Summary
The synovial joint forms from a pool of progenitor cells in the future region of the joint, the interzone. Expression of Gdf5 and Wnt9a has been used to mark the earliest cellular processes in the formation of the interzone and the progenitor cells. However, lineage specification and progression toward the different tissues of the joint are not well understood. Here, by lineage-tracing studies we identify a population of Lgr5+ interzone cells that contribute to the formation of cruciate ligaments, synovial membrane, and articular chondrocytes of the joint. This finding is supported by single-cell transcriptome analyses. We show that Col22a1, a marker of early articular chondrocytes, is co-expressed with Lgr5+ cells prior to cavitation as an important lineage marker specifying the progression toward articular chondrocytes. Lgr5+ cells contribute to the repair of a joint defect with the re-establishment of a Col22a1-expressing superficial layer.
Keywords: Lgr5, Col22a1, Gdf5, synovial joint, joint development, progenitor, articular chondrocytes, joint formation, joint lineage, single-cell transcriptome
Graphical Abstract
Highlights
-
•
Lgr5 marks multipotent progenitors and onset of lineage divergence in joint formation
-
•
Committed articular chondrocyte progenitor cells co-express Lgr5 and Col22a1
-
•
ECM niche of the juvenile articular superficial surface is rich in COLXXII and CILP
-
•
Lgr5+ interzone cells contribute to the repair of the superficial zone of joint defects
Chan and colleagues show that Lgr5 marks a subset of progenitor cells in the developing synovial joints, contributing to the formation of ligaments and articular cartilage. Specific to the articular cartilage lineage are committed progenitors co-expressing Lgr5 and Col22a1 that become Col22a1-expressing juvenile articular cartilage chondrocytes. These cells have high capacity in repairing the superficial zone of a joint defect.
Introduction
Skeletal movement is facilitated by synovial joints comprising articular cartilage encased in a capsule. Healthy articular cartilage minimizes friction and shock impacts, but wears with aging, and repair is inefficient (Hunter, 1995). Tissue repair involves the recruitment/activation of local progenitor cells, often recapitulating cellular differentiation similar to embryonic development. Synovial joints form through dedifferentiation of chondrocytes at the site of future joints (Craig et al., 1987), defined as “interzones,” marked by early expression of Gdf5 and Wnt9a (Guo et al., 2004, Hartmann and Tabin, 2001), with progenitor properties (Koyama et al., 2008, Kozhemyakina et al., 2015). Studies using Gdf5-Cre (Koyama et al., 2008, Rountree et al., 2004) and inducible Gdf5-CreERT2 mice (Decker et al., 2017, Shwartz et al., 2016) confirmed Gdf5-expressing cells contribute to the formation of articular chondrocytes and other joint-related cells. Furthermore, expansion of Gdf5+ cells in the interzone can come from recruitment of surrounding Sox9+/Gdf5-mesenchymal cells (Shwartz et al., 2016). Regional localization of Gdf5+ cells (central or peripheral) in the interzone may influence differentiation of joint cell lineages (Decker et al., 2017). Thus, the origin, timing, and location of interzone cells may determine cell fate in the joint tissues.
Lgr5, a member of the G-protein-coupled receptor family and a Wnt signaling target gene, is a stem cell marker for highly proliferative progenitor cells in the small intestine, colon, hair follicle, mammary gland, and ovary (Leung et al., 2018). Collagen XXII (COLXXII), encoded by the Col22a1 gene, is an extracellular matrix (ECM) protein localized at the articular cartilage-synovial fluid junction (Koch et al., 2004). Its function is not well understood. A role as a negative regulator of chondrocyte hypertrophy through interacting with β1-integrin was proposed (Zwolanek et al., 2014). Here, we identify a population of Lgr5-expressing cells in the interzone of developing joints that contribute to the formation of the articular cartilage, cruciate ligaments, and meniscus. We show that Col22a1 is expressed by Lgr5+ interzone cells prior to the cavitation process, supporting a lineage progression from Lgr5+ interzone cells to Lgr5+/Col22a1+ double-positive cells as committed progenitors for Col22a1+ juvenile chondrocytes at the articular surface.
Results
Lgr5 as a Novel Marker for Distinct Cells in Developing Synovial Joints
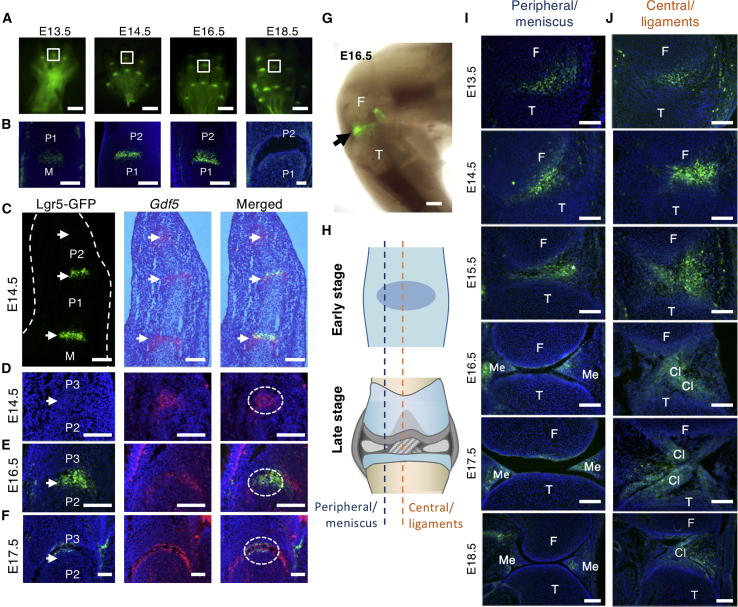
As interzone cells are progenitor cells, we screened these cells with a panel of stem cell markers and detected Lgr5 expression by qRT-PCR (Figure S1). Using GFP expression in Lgr5-eGFP-IRES-CreERT2 (Lgr5-GFP) mice, we confirmed Lgr5 as a marker of interzone cells. Lgr5-GFP is a null allele, with GFP expression replacing Lgr5 (Barker et al., 2007). Mice heterozygous for this allele are normal and viable, while homozygous mice die perinatally (Barker et al., 2007). However, we observed no abnormalities in limb development or synovial joint formation in homozygotes (Figure S2). All analyses of Lgr5/GFP expression in synovial joints were carried out in mice heterozygous for this allele. Digit joints develop proximodistally, providing information on progression. By whole-mount analysis of Lgr5-GFP mice, we detected GFP in digit joints from embryonic day 13.5 (E13.5) to E18.5 (Figure 1A). At E13.5, the proximal M/P1 joint is clearly positive for GFP, whereas the P1/P2 joints show only a faint signal and no signal for the P2/P3 joints (Figure 1A), which was confirmed by histological analysis (Figure 1B). In the M/P1 joint of digit III, signal can be detected at E13.5 as a “salt and pepper” pattern in cells of the interzone, which becomes more intense and uniformly distributed in the center of the interzone from E14.5. With cavitation, Lgr5+ cells are detected in the flanking regions of future articular cartilage, and the intensity and number of expressing cells decrease substantially.
Figure 1.
Expression of Lgr5 in the Developing Digit and Knee Joints
(A) Whole-mount images of hind paws from Lgr5-GFP embryos (E13.5 to E18.5). Scale bars, 1 mm.
(B) Sagittal sections of the boxed areas in (A) illustrating the expression of Lgr5 (GFP).
(C) Immunostaining for GFP (green) and in situ hybridization for Gdf5 (red) of adjacent sagittal sections from digit III of E14.5 hind paw, showing Lgr5 expression is sequential to Gdf5 in development. Lgr5 demarcates the center of the Gdf5-expressing domain in P1/P2 and M/P1 interzone.
(D–F) Higher magnifications of the P2/P3 joint showing Gdf5 expression but not Lgr5 at E14.5 (D), and its temporal expression in development (circled), as shown in the same joint at E16.5 (E) and E17.5 (F).
(G) Whole-mount image of the knee from an E16.5 embryo. Scale bars, 500 μm.
(H) Illustrations showing the positions and structures of the section chosen for analysis.
(I and J) Lgr5 expression during articular cartilage/meniscus (I) and cruciate ligament (J) formation from E13.5 to E18.5.
M, metacarpal; P1, proximal phalange; P2, middle phalange; P3, distal phalange; F, femur; T, tibia; Ac, articular cartilage. Scale bars (B) to (F), (I), and (J) represent 100 μm.
Lgr5 Expression Begins after Gdf5 Expression in Digit Joint Formation
Gdf5 is a marker for interzone cells (Merino et al., 1999, Storm and Kingsley, 1999). We compared the expression of Lgr5 with that of Gdf5 in adjacent sections (Figures 1C–1F) in digit III. Gdf5 is expressed in the P2/3 interzone, the last joint formed at E14.5 (Figure 1C), but not Lgr5 (Figure 1C), indicating a later onset. Both Gdf5 and Lgr5 are expressed in the more proximal P1/P2 and M/P1 joints. Interestingly, Lgr5 expression is localized to a subset of interzone cells central to the Gdf5 expression margin of each joint (Figure 1D). At E16.5, just before cavitation, Gdf5 expression persists in a region of the interzones in a distinct horseshoe shape (Figure 1E), with Lgr5+ cells localized to the center of the horseshoe (Figure 1F, circle) with distinct Gdf5+ flanking cells. With cavitation, the number of Lgr5+ cells decreases, whereas some Gdf5+ cells are maintained at the articular surface (Figure 1F). Thus, Lgr5 marks a subset of Gdf5-expressing cells in the interzone with a distinct temporal and spatial pattern in joint development.
Lgr5 Expression in the Developing Knee Joint
The knee joint is more complex, with additional structures of the meniscus and cruciate ligaments. Specific Lgr5 expression can be seen from whole-mount imaging at E16.5 (Figure 1G). We examined histological sections at the peripheral (Figure 1I) and central (Figure 1J) regions of the developing joint from E13.5 to E18.5 as indicated in Figure 1H. Lgr5 is expressed as early as E13.5 in the interzone, before formation of the meniscus, articular cartilage, and cruciate ligaments. From E16.5, concomitant with early-stage cavitation and formation of the meniscus and cruciate ligaments, to maturation at E18.5, Lgr5 expression becomes restricted and weaker at the future articular surfaces of the knee joint (Figure 1I, peripheral sections). However, at this stage, many Lgr5+ cells become evident in the developing lateral and medial meniscus (Figure 1I, peripheral sections). These Lgr5+ cells are localized to the “lip” or narrow regions of the meniscus. Postnatally, Lgr5 expression diminishes with little or no detection in cells of the articular cartilage or the meniscus by day 10 (P10) (Figure S4C). Formation of the cruciate ligaments also starts within the interzone. Strong Lgr5 expression is detected in cruciate ligaments (Figure 1J, central sections), throughout the length of the ligaments from the base at the insertion site and into the cartilage element (Figure 1J).
Lgr5+ Interzone Cells Are Progenitors for Interior Structures of the Knee Joint
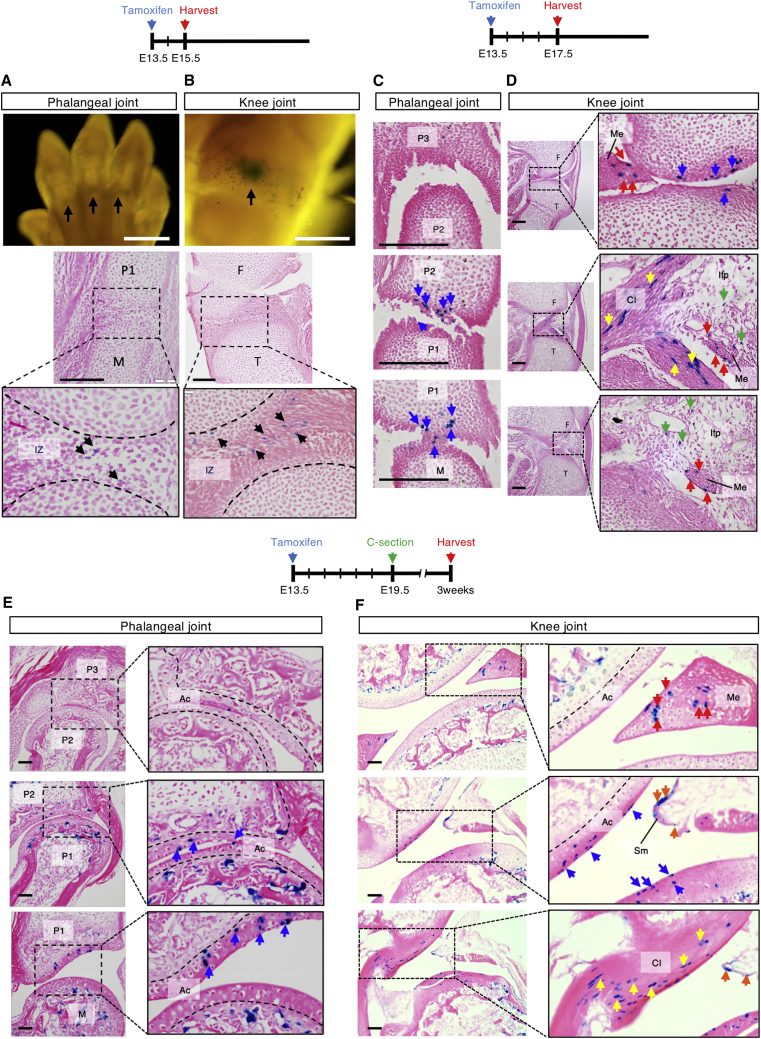
We used Lgr5-eGFP-IRES-CreERT2 mice to tag and trace Lgr5+ cells in the developing joints. A single injection of tamoxifen at E13.5 into Lgr5-GFP; Rosa26-LacZ (R26R) pregnant mice showed β-galactosidase-labeled (LacZ+) cells in the digit (Figure 2A) and knee (Figure 2B) joint interzones at E15.5. At E17.5, descendants of Lgr5+ cells persisted at cavitation (Figure 2C), and at P21 digit joints showed Lgr5+ descendants throughout the full thickness of the articular cartilage (Figure 2E). In the developing knee, descendants of Lgr5+ cells can be detected at E17.5 near the surface of the articular cartilage (Figure 2D, blue arrows), the meniscus (Figure 2D, red arrows), intrapatellar fat pad (Figure 2D, green arrows), and the developing cruciate ligaments (Figure 2D, yellow arrows). They can also be detected when traced to P21 (Figure 2F). Interestingly, many more descendants of Lgr5+ cells were detected in the ligaments than expected from the limiting labeling of interzone cells at E15.5, suggesting proliferation of cells in this lineage. Thus, Lgr5+ cells in the ligament are derived from Lgr5+ interzone cells that persisted to P21 (Figure 2F), and can be located in the synovial membrane (Figure 2F, orange arrows). Together, our findings support the notion that Lgr5+ interzone cells are progenitor cells contributing to all structures of joints.
Figure 2.
Fate of Lgr5+ Interzone Cells in Developing Synovial Joints
Pregnant mice carrying Lgr5-GFP;R26R embryos were injected with tamoxifen at E13.5, and digit and knee joints of the offspring were collected at E15.5 (A and B), E17.5 (C and D), and 3 weeks postnatal (E and F) for analysis, respectively.
(A and B) Cells in the interzone (IZ) are tagged as shown in embryos at E15.5 in whole mount (scale bars, 1 mm) and sagittal section stained for LacZ+ cells (black arrows; scale bars, 100 μm).
(C–F) Descendant cells at the different stages of cell tracing at the articular surface (Ac) in both digit and knee joints (blue arrows). In the knee, LacZ+ cells are also detected in the meniscus (Me; red arrows), the cruciate ligaments (Cl; yellow arrows), the infrapatellar fat pad (Ifp; green arrows), and the synovial membrane (Sm; orange arrows). Scale bars, 100 μm.
Col22a1, a Marker in the Articular Chondrocyte Lineage
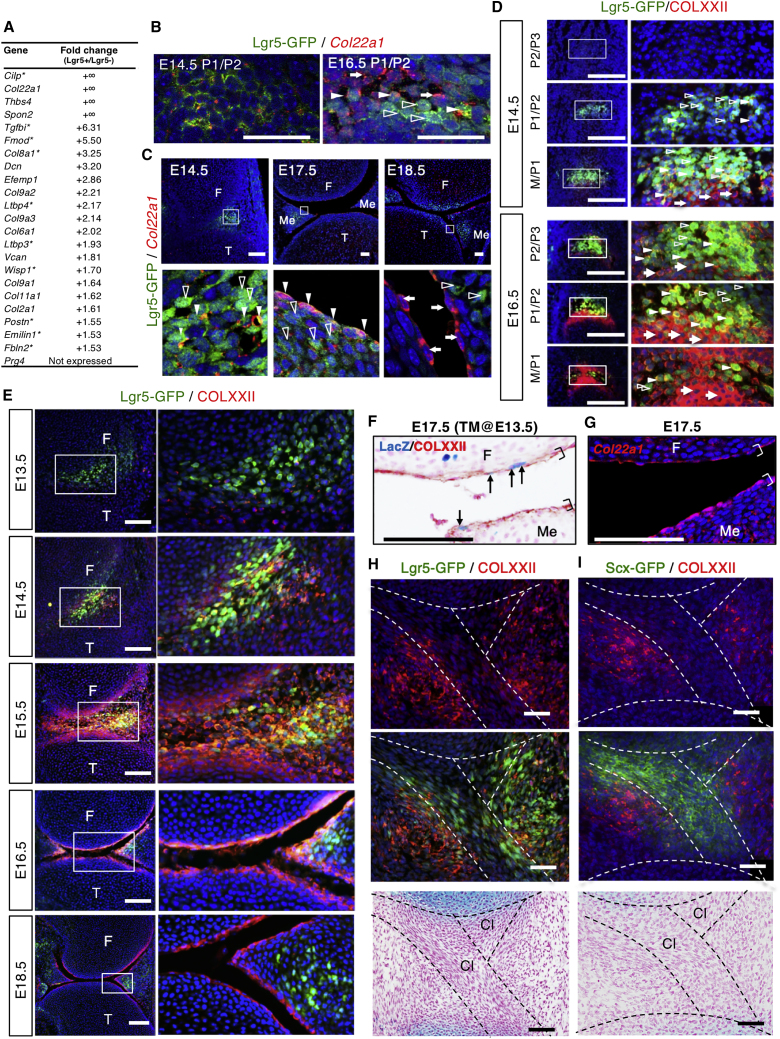
To better understand Lgr5+ cells, we performed transcriptome profiling using RNA sequencing (RNA-seq). We used fluorescence-activated cell sorting (FACS) to isolate three cell types: Sox9-GFP+ cells from metacarpal cartilage anlagen of the condensed mesenchyme at E13.5 from a Sox9IRES-eGFP/+ (Sox9-GFP) embryo, Lgr5+ (GFP+) cells from developing digit joint interzones at E14.5, and Lgr5− (GFP−) cells from the surrounding tissues of the interzone from Lgr5GFP/+ embryos (Figures S3A–S3C). With the cutoff set at fragments per kilobase of exon per million fragments mapped (FPKM) ≥5, ∼8,000 genes were identified in each pool: 7,356 were common to all three datasets; some were common to two sets, and each set contained uniquely expressed genes (Figure S3E). The most commonly expressed genes specific to the Lgr5+ bulk dataset included transcription factors (Glis1, Barx2, and Pknox2) and ECM proteins (Cilp and Col22a1). Several transcription factors reported to be related to joint formation were either specifically or more strongly expressed in Lgr5+ cells from the digit joint, such as Gata3 (Singh et al., 2018), Barx1/2 (Makarenkova and Meech, 2012), Irx1/2 (Zulch et al., 2001), and Sox5/6 (Dy et al., 2010) (Figure S3E and Tables S1–S3). Known pathways regulating interzone differentiation such as WNT, TGFβ, and MAPK (Decker et al., 2014, Gunnell et al., 2010) are also enriched in our Lgr5+ dataset (Figure S3F and Table S4). Our data agree with descriptions of interzone cells in the literature, confirming the quality of our dataset.
Next, we looked for new information and potential markers in the lineage progression to articular chondrocytes. Analysis of ECM environment of Lgr5+ cells can provide an indication of lineage progression with a change in the cell niche. Cilp and Col22a1 are the top differentially expressed ECM genes in Lgr5+ cells (Figure 3A), with Col22a1 specific to Lgr5+ cells (Figure S3E). The overall complement of ECM genes in Lgr5+ cells indicates a chondroprogenitor phenotype not yet expressing Comp or Prg4 (Figure 3A). Thus, Cilp and Col22a1 are potential lineage progression markers of ECM cells. We focus on Col22a1 (COLXXII), as it is localized to tissue junctions and detected at the superficial surface of a mature synovial joint (Koch et al., 2004). To compare the expression of Lgr5 and Col22a1 during joint development, we used in situ hybridization for Col22a1 (Figures 3B and 3C) and immunostaining for COLXXII (Figures 3D and 3E) and GFP (Lgr5) proteins from E14.5 to P0 in digit and knee joints. In E14.5 P1/P2 digit joint, Col22a1/COLXXII expression in the Lgr5+ interzone region was limited (Figures 3B and 3D), whereas a higher level of COLXXII was observed in the more mature M/P1 joint (Figure 3D). This indicates that the onset of Col22a1 expression is later than Lgr5. Before cavitation, many cells co-express Lgr5 and COLXXII (Figure 3D). With cavitation (E16.5), there are fewer Lgr5+ cells and more COLXXII-expressing Lgr5− cells (Figure 3D, E16.5). Using the P1/P2 joint at E16.5 as an example, we clearly identified cells that express Lgr5 but not Col22a1 (Figure 3D, open arrowheads), double-positive cells (Figure 3D, solid arrowheads), and many cells expressing only COLXXII (Figure 3D, solid arrows). As COLXXII is an ECM protein, we define double-positive cells as cells that are GFP+ (intracellular) with pericellular staining for COLXXII. This is supported by direct co-localization of Col22a1 mRNA in Lgr5-expressing cells (Figure 3B). By E18.5, there were only a few Lgr5+ cells, which are likely to be double positive for COLXXII as they are embedded in a COLXXII-enriched ECM layer (Figure S4A). At this stage, COLXXII-expressing cells marked the surface of the future articular cartilage. By birth (P0), a distinct thin layer of COLXXII-containing ECM became apparent, and the proximal/distal expression difference was no longer evident (Figure S4B). Similarly, at P10 (Figure S4C), Lgr5+ cells were no longer detectable along the entire surface of the digit joint.
Figure 3.
Dynamic Spatiotemporal Expression of Lgr5 and Collagen XXII in Knee Joint Development
(A) Matrix genes with higher expression in Lgr5+ compared with Lgr5-from digit bulk transcriptome. “+∞” indicates that a gene is not expressed in the Lgr5− cell population. Genes with asterisks indicate that expression data for the developing joint are available in Eurexpress.
(B and C) Co-expression of Lgr5 (GFP) and Col22a1 mRNA in digit (B; scale bars, 50 μm) and knee joints (C; scale bars, 100 μm); boxed areas are shown in the bottom panels. Cells positive to Lgr5-GFP only are indicated with open white arrowheads, double positive to Lgr5-GFP/Col22a1 with solid white arrowheads, and cells expressing only Col22a1 with solid arrows.
(D) Double fluorescent immunostaining of Lgr5-GFP (green) and COLXXII (red) on sagittal sections of the developing knees of Lgr5-GFP embryos at E14.5 and E18.5 (see also Figures S4A and S4B). Images of the boxed areas are shown on the right panels. Formation of the articular cartilage progresses from Lgr5+ cells (open white arrowheads), to cells expressing both Lgr5 and COLXXII (solid white arrowheads), to cells expressing only COLXXII (solid arrows). Scale bars, 200 μm.
(E) Co-localization of Lgr5 and COLXXII in developing articular cartilage and meniscus. COLXXII matrix layer condenses to a very narrow region at the superficial surface of the fully developed articular cartilage and meniscus. Boxed regions are shown in the right panels. Scale bars, 200 μm.
(F) LacZ+ descendant cells of Lgr5 (arrows), with tamoxifen (TM) injected at E13.5 and harvested at E17.5 can be detected at the COLXXII+ superficial layer (brackets) of the articular cartilage and meniscus. Scale bars, 100 μm.
(G–I) All cells in this layer (G; brackets) express Col22a1 in a comparable section. Co-localization of COLXXII with Lgr5 (GFP) (H) and Scx (GFP) (I). Histology of the same sections stained with Alcian blue and nuclear fast red (H and I, lower panels). F, femur; T, tibia; Cl, cruciate ligament. Scale bars, 50 μm.
See also Figures S3 and S4.
In the knee, a similar expression relationship is observed (Figures 3C and 3E). Lgr5 is expressed at E13.5 (Figure 3E) and E14.5 (Figures 3C and 3E) as the interzone cells condense to form the articular cartilage and meniscus. Lgr5+ cells first mark the structure of the future meniscus at E13.5, followed by the condensation of COLXXII-expressing cells along the superficial layer from E15.5 to E18.5 (Figure 3E). Differentiation to superficial meniscus cells is also likely to involve a transition from Lgr5- to COLXXII-expressing cells. Indeed, Lgr5/COLXXII double-positive cells were detected at the boundary by immunostaining at E15.5 (Figure 3E) and by in situ hybridization at E17.5 (Figure 3C). By E18.5 (Figures 3C and 3E), a distinct layer of COLXXII-containing matrix was present, with some Lgr5+ cells in the deeper region of the meniscus. Similar to digit joints, Lgr5+ cells were no longer detectable at P10, but the COLXXII layer persisted (Figure S4C). At E17.5, we detected Lgr5-descendant cells (LacZ+) labeled on E13.5 in the COLXXII-containing superficial layer (Figure 3F) where Col22a1 transcripts were also detected (Figure 3G), indicating that some of the cells in this layer come from Lgr5+ interzone cells. Furthermore, cells embedded in this layer interact with the ECM as indicated by the clustering of β1-integrin into focal adhesions (Figure S4D).
Because Lgr5 is also expressed in the developing ligaments, we analyzed its expression/localization relationship with COLXXII at E15.5, when the cruciate ligaments start to form (Figures 3H and 3I). Interestingly, while Lgr5+ cells are detected throughout the central region of the interzone, COLXXII expression is localized to the flanking regions outside of the developing ligaments (Figure 3H). Furthermore, analysis of COLXXII expression in a Scx-GFP mouse showed distinct expression of Scx (GFP) restricted within the developing ligament and not in the flanking COLXXII-positive regions of the interzone (Figure 3I). This supports lineage divergence of Lgr5+ progenitors to ligament cells and articular chondrocytes, with mutually exclusive expression patterns, and that there is a distinct ECM niche for these two lineages. A COLXXII-containing niche would support the formation of articular cartilage and meniscus.
Distinct Signatures of Lgr5+ Interzone Cells for Chondrocyte or Ligament Lineages
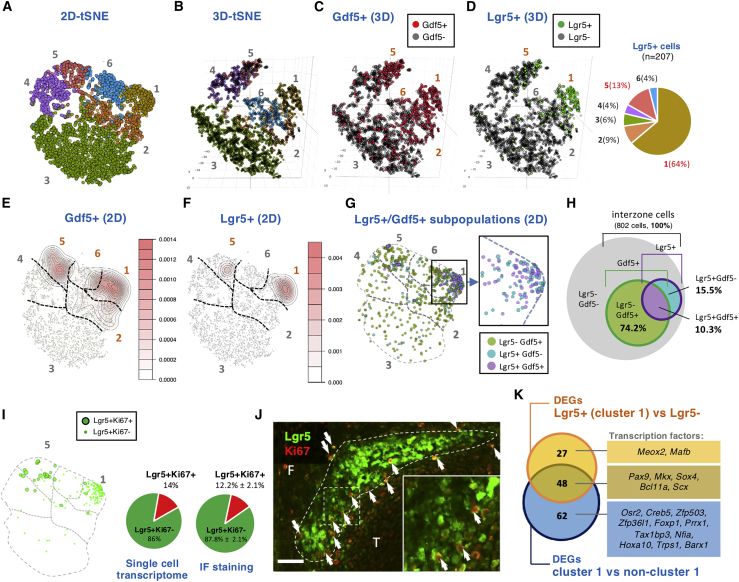
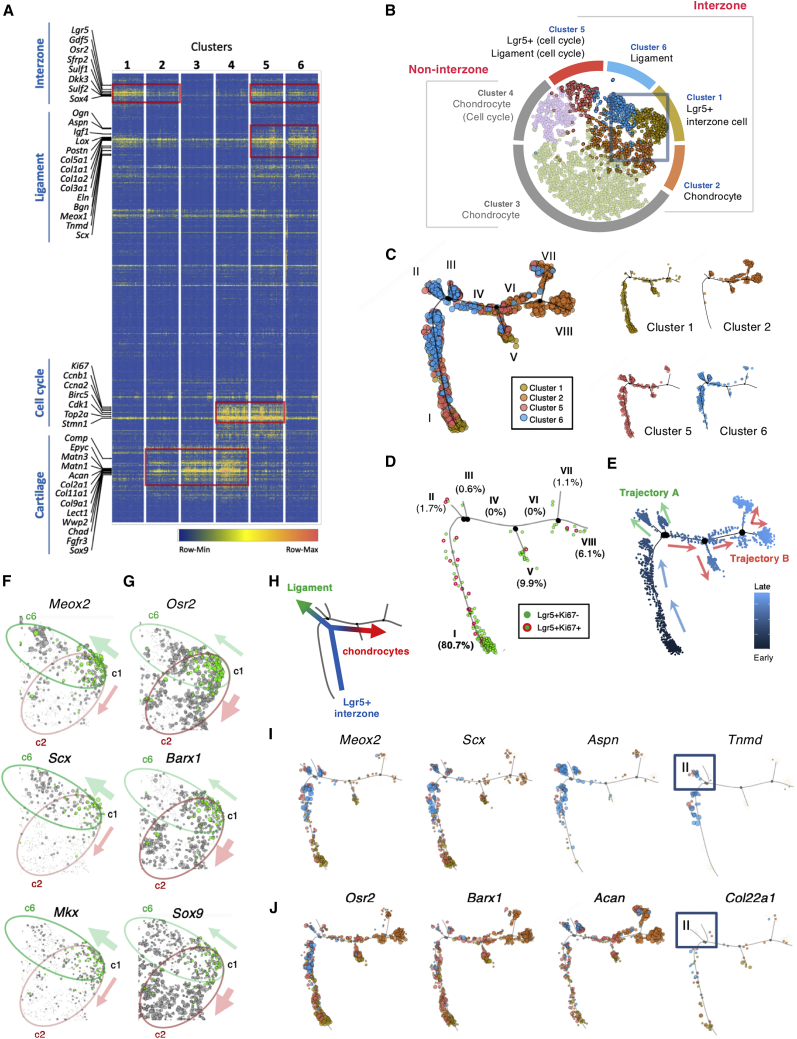
To investigate the relationships of different cell populations in the developing knee joint, we performed single-cell RNA-seq analysis of cells from knee interzone regions of Lgr5GFP/+ embryos at E14.5. After exclusion of blood cells, 5,460 interzone and surrounding cells were sequenced (Table S5). T-distributed stochastic neighbor embedding (tSNE) analysis grouped cells with similar expression profiles into six clusters. When the tSNE map was viewed in two dimensions (2D-tSNE), clusters 4 and 5 appeared to be distant from the main clusters (1, 2, 3, 6) (Figure 4A). The 3D view (3D-tSNE) showed a horseshoe shape, with clusters 1 and 5 at the tips of the horseshoe (Figure 4B). Gdf5, a marker for all interzone cells, is expressed in cells scattered throughout the six clusters (Figure 4C), but appeared concentrated within clusters 1, 2, 5, and 6 (Figure 4E). A total of 207 Lgr5-expressing cells were identified among cells expressing the endogenous Lgr5 (n = 94), the Lgr5-eGFP-CreERT2 allele (n = 87), or both (n = 26). Interestingly, Lgr5 expression is concentrated in clusters 1 (64% of all Lgr5+ cells) and 5 (13%) at the tips of the horseshoe (Figure 4D), and is well illustrated in the 2D-tSNE heatmap (Figure 4F). Other Lgr5+ cells are scattered throughout the cluster map (Figure 4D).
Figure 4.
Properties and Molecular Signatures of Lgr5+ Cells in E14.5 Knee Joint Interzone Revealed by Single-Cell Transcriptome
(A and B) Gene expression from 5,460 cells was analyzed using tSNE plots in 2D (A) or 3D (B), with six major clusters identified.
(C and D) 3D view for the distribution of Gdf5+ (C) and Lgr5+ (D) cells. The relative distribution of Lgr5 expression in the different clusters is presented as a pie chart in (D).
(E and F) Contour plots illustrating the density of cells expressing (F) Lgr5 shown in (B), and (E) Gdf5 shown in (C), with the corresponding colored density chart.
(G) 2D-tSNE showing the distribution of Lgr5+ among Gdf5+ cells. Higher magnification of the boxed area in cluster 1 is shown on the right panel, highlighting the relative Lgr5+/Gdf5− (cyan) and Lgr5+/Gdf5+ (purple) cells.
(H) Venn diagram illustrating the distribution and percentages of Lgr5−/Gdf5+ (green), Lgr5+/Gdf5− (cyan), and Lgr5+/Gdf5+ (purple) subpopulation of cells.
(I) 2D-tSNE plot highlighting the distribution of Lgr5+/Ki67+ in cluster 5, relative to the dominance of Lgr5+/Ki67− cells in cluster 1, and the percentage of Lgr5+/Ki67+ shown in a pie chart.
(J) Double immunofluorescence detection of Lgr5-GFP- and Ki67-expressing cells in an E14.5 knee joint. The peripheral of the Lgr5 cell population is marked with a dotted line, and arrows indicate the double-positive cells with the boxed region shown in higher magnification. The relative percentage of Lgr5+/Ki67+, determined from three embryos, is also shown in a pie chart. F, femur; T, tibia. Scale bars, 20 μm.
(K) Venn diagram showing the DEGs of cluster 1 versus non-cluster 1 (n = 110, blue circle) and DEGs Lgr5+ versus Lgr5− cells (n = 75, yellow circle). There are 48 common DEGs identified with these two gene lists. Transcription factors in each sector are shown.
Next, we assessed the relationship between Lgr5+ and Gdf5+ cells. We expected Lgr5+ cells to be Gdf5+ from our in vivo analysis at E14.5 (Figures 1C and 1D). We observed Gdf5+/Lgr5− and Gdf5+/Lgr5+ cells, but also Gdf5−/Lgr5+ cells (Figures 4G and 4H). Differentially expressed gene (DEG) analysis of Lgr5+/Gdf5− and Lgr5+/Gdf5+ cells found the only difference was the absence or presence of Gdf5 expression (Figure S5A). Thus, these two cell populations are the same, and the difference is likely due to a “dropout” event in single-cell RNA-seq (Kharchenko et al., 2014) for Gdf5 if its expression was low in some Lgr5+ cells. Similar DEG analysis of clusters 1 and 5 of Lgr5+ cells showed that the key difference was additionally expressed genes in cluster 5 that mapped to Gene Ontology terms corresponding to cell-cycle events (Figures S5B and S5C). We selected Ki67 as one of the most differentially expressed cell-cycle genes and mapped its expression in the 2D-tSNE profiles. A total of 14% of the Lgr5+ cells in all clusters expressed Ki67; most of them are in cluster 5 but not in cluster 1 (Figure 4I). Similarly, we found that 12.2% ± 2.1% (n = 3) of Lgr5+ cells in the E14.5 knee interzone region expressed Ki67; these cells were located around the periphery of the Lgr5+ interzone region (Figure 4J).
As most Lgr5+ cells were in cluster 1, we conducted a DEG analysis of cluster 1 against the other five clusters. We identified 110 DEGs (Figure 4K and Table S6), including transcription factors related to joint development such as Osr2, Trps1, Barx1, and Sox4. When we increased the stringency by comparing Lgr5+ cells in cluster 1 with Lgr5− cells in the whole population, we identified 75 DEGs (Table S6), 48 of which overlapped with the previous gene set including the transcription factors Mkx and Scx. Twenty-seven genes were specifically expressed in Lgr5+ cells, including the transcription factor Meox2 (Figure 4K). Together, these analyses described distinct signatures of Lgr5+ cells in the interzone for the chondrocyte and ligament lineages.
Trajectories of Joint Lineage Specification
To annotate these clusters, we clustered the top 5% of the dispersed genes (genome-wide of all 5,460 cells) into a heatmap. Gene modules associated with interzone, ligament, cell cycle, and cartilage were identified (Figure 5A). As expected from the 2D-tSNE heatmap for Gdf5 expression (Figure 4E), cells in clusters 1, 2, 5, and 6 expressed genes associated with interzone cells, including Gdf5, Osr2, Sfrp2, Sulf1, and Sox4. Relative to other clusters, cells in clusters 5 and 6 expressed higher levels of genes related to ligament, such as Lox, Col1a1, Aspn, and Scx. The major difference between cluster 5 and cluster 6 was the high abundance of cell-cycle-related genes in cluster 5, such as Ki67, Ccna2, Birc5, and Top2a, which are also expressed in cluster 4. Cells in clusters 2, 3, and 4 expressed cartilage-related genes such as Epyc, Matn1, Acan, Lect1, and Sox9. When we analyzed the same dataset with principal component analysis, we found similar clustering of interzone and non-interzone cells as well as similar cell-cycle status of cells in these clusters (Figures S5D–S5G). The expression profile in each of the clusters agreed with the violin plots of representative genes for cartilage and ligament, and cell-cycle genes (Figure S5H). Thus, clusters 1, 2, 5, and 6 are considered “interzone clusters,” whereas 3 and 4 are defined as “non-interzone clusters” (Figure 5B). Cluster 1 is enriched with Lgr5+/Gdf5+ interzone cells and is mapped between the clusters with the ligament (cluster 6) and chondrocyte (cluster 2) signatures, with some “mixing” of cells at the borders, implicating a potential divergence of the cluster 1 interzone cells into either chondrocytes or ligament lineages (Figure 5B).
Figure 5.
Lineage Trajectories of Knee Joint
(A) Heatmap showing the clustering of the top 5% dispersed genes (genome-wide), from the profiles of 200 cells randomly selected from each cluster as a representative. Gene clusters related to joint development, ligament, cell cycle, and cartilage were identified.
(B) Summary diagram of the property/signature of each cluster, with clusters 1, 2, 5, and 6 defined as interzone cells.
(C) Pseudo-timeline analysis of cells from clusters 1, 2, 5, and 6 identified eight cell states, and the distribution of cells from individual cluster and a combined map along the pseudo-timeline is shown.
(D) Distribution of all Lgr5+ cells along the pseudo-timeline, and relative percentages distributed in each of the cell state indicated, together with proliferative Lgr5+/Ki67+ cells.
(E–G) The pseudo-timeline (E) predicted a gradient of “early” (dark blue) to late (light blue) cells, with a major divergence to two trajectories A and B, with arrows indicating the predicted direction. Expression distribution of ligament (F) and chondrocyte (G) genes are mapped onto the 2D tSNE. The size of each circle is a relative reflection of the gene expression level; Lgr5+ cells are indicated as green.
(H) Illustration showing the major lineage divergence of Lgr5+ interzone cells.
(I and J) Distribution of cells expressing ligament (I) and cartilage (J) genes along the pseudo-timeline. The boxes highlight a specific comparison of cells in state II expressing a mature ligament marker Tnmd but not Col22a1.
To study the cell-lineage divergence, we performed a pseudo-timeline analysis focusing on the interzone clusters (1, 2, 5, and 6) that mapped into eight potential cell states (Figure 5C). Cells in cluster 1 mapped mainly in state I with some cells scattered throughout other states. Cells in clusters 5 and 6 with the ligament signature were distributed in states I, II, and III, while cells in cluster 2 with the chondrocyte signature were distributed primarily in cell states V, VI, VII, and VIII (Figure 5C). State IV contained a mixture of cells from all four clusters. Next, we assessed the distribution of Lgr5+/Ki76+ cells and showed that they are distributed within Lgr5+/Ki67− cells, but most Lgr5+ cells mapped to state I (Figure 5D), which represents the most primitive/young state as predicted in the pseudo-timeline (Figure 5E). Two major trajectories branched from state I: trajectory A consisted of cells in states II/III (Figure 5E, green arrows) and trajectory B included cells in states V/VI/VII/VIII (Figure 5E, red arrows).
To characterize the trajectories, we assessed expression of the differentially expressed transcription factors identified (Figure 4K). Scx, Meox2, and Mkx are expressed in ligament cells, and their expression is enriched in cells of clusters 1 and 6 (Figure 5F, green oval) but less in cells of cluster 2 (Figure 5F, pink oval). Osr2 and Barx1 are expressed in cartilage development and their expression is enriched in clusters 1 and 2, but less in cluster 6 (Figure 5G). Sox9, a key chondrogenic transcription factor, is not differentially expressed in cluster 1 or among Lgr5+ cells: overall, its expression is upregulated in cluster 2 but not in cluster 6 (Figure 5G). This supports a divergence of cells in cluster 1 from a “bipotential” state that can differentiate along the ligament or the articular cartilage lineage (Figure 5H). Next, we mapped the transcription factors and ECM genes specific to ligament (Figure 5I) and cartilage (Figure 5J) onto the pseudo-timeline and found them to be enriched in the cell states that supported the relevant tissue trajectory (Figure 5E). Based on the algorithm for the trajectory, some cells co-expressed both ligament and cartilage transcription factors prior to the lineage divergence. For example, cells from cluster 6 distributed at the end of state I expressed both Meox2 (blue circles in Figure 5I, left panel) and Osr2 (blue circles in Figure 5J, left panel). In support of the in vivo data (Figure 3H), state II cells in trajectory A did not express Col22a1 but did express the mature ligament marker Tnmd (Figures 5I and 5J, blue boxes). Col22a1 was expressed in cells of trajectory B, supporting our hypothesis that Col22a1 marks the chondrocyte lineage as distinct from the ligament lineage. Thus, our single-cell transcriptome data, together with our lineage-tracing experiment, showed Lgr5+ interzone cells are likely to be multipotent and represent a stage of joint formation at which the lineage divides into ligament and articular chondrocytes (Figure 5H).
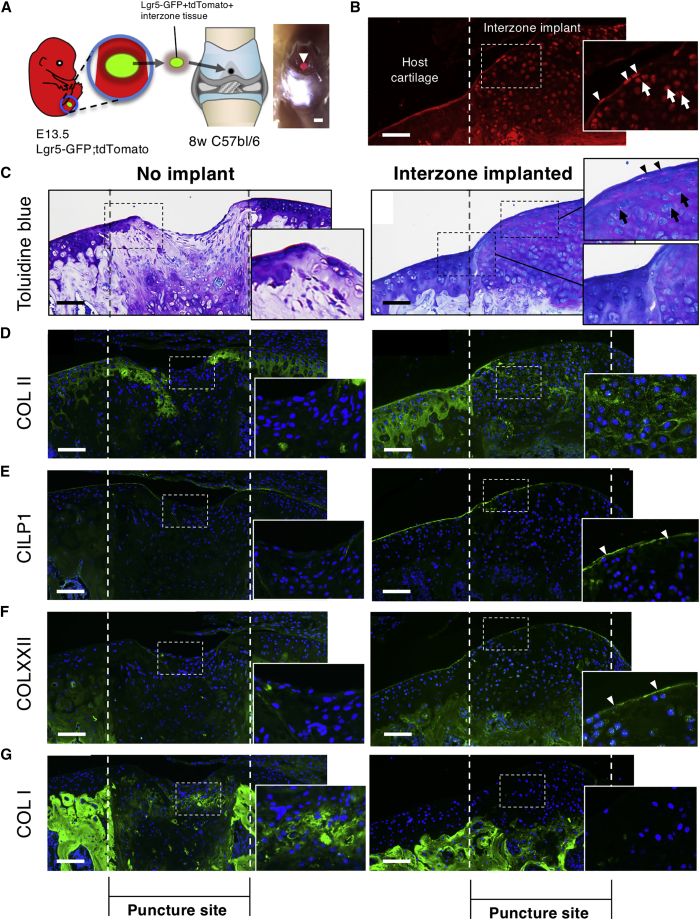
Lgr5+ Interzone Tissue Repairs Cartilage Lesion
Lgr5+ interzone cells could be suitable for the repair of cartilage, as they are “primitive” and multipotent. To test this capacity, we dissected the Lgr5-GFP+ interzone tissue from E13.5 Lgr5-GFP;ROSA-tdTomato embryos and transplanted it to a full-thickness needle-puncture lesion at the trochlear groove of the knee of 8-week-old mice (n = 3) (Figures 6A and S6B). Fifteen days post puncture, tdTomato+ cells were detected in the lesion, as differentiating round chondrocytes in the intermediate and deep zones of articular cartilage (Figures 6B and 6C, arrows; control section is shown in Figure S6A) and flattened cells in the superficial zone (Figures 6B and 6C, arrowheads). The healing lesion contained cells that originated from the transplanted tissue, which produced proteoglycan-enriched matrix (Figure 6C) and COL II deposition (Figure 6D). The new tissue integrated well with the host cartilage (Figure 6C, box) with a new superficial layer containing COLXXII and CILP1 intercalated with the superficial zone of the host articular cartilage (Figures 6E and 6F). The implanted tissue suppressed expression of COL I (Figure 6G) in the lesion, which could result in unwanted fibrosis during cartilage repair. Together, our findings suggest that Lgr5+ interzone cells have repair capacity for articular cartilage.
