Abstract
Introduction
The success rate of weight loss maintenance is limited. Therefore, the purpose of this study is to investigate the maintenance of weight loss and immunometabolic health outcomes after diet-induced weight loss followed by 1-year treatment with a glucagon-like peptide-1 receptor agonist (liraglutide), physical exercise or the combination of both treatments as compared with placebo in individuals with obesity.
Methods and analysis
This is an investigator-initiated, randomised, placebo-controlled, parallel group trial. We will enrol expectedly 200 women and men (age 18–65 years) with obesity (body mass index 32–43 kg/m2) to adhere to a very low-calorie diet (800 kcal/day) for 8 weeks in order to lose at least 5% of body weight. Subsequently, participants will be randomised in a 1:1:1:1 ratio to one of four study groups for 52 weeks: (1) placebo, (2) exercise 150 min/week+placebo, (3) liraglutide 3.0 mg/day and (4) exercise 150 min/week+liraglutide 3.0 mg/day. The primary endpoint is change in body weight from randomisation to end-of-treatment.
Ethics and dissemination
The trial has been approved by the ethical committee of the Capital Region of Denmark and the Danish Medicines Agency. The trial will be conducted in agreement with the Declaration of Helsinki and monitored to follow the guidelines for good clinical practice. Results will be submitted for publication in international peer-reviewed scientific journals.
Trial registration number
2015-005585-32
Keywords: weight loss, weight loss maintenance, liraglutide, exercise, obesity, GLP-1 receptor agonist
Strengths and limitations of this study.
First randomised controlled trial investigating the combined and individual effects of liraglutide and exercise to maintain diet-induced weight loss in individuals with obesity.
Direct comparison of liraglutide and exercise on weight loss maintenance and immunometabolic health.
Applying state-of-the-art methodologies, the study may identify novel targets for sustainable immunometabolic health-promoting weight loss strategies.
Introduction
Obesity is associated with increased risk of developing cardiovascular disease and type 2 diabetes (T2D), along with increased risk of all-cause mortality.1 2 Obesity management guidelines recommend weight loss of more than 5% of initial body weight to improve cardiometabolic risk factors, with greater weight loss producing greater benefits.3 4 A 5%–10% weight loss improves lipid profile (~20% reduction in triglycerides, ~15% reduction in low-density lipoprotein cholesterol, ~8% increase in high-density cholesterol levels),1 4 5 reduces systolic and diastolic blood pressure (~5 and ~4 mm Hg, respectively),3 6 reduces glycated haemoglobin (HbA1c)3 4 and improves insulin sensitivity.7–9 However, weight regain reverse these health benefits.10 11 Furthermore, intentional weight loss is typically followed by a 30%–50% regain of lost weight within the first year.12–14 The main biological reasons for the rapid weight regain seem to be that weight loss causes a decrease in total energy expenditure to a degree that is greater than predicted from the decrease in fat and lean mass15 16 in combination with increased appetite in the weight-reduced state.17 18
Increasing energy expenditure by increasing physical activity is the first-line lifestyle modification in the treatment of obesity along with reducing food intake. For exercise interventions targeting general public health recommendations (at least 150 min/week of moderate-intensity aerobic exercise), the associated weight loss is often modest (0%–3%) without concomitant calorie restriction.19–21 However, independent of weight loss, increasing physical activity improves body composition, glycaemic control, low-grade inflammatory profile and cardiorespiratory fitness in individuals with overweight and obesity.22–25 In addition, exercise may preserve lean mass during weight loss26 and thereby counteract the associated decrease in resting metabolic rate27 which may explain the observation that individuals performing regular exercise have less body weight regain after weight loss compared with participants that do not exercise.28 29
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted from enteroendocrine L-cells in the gut after food intake. GLP-1 dose-dependently stimulates glucose-dependent insulin secretion thereby lowering blood glucose and reduces appetite and thereby food intake.30 31 32 33 Treatment for 56 weeks with the GLP-1 receptor agonist (GLP-1 RA), liraglutide (3.0 mg), as an adjunct to regular diet and physical activity recommendations has been shown to improve glycaemic control and induce moderate weight loss of 4.0% in patients with T2D34 and 5.4% in non-diabetic individuals with overweight or obesity35 compared with placebo. In addition, liraglutide has been shown to maintain a diet-induced weight loss over 56 weeks36 and maintain very low-calorie diet (VLCD)-induced improvements of fasting plasma glucose and triglycerides and prevent bone loss over 52 weeks of weight loss maintenance superior to similar diet-induced weight loss maintenance in obese non-diabetic individuals.18 37
Obesity is associated with chronic low-grade inflammation38–40 which is linked to the development of atherosclerosis and insulin resistance.41–43 Physically active individuals have lower inflammatory biomarker concentrations than their inactive counterparts,24 possibly explained by anti-inflammatory effects of an acute bout of exercise44 and lower levels of visceral adipose tissue.45 GLP-1 has also emerged as an immunomodulatory agent.46 47 In mice, GLP-1 RA administration reduces macrophage accumulation and inflammatory markers in the arterial wall,48 adipose tissue49 and heart.50 Similarly, GLP-1 RAs have shown anti-inflammatory effects in human coronary artery endothelial cells and aortic endothelial cells.51 In humans with T2D, short-term GLP-1 RA treatment exerts anti-inflammatory actions, reflected by reduced levels of the macrophage activation molecule sCD16352 and reduced production of several proinflammatory markers, such as tumor necrosis factor-α, interleukin (IL)-1β and IL-6 in peripheral blood mononuclear cells.52 53 Another study showed no improvement of obesity-associated adipose tissue dysfunction in T2D patients after GLP-1RA treatment.54 One year treatment with GLP-1 RAs reduces the inflammation marker, high-sensitivity C-reactive protein, in overweight and obese individuals35 and T2D patients.55 Notably, in patients with T2D and high cardiovascular disease risk, GLP-1 RAs reduced the rate of occurrence of first major cardiovascular event and mortality.56 57
Thus, both physical activity and GLP-1 RA treatment seem to facilitate weight loss maintenance, improve metabolic health and reduce systemic inflammation. However, diet-induced weight loss decreases energy expenditure and increases appetite. We hypothesise that the combination of physical activity and liraglutide treatment improves weight loss maintenance and immunometabolic health since the decreased energy expenditure is targeted with exercise and the increased appetite with liraglutide.
