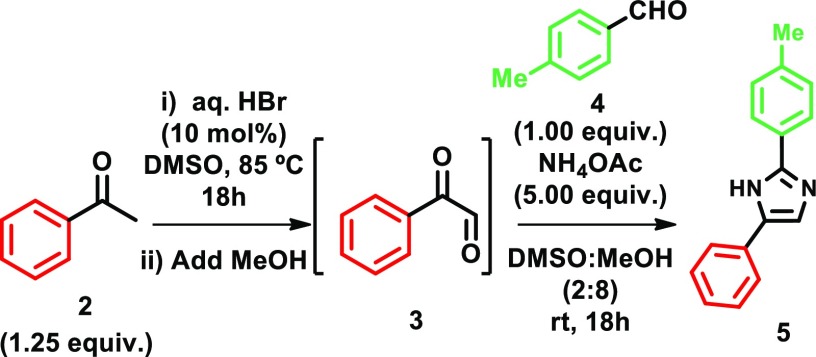
Initially, we investigated the possibility of obtaining the 2,4(5)-NH imidazole 5 employing the sequential oxidation–condensation protocol with acetophenone (2) and p-tolualdehyde (4) as representative carbonyl substrates. After extensive optimization, it was found that formation of glyoxal 3 from acetophenone (2) could be achieved, employing a catalytic amount (10 mol%) of aqueous HBr in DMSO at 85 °C. After addition of a MeOH/DMSO (6:4 v/v) solution of glyoxal to a mixture of p-tolualdehyde (4) and NH4OAc in MeOH, the desired imidazole was isolated in 69% yield (Table 1, entry 1).
Table 1. Optimization of the Reaction Conditions for the Synthesis of Disubstituted Imidazole 5.
| Entry | Changes from the conditions described above | Yieldb |
|---|---|---|
| 1 | none | 69 (69) |
| 2 | 2 (1.00 equiv), aq HBr (200 mol %), 60 °C, 24 h | 48 |
| 3 | 2 (1.00 equiv), aq HBr (50 mol %), 60 °C, 72 h | 57 |
| 4 | 2 (1.00 equiv), aq HBr (50 mol %), 85 °C, 12 h | 55 |
| 5 | 2 (1.00 equiv), aq HBr (10 mol %), 85 °C, 18 h | 61 |
| 6 | no aq HBr | 0 |
| 7 | DMSO/MeOH (7:3)c | 45 |
| 8 | DMSO/EtOH (2:8)c | 49 |
| 9 | DMSO/MeOH/DMF (2:3:5)c | 45 |
| 10 | DMSO/MeOH/PhMe (2:3:5)c | 47 |
| 11 | with isolation of 3 (stepwise procedure) | (52) |
Oxidation step performed using acetophenone (2) (Table 1), aqueous HBr (48% w/w, 8.9 M) (Table 1), and DMSO (0.50 M). Condensation step performed by slow addition (30 min) of glyoxal 3 solution in DMSO/MeOH (4:6, v/v, 0.19 M relative to acetophenone 2) to a mixture of tolualdehyde (4) (0.3 mmol) and NH4OAc (1.5 mmol) in MeOH (1.5 mL, 0.2 M). Final solvent composition: DMSO/MeOH (8:2).
Yield after workup as determined by 1H NMR analysis of the crude reaction mixture with 1,3,5-trimethoxybenzene as the internal standard. Isolated yield given in (parenthesis).
Final solvent composition
Decreasing HBr loading (entries 2–5) resulted in longer reaction times (oxidation step) with some improvement in the yield. Importantly, an increase in reaction temperature did not have an impact on the yield (entries 4 and 5), but the oxidation reaction proceeded faster. When the reaction was carried out in the absence of HBr (entry 6), neither glyoxal 3 nor imidazole 5 were observed. When the amount of acetophenone was increased (entry 1), a better yield was observed and 1.25 equiv was selected as the optimum amount. Changing from MeOH to EtOH (oxidation step, entry 8) or adding polar aprotic (DMF and DMSO, entries 7 and 9) and apolar (PhMe, entry 10) solvent in the condensation step did not provide better yields. (See Table S1 for all conditions employed.)
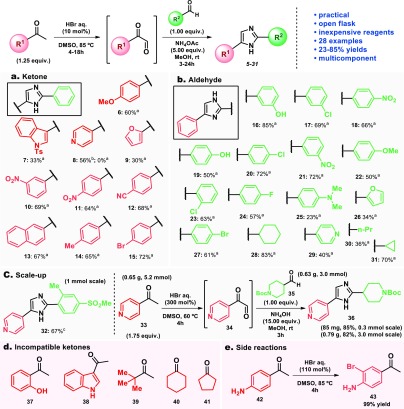
The substrate scope (Scheme 1) was then explored using different methyl ketones and aldehydes. The transformation proved to tolerate well the electronic properties of the substituted acetophenones employed. Notably, substituted acetophenones bearing electron-donating (6, 14), electron-withdrawing (10, 11, 12, 15), electron-neutral (13), and pyridine (8) were good substrates for this transformation, and products were isolated in yields ranging from 56 to 72% (Scheme 1a). However, the 3-indole and 2-furyl derivatives 7 and 9, respectively, performed poorly under standard conditions while 2-hydroxyacetophenone (37) did not show reactivity even when higher amounts of HBr (300 mol %) were employed. For 3-acetylindole (38), it was necessary to increase the HBr loading to 300 mol % at 85 °C to accomplish consumption of the starting material, but the imidazole product was not obtained under these conditions. Saturated ketones (40 and 41) were consumed under standard oxidation conditions without formation of the imidazole product (Scheme 1d). Interestingly, when 4-aminoacetophenone (42) was reacted with 110 mol % of HBr, the brominated side product 43 was obtained (Scheme 1e).
Scheme 1. Scope of the Oxidation–Condensation Approach to 2,4(5)-Disubstituted Imidazoles.
Reaction scale: 0.30 mmol. Reactions performed employing the ketone (1.25 equiv), aldehyde (1.00 equiv), NH4OAc (5.00 equiv), DMSO (0.75 mL), MeOH (2.75 mL). Yields described correspond to isolated yields after column cromatography. Ketone (1.25 equiv), 48% aq HBr (10 mol %), DMSO, 85 °C, 18 h then aldehyde (1.00 equiv), NH4OAc (5.00 equiv), MeOH, rt, 24 h.
Ketone (1.25 equiv), 48% aq HBr (300 mol %), DMSO, 85 °C, 8 h then aldehyde (1.00 equiv), NH4OAc (5.00 equiv), MeOH, rt, 24 h.
Ketone (1.75 equiv), 48% aq HBr (300 mol %), DMSO, 85 °C, 18 h then aldehyde (1.00 equiv), NH4OAc (10.00 equiv), MeOH, rt, 24 h.
Considering the aldehyde scope, benzaldehydes bearing electron-withdrawing groups (17, 18, 20, 21, 23, 24, 27), such as halides and nitro groups, performed better than those bearing electron-donating groups (19, 22) with the exception of the phenolic derivative 16, which was isolated in 85% yield. This behavior might be due to the electron distribution in the aromatic ring of the substituted benzaldehyde, which is more reactive when electron-withdrawing groups are present. Interestingly, saturated cyclic aldehydes such as cyclopropyl (31) and cyclohexyl (28) carboxyaldehydes were good substrates for this transformation (70% and 83% yield, respectively), although n-butyraldehyde derivative 30 was isolated in only 36%. Overall, imidazoles 16–31 from the aldehyde scope were isolated in yields ranging from 23 to 85% from the corresponding aldehydes (Scheme 1b). The disubstituted imidazole 32 was obtained in 67% yield after optimization of the reaction conditions for this specific substrate. (See Table S2.) It was also possible to employ the commercially available Boc-protected aldehyde 35 under slightly modified conditions using NH4OH as a basic ammonia source to neutralize HBr in order to avoid unwanted deprotection. The disubstituted imidazole 36 was isolated in 85% yield in a 0.3 mmol scale, and the reaction proved to be scalable in a 3.0 mmol scale, affording 36 in 82% yield. (Scheme 1c). This one-pot approach for disubstituted imidazoles has the following advantages when compared to a stepwise procedure: (1) avoids glyoxal isolation, which can be troublesome;23 (2) starts from ubiquitous and/or easily accessible starting materials; (3) employs aqueous HBr as the catalyst and DMSO as the oxidant, and (4) is amenable to scale-up. On the other hand, the 4(5)-position of the imidazole ring is restricted to aryl substitutents and is not compatible with acid-sensitive substrates, such as indoles. (Scheme 1d).
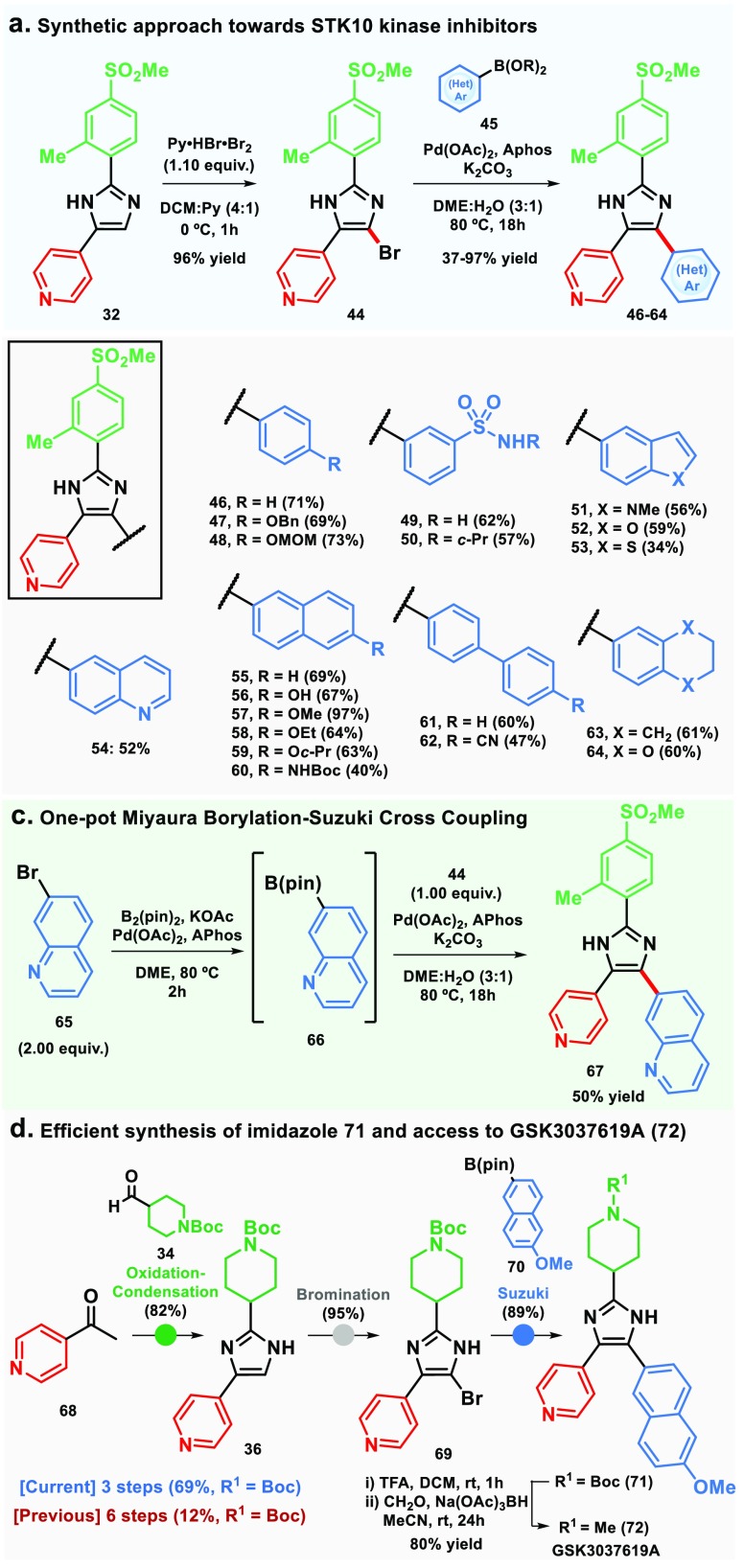
To show further applicability of the method, disubstituted imidazole 32 was functionalized at C-5 position of the imidazole ring to afford a small library of pyridyl imidazoles inhibitors for testing against STK10 and SLK kinases.15 This was accomplished by bromination of the 2,4-disubstituted imidazole2432, followed by Suzuki–Miyaura cross-coupling25 with boronic acids or esters (Scheme 2a). In this case, nineteen 2,4,5-trisubstituted imidazoles 46–64 were obtained in yields ranging from 37 to 97% from the common intermediate 44 (Scheme 2b). Interestingly, it was possible to perform a one-pot Miyaura borylation and Suzuki cross-coupling starting from bromide 65 to access trisubstituted imidazole 67 in 52% yield (Scheme 2c). Moreover, the trisubstituted imidazole 71, which was synthesized by Yudin in six steps (12% overall yield),11 could be accessed in three steps (69% overall yield) from 4-acetylpyridine (68) employing the same strategy as for imidazoles 46–64 (Scheme 2d). From this advanced intermediate 71, the known inhibitor GSK3037619A (72) could be synthesized in a one-pot procedure in 80% yield (Scheme 2d).
Scheme 2. Synthesis of 2,4,5-Trisubstituted Imidazole STK10 Kinase Inhibitors.
Compounds 57 (R = OMe) and 59 (R = Oc-Pr) were subjected to binding displacement assays11 against STK10 and SLK kinases and displayed Ki values of 146 and 700 nM, respectively, against STK10, and 180 and 230 nM, respectively, for SLK. The weaker binding of the cyclopropyl derivative to STK10 might be explained by a more significant space restriction in the hydrophobic pocket of STK10 to bulkier substituents at the 6-position compared to SLK.
In conclusion, we developed an improved one-pot procedure for the synthesis of 2,4(5)-disubstituted NH-imidazoles, employing widely available starting materials such as methyl ketones and aldehydes, and demonstrated the utility of the methodology by using it as a key step in a short, modular, and divergent synthetic route to 2,4,5-trisubstituted pyridyl-imidazole inhibitors of the STK10 kinase and for the synthesis of the GSK3037619A in 4 steps (55% overall yield). This approach enabled rapid exploration of the SAR at the C-5 position of the imidazole ring and permits regioselective variation at the C-2 and C-4 positions for future exploration.