Figure 6.
Capability of Lgr5+ Interzone Tissue to Repair Cartilage Lesion
(A) Experimental design for the isolation of Lgr5+ interzone tissue (labeled with both GFP and tdTomato) and transplantation into articular cartilage defect for repair. The right panel shows the tdTomato+ tissue inside the lesion directly after transplantation. Scale bars, 1 mm.
(B) Implanted interzone tissue (tdTomato+) integrated with the host cartilage and differentiated into cells in articular cartilage and flattened cells in the superficial layer (arrowheads), 15 days post implantation.
(C–G) Histological (C) and ECM marker (D–G) analyses performed on lesion with and without Lgr5+ implantation after 15 days. High magnifications of the boxed areas are shown in the insets.
Scale bars, 50 μm. See also Figure S6.
Discussion
The diverse tissues of the synovial joint derive from mesenchymal cells in the developing interzone, most or all of which express Gdf5 (Koyama et al., 2008). Tracing studies in a Gdf5-CreERT2 mouse showed that after the initial dedifferentiation of chondrogenic cells to Gdf5+ interzone cells, further expansion of the Gdf5+ region is primarily through recruitment of regional mesenchymal cells rather than proliferation of Gdf5+ interzone cells (Shwartz et al., 2016). Furthermore, Shwartz et al. (2016) proposed that cells within the interzone have contextual cues, and their ability to produce distinct joint tissues is governed by positional effects. Here, we identified a new subset of Gdf5-expressing interzone cells, distinguished by co-expression of Lgr5, a known marker for highly proliferative stem cells (Leung et al., 2018), although cell division is rare within the interzone (Shwartz et al., 2016). Significantly, this subset of Lgr5-expressing cells in the Gdf5+ population can also give rise to the diverse tissues of the joint, including the synovial membrane, cruciate ligaments, meniscus, and articular cartilage.
Lgr5+ Interzone Cells Contribute to the Making of the Diverse Joint Structures
The Lgr5+ cell pool emerges shortly after the initial appearance of Gdf5-expressing cells marking the future joint site. Therefore, Lgr5+ cells are likely to be derived from Gdf5+ cells that have undergone dedifferentiation, consistent with cell-fate mapping findings using Gdf5-Cre mice (Koyama et al., 2008), and that the progressive differentiation from a Gdf5+ to a Lgr5+ cell is a sequential event. In the digit joints, because Lgr5+ cells arise and remain localized within the center of the interzone, incoming Gdf5+ cells proposed in the continuous influx model (Shwartz et al., 2016) would be the peripheral to the central pool of Lgr5+ cells. Our single-cell transcriptome showed that the Lgr5+ pool expands in part through differentiation and proliferation of the peripheral interzone cells, shown by the presence of Lgr5+/Ki67+ cells in the developing joint, and a population of Lgr5+ cells that differ only in the expression of active cell-cycle-related genes in the tSNE map of cell clusters. Other mechanisms might exist in the more complex knee interzone.
In our mapping study, activation of the LacZ at E13.5 would tag this early pool of Lgr5+ cells, and we observed that they can contribute to all structures of the joint. Having only a few Lgr5-expressing cells tagged from a single tamoxifen injection allows us to assess the level of contribution to the different structures of the joint. In the developing knee joint, there were no observable differences in the contribution of Lgr5+ cells to the articular cartilage, meniscus, and synovium. Interestingly, many more descendant cells are found in the cruciate ligaments, suggesting that they have proliferated. Furthermore, Lgr5 continues to be expressed in cells of the developing ligament, consistent with expansion of the tagged Lgr5+ cells from the E13.5 interzone with numerous descendant cells in the ligament at E17.5. This is supported by the identification of a ligament cell cluster with active cell-cycle genes in the tSNE map of the single-cell transcriptome data from an E14.5 interzone.
Lgr5+ Cells Are Poised Progenitors at the Onset of Lineage Divergence
Consistent with the in vivo analyses, the transcriptomic data from both the bulk and single-cell analyses support Lgr5+ cells as progenitors “poised” for differentiation along the articular chondrocyte or ligament lineages. Using the clusters representing the interzone cells to derive a pseudo-timeline, we identified eight cell states, placing cluster 1 with the bulk of the Lgr5+ cells as the most “primitive” state I of “poised” progenitor cells (Figure 5D). Many Lgr5+ cells in state I express gene signatures for both ligament and chondrocyte, then specialize into the more distinct signature for the ligament or chondrocyte lineages. Changes in the balance of lineage-specifying transcription factors may control the divergence.
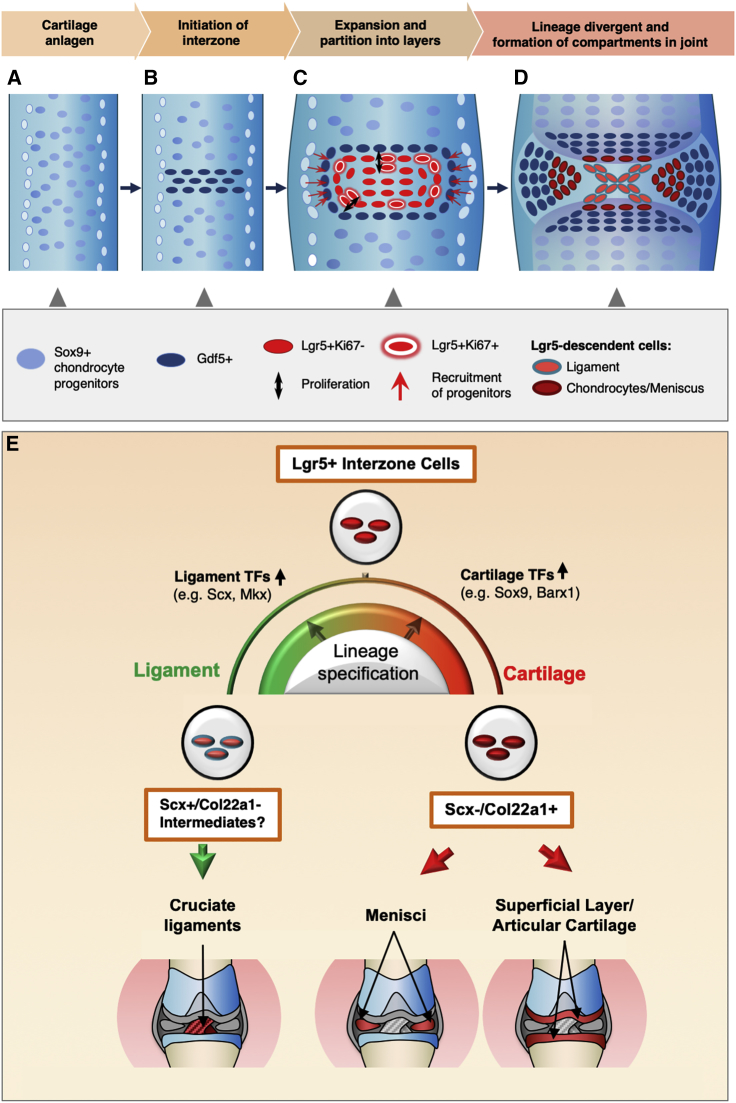
Interestingly, about 10% of Lgr5+ cells are found in state V, predicted to be derived from state I, and the reason for this is unclear. It is possible that these are from cells recruited differently in joint development (Shwartz et al., 2016), and this cannot be excluded. Multiple cell states exist within each of the ligament or chondrocyte trajectories, suggesting that there are subclasses of cells within each lineage, possibly related to their position along the cruciate ligament, or differences between chondrocytes residing in the articular or meniscus regions. For example, a multipotent Scx+/Sox9+ progenitor cell pool at the chondro-tendinous/ligamentous junction gives rise to tenocytes/ligamentocytes and chondrocytes (Sugimoto et al., 2013). We propose that there could be positional values for these cells within the developing knee joint, as depicted in a model based on our current findings (Figure 7).
Figure 7.
Model for the Contribution and Divergence of Progenitor Cells in Joint Formation
A schematic diagram showing the developmental processes of a knee joint.
(A) Formation of the cartilage anlagen containing Sox9-expressing chondrocytes.
(B) Dedifferentiation of Sox9+ chondrocytes to Gdf5+ interzone cells at the presumptive joint site.
(C) Expansion of the interzone through recruitment of mesenchymal cells from the surrounding and limited proliferation of peripheral Lgr5+ cells, and the partitioning of the interzone into specific regions.
(D) Lgr5+ interzone cells undergo lineage divergent and differentiate into cells for the different tissues in a mature joint.
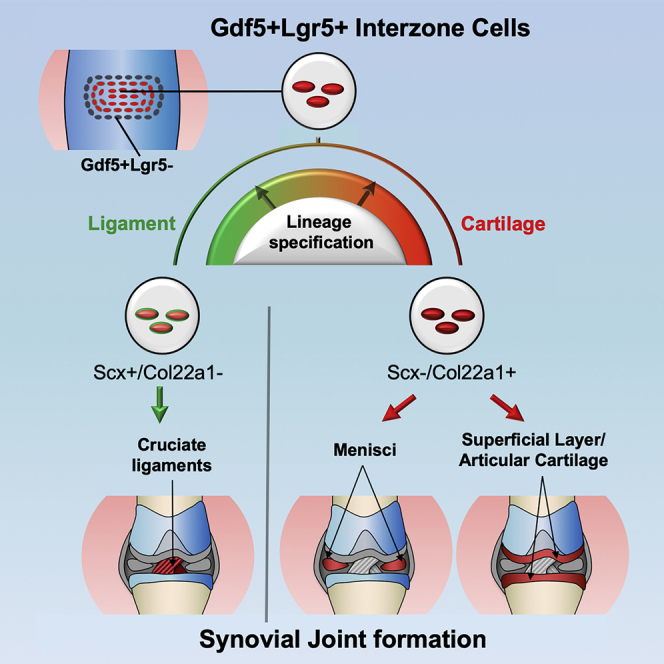
(E) Lgr5+ interzone cells are multipotent progenitors contributing to all interior structures of the knee joint including cruciate ligaments, articular cartilage, and menisci. Lgr5+/Scx−/Col22a1+ cells are committed progenitors for articular chondrocyte lineage and menisci, whereas Lgr5+/Scx+/Col22a1− cells are restricted within the ligament lineage.
Role of Lgr5 and Wnt Signaling in Joint Development
Although Lgr5-GFP homozygous mice are perinatal lethal (Barker et al., 2007), they show no histological abnormality in joints (Figure S2). This might be due to functional redundancy among the three LGR proteins (LGR4, LGR5, and LGR6) (Ruffner et al., 2012). Lgr4 is expressed in our transcriptome dataset, although Lgr6 is not. Lgr5 expression may reflect a positional effect at an appropriate level of Wnt ligands for its activation, and the potentiation of Wnt signaling by Lgr5 in turn sustains a higher signaling level through interaction with R-spondins (de Lau et al., 2011), necessary for the progression to the next stage, poised for the formation of other structures and cavitation.
Col22a1 Marks an Articular Cartilage Lineage from Lgr5-Expressing Interzone Cells
Decreasing Lgr5 expression with cavitation and in cells at the juvenile articular surface is consistent with the need to reduce anti-chondrogenic Wnt signal for articular chondrocyte differentiation. We know very little about the molecular control of this lineage. Chondrogenic bone morphogenetic protein signaling plays an important role (Rountree et al., 2004), supported by the continuing expression of Gdf5 while Lgr5 decreases, tipping the balance to chondrogenesis, and our identification of Col22a1 as an intermediate marker for this lineage and cells co-expressing Col22a1 and Lgr5. Col22a1/COLXXII expression started proximally, but then occurred close to the margins of the future articular surface within the developing interzone. This is best illustrated in the less complex digit joints, and is consistent with the proposed model whereby the developing interzone is organized into three layers, with the two outer layers containing cells committed to become articular chondrocytes, and cells in the middle layer contributing to other structures or undergoing apoptosis (Mitrovic, 1977). The COLXXII-expressing cell layers within the interzone are broad just prior to cavitation. Overlap between the COLXXII-expressing regions and Lgr5+ cells at the earliest stage of cavitation suggests that most Col22a1-expressing cells are derived from Lgr5+ cells. Interestingly, the Lgr5+/Col22a1+ co-expressing cells are primarily localized to the mid region of the articular surface throughout the cavitation process, with Col22a1+/Lgr5− cells occupying the flanking regions along the entire surface of the joint. If Col22a1-expressing cells are derived from Lgr5+ cells, expansion of the articular surface is mediated through cellular differentiation at the midpoint contributing to the lateral growth of the joint surface. Indeed, this may have been reflected in the multiple cell states identified in the chondrocyte trajectory from the pseudo-timeline prediction of the single-cell transcriptome (Figures 5D and 5E).
We propose a model whereby cells express Gdf5, progress to Gdf5+/Lgr5+ double-positive progenitor cells, then Lgr5+/Col22a1+ double-positive committed articular chondrocyte progenitors, and finally Col22a1-expressing juvenile articular chondrocytes (Figure 7). The continuous influx model invokes an expansion of the Gdf5+ interzone cell pool from recruitment (Shwartz et al., 2016). We propose that a spatial effect of the various pools of committed progenitors dictates the positioning of the joint structures. Single-cell transcriptome with more cells from additional developmental time points should provide key markers for validation, and further insights into the molecular controls and subdivision of cell states.
Capacity of Lgr5+ Cells in Cartilage Repair: Re-establishment of the Collagen XXII-Containing Superficial Layer
The superficial zone contains cells producing Lubricin/Prg4 for joint lubrication (Kozhemyakina et al., 2015) and progenitor cells (Dowthwaite et al., 2004). Recent cell-tracing studies identified self-renewing progenitor cells at the superficial zone of the mouse articular cartilage that may undergo symmetric and asymmetric expansion in juvenile joints (Decker et al., 2017, Li et al., 2017). Our analysis showed that COLXXII demarcates the outermost surface of the joint. Cells embedded in the COLXXII-containing layer have a distinct flattened morphology with direct interaction with the ECM, shown by the presence of focal adhesions with clustering of β1-integrin receptors. These cells might provide a special niche for cell maintenance and act as a source of progenitors. Finally, we showed that Lgr5+ cells collected from an E13.5 embryonic knee joint can repair a full-thickness articular cartilage defect. Importantly, a COLXXII-containing superficial layer was re-established in the healing defect, which quickly integrated with the host cartilage, indicating that these Lgr5+ cells are candidates for cell-based therapy for cartilage and ligament trauma or associated degenerative diseases.
Experimental Procedures
Mouse Strains
Lgr5-eGFP-IRES-CreERT2 (Lgr5-GFP) (Barker et al., 2007), ROSA26-LacZ (R26R) reporter (Soriano, 1999), and Scx-GFP (Pryce et al., 2007) mice were maintained in C57bl/6 background. PCR primers for genotyping these mice are listed in Table S7. All animal works were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong.
Real-Time qPCR
mRNA was extracted from E14.5 digit interzone and surrounding non-interzone regions using TRIzol reagent (Thermo Fisher). cDNA was generated using the PrimeScript RT reagent kit (Clontech). qPCR was performed using LightCycler480 SYBR Green I Master kit (Roche). All primers are listed in Table S7.
In Situ Hybridization and Immunohistochemistry
In situ hybridization was performed as previously described (Gao et al., 2009), using [α-35S]uridine triphosphate-labeled riboprobes for Gdf5. Fluorescence in situ hybridization was performed as previously described (Shwartz and Zelzer, 2014), using a digoxigenin-labeled probe for Col22a1. For immunohistochemistry, sections were incubated with primary antibodies for goat anti-GFP (ab6673), guinea pig anti-COLXXII (produced by Dr. Manuel Koch), sheep anti-CILP1 (R&D Systems), and rabbit anti-Ki67 (Abcam ab1558), and detected using the relevant secondary antibodies (Alexa Fluor 488 anti-rabbit [Thermo Fisher], Alexa Fluor 488 anti-goat [Abcam], Alexa Fluor 488 anti-sheep [Abcam], and Cy3 anti-guinea pig immunoglobulin G [Jackson ImmunoResearch]).
Lineage Tracing and X-gal Staining
Tamoxifen (Sigma) was administered by intraperitoneal injection (0.2 mg/g body weight) to pregnant mice carrying Lgr5-GFP/R26R embryos at E13.5. Limbs were processed for whole-mount X-gal staining to detect LacZ activity. Three-week-old samples were decalcified overnight in 0.5 M EDTA (pH 7.5); 7-μm paraffin sections were counterstained with eosin.
Imaging
Images were acquired using an Axioplan 2 microscope (Zeiss) with SPOT-camera or the Leica TCS SPE Live confocal microscope. The contrast and color of some images were adjusted with the “brightness/contrast” and “hue/saturation” functions in Adobe Photoshop CS. No further modifications of the images were made.
Bulk Transcriptome Analysis
Sox9+ cells were isolated from digits of E13.5 Sox9-GFP embryos (Nakamura et al., 2011). Lgr5+ and surrounding non-Lgr5 (Lgr5−) cells were isolated from forelimb digit interzones of E14.5 Lgr5GFP/+ mice (Figures S3A–S3C). Cells were released with a mixture of TrypLE Express (Gibco) and 0.1% DNase I for 20 min, filtered through a 40 μm cell strainer, and sorted by FACS using an Aria I flow cytometer (BD Biosciences). Total RNA was extracted using a mirVana miRNA Isolation Kit (Ambion) and cDNA libraries were constructed with 10 ng of RNA using a SMARTer Ultra Low Input Kit (v3, Illumina), and sequenced using the Illumina HiSeq 1500 platform (Center for Genomic Sciences, The University of Hong Kong). cDNA fragment sequences were aligned to mouse genome (mm10) using the HISAT program (Kim et al., 2015). FPKM values were generated for comparison (Figure S3D). Genes with FPKM ≥ 5 were considered as expressing genes. Pathway and expression analyses were performed with DAVID (Huang da et al., 2009) and Eurexpress (Diez-Roux et al., 2011) databases, respectively. Datasets have been uploaded to the Gene Expression Omnibus for public access (GEO: GSE110281).