Objectives
The objectives of this study are to investigate the maintenance of weight loss and immunometabolic health outcomes over 52 weeks with liraglutide treatment, physical exercise and the combination in individuals with obesity after a VLCD.
Methods and analysis
Participants, interventions and endpoints
Trial design
This study protocol describes an investigator-initiated, randomised, placebo-controlled, parallel group trial, the S-LiTE trial (acronym for ‘Synergy effect of the appetite hormone GLP-1 (LiragluTide) and Exercise on maintenance of weight loss and health after a low calorie diet’). The trial is double-blinded with regards to study medication but not exercise intervention. The study design is outlined in figure 1.
Figure 1.
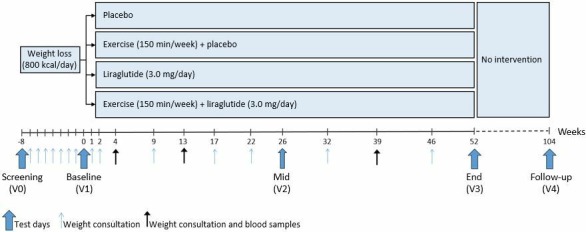
Study design.
Study setting
All examinations in the trial will be carried out at Department of Endocrinology, Hvidovre University Hospital, and Department of Biomedical Sciences, University of Copenhagen, Denmark.
Study status
Recruitment of participants was initiated in September 2016. Last participant last visit is planned for November 2020.
Participants and recruitment
We will enrol expectedly 200 participants. Eligible participants are adults (age 18–65 years) with obesity (body mass index (BMI) 32–43 kg/m2) and no known serious chronic illness (including type 1 and 2 diabetes). Inclusion and exclusion criteria are listed in box 1. Recruitment will be done via local newspapers, online media and flyers from Department of Endocrinology, Hvidovre University Hospital, and Department of Biomedical Sciences, University of Copenhagen. Individuals who agree to participate will be invited to a pre-screening that includes screening of the study eligibility criteria before being finally included in the study. Withdrawn subjects will not be replaced. Re-screening is allowed within the recruitment period.
Box 1. Eligibility criteria for participants in the S-LiTE trial.
Inclusion criteria
BMI: 32–43 kg/m2.
Age: 18–65 years.
Safe contraceptive method or menopause for women.
Exclusion criteria
Patients diagnosed with any known serious chronic illness, including type 1 or 2 diabetes (or a randomly measured fasting plasma glucose >7 mmol/L).
Angina pectoris, coronary heart disease or congestive heart failure (NYHA Class III-IV).
Severe renal impairment (creatinine clearance (GFR) <30 mL/min).
Severe hepatic impairment.
Inflammatory bowel disease.
Gastroparesis.
Cancer.
Chronic obstructive lung disease.
Psychiatric disease, a history of major depressive or other severe psychiatric disorders.
The use of medications that cause clinically significant weight gain or loss.
Previous bariatric surgery.
A history of idiopathic acute pancreatitis.
A family or personal history of multiple endocrine neoplasia type 2 or familial medullary thyroid carcinoma.
Osteoarthritis, which is judged to be too severe to manage the exercise programme.
Pregnancy, expecting pregnancy or breast feeding.
Allergy to any of the ingredients of the study medication: liraglutide, disodium phosphate dihydrate, propylene glycol, phenol, hydrochloric acid and sodium hydroxide.
Regular exercise training at high intensity (eg, spinning)>2 hours per week.
BMI, body mass index; GFR, glomerular filtration rate; GLP, glucagon-like peptide-1; NYHA, New York Heart Association; S-LiTE, Synergy effect of the appetite hormone GLP-1 (LiragluTide) and Exercise on maintenance of weight loss and health after a low calorie diet.
Participant involvement
The design of the study was partly inspired by qualitative interviews of a similar participant group performed by an anthropologist.58 On completion of data analyses, results will be disseminated to all participants as a lay summary of the main findings.
Interventions
Diet-induced weight loss
Initially, all participants will undergo 8 weeks with a VLCD (Cambridge Weight Plan, 800 kcal/day) with the objective to lose at least 5% of body weight. Although some benefits may be evident already at modest weight loss of 2%–3% (eg, triglycerides and HbA1c),3 a ≥5% cut-off is chosen because it is associated with improved cardiovascular disease risk factors59 and T2D prevention60 and thus generally considered a clinically meaningful weight loss.3 20 61 Participants will be instructed to eat four meal replacements per day containing approximately 200 kcal and to only drink water and non-caloric beverages. Participants who have lost at least 5% of body weight after the 8-week weight loss phase will be randomised to one of the four study groups: 52 weeks of treatment with (1) placebo, (2) exercise+placebo, (3) liraglutide or (4) exercise+liraglutide. After randomisation, participants will undergo a 4-week phase-out plan with three daily Cambridge meal products and one regular meal the first week and two daily Cambridge meal products and two regular meals the three subsequent weeks.
Liraglutide or placebo
The GLP-1 RA, liraglutide (3.0 mg) (Saxenda, Novo Nordisk A/S, Bagsværd, Denmark) or placebo will be administrated once daily as subcutaneous injections in the abdomen or thigh. The starting dose is 0.6 mg with weekly increments of 0.6 mg until 3.0 mg is achieved. The titration procedure will be prolonged for participants who do not tolerate fast up-titration. Participants who do not tolerate the 3.0 mg dose may in special circumstances stay at lower dose (2.4 mg). However, the aim is to reach 3.0 mg for all study participants.