Experimental Section
General Information
Unless stated otherwise, the synthesis of 2,4-disubstituted imidazoles was performed using an undistilled solvent, without any precaution to exclude air and moisture, in 5 mL vials, and the mixture was stirred with Teflon-coated magnetic bars (1 cm × 0.5 cm). Suzuki couplings for the preparation of 2,4,5-trisubstituted imidazoles were performed under a nitrogen atmosphere in 100 mm × 13 mm (9 mL) culture tubes and were stirred with Teflon-coated magnetic bars (1 cm × 0.5 cm). Dry dimethoxyethane (DME, 99.5%) and dry dimethylformamide (DMF, 99.5%) were purchased from Sigma-Aldrich and stored under 3 Å molecular sieves and nitrogen-purged before use. Dichloromethane (DCM) and triethylamine (Et3N) were pretreated with calcium hydride and distilled before use. Pyridine was distilled from calcium hydride and stored over 4 Å molecular sieves. Tetrahydrofuran was dried over 4 Å molecular sieves and distilled from sodium metal and benzophenone before use. All other solvents and commercial reagents were used as supplied without further purification unless stated otherwise. All reactions involving heating were carried out using aluminum blocks and a contact thermometer. Reactions were monitored by thin-layer chromatography (silica gel 60 F254 in aluminum foil, Merck), and visualization was achieved under UV light (254 nm) followed by staining in potassium permanganate (KMnO4), Dragendorff stain (Dragendorff), dinitrophenylhydrazine stain (DNFH), p-anisaldehyde stain (p-ASD), or curcumin stain and heating. Silica gel 60 F254 (200–400 Mesh, Merck) was used for purifications by standard flash column chromatography. NMR spectra were recorded on a Bruker Avance DPX 250 MHz (250 MHz 1H, 63 MHz 13C), Bruker Avance III 400 (400 MHz 1H, 101 MHz 13C), Bruker Avance III 500 (500 MHz 1H, 126 MHz 13C), or Bruker Avance III 600 (600 MHz 1H, 151 MHz 13C) unit. The chemical shifts are expressed in parts per million (ppm) relative to the residual solvent signal as an internal reference ([1] CDCl31H RMN = 7.26, 13C RMN = 77.16; [2] DMSO-d61H RMN = 2.50, 13C RMN = 39.52; [3] acetone-d6: 1H RMN = 2.05, 13C RMN = 206.26; [4] methanol-d4: 1H RMN = 3.31, 13C RMN = 49.00). Multiplicities are reported with the following symbols: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and multiples thereof. High-resolution mass spectra (ESI) were acquired on an Xevo Q-Tof mass spectrometer (Waters, Manchester, U.K.) equipped with a nanoESI type ionization source. IR spectra were recorded using a Thermo Scientific Nicolet IS5 spectrometer, using Thermo Scientific ID3 ATR. Melting points were recorded on a MP50 Metler-Toledo melting point apparatus and are uncorrected. STK10 and SLK binding displacement assays were performed as previously described.11
Optimization of the Reaction Conditions
5-Phenyl-2-(p-tolyl)-1H-imidazole (5)
A 6 mL vial was charged with acetophenone 2 (46.0 mg, 0.375 mmol, 1.25 equiv), DMSO (0.75 mL, 0.5 M), concentrated aqueous HBr (48% w/w, 8.9 M) (4.24 μL, 0.03 mmol, 10 mol %), deionized water (71 μL), and a magnetic stirr bar under air. The reaction mixture was stirred in a preheated aluminum block at 85 °C and was followed by TLC analysis (30% EtOAc/hexane, p-ASD). After consumption of the starting material, the reaction mixture was cooled to room temperature and diluted with MeOH (1.25 mL, 0.19 M, final concentration relative to acetophenone (2), 2:8 mixture of DMSO/MeOH). This stock DMSO/MeOH solution was added dropwise over 30 min via syringe to a 6 mL vial containing p-tolualdehyde (4) (37.0 mg, 0.30 mmol, 1.00 equiv), NH4OAc (116 mg, 1.50 mmol, 5.00 equiv), and MeOH (1.5 mL, 0.2 M in relation to 4). The reaction mixture was stirred at room temperature for 18 h and then poured directly into a separatory funnel containing a mixture of saturated NaHCO3 and saturated Na2S2O3 (1:1, 1 × 20 mL) and EtOAc (10 mL). The phases were separated, and the aqueous phase was extracted with EtOAc (5 × 5 mL). The organic phases were combined, washed with saturated NaCl solution (1 × 5 mL), dried over Na2SO4, filtered, and concentrated in the rotaevaporator. The residue was diluted with EtOAc (5 mL), and a 1 mL aliquot was taken and concentrated in vacuo. To this were added 1,3,5-trimethoxybenzene (10.2 mg, 0.06 mmol) and acetone-d6 (0.6 mL), and the sample was analyzed by 1H NMR. The crude mixtures were combined and purification of the residue by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 5 as a white solid (69% yield, 48.0 mg, 0.21 mmol): Rf = 0.30 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.55 (s, 1H), 7.89 (d, J = 7.9 Hz, 2H), 7.86 (d, J = 7.6 Hz, 2H), 7.72 (s, 1H), 7.36 (t, J = 7.5 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H), 7.20 (t, J = 7.2 Hz, 1H), 2.34 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 146.0, 140.9, 137.5, 134.8, 129.3, 128.4, 126.1, 125.2, 124.9, 124.4, 114.0, 20.9. Spectroscopic data are in accordance with the literature.26
Ketone Scope: General Procedure A
A 6 mL vial was charged with the corresponding acetophenone (0.375 mmol, 1.25 equiv), DMSO (0.75 mL, 0.5 M), concentrated aqueous HBr (48% w/w, 8.9 M) (4.24 μL, 0.03 mmol, 10 mol %), deionized water (71 μL), and a magnetic stir bar under air. The reaction mixture was stirred in a preheated aluminum block at 85 °C and was followed by TLC analysis (EtOAc/hexane, p-ASD). After consumption of the starting material, the reaction mixture was cooled to room temperature and diluted with MeOH (1.25 mL, 0.19 M, final concentration relative to the corresponding acetophenone, 4:6 mixture of DMSO/MeOH). This stock DMSO/MeOH solution was added dropwise over 30 min via syringe to a 6 mL vial containing benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv), NH4OAc (116 mg, 1.50 mmol, 5.00 equiv), and MeOH (1.5 mL, 0.2 M in relation to benzaldehyde). The reaction mixture was stirred at room temperature for 18 h and then poured directly into a separatory funnel containing a mixture of saturated NaHCO3 and saturated Na2S2O3 (1:1, 1 × 20 mL) and EtOAc (10 mL). The phases were separated, and the aqueous phase was extracted with EtOAc (5 × 5 mL). The organic phases were combined, washed with saturated NaCl solution (1 × 5 mL), dried over Na2SO4, filtered, and concentrated in the rotaevaporator. The residue was purified by silica gel column chromatography.
Aldehyde Scope: General Procedure B
A 6 mL vial was charged with acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv), DMSO (0.75 mL, 0.5 M), concentrated aqueous HBr (48% w/w, 8.9 M) (4.24 μL, 0.0375 mmol, 10 mol %), deionized water (71 μL), and a magnetic stir bar under air. The reaction mixture was stirred in a preheated aluminum block at 85 °C and was followed by TLC analysis (EtOAc/hexane, p-ASD). After consumption of the starting material, the reaction mixture was cooled to room temperature and diluted with MeOH (1.25 mL, 0.19 M, final concentration relative to the corresponding acetophenone, 4:6 mixture of DMSO/MeOH). This stock DMSO/MeOH solution was added dropwise over 30 min via syringe to a 6 mL vial containing the corresponding aldehyde (0.30 mmol, 1.00 equiv), NH4OAc (116 mg, 1.50 mmol, 5.00 equiv), and MeOH (1.5 mL, 0.2 M in relation to the aldehyde). The reaction mixture was stirred at room temperature for 18 h and then poured directly into a separatory funnel containing a mixture of saturated NaHCO3 and saturated Na2S2O3 (1:1, 1 × 20 mL) and EtOAc (10 mL). The phases were separated, and the aqueous phase was extracted with EtOAc (5 × 5 mL). The organic phases were combined, washed with saturated NaCl solution (1 × 5 mL), dried over Na2SO4, filtered, and concentrated in the rotaevaporator. The residue was purified by silica gel column chromatography.
4-(4-Methoxyphenyl)-2-phenyl-1H-imidazole (6)
The title compound was prepared according to general procedure A, using 4′-methoxyacetophenone (58.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 6 as a pale yellow solid (60% yield, 44.0 mg, 0.18 mmol): Rf = 0.12 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.55 (br s, 1H), 7.99 (d, J = 7.4 Hz, 2H), 7.78 (d, J = 8.7 Hz, 2H), 7.63, (d, J = 1.4 Hz, 1H), 7.46 (t, J = 7.8 Hz, 2H), 7.35 (t, J = 7.3 Hz, 1H), 6.95 (d, J = 8.7 Hz, 2H), 3.77 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 158.3, 157.9, 146.3, 145.5, 141.0, 130.7, 128.7, 128.6, 128.0, 127.5, 125.9, 125.6, 125.5, 125.0, 124.8, 114.3, 113.9, 113.0, 55.2, 55.0; νmax (cm–1, thin film, ATR) 2925 (br), 1602 (s), 1517 (w), 1480 (w), 1443 (w), 1312 (s), 1213 (w), 1147 (s), 1109 (m), 1075 (w), 1075 (w), 999 (w), 958 (m), 877 (w), 768 (s), 762 (s), 744 (s), 733 (s), 700 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C16H15N2O 251.1184, found 251.1173; mp 175.0–177.8 °C (EtOAc) (lit. 170–174 °C). Spectroscopic data are in accordance with the literature.27
3-(2-Phenyl-1H-imidazol-5-yl)-1-tosyl-1H-indole (7)
The title compound was prepared according to general procedure A, using 1-(1-tosyl-1H-indol-3-yl)ethanone (S2) (58.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (17 cm × 20 mm, gradient elution, 0% → 60%, 5% increases, 45 mL runs, 15 mL fractions), yielded 7 as a white solid (33% yield, 44.0 mg, 0.11 mmol): Rf = 0.17 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (600 MHz, DMSO-d6/D2O/TFA) δ 8.54 (s, 1H), 8.30 (s, 1H), 8.10–8.07 (m, 2H), 8.01 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 8.5 Hz, 2H), 7.71–7.68 (m, 3H), 7.49 (t, J = 7.8 Hz, 1H), 7.44–7.39 (m, 3H), 2.30 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6/D2O/TFA) δ 146.6, 144.5, 134.5, 133.8, 132.8, 130.8, 129.9, 127.5, 127.2, 127.0, 126.4, 126.3, 125.6, 124.8, 123.1, 121.0, 117.3, 113.8, 109.8, 21.3; νmax (cm–1, thin film, ATR) 2847 (br), 1594 (w), 1460 (w), 1445 (m), 1396 (w), 1376 (m), 1304 (w), 1279 (w), 1176 (s), 1133 (m), 1113 (m), 1092 (m), 1050 (w), 1024 (w), 985 (m), 966 (w), 903 (w), 817 (w), 746 (s), 709 (s), 688 (s), 660 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C24H20N3O2S 414.1276, found 414.1264; mp 249.0 °C (dec).
4-(2-Phenyl-1H-imidazol-5-yl)pyridine (8)
The title compound was prepared according to general procedure A, using 4-acetylpyridine (68) (47.0 mg, 0.375 mmol, 1.25 equiv) ,benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv) and 48% aq. HBr (300 mol%). Purification by silica gel chromatography, eluting with MeOH in DCM (19 cm × 20 mm, gradient elution, 0% → 6%, 0.5% increases, 30 mL runs, 7 mL fractions), yielded 8 as a yellow solid (56% yield, 37.0 mg, 0.17 mmol): Rf = 0.18 (EtOAc, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.90 (br s, 1H), 8.53 (d, J = 5.0 Hz, 2H), 8.10–7.98 (m, 3H), 7.80 (d, J = 6.0 Hz, 2H), 7.49 (t, J = 7.4 Hz, 2H), 7.39 (t, J = 7.4 Hz, 2H); 13C{1H} NMR (125 MHz, DMSO-d6) δ 150.3, 147.2, 142.1, 139.1, 130.7, 129.3, 129.0, 125.6, 119.3, 117.9; νmax (cm–1, thin film, ATR) 2923, 1601, 1571, 1493, 1458, 1424, 1159, 1093, 999, 950, 821, 838, 780, 774, 712, 705, 694, 685, 677; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C14H12N3 222.1031, found 222.1037; mp 209.5–210.6 °C (lit. 212–214 °C). Spectroscopic data are in accordance with the literature.10
4-(Furan-2-yl)-2-phenyl-1H-imidazole (9)
The title compound was prepared according to general procedure A, using 4-acetylfuran (41.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 35%, 5% increases, 30 mL runs, 7 mL fractions), yielded a yellow oil, which was triturated with 5% DCM/hexanes to yield 9 as a white solid (30% yield, 19.0 mg, 0.09 mmol): Rf = 0.33 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.71 (br s, 1H), 7.97 (d, J = 7.3, 2H), 7.62 (s, 1H), 7.52 (s, 1H), 7.46 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H) 6.59 (s, 1H), 6.53 (s, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 150.3, 146.0, 141.1, 133.9, 130.3, 128.7, 128.3, 125.0, 113.9, 111.4, 103.7; νmax (cm–1, thin film, ATR) 3130 (w), 2739 (w, br), 1560 (w), 1494 (w), 1460 (w), 1407 (w), 1297 (w), 1212 (w), 1160 (m), 1143 (m), 1092 (w), 1068 (w), 1011 (m), 969 (m), 889 (m), 786 (s), 741 (s), 719 (s), 695 (s), 681 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C13H11N2O 211.0871, found 211.0878; mp 145.4–148.7 °C (EtOAc) (lit. 154–156 °C (EtOH)).
5-(3-Nitrophenyl)-2-phenyl-1H-imidazole (10)
The title compound was prepared according to general procedure A, using 3′-nitroacetophenone (63.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 35%, 5% increases, 30 mL runs, 7 mL fractions), yielded 10 as a bright yellow solid (69% yield, 55.0 mg, 0.21 mmol): Rf = 0.17 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.87 (br s, 1H), 8.68 (s, 1H), 8.30 (d, J = 7.7 Hz, 1H), 8.10–8.00 (m, 4H) 7.67 (t, J = 8.0 Hz, 1H), 7.49 (t, J = 7.6 Hz, 2H), 7.40 (t, J = 7.2 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 148.4, 146.5, 138.9, 136.5, 130.5, 130.2, 130.0, 128.8, 128.4, 125.1, 120.7, 118.4, 116.3; νmax (cm–1, thin film, ATR) 3383 (m), 1561 (w), 1541 (w), 1516 (s), 1290 (w), 1118 (m), 1103 (w), 893 (m), 872 (w), 821 (m), 782 (s), 745 (s), 737 (s), 718 (s), 695 (s), 687 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12N3O2 266.0930, found 266.0933; mp 183.7–185.3 °C (EtOAc) (lit. 181.1–183.9 °C). Spectroscopic data are in accordance with the literature.28
5-(4-Nitrophenyl)-2-phenyl-1H-imidazole (11)
The title compound was prepared according to general procedure A, using 4′-nitroacetophenone (63.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 35%, 5% increases, 30 mL runs, 7 mL fractions), yielded 11 as a bright yellow solid (64% yield, 51.0 mg, 0.19 mmol): Rf = 0.20 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.97 (br s, 1H), 8.25 (d, J = 8.8 Hz, 2H), 8.14–8.10 (m, 3H), 8.30 (d, J = 7.8 Hz, 2H), 7.49 (t, J = 7.6 Hz, 2H), 7.40 (t, J = 7.4 Hz, 2H); 13C{1H} NMR (125 MHz, DMSO-d6) δ 147.0, 145.3, 141.4, 139.1, 130.1, 128.8, 125.1, 124.8, 124.1, 118.0; νmax (cm–1, ATR): 3352, 2359, 2344, 1598, 1506, 1489, 1458, 1333, 1178, 1131, 1109, 945, 858, 791, 780, 753, 717, 696; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12N3O2 266.0930, found 266.0929; mp 187.2–188.7 °C (EtOAc) (lit. 190–191 °C), turned brown upon heating. Spectroscopic data are in accordance with the literature.29
4-(2-Phenyl-1H-imidazol-5-yl)benzonitrile (12)
The title compound was prepared according to general procedure A, using 4-acetylbenzonitrile (55.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 60%, 5% increases, 12 × 30 mL runs, then 10% increases, 2 × 30 mL runs, 10 mL fractions), yielded 12 as a yellow solid (68% yield, 50.0 mg, 0.20 mmol): Rf = 0.18 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.88 (br s, 1H), 8.10–7.97 (m, 5H), 7.82 (d, J = 8.2 Hz, 2H), 7.49 (t, J = 7.7 Hz, 2H), 7.39 (t, J = 7.5 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 146.6, 139.4, 139.2, 132.5, 130.2, 128.8, 125.0, 124.8, 119.3, 117.1, 108.0; νmax (cm–1, thin film, ATR) 3294 (m), 2923 (w), 2851 (w), 2539 (w), 2226 (m), 1604 (m), 1539 (w), 1491 (w), 1458 (w), 1416 (w), 1133 (m), 945 (w), 849 (m), 728 (s), 699 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C16H12N3 246.1031, found 246.1032; mp 209.0–211.8 °C (EtOAc)
5-(Naphthalen-2-yl)-2-phenyl-1H-imidazole (13)
The title compound was prepared according to general procedure A, using 2′-acetonaphtone (64.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with MeOH in DCM (18 cm × 15 mm, gradient elution, 0% → 5%, 0.5% increases, 30 mL runs, 7 mL fractions), yielded 13 as a pale yellow solid (67% yield, 54.0 mg, 0.20 mmol): Rf = 0.33 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.73 (br s, 1H), 8.38 (s, 1H), 8.10–8.00 (m, 3H), 7.97–7.85 (m, 4H), 7.54–7.48 (m, 3H), 7.45 (t, J = 7.5 Hz, 1H), 7.38 (t, J = 7.4 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 146.1, 141.0, 133.4, 132.2, 131.9, 130.5, 128.7, 128.2, 127.9, 129.7, 127.6, 126.3, 125.2, 125.0, 123.7, 121.8, 115.0; νmax (cm–1, thin film, ATR) 2850, 1630, 1602, 1572, 1500, 1484, 1464, 1454, 1401, 1263, 1138, 1126, 1070, 891, 859, 820, 792, 784, 748, 693; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C19H15N2 271.1235, found 271.1231; mp 223.9–225.0 °C (MeOH/DCM).
5-(4-Methylphenyl)-2-phenyl-1H-imidazole (14)
The title compound was prepared according to general procedure A, using 4′-methylacetophenone (53.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 25%, 5% increases, 30 mL runs, 7 mL fractions), yielded 14 as a pale yellow solid (65% yield, 46.0 mg, 0.20 mmol): Rf = 0.37 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.60 (br s, 1H), 8.00 (d, J = 7.1 Hz, 2H), 7.80–7.64 (m, 3H), 7.46 (t, J = 7.8 Hz, 2H), 7.35 (t, J = 7.3 Hz, 1H), 7.18 (d, J = 7.0 Hz, 2H), 2.31 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 145.6, 141.2, 135.2, 131.9, 130.7, 129.4, 129.0, 128.7, 128.0, 124.9, 124.4, 113.7, 20.8; νmax (cm–1, thin film, ATR) 2985, 1606, 1576, 1498, 1458, 1399, 1137, 1084, 962, 823, 803, 786, 721, 710, 695; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C16H15N2 235.1235, found 235.1222; mp 172.8–174.0 °C (EtOAc) (lit 179 °C [benzene]), turned violet upon heating. Spectroscopic data are in accordance with the literature30,31
5-(4-Bromophenyl)-2-phenyl-1H-imidazole (15)
The title compound was prepared according to general procedure A, using 4′-bromoacetophenone (75.0 mg, 0.375 mmol, 1.25 equiv) and benzaldehyde (32.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 25%, 5% increases, 30 mL runs, 7 mL fractions), yielded 15 as a pale yellow solid (72% yield, 65.0 mg, 0.22 mmol): Rf = 0.21 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.72 (br s, 1H), 7.99 (d, J = 7.5 Hz, 2H), 7.86–7.80 (m, 3H), 7.55 (d, J = 8.3 Hz, 2 H), 7.47 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H); 13C{1H} NMR (125 MHz, DMSO-d6) δ 146.1, 139.9, 134.0, 131.4, 130.4, 128.8, 128.3, 126.4, 125.0, 118.9, 115.0; νmax (cm–1, thin film, ATR) 2925 (br), 2360 (w), 1602 (s), 1517 (w), 1480 (w), 1443 (w), 1312 (s), 1213 (w), 1147 (s), 1109 (m), 1075 (w), 1075 (w), 999 (w), 958 (m), 877 (w), 768 (s), 762 (s), 744 (s), 733 (s), 700 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12N2Br 299.0184, 301.0164, found 299.0180, 301.0167; mp 169.2–172.5 (EtOAc) (lit. 169–171 °C), turned brown upon heating. Spectroscopic data are in accordance with the literature.28,30
3-(5-Phenyl-1H-imidazol-2-yl)phenol (16)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 3-hydroxybenzaldehyde (37.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 16 as a white yellow solid (85% yield, 60.0 mg, 0.25 mmol): Rf = 0.30 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (400 MHz, MeOD-d4) δ 7.76 (dd, J = 1.1, 8.3 Hz, 2H), 7.44 (s, 1H), 7.41–7.36 (m, 4H), 7.30–7.23 (m, 2H), 6.83 (ddd, J = 1.1, 2.4, 8.0 Hz, 1H); 13C{1H} NMR (126 MHz, MeOD-d4) δ 159.1, 149.0, 132.7, 131.0, 129.7, 128.0, 126.1, 117.9, 116.9, 113.7 (note that, due to slow relaxation, some 13C{1H} NMR signals were difficult to identify;28 concerning this compound, three signals are missing); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H13N2O 237.1028, found 237.1011.
2-(3-Chlorophenyl)-5-phenyl-1H-imidazole (17)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 3-chlorobenzaldehyde (42.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 17 as a pale yellow solid (69% yield, 53.0 mg, 0.21 mmol): Rf = 0.40 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.80 (s, 1H), 8.06 (s, 1H), 7.97 (d, J = 7.5 Hz, 1H), 7. 91–7.76 (m, 3H), 7.50 (t, J = 7.9 Hz, 1H), 7.42 (ddd, J = 8.0, 2.1, 0.9 Hz, 1H), 7.38 (t, J = 7.3 Hz, 2H), 7.22 (t, J = 6.9 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 144.4, 141.4, 133.6, 132.5, 130.7, 128.9, 128.5, 127.8, 127.0, 126.4, 124.4, 123.4, 114.9; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12ClN2 255.0689, 257.0663, found 255.0693, 257.0670. Spectroscopic data are in accordance with the literature.23
2-(4-Nitrophenyl)-5-phenyl-1H-imidazole (18)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-nitrobenzaldehyde (46.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 18 as an orange solid (66% yield, 53.0 mg, 0.21 mmol): Rf = 0.20 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 13.13 (s, 1H), 8.34 (d, J = 8.9 Hz, 2H), 8.23 (d, J = 8.8 Hz, 2H), 7.93 (s, 1H), 7.89 (d, J = 7.6 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.24 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (63 MHz, DMSO-d6) δ 146.5, 143.8, 142.4, 136.3, 134.1, 128.5, 126.7, 125.5, 124.5, 124.3, 116.3; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12N3O2 266.0930, found 266.0933. Spectroscopic data are in accordance with the literature.32
4-(4-Phenyl-1H-imidazol-2-yl)phenol (19)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-hydroxybenzaldehyde (37.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 19 as a yellow solid (50% yield, 36.0 mg, 0.15 mmol): Rf = 0.30 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (250 MHz, DMSO-d6) δ 12.34 (s, 1H), 9.68 (s, 1H), 7.90–7.71 (m, 4H), 7.65 (s, 1H), 7.35 (t, J = 7.6 Hz, 2H), 7.18 (t, J = 7.2 Hz, 1H), 6.84 (d, J = 8.5 Hz, 2H); 13C{1H} NMR (63 MHz, DMSO-d6) δ 157.6, 146.4, 140.5, 134.9, 128.4, 126.5, 126.0, 124.3, 122.0, 115.4, 113.3; νmax (cm–1, thin film, ATR) 3221, 2926, 1773,1701, 1609, 1541, 1496, 1460, 1367, 1275, 1175, 1099, 1029, 948, 908, 837, 761, 738, 694, 661, 635; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H13N2O 237.1028, found 237.1013; mp 227 °C (dec).
2-(3-Chlorophenyl)-5-phenyl-1H-imidazole (20)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-chlorobenzaldehyde (42.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 20 as a pale yellow solid (72% yield, 55.0 mg, 0.22 mmol): Rf = 0.40 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.74 (s, 1H), 8.01 (d, J = 8.5 Hz, 2H), 7.86 (d, J = 7.4 Hz, 2H), 7.79 (d, J = 1.8 Hz, 1H), 7.54 (d, J = 8.5 Hz, 2H), 7.37 (t, J = 7.7 Hz, 2H), 7.21 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 144.8, 141.3, 134.5, 132.6, 129.4, 128.8, 128.4, 126.5, 126.3, 124.4, 114.7; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12ClN2 255.0689, 257.0663, found 255.0694, 257.0677. Spectroscopic data are in accordance with the literature.32
2-(3-Nitrophenyl)-5-phenyl-1H-imidazole (21)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 3-nitrobenzaldehyde (46.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 21 as a yellow solid (72% yield, 53.0 mg, 0.21 mmol): Rf = 0.20 (30% EtOAc/hexane, Dragendorff stain); 1H NMR (250 MHz, DMSO-d6) δ 13.06 (s, 1H), 8.84 (s, 1H), 8.43 (d, J = 7.8 Hz, 1H), 8.20 (dd, J = 8.1, 2.1 Hz, 1H), 7.93–7.91 (m, 4H), 7.39 (t, J = 7.5 Hz, 2H), 7.24 (t, J = 7.0 Hz, 1H); 13C{1H} NMR (63 MHz, DMSO-d6) δ 148.4, 143.8, 141.7, 134.2, 132.1, 130.9, 130.5, 128.5, 126.5, 124.5, 122.5, 119.2, 115.4; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12N3O2 266.0930, found 266.0950.
2-(4-Methoxyphenyl)-5-phenyl-1H-imidazole (22)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and p-anisaldehyde (41.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 40%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 22 as a white solid (50% yield, 38.0 mg, 0.15 mmol): Rf = 0.30 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (400 MHz, DMSO-d6/D2O/TFA) δ 7.99 (s, 1H), 7.95 (d, J = 8.8 Hz, 2H), 7.80 (d, J = 7.6 Hz, 2H), 7.50 (t, J = 7.5 Hz, 2H), 7.43 (t, J = 7.2 Hz, 1H), 7.16 (d, J = 8.8 Hz, 2H), 3.82 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6/D2O/TFA) δ 163.0, 145.3, 133.9, 130.1, 130.0, 129.8, 127.2, 126.4, 116.3, 115.7, 115.6, 56.3; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C16H15N2O 251.1184, found 251.1186.
2-(2-Chlorophenyl)-5-phenyl-1H-imidazole (23)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 2-chlorobenzaldehyde (42.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 23 as a white solid (63% yield, 48.0 mg, 0.19 mmol): Rf = 0.50 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.43 (s, 1H), 7.78 (d, J = 2.0 Hz, 1H), 7.88–7.84 (m, 3H), 7.58 (dd, J = 1.9, 7.2 Hz, 1H), 7.48–7.43 (m, 2H), 7.37 (t, J = 7.6 Hz, 2H), 7.21 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 143.3, 140.8, 134.5, 131.2, 130.8, 130.2, 130.0, 129.9, 128.4, 127.3, 126.3, 124.4, 114.5; νmax (cm–1, thin film, ATR) 3059, 1708, 1607, 1567, 1482, 1453, 1111, 1086, 1049, 946, 694; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12ClN2 255.0689, 257.0663, found 255.0690, 257.0670; mp 161.0–162.0 °C (EtOAc).
2-(4-Fluorophenyl)-5-phenyl-1H-imidazole (24)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-fluorobenzaldehyde (38.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 24 as a pale yellow solid (57% yield, 41.0 mg, 0.17 mmol): Rf = 0.30 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.65 (s, 1H), 8.04 (dd, J = 8.6, 5.5 Hz, 2H), 7.86 (d, J = 7.4 Hz, 2H), 7.76 (s, 1H), 7.37 (t, J = 7.6 Hz, 2H), 7.32 (t, J = 8.8 Hz, 2H), 7.21 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 162.0 (d, 1JCF = 245.1 Hz), 145.1, 141.1, 134.6, 128.5, 127.30 (d, 4JCF = 2.5 Hz, 1C), 127.04 (d, 3JCF = 8.4 Hz), 126.2, 124.4, 115.74 (d, 2JCF = 22.1 Hz), 114.3; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12FN2 239.0984, found 239.0985. Spectroscopic data are in accordance with the literature.33
N,N-Dimethyl-4-(5-phenyl-1H-imidazol-2-yl)aniline (25)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-(dimethylamino)benzaldehyde (46.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 30% → 50%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 25 as a yellow solid (23% yield, 18.0 mg, 0.07 mmol): Rf = 0.40 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (400 MHz, DMSO-d6/D2O/TFA) δ 7.88 (s, 1H), 7.83 (d, J = 9.1 Hz, 2H), 7.77 (d, J = 7.1 Hz, 1H), 7.49 (t, J = 7.5 Hz, 2H), 7.42 (t, J = 7.4 Hz, 1H), 6.85 (d, J = 9.2 Hz, 2H), 2.98 (s, 6H); 13C{1H} NMR (101 MHz, DMSO-d6/D2O/TFA) δ 153.1, 146.4, 133.3, 130.0, 129.0, 127.5, 126.3, 115.7, 112.7, 109.5, 40.4; νmax (cm–1, thin film, ATR) 2919, 2850, 1615, 1545, 1500, 1443, 1396, 1363, 1227, 1202, 1170, 945, 820, 760, 738, 695; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C17H18N3 264,1501, found 264,1502; mp 142.0–145.0 °C (EtOAc).
2-(Furan-2-yl)-5-phenyl-1H-imidazole (26)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and furfural (29.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 20%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 26 as a white solid (34% yield, 22.0 mg, 0.11 mmol): Rf = 0.10 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (400 MHz, DMSO-d6/D2O/TFA) δ 8.00 (s, 1H), 7.99 (s, 1H), 7.78 (d, J = 7.5 Hz, 2H), 7.50 (t, J = 7.5 Hz, 2H), 7.46–7.41 (m, 2H), 6.80 (dd, J = 3.3, 1.5 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6/D2O/TFA) δ 147.8, 138.8, 136.7, 134.0, 130.3, 130.1, 127.1, 126.3, 116.4, 115.5, 113.9; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C13H11N2O 211.0871, found 211.0871. Spectroscopic data are in accordance with the literature34
2-(4-Bromophenyl)-5-phenyl-1H-imidazole (27)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-bromobenzaldehyde (56.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 30%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 27 as a white solid (61% yield, 55.0 mg, 0.18 mmol): Rf = 0.20 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.75 (s, 1H), 7.94 (d, J = 8.5 Hz, 2H), 7.86 (d, J = 7.2 Hz, 2H), 7.79 (s, 1H), 7.67 (d, J = 8.5 Hz, 2H), 7.37 (t, J = 7.7 Hz, 2H), 7.21 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 144.8, 141.3, 134.5, 131.7, 129.8, 128.5, 126.9, 126.3, 124.4, 121.2, 114.7; νmax (cm–1, thin film, ATR) 3069, 1703, 1603, 1486, 1466, 1452, 1431, 1364, 1298, 1269, 1228, 1143, 1085, 1971, 1010, 949, 911, 830, 729, 694; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H12BrN2 299.0184, 301.0164, found 299.0186, 301.0171; mp 196.0–198.0 °C (EtOAc).
2-Cyclohexyl-5-phenyl-1H-imidazole (28)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and cyclohexanecarboxaldehyde (34.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 40%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 28 as a white solid (83% yield, 56.0 mg, 0.25 mmol): Rf = 0.20 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, CDCl3) δ 8.35 (br s, 1H), 7.66 (d, J = 7.3 Hz, 2H), 7.32 (t, J = 7.7 Hz, 2H), 7.22–7.17 (m, 2H), 2.74 (tt, J = 12.0, 3.5 Hz, 1H), 1.98 (d, J = 11.7 Hz, 2H), 1.75 (d, J = 13.2 Hz, 2H), 1.66 (d, J = 12.6 Hz, 1H), 1.50 (dq, J = 12.4, 3.1 Hz, 2H), 1.32–1.12 (m, 3H); 13C{1H} NMR (126 MHz, CDCl3) δ 153.9, 137.1, 133.1, 128.7, 126.7, 124.9, 115.7, 38.1, 32.2, 26.2, 25.9; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C15H19N2 227.1548, found 227.1558. Spectroscopic data are in accordance with the literature.33−35
4-(5-Phenyl-1H-imidazol-2-yl)pyridine (29)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and 4-pyridinecarboxaldehyde (33.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 50% → 100%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 29 as a pale yellow solid (40% yield, 26.5 mg, 0.12 mmol): Rf = 0.01 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (400 MHz, DMSO-d6/D2O/TFA) δ 8.81 (d, J = 6.6 Hz, 2H), 8.40 (d, J = 6.6 Hz, 2H), 8.04 (s, 1H), 7.85 (d, J = 7.6 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.34 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6/D2O/TFA) δ 143.5, 143.2, 142.0, 141.6, 131.4, 129.8, 129.1, 125.9, 122.2, 122.0; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C14H12N3 222.1031, found 222.1030. Spectroscopic data are in accordance with the literature.23
5-Phenyl-2-propyl-1H-imidazole (30)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and butyraldehyde (23.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 30% → 70%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 30 as a white solid (36% yield, 20.0 mg, 0.11 mmol): Rf = 0.10 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (500 MHz, CDCl3) δ 7.84 (s, 1H), 7.67 (d, J = 7.4 Hz, 2,H), 7.33 (t, J = 7.7 Hz, 2H), 7.23–7.17 (m, 2H), 2.68 (t, J = 7.6 Hz, 2H), 1.70 (sx, J = 7.5 Hz, 2H), 0.89 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (126 MHz, CDCl3) δ 149.7, 137.6, 133.1, 128.8, 126.8, 124.8, 115.5, 30.6, 22.2, 13.9; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C12H15N2 187.1235, found 187.1243. Spectroscopic data are in accordance with the literature.36,37
2-Cyclopropyl-5-phenyl-1H-imidazole (31)
The title compound was prepared according to general procedure B, using acetophenone (46.0 mg, 0.375 mmol, 1.25 equiv) and cyclopropanecarboxaldehyde (23.0 mg, 0.30 mmol, 1.00 equiv). Purification by silica gel chromatography, eluting with EtOAc in hexanes (19 cm × 20 mm, gradient elution, 0% → 40%, 5% increases, 50 mL runs, 5–10 mL fractions), yielded 31 as a white solid (70% yield, 39.0 mg, 0.21 mmol): Rf = 0.10 (30% EtOAc/hexane, UV, Dragendorff stain); 1H NMR (400 MHz, DMSO-d6/D2O/TFA) δ 7.76 (s, 1H), 7.67 (d, J = 7.4 Hz, 2H), 7.46 (t, J = 7.5 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H), 2.30–2.20 (m, 1H), 1.28–1.20 (m, 2H), 1.18–1.11 (m, 2H); 13C{1H} NMR (101 MHz, DMSO-d6/D2O/TFA) δ 150.9, 132.5, 130.0, 129.9, 127.3, 125.9, 114.7, 9.7, 7.5; νmax (cm–1, thin film, ATR) 3034, 2910, 1606, 1566, 1545, 1524, 1483, 1451, 1425, 1313, 1166, 1135, 1090, 1027, 1005, 881, 756, 727, 693; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C12H13N2 185.1079, found 185.1080; mp 160.0–162.0 °C (EtOAc).
4-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (32)
A 10 mL round-bottom flask was charged with 4-acetylpyridine (68) (219 mg, 1.70 mmol, 1.70 equiv), a magnetic stir bar, and DMSO (3.5 mL, 0.5 M) under air, and concentrated aq HBr (48% w/w, 8.9 M) (595 mL, 5.25 mmol, 3.0 equiv) was added dropwise. The reaction mixture was stirred in a preheated oil bath at 60 °C for 8 h. After consumption of the starting material, indicated by TLC analysis (EtOAc, p-ASD), the reaction mixture was left to reach room temperature and MeOH (5.7 mL, 0.19 M) was added. This reaction mixture was added dropwise over 30 min via syringe to a solution of 2-methyl-4-(methylsulfonyl)benzaldehyde (S5) (198 mg, 1.00 mmol, 1.00 equiv) and NH4OAc (771 mg, 10.0 mmol, 10.0 equiv) in MeOH (5 mL, 0.2 M in relation to S5) at room temperature. The reaction mixture was stirred at room temperature for 18 h, and the solvent was removed in the rotaevaporator; the residue was diluted with 10% MeOH/DCM (10 mL) and poured into a separatory funnel containing saturated NaHCO3 (1 × 40 mL) and 10% MeOH/DCM (1 × 15 mL). The phases were separated, and the aqueous phase was extracted with 10% MeOH/DCM (7 × 10 mL). The organic phases were combined, dried over MgSO4, filtered, and concentrated in the rotaevaporator. Purification by silica gel chromatography, eluting with MeOH in DCM (gradient elution 5% → 9%), yielded 31 as a pale yellow solid (67% yield, 210 mg, 0.67 mmol): Rf = 0.17 (EtOAc, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.92 (br s, 1H), 8.54 (d, J = 5.9 Hz, 2H), 8.14 (s, 1H), 7.94–7.90 (m, 2H), 7.85 (d, J = 8.8 Hz, 1H), 7.81 (d, J = 5.9 Hz, 2H), 3.26 (s, 3H), 2.75 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 149.9, 145.4, 141.5, 140.0, 138.8, 137.6, 134.1, 129.6, 128.9, 124.4, 118.8, 117.7, 43.5, 21.4; νmax (cm–1, ATR): 2673, 1607, 1302, 1150, 1106, 1077, 1004, 965, 950, 828, 763, 739, 709, 690; HRMS (ESI+/TOF) m/z [M + H]+ calcd for C16H16N3O2S 314.0963, found 314.0938; mp 225.4–227.3 °C (MeOH/DCM).
tert-Butyl 4-(5-(Pyridin-4-yl)-1H-imidazol-2-yl)piperidine-1-carboxylate (36)
A 50 mL round-bottom flask was charged with 4-acetylpyridine (68) (645 mg, 5.16 mmol, 1.75 equiv), a magnetic stir bar, and DMSO (10.8 mL, 0.5 M) under air, and concentrated aq HBr (48% w/w, 8.9 M) (1.75 mL, 15.5 mmol, 3.0 equiv) was added dropwise. The reaction mixture was stirred in a preheated oil bath at 60 °C for 4 h. After consumption of the starting material, indicated by TLC analysis (EtOAc, p-ASD), the reaction mixture was left to reach room temperature and MeOH (18.3 mL, 0.18 M relative to 4-acetylpyridine) was added. This reaction mixture was added dropwise over 30 min via syringe to a solution of 1-(tert-butoxycarbonyl)-4-piperidinecarboxaldehyde (35) (629 mg, 2.95 mmol, 1.00 equiv) and NH4OH (6.4 mL, 44.3 mmol, 15.0 equiv) in MeOH (14.8 mL, 0.2 M in relation to 35) at room temperature. The reaction mixture was stirred at room temperature for 4 h and poured into a separatory funnel containing saturated NaHCO3 (1 × 40 mL) and EtOAc (1 × 40 mL). The phases were separated, and the aqueous phase was extracted with EtOAc (3 × 40 mL). The organic phases were combined, dried over Na2SO4, filtered, and concentrated in the rotaevaporator. Purification by silica gel chromatography, eluting with EtOH/EtOAc/NH4OH/hexane (11:34:5:50) (18 cm × 40 mm, isocratic elution, (11:34:5:50) EtOH/EtOAc/NH4OH/hexane, 1 L run, 20 mL fractions), yielded 36 as a white solid (82% yield, 793 mg, 2.40 mmol): Rf = 0.40 (EtOH/EtOAc/NH4OH/hexane (11:34:5:50), UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 12.10 (br s, 1H), 8.46 (d, J = 6.0 Hz, 2H), 7.77 (br s, 1H), 7.66 (d, J = 6.0 Hz, 2H), 3.99 (d, J = 12.4 Hz, 2H), 2.96–2.78 (m, 3H), 1.90 (dd, J = 13.0, 2.3 Hz, 2H), 1.59 (dq, J = 12.3, 3.9 Hz, 2H), 1.41 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 153.9, 152.1, 149.7, 118.5, 78.6, 43.2, 35.2, 30.4, 28.1 (note that, due to slow relaxation, some 13C{1H} NMR signals were not identified in the spectra;28 specifically, the 13C{1H} NMR data for compound 36 lacks three of the 12 expected signals); νmax (cm–1, thin film, ATR) 2867 (br), 1690 (s), 1603 (s), 1553 (w), 1429 (m), 1363 (w), 1285 (w), 1248 (w), 1230 (w), 1212 (w), 1173 (s), 1151 (m), 1126 (m), 1038 (w), 1004 (m), 942 (w), 876 (w), 766 (s), 720 (w), 686 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C18H25N4O2 329.1978, found 329.1964; mp 215.0 °C (dec)
1-(4-Amino-3-bromophenyl)ethenone (43)
A 6 mL vial was charged with the 4′-aminoacetophenone (51.0 mg, 0.375 mmol, 1.00 equiv), DMSO (0.75 mL, 0.5 M), concentrated aqueous HBr (48% w/w, 8.9 M) (47 μL, 0.41 mmol, 110 mol %), deionized water (47 μL), and a magnetic stir bar under air. The reaction mixture was stirred in a preheated aluminum block at 85 °C and was followed by TLC analysis (30% EtOAc/hexane, p-ASD). The reaction mixture was poured directly into a separatory funnel containing a mixture of saturated NaHCO3 and saturated Na2S2O3 (1:1, 1 × 20 mL) and EtOAc (10 mL). The phases were separated, and the aqueous phase was extracted with EtOAc (5 × 5 mL). The organic phases were combined, washed with saturated NaCl solution (1 × 5 mL), dried over Na2SO4, filtered,, and concentrated in the rotaevaporator. Purification by silica gel chromatography, eluting with EtOAc in hexanes (18 cm × 15 mm, gradient elution, 0% → 35%, 5% increases, 30 mL runs, 10 mL fractions), yielded 33 as a pale yellow solid (99% yield, 64.0 mg, 0.30 mmol): Rf = 0.53 (30% EtOAc/hexane, p-ASD); 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J = 1.8 Hz, 1H), 7.73 (dd, J = 8.4, 1.8 Hz, 1H), 6.74 (d, J = 8.4 Hz, 1H), 4.60 (br s, 2H), 2.49 (s, 3H); 13C{1H} NMR (126 MHz, CDCl3) δ 195.5, 148.5, 133.9, 129.5, 128.9, 114.3, 108.3, 26.2. Spectroscopic data are in accordance with the literature.38
4-(4-Bromo-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (44)
Following a modified literature procedure,24 a 25 mL round-bottom flask was charged with 32 (595 mg, 1.90 mmol, 1.00 equiv), dry DCM (8.4 mL), dry pyridine (2.1 mL),and a magnetic stir bar under an inert atmosphere. The RBF was covered with aluminum foil, and the reaction mixture was cooled to 0 °C in an ice/water bath and stirred for 15 min. Solid Py·HBr·Br2 (pyridinium hydrobromide perbromide, 743 mg, 2.09 mmol, 1.10 equiv) was added in portions, by briefly removing the Suba seal, and the reaction mixture was stirred at 0 °C for 1 h. After consumption of the starting material, indicated by TLC analysis (100% EtOAc, Dragendorff), the solvent was removed in the rotaevaporator. The residue was partitioned between 1 M aq NaHSO3 (1 × 75 mL) and 10% MeOH/DCM (1 × 60 mL). The phases were separated, and the aqueous layer was extracted with 10% MeOH/DCM (3 × 60 mL). The organic phases were combined, dried over MgSO4, filtered, and concentrated in the rotaevaporator. The residue was triturated with hexanes, filtered, and washed with hexanes until all pyridine was removed, indicated by TLC analysis, and dried in vacuo to afford 44 as a yellow solid (96% yield, 716 mg, 1.83 mmol): Rf = 0.47 (EtOAc, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 13.32 (s, 1H), 8.70 (s, 2H), 8.00–7.79 (m, 5H), 3.28 (s, 3H), 2.65 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 150.5, 146.7, 141.3, 138.6, 136.1, 133.7, 130.4, 129.9, 126.6, 124.8, 120.8, 116.0, 43.9, 21.3; νmax (cm–1, thin film, ATR) 2765 (br), 1606 (s), 1573 (w), 1533 (w), 1491 (w), 1448 (w), 1422 (w), 1301 (s), 1222 (w), 1205 (w), 1150 (s), 1105 (m), 1077 (m), 1004 (m), 986 (w), 964 (m), 950 (m), 892 (w), 875 (w), 828 (s), 762 (s), 739 (m), 708 (w), 699 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C16H15BrN3O2S 392.0068, 394.0049, found 392.0053, 394.0034; mp 225.0 °C (dec), turned brown at 210.0 °C.
