Abstract
The growth factor bone morphogenetic protein 2 (BMP2) plays an important role in bone development and repair. Despite the positive effects of BMP2 in fracture healing, its use is associated with negative side effects and poor cost effectiveness, partly due to the large amounts of BMP2 applied. Therefore, reduction of BMP2 amounts while maintaining efficacy is of clinical importance. As nitric oxide (NO) signaling plays a role in bone fracture healing and an association with the BMP2 pathway has been indicated, this study aimed to investigate the relationship of BMP2 and NO pathways and whether NO can enhance BMP2-induced signaling and osteogenic abilities in vitro. To achieve this, the stable BMP reporter cell line C2C12BRELuc was used to quantify BMP signaling, and alkaline phosphatase (ALP) activity and gene expression were used to quantify osteogenic potency. C2C12BRELuc cells were treated with recombinant BMP2 in combination with NO donors and substrate (Deta NONOate, SNAP & L-Arginine), NOS inhibitor (LNAME), soluble guanylyl cyclase (sGC) inhibitor (LY83583) and activator (YC-1), BMP type-I receptor inhibitor (LDN-193189), or protein kinase A (PKA) inhibitor (H89). It was found that the NOS enzyme, direct NO application, and sGC enhanced BMP2 signaling and improved BMP2 induced osteogenic activity. The application of a PKA inhibitor demonstrated that BMP2 signaling is enhanced by the NO pathway via PKA, underlining the capability of BMP2 in activating the NO pathway. Collectively, this study proves the ability of the NO pathway to enhance BMP2 signaling.
Keywords: C2C12, BMP2, nitric oxide, signaling, crosstalk
1. Introduction
Bone morphogenetic proteins (BMPs) provide approximately 20 members to the transforming growth factor beta (TGFβ) superfamily. BMPs are archetypal growth factors capable of inducing diverse cellular responses such as proliferation, survival, apoptosis, differentiation, and migration, and play an important role in the development and homeostasis of bone [1]. BMPs act not only in the skeleton, but also have roles in other tissue types, for example, in tendons [2], nerve synapses [3], and endothelial cells [4].
BMPs have become clinically relevant [5], particularly in the orthopedic discipline [6,7,8], where BMP2 is licensed for the treatment of open tibia fractures [9]. Clinical trials highlight the ability of BMP2 to improve health outcomes of patients with open tibia shaft fracture by significantly reducing healing times, complications, infections, further operations, and hardware failures compared to the standard care group [10,11]. A clinical trial of rhBMP2 in the treatment of open tibia fractures did not find a clear improvement in fracture healing. However, rhBMP2 reduced the failure rates of surgical interventions, therefore reducing the need for further clinical interventions [8].
With current clinical applications of BMP2 being 1000 times greater than physiological levels, it is not surprising that negative effects, such as poor bone structure and excess inflammation, have become apparent [12,13,14]. Clinical trials have indicated issues with the effectiveness of BMP2 and negative effects, such as increased signs of edema and deep venous thrombosis [15] or prolonged inflammatory response (wound drainage) [16]. The Orthopedic Research Society Forum [14] stated that the excessive clinical doses of rhBMP2 (compared to normal physiological amounts) could be the underlining issue in negative outcomes and that there is a need to refine and reduce rhBMP2 application in the clinic. One possibility is to optimize BMP2 treatment through the enhancement of BMP-signaling by modulating this pathway.
BMP2 binds dimeric BMP receptor type 1 (BMPR1a, BMPR1b) and type 2 (BMPR2, ActR2A, ActR2B), thus forming hetero-tetrameric receptor complex [17,18]. The affinities between BMP2 and the various receptors differ, generating complexity and redundancy in BMP signaling [19].
The long C-terminal cytoplasmic tail of the type 2 receptor BMPR2 contains valuable binding sites for adapter proteins [20,21,22]. This extra binding allows for interactions with additional proteins that lead to modulations of signaling processes such as CD117, tribbles 3 (Trb-3), protein phosphatase 2 (PP2), protein kinase A (PKA), and cGMP dependent kinase I (cGKI) [23,24,25,26,27].
The NO pathway effector kinase, cGKI, was shown to play a role at many points of BMP signaling. cGKI associates with and phosphorylates the BMPR2 tail-domain [28]. Once the receptor is activated by ligand binding, cGKI leaves the receptor to associate with and enhance the action of SMAD transcription factors [26,29]. In pulmonary arterial endothelial cells, PKA was described as a crucial link between the NO- and BMP-pathway [27]. Endothelial cells treated with BMP2/4 activate PKA via BMPR2 which, in turn, mediates phosphorylation of the endothelial NO synthase (eNOS) [27]. Here, we asked whether there is an association between the NO- and BMP2-pathways in the context of BMP2-mediated osteo-inductive activity. This might have important implications for improvement of BMP2-based therapeutics.
The NO pathway has been associated with osteo-inductive activities. The application of a NOS inhibitor (LNAME) to rats following bone fracture led to reduction in bone healing, which was recovered by direct NO donation [30]. The application of nitrates (e.g., nitroglycerin) has been shown to reduce osteopenia and osteoporosis in animal models [31,32,33]. In clinical trials, nitrate-treated female osteoporotic patients showed an increase in osteogenic markers and bone density [34,35,36]. This association between the NO and BMP2 pathway could prove valuable in improving BMP2-mediated osteogenic activities. For this purpose, the BMP2 sensitivity [37] and osteogenic features [38,39,40] of the mouse C2C12 myoblast cell line were exploited to investigate whether the NO pathway combines and enhances the effects of the BMP2 pathway, and if the BMP2 pathway is capable of modulating the NO pathway.
2. Materials and Methods
2.1. Cell Culture
The C2C12BRELuc reporter cell line [41] was generated by the Inman group and used in this study. In this cell line, the luciferase (Luc) reporter is under the control of the BMP response elements (BRE) of the Id1 gene. C2C12BRELuc were cultivated in culture medium (Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin, all from Biochrom AG, Berlin, Germany). C2C12BRELuc cells were passaged twice per week in a 175 or 225 cm2 cell culture flask. The cultivation media was supplemented with the antibiotic G418 (0.7 mg/mL) to select for the BREluc-positive C2C12BRELuc cells in accordance with previous studies [41].
C2C1BRELuc cells were sub-cultivated at a ratio of 2.5–5 × 104 cells per mL of culture medium. DMEM, antibiotics, FBS, and trypsin (Biochrom AG, Berlin, Germany) were used in cell cultivation and passaging. To ensure the viability and to calculate cell number, the metabolic activity of cells was calculated through Presto Blue® assay (Invitrogen by Life Technologies Co., Carlsbad, CA, USA). BMP2 was purchased from Osteogenetics (Würzburg, Germany). H89 was purchased from AbCam (Cambridge, UK). Deta NONOate, SNAP, YC-1, LY83583, LDN-193189, and IBMX were purchased from Cayman Chemical (Ann Arbor, MI, USA).
2.2. BMP Reporter Assay
The experimental setup utilized 0.1% FCS, as it allows C2C12 cells to respond rapidly to BMP2. Cells were seeded at a density of 2.5 × 104 cells per well in culture media into 48-well cell culture dishes overnight. The medium was exchanged to Dulbecco's Modified Eagle Medium (DMEM) without l-Arginine (GIBCO® by Life Technologies GmbH, Darmstadt, Germany) supplemented with 0.1% FCS and 1% Pen/Strep for starvation for three hours. Subsequently, medium was changed to the appropriate stimulation medium for the stated period of time (see Table 1 for experimental setup). The medium was removed after the stimulation period and the cells were lysed in 100 μL lysis buffer (1:5 Cell Lysis Reagent diluted in ddH2O; Promega, Mannheim, Germany), frozen at −20 °C, and subsequently thawed at room temperature on a shaker. The cell lysate (20 µL) was then transferred to a 96-well plate and 50 µL of Luciferase buffer (Promega, Mannheim, Germany) was added by automatic injection using a luminometer (Mithras LB-940, Berthold Technologies, Bad Wildbad, Germany). Quantification was determined as relative light units (RLU). For protein normalization, the protein content of lysates was determined with the Pierce™ Coomassie assay (Thermo Fisher Scientific, Darmstadt, Germany) and Bovine Serum Album (BSA) as standard. The assay was carried out following manufactures instruction. Standards were made in PBS (or appropriate experimental buffer) and samples were diluted 1:2 in PBS and measured in triplicates.
Table 1.
Experimental setups for this study.
| Method | Pre-Stimulation | Stimulation | |||
|---|---|---|---|---|---|
| Media | Additional Treatment | Media & FCS | Added Factors | Time (h) | |
| Reporter activity | DMEM without Arginine, 0.1% FCS | Inhibitors: LNAME, LY83583, H89, LDN final hour | DMEM without Arginine, 0.1% FCS | Arginine, Deta, SNAP and BMP2 | 24 6 |
| ALP activity | DMEM/HAMs, 1% FCS | Inhibitors: LNAME, LY83583 final hour |
DMEM/HAMs, 1% FCS | Arginine, Deta, and BMP2 | 72 |
| NO production | DMEM High Glucose, 1% FCS | DAF-2, 1 h | DMEM High Glucose, 1% FCS | Deta, and BMP2 | 2 |
| cGMP ELISA | DMEM without Arginine, 1% FCS |
IBMX final 30 min | DMEM without Arginine, 1% FCS | Deta, and BMP2 | 1 |
| SMAD-1 ELISA | DMEM without Arginine, 0.1% FCS | Inhibitors: LDN, final hour |
DMEM without Arginine, 0.1% FCS | Arginine and BMP2 | 0.5 |
| Gene Expression Id1,2,3 |
DMEM without Arginine, 0.1% FCS | - | DMEM without Arginine, 0.1% FCS | Arginine, and BMP2 | 6 |
| Differentiation (Gene Expression OPG, Runx, Col1a1) |
DMEM without Arginine, 1% FCS | - | DMEM without Arginine, 1% FCS | Arginine and BMP2 | 144 |
2.3. Alkaline Phosphatase Activity (ALP) Assay
C2C12BRELuc cells were seeded at a density of 2 × 104 per well in a 24-well plate and incubated in DMEM High Glucose (Biochrom AG, Berlin, Germany) supplemented with 10% FCS and 1% Pen/Strep. Prior to starvation, the cell number was quantified using Presto Blue® Assay. After starvation with 1% FCS, 1% Pen/Strep in DMEM/Ham’s F-12 (Biochrom AG, Berlin, Germany) for three hours, cells were stimulated with the same medium for three days (Table 1). Following stimulation, the medium was aspirated and cells were washed with pre-warmed PBS. ALP activity was analyzed by the addition of 1 mL of ALP buffer (50 mM glycine, 100 mM TRIZMA base, 1 mM MgCl2, pH 10.5) containing 1.3 mg pNPP (Sigma-Aldrich, Hamburg, Germany) per well. After incubation for one hour at 37 °C, 180 µL medium was transferred to a clear 96-well plate in triplicates and the absorbance was quantified at 405 nm using a spectrophotometer.
2.4. Gene Expression Analysis
For expression analysis of inhibitor of differentiation (Id), 1, 2, and 3, 5 × 105 cells were seeded to six-well cell culture dishes in culture medium. The next day, cells were starved for three hours in starving medium. The cells were then stimulated for six hours (Table 1).
Osteogenic differentiation was determined by expression analysis of osteoprotegerin (OPG), collagen type I alpha 1 chain (Col1a1), and runt-related transcription factor 2 (Runx2). A total of 1.2 × 105 cells was cultivated in culture medium overnight and starved with DMEM without Arginine (supplemented with 1% FCS and 1% antibiotics) for three hours. Stimulation was done for six days with a complete medium change after three days (Table 1).
RNA isolation was carried out using the NucleoSpin® RNA kit (Macherey-Nagel, Düren, Germany) following the instructions. The RNA was dissolved in 30 µL RNAase Free H2O and RNA concentration was measured using a spectrophotometer.
Typically, 500 ng RNA was translated into cDNA using qScript cDNA SuperMix (Quantabio, Beverly, MA, USA) according to the manufacturer’s protocol. The mRNA expression of the genes of interest was quantified using quantitative real-time polymerase chain reaction (qRT-PCR). The reaction mixture was composed of 25–50 ng cDNA with 10 µM primers (see Table 2, TIB-MOLBIOL Syntheselabor GmbH, Berlin, German), nuclease-free H2O, and PerfeCTa SYBR® Green SuperMix (Quantabio, Beverly, MA, USA). Next, 15 µL of the reaction mixture was transferred to a white 96-well qRT-PCR plate and LightCycler 480 System (Roche, Mannheim, Germany) was used. The amplification protocol included 40 cycles with an annealing temperature at 60 °C, followed by a melting curve protocol until 95 °C with a ramp rate of 0.11 °C/sec. The efficiency was tested for all primers and the ΔCt method with efficiency correction was used to calculate the relative gene expression to the housekeeping gene hypoxanthine-guanine phosphoribosyl-transferase (HPRT). HPRT was chosen as a housekeeping gene as it was most constant over the different stimulation conditions compared to other housekeeping genes such as Actin (NM_009608.3), Eukaryotic Translation Elongation Factor 1 alpha 2 (NM_010106), Ribosomal protein L13 (NM_016738.5), 18S rRNA (NR_003278.3), and Glycerinaldehyd-3-phosphat-Dehydrogenase (NM_001289726.1). For exemplary melting curves of qRT-PCR see Figure S1.
Table 2.
Murine primers for quantitative real-time polymerase chain reaction (qRT-PCR).
| Gene | Accession Number | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|---|
| HPRT | NM_000194 | TTGCTGACCTGCTGGATTAC | AACTTTTATGTCCCCCGTTG |
| Id1 | NM_010495.3 | ACGACATGAACGGCTGCTACT | GCTCACTTTGCGGTTCTGG |
| Id2 | NM_010496.3 | TATCAGCCATTTCACCAGGAG | TGTGAAAAGGCAAAGTCTGCT |
| Id3 | NM_008321 | AAGGACAAGAGGAGCTTTTGC | GCGTTGAGTTCAGGGTAAGTG |
| OPG | NM_002546.3 | TGCAGTACGTCAAGCAGGAG | CCCATCTGGACATCTTTTGC |
| Col1a1 | NM_000088.3 | TGACCTCAAGATGTGCCACT | ACCAGACATGCCTCTTGTCC |
| Runx2 | NR_103532 | GCCCCCAAACAGTATCTTGA | GCCTGAAGTGAGGTTTTAGGC |
2.5. NO Quantification
NO quantification protocol was modified from Hinchee-Rodriguez et al. 2013 [42]. C2C12BRELuc cells were seeded at a density of 6 × 104 cells per well of a 24-well plate with DMEM High Glucose 10% FCS and 1% Pen/Strep and incubated overnight. Following starvation, cells were loaded for one hour with 5 µM DAF-2 (Biomol GmbH, Hamburg, Germany) in serum-free PBS. Subsequently, cells were stimulated for two hours in DMEM High Glucose supplemented with 1% FCS and 1% Pen/Strep. Following stimulation, cells were washed twice with warm PBS and fluorescence was quantified using a spectrophotometer at 485 nm excitation and 538 nm emission. The direct NO donor DetaNONOate was employed as a positive NO control.
2.6. cGMP ELISA
C2C12BRELuc cells were seeded in six-well plates with 6 × 105 cells per well in culture medium overnight. Three hours of starvation and one hour of stimulation were carried out in DMEM without Arginine with 1% FCS. The inhibitor of cGMP degradation IBMX (500 µM) was added during starvation and stimulation to all samples. Post-stimulation, cells were washed with cold PBS, aspirated, and 500 µL 0.1 M HCL was added. After 10 min incubation at room temperature, the plate was stored at −20 °C. Subsequent thawing was followed by scrapping of cells. The lysates were then aliquoted into protein LoBind tubes (Eppendorf, Hamburg, Germany) and centrifuged at 600× g for 10 min at 4 °C.
The cGMP ELISA (NewEast Biosciences, King of Prussia, PA, USA) assay was carried out following the standard protocol from the manufacturer and absorption was measured at 450 nm for HRP and 570 nm reference using a spectrophotometer. The normalization of samples was carried out using Coomassie assay.
2.7. SMAD-1 ELISA
C2C12BRELuc cells were seeded with 5 × 104 cells per well in a 24-well plate and incubated in culture medium overnight. The next day, cells were starved in DMEM without Arginine, supplemented with 0.1% FCS and 1% antibiotics for three hours. One hour before starving ended and during stimulation, the BMP type-I receptor inhibitor LDN was supplemented to the inhibitor group. Stimulation was carried out with the same medium supplemented with BMP2 and Arginine (Table 1) for 30 min.
Phosphorylated and total SMAD1 were measured with the SMAD1 InstantOne ELISA™ kit, Invitrogen by Life Technologies Co., Carlsbad, CA, USA. Cells were incubated with Cell Lysis Mix (1:5 Cell Lysis Reagent diluted in ddH2O, provided in the kit) for 10 min at room temperature and lysates were transferred to protein LoBind tubes. Samples were stored on ice and ELISA was directly carried out after sample taking following manufacturers instruction. Absorption was measured with a spectrophotometer at 450 nm with reference at 570 nm. Phosphorylated SMAD1 was normalized to total SMAD1.
2.8. Statistics
All experiments were done in triplicate and repeated at least once (except for the PCR). The statistical analyses were performed using IBM® SPSS® Statistics version 22. All graphs were visualized as boxplots. The mild outliers are marked with a circle and extreme outliers are marked with a star. The nonparametric Kruskal-Wallis test was used to determine if there were statistically significant differences between all groups. In cases of significances during Kruskal-Wallis tests, a comparison between two groups was made by Mann-Whitney U test. The significances are given in the figures and are assigned if p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
3. Results
C2C12 is a myoblast precursor cell line, which is responsive to BMPs [37] and possess osteogenic features [38,39,40]. The stable transfection of C2C12 cells with a luciferase (Luc) reporter composed of SMAD-binding BMP response elements (BRE) has provided a valuable tool in the quantification of BMP activity [41]. In order to investigate the possible NO-BMP crosstalk, Arginine-free DMEM was used to have better control over the NO pathway.
3.1. BMP2-Mediated Signaling and Osteogenic Activity Can be Enhanced by Arginine Via NOS
3.1.1. Arginine Supports BMP2-Mediated Activity
Arginine, a substrate of the NOS enzyme, was investigated to determine its effect on BMP2 signaling. After 24 h of stimulation, C2C12BRELuc cells showed no increase in reporter activity in response to supplementation of Arginine alone (Figure 1A). Arginine supplementation in combination with 1 nM or 5 nM BMP2 demonstrated a significant increase in BMP2-induced BMP reporter activity (Figure 1B,C). Co-stimulation of the cells with 1 nM BMP2 and Arginine was as effective as stimulation with 5 nM BMP2 alone. The expression of the immediate BMP2 response genes Id1, Id2, and Id3 was examined following six hours of stimulation with 1 nM BMP2 with or without 1 mM Arginine (Figure 1D). The stimulation with BMP2 increased expression of the target genes as expected, which was further stimulated by the application of the NOS substrate Arginine (Figure 1D).
Figure 1.
Bone morphogenetic protein (BMP) reporter activity and BMP target gene expression after stimulation with the nitric oxide synthase (NOS) substrate Arginine. C2C12BRELuc cells stimulated for 24 h in Arginine-free Dulbecco's Modified Eagle Medium (DMEM) with (A) 0.1 to 7.5 mM Arginine alone or in combination with (B) 1 nM BMP2 or (C) 5 nM BMP2. (D) C2C12BRELuc cells were stimulated for six hours in Arginine-free DMEM with 1 nM BMP2, with or without 1 mM Arginine. The gene expression of Id1, Id2, and Id3 was investigated. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Stars and circles are outlying values. Number of replications: (A) n = 12; (B) n = 6; (C) n = 9; (D) n = 3.
3.1.2. Arginine Support of BMP2 Activity is Mediated by NOS
To further demonstrate that the application of Arginine supports BMP2-mediated SMAD signaling via the NO pathway, the inhibitor of the NOS enzyme (LNAME) was applied to C2C12BRELuc cells. The application of LNAME (250 µM–10000 µM; high concentration in accordance to [43]) alone had no effect on the BMP reporter activity (Figure 2A). The Arginine-enhanced BMP2 signaling was significantly reduced by LNAME (5,000 µM and 10,000 µM) (Figure 2B). ALP activity was utilized to investigate the osteoinductive effect of BMP2. For this assay, cells were cultured for three days. The application of LNAME at 10 and 100 µM reduced significantly the BMP2-mediated ALP activity (Figure 2C). Subsequently, LNAME inhibition of BMP2-mediated ALP activity was recovered by the direct application of direct NO donor Deta NONOate (Figure 2D).
Figure 2.
BMP reporter and alkaline phosphatase activity (ALP) activity following the inhibition of NOS enzyme by NOS inhibitor (LNAME): C2C12BRELuc cells were stimulated for 24 h with (A) 250-10,000 µM LNAME or (B) 5 nM BMP2 and 1 mM Arginine in combination with 250-10,000 µM LNAME. ALP activity was normalized to cell number of cells stimulated for 72 h with 10 nM BMP2 with (C) NOS inhibitor LNAME (0.01 to 100 µM) and (D) following recovery of 10 µM or 100 µM LNAME inhibition with the direct NO donor. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**) or p < 0.001 (***). Stars and circles are outlying values. Abbreviation: Deta: Deta NONOate. Number of replications: (A–D) n = 6.
3.2. BMP2-Mediated Signaling and Osteogenesis Can be Enhanced by Direct NO Donor
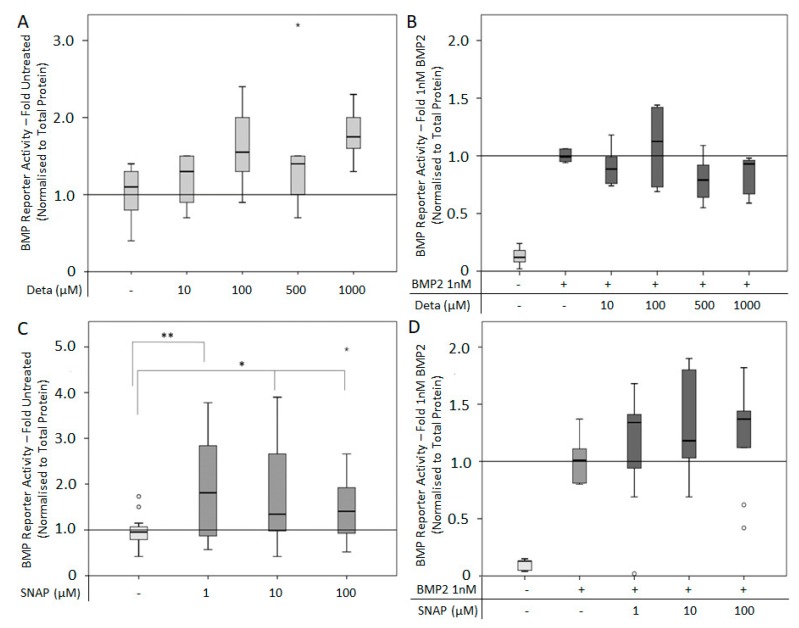
The NO donors Deta NONOate and SNAP release NO spontaneously at different rates. Deta NONOate has a half-life of 20 h, whereas SNAP has a half-life of six hours. The use of Deta NONOate (10–1000 μM) alone or in combination with 1 nM BMP2 for six hours had no significant effect on BMP reporter activity (Figure 3A,B). Application of SNAP (1–100 μM) alone significantly increased BMP reporter activity compared to untreated controls (Figure 3C), while co-application of SNAP (1–100 μM) with 1 nM BMP2 had no significant effect on reporter activity compared to BMP2 alone (Figure 3D).
Figure 3.
BMP reporter activity after stimulation with the NO donors Deta NONOate and SNAP for six hours: C2C12BRELuc cell were stimulated in Arginine-free DMEM with (A) 10–1000 µM Deta NONOate or (B) in combination with 1 nM BMP2, with (C) 1-100 µM SNAP or (D) SNAP in combination with 1 nM BMP2. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviations: Deta = Deta NONOate. Stars and circles are outlying values. Number of replications (A,B) n = 6; (C) n = 18; (D) n = 9.
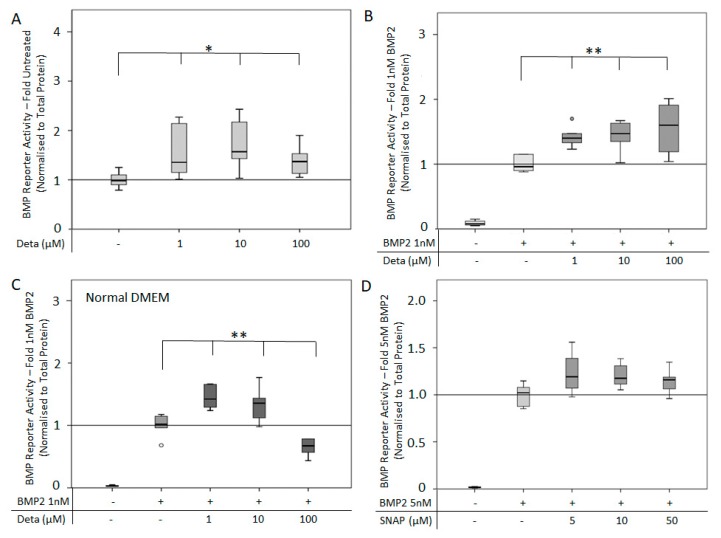
Due to the fact that no effect was observed after six hours, the experiment was repeated with a stimulation period of 24 h. For this 24 h timepoint, Deta NONOate alone (1–100 μM) stimulated BMP reporter activity (Figure 4A). The combined use of Deta NONOate (1–100 μM) and 1 nM BMP2 significantly increased BMP reporter activity compared to BMP2 alone in a dose-dependent manner (Figure 4B). Interestingly, in Arginine-containing media, a lower dose of Deta NONOate (1 and 10 μM) with 1 nM BMP2 had an additional effect on the cells, while a high concentration of Deta NONOate (100 μM) and 1nM BMP2 was inhibitory (Figure 4C). The combination of SNAP (5–50 μM) with 1 nM BMP2 had no effect on reporter activity compared to BMP2 alone (Figure 4D).
Figure 4.
BMP reporter activity after stimulation with the NO donors Deta NONOate and SNAP for 24-h: C2C12BRELuc cells stimulated in Arginine-free DMEM with (A) 1–100 µM Deta NONOate, (B) and in combination 1 nM BMP2. (C) Cells stimulated in normal DMEM containing Arginine with 1 to 100 µM Deta NONOate and 1 nM BMP2. (D) Stimulation in Arginine-free DMEM with 5–50 µM SNAP in combination with 5 nM BMP2. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviation: Deta = Deta NONOate. Circles are outlying values. Replication (A–D) n = 6.
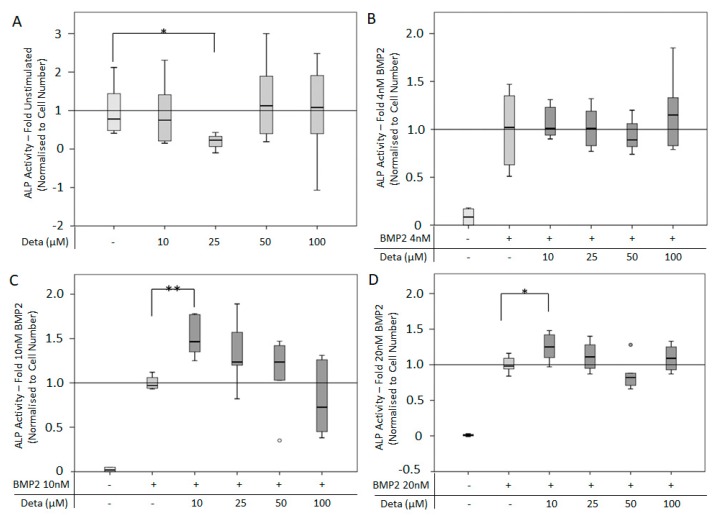
The ALP activity of C2C12BRELuc cells was used as an outcome measure of osteogenic differentiation. Since Arginine-free media was unable to mediate an increase of ALP activity in response to BMP2, cells were cultured in DMEM-HAMs with 1% FCS [44]. Stimulation of cells with Deta NONOate (10–100 µM) for 72 h showed no significant difference in ALP activity compared to untreated cells except for a reduction of activity by 25 µM Deta NONOate (Figure 5A). The co-stimulation of cells with different concentrations of BMP2 (4, 10, 20 nM) and Deta NONOate (10–100 µM) revealed a complex relationship depending on the concentration combinations. Only the low Deta NONOate concentration (10 µM) in combination with 10 and 20 nM BMP2 significantly stimulated ALP activity (Figure 5B–D). The stimulatory effect was more pronounced at 10 nM BMP2 than at 20 nM BMP2.
Figure 5.
ALP activity after NO supplemented BMP2 stimulation: ALP activity normalized to cell number of C2C12BRELuc cells stimulated with direct NO donor Deta NONOate (10–100 µM) (A) without BMP2, (B) with 4 nM BMP2, (C) with 10 nM BMP2, or (D) with 20 nM BMP2. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviation: Deta = Deta NONOate. Circles are outlying values. Replications (A,B) n = 6.
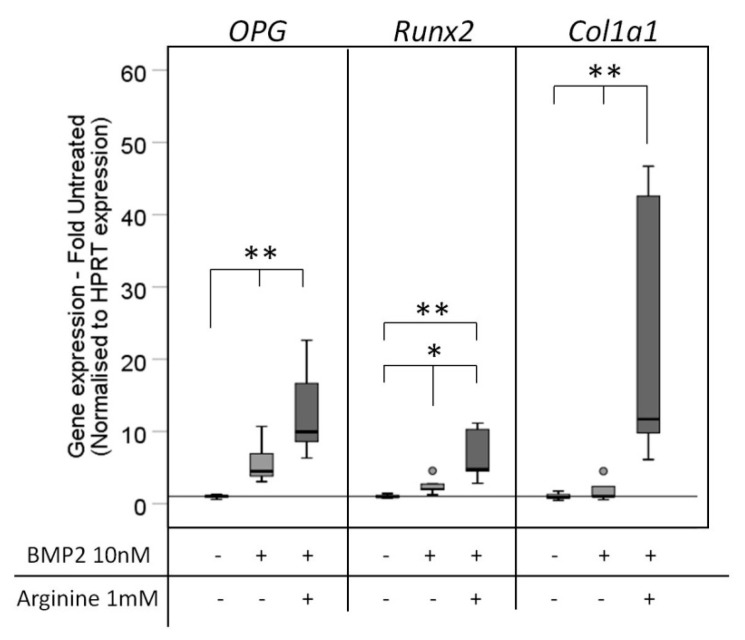
The effect of NO donation on BMP mediated osteogenic differentiation was further tested in a six-day stimulation experiment in Arginine-free DMEM supplemented with BMP2 with and without Arginine. BMP significantly stimulated the expression of OPG and Runx2 compared to the unstimulated control, while Col1a1 expression was not affected. Further Arginine supplementation significantly enhanced the BMP-mediated osteogenic differentiation as determined by Runx2, Col1a1 expression, without reaching significant differences in OPG expression (Figure 6).
Figure 6.
Osteogenic gene expression after stimulation with the NOS substrate Arginine. C2C12BRELuc cells were stimulated for six days in Arginine-free DMEM with 10 nM BMP2 alone or in combination with 1 mM Arginine. The gene expression of OPG, Runx2, and Col1a1 was investigated by qRT-PCR. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Circles are outlying values. Number of replications: n = 6.
Taken together, BMP signaling, as well as BMP-induced ALP activity and expression of osteogenic markers of C2C12 cells, can further be enhanced by a NO donor.
3.3. NO Receptor, sGC, Assists in BMP2 Induced Signaling
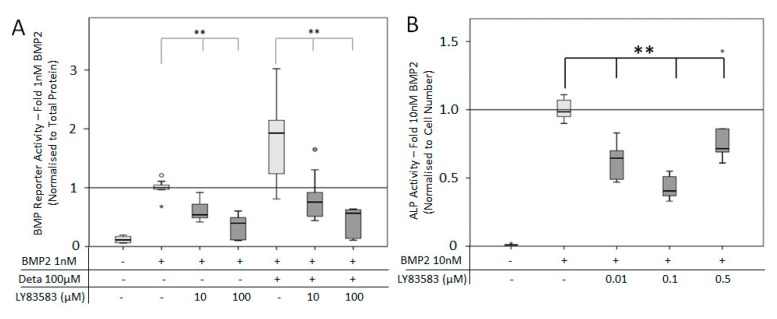
The NO pathway relies on the NO receptor, soluble guanylyl cyclase (sGC), as part of the signal transduction pathway. Therefore, understanding the effects of inhibition (LY83583, Figure 7) and NO-independent activation (YC-1, Figure 7) of this receptor is important to demonstrate the effect of the NO pathway on supporting BMP2 signaling activity. C2C12BRELuc cells were treated with LY83583 and 1 nM BMP2, with and without 100 μM Deta NONOate (Figure 7A). Both concentrations of LY83583 (10 and 100 μM) significantly reduced BMP2 signaling without a further effect of direct NO application (Figure 7A). The addition of LY83583 (0.01–0.5 μM) reduced significant the BMP induced ALP activity (Figure 7B). At the three-day period required for ALP activity, high concentrations of LY83538 (10–100 μM) resulted in a decrease in cell number, either due to the longer time or change in media type.
Figure 7.
BMP reporter and ALP activity after inhibition of soluble guanylyl cyclase (sGC): C2C12BRELuc cell line was stimulated for 24 h in DMEM without Arginine (A) with 10 or 100 µM LY83583 in the presence of 1 nM BMP2 with and without 100 µM Deta NONOate. (B) ALP activity normalized to cell number after stimulation for 72 h in DMEM/Hams with 10 nM BMP2 and sGC inhibitor LY83583 (0.01 to 0.5 µM). Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviation: Deta = Deta NONOate. Stars and circles are outlying values. Replications (A) n = 9, (B) n = 6.
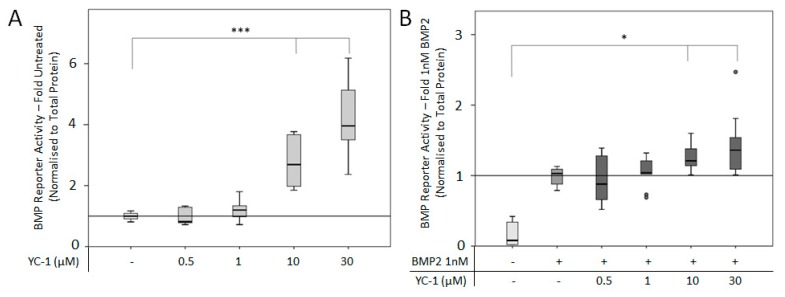
To further investigate the direct connection between sGC and BMP2-mediated signaling, the NO-independent activator YC-1 was applied to C2C12BRELuc cells in combination with 1 nM BMP2 (Figure 8). The addition of 10 and 30 µM YC-1 alone significantly increased BMP reporter activity (Figure 8A). A similar trend was seen when additional BMP2 was added to the cultures (Figure 8B).
Figure 8.
BMP reporter activity following NO independent activation of sGC: C2C12BRELuc reporter cell line stimulated for 24 h in DMEM without Arginine with (A) 0.5-30 µM YC-1 and (B) in combination with 1 nM BMP2. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.001 (***). Circles are outlying values. Replications (A, B) n = 9.