Physical exercise
The exercise intervention follows the global recommendations from the WHO of 150 min of moderate-intensity aerobic physical activity throughout the week or 75 min of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate- and vigorous-intensity activity.62 The intervention consists of four sessions per week for a total of 150 min/week. Two sessions per week will be performed under supervision of the study staff and two sessions will be performed individually. A heart rate monitor (Polar A300, Polar Electro Oy, Kempele, Finland) will be worn during all planned exercise sessions. Supervised sessions will consist of structured exercise for a duration of 45 min. Of this, 30 min will be interval-based spinning and 15 min will be circuit training focusing on large muscle groups. Individual exercise sessions will include aerobic exercise such as cycling, rowing or elliptical training as well as brisk walking and/or cycling to work. Participants will be advised to primarily perform non-weight bearing activities. The target aerobic exercise intensity is 80% of maximal heart rate. Participants randomised to an exercise group will undergo a 6-week ramp-up phase with one session in weeks 1 and 2, two sessions in weeks 3 and 4, and three sessions in weeks 5 and 6 before exercising four times per week from weeks 7 to 52. If the planned ramp-up phase with exercise is not possible (eg, due to side effects of study medication or joint pain), ramp-up will proceed more slowly. Participants not randomised to exercise will be instructed to maintain habitual physical activity according to level before entering the trial.
Liraglutide and physical exercise
Combination of the two interventions described above.
The trial will end at week 52 after randomisation where liraglutide 3.0 mg/placebo and exercise treatment will be discontinued. One year after the intervention, the participants will be invited for a follow-up visit.
Criteria for discontinuing/modifying allocated interventions
Participants may withdraw from the intervention at any time. Withdrawn participants will be invited for the planned examinations at week 52 unless written consent is withdrawn. Participants may be withdrawn from the trial at the discretion of the investigator due to a safety concern or a serious violation of the protocol. Participants will be withdrawn in occurrence of pregnancy or at the intention to become pregnant.
Endpoints
Primary endpoint
The primary endpoint is change in body weight from after the initial weight loss phase (baseline/V1) to end of treatment after 52 weeks (end/V3).
Secondary endpoint
The secondary endpoints are changes in body composition (lean/fat mass ratio) and metabolic health (glucose tolerance, lipid status, waist circumference, blood pressure) from V1 to V3.
Explorative endpoints
Explorative endpoints include changes from V1 to V3 in the following parameters: meal-related appetite hormone response; physical fitness and determination of daily physical activity and sleep; systemic markers of immunometabolism; endothelial function; immunometabolic changes in the subcutaneous adipose tissue; gene expression profile of circulating inflammatory cells; bone health; food preferences and subjective appetite sensation; faecal bacterial composition; plasma metabolomics and proteomics; epigenetics of spermatozoa.
Sample size calculation
Sample size is calculated in relation to body weight. In our previous weight loss maintenance study,18 the response within each treatment group was normally distributed with SD of 5.5 kg. Thus, with expectedly 30 participants completing each study arm we will be able to detect a true difference of 4 kg between groups with a power of 0.8, assuming a two-sided α-level of 0.05. In our previous study, 10% did not complete the initial weight loss phase.18 Thus, with expectedly 200 enrolled study participants and an expected dropout rate of 25% after randomisation, we expect to have at least 30 participants from each study arm to complete the trial.
Assignment of intervention
Treatment allocation
After the initial 8-week VLCD phase, participants will be randomised after the test day to one of the four study groups in a 1:1:1:1 ratio in accordance with a subject randomisation list (SRL) provided by Novo Nordisk (NN). A non-blinded study nurse (not otherwise associated with the trial) will allocate study participants according to the SRL. Randomisation will be stratified by sex (male/female) and age (below/above 40 years). NN will provide a total dispensing unit number list (TDL). The non-blinded study nurse will allocate trial medication using the TDL by matching a six-digit dispensing unit number (DUN) to the correct treatment. Each box of study medication will be labelled with a unique DUN. The DUN alone is not unblinding. Thus, dispensing of trial medication to subjects can be carried out by blinded trial staff by selecting the DUN provided by the non-blinded study nurse.
Unblinding
The SRL and the TDL are stored on site at Hvidovre Hospital with restricted access only to the designated non-blinded study nurse. Study ID of the participant is matched with the SRL and TDL which reveals if trial medication is liraglutide or placebo. Preferably, the non-blinded study nurse will perform any unblinding of study participants. However, if needed, all trial staff (sponsor, investigator and sub-investigators) can get access to the SRL and TDL and perform the unblinding procedure. Unblinding can be performed under the following circumstances: treatment of a participant in a medical emergency that requires knowledge of treatment allocation; treatment of a participant for an adverse event (AE); in the event of a suspected unexpected serious adverse reaction (SUSAR); in the event that the participant's study medication is accidentally taken by a member of their household, for example, a child; for the submission of trial data to the Data Monitoring and Safety Committee for the monitoring of safety and/or efficacy.
Data collection, management and analysis
Study visits
Identical test days will take place before the initial weight loss phase (screening/V0), after initial weight loss (baseline/V1) and after 52 weeks of treatment (end/V3) (figure 1). Furthermore, a visit will be performed after 26 weeks of treatment (mid/V2). An overview of performed assessments is provided in table 1. During the weight loss phase, weekly consultations will be conducted to assess compliance to the VLCD, including measurement of body weight and handing out Cambridge meal products. During the 52-week weight maintenance phase, weight consultations, including assessment of AEs, will be conducted at weeks 1, 2, 3, 4, 9, 13, 17, 22, 32, 39 and 46. Consultations at weeks 4, 13 and 39 will include collection of fasting blood samples, measurement of hip and waist circumference, blood pressure and resting heart rate. Finally, participants will be invited to complete a post-trial unsupervised follow-up visit (V4) 1 year after intervention completion.