Single-Cell Transcriptome Analysis
The knee interzone and surrounding cartilage tissues were dissected with the aid of GFP expression from E14.5 Lgr5GFP/+ embryos. Dissociated cells were pooled for single-cell RNA sequencing with 10X Genomics Chromium Single Cell Controller for encapsulations (10X Genomics). cDNA libraries were prepared according to the manufacturer's instructions (Chromium Single Cell 3′ Reagent Kits v2 and Chromium Single Cell A Chip Kit), then sequenced on the Illumina HiSeq 1500 platform. The raw data were processed with the Cell-Ranger pipeline (version 2.1.0; 10X Genomics) for alignment to mm10, quantification of UMIs (unique molecular identifiers), and dimension-reduction (tSNE) analysis. Data were aligned to CreERT2 sequence to assess the expression of the Lgr5-eGFP-CreERT2 allele. Cells expressing Lgr5 and/or Lgr5-eGFP-CreERT2 alleles are considered as Lgr5+ cells. Clustering and pseudo-timeline analyses were performed with Cell-Ranger and Monocle2, respectively. Details of the parameter settings are shown in Table S5.
Cartilage Puncture and Repair with Lgr5+ Interzone Tissue
Lgr5-GFP mice were crossed with ROSA26-tdTomato mice to generate double heterozygous Lgr5-GFP;tdTomato embryos. GFP+ tissue was dissected from the forming knee joint of E13.5 embryos and transplanted to a lesion punctured with a 27-gauge needle at the trochlear groove of 8-week-old C57bl/6 mice (n = 3, Figure 6A). Punctured animals without tissue transplant represented the control.
Author Contributions
C.F., W.C.W.C., Y.L. and D.C. designed the experiments, analyzed the data, and wrote the manuscript. C.F., W.C.W.C., and Y.L. performed in vivo experiments. W.C.W.C. was involved in the generation and analysis of the bulk and transcriptome and single-cell transcriptome data. X.W., V.C.W.N., and J.C.Y. assisted in mouse embryo harvesting and data collection. B.N. assisted in the bulk transcriptome data analysis. P.C. assisted in the single-cell transcriptome analysis. K.S.E.C., S.S., S.M., M.K., and H.H.N. participated in the experimental design, data interpretation, and revision of the manuscript.
Acknowledgments
This work was supported by grants from the Hong Kong Research Grants Council (HKU761113M, C7030-18GF, and T12-708/12-N), the Ministry of Science and Technology of the People's Republic of China: National Strategic Basic Research Program (“973”) 2014CB942901, German Research Foundation FOR2722, and the S.Y. and H.Y. Cheng Professor endowment in Stem Cell Biology and Regenerative Medicine.
Published: September 12, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.08.006.
Accession Numbers
The accession number for the bulk transcriptome datasets reported in this paper is GEO: GSE110281. The accession number for the single-cell transcriptome dataset reported in this paper is GEO: GSE130919.
Supplemental Information
References
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Craig F.M., Bentley G., Archer C.W. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987;99:383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T.Y., Koo B.K., Li V.S.W., Teunissen H., Kujala P., Haegebarth A., Peters P.J., van de Wetering M. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–U257. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Decker R.S., Koyama E., Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R.S., Um H.B., Dyment N.A., Cottingham N., Usami Y., Enomoto-Iwamoto M., Kronenberg M.S., Maye P., Rowe D.W., Koyama E. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017;426:56–68. doi: 10.1016/j.ydbio.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux G., Banfi S., Sultan M., Geffers L., Anand S., Rozado D., Magen A., Canidio E., Pagani M., Peluso I. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowthwaite G.P., Bishop J.C., Redman S.N., Khan I.M., Rooney P., Evans D.J., Haughton L., Bayram Z., Boyer S., Thomson B. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Dy P., Smits P., Silvester A., Penzo-Mendez A., Dumitriu B., Han Y., de la Motte C.A., Kingsley D.M., Lefebvre V. Synovial joint morphogenesis requires the chondrogenic action of Sox5 and Sox6 in growth plate and articular cartilage. Dev. Biol. 2010;341:346–359. doi: 10.1016/j.ydbio.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Hu J., Stricker S., Cheung M., Ma G., Law K.F., Witte F., Briscoe J., Mundlos S., He L. A mutation in Ihh that causes digit abnormalities alters its signalling capacity and range. Nature. 2009;458:1196–1200. doi: 10.1038/nature07862. [DOI] [PubMed] [Google Scholar]
- Gunnell L.M., Jonason J.H., Loiselle A.E., Kohn A., Schwarz E.M., Hilton M.J., O'Keefe R.J. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J. Bone Miner. Res. 2010;25:1784–1797. doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.Z., Day T.F., Jiang X.Y., Garrett-Beal L., Topol L., Yang Y.Z. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C., Tabin C.J. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin. Orthop. Relat. Res. 1995;317:3–6. [PubMed] [Google Scholar]
- Kharchenko P.V., Silberstein L., Scadden D.T. Bayesian approach to single-cell differential expression analysis. Nat. Methods. 2014;11:740–742. doi: 10.1038/nmeth.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., Schulze J., Hansen U., Ashwodt T., Keene D.R., Brunken W.J., Burgeson R.E., Bruckner P., Bruckner-Tuderman L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004;279:22514–22521. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E., Shibukawa Y., Nagayama M., Sugito H., Young B., Yuasa T., Okabe T., Ochiai T., Kamiya N., Rountree R.B. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E., Zhang M., Ionescu A., Ayturk U.M., Ono N., Kobayashi A., Kronenberg H., Warman M.L., Lassar A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015;67:1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C., Tan S.H., Barker N. Recent advances in Lgr5(+) stem cell research. Trends Cell Biol. 2018;28:380–391. doi: 10.1016/j.tcb.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Li L., Newton P.T., Bouderlique T., Sejnohova M., Zikmund T., Kozhemyakina E., Xie M., Krivanek J., Kaiser J., Qian H. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017;31:1067–1084. doi: 10.1096/fj.201600918R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova H.P., Meech R. Barx homeobox family in muscle development and regeneration. Int. Rev. Cell Mol. Biol. 2012;297:117–173. doi: 10.1016/B978-0-12-394308-8.00004-2. [DOI] [PubMed] [Google Scholar]
- Merino R., Macias D., Ganan Y., Economides A.N., Wang X., Wu Q., Stahl N., Sampath K.T., Varona P., Hurle J.M. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev. Biol. 1999;206:33–45. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- Mitrovic D.R. Development of the metatarsophalangeal joint of the chick embryo: morphological, ultrastructural and histochemical studies. Am. J. Anat. 1977;150:333–347. doi: 10.1002/aja.1001500207. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yamamoto K., He X., Otsuki B., Kim Y., Murao H., Soeda T., Tsumaki N., Deng J.M., Zhang Z. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat. Commun. 2011;2:251. doi: 10.1038/ncomms1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce B.A., Brent A.E., Murchison N.D., Tabin C.J., Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- Rountree R.B., Schoor M., Chen H., Marks M.E., Harley V., Mishina Y., Kingsley D.M. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:1815–1827. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffner H., Sprunger J., Charlat O., Leighton-Davies J., Grosshans B., Salathe A., Zietzling S., Beck V., Therier M., Isken A. R-Spondin potentiates Wnt/beta-catenin signaling through orphan receptors LGR4 and LGR5. PLoS One. 2012;7:e40976. doi: 10.1371/journal.pone.0040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwartz Y., Zelzer E. Nonradioactive in situ hybridization on skeletal tissue sections. Methods Mol. Biol. 2014;1130:203–215. doi: 10.1007/978-1-62703-989-5_15. [DOI] [PubMed] [Google Scholar]
- Shwartz Y., Viukov S., Krief S., Zelzer E. Joint development involves a continuous influx of Gdf5-positive cells. Cell Rep. 2016;15:2577–2587. doi: 10.1016/j.celrep.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.N.P., Yadav U.S., Azad K., Goswami P., Kinare V., Bandyopadhyay A. NFIA and GATA3 are crucial regulators of embryonic articular cartilage differentiation. Development. 2018;145 doi: 10.1242/dev.156554. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Storm E.E., Kingsley D.M. GDF5 coordinates bone and joint formation during digit development. Dev. Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y., Shukunami C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- Zulch A., Becker M.B., Gruss P. Expression pattern of Irx1 and Irx2 during mouse digit development. Mech. Dev. 2001;106:159–162. doi: 10.1016/s0925-4773(01)00411-7. [DOI] [PubMed] [Google Scholar]
- Zwolanek D., Veit G., Eble J.A., Gullberg D., Ruggiero F., Heino J., Meier M., Stetefeld J., Koch M. Collagen XXII binds to collagen binding integrins via the novel motifs GLQGER and GFKGER. Biochem. J. 2014;459:217–227. doi: 10.1042/BJ20130642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.