3.4. SMAD Signaling after BMP and NO Stimulation
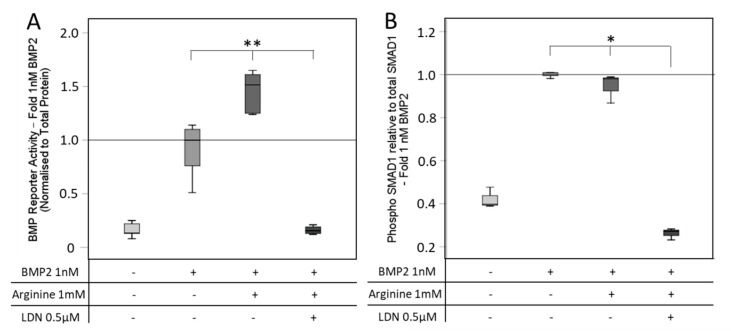
The addition of the BMP type-I receptor inhibitor LDN, which inhibits BMP-mediated SMAD signaling, led to a decrease of BMP reporter activity, irrespective of the presence of the NOS substrate L-Arginine (Figure 9A). Analyzing the phosphorylation of SMAD1, this effect could be underlined, as the inhibitor LDN significantly reduced the SMAD1 phosphorylation. Arginine supplementation to BMP2 had no additional effect on the SMAD1 phosphorylation under the tested conditions (Figure 9B).
Figure 9.
BMP reporter activity and SMAD1 phosphorylation after inhibition of the SMAD pathway. (A) C2C12BRELuc cells were stimulated for 6 h in Arginine-free DMEM with 1 nM BMP2 with or without 1 mM Arginine and 0.5 µM LDN. (B) C2C12BRELuc cells were stimulated for 30 min in Arginine-free DMEM with 1 nM BMP2 with or without 1 mM Arginine and 0.5 µM LDN. SMAD1 phosphorylation was measured with the SMAD1 InstantOne ELISA™ kit and normalized to total SMAD1. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviations: LDN: LDN-193189 (A) n = 6; (B) n = 3.
3.5. PKA Connects BMP2 and NO Pathway for BMP2 Signaling
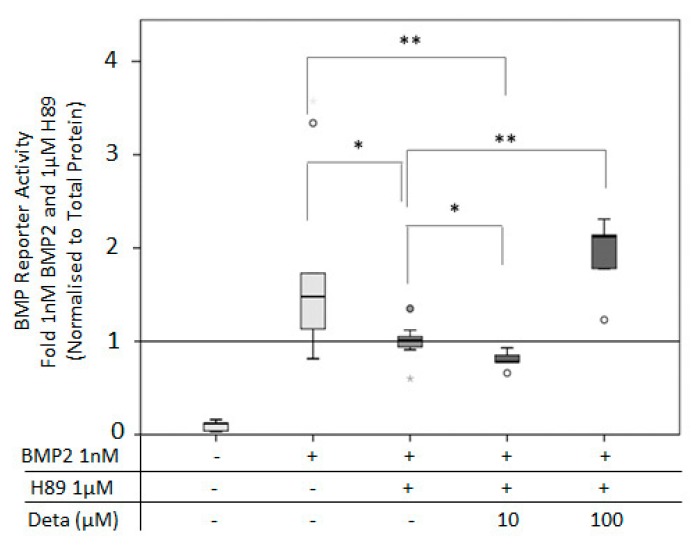
A PKA-mediated link between the BMP2 and NO pathway has been described [27]. To investigate this connection, the PKA inhibitor H89 was used. Application of 1 µM H89 significantly reduced BMP2-induced signaling. The inhibition of BMP2 signaling was rescued by the application of 100 µM Deta NONOate (Figure 10).
Figure 10.
BMP reporter activity after inhibition of protein kinase A (PKA) by H89: BMP reporter activity of C2C12BRELuc cells stimulated for 6 h with 1 nM BMP2, 1 µM H89, 10 and 100 µM Deta NONOate. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviation: Deta = Deta NONOate. Circles are outlying values. Replications n = 6.
3.6. BMP2 Can Induce NO Pathway Activity
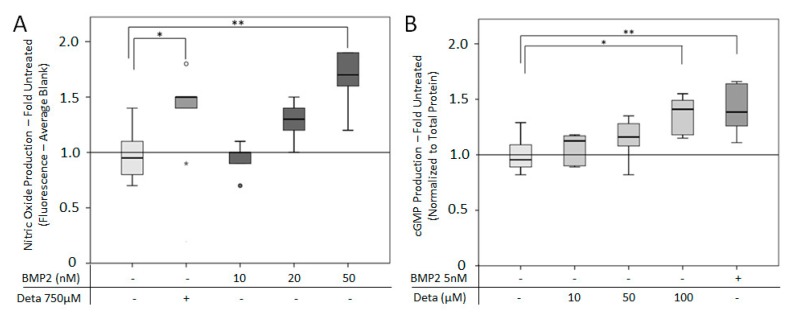
We hypothesized that BMP2 is able to activate the NO pathway. To prove this, NO and cGMP production was examined in response to BMP2 (Figure 11). The quantification of NO was carried out using the fluorescent NO reactive DAF-2. The addition of 10–50 nM BMP2 led to a dose-dependent increase in NO production, but only 50 nM BMP2 was significantly higher compared to the untreated group (Figure 11A) and comparable to the NO donor Deta NONOate. Stimulation of cells with 5 nM BMP2 significantly increased the cGMP production compared to the untreated group. The BMP2-induced cGMP production was comparable to the amount induced by 100 µM Deta NONOate (Figure 11B).
Figure 11.
Treating C2C12BRELuc cells with BMP2 stimulated NO signaling. (A) NO production quantified by DAF-2-mediated fluorescence and fold comparison to untreated group. C2C12BRELuc cells were stimulated for 2 h with 750 µM Deta NONOate or 10, 20, or 50 nM BMP2. (B) cGMP production in cells after 1 h stimulation with 10, 50, or 100 µM Deta NONOate or 5 nM BMP2 given as fold comparison to untreated groups. Statistics: Kruskal–Wallis Test followed by Mann-Whitney U test. Mann-Whitney U test significances between samples were assigned if p < 0.05 (*) or p < 0.01 (**). Abbreviation: Deta: = Deta NONOate. Stars and circles indicate outlying values. Replications (A, B) n = 6.
4. Discussion
This study aimed to investigate the molecular association between the BMP2 and NO pathways and to gain further understanding of the ability of the NO pathway to enhance BMP2-mediated signaling and osteoinductive activities. To this end, BMP2 was applied in combination with various compounds that stimulate or inhibit the NO pathway in C2C12 cells, and BMP2 reporter activity and osteoinduction (ALP activity) was measured. By demonstrating that the NO pathway can enhance BMP2-mediated signaling and osteoinduction, there are opportunities to enhance BMP2-based therapeutics, e.g., in fracture treatment, in turn filling the current need for increased BMP2 therapeutic efficiency [45].
Utilizing Arginine-free DMEM as a cell culture medium allowed for better control of NO signaling. Therefore, this study clearly demonstrated that NO production by the NOS enzyme can enhance BMP2-mediated signaling. Supplementation of BMP2 stimulation with Arginine enhanced BMP2-mediated reporter activity and the expression of BMP2 target genes Id1, Id2, and Id3 after 6 h of stimulation. LNAME inhibition of the NOS enzyme confirmed the NO-BMP2 link. However, high concentrations of LNAMEs were required (5 to 10 mM) to reduce BMP2-mediated signaling. The reason could be the BMP2-mediated activation of the NOS enzyme [27] which, in turn, acts as a competitor to the inhibition by LNAME.
The addition of the direct NO donors SNAP and Deta NONOate for a short period of time (6 h) had no effect on BMP2-mediated signaling. However, SNAP addition without BMP2 was able to induce BMP reporter activity. At a longer period of time (24 h), Deta NONOate enhanced BMP2-mediated signaling, whereas SNAP did not. This provides further evidence that the NO pathway can enhance BMP2-mediated signaling. These time-dependent effects of direct NO donors are well-known due to the variable half-life of the NO donors [46,47,48].
Investigating the phosphorylation of SMAD1, a clear effect of BMP2 was seen and the phosphorylation was reduced by the BMP-receptor inhibitor LDN. As Arginine was not able to recover the inhibition of the BMP2 pathway, NO supplementation might require a functional BMP pathway. The reason for the ineffectiveness of Arginine on the additional stimulation of SMAD1 phosphorylation under BMP2 exposure cannot be explained, although a significant supplementary effect on the reporter activity was detected. Further studies are necessary to elucidate if concentration and time dependency might be responsible.
In this study, the supplementary role of the NO receptor sGC in BMP2-mediated signaling was investigated using the chemical inhibitor LY83583 and the NO independent activator YC-1. Application of the sGC inhibitor LY83583 reduced BMP2-mediated signaling, while addition of YC-1 had a supportive effect on the BMP2-mediated signaling. Taken together, these results demonstrate a role for the NO receptor, sGC, in supporting BMP2-mediated signaling.
To understand if the NO pathway is able to support the early osteogenic differentiation in C2C12 cells, ALP activity and expression of osteogenic markers were investigated. As Arginine-free DMEM did not allow the induction of ALP activity in C2C12 cells, a media containing Arginine was required to investigate BMP2-mediated ALP activity. Therefore, Arginine supplementation could not be used, and the role of NO in BMP2-mediated ALP induction was investigated by the application of direct NO donors and NOS inhibitors. The application of NOS inhibitor (LNAME) in combination with BMP2 inhibited the BMP2-mediated ALP activity, which was partially recovered by the addition of the direct NO donor Deta NONOate. The osteogenic differentiation was further investigated by gene expression analysis of the osteogenic markers OPG, Runx2, and Col1a1. The analysis revealed that the NO substrate Arginine was able to enhance the osteogenic differentiation compared to BMP2 alone. Altogether, this highlights the role of the NOS enzyme and NO pathway in supporting BMP2-mediated early osteogenic activity, and that the addition of NO donors can help to enhance BMP2-mediated activity.
There are dosing effects between BMP2 and Deta NONOate in the regulation of ALP activity. This work demonstrated that the BMP2-mediated ALP activity of Deta NONOate was only achieved at higher BMP2 concentrations (10 and 20 nM). In addition, while Deta NONOate was supplementary at low concentrations (10 µM), it lost effectiveness and became inhibitory with increasing concentrations. It is interesting to note a parallel effect seen in stretching experiments of C2C12 cells: 10 µM Deta NONOate enhanced stretch induced proliferation, whereas 50 or 100 µM had no effect [49]. Therefore, further studies are needed to determine the NO donor concentration required to achieve the most effective activity, as well as to elucidate the mechanism of adverse effects with increasing NO concentration.
The negative effects of NO on cell vitality were previously described [50,51,52,53]. Particularly, in C2C12 cells, the NO donor SNP reduced the cell number and increased the apoptotic markers p53 and caspase-3 in a concentration- and time-dependent manner [54]. However, in differentiated C2C12 cells treated with 0.35 to 3 mM Deta NONOate for 24 h, viability studies found that at the lowest concentration (0.35 mM), cell viability was 82.1%, while at the highest concentration (3 mM), cell viability dropped to 41.5% [55]. Over 24 h Deta NONOate was able to enhance stretch-induced proliferation of C2C12 at 10 µM, but this was lost at higher concentrations (50–100 µM) and did not reduce proliferation compared to control [49]. In the C2C12BRELuc cells used in this study, the application of 10 to 100 µM Deta NONOate had little to no negative effects on C2C12BRELuc viability over 24 h. A decrease in p53, however, might also be associated with osteogenic differentiation as reported earlier for mouse bone marrow-derived mesenchymal cells [56]. The authors found that p53 indirectly suppresses Runx2 expression.
In clinical use, a high concentration of BMP2 is applied, possibly contributing to the negative side effects seen in patients [57,58]. The ability of Arginine, YC-1, and Deta NONOate to supplement BMP2-mediated signaling and early osteogenic differentiation provides an opportunity to reduce the amount of BMP2 applied in the clinic while maintaining the osteoinductive effects. As NO pathway activators (such as nitroglycerine) have been previously used in clinical trials as an orthopedic therapy [34,35,59,60], this should provide a valuable starting point for future investigations into compounds that can be applied clinically in combination with BMP2.
Furthermore, it was noticed that Deta NONOate-, SNAP-, and YC-1-mediated activation of the NO pathway led to a BMP2-independent activation of BMP reporter activity. This could be an indication of an increase in BMP2 expression, as shown in cultured embryonic heart cells in response to NO pathway activation [61], or though osteoinduction as seen in MC3T3 cells [62,63].
It was previously shown that PKA connects BMP2 signaling with the activation of eNOS through phosphorylation in pulmonary cells [27]. This possible link was investigated now in a more osteogenic context. The application of the PKA inhibitor H89 led to inhibition of BMP2-mediated signaling, which was recovered by the addition of the direct NO donor, Deta NONOate. This underlines a connection between BMP2, PKA, and the NO pathway. This finding is in agreement with results reported in previous work, where it was shown that PKA activation itself leads to osteoinduction in MC3T3 cells [64,65], also involving the PKA/CREB signaling [66].
This study showed the ability of the BMP2 pathway to activate the NO pathway through an increase of NO and cGMP production in C2C12 cells. BMP2-stimulated NO production was clearly dose-dependent, with NO production increasing with higher BMP2 concentrations. This is in line with previous studies, where increasing BMP2 concentration increased the NO production in chondrocytes [67]. In endothelial cells, BMP2 enhanced the NOS activity. However, the BMP2 dosage effects on NOS activity was not investigated [27]. This study required a high concentration (50 nM) of BMP2 to significantly increase NO production compared to other studies [27,67], which was partially due to the short stimulatory time required for DAF-2-mediated NO quantification (2 h) compared to the longer times point of Griess assay (4 d) used for BMP2 treated chondrocytes [67]. The BMP2-mediated activation of the NO pathway also increased the cGMP level. This builds on previous studies, which have focused on the association of the BMP2 signaling with the cGMP-activated cGKI. It was demonstrated that the addition of 8-br-cGMP increased cGKI binding of the BMPR2 tail domain [26], but previous studies have overlooked whether addition of BMP2 leads to an increase in cGMP production. Collectively, this and previous work [26,27,67] suggest a possible positive reinforcement of BMP2 signaling, whereby BMP2 is enhanced by the NO pathway and BMP2 itself can activate the NO pathway.
This work provided evidence that the NO pathway is capable of enhancing BMP2-mediated signaling and early osteogenic differentiation, which can have implications for the application of BMP2 in the clinic. Further, it was demonstrated that PKA connects BMP2 and NO signaling. This investigation has solidified the role of NO in activating the BMP2 pathway by demonstrating this in the C2C12 cells. Further studies should investigate the effect on osteogenic differentiation in vitro and in vivo by addressing the effect on mineralization in vitro and ectopic, as well as heterotopic bone formation in vivo.
5. Conclusions
The presented data show that there is a crosstalk between the NO and BMP signaling pathway and that NO leads to enhanced BMP activity (summarized in Figure 12). The NO pathway is linked to the BMP pathway via sGC, with PKA mediating the link between BMP2 and NO pathway.
Figure 12.
Graphical summary of perceived crosstalk of BMP2-mediated signaling and the NO pathway. Figure image composed from graphics taken from Servier Medical Art.
Acknowledgments
I would like to acknowledge the kind gift of C2C12BRELuc cells from Gareth Inman of the Beatson Institute, Glasgow UK. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Abbreviations
| 18S | ribosomal unit 18S | LDN | LDN-193189 |
| ALP | Alkaline Phosphatase | Luc | Luciferase |
| AMHR | anti-Mullerian hormone receptor type | MNE | mean normalized expression |
| Arginine BMP |
L-Arginine Bone Morphogenic Protein |
nNOS | neuronal Nitric Oxide Synthase |
| BMPR | BMP receptor | NO OPG |
Nitric Oxide Osteoprotegerin |
| BMPR2-LF/-SF | BMP receptor - long form/- short form | PAEC | pulmonary arterial endothelial cells |
| BRE | BMP response elements | PBS | Phosphate Buffered Saline |
| cGKI/II | cGMP dependent kinase I/II | PDE | phosphodiesterase’s |
| cGMP | cyclic guanosine monophosphate | Pen/Strep | Penicillin-Streptomycin |
| CO2 Col1a1 |
Carbon Dioxide Type 1 collagen |
PKA | Protein Kinase A |
| Co-SMAD | common mediator SMAD | pNPP | 4-nitrophenyl phosphate |
| CREB | cAMP response element-binding - | PP2 | protein phosphatase 2 |
| CT | Crossing Threshold | rhBMP2 | recombinant human BMP2 |
| DAF-2T | 4,5-Diaminofluorescein triazole | RLU | relative light units |
| Deta | Deta NONOate | R-SMAD | receptor regulated SMAD |
| DMEM | Dulbecco Modified Eagle Medium | Runx2 | Runt-related transcription factor 2 |
| ECM | extracellular matrix | sGC | soluble Guanylyl Cyclase |
| ELISA | Enzyme Linked Immunosorbent Assay | SMAD | Small Mothers Against Decapentaplegic |
| eNOS | Endothelial Nitric Oxide Synthase | TF | Transcription Factors |
| Exp. | Experiment | TGFβ | Transforming Growth Factor β |
| FCS | Fetal Calf Serum | ||
| Id1,2,3 | Inhibitor of Differentiation 1,2,3 |
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/8/10/1273/s1, Figure S1: Supplementary figure for melting curves of qRT-PCR.
Author Contributions
Conceptualization: B.W., P.K.; Methodology: C.D., S.M., and N.B.; Formal Analysis: C.D.; Investigation: C.D.; Data Curation: C.D.; Writing-Original Draft Preparation: C.D.; Writing-Review & Editing: B.W., and F.K.-S.; Supervision: B.W., F.K.-S., and P.K.; Project Administration: B.W., F.K.-S. and C.D.; Funding Acquisition: B.W. and C.D.