Table 1.
Overview of study visits
| Visit | Prescreening | V0 | V1 | V2 | V3 | V4 |
| Time point (week) | – | -8 | 0 | 26 | 52 | 104 |
| Informed consent | X | |||||
| Anamnesis | X | |||||
| Inclusion/exclusion criteria | X | |||||
| Demographics | X | |||||
| Pregnancy test | X | X | X | |||
| Adverse events | X | X | X | X | ||
| Body weight | X | X | X | X | X | X |
| Waist and hip circumference | X | X | X | X | X | |
| Blood pressure and heart rate | X | X | X | X | X | |
| Fasting blood samples | X | X | X | X | X | |
| 7-day accelerometry | X | X | X | X | X | |
| Adipose tissue biopsy | X | X | X | X | ||
| DXA scan | X | X | X | X | ||
| Liquid meal test | X | X | X | |||
| Faecal, urine, saliva and semen | X | X | X | |||
| FMD | X | X | X | |||
| ECG | X | X | X | |||
| Physical fitness testing | X | X | X | |||
| Questionnaires | X | X | X | X | ||
| Food preference test | X | X | X | X |
DXA, dual-energy X-ray absorptiometry; FMD, flow-mediated dilation.
Criteria for assessments
Participants must be fasting for minimum 10 hours prior to test days, including foods, liquids and medication (except study medication).
Study medication should preferably be taken on the morning of the tests.
Exercise should not be performed the day before or in the morning before tests.
Assessments
Anthropometrics
Body weight will be measured on a digital scale (TANITA WB-110MA, Tokyo, Japan) to the nearest 0.1 kg without shoes and wearing light clothes. Waist circumference, the midpoint between lowest rib and iliac crest, and hip circumference, the level of the great trochanters, will be measured in duplicate to the nearest 0.1 cm after gentle expiration.
DXA scan
Dual-energy X-ray absorptiometry (DXA) scans will be performed in fasting state to measure body fat mass, fat free mass and bone density (Hologic Discovery A, Hologic, Bedford, USA).
Blood pressure
Blood pressure and resting heart rate will be measured in duplicate from the non-dominant arm with a digital blood pressure monitor (Microlife BP A3 plus, Widnau, Switzerland) in sitting position after at least 5 min of rest.
Fasting blood samples
Fasting blood samples will be collected to measure circulating biomarkers of metabolic health, appetite hormones, immune markers, plasma proteomics and plasma metabolomics. Furthermore, peripheral blood mononuclear cells will be isolated and DNA will be collected. A set of standard samples will be collected and analysed on the same day for participants’ safety, including: haemoglobin, free calcium, creatinine and estimated glomerular filtration rate, potassium, sodium, C-reactive protein, alanine aminotransferase, amylase, alkaline phosphatase, vitamin D, glycated haemoglobin, parathyroid hormone, thyrotropin and blood lipids.
Adipose tissue biopsy
Subcutaneous abdominal adipose tissue biopsies (~1 g) will be obtained by needle aspiration under local anaesthesia using 5–10 mL 0.5% lidocaine. From adipose tissue biopsies, gene expression will be determined from reverse transcription-qPCR: proinflammatory and anti-inflammatory adipocytokines, adipocyte differentiation markers and markers of macrophage infiltration. The immune cells of the adipose tissue will be isolated to evaluate macrophage sup-populations and activation status (single cell analysis).
Meal test
After fasting blood sampling, a liquid meal (Nutricia Nutridrink, 600 kcal, 49.0 E% from carbohydrates, 35.2 E% from fat and 15.8 E% from protein) will be ingested over 15 min, and blood samples will be collected continuously every 15 min for the first hour and every 30 min for the next 2 hours to measure circulating biomarkers of metabolic health, appetite hormones, immune markers, plasma proteomics and plasma metabolomics. During the meal test, appetite sensation will be assessed after each blood sample using a visual analogue scale.63
Faeces, urine, saliva and semen
Faeces samples will be collected to investigate faecal bacterial composition. Semen will be collected (if relevant) to investigate epigenetics of the spermatozoa. Additionally, urine and saliva samples will be collected.
Endothelial function
Flow-mediated dilation (FMD) of the brachial artery will be measured to assess endothelial function.64 FMD, also known as endothelium-dependent vasodilation, is the vasodilatory response of the brachial artery to increased shear stress and reflects the ability of vascular endothelium to produce nitric oxide. The brachial artery will be scanned with high resolution ultrasound imaging using a linear probe at rest and during hyperaemia. Hyperaemia will be induced by inflation and deflation of a sphygmomanometer cuff around the forearm, distal to the site scanned with ultrasound. FMD is calculated as the percentage change of the brachial artery diameter from rest to 60 s after the cuff is released. To assess the endothelium-independent vasodilation, nitroglycerine is given sublingually (0.4 mg) with the diameter of the brachial artery measured before and 5 min after drug administration. Carotid intima-media thickness will also be measured using ultrasound.
Electrocardiography
ECG will be performed to assess safety concerns related to study participation.
Physical fitness
Measurement of physical fitness will include three components: (1) cardiorespiratory fitness (peak oxygen consumption) will be assessed with an incremental maximal cycle protocol performed on an electromagnetically braked cycle ergometer (Corival, Lode Medical Technology, The Netherlands) with continuously determined oxygen consumption and carbon dioxide production (MasterScreen CPX, CareFusion, Germany). After a warm-up protocol, workload will be increased every minute (20 and 25 watt for females and males, respectively) until attainment of peak oxygen consumption based on the following criteria: plateau in oxygen consumption, respiratory exchange ratio above 1.15 or attainment of age-predicted maximal heart rate (220 minus age) and voluntary exhaustion.65 (2) Physical functioning will be measured as time to ascend and descend an 11-step stairway twice. (3) Maximal strength will be measured as isometric maximal voluntary contraction force of the dominant thigh using a dynamometer chair (Good Strength, Metitur Oy, Jyväskylä, Finland).66 67
Questionnaires
Participants will answer five questionnaires to determine self-rated quality of life (The Short Form (36) Health Survey68), eating habits (three-factor eating questionnaire69), physical activity (International Physical Activity Questionnaire70), sleep quality (Pittsburgh Sleep Quality Index71) and self-efficacy (General Self-Efficacy Scale72).
Food preferences
Food preferences and food reward responses will be measured in fasted state and postprandially with the Leeds Food Preference Questionnaire.73 This is a computerised task where standardised pictures of 20 typical Danish food items are shown in the categories: high fat savoury, low fat savoury, high fat sweet and low fat sweet. Participants are instructed to rate each individual food item according to liking and to systematically choose the food items they prefer the most. Speed of choices is covertly assessed as an index of implicit wanting.
Free-living assessment of physical activity and sleep
An accelerometer device (GENEActiv, ActivInsights Ltd, UK) will be worn on the wrist for seven consecutive days and nights with concomitant sleep registration (time for going to and out of bed) to assess habitual physical activity and sleep duration.
Data management
Participants will be identified by study ID. Study data are collected and managed using the REDCap secure web-based system74 hosted by the Capital Region of Denmark, where electronic case report forms (CRF) have been created. During the trial, data will be entered directly into REDCap by study personnel. Extraction procedures will be performed by investigators or sponsor. Laboratory data will be transferred electronically from the laboratory performing clinical analyses and will be archived in secured hard drives with backup. All biological material (blood, adipose tissue, faeces, urine, saliva and semen) obtained from study participants will be kept in a research bio-bank. Samples will be labelled with study ID. The bio-bank allows for analyses of samples to be performed simultaneously to avoid large instrumental variations. The research bio-bank will be terminated no later than 1 August 2036. After this date, the remains of the material will be transferred to a regular bio-bank in Denmark. In order to access this material, a new protocol must be approved by an ethics committee. The Danish Data Protection Agency has been notified about the trial and the trial adheres to the Data Protection Act which requires data to be anonymised as soon as it is practical to do so.
Data analysis plan
Analyses will be based on two defined analysis sets: an intention-to-treat (ITT) analysis set and a per-protocol (PP) analysis set. ITT includes all randomised participants exposed to at least one dose of trial product or exercise that have completed the visit at week 4. PP will include all participants who complete the 52 week intervention with ≥75% compliance to the interventions. Safety analysis will be performed on the ITT analysis set. Two-tailed tests will be performed and the significance level will be set to α=0.05.
Primary endpoint
The change from V1 to V3 in body weight (kg) will be analysed using a general linear model with treatment group as explanatory variable and baseline weight or BMI, age, sex and initial weight loss (if relevant) as covariates. We will use drug treatment (liraglutide, placebo), exercise (yes, no) and their interaction to investigate the effects of drug and exercise. Adequacy of model assumptions will be assessed using graphical models, and outcome variables may be logarithmically transformed if considered necessary to meet the assumptions of linearity, variance homogeneity and/or normality of residuals. The objective of the analysis is to determine whether the effect on body weight of liraglutide 3.0 mg in combination with exercise programme is different from (1) placebo and (2) to either treatments alone, and to quantify the extend of this difference. Weight change over time will also be assessed by repeated measures analyses as appropriate (linear mixed model with an appropriate covariance structure and a suitably described effect over time). Blinding of study medication allocation will be kept for investigators until analysis of the primary endpoint has been completed.
Secondary and exploratory endpoints
For secondary and exploratory endpoints, general linear models will be used to test for differences. The change from V1 to V3 will be analysed using general linear models with treatment group as explanatory variable and baseline weight or BMI, age, sex and initial weight loss (if relevant) as covariates. Longitudinal data will also be analysed using linear mixed models as described above.
Auditing
The Good Clinical Practice (GCP) unit of the University Hospitals of Copenhagen will perform on-site audits minimum once yearly to ensure that the trial adhere to the guidelines for good clinical practice provided by the International Council for Harmonisation (ICH).
Discussion
Obesity prevalence has increased dramatically in the past decades,75 and the high recidivism rates after intentional weight loss13 29 emphasise a strong need for new effective strategies to promote sustainable weight loss maintenance in individuals with obesity. The present protocol describes the first randomised controlled trial to investigate GLP-1 RA treatment combined with physical exercise in the context of long-term weight loss maintenance. The results have the potential to reveal a novel approach of combined lifestyle and pharmacological intervention that may provide substantial improvements of body weight, body composition and immunometabolic health in obesity. Additionally, by applying state-of-the-art methodologies, the study may identify novel targets for future immunometabolic health-promoting weight loss interventions.
Ethics and dissemination
Harms
All AEs will be collected from the first drug administration and in all following contacts with participants throughout the trial. A serious AE (SAE) is defined as any untoward medical occurrence that results in death, is life-threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity, or is a congenial anomaly or birth defect. Once yearly, sponsor will send a report regarding SARs occurring in the trial and safety of the study participants with regard to continuation of the trial to the Danish Medicines Agency and the Ethics Committee. In case of any deadly or life-threatening SUSAR, sponsor will immediately (and no later than 7 days after becoming aware) notify the Danish Medicines Agency and the Ethics Committee. No later than 8 days after reporting of a SUSAR, sponsor will notify the Danish Medicines Agency and the Ethics Committee of all relevant information about sponsor’s and investigator’s follow-up of the SUSAR. All other SUSARs will be reported to the Danish Medicines Agency and the Ethics Committee no later than 15 days after sponsor becoming aware of this. All AEs and SAEs will be noted in the CRF and recorded in the End-of-Trial Form to the Danish Medicines Agency and in the report to the Ethics Committee (if requested) no later than 90 days after trial completion.
Ethical considerations
Liraglutide 3.0 mg will be given with injection pen. Liraglutide 3.0 mg is an approved drug and the dosage will be kept within the approved maximum (3.0 mg). Liraglutide 3.0 mg is safe but may cause transient nausea during the first weeks. Other side effects include dizziness, insomnia (transient) and gall stones. Uncommon/rare side effects include dehydration, inflamed gall bladder, allergic reactions and reduced kidney function. Placebo injections should not cause any discomfort. There should not be any discomfort to the injection if performed as prescribed. The Cambridge Weight Plan is used in daily clinical practice and is considered safe but may cause constipation, fatigue, headache and dizziness. The exercise programme does not exceed the recommendations from WHO.76 However, participants are not habitual exercisers. Therefore, careful considerations regarding the ramp-up of the exercise intervention will be given. Furthermore, most of the exercise is non-weight bearing activity in an attempt to limit potential injuries associated with the exercise intervention. The discomfort associated with the planned assessments is considered minimal. Applying a peripheral venous catheter for blood samples collection can cause transient discomfort, irritation and redness around the puncture site. Some discomfort might be experienced when applying local anaesthesia for the adipose tissue biopsy. The biopsy itself should only cause minimal discomfort. DXA scans use radiation with a radiation dose of approximately 0.02 mSv per examination. This dose is very low compared with the background radiation in Denmark (approximately 3 mSv/year). The FMD and carotid intima-media thickness ultrasound scans do not use radiation. Applying nitroglycerine may cause transient headache, dizziness, decreased blood pressure and increased heart rate. Half-life of nitroglycerine is 1–3 min. Nitroglycerine will not be used if systolic blood pressure is under 100 mm Hg. The risks that are associated with this study are assessed as minimal. By participating in this project, the participants will contribute with new important knowledge about the interaction between GLP-1 and exercise and their importance for weight loss maintenance and metabolic health. Overall, we consider that any potential risks and side effects are outweighed by the advantages of participation. The trial will be carried out in accordance with the Declaration of Helsinki and adhere to the GCP-ICH guidelines. After careful written and oral information about the trial and associated risks have been given, a written informed consent form will be obtained by investigator or sub-investigators before any study-related activities are performed. An English translation of the informed consent form is provided in online supplementary file 1.
bmjopen-2019-031431supp001.pdf (27.7KB, pdf)
Dissemination plan
All study results (positive, negative and inconclusive) will be presented at international scientific conferences as oral presentations or poster presentations. Furthermore, results will be published in international peer-reviewed scientific journals.
Protocol version
The study protocol was approved on 30 June 2016. The present manuscript details the latest version of the protocol (version 9) approved on 6 October 2018.
Supplementary Material
Footnotes
SBKJ, JRL and CJ contributed equally.
Contributors: SST formulated, initiated and designed the study. BMS, JJH, SM, MR, CJ and JRL contributed to the overall study design. SST, SBKJ, JRL, CJ, CRJ and LMO contributed to detailed description of interventions, assessments and/or data analysis plan. SBKJ, JRL, CJ and SST drafted the manuscript. All authors have contributed to and approved the final version of the manuscript. Authorship eligibility will follow the Vancouver guidelines.
Funding: This work is supported by an excellence grant from the Novo Nordisk Foundation (NNF16OC0019968), a Novo Nordisk Foundation Center for Basic Metabolic Research synergy grant and a grant from Helsefonden. The GLP-1 RA (liraglutide 3.0 mg) and placebo are provided by Novo Nordisk. Cambridge weight plan diet products for the initial 8 weeks weight loss programme are provided by Cambridge weight plan. JRL is funded by a PhD grant from Faculty of Health and Medical Sciences, University of Copenhagen. CJ is funded by a PhD grant from Danish Diabetes Academy.
Disclaimer: The planning and conduct of the study, interpretation of data, and writing of manuscripts are completely independent of the funders.
Competing interests: SST has received research grants from Novo Nordisk. SM has received research grants from Novo Nordisk and Boehringer Ingelheim; lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi Aventis; and is a member of advisory boards of AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Squibb, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi Aventis. JJH has served on advisory panels for GlaxoSmithKline, Novo Nordisk, Zealand Pharma, AstraZeneca, MSD, Intarcia and Hanmi and as a consultant for Novo Nordisk, and has received research support from Merck, Sharp & Dohme.
Patient consent for publication: Not required.
Ethics approval: The trial is approved by the Ethical Committee of the Capital Region of Denmark (H-16027082) and the Danish Medicines Agency (EudraCT Number: 2015-005585-32).
.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Akram D, Astrup A, Atinmo T, et al. . Obesity: preventing and managing the global epidemic. Tech Rep Ser 2000. [PubMed] [Google Scholar]
- 2. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med 2017;5 10.21037/atm.2017.03.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jensen MD, Ryan DH, Apovian CM, et al. . AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation 2013;2014:S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garvey WT, Mechanick JI, Brett EM, et al. . American association of clinical endocrinologists and American College of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22:1–203. 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 5. Nordmann AJ, Nordmann A, Briel M, et al. . Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:285–93. 10.1001/archinte.166.3.285 [DOI] [PubMed] [Google Scholar]
- 6. Weiss EP, Albert SG, Reeds DN, et al. . Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am J Clin Nutr 2016;104:576–86. 10.3945/ajcn.116.131391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss EP, Reeds DN, Ezekiel UR, et al. . Circulating cytokines as determinants of weight loss-induced improvements in insulin sensitivity. Endocrine 2017;55:153–64. 10.1007/s12020-016-1093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magkos F, Fraterrigo G, Yoshino J, et al. . Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23:591–601. 10.1016/j.cmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trussardi Fayh AP, Lopes AL, Fernandes PR, et al. . Impact of weight loss with or without exercise on abdominal fat and insulin resistance in obese individuals: a randomised clinical trial. Br J Nutr 2013;110:486–92. 10.1017/S0007114512005442 [DOI] [PubMed] [Google Scholar]
- 10. Kroeger CM, Hoddy KK, Varady KA. Impact of weight regain on metabolic disease risk: a review of human trials. J Obes 2014;2014:1–8. 10.1155/2014/614519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas TR, Warner SO, Dellsperger KC, et al. . Exercise and the metabolic syndrome with weight regain. J Appl Physiol 2010;109:3–10. 10.1152/japplphysiol.01361.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barte JCM, Ter Bogt NCW, Bogers RP, et al. . Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev 2010;11:899–906. 10.1111/j.1467-789X.2010.00740.x [DOI] [PubMed] [Google Scholar]
- 13. Anderson JW, Konz EC, Frederich RC, et al. . Long-Term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74:579–84. 10.1093/ajcn/74.5.579 [DOI] [PubMed] [Google Scholar]
- 14. Curioni CC, Lourenço PM. Long-Term weight loss after diet and exercise: a systematic review. Int J Obes 2005;29:1168–74. 10.1038/sj.ijo.0803015 [DOI] [PubMed] [Google Scholar]
- 15. Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes 2010;34:S47–55. 10.1038/ijo.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–8. 10.1056/NEJM199503093321001 [DOI] [PubMed] [Google Scholar]
- 17. Iepsen EW, Lundgren J, Holst JJ, et al. . Successful weight loss maintenance includes long-term increased meal responses of GLP-1 and PYY3–36. Eur J Endocrinol 2016;174:775–84. 10.1530/EJE-15-1116 [DOI] [PubMed] [Google Scholar]
- 18. Iepsen EW, Lundgren J, Dirksen C, et al. . Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes 2015;39:834–41. 10.1038/ijo.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swift DL, Johannsen NM, Lavie CJ, et al. . The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 2014;56:441–7. 10.1016/j.pcad.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donnelly JE, Blair SN, Jakicic JM, et al. . American College of sports medicine position stand. appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;41:459–71. 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 21. Shaw KA, Gennat HC, O'Rourke P, et al. . Exercise for overweight or obesity. Cochrane Database Syst Rev 2006;73 10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nordby P, Auerbach PL, Rosenkilde M, et al. . Endurance training per se increases metabolic health in young, moderately overweight men. Obesity 2012;20:2202–12. 10.1038/oby.2012.70 [DOI] [PubMed] [Google Scholar]
- 23. Bruce CR, Thrush AB, Mertz VA, et al. . Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 2006;291:E99–107. 10.1152/ajpendo.00587.2005 [DOI] [PubMed] [Google Scholar]
- 24. Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clinica Chimica Acta 2010;411:785–93. 10.1016/j.cca.2010.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavie CJ, Arena R, Swift DL, et al. . Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res 2015;117:207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calbet JAL, Ponce-González JG, Calle-Herrero JdeL, et al. . Exercise preserves lean mass and performance during severe energy deficit: the role of exercise volume and dietary protein content. Front Physiol 2017;8:1–13. 10.3389/fphys.2017.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and weight loss. Sport Med 2006;36:239–62. [DOI] [PubMed] [Google Scholar]
- 28. Jakicic JM, Marcus BH, Lang W, et al. . Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med 2008;168:1550 10.1001/archinte.168.14.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wing RR, Phelan S. Long-Term weight loss maintenance. Am J Clin Nutr 2005;82:222S–5. 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
- 30. Flint A, Raben A, Astrup A, et al. . Glucagon-Like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515–20. 10.1172/JCI990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holst JJ, Deacon CF, Vilsbøll T, et al. . Glucagon-Like peptide-1, glucose homeostasis and diabetes. Trends Mol Med 2008;14:161–8. 10.1016/j.molmed.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 32. Torekov SS, Holst JJ, Ehlers MR. Dose response of continuous subcutaneous infusion of recombinant glucagon-like peptide-1 in combination with metformin and sulphonylurea over 12 weeks in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014;16:451–6. [DOI] [PubMed] [Google Scholar]
- 33. Torekov SS, Kipnes MS, Harley RE, et al. . Dose response of subcutaneous GLP-1 infusion in patients with type 2 diabetes. Diabetes Obes Metab 2011;13:639–43. [DOI] [PubMed] [Google Scholar]
- 34. Davies MJ, Bergenstal R, Bode B, et al. . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the scale diabetes randomized clinical trial. J Am Med Assoc 2015;314:687–99. [DOI] [PubMed] [Google Scholar]
- 35. Pi-Sunyer X, Astrup A, Fujioka K, et al. . A randomized, controlled trial of 3.0 Mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 36. Wadden TA, Hollander P, Klein S, et al. . Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the scale maintenance randomized study. Int J Obes 2013;37:1443–51. 10.1038/ijo.2013.120 [DOI] [PubMed] [Google Scholar]
- 37. Iepsen EW, Lundgren JR, Hartmann B, et al. . Glp-1 receptor agonist treatment increases bone formation and prevents bone loss in Weight-Reduced obese women. J Clin Endocrinol Metab 2015;100:2909–17. [DOI] [PubMed] [Google Scholar]
- 38. Stępień M, Stępień A, Wlazeł RN, et al. . Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: a comparative pilot study. Lipids Health Dis 2014;13:10–13. 10.1186/1476-511X-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panagiotakos D, Pitsavos C, Yannakoulia M, et al. . The implication of obesity and central fat on markers of chronic inflammation: the Attica study. Atherosclerosis 2005;183:308–15. 10.1016/j.atherosclerosis.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 40. Geyer PE, Wewer Albrechtsen NJ, Tyanova S, et al. . Proteomics reveals the effects of sustained weight loss on the human plasma proteome. Mol Syst Biol 2016;12:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–43. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 42. Duncan BB, Schmidt MI, Pankow JS, et al. . Low-Grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis risk in Communities study. Diabetes 2003;52:1799–805. 10.2337/diabetes.52.7.1799 [DOI] [PubMed] [Google Scholar]
- 43. Vozarova B, Weyer C, Lindsay RS, et al. . High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002;51:455–61. 10.2337/diabetes.51.2.455 [DOI] [PubMed] [Google Scholar]
- 44. Starkie R, Ostrowski SRYE, Jauffred S, et al. . Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J 2003;17:884–6. 10.1096/fj.02-0670fje [DOI] [PubMed] [Google Scholar]
- 45. Gleeson M, Bishop NC, Stensel DJ, et al. . The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607–15. 10.1038/nri3041 [DOI] [PubMed] [Google Scholar]
- 46. Torekov SS. Glucagon-Like peptide-1 receptor agonists and cardiovascular disease: from leader to EXSCEL. Cardiovasc Res 2018;114:e70–1. 10.1093/cvr/cvy124 [DOI] [PubMed] [Google Scholar]
- 47. Insuela DBR, Carvalho VF. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur J Pharmacol 2017;812:64–72. 10.1016/j.ejphar.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 48. Arakawa M, Mita T, Azuma K, et al. . Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010;59:1030–7. 10.2337/db09-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee Y-S, Park M-S, Choung J-S, et al. . Glucagon-Like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012;55:2456–68. 10.1007/s00125-012-2592-3 [DOI] [PubMed] [Google Scholar]
- 50. Noyan-Ashraf MH, Shikatani EA, Schuiki I, et al. . A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 2013;127:74–85. 10.1161/CIRCULATIONAHA.112.091215 [DOI] [PubMed] [Google Scholar]
- 51. Garczorz W, Gallego-Colon E, Kosowska A, et al. . Exenatide exhibits anti-inflammatory properties and modulates endothelial response to tumor necrosis factor α-mediated activation. Cardiovasc Ther 2018;36:e12317 10.1111/1755-5922.12317 [DOI] [PubMed] [Google Scholar]
- 52. Hogan AE, Gaoatswe G, Lynch L, et al. . Glucagon-Like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 2014;57:781–4. 10.1007/s00125-013-3145-0 [DOI] [PubMed] [Google Scholar]
- 53. Chaudhuri A, Ghanim H, Vora M, et al. . Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012;97:198–207. 10.1210/jc.2011-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pastel E, McCulloch LJ, Ward R, et al. . Glp-1 analogue-induced weight loss does not improve obesity-induced at dysfunction. Clin Sci 2017;131:343–53. 10.1042/CS20160803 [DOI] [PubMed] [Google Scholar]
- 55. Bunck MC, Diamant M, Eliasson B, et al. . Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734–7. 10.2337/dc09-2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marso SP, Daniels GH, Brown-Frandsen K, et al. . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marso SP, Bain SC, Consoli A, et al. . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 58. Christensen BJ, Iepsen EW, Lundgren J, et al. . Instrumentalization of eating improves weight loss maintenance in obesity. Obes Facts 2017;10:633–47. 10.1159/000481138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wing RR, Lang W, Wadden TA, et al. . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–6. 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Knowler WC, Barrett-Connor E, Fowler SE, et al. . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015;23:2319–20. 10.1002/oby.21358 [DOI] [PubMed] [Google Scholar]
- 62. World Health Organization Physical Activity and Adults - Recommended levels of physical activity for adults aged 18 - 64 years. Available: https://www.who.int/dietphysicalactivity/factsheet_adults/ [Accessed 8 Apr 2019].
- 63. Flint A, Raben A, Blundell JE, et al. . Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes 2000;24:38–48. 10.1038/sj.ijo.0801083 [DOI] [PubMed] [Google Scholar]
- 64. Tousoulis D, Antoniades C, Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non-invasive techniques. Heart 2005;91:553–8. 10.1136/hrt.2003.032847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beltz NM, Gibson AL, Janot JM, et al. . Graded Exercise Testing Protocols for the Determination of VO2 max: Historical Perspectives, Progress, and Future Considerations. J Sports Med 2016;2016:1–12. 10.1155/2016/3968393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Curb JD, Ceria-Ulep CD, Rodriguez BL, et al. . Performance-Based measures of physical function for high-function populations. J Am Geriatr Soc 2006;54:737–42. 10.1111/j.1532-5415.2006.00700.x [DOI] [PubMed] [Google Scholar]
- 67. Ruhdorfer AS, Dannhauer T, Wirth W, et al. . Thigh muscle cross-sectional areas and strength in knees with early vs knees without radiographic knee osteoarthritis: a between-knee, within-person comparison. Osteoarthritis Cartilage 2014;22:1634–8. 10.1016/j.joca.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ware JE, Sherbourne CD, Donald C. The mos 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 69. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83. 10.1016/0022-3999(85)90010-8 [DOI] [PubMed] [Google Scholar]
- 70. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport 2000;71:114–20. 10.1080/02701367.2000.11082794 [DOI] [PubMed] [Google Scholar]
- 71. Buysse DJ, Reynolds CF, Monk TH, et al. . The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 72. Schwarzer R, Jerusalem M. Generalized Self-Efficacy scale. In: Measures in health psychology: A user’s portfolio. Causal and control beliefs 1995:35–7. [Google Scholar]
- 73. Finlayson G, King N, Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite 2008;50:120–7. 10.1016/j.appet.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 74. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. World Health Organization Obesity and overweight. who fact sheet, 2017. Available: http//wwwwhoint/mediacentre/factsheets/fs311/en/ [Accessed 14 Jan 2018].
- 76. World Health Organization Global recommendations on physical activity for health, 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031431supp001.pdf (27.7KB, pdf)