Suzuki–Miyaura Cross-Coupling: General Procedure C
A culture tube (13 mm × 100 mm, 9 mL) was charged with the corresponding bromo-imidazole (0.10 mmol, 1.00 equiv), corresponding boronic ester or boronic acid (0.125 mmol, 1.25 equiv) and a magnetic stir bar under inert atmosphere. Then, degassed DME (0.5 mL) was added followed by addition of a premixed solution of Pd(OAc)2 (10 mol %) and Aphos (24 mol %) in degassed DME (0.25 mL). The reaction mixture was stirred for 5 min at room temperature and then 1.2 M aqueous K2CO3 (0.25 mL, 3.00 equiv) degassed solution was added and the mixture was stirred for additional 5 min. After this time, the reaction mixture was stirred in a preheated aluminum block at 80 °C for 18 h. After consumption of the starting material, indicated by TLC analysis (7% EtOH/CHCl3, Dragendorff), the reaction mixture was allowed to reach room temperature and it was diluted with 10% MeOH/DCM (∼7 mL), filtered through a pad (20 mm diameter) composed of Celite (top, 1 cm) and silica gel (bottom, 3 cm). The pad was washed with 10% MeOH/DCM (25–50 mL) and the filtrate was concentrated under in the reduced pressure. The crude product was adsorbed over basic alumina and purification was performed by silica gel column chromatography.
4-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-4-phenyl-1H-imidazol-5-yl)pyridine (46)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), phenylboronic acid (15.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 4%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using EtOAc (4 cm × 30 mm, isocratic elution, 100% EtOAc, 150 mL run, 10 mL fractions), yielded 46 as a white solid (71% yield, 28.0 mg, 0.07 mmol): 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.66 (d, J = 7.0 Hz, 2H), 8.05 (d, J = 7.0 Hz, 2H), 7.99 (d, J = 8.2 Hz 1H), 7.93 (d, J = 1.1 Hz, 2H), 7.87 (dd, J = 8.2, 1.5 Hz, 1H), 7.66–7.61 (m, 2H), 7.60–7.55 (m, 2H), 3.24 (s, 3H), 2.75 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.9, 146.8, 141.5, 141.1, 138.9, 137.3, 133.5, 131.5, 130.3, 130.2, 129.9, 129.8, 129.5, 129.5, 124.8, 122.4, 43.8, 21.5; νmax (cm–1, thin film, ATR) 3084 (br), 2928 (w), 1601 (s), 1501 (w), 1486 (w), 1444 (w), 1327 (m), 1303 (s), 1214 (w), 1147 (s), 1108 (m), 1074 (m), 999 (w), 962 (m), 951 (m), 879 (w), 832 (s), 777 (m), 762 (s), 742 (s), 702 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C22H20N3O2S 390.1276, found 390.1273; mp 265.0 °C (dec).
4-(4-(4-(Benzyloxy)phenyl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (47)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 4-benzyloxyphenylboronic acid (29.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 47 as a pale yellow solid (69% yield, 34.0 mg, 0.07 mmol): 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.57 (d, J = 7.0 Hz, 2H), 8.04 (d, J = 7.0 Hz, 1H), 7.93–7.88 (m, 2H), 7.84 (dd, J = 8.1, 1.6 Hz, 1H), 7.52 (d, J = 8.7 Hz, 2H), 7.44 (d, J = 7.5 Hz, 2H), 7.38 (t, J = 7.4 Hz, 2H), 7.32 (t, J = 7.2 Hz, 1H), 7.16 (d, J = 8.8 Hz, 2H), 5.14 (s, 2H), 3.20 (s, 3H), 2.67 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 160.3, 151.0, 146.9, 141.7, 141.5, 139.5, 137.6, 137.3, 133.6, 131.4, 131.0, 130.8, 130.2, 129.4, 129.0, 128.6, 125.3, 122.9, 121.6, 116.5, 70.3, 44.1, 21.5; νmax (cm–1, thin film, ATR) 3041 (w), 2921 (w), 1732 (w), 1605 (s), 1513 (m), 1488 (w), 1469 (w), 1445 (w), 1303 (m), 1289 (m), 1243 (m), 1151 (s), 1143 (s), 1072 (w), 974 (m), 831 (s), 808 (w), 767 (s), 742 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C29H26N3O3S 496.1695 466.1589, found 496.1688; mp 245.0–248.5 °C (MeOH/DCM), turned brown upon heating
4-(4-(4-(Methoxymethoxy)phenyl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (48)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 4-(methoxymethoxy)phenyl boronic acid (23.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20.0 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 48 as a white solid (73% yield, 33.0 mg, 0.07 mmol): 1H NMR (500 MHz, DMSO-d6/D2O) δ 8.38 (d, J = 4.7 Hz, 2H), 7.89–7.83 (m, 2H), 7.80 (d, J = 8.53, 1H), 7.50 (d, J = 4.7 Hz, 2H), 7.42 (d, J = 8.53, 2H) 7.10 (d, J = 8.15 Hz, 2H), 5.20 (s, 2H), 3.37 (s, 3H), 3.19 (s, 3H), 2.66 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O) δ 157.8, 150.0, 145.7, 143.2, 140.6, 139.0, 134.9, 134.4, 131.9, 130.9, 130.5, 130.1, 125.1, 123.8, 121.8, 117.4, 94.5, 56.6, 44.2, 21.6 (note that extra signals in the 13C{1H} NMR spectra are due to the presence of tautomers); νmax (cm–1, thin film, ATR) 2925 (w), 1600 (s), 1513 (m), 1491 (w), 1444 (w), 1309 (m), 1238 (m), 1214 (w), 1200 (w), 1143 (s), 1108 (m), 1000 (m), 970 (s), 955 (m), 918 (w), 834 (s), 761 (s), 741 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C24H24N3O4S 450.1487, found 450.1467; mp 225.0–226.4 °C (MeOH/DCM).
3-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)benzenesulfonamide (49)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), (3-aminosulfonylphenyl)boronic acid (26.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by preparative TLC, eluting with EtOH in CHCl3 (20 cm × 20 cm plate, 10% EtOH/CHCl3, two runs), yielded 49 as a white solid (62% yield, 29.0 mg, 0.06 mmol): 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.66 (d, J = 6.2 Hz, 2H), 8.06–8.01 (m, 3H), 7.99–7.90 (m, 3H), 7.89–7.84 (m, 2H), 7.75 (t, J = 7.7, 1H), 3.23 (s, 3H), 2.73 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.5, 147.5, 145.2, 141.8, 141.3, 139.1, 136.0, 133.6, 133.0, 131.8, 130.8, 130.6, 130.4, 130.0, 127.1, 126.5, 125.0, 123.0, 43.9, 21.5; νmax (cm–1, thin film, ATR) 3296 (br), 2931 (w), 1606 (m), 1479 (w), 1410 (w), 1342 (m), 1303 (m), 1205 (w), 1161 (s), 1156 (s), 1118 (w), 1079 (w), 1108 (w), 976 (w), 859 (w), 833 (m), 806 (w), 764 (m), 746 (m), 690 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C22H21N4O4S2 469.1004, found 469.0997; mp 234.0–236.2 °C (EtOH/CHCl3).
N-Cyclopropyl-3-(2-(2-methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)benzenesulfonamide (50)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 3-(cyclopropylsulfamoyl)phenylboronic acid (31.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 3% → 8%, 0.5% increases, 20 mL runs, 3–4 mL fractions), yielded 50 as a white solid (57% yield, 29.0 mg, 0.06 mmol): 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.63 (d, J = 6.8 Hz, 2H), 8.02 (d, J = 6.8 Hz, 2H), 7.97–7.84 (m, 6H), 7.79 (t, J = 7.7 1H), 3.21 (s, 3H), 2.70 (s, 3H). 2.15–2.09 (m, 1H), 0.51–0.45 (m, 2H), 0.41–0.36 (m, 2H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.6, 147.7, 141.9, 141.7, 141.5, 139.4, 136.1, 133.8, 133.7, 131.9, 131.3, 130.9, 130.8, 130.1, 128.6, 127.7, 125.2, 123.4, 44.1, 24.7, 21.5, 5.9; νmax (cm–1, thin film, ATR) 3077 (br), 2925 (w), 2835 (w), 1608 (m), 1539 (w), 1475 (w), 1413 (w), 1334 (m), 1318 (m), 1222 (w), 1161 (s), 1119 (w), 1103 (w), 1030 (w), 1008 (w), 961 (m), 890 (w), 836 (m), 765 (w), 695 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C25H25N4O4S2 509.1317, found 509.1317; mp 212.7–215.7 °C (EtOH/CHCl3).
1-Methyl-5-(2-(2-methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)-1H-indole (51)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), N-methylindole-5-boronic acid (22.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 41 as a white solid (56% yield, 25.0 mg, 0.06 mmol): Rf = 0.45 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (400 MHz, DMSO-d6/D2O/TFA) δ 8.56 (d, J = 7.0 Hz, 2H), 8.04 (d, J = 7.0 Hz, 2H), 7.97 (d, J = 8.2 Hz, 1H), 7.92 (d, J = 1.4 Hz, 1H), 7.86 (dd, J = 1.6, 8.2 Hz, 1H), 7.83 (d, J = 1.2 Hz, 1H), 7.60 (d, J = 8.5 Hz, 1H), 7.41 (d, J = 3.0 Hz, 1H), 7.34 (dd, J = 8.5, 1.5 Hz, 1H), 6.54 (d, J = 3.0 Hz, 1H), 3.82 (s, 3H), 3.22 (s, 1H), 2.73 (s, 3H) (note that the signal at δ 8.09 ppm corresponds to residual CHCl3 in the sample); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 151.1, 146.5, 141.5, 141.3, 139.5, 139.2, 137.5, 133.5, 131.8, 130.8, 130.6, 130.1, 129.0, 125.1, 122.5, 122.4, 122.0, 119.6, 111.4, 44.0, 33.2, 21.5 (note that signal at δ 79.5 ppm corresponds to residual CHCl3 in the sample and one carbon signal missing in the spectra); νmax (cm–1, thin film, ATR) 2914 (w), 2683 (br), 1603 (s), 1507 (w), 1485 (w), 1441 (w), 1430 (w), 1378 (w), 1309 (s), 1286 (w), 1243 (w), 1210 (w), 1154 (s), 1112 (m), 1090 (m), 1071 (w), 1003 (w), 964 (m), 951 (m), 893 (w), 832 (s), 815 (w), 763 (m), 741 (m), 730 (m), 701 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C25H23N4O2S 443.1542, found 443.1529; mp 294.0 °C (dec).
4-(4-(Benzofuran-5-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (52)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), benzofuran-5-boronic acid (21.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 52 as a white solid (59% yield, 26.0 mg, 0.06 mmol): Rf = 0.45 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.57 (d, J = 6.9 Hz, 1H), 8.04–8.00 (m, 3H), 7.95 (d, J = 8.2 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.86 (dd, J = 8.5, 1.4 Hz, 1H), 7.76 (d, J = 8.5 Hz, 1H), 7.52 (dd, J = 8.4, 1.8 Hz, 1H), 7.03 (d, J = 1.4 Hz, 1H), 3.21 (s, 3H), 2.72 (s, 3H) (note that the signal at δ 8.09 ppm corresponds to residual CHCl3 in the sample); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 155.6, 151.0, 147.9, 146.9, 141.6, 141.4, 139.4, 138.0, 133.7, 131.4, 130.7, 130.1, 128.9, 126.2, 125.2, 124.3, 123.1, 122.8, 113.1, 107.8, 44.1, 21.6 (note that the signal at δ 79.5 ppm corresponds to residual CHCl3 in the sample); νmax (cm–1, thin film, ATR) 2925 (w), 1601 (s), 1457 (w), 1444 (w), 1307 (m), 1210 (w), 1196 (w), 1150 (s), 1107 (m), 1086 (w), 1070 (w), 956 (m), 869 (w), 833 (m), 763 (s), 743 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C24H20N3O3S 430.1225, found 430.1207; mp 232.0–233.4 °C (MeOH/DCM).
4-(4-(Benzo[b]thiophen-5-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (53)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 2-(benzo[b]thiophen-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane39 (S6) (33.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 53 as a yellow solid (34% yield, 15.0 mg, 0.03 mmol): Rf = 0.45 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.56 (d, J = 6.9 Hz, 2H), 8.16–8.12 (m, 2H), 8.04 (d, J = 6.9 Hz, 2H), 7.95 (d, J = 8.2 Hz, 1H), 7.85 (dd, J = 8.1, 1.6 Hz, 1H) 7.81 (d, J = 5.4 Hz, 1H), 7.54 (dd, J = 8.5, 1.6 Hz, 1H), 7.52 (d, J = 5.4 Hz, 1H), 3.21 (s, 3H), 2.71 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 151.1, 147.2, 141.7, 141.4, 141.3, 140.8, 139.5, 137.9, 133.8, 131.5, 130.8, 130.2, 129.9, 125.6, 125.5, 125.3, 125.1, 125.0, 124.5, 123.0, 44.1, 21.6; νmax (cm–1, thin film, ATR) 2919 (w), 2853 (w), 1602 (s), 1488 (w), 1434 (m), 1427 (w), 1304 (s), 1213 (w), 1201 (w), 1142 (s), 1103 (m), 1072 (w), 1049 (w), 992 (w), 975 (m), 955 (m), 835 (m), 816 (m), 766 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C24H20N3O2S2 446.0991, found 446.0985; mp 274.0 °C (dec).
4-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-5-(pyridin--4-yl)-1H-imidazol-4-yl)quinoline (54)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), quinoline-6-boronic acid (22.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 8%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 8%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 54 as a white solid (52% yield, 23.0 mg, 0.05 mmol): Rf = 0.42 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 9.22 (d, J = 5.1 Hz, 1H), 9.12 (d, J = 8.3 Hz, 1H), 8.62–8.56 (m, 3H), 8.33 (d, J = 8.8 Hz, 1H), 8.27 (dd, J = 8.9, 1.4 Hz, 1H), 8.11–8.05 (m, 3H), 7.96 (d, J = 8.2 Hz, 1H), 7.92 (s, 1H), 7.87 (d, J = 8.1 Hz, 1H), 3.21 (s, 3H), 2.72 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.4, 148.3, 147.2, 146.9, 142.0, 141.6, 139.6, 139.1, 135.8, 135.3, 133.9, 132.4, 131.3, 130.9, 130.3, 130.0, 129.6, 125.3, 123.8, 123.6, 123.4, 44.2, 21.6; νmax (cm–1, thin film, ATR) 1729 (w), 1598 (m), 1510 (w), 1490 (w), 1304 (m), 1141 (s), 1103 (w), 1073 (w), 954 (m), 883 (w), 836 (m), 765 (m), 743 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C25H21N4O2S441.1385, found 441.1372; mp 225.0–227.0 °C (dec), turned brown at 160.0 °C.
6-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-4-(naphthalen-2-yl)-1H-imidazol-5-yl)pyridine (55)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 2-naphthaleneboronic acid (22.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 55 as a white solid (69% yield, 31.0 mg, 0.07 mmol): Rf = 0.42 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.59 (d, J = 6.9 Hz, 2H), 8.21 (s, 1H), 8.09–8.04 (m, 3H), 8.02–7.95 (m, 3H), 7.92 (s, 1H), 7.87 (dd, J = 8.1, 1.2 Hz, 1H), 7.66 (dd, J = 8.4, 1.6 Hz, 1H), 7.64–7.57 (m, 2H), 3.22 (s, 3H), 2.74 (s, 3H) (note that the signal at δ 8.09 ppm corresponds to residual CHCl3 in the sample); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 151.1, 147.3, 141.7, 141.3, 139.3, 137.5, 133.8, 133.8, 133.5, 131.8, 130.7, 130.1, 129.8, 129.2, 129.0, 128.5, 128.2, 127.9, 126.9, 126.8, 125.1, 123.0, 44.1, 21.6 (note that the signal at δ 79.5 ppm corresponds to residual CHCl3 in the sample); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C26H22N3O2S 440.1433, found 440.1418.
6-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)naphthalen-2-ol (56)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)naphthalen-2-ol (S7) (34.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 4% → 9%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 4% → 9%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 56 as a pale yellow solid (67% yield, 30.0 mg, 0.07 mmol): Rf = 0.28 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.58 (d, J = 6.7 Hz, 2H), 8.10–8.04 (m, 3H), 7.97 (d, J = 8.1 Hz, 1H), 7.92 (s, 1H), 7.88–7.81 (m, 3H) 7.54 (dd, J = 8.5, 1.2 Hz, 1H), 7.22 (d, J = 1.9 Hz, 1H), 7.16 (dd, J = 8.8, 2.2 Hz, 1H), 3.21 (s, 3H), 2.72 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 157.0, 151.1, 147.1, 141.6, 141.4, 139.4, 138.0, 135.7, 133.7, 131.4, 131.0, 130.7, 130.2, 129.2, 128.3, 128.0, 127.1, 125.2, 123.5, 122.8, 120.3, 109.5, 44.1, 21.6; νmax (cm–1, thin film, ATR) 3221 (br), 2927 (w), 2851 (w), 1626 (w), 1608 (s), 1572 (w), 1436 (w), 1396 (w), 1305 (s), 1250 (w), 1211 (m), 1163 (w), 1144 (s), 1124 (w), 1114 (m), 1038 (m), 1013 (w), 1001 (w), 947 (m), 915 (w), 878 (s, 837 (m), 829 (m), 820 (w), 767 (s); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C26H22N3O3S 456.1382, found 456.1358; mp 250.0 °C (dec).
4-(4-(6-Methoxynaphthalen-2-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (57)
The title compound was prepared according to general procedure C, using 44 (79.0 mg, 0.20 mmol, 1.00 equiv), 6-methoxy-2-naphthaleneboronic acid (53.0 mg, 0.25 mmol, 1.25 equiv), Pd(OAc)2 (4.6 mg, 10 mol %), Aphos (13.4 mg, 24 mol %), K2CO3 (83 mg, 0.06 mmol, 3.00 equiv), degassed DME (1.5 mL), and distilled H2O (0.5 mL). Purification by silica gel chromatography, eluting with EtOH in DCM (21 cm × 20 mm, gradient elution, 0% → 8%, 0.5% increases, 20 mL runs, 3–4 mL fractions), yielded 57 as a white solid (97% yield, 91.0 mg, 0.19 mmol): Rf = 0.37 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (DMSO-d6/D2O/TFA) δ 8.58 (d, J = 7.0 Hz, 2H), 8.12 (s, 1H), 8.05 (d, J = 7.0 Hz, 2H), 7.98 (d, J = 1.7 Hz, 1H), 7.96 (d, J = 2.2 Hz, 1H), 7.92 (s, 1H), 7.89 (d, J = 9.0 Hz, 1H), 7.86 (dd, J = 8.2, 1.5 Hz, 1H), 7.60 (dd, J = 8.5, 1.5, 1H), 7.40 (d, J = 2.3 Hz, 1H), 7.23 (dd, J = 9.0, 2.5 Hz, 1H), 3.88 (s, 3H), 3.22 (s, 3H), 2.73 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 159.1, 151.1, 147.1, 141.6, 141.3, 139.3, 137.8, 135.4, 133.7, 131.6, 130.7, 130.6, 130.1, 129.0, 129.0, 128.7, 127.2, 125.1, 124.4, 122.8, 120.3, 106.8, 56.1, 44.1, 21.6; νmax (cm–1, thin film, ATR) 3125 (br), 1629 (w), 1600 (s), 1498 (w), 1302 (s), 1263 (m), 1205 (m), 1147 (s), 1110 (m), 1070 (w), 953 (m), 859 (m), 835 (m), 767 (m), 740 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C27H24N3O3S 470.1538, found 470.1551; mp 256.0 °C (dec), turned brown at 254.0 °C.
4-(4-(6-Ethoxynaphthalen-2-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (58)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 2-(6-ethoxynaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (S9) (37.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (16 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 58 as a white solid (64% yield, 31.0 mg, 0.06 mmol): Rf = 0.43 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.58 (d, J = 6.8 Hz, 2H), 8.11 (s, 1H), 8.06 (d, J = 6.8 Hz, 2H), 7.97 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 8.6 Hz, 1H) 7.92 (s, 1H), 7.90–7.84 (m, 2H), 7.59 (dd, J = 8.5, 1.4 Hz, 1H), 7.38 (d, J = 1.9 z, 1H), 7.21 (dd, J = 8.9, 2.3 Hz, 1H), 3.22 (s, 3H), 2.73 (s, 3H), 1.38 (t, J = 7.0 Hz, 3H) (note that CH2 of the ethoxy group is not observed due to superposition of HOD signal); 1H NMR (500 MHz, DMSO-d6) δ 13.10 (s, 1H), 8.45 (d, J = 4.8 Hz, 2H), 8.09 (s, 1H), 8.04 (d, J = 8.1 Hz, 1H), 7.95–7.85 (m, 4H), 7.61–7.50 (m, 3H), 7.40 (d, J = 2.2 Hz, 1H), 7.23 (dd, J = 8.9, 2.5 Hz, 1H), 4.19 (q, J = 7.0 Hz, 2H), 3.28 (s, 3H), 2.82 (s, 3H), 1.43 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 158.3, 151.1, 147.1, 141.6, 141.3, 139.3, 137.8, 135.4, 133.7, 131.6, 130.68, 130.65, 130.1, 129.0, 128.9, 128.6, 127.2, 125.1, 124.3, 122.8, 120.6, 107.4, 64.2, 44.1, 21.6, 15.1; 13C{1H} NMR (126 MHz, DMSO-d6/D2O) δ 157.3, 149.7, 145.1, 142.2, 140.0, 137.9, 134.3, 134.2, 134.0, 131.6, 129.7, 129.6, 129.3, 128.3, 127.9, 127.4, 127.1, 125.1, 124.4, 120.8, 119.7, 106.7, 63.3, 43.5, 21.5, 14.7; νmax (cm–1, thin film, ATR) 3033 (br), 2928 (w), 1631 (w), 1600 (s), 1497 (w), 1442 (w), 1400 (w), 1319 (m), 1300 (m), 1261 (m), 1207 (w), 1144 (s), 1094 (m), 1041 (m), 994 (m), 834 (m), 768 (s), 742 (s), 700 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C28H26N3O3S 484.1695, found 484.1697; mp 250.0 °C (dec).
4-(4-(6-Cyclopropoxynaphthalen-2-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (59)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 2-(6-cyclopropoxynaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane40 (S11) (37.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 59 as a white solid (63% yield, 31.0 mg, 0.06 mmol): Rf = 0.33 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 13.10 (s, 1H), 8.65–8.39 (m, 2H), 8.15–7.76 (m, 6H), 7.68–7.48 (m, 4H), 7.87–7.81 (m, 3H), 7.29–7.13 (m, 1H), 4.00 (s, 1H), 3.28 (s, 3H), 2.82 (s, 3H), 0.92–0.85 (m, 2H), 0.79–0.71 (m, 2H); 1H NMR (600 MHz, DMSO-d6/D2O/TFA) δ 8.67 (d, J = 7.0 Hz, 2H), 8.19 (s, 1H), 8.07 (d, J = 7.0 Hz, 2H), 8.04 (d, J = 8.2 Hz, 1H), 8.02 (d, J = 8.6 Hz, 1H), 7.96 (s, 1H), 7.93 (d, J = 9.0 Hz, 1H), 7.90 (dd, J = 1.5, 8.2 Hz, 1H), 7.68–7.65 (m, 2H), 7.27 (dd, J = 2.4, 8.9 Hz, 1H), 3.27 (s, 3H), 2.80 (s, 3H), 0.92–0.87 (m, 2H), 0.75–0.72 (m, 2H) (note that the CH of the cyclopropoxy group is not observed due to superposition of HOD signal); 13C{1H} NMR (126 MHz, DMSO-d6) δ 157.4, 149.7, 145.1, 142.1, 140.0, 137.9, 134.4, 134.0, 129.7, 129.5, 129.2, 128.6, 127.8, 127.4, 127.1, 125.3, 124.3, 120.7, 119.3, 108.0, 51.0, 43.5, 21.5, 6.0 (note that two carbon signals in the 13C{1H} NMR are missing); 13C{1H} NMR (151 MHz, DMSO-d6/D2O/TFA) δ 158.0, 150.9, 146.7, 141.4, 140.9, 138.6, 137.4, 134.8, 133.4, 131.6, 130.2, 129.9, 129.8, 128.8, 128.7, 128.2, 127.0, 124.7, 124.4, 122.1, 119.8, 108.3, 51.4, 43.6, 21.5, 6.2; νmax (cm–1, thin film, ATR) 3038 (w), 2927 (w), 1629 (w), 1603 (s), 1573 (w), 1494 (w), 1445 (w), 1354 (w), 1304 (m), 1260 (m), 1216 (m), 1149 (s), 1120 (w), 1107 (m), 1074 (w), 996 (w), 986 (s), 966 (w), 953 (m), 872 (w), 836 (s), 804 (w), 764 (s), 742 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C29H26N3O3S 496.1695, found 496.1715; mp 268.0 °C (dec).
tert-Butyl (6-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)naphthalen-2-yl)carbamate (60)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), tert-butyl (6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)naphthalen-2-yl)carbamate41 (S13) (46.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 60 as a yellow solid (40% yield, 22.0 mg, 0.04 mmol): Rf = 0.47 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6) δ 13.09 (s, 1H), 9.67 (s, 1H), 8.45 (d, J = 3.6 Hz, 2H), 8.17 (s, 1H), 8.10–8.00 (m, 2H), 7.95–7.82 (m, 4H), 7.63–746 (m, 4H), 3.27 (s, 3H), 2.81 (s, 3H), 1.52 (s, 9H) (note that minor peaks in the 1H NMR are due to the presence of a tautomers in the sample); 13C{1H} NMR (126 MHz, DMSO-d6) δ 152.9, 150.0, 149.7, 145.2, 142.2, 140.1, 138.1, 137.9, 134.4, 133.9, 133.4, 131.5, 129.6, 129.5, 129.3, 129.0, 128.7, 127.7 (2×), 127.0, 125.7, 124.4, 122.0, 120.8, 120.3, 113.4, 79.5, 43.5, 28.2, 21.5 (note that extra peaks in the 13C{1H} NMR are due to the presence of tautomers in the sample); νmax (cm–1, thin film, ATR) 2925 (w), 2848 (w), 1724 (m), 1712 (m), 1603 (s), 1494 (w), 1367 (w), 1305 (m), 1238 (m), 1150 (s), 1108 (w), 1052 (w), 1025 (w), 958 (m), 884 (m), 835 (m), 764 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C31H31N4O4S 555.2066, found 555.2047; mp 180.0 °C (dec).
4-(5-([1,1′-Biphenyl]-4-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-4-yl)pyridine (61)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 4-biphenylboronic acid (25.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 4%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 4.5%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 61 as a pale yellow solid (60% yield, 28.0 mg, 0.06 mmol): Rf = 0.42 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.60 (d, J = 6.9 Hz, 2H), 8.09 (d, J = 6.9 Hz, 2H), 7.94 (d, J = 8.2 Hz, 1H), 7.91 (s, 1H) 7.87–7.81 (m, 3H), 7.74–7.68 (m, 4H), 7.49 (t, J = 7.7 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.9, 147.3, 142.3, 141.8, 141.5, 139.8, 139.6, 137.3, 133.6, 131.5, 130.9, 130.4, 130.2, 130.0, 129.0, 128.4, 128.3, 127.5, 125.3,123.3, 44.2, 21.6; νmax (cm–1, thin film, ATR) 2925 (br), 2360 (w), 1602 (s), 1517 (w), 1480 (w), 1443 (w), 1312 (s), 1213 (w), 1147 (s), 1109 (m), 1075 (w), 1075 (w), 999 (w), 958 (m), 877 (w), 768 (s), 762 (s), 744 (s), 733 (s), 700 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C28H24N3O2S 466.1589, found 466.1571; mp 241.0 °C (dec).
4′-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)-[1,1′-biphenyl]-4-carbonitrile (62)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-carbonitrile (39.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 4%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 5%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 62 as a white solid (47% yield, 23.0 mg, 0.05 mmol); Rf = 0.35 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.61 (d, J = 6.8 Hz, 1H), 8.10 (d, J = 6.8 Hz, 1H), 7.97–7.88 (m, 8H), 7.86 (d, J = 8.1 Hz, 1H), 7.75 (d, J = 8.3 Hz, 1H), 3.21 (s, 1H), 2.71 (s, 1H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 151.0, 147.5, 144.4, 141.8, 141.4, 140.1, 139.4, 136.9, 133.7, 133.7, 131.8, 130.8, 130.5, 130.2, 129.9, 128.7, 128.4, 125.2, 123.2, 119.7, 111.2, 44.1, 21.6; νmax (cm–1, thin film, ATR) 3083 (br), 2846 (br), 2359 (w), 2225 (w), 1604 (m), 1499 (w), 1410 (w), 1310 (m), 1301 (m), 1150 (s), 1111 (m), 1077 (w), 1004 (w), 972 (w), 959 (w), 880 (w), 825 (s), 765 (m), 765 (m), 745 (m), 715 (w), 693 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C29H23N4O2S 491.1542, found 491.1530; mp 255.0 °C (dec).
4-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-4-(5,6,7,8-tetrahydronaphthalen-2-yl)-1H-imidazol-5-yl)pyridine (63)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 4,4,5,5-tetramethyl-2-(5,6,7,8-tetrahydronaphthalen-2-yl)-1,3,2-dioxaborolane (S15) (33.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 5.5%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 5.5%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 63 as a white solid (61% yield, 27.0 mg, 0.06 mmol): Rf = 0.33 (7% EtOH/CHCl3, UV, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.61 (d, J = 6.6 Hz, 2H), 8.08 (d, J = 6.8 Hz, 2H), 7.94 (d, J = 8.3 Hz, 1H), 7.91 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.32 (s, 1H), 7.29 (d, J = 7.9 Hz, 1H), 7.22 (d, J = 7.9 Hz, 1H), 3.22 (s, 3H), 2.80–2.73 (m, 4H), 2.71 (s, 3H), 1.78–1.72 (m, 4H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 151.2, 146.8, 141.5, 141.2, 139.6, 139.1, 138.6, 137.8, 133.6, 131.2, 130.6, 130.5, 130.0, 129.9, 126.6, 126.4, 125.0, 122.6, 44.0, 29.3, 29.2, 23.0, 21.5 (note that the signal at δ 23.0 ppm in the 13C{1H} NMR corresponds to two carbons from the tetrahydronaphthalene moiety); νmax (cm–1, thin film, ATR) 2935 (w), 2856 (w), 1599 (s), 1429 (w), 1309 (s), 1212 (w), 1147 (s), 1106 (m), 1076 (w), 998 (w), 963 (w), 952 (w), 871 (w), 827 (m), 808 (w), 765 (s), 738 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C26H26N3O2S 444.1746, found 444.1761; mp 252.0 °C (dec).
4-(4-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-2-(2-methyl-4-(methylsulfonyl)phenyl)-1H-imidazol-5-yl)pyridine (64)
The title compound was prepared according to general procedure C, using 44 (39.0 mg, 0.10 mmol, 1.00 equiv), 1,4-benzodioxane-6-boronic acid (25.0 mg, 0.125 mmol, 1.25 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 5%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (15 cm × 10 mm, gradient elution, 0% → 6%, 0.5% increases, 20 mL runs, 7 mL fractions), yielded 64 as a pale yellow solid (60% yield, 27.0 mg, 0.06 mmol): Rf = 0.37 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 8.59 (d, J = 7.0 Hz, 2H), 8.06 (d, J = 7.0 Hz, 2H), 7.91 (d, J = 8.2 Hz, 1H), 7.89 (d, J = 1.4, 1H) 7.84 (dd, J = 8.2, 1.8 Hz, 1H), 7.10 (d, J = 2.1, 1H), 7.05 (dd J = 8.3, 2.1 Hz, 1H), 7.00 (d, J = 8.3 Hz, 1H), 4.30–4.25 (m, 4H), 3.20 (s, 3H), 2.67 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.9, 146.8, 145.6, 144.6, 141.7, 141.5, 139.5, 137.2, 133.5, 130.9, 130.8, 130.2, 125.2, 123.0, 123.0, 122.1, 118.9, 118.4, 65.1, 64.9, 44.1, 21.5; νmax (cm–1, thin film, ATR) 2668 (br), 2360 (w), 1603 (s), 1541 (w), 1512 (w), 1489 (w), 1461 (w), 1442 (w), 1311 (s), 1287 (s), 1253 (m), 1154 (s), 1112 (w), 1097 (w), 1063 (s), 1049 (w), 1006 (w), 977 (w), 965 (w), 951 (m), 931 (w), 893 (w), 875 (w), 865 (m), 841 (w), 830 (s), 764 (m), 741 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C24H22N3O4S 448.1331, found 448.1315; mp 299.0 °C (dec).
One-Pot Miyaura Borylation–Suzuki Coupling
7-(2-(2-Methyl-4-(methylsulfonyl)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)quinoline (67)
A culture tube (13 mm × 100 mm, 9 mL) was charged with 7-bromoquinoline (65) (42.0 mg, 0.20 mmol, 2.00 equiv), B2(pin)2 (80.0 mg, 0.30 mmol, 3.00 equiv), KOAc (59.0 mg, 0.60 mmol, 6.00 equiv), and a magnetic stir bar under nitrogen. Degassed DME (0.15 mL) was added followed by a premixed solution of Pd(OAc)2 (2.4 mg, 0.011 mmol, 5 mol % relative to 65) and Aphos (7.1 mg, 0.025 mmol, 12% relative to 65) in DME (0.35 mL). The reaction mixture was stirred in a preheated aluminum block at 80 °C for 2 h. After consumption of the starting material, indicated by TLC analysis (30% EtOAc/hexane, KMnO4), the reaction mixture was cooled to room temperature, the culture tube was opened under a nitrogen flow, and 44 (39.0 mg, 0.10 mmol, 1.00 equiv) was added followed by addition of a premixed solution of Pd(OAc)2 (1.2 mg, 0.005 mmol, 5 mol % relative to 44) and Aphos (3.6 mg, 0.01 mmol, 12 mol % relative to 44) in DME (0.15 mL). Then, DME (0.10 mL) and 1.2 M K2CO3 aqueous solution (0.25 mL, 0.30 mml, 3.00 equiv) were added, and the reaction mixture was purged with nitrogen for 5 min. The reaction mixture was stirred in a preheated aluminum block at 80 °C for 18 h. After consumption of 44, indicated by TLC analysis (7% EtOH/CHCl3, Dragendorff), the reaction mixture was allowed to reach room temperature and it was diluted with 10% MeOH/DCM (∼7 mL), filtered through a pad (20 mm diameter) composed of Celite (top, 1 cm) and silica gel (bottom, 3 cm). The pad was washed with 10% MeOH/DCM (25–50 mL), and the filtrate was concentrated under reduced pressure. The crude product was adsorbed over basic alumina and purified by silica column chromatography, eluting with EtOH in CHCl3 (21 cm × 10 mm, gradient elution, 0% → 7%, 0.5% increases, 20 mL runs, 3–4 mL fractions) followed by repurification in silica gel using MeOH in DCM eluent (21 cm × 10 mm, gradient elution, 0% → 8%, 0.5% increases, 20 mL runs, 7 mL fractions), to yield 67 as a white solid (50% yield, 22.0 mg, 0.05 mmol): Rf = 0.45 (7% EtOH/CHCl3, Dragendorff stain); 1H NMR (500 MHz, DMSO-d6/D2O/TFA) δ 9.22 (d, J = 5.2 Hz, 1H), 9.12 (d, J = 8.4 Hz, 1H), 8.66 (d, J = 6.8 Hz, 2H), 8.44 (s, 1H), 8.41 (d, J = 8.6 Hz, 1H), 8.13 (d, J = 6.8 Hz, 2H), 8.09 (d, J = 8.5 Hz, 1H), 8.07–8.03 (m, 1H), 7.99 (d, J = 8.1 Hz, 1H), 7.94 (s, 1H), 7.89 (d, J = 8.1 Hz, 1H), 3.23 (s, 3H), 2.74 (s, 3H); 13C{1H} NMR (126 MHz, DMSO-d6/D2O/TFA) δ 150.0, 148.5, 147.3, 146.0, 142.1, 141.5, 139.7, 139.5, 135.9, 135.5, 133.8, 132.5, 131.0, 130.8, 130.2, 130.2, 129.3, 125.2, 124.1, 123.3, 122.0, 44.1, 21.5; νmax (cm–1, thin film, ATR) 2922 (w), 2845 (w), 1614 (w), 1584 (w), 1509 (w), 1490 (w), 1449 (w), 1303 (s), 1210 (w), 1155 (m), 1141 (s), 1104 (m), 1073 (w), 975 (w), 958 (w), 880 (m), 837 (s), 765 (s), 742 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C25H21N4O2S 441.1385, found 441.1372; mp 300.0 °C (dec).
tert-Butyl 4-(4-Bromo-5-(pyridin-4-yl)-1H-imidazol-2-yl)piperidine-1-carboxylate (69)
Following a modified literature procedure,24 a 25 mL round-bottom flask was charged with 36 (437 mg, 1.33 mmol, 1.00 equiv), dry DCM (5.9 mL), dry pyridine (1.5 mL), and a magnetic stir bar under an inert atmosphere. The reaction flask was covered with aluminum foil, and the reaction mixture was cooled to 0 °C in an ice/water bath and stirred for 15 min. Solid Py·HBr·Br2 (pyridinium hydrobromide perbromide, 520 mg, 1.46 mmol, 1.10 equiv) was added in portions, by briefly removing the Suba seal, and the reaction mixture was stirred at 0 °C for 1 h. After consumption of the starting material, indicated by TLC analysis (100% EtOAc, Dragendorff), the solvent was removed in the rotaevaporator. The residue was partitioned between 1 M aq NaHSO3 (1 × 30 mL) and CHCl3 (1 × 30 mL). The phases were separated, and the aqueous layer was extracted with CHCl3 (3 × 15 mL). The organic phases were combined, dried over MgSO4, filtered, and concentrated in the rotaevaporator. Purification by silica gel chromatography, eluting with MeOH in CHCl3 (13 cm × 30 mm, gradient elution, 4% → 6%, 0.5% increases, 80 mL runs, 20 mL fractions) followed by repurification in silica gel using EtOAc/EtOH (3:1) in hexanes (13 cm × 30 mm, isocratic elution, 50% EtOAc/EtOH (3:1)/hexane, 400 mL run, 20 mL fractions), yielded a light yellow gum, to which precipitation was induced with pentane to afford 69 as a pale yellow solid (95% yield, 513 mg, 1.26 mmol): Rf = 0.37 (50% EtOAc:EtOH (3:1)/hexaneanes, UV, Dragendorff): Rf = 0.17 (5% MeOH/DCM, UV, Dragendorff); 1H NMR (500 MHz, CDCl3) δ 12.10 (br s, 1H), 8.52 (s, 2H), 7.76 (s, 2H), 4.14 (d, J = 11.4 Hz, 2H), 2.97–2.88 (m, 1H), 2.88–2.68 (m, 2H), 1.92 (d, J = 11.5 Hz, 2H), 1.84–1.64 (m, 2H), 1.43 (s, 9H); 13C{1H} NMR (126 MHz, CDCl3) δ 154.8, 153.2, 149.6, 120.4, 80.3, 43.9, 36.7, 30.8, 28.6 (note that, due to slow relaxation, some 13C{1H} NMR signals were not identified in the spectra;28 specifically, the 13C{1H} NMR data for compound 69 lacks three of the 12 expected signals) νmax (cm–1, thin film, ATR) 2875 (br), 1679 (s), 1603 (s), 1580 (w), 1519 (w), 1367 (m), 1276 (m), 1233 (s), 1164 (s), 1125 (m), 1063 (w), 1045 (w), 1003 (m), 981 (m), 935 (m), 874 (w), 821 (m), 723 (w), 693 (m); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C18H24BrN4O2 407.1083, 409.1064, found 407.1051, 409.1126; mp 197.0 °C (dec).
tert-Butyl 4-(4-(6-methoxynaphthalen-2-yl)-5-(pyridin-4-yl)-1H-imidazol-2-yl)piperidine-1-carboxylate (71)
The title compound was prepared according to general procedure C, using 69 (41.0 mg, 0.10 mmol, 1.00 equiv), 2-(6-methoxynaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (S16) (52.0 mg, 0.175 mmol, 1.75 equiv), Pd(OAc)2 (2.3 mg, 10 mol %), and Aphos (6.7 mg, 24 mol %). Purification by silica gel chromatography, eluting with EtOH in CHCl3 (20 cm × 15 mm, gradient elution, 0% → 4.5%, 0.5% increases, 25 mL runs, 5 mL fractions then isocratic elution, 4.5% EtOH/CHCl3, 50 mL run, 5 mL fractions), yielded 71 as a pale yellow solid (89% yield, 43.0 mg, 0.89 mmol): Rf = 0.40 (7% EtOH/CHCl3, UV, Dragendorff stain); 1H NMR (250 MHz, CDCl3) δ 10.07 (br s, 1H), 8.41 (d, J = 5.2 Hz, 2H), 7.90–7.77 (m, 1H), 7.72 (d, J = 8.6 Hz, 1H), 7.67 (d, J = 8.8 Hz, 1H), 7.59–7.36 (m, 3H), 7.21–7.11 (m, 2H), 4.30–4.12 (m, 2H), 3.93 (s, 3H), 3.00 (tt, J = 11.7, 3.6 Hz, 1H), 2.92–2.73 (m, 2H), 2.11–1.95 (m, 2H), 1.77 (dq, J = 3.7, 12.4 Hz, 1H), 1.45 (s, 9H); 13C{1H} NMR (126 MHz, CDCl3) δ 158.5, 154.8, 151.3, 150.2, 149.8, 142.8, 134.4, 133.6, 129.6, 129.4, 129.0, 127.7, 127.2, 127.1, 126.8, 125.8, 121.5, 121.3, 119.8, 105.9, 79.9, 55.5, 36.5, 31.0, 29.8, 28.6; νmax (cm–1, thin film, ATR) 2930 (br), 1693 (s), 1601 (s), 1536 (w), 1418 (m), 1391 (w), 1366 (w), 1273 (m), 1249 (w), 1210 (m), 1165 (s), 1123 (m), 1085 (m), 1030 (w), 1007 (w), 99 (w), 857 (w), 831 (m), 693 (w), 667 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C29H33N4O3 485.2553, found 485.2537; mp 193.0 °C (dec). Spectroscopic data are in accordance with the literature.11
Synthesis of GSK3037619A (72)
N-Boc piperidine-substituted imidazole 71 (12.0 mg, 0.03 mmol, 1.0 equiv) was dissolved in DCM (0.25 mL, 0.1 M) under a nitrogen atmosphere. Trifluoroacetic acid (39 μL, 0.50 mmol, 20 equiv) was added, and the reaction mixture was allowed to stir for 1 h and was followed by TLC (10% MeOH/NH4OH (10:1)/DCM). After consumption of the starting material, the solvent and excess trifluoroacetic acid were removed in vacuo and the residue was dissolved in anhydrous MeCN (1 mL, 0.03 M) under a nitrogen atmosphere. Then Et3N (5.3 μL, 0.04 mmol, 1.5 equiv) was added followed by a 37% aqueous formaldehyde solution (14 μL, 0.19 mmol, 7.5 equiv), and the reaction mixture was left to stir for 1 h at room temperature. Na(OAc)3BH (14.0 mg, 0.06 mmol, 2.5 equiv) was added, and the reaction mixture was stirred for 18 h. The solvent was removed under reduced pressure, and the residue was diluted in 10% MeOH/NH4OH (10:1)/CHCl3, filtered through a short (1 cm × 15 mm) pad of silica gel, which was washed with 10% MeOH/NH4OH (10:1)/CHCl3 until the product was eluted completely. The solvent was concentrated, resulting in a yellow residue. Purification by silica gel chromatography, eluting with MeOH/NH4OH (10:1) in CHCl3 (4 cm × 15 mm, isocratic elution, 10% MeOH/NH4OH (10:1)/CHCl3, 50 mL run, 2 mL fractions), yielded a white solid, which was triturated with Et2O/hexanes (2:8) (3 × 5 mL) to afford 72 as a white solid (80% yield, 8 mg, 0.02 mmol): Rf = 0.40 (10% MeOH/NH4OH (10:1)/CHCl3, UV, Dragendorff stain); 1H NMR (600 MHz, CD3OD) δ 8.36 (d, J = 6.2 Hz, 2H), 7.88 (s, 1H), 7.82 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 8.9 Hz, 1H), 7.49 (d, J = 6.2 Hz, 2H), 7.43 (dd, J = 1.7, 8.6 Hz, 1H), 7.28 (d, J = 2.5 Hz, 1H), 7.17 (dd, J = 9.0, 2.7 Hz, 1H), 3.93 (s, 3H), 3.02 (d, J = 11.8 Hz, 2H), 2.87 (tt, J = 12.0, 3.9, Hz, 1H), 2.34 (s, 3H), 2.19 (dt, J = 11.9, 2.0 Hz, 2H), 2.06 (d, J = 11.1 Hz, 2H), 1.97 (dq, J = 12.6, 3.4 Hz, 2H); 13C{1H} NMR (151 MHz, CD3OD) δ 159.9, 154.1, 150.0, 136.0, 130.6, 130.3, 128.6, 128.6, 127.9, 123.0, 120.6, 106.8, 56.4, 55.8, 46.4, 36.9, 31.7; νmax (cm–1, thin film, ATR) 3010 (br), 2939 (w), 2848 (w), 2792 (w), 1630 (w), 1601 (s), 1535 (w), 1493 (w), 1465 (w), 1379 (w), 1270 (m), 1209 (w), 1181 (w), 1164 (w), 1127 (w), 1066 (w), 1029 (w), 994 (w), 832 (w), 753 (w), 695 (w); HRMS (ESI+/TOF) m/z [M + H]+ calcd for C25H27N4O 399.2185, found 399.2201; mp 262.0 °C (dec).
Acknowledgments
We thank the Brazilian agencies FAPESP (2013/07607-8 and 2019/13104-5) and CNPq (131263/2017-0) for financial support. The SGC is a registered charity (1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada, Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD grant no. 115766], Janssen, Merck KGaA Darmstadt Germany, MSD, Novartis Pharma AG, Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda, and Wellcome Trust [106169/ZZ14/Z]. We thank Dr. Ricardo Serafim for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.9b01844.
1H and 13C{1H} NMR spectra for compounds 5–31, 32, 36, 43, 44, 46–64, 67, 69, 71, and 72, optimization tables, and synthetic procedures for compounds S1–S16 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Blakemore D. C.; Castro L.; Churcher I.; Rees D. C.; Thomas A. W.; Wilson D. M.; Wood A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10 (4), 383–394. 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Organic Synthesis toward Small-Molecule Probes and Drugs. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (17), 6699–6702. 10.1073/pnas.1103205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. B.; Long T. E. Imidazole- and Imidazolium-Containing Polymers for Biology and Material Science Applications. Polymer 2010, 51 (12), 2447–2454. 10.1016/j.polymer.2010.02.006. [DOI] [Google Scholar]
- Green M. D.; Long T. E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym. Rev. 2009, 49 (4), 291–314. 10.1080/15583720903288914. [DOI] [Google Scholar]
- Amarasekara A. S. Acidic Ionic Liquids. Chem. Rev. 2016, 116 (10), 6133–6183. 10.1021/acs.chemrev.5b00763. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Peng X.-M.; Damu G. L. V.; Geng R.-X.; Zhou C.-H. Comprehensive Review in Current Developments of Imidazole-Based Medicinal Chemistry. Med. Res. Rev. 2014, 34 (2), 340–437. 10.1002/med.21290. [DOI] [PubMed] [Google Scholar]
- Sakemi S.; Sun H. H. Nortopsentins A, B, and C. Cytotoxic and Antifungal Imidazolediylbis[Indoles] from the Sponge Spongoaorites Ruetzleri. J. Org. Chem. 1991, 56 (13), 4304–4307. 10.1021/jo00013a044. [DOI] [Google Scholar]
- Rani N.; Sharma A.; Singh R. Trisubstituted Imidazole Synthesis: A Review. Mini-Rev. Org. Chem. 2014, 12 (1), 34–65. 10.2174/1570193X11666141028235010. [DOI] [Google Scholar]
- Bellina F.; Rossi R. Regioselective Functionalization of the Imidazole Ring via Transition Metal-Catalyzed C-N and C-C Bond Forming Reactions. Adv. Synth. Catal. 2010, 352 (8), 1223–1276. 10.1002/adsc.201000144. [DOI] [Google Scholar]
- Liverton N. J.; Butcher J. W.; Claiborne C. F.; Claremon D. A.; Libby B. E.; Nguyen K. T.; Pitzenberger S. M.; Selnick H. G.; Smith G. R.; Tebben A.; et al. Design and Synthesis of Potent, Selective, and Orally Bioavailable Tetrasubstituted Imidazole Inhibitors of P38 Mitogen-Activated Protein Kinase. J. Med. Chem. 1999, 42 (12), 2180–2190. 10.1021/jm9805236. [DOI] [PubMed] [Google Scholar]
- Lee C. F.; Holownia A.; Bennett J. M.; Elkins J. M.; St. Denis J. D.; Adachi S.; Yudin A. K. Oxalyl Boronates Enable Modular Synthesis of Bioactive Imidazoles. Angew. Chem., Int. Ed. 2017, 56 (22), 6264–6267. 10.1002/anie.201611006. [DOI] [PubMed] [Google Scholar]
- Selig R.; Goettert M.; Schattel V.; Schollmeyer D.; Albrecht W.; Laufer S. A Frozen Analogue Approach to Aminopyridinylimidazoles Leading to Novel and Promising P38 MAP Kinase Inhibitors. J. Med. Chem. 2012, 55 (19), 8429–8439. 10.1021/jm300852w. [DOI] [PubMed] [Google Scholar]
- Günther M.; Lategahn J.; Juchum M.; Döring E.; Keul M.; Engel J.; Tumbrink H. L.; Rauh D.; Laufer S. Trisubstituted Pyridinylimidazoles as Potent Inhibitors of the Clinically Resistant L858R/T790M/C797S EGFR Mutant: Targeting of Both Hydrophobic Regions and the Phosphate Binding Site. J. Med. Chem. 2017, 60 (13), 5613–5637. 10.1021/acs.jmedchem.7b00316. [DOI] [PubMed] [Google Scholar]
- Tan J.; Chen Y.; Li H.; Yasuda N. Suzuki–Miyaura Cross-Coupling Reactions of Unprotected Haloimidazoles. J. Org. Chem. 2014, 79 (18), 8871–8876. 10.1021/jo501326r. [DOI] [PubMed] [Google Scholar]
- Elkins J. M.; Fedele V.; Szklarz M.; Abdul Azeez K. R.; Salah E.; Mikolajczyk J.; Romanov S.; Sepetov N.; Huang X. P.; Roth B. L.; et al. Comprehensive Characterization of the Published Kinase Inhibitor Set. Nat. Biotechnol. 2016, 34 (1), 95–103. 10.1038/nbt.3374. [DOI] [PubMed] [Google Scholar]
- Belkina N. V.; Liu Y.; Hao J.-J.; Karasuyama H.; Shaw S. LOK Is a Major ERM Kinase in Resting Lymphocytes and Regulates Cytoskeletal Rearrangement through ERM Phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (12), 4707–4712. 10.1073/pnas.0805963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi S.; Moriguchi T.; Kuida K.; Endo J.; Semba K.; Nishida E.; Karasuyama H. LOK Is a Novel Mouse STE20-like Protein Kinase That Is Expressed Predominantly in Lymphocytes. J. Biol. Chem. 1997, 272 (36), 22679–22684. 10.1074/jbc.272.36.22679. [DOI] [PubMed] [Google Scholar]
- Viswanatha R.; Ohouo P. Y.; Smolka M. B.; Bretscher A. Local Phosphocycling Mediated by LOK/SLK Restricts Ezrin Function to the Apical Aspect of Epithelial Cells. J. Cell Biol. 2012, 199 (6), 969–984. 10.1083/jcb.201207047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo J.; Toyama-Sorimachi N.; Taya C.; Kuramochi-Miyagawa S.; Nagata K.; Kuida K.; Takashi T.; Yonekawa H.; Yoshizawa Y.; Miyasaka N.; et al. Deficiency of a STE20/PAK Family Kinase LOK Leads to the Acceleration of LFA-1 Clustering and Cell Adhesion of Activated Lymphocytes. FEBS Lett. 2000, 468 (2–3), 234–238. 10.1016/S0014-5793(00)01219-9. [DOI] [PubMed] [Google Scholar]
- Johnson N. W.; Semones M.; Adams J. L.; Hansbury M.; Winkler J. Optimization of Triarylimidazoles for Tie2: Influence of Conformation on Potency. Bioorg. Med. Chem. Lett. 2007, 17 (20), 5514–5517. 10.1016/j.bmcl.2007.08.052. [DOI] [PubMed] [Google Scholar]
- Niculescu-Duvaz D.; Niculescu-Duvaz I.; Suijkerbuijk B. M. J. M.; Ménard D.; Zambon A.; Davies L.; Pons J. F.; Whittaker S.; Marais R.; Springer C. J. Potent BRAF Kinase Inhibitors Based on 2,4,5-Trisubstituted Imidazole with Naphthyl and Benzothiophene 4-Substituents. Bioorg. Med. Chem. 2013, 21 (5), 1284–1304. 10.1016/j.bmc.2012.12.035. [DOI] [PubMed] [Google Scholar]
- Floyd M. B.; Du M. T.; Fabio P. F.; Jacob L. A.; Johnson B. D. The Oxidation of Acetophenones to Arylglyoxals with Aqueous Hydrobromic Acid in Dimethyl Sulfoxide. J. Org. Chem. 1985, 50 (25), 5022–5027. 10.1021/jo00225a004. [DOI] [Google Scholar]
- Zuliani V.; Cocconcelli G.; Fantini M.; Ghiron C.; Rivara M. A Practical Synthesis of 2,4(5)-Diarylimidazoles from Simple Building Blocks. J. Org. Chem. 2007, 72 (12), 4551–4553. 10.1021/jo070187d. [DOI] [PubMed] [Google Scholar]
- Vernier J.-M.; O’Connor P.; Ripka W.; Matthews D.; Pinkerton A.; Bounaud P.-Y.; Hopkins S.. RAF Kinase Inhibitors. WO2011085269(A1), 2011.
- Tan J.; Chen Y.; Li H.; Yasuda N. Suzuki–Miyaura Cross-Coupling Reactions of Unprotected Haloimidazoles. J. Org. Chem. 2014, 79 (18), 8871–8876. 10.1021/jo501326r. [DOI] [PubMed] [Google Scholar]
- Rivara M.; Zuliani V. In Vivo Screening of Diarylimidazoles as Anticonvulsant Agents. Med. Chem. Res. 2012, 21 (11), 3428–3434. 10.1007/s00044-011-9869-9. [DOI] [Google Scholar]
- Bellina F.; Cauteruccio S.; Di Fiore A.; Marchetti C.; Rossi R. Highly Selective Synthesis of 4(5)-Aryl-, 2,4(5)-Diaryl-, and 4,5-Diaryl-1H-Imidazoles via Pd-Catalyzed Direct C-5 Arylation of 1-Benzyl-1H-Imidazole. Tetrahedron 2008, 64 (26), 6060–6072. 10.1016/j.tet.2008.01.051. [DOI] [Google Scholar]
- Chen X. Y.; Englert U.; Bolm C. Base-Mediated Syntheses of Di- and Trisubstituted Imidazoles from Amidine Hydrochlorides and Bromoacetylenes. Chem. - Eur. J. 2015, 21 (38), 13221–13224. 10.1002/chem.201502707. [DOI] [PubMed] [Google Scholar]
- Bunge K.; Huisgen R.; Raab R.; Sturm H. J. 1.3-Dipolare Cycloadditionen, 64. Weitere Umsetzungen von Nitril-Yliden Mit Hetero-Mehrfachbindungen. Chem. Ber. 1972, 105 (4), 1307–1323. 10.1002/cber.19721050422. [DOI] [Google Scholar]
- Shi S.; Xu K.; Jiang C.; Ding Z. ZnCl 2 -Catalyzed [3 + 2] Cycloaddition of Benzimidates and 2 H-Azirines for the Synthesis of Imidazoles. J. Org. Chem. 2018, 83 (23), 14791–14796. 10.1021/acs.joc.8b02437. [DOI] [PubMed] [Google Scholar]
- Neunhoeffer H.; Lehmann B.; Ewald H. Zur Chemie Der 1,2,4-Triazine, VIII. Struktur Eines Reaktionsproduktes von 3-(P-Tolyl)-1,2,4-triazinen Mit Acetylendicarbonsäure-dimethylester. Justus Liebigs Ann. Chem. 1977, 1977 (9), 1421–1428. 10.1002/jlac.197719770903. [DOI] [Google Scholar]
- Bellina F.; Cauteruccio S.; Rossi R. Efficient and Practical Synthesis of 4(5)-Aryl-1 H -Imidazoles and 2,4(5)-Diaryl-1 H -Imidazoles via Highly Selective Palladium-Catalyzed Arylation Reactions. J. Org. Chem. 2007, 72 (22), 8543–8546. 10.1021/jo701496p. [DOI] [PubMed] [Google Scholar]
- Shimbayashi T.; Okamoto K.; Ohe K. Synthesis of Imidazoles and Pyrimidines Using Palladium-Catalyzed Decarboxylative Intramolecular Condensation of 1,2,4-Oxadiazol-5(4 H)-Ones. Synlett 2014, 25 (13), 1916–1920. 10.1055/s-0034-1378320. [DOI] [Google Scholar]
- Zuliani V.; Fantini M.; Nigam A.; Stables J. P.; Patel M. K.; Rivara M. Anticonvulsant Activity of 2,4(1H)-Diarylimidazoles in Mice and Rats Acute Seizure Models. Bioorg. Med. Chem. 2010, 18 (22), 7957–7965. 10.1016/j.bmc.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivara M.; Baheti A. R.; Fantini M.; Cocconcelli G.; Ghiron C.; Kalmar C. L.; Singh N.; Merrick E. C.; Patel M. K.; Zuliani V. 2,4(5)-Diarylimidazoles: Synthesis and Biological Evaluation of a New Class of Sodium Channel Blockers against HNav1.2. Bioorg. Med. Chem. Lett. 2008, 18 (20), 5460–5462. 10.1016/j.bmcl.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Donohoe T. J.; Kabeshov M. A.; Rathi A. H.; Smith I. E. D. Direct Preparation of Thiazoles, Imidazoles, Imidazopyridines and Thiazolidines from Alkenes. Org. Biomol. Chem. 2012, 10 (5), 1093–1101. 10.1039/C1OB06587D. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhang P.; Jiang M.; Yang H.; Zhao Y.; Fu H. Visible Light as a Sole Requirement for Intramolecular C(Sp3)-H Imination. Org. Lett. 2017, 19 (8), 1994–1997. 10.1021/acs.orglett.7b00533. [DOI] [PubMed] [Google Scholar]
- Nery M.; Azevedo M.; Cardoso J.; Slana G.; Lopes R.; Lopes C. A New Chemoselective Synthesis of Brombuterol. Synthesis 2007, 2007 (10), 1471–1474. 10.1055/s-2007-966044. [DOI] [Google Scholar]
- Taylor N. J.; Emer E.; Preshlock S.; Schedler M.; Tredwell M.; Verhoog S.; Mercier J.; Genicot C.; Gouverneur V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139 (24), 8267–8276. 10.1021/jacs.7b03131. [DOI] [PubMed] [Google Scholar]
- Vidadala R. S. R.; Rivas K. L.; Ojo K. K.; Hulverson M. A.; Zambriski J. A.; Bruzual I.; Schultz T. L.; Huang W.; Zhang Z.; Scheele S.; et al. Development of an Orally Available and Central Nervous System (CNS) Penetrant Toxoplasma Gondii Calcium-Dependent Protein Kinase 1 (TgCDPK1) Inhibitor with Minimal Human Ether-a-Go-Go-Related Gene (HERG) Activity for the Treatment of Toxoplasmosis. J. Med. Chem. 2016, 59 (13), 6531–6546. 10.1021/acs.jmedchem.6b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumiec G. R.; Cai L.; Lu S.; Pike V. W. Quinuclidine and DABCO Enhance the Radiofluorination of 5-Substituted 2-Halopyridines. Eur. J. Org. Chem. 2017, 2017 (45), 6593–6603. 10.1002/ejoc.201700970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.