Funding
Berlin-Brandenburg Center for Regenerative Therapies (BCRT, BMBF FKZ1315848A), and Berlin-Brandenburg School for Regenerative Therapies (BSRT, DFG Graduate School 203) supported and funded the research conducted by this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Wu M., Chen G., Li Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klatte-Schulz F., Giese G., Differ C., Minkwitz S., Ruschke K., Puts R., Knaus P., Wildemann B. An investigation of BMP-7 mediated alterations to BMP signalling components in human tenocyte-like cells. Sci. Rep. 2016;6:29703. doi: 10.1038/srep29703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao L., Michalski N., Kronander E., Gjoni E., Genoud C., Knott G., Schneggenburger R. BMP signaling specifies the development of a large and fast CNS synapse. Nat. Neurosci. 2013;16:856–864. doi: 10.1038/nn.3414. [DOI] [PubMed] [Google Scholar]
- 4.Dyer L.A., Pi X., Patterson C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol. Metab. 2014;25:472–480. doi: 10.1016/j.tem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali I.H., Brazil D.P. Bone morphogenetic proteins and their antagonists: Current and emerging clinical uses. Br. J. Pharmacol. 2014;171:3620–3632. doi: 10.1111/bph.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alt V., Borgman B., Eicher A., Heiss C., Kanakaris N.K., Giannoudis P.V., Song F. Effects of recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) in grade III open tibia fractures treated with unreamed nails—A clinical and health-economic analysis. Injury. 2015;46:2267–2272. doi: 10.1016/j.injury.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Lyon T.R., Pollak A.N., Valentin-Opran A., Jones A.L., Bucholz R.W., Bosse M.J., Mirza S.K., Webb L.X., Golden J.D. Recombinant Human BMP-2 and Allograft Compared with Autogenous Bone Graft for Reconstruction of Diaphyseal Tibial Fractures with Cortical Defects. J. Bone Jt. Surg. 2006;88:1431–1441. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 8.Wei S., Cai X., Huang J., Xu F., Liu X., Wang Q. Recombinant Human BMP-2 for the Treatment of Open Tibial Fractures. Orthopedics. 2012;35:e847–e854. doi: 10.3928/01477447-20120525-23. [DOI] [PubMed] [Google Scholar]
- 9.Barcak E.A., Beebe M.J. Bone Morphogenetic Protein: Is there Still a Role in Orthopedic Trauma in 2017? Orthop. Clin. North Am. 2017;48:301–309. doi: 10.1016/j.ocl.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Aro H.T., Govender S., Patel A.D., Hernigou P., De Gregorio A.P., Popescu G.I., Golden J.D., Christensen J., Valentin A. Recombinant Human Bone Morphogenetic Protein-2: A Randomized Trial in Open Tibial Fractures Treated with Reamed Nail Fixation. J. Bone Jt. Surg. 2011;93:801–808. doi: 10.2106/JBJS.I.01763. [DOI] [PubMed] [Google Scholar]
- 11.Govender S., Csimma C., Genant H.K., Valentin-Opran A., Amit Y., Arbel R., Aro H., Atar D., Bishay M., Börner M.G., et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. 2002;84:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 12.TerMaat M., Boer F.D., Bakker F., Patka P., Haarman H. Bone Morphogenetic ProteinsDevelopment and Clinical Efficacy in the Treatment of Fractures and Bone Defects. J. Bone Jt. Surg. 2005;87:1367–1378. doi: 10.2106/JBJS.D.02585. [DOI] [PubMed] [Google Scholar]
- 13.Zara J.N., Siu R.K., Zhang X., Shen J., Ngo R., Lee M., Li W., Chiang M., Chung J., Kwak J., et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng. Part A. 2011;17:1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton T. Lessons Learned: What the BMP Trials Controversy Has Taught Us: Lessons Learned: What the BMP Trials Controversy Has Taught Us. [(accessed on 12 March 2018)]; Available online: https://www.aaos.org/News/The_Daily_Edition_of_AAOS_Now/2012/Thursday,_February_9/AAOS9_2_9/?ssopc=1.
- 15.Lyon T., Scheele W., Bhandari M., Koval K.J., Sanchez E.G., Christensen J., Valentin A., Huard F. Efficacy and safety of recombinant human bone morphogenetic protein-2/calcium phosphate matrix for closed tibial diaphyseal fracture: A double-blind, randomized, controlled phase-II/III trial. J. Bone Jt. Surg. 2013;95:2088–2096. doi: 10.2106/JBJS.L.01545. [DOI] [PubMed] [Google Scholar]
- 16.Chan D.S., Garland J., Infante A., Sanders R.W., Sagi H.C. Wound Complications Associated with Bone Morphogenetic Protein-2 in Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2014;28:599–604. doi: 10.1097/BOT.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 17.Heldin C.-H., Miyazono K., Dijke P.T. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 19.Yadin D., Knaus P., Mueller T.D., Information P.E.K.F.C. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016;27:13–34. doi: 10.1016/j.cytogfr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu F., Ventura F., Doody J., Massague J. Human type II receptor for bone morphogenic proteins (BMPs): Extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 1995;15:3479–3486. doi: 10.1128/MCB.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa T., Nohno T., Saito T., Hosokawa K., Noji S., Wolsing D.H., Rosenbaum J.S. Identification of a Human Type II Receptor for Bone Morphogenetic Protein-4 That Forms Differential Heteromeric Complexes with Bone Morphogenetic Protein Type I Receptors. J. Biol. Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig B.L., Imamura T., Okadome T., Cox G.N., Yamashita H., Dijke P.T., Heldin C.H., Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtsson L., Schwappacher R., Roth M., Boergermann J.H., Hassel S., Knaus P. PP2A regulates BMP signalling by interacting with BMP receptor complexes and by dephosphorylating both the C-terminus and the linker region of Smad1. J. Cell Sci. 2009;122:1248–1257. doi: 10.1242/jcs.039552. [DOI] [PubMed] [Google Scholar]
- 24.Chan M.C., Nguyen P.H., Davis B.N., Ohoka N., Hayashi H., Du K., Lagna G., Hata A. A Novel Regulatory Mechanism of the Bone Morphogenetic Protein (BMP) Signaling Pathway Involving the Carboxyl-Terminal Tail Domain of BMP Type II Receptor. Mol. Cell. Biol. 2007;27:5776–5789. doi: 10.1128/MCB.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassel S., Yakymovych M., Hellman U., Rönnstrand L., Knaus P., Souchelnytskyi S. Interaction and functional cooperation between the serine/threonine kinase bone morphogenetic protein type II receptor with the tyrosine kinase stem cell factor receptor. J. Cell. Physiol. 2006;206:457–467. doi: 10.1002/jcp.20480. [DOI] [PubMed] [Google Scholar]
- 26.Schwappacher R., Weiske J., Heining E., Ezerski V., Marom B., Henis Y.I., Huber O., Knaus P. Novel crosstalk to BMP signalling: cGMP-dependent kinase I modulates BMP receptor and Smad activity. EMBO J. 2009;28:1537–1550. doi: 10.1038/emboj.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangopahyay A., Oran M., Bauer E.M., Wertz J.W., Comhair S.A., Erzurum S.C., Bauer P.M. Bone Morphogenetic Protein Receptor II Is a Novel Mediator of Endothelial Nitric-oxide Synthase Activation*. J. Biol. Chem. 2011;286:33134–33140. doi: 10.1074/jbc.M111.274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassel S., Eichner A., Yakymovych M., Hellman U., Knaus P., Souchelnytskyi S. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1346–1358. doi: 10.1002/pmic.200300770. [DOI] [PubMed] [Google Scholar]
- 29.Rangaswami H., Schwappacher R., Marathe N., Zhuang S., Casteel D.E., Haas B., Chen Y., Pfeifer A., Kato H., Shattil S., et al. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci. Signal. 2010;3:ra91. doi: 10.1126/scisignal.2001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broulik P.D., Rosenkrancová J., Ruzicka P., Sedlacek R. Effects of Triiodothyronine and Estrogen Administration on Bone Mass, Mineral Content and Bone Strength in Male Rats. Horm. Metab. Res. 2003;35:527–531. doi: 10.1055/s-20034-2653. [DOI] [PubMed] [Google Scholar]
- 31.Wimalawansa S., De Marco G., Gangula P., Yallampalli C., Wimalawansa P.S. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone. 1996;18:301–304. doi: 10.1016/8756-3282(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 32.Wimalawansa S.J. Restoration of Ovariectomy-Induced Osteopenia by Nitroglycerin. Calcif. Tissue Int. 2000;66:56–60. doi: 10.1007/s002230050011. [DOI] [PubMed] [Google Scholar]
- 33.Wimalawansa S., Chapa M., Yallampalli C., Zhang R., Simmons D., Wimalawansa P.S. Prevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male rats. Bone. 1997;21:275–280. doi: 10.1016/S8756-3282(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 34.Wimalawansa S.J. Nitroglycerin Therapy Is as Efficacious as Standard Estrogen Replacement Therapy (Premarin) in Prevention of Oophorectomy-Induced Bone Loss: A Human Pilot Clinical Study. J. Bone Miner. Res. 2000;15:2240–2244. doi: 10.1359/jbmr.2000.15.11.2240. [DOI] [PubMed] [Google Scholar]
- 35.Jamal S.A., Hamilton C.J., Eastell R., Cummings S.R. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: A randomized trial. JAMA. 2011;305:800–807. doi: 10.1001/jama.2011.176. [DOI] [PubMed] [Google Scholar]
- 36.Jamal S.A., Browner W.S., Bauer D.C., Cummings S.R. Intermittent Use of Nitrates Increases Bone Mineral Density: The Study of Osteoporotic Fractures. J. Bone Miner. Res. 1998;13:1755–1759. doi: 10.1359/jbmr.1998.13.11.1755. [DOI] [PubMed] [Google Scholar]
- 37.Boergermann J., Kopf J., Yu P., Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int. J. Biochem. Cell Biol. 2010;42:1802–1807. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sondag G.R., Salihoglu S., Lababidi S.L., Crowder D.C., Moussa F.M., Abdelmagid S.M., Safadi F.F. Osteoactivin Induces Transdifferentiation of C2C12 Myoblasts Into Osteoblasts. J. Cell. Physiol. 2014;229:955–966. doi: 10.1002/jcp.24512. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K.-I., Matsumoto E., Higashimaki Y., Katagiri T., Sugimoto T., Seino S., Kaji H. Role of Osteoglycin in the Linkage between Muscle and Bone. J. Biol. Chem. 2012;287:11616–11628. doi: 10.1074/jbc.M111.292193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee M.H., Javed A., Kim H.J., Shin H.I., Gutierrez S., Choi J.Y., Rosen V., Stein J.L., Van Wijnen A.J., Stein G.S., et al. Transient upregulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor beta1 in C2C12 myogenic cells coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J. Cell. Biochem. 1999;73:114–125. doi: 10.1002/(SICI)1097-4644(19990401)73:1<114::AID-JCB13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Herrera B., Inman G.J. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinchee-Rodriguez K., Garg N., Venkatakrishnan P., Roman M.G., Adamo M.L., Masters B.S., Roman L.J. Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem. Biophys. Res. Commun. 2013;435:501–505. doi: 10.1016/j.bbrc.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R., Jiao H., Zhao J., Wang X., Lin H. L-Arginine Enhances Protein Synthesis by Phosphorylating mTOR (Thr 2446) in a Nitric Oxide-Dependent Manner in C2C12 Cells. Oxidative Med. Cell. Longev. 2018;2018:1–13. doi: 10.1155/2018/7569127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strobel C., Bormann N., Kadow-Romacker A., Schmidmaier G., Wildemann B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J. Control. Release. 2011;156:37–45. doi: 10.1016/j.jconrel.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Garrison K.R., Shemilt I., Donell S., Ryder J.J., Mugford M., Harvey I., Song F., Alt V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2010;2010:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrero R., Rodríguez-Pascual F., Miras-Portugal M.T., Torres M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: Correlation with nitric oxide production. Br. J. Pharmacol. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keefer L.K., Nims R.W., Davies K.M., Wink D.A. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. In: Packer L., Cadenas E., editors. Methods in Enzymology. Academic Press; Cambridge, MA, USA: 1996. pp. 281–293. [DOI] [PubMed] [Google Scholar]
- 48.Wang P.G., Xian M., Tang X., Wu X., Wen Z., Cai T., Janczuk A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002;102:1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 49.Soltow Q.A., Lira V.A., Betters J.L., Long J.H.D., Sellman J.E., Zeanah E.H., Criswell D.S. Nitric oxide regulates stretch-induced proliferation in C2C12 myoblasts. J. Muscle Res. Cell Motil. 2010;31:215–225. doi: 10.1007/s10974-010-9227-4. [DOI] [PubMed] [Google Scholar]
- 50.Babich H., Zuckerbraun H.L., Hirsch S.T., Blau L. In vitro Cytotoxicity of the Nitric Oxide Donor, S -Nitroso- N -acetyl-peraciUamine, towards Cells from Human Oral Tissue. Pharmacol. Toxicol. 1999;84:218–225. doi: 10.1111/j.1600-0773.1999.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 51.Pervin S., Singh R., Gau C.L., Edamatsu H., Tamanoi F., Chaudhuri G. Potentiation of nitric oxide-induced apoptosis of MDA-MB-468 cells by farnesyltransferase inhibitor: Implications in breast cancer. Cancer Res. 2001;61:4701–4706. [PubMed] [Google Scholar]
- 52.Dubey M., Nagarkoti S., Awasthi D., Singh A.K., Chandra T., Kumaravelu J., Barthwal M.K., Dikshit M. Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis. 2016;7:e2348. doi: 10.1038/cddis.2016.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makeeva N., Roomans G.M., Welsh N. Role of TAB1 in nitric oxide-induced p38 activation in insulin-producing cells. Int. J. Biol. Sci. 2006;3:71–76. doi: 10.7150/ijbs.3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee M.-H., Jang M.-H., Kim E.-K., Han S.-W., Cho S.-Y., Kim C.-J. Nitric oxide induces apoptosis in mouse C2C12 myoblast cells. J. Pharmacol. Sci. 2005;97:369–376. doi: 10.1254/jphs.FPJ04017X. [DOI] [PubMed] [Google Scholar]
- 55.Makris A.C., Sotzios Y., Zhou Z., Makropoulou M., Papapetropoulos N., Zacharatos P., Pyriochou A., Roussos C., Papapetropoulos A., Vassilakopoulos T. Nitric Oxide Stimulates Interleukin-6 Production in Skeletal Myotubes. J. Interf. Cytokine Res. 2010;30:321–327. doi: 10.1089/jir.2009.0022. [DOI] [PubMed] [Google Scholar]
- 56.He Y., De Castro L.F., Shin M.H., Dubois W., Yang H.H., Jiang S., Mishra P.J., Ren L., Gou H., Lal A., et al. p53 Loss Increases the Osteogenic Differentiation of Bone Marrow Stromal Cells. Stem Cells. 2015;33:1304–1319. doi: 10.1002/stem.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pradhan B.B., Bae H.W., Dawson E.G., Patel V.V., Delamarter R.B. Graft Resorption with the Use of Bone Morphogenetic Protein: Lessons from Anterior Lumbar Interbody Fusion Using Femoral Ring Allografts and Recombinant Human Bone Morphogenetic Protein-2. Spine. 2006;31:E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 58.Lee K.-B., Johnson J.S., Song K.-J., Taghavi C.E., Wang J.C. Use of autogenous bone graft compared with RhBMP in high-risk patients: A comparison of fusion rates and time to fusion. Clin. Spine Surg. 2013;26:233–238. doi: 10.1097/BSD.0b013e3182440162. [DOI] [PubMed] [Google Scholar]
- 59.Drescher W., Beckmann R., Kasch R., Pufe M., Knobe M., Kweider N., Hassenpflug J., Tingart M., Pufe T., Kadyrov M. Nitrate patch prevents steroid-related bone necrosis. J. Orthop. Res. 2011;29:1517–1520. doi: 10.1002/jor.21420. [DOI] [PubMed] [Google Scholar]
- 60.Nichols S.P., Storm W.L., Koh A., Schoenfisch M.H. Local delivery of nitric oxide: Targeted delivery of therapeutics to bone and connective tissues. Adv. Drug Deliv. Rev. 2012;64:1177–1188. doi: 10.1016/j.addr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Lu X., Xiang F.-L., Lu M., Feng Q. Nitric Oxide Synthase-3 Promotes Embryonic Development of Atrioventricular Valves. PLoS ONE. 2013;8:e77611. doi: 10.1371/journal.pone.0077611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanazawa I., Yamaguchi T., Yano S., Yamauchi M., Sugimoto T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem. Biophys. Res. Commun. 2008;375:414–419. doi: 10.1016/j.bbrc.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 63.Kanazawa I., Yamaguchi T., Yano S., Yamauchi M., Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am. J. Physiol. Metab. 2009;296:E139–E146. doi: 10.1152/ajpendo.90677.2008. [DOI] [PubMed] [Google Scholar]
- 64.Siddappa R., Martens A., Doorn J., Leusink A., Olivo C., Licht R., Van Rijn L., Gaspar C., Fodde R., Janssen F., et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo K.W.-H., Kan H.M., Ashe K.M., Laurencin C.T. The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J. Tissue Eng. Regen. Med. 2011;6:40–48. doi: 10.1002/term.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H., Li L., Dong Q., Wang Y., Feng Q., Ou X., Zhou P., He T., Luo J. Activation of PKA/CREB Signaling is Involved in BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2015;37:548–562. doi: 10.1159/000430376. [DOI] [PubMed] [Google Scholar]
- 67.Smith R.L., Lindsey D.P., Dhulipala L., Harris A.H.S., Goodman S.B., Maloney W.J. Effects of intermittent hydrostatic pressure and BMP-2 on osteoarthritic human chondrocyte metabolism in vitro. J. Orthop. Res. 2011;29:361–368. doi: 10.1002/jor.21250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.