Abstract
Objective
This study aimed to promote an understanding of spontaneous pneumothorax by analysing the prevalence rate and medical service use by patients with spontaneous pneumothorax according to sociodemographic characteristics.
Design
A 12-year nationwide study.
Setting
Data obtained from the Korean National Health Insurance Service Sharing Service.
Participants
A total of 4658 participants who used medical services due to spontaneous pneumothorax between 2002 and 2013 in Korea.
Outcome measures
For those diagnosed with spontaneous pneumothorax, use of medical services, hospitalisation data, sociodemographics, comorbidity, treatment administered and medication prescribed were recorded.
Results
The annual prevalence of spontaneous pneumothorax ranged from 39 to 66 per 100 000 individuals, while the prevalence of hospitalisation due to spontaneous pneumothorax ranged from 18 to 36 per 100 000 individuals. The prevalence rate of spontaneous pneumothorax in Korea has increased since 2002. The male to female ratio was approximately 4–10:1, with a higher prevalence rate in men. By age, the 15–34 years old group, and particularly those aged 15–19 years old, showed the highest prevalence rate; the rate then declined before increasing again for those aged 65 years or older. In total, 47%–57% of patients with spontaneous pneumothorax underwent hospitalisation. The average number of rehospitalisations due to pneumothorax was 1.56 per person, and more than 70% of recurrences occurred within 1 year. Chronic obstructive pulmonary disease was the most common comorbidity. The average treatment period was 11 days as an outpatient and 14 days in-hospital. The average medical costs were $94.50 for outpatients and $2523 for hospital admissions. The most common treatment for spontaneous pneumothorax was oxygen inhalation and thoracostomy, and the most commonly prescribed medications were analgesics, antitussives and antibiotics.
Conclusions
We here detailed the epidemiology and treatments for spontaneous pneumothorax in Korea. This information can contribute to the understanding of spontaneous pneumothorax.
Keywords: epidemiology, longitudinal cohort, National Health Insurance Service-Sample Cohort Database (NHIS-SCD), prevalence, primary spontaneous pneumothorax
Strengths and limitations of this study.
Large-scale, long-term (12 years) follow-up data sets, representing the Korean population, were analysed.
Inpatient and outpatient costs of treatment for spontaneous pneumothorax were calculated.
Data on detailed medical service use, such as medication, procedures and surgery undertaken due to spontaneous pneumothorax, were recorded and analysed.
Due to a lack of relevant information in the claims data (eg, medical treatments are not necessarily covered by insurance), not all relevant treatments data could be considered.
This study could not distinguish whether medical service use was due to new occurrences or follow-up of existing spontaneous pneumothorax.
Introduction
The pulmonary system is important because it plays a crucial role in the oxygenation of the blood.1 In pneumothorax, air or gas pools in the interpleural cavity,2 leading to lung collapse and impaired pulmonary function.3 Pneumothorax is categorised into spontaneous pneumothorax (SP) or traumatic pneumothorax, depending on the aetiology, and SP is subcategorised into primary spontaneous pneumothorax (PSP), occurring in the absence of underlying lung-related comorbidities, and secondary spontaneous pneumothorax (SSP), occurring in the presence of underlying lung-related comorbidities.4
The prevalence rate of SP per 100 000 individuals reportedly ranges from 1.2 to 9.8 in women and from 7.4 to 24.0 in men; the prevalence rate of hospitalised SP per 100 000 individuals is about 17–22 in men, 6–7 in women and 14–23 overall.5–9 Although SP has a relatively low prevalence rate, it is clinically important10 as relapse rates are high (20%–40%, depending on duration)7 11 12; hospitalisation is often required, influencing work productivity. Furthermore, patients with pneumothorax may be at high risk of stress.13
However, there is a paucity of research on SP.14 There is a lack of knowledge about the modalities of management and the treatments of SP.15 Given its low prevalence rate, a large-scale research at the national level is needed to evaluate the epidemiology of SP. Although large-scale studies have been performed in the UK, France and Germany, these have provided limited epidemiological information.6–8
Thus, we aimed to determine the prevalence rate of SP and medical service use of patients treated for SP, according to sociodemographic characteristics, by implementing the National Sample Cohort (NSC) data from the National Health Insurance Service (NHIS) of Korea, to reformulate a more accurate understanding of the prevalence, treatment and management of SP in the country.
Methods
Database
Twelve years of NHIS-NSC data (for the period 2002–2013 period) were analysed. The National Health Insurance (NHI) system in Korea is a single-payer system to which 98% of citizens belong. Under this system, a medical provider requests reimbursement from a third-person payer (the NHIS) when a patient uses a medical service.16 17 The NHIS-NSC is a population-based cohort built by the NHIS. In 2002, a cohort was extracted by systematic stratified random sampling from a pool of 47 851 928 individuals, excluding those who were not enrolled in the NHI (income level of 0) and foreigners.18 The cohort was randomly selected from 1476 strata were constructed by age group, sex, participant’s eligibility status and income level. Finally, 2.2% of this pool (1 025 340 individuals) were extracted and data from newborns were added each year to maintain the sample size.18
Study population and sampling
Our study was performed on 4658 patients diagnosed with SP in the 12 years between January 2002 and December 2013 in Korea. Patients with SP were defined as those who used a medical service at least once for main or secondary diseases with the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) codes J93.0 (spontaneous tension pneumothorax), J93.1 (other spontaneous pneumothorax), J93.8 (other pneumothorax) or J93.9 (pneumothorax, unspecified). S27 (injury of other and unspecified intrathoracic organs) and S27.0X (traumatic pneumothorax) were excluded because the present analysis was only for patients with SP.
Main descriptive variables
Prevalence rate of SP
For each year, the frequency per 100 000 individuals and the frequency of patients who used medical services at least once for SP as the major or secondary diagnosis were determined. Sociodemographic characteristics are shown by sex, age, income level and insurance type. Based on previous studies,6 8 age was subdivided as follows: <15, 15–34, 35–64 and ≥65 years. Income level was divided into low (0, 1, 2 or 3 out of 0–10), medium (4, 5, 6 or 7) or high (8, 9 or 10). Insurance type was divided into region-based enrollee, job-based enrollee and medical assistance recipient.
For each year, the prevalence of SP per 100 000 individuals and the frequency of patients who were hospitalised due to SP were analysed. In addition, the prevalence rate of SSP was analysed. SSP was operationally defined as patients with SP with a diagnosis of an underlying lung disease such as chronic obstructive pulmonary disease (COPD), pneumonia, interstitial lung disease, lung cancer, asthma and lung abscess within a year before the first date of SP onset. Patients who used hospitalisation services at least once were counted; two or more hospitalisations were not counted more than once. The hospitalisation rate among patients with pneumothorax was calculated using the same standards: the denominator was the number of patients who used medical services at least once due to SP in a given year, by sex and age, and the numerator was the number of patients who used hospitalisation services at least once.
Hospitalisation of patients with SP by year
The frequency of rehospitalisation due to SP is shown by time period. The ratios of the total rehospitalisation number to rehospitalisation in a specific time period are also shown. The number of rehospitalisations counted all hospitalisations (other than the first time) and allowed for duplication for each patient. To calculate the number of hospitalisations per person, the average, SD, minimum and maximum number of hospitalisations per time period were calculated. For the number of hospitalisations per person, first-time hospitalisations were also included. The interval between hospitalisations was calculated as the interval between the dates of two admissions.
Comorbidities in patients with SP
Diseases were counted as comorbidities if they were diagnosed as the major or secondary disease within 1 year before the first date of SP onset. Patients who were given codes for frequent comorbidities, that is, COPD, pneumonia, interstitial lung disease, lung cancer, asthma and lung abscess, were counted; patients with multiple comorbid conditions were counted more than once.8 19 The Charlson Comorbidity Index (CCI) was categorised into normal and CCI 0, 1, 2 and ≥3.20–22 The ICD-10 codes of comorbidities are shown in online supplementary table 1.
bmjopen-2018-028624supp005.pdf (118.3KB, pdf)
Treatment period and medical costs of patients with SP
The total number of follow-ups within 60 days after medical service use due to new SP onset, the number of follow-ups that occurred within a specified duration and the comparative ratio of follow-ups within 60 days were analysed. The mean, minimum and maximum number of follow-ups, including the first medical service use, were analysed. For hospitalisation service users, the follow-up duration was calculated based on the date of discharge. The number of follow-ups in a specified period after discharge and the average number of follow-ups are shown.
Additionally, the average follow-up duration and the average medical costs for each new SP onset were also calculated, as was the average length of hospitalisation for patients. Given that we analysed administrative data, it was necessary to distinguish between medical service use due to new SP onset and that due to follow-up. Based on previous research indicating that air leaks from SP generally stop within approximately 15 days and that X-ray follow-up is recommended 2–4 weeks after discharge,7 23 screening analysis results and discussions with medical experts in Korea, SP follow-up duration was determined as 60 days. New SP onset was defined as cases in which no medical service use (due to SP) was recorded in the previous 60 days. When one patient experienced multiple new occurrences of SP, repeated counting was allowed.
The medical costs of patients with SP included the average medical cost per person. Medical costs determined to be eligible for reimbursement by the Health Insurance Review and Assessment Service out of treatment costs were indicated in the submitted insurance claims statement. Medical costs are the sum of benefits reimbursed by the insurer (NHIS) to the medical care institutions and self-payment costs paid by the beneficiary (patient). Each patient’s medical costs were calculated as the sum of costs listed on their claims. The average medical costs were the amount of total medical expenses for 1 year divided by the number of patients.
Treatment and medication for patients with SP
In terms of treatment of patients with SP, the most frequent treatment with a code that corresponds to the ‘Procedure/surgery’ category in the statement listing SP as the major or secondary diagnosis was given. All statements prescribing the corresponding treatment were counted, and duplication of treatments and patients was allowed. Treatments were categorised as non-surgical treatments and surgical treatments. Non-surgical treatment included oxygen inhalation, nebuliser treatment of the lower airway, suction drainage or tracheostomy suction, and tracheal intubation, and surgical treatment included thoracostomy, wedge resection of the lung, resection of bullae and pleurodesis. The criteria distinguishing between surgical and non-surgical treatments were in accordance with the classification of the claims data. Percentages were calculated by having the denominator as codes that correspond to the ‘Procedure/surgery’ category, excluding costs of materials, drugs used during treatment and list of tests. For medications, the most frequent treatment belonging to the code that corresponds to the ‘Medication’ category in the diagnostic statement was given. Among frequently prescribed drugs, digestants were excluded. Similar to ‘Procedure/surgery’, all statements that prescribed the corresponding drug were counted, and drug and patient duplication was allowed. Medication data were limited to cases of inpatient prescription, while outpatient prescriptions were excluded. Based on the fifth Anatomical Therapeutic Chemical Classification System level,11 12 24 notation of the medication taken was indicated by the five-step code of the chemical name, and the three drugs that did not undergo the five-step classification were indicated by their ingredients.
Statistical analysis
The total annual prevalence rate of SP and that of hospitalisation due to SP is shown in frequency and frequency per 100 000 individuals. It was calculated by dividing the corresponding frequency by the total population that fits in the corresponding range and multiplying by 100 000. Categorical variables, such as the sociodemographic characteristics, number of rehospitalisations per period, comorbidities, CCI level, and follow-up frequency per period, treatment and drugs for the total number of patients with SP for 12 years, are shown as frequencies and percentages. Continuous variables, such as number of hospitalisations, CCI value, total follow-up frequency, length of hospitalisation in days and medical costs, are reflected by calculating the average, SD, minimum and maximum values. Logistic regression analysis was performed including age, sex, income, type of insurance and comorbidity to identify factors influencing hospitalisation rate. Data cleaning and analyses were conducted using the SAS V.9.4 statistical package.
Patient and public involvement statement
Patients and the public were not involved in the design. Encrypted and published data were used in this study. Thus, there was no involvement of patients in this study.
Results
Sociodemographic characteristics of patients with SP
A flow chart that summarises the selection of patients is shown in online supplementary figure 1. It shows that 4658 patients used medical services for SP as their major or secondary diagnosis at least once during 2002–2013. The sociodemographic characteristics of patients with SP are shown in table 1. The male to female ratio was about 4.4:1. Most patients were 15–34 years old, while those below 15 years were the least affected. Most were in the high-income group, while the low-income group used medical services the least. Job-based enrollees were the most common category.
Table 1.
Sociodemographic characteristics of patients with SP
| All SP (N=4658) |
%* | |
| Sex | ||
| Male | 3796 | 81.5 |
| Female | 862 | 18.5 |
| Age (years) | ||
| <15 | 123 | 2.6 |
| 15–34 | 2407 | 51.7 |
| 35–64 | 1374 | 29.5 |
| ≥65 | 754 | 16.2 |
| Income range | ||
| Low (0–3) | 1037 | 22.3 |
| Medium (4–7) | 1725 | 37.0 |
| High (8–10) | 1896 | 40.7 |
| Insurance type | ||
| Region | 1741 | 37.4 |
| Job | 2804 | 60.2 |
| Medical assistance | 113 | 2.4 |
*%=n/4658×100.
SP, spontaneous pneumothorax.
bmjopen-2018-028624supp001.pdf (52.9KB, pdf)
Prevalence rate of SP
The annual prevalence rate of SP (patients with SP per 100 000 individuals) by sex ranged from 39 to 66 per 100 000 individuals, depending on the year (figure 1). The prevalence rate of SP consistently increased from 2002 to 2011 and decreased slightly thereafter. Among men, the rate was 63 per 100 000 individuals in 2002, and then increased consistently to 109 per 100 000 individuals in 2011. Thereafter, it decreased slightly, but the rate still exceeded 100 per 100 000 individuals in 2012 and 2013. Among women, the rate was 15 per 100 000 individuals in 2002, and remained essentially constant at 15–18 per 100 000 individuals, except for an increase to 23 per 100 000 individuals in 2011. The male to female ratio slowly increased from 4.2:1 in 2002, to a ratio of 6.5:1 in 2010.
Figure 1.
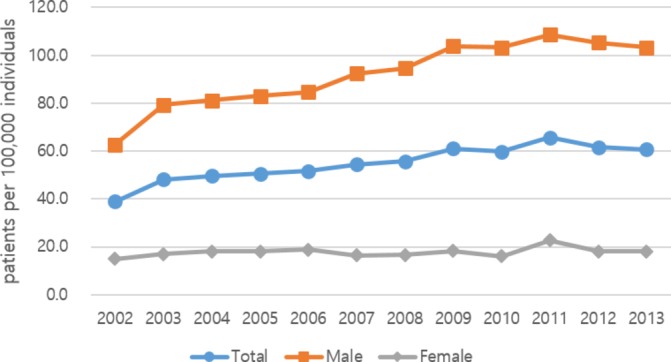
Annual prevalence rate of spontaneous pneumothorax, by sex (per 100 000 individuals).
In all years, except 2002, the SP prevalence rate was highest in the 15–34 years old group, followed by the ≥65 years old, 34–64 years old and <15 years old groups (figure 2). The prevalence rate for those in the 15–34 years old group was 64.8 per 100 000 individuals in 2002, which then increased to 133.2 per 100 000 individuals in 2009. The rate in the ≥65 years old group also increased, from 69.1 per 100 000 individuals in 2002 to 93.5 per 100 000 individuals in 2013.
Figure 2.
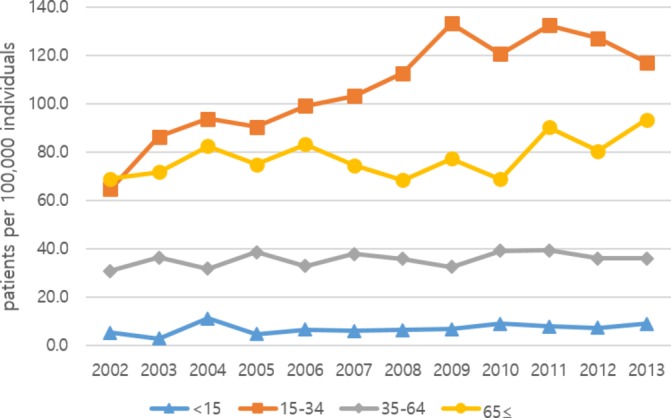
Annual prevalence rate of spontaneous pneumothorax, by age (per 100 000 individuals).
The prevalence rate of SP by sex, age, income level and insurance type is summarised in online supplementary table 2. To examine the detailed distribution by age, age groups were further subdivided into 5-year increments. The prevalence rate tended to be highest at 15–19 years, decreased to 40–49 years, and then peaked again at 65–74 years before decreasing again (online supplementary figure 2). The prevalence rate was slightly higher in the high-income group, but remained low among medical assistance recipients, although this rate increased after 2011 to a rate higher than that of health insurance enrollees.
bmjopen-2018-028624supp002.pdf (1.1MB, pdf)
The prevalence rates of patients with SP who were hospitalised are shown in figures 3 and 4. The rate of hospitalisation was 18.3 per 100 000 individuals in 2002, increasing to 36.2 per 100 000 individuals in 2011 before decreasing in 2012. The rate of hospitalisation in men was 31.4 per 100 000 individuals in 2002, which increased to 61.0 per 100 000 individuals in 2011. Among women, the hospitalisation rate was 5.3 per 100 000 individuals in 2002 and 11.3 per 100 000 in 2011. This rate decreased for both sexes in 2012. The rate tended to increase up to 2012 for men, but there was no consistent trend for women. The male to female ratio of hospitalisation was 6:1 on average. By age, hospitalisation in the 15–34 years age group was the highest at 33–75 per 100 000 individuals, followed by the ≥65 years old group and the 35–64 years old group. The rate was lowest for the <15 years old group, at 1–3 per 100 000 individuals. The prevalence of hospitalised patients with SP among the total population, by age and sex, is shown in online supplementary table 3.
Figure 3.
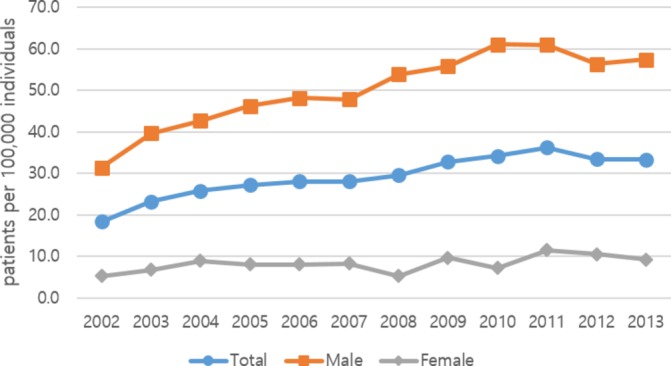
Annual prevalence rate of hospitalisation due to spontaneous pneumothorax, by sex (per 100 000 individuals).
Figure 4.
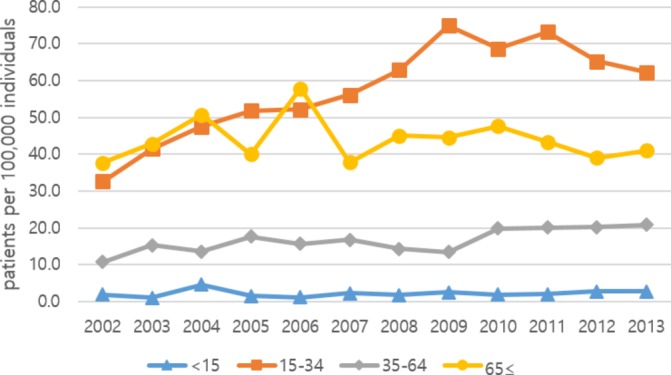
Annual prevalence rate of hospitalisation due to spontaneous pneumothorax, by age (per 100 000 individuals).
The prevalence rates of SSP ranged from 10.5 to 12.1 per 100 000 individuals, from 8.5 to 9.6 in men and from 1.4 to 2.7 in women (online supplementary figure 3).
bmjopen-2018-028624supp003.pdf (269.7KB, pdf)
Hospitalisation of patients with SP by year
Within the sample of patients with SP, the proportion of those receiving hospital treatments was defined as the hospitalisation treatment rate. The number of patients who used the hospitalisation service for SP in 2002 was 188 (47.2%) (table 2). The rate of hospitalisation treatment slowly increased thereafter up to 2010 and then decreased slightly. No sex-related differences in hospitalisation treatment rates were observed. However, for age-related differences, the ≥65 years old group had the highest hospitalisation treatment rate, while the <15 years old group had the lowest rate. In addition, the results of the logistic regression analysis model showed that the hospitalisation rate was influenced by sex, age and underlying lung disease (online supplementary table 4).
Table 2.
Rate of hospitalisation treatment for patients with SP
| Total SP (n) | Total HP (n) | HR (%)* | Sex | Age (years) | |||||||||||
| Male | Female | <15 | 15–34 | 35–64 | ≥65 | ||||||||||
| n | HR* | n | HR* | n | HR* | n | HR* | n | HR* | n | HR* | ||||
| 2002 | 398 | 188 | 47.2 | 161 | 50.0 | 27 | 35.5 | 4 | 36.4 | 111 | 53.1 | 43 | 35.0 | 30 | 54.5 |
| 2003 | 490 | 236 | 48.2 | 202 | 50.0 | 34 | 39.5 | 2 | 33.3 | 135 | 49.1 | 62 | 42.2 | 37 | 59.7 |
| 2004 | 504 | 262 | 52.0 | 217 | 52.7 | 45 | 48.9 | 9 | 40.9 | 151 | 54.7 | 56 | 42.7 | 46 | 61.3 |
| 2005 | 514 | 276 | 53.7 | 235 | 55.7 | 41 | 44.6 | 3 | 33.3 | 161 | 59.2 | 74 | 45.7 | 38 | 53.5 |
| 2006 | 518 | 281 | 54.2 | 241 | 56.8 | 40 | 42.6 | 2 | 16.7 | 156 | 54.7 | 66 | 47.5 | 57 | 69.5 |
| 2007 | 555 | 286 | 51.5 | 244 | 51.8 | 42 | 50.0 | 4 | 36.4 | 169 | 56.3 | 73 | 44.2 | 40 | 50.6 |
| 2008 | 557 | 296 | 53.1 | 270 | 57.0 | 26 | 31.3 | 3 | 27.3 | 183 | 57.7 | 62 | 39.7 | 48 | 65.8 |
| 2009 | 610 | 327 | 53.6 | 279 | 53.8 | 48 | 52.7 | 4 | 36.4 | 215 | 58.0 | 59 | 41.3 | 49 | 57.6 |
| 2010 | 598 | 342 | 57.2 | 306 | 59.1 | 36 | 45.0 | 3 | 21.4 | 196 | 59.4 | 89 | 50.6 | 54 | 69.2 |
| 2011 | 661 | 364 | 55.1 | 307 | 56.1 | 57 | 50.0 | 3 | 25.0 | 208 | 57.1 | 82 | 45.8 | 71 | 67.0 |
| 2012 | 623 | 338 | 54.3 | 285 | 53.6 | 53 | 58.2 | 4 | 36.4 | 184 | 52.9 | 83 | 50.3 | 67 | 67.7 |
| 2013 | 616 | 338 | 54.9 | 291 | 55.5 | 47 | 51.1 | 4 | 30.8 | 175 | 55.4 | 86 | 51.5 | 73 | 60.8 |
*Hospitalisation rate (%)=n/total number of patients with SP×100.
HP, hospitalised patients; HR, hospitalisation rate; SP, spontaneous pneumothorax.
The frequency and proportion of rehospitalisation of patients with SP are shown in online supplementary table 5. In total, 3005 patients used hospital services at least once due to SP between 2002 and 2013. Among these, 1683 were rehospitalised, with 60.7% of all rehospitalisations occurring within 6 months and 71.4% within 1 year. The average number of hospitalisations per person overall was 1.56, at an average interval of 223.07 days.
Comorbidities in patients with SP
Lung-related comorbidities in patients with SP by age are shown in table 3. For the <15 years old group, there were no particular comorbidities. COPD was the most common comorbidity, followed by pneumonia and asthma.
Table 3.
Comorbidities of patients with SP
| 15–34 years | 35–64 years | ≥65 years | Total | |||||
| n | %* | n | %† | n | %‡ | n | %§ | |
| Total | 2407 | 1374 | 497 | 4658 | ||||
| COPD | 273 | 11.3 | 277 | 20.2 | 287 | 57.7 | 837 | 18.0 |
| Pneumonia | 102 | 4.2 | 109 | 7.9 | 150 | 30.2 | 361 | 7.8 |
| Interstitial | 4 | 0.2 | 17 | 1.2 | 13 | 2.6 | 34 | 0.7 |
| Lung cancer | 11 | 0.5 | 72 | 5.2 | 67 | 13.5 | 150 | 3.2 |
| Asthma | 63 | 2.6 | 111 | 8.1 | 174 | 35.0 | 348 | 7.5 |
| Lung abscess | 1 | 0.0 | 8 | 0.6 | 0 | 0.0 | 9 | 0.2 |
| CCI | ||||||||
| Mean | 0.25 | 0.69 | 1.31 | 0.47 | ||||
| 0 | 1823 | 75.7 | 708 | 51.5 | 173 | 34.8 | 2704 | 58.1 |
| 1 | 468 | 19.4 | 420 | 30.6 | 305 | 61.4 | 1193 | 25.6 |
| 2 | 45 | 1.9 | 165 | 12.0 | 166 | 33.4 | 376 | 8.1 |
| 3 or more | 5 | 0.2 | 65 | 4.7 | 99 | 19.9 | 159 | 3.4 |
*%=n/2407×100.
†%=n/1374×100.
‡%=n/496×100.
§%=n/4658×100.
CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; SP, spontaneous pneumothorax.
COPD was more common in the ≥65 years old group than in the younger age groups. Fewer patients among the 15–34 years old group had asthma compared with pneumonia. The prevalence of all comorbidities other than lung abscess was highest in the ≥65 years old group. The lung cancer rate was lower in the 15–34 years old and 35–64 years old groups than in the ≥65 years old group.
The average CCI value of patients with SP was 0.47 and increased with increasing age. The number of patients with a CCI value of 0 decreased with increasing age, while that of patients with a CCI value of ≥3 increased with increasing age.
Treatment period and medical costs of patients with SP
There were 4157 outpatient service users among patients with first-time SP (no use of medical service for SP for 60 days prior) from 2002 to 2013 (table 4). Among these, 3035 required follow-up within 60 days of the first onset of SP. Most follow-ups occurred within 30 days. The average number of follow-ups in each time period including the first-time visit was found to be 1.14 days within 7 days, 1.33 days within 14 days, 1.54 days within 30 days, and 1.73 days within 60 days. The average length of time between follow-ups was 11.07 days, and the average medical cost per person was $94.5 (106 766 Korean won).
Table 4.
Outpatient or inpatient treatment period and medical costs of spontaneous pneumothorax
| FU frequency during the period | Total FU frequency (including first visit) | ||||||
| Cumulative frequency | Total FU frequency (within 60 days) Comparative ratio |
Mean | SD | Minimum | Maximum | ||
| Outpatient FU | |||||||
| First-time outpatient use | 4157 | ||||||
| Within 7 days | 582 | 19.2 | 1.14 | 0.42 | 1 | 6 | |
| Within 14 days | 1372 | 45.2 | 1.33 | 0.63 | 1 | 8 | |
| Within 30 days | 2245 | 74.0 | 1.54 | 0.84 | 1 | 13 | |
| Within 60 days | 3035 | 100.0 | 1.73 | 1.07 | 1 | 13 | |
| Average follow-up period | 11.07 | 16.55 | 0 | 60 | |||
| Average medical costs (US$)* | 94.5 | 141.5 | 0.9 | 2673.6 | |||
| Hospitalisation FU | |||||||
| First-time hospitalisation use | 3005 | ||||||
| Within 7 days | 180 | 30.0 | 1.06 | 0.24 | 1 | 3 | |
| Within 14 days | 301 | 50.1 | 1.10 | 0.31 | 1 | 3 | |
| Within 30 days | 451 | 75.0 | 1.15 | 0.40 | 1 | 5 | |
| Within 60 days | 601 | 100.0 | 1.20 | 0.48 | 1 | 7 | |
| Average number of days of hospitalisation | 14.19 | 18.25 | 0 | 601 | |||
| Average follow-up period | 3.67 | 10.67 | 0 | 60 | |||
| Average medical costs (US$)* | 2523.0 | 2692.2 | 45.9 | 48 967.3 | |||
*The cost of items determined to be eligible for reimbursement by the Health Insurance Review and Assessment Service out of the total treatment amount was indicated in the submitted insurance claims statement. It was converted from Korean won to US dollars, according to the exchange rate on 12 October 2018 (US$1.00=1130 Korean won).
FU, follow-up.
The number of hospitalisations for first cases of SP was 3005, of which 601 required follow-up within 60 days of discharge. Most follow-ups occurred within 30 days, and the proportion of follow-ups occurring in the first week was higher for inpatients than for outpatient service users. The average number of follow-ups was lower for inpatients than for outpatient service users. The average length of hospitalisation was 14.19 days. The average follow-up period after discharge was 3.67 days, and the average medical costs for each hospitalisation were markedly higher than those of outpatients.
Treatment and medication for patients with SP
Treatment and medication details are shown in table 5. The most commonly received non-surgical treatment for patients with SP was oxygen inhalation, followed by suction drainage or tracheostomy suction. Thoracostomy was the most common surgical treatment, followed by lung wedge resection. The frequency of other surgical treatments was low. The rate of surgery for SP varied by year, but was estimated at about 40%. It was highest at 47.5% in 2010 and slightly decreased thereafter. By sex, the rate of surgery was higher in men than in women (online supplementary figure 4).
Table 5.
Treatments and drugs used for patients with spontaneous pneumothorax
| Treatment | Drugs | ||||
| n | %* | n | %† | ||
| Total count of prescription statements | 21 107 | 37 488 | |||
| Non-surgical treatments | Analgesics | ||||
| Oxygen inhalation | 4351 | 20.6 | Paracetamol | 1039 | 2.8 |
| Nebuliser treatment of lower airway | 534 | 2.5 | Codeine, combinations excluding psycholeptics | 878 | 2.3 |
| Suction drainage or tracheostomy suction, etc | 2481 | 11.8 | Tramadol and paracetamol | 832 | 2.2 |
| Tracheal intubation | 81 | 0.4 | Propionic acid derivatives | 784 | 2.1 |
| Surgical treatments | Aceclofenac | 455 | 1.2 | ||
| Thoracostomy | 3132 | 14.8 | Antitussives | ||
| Wedge resection of lung | 1304 | 6.2 | Acetylcysteine | 672 | 1.8 |
| Resection of bullae | 125 | 0.6 | Xanthines | 635 | 1.7 |
| Pleurodesis | 27 | 0.1 | Bromhexine | 789 | 2.1 |
| Apicolysis, pleurolysis | 15 | 0.1 | Ambroxol | 553 | 1.5 |
| Lobectomy of lung | 13 | 0.1 | Antibiotics | ||
| Pleurectomy | 12 | 0.1 | Cefixime | 374 | 1.0 |
| Segmentectomy of lung | 10 | 0.0 | Third-generation cephalosporins | 305 | 0.8 |
| Pleural decortication | 8 | 0.0 | |||
| Primary thoracoplasty | 4 | 0.0 | |||
*%=n/21 107.
†%=n/37 488.
bmjopen-2018-028624supp004.pdf (262.3KB, pdf)
Medication commonly prescribed for SP could be categorised into analgesics, antitussives and antibiotics, when excluding digestants. Among analgesics, paracetamol, codeine combinations excluding psycholeptics, and tramadol as well as paracetamol combinations were prescribed at similar rates. Among antitussives, the most prescribed was bromhexine. Among antibiotics, cefixime was the most frequently prescribed.
Discussion
The annual prevalence of SP ranged from 39 to 66 per 100 000 individuals. The male to female ratio was approximately 4–10:1, and the 15–34 years old group, particularly those aged 15–19 years old, showed the highest prevalence rate. About 47%–57% of patients with SP underwent hospitalisation. COPD was the most common comorbidity. The average treatment period was 11 days as an outpatient and 14 days in-hospital. The average medical costs were $94.50 for outpatients and $2523 for hospital admissions. The most common treatment for SP was oxygen inhalation and thoracostomy, and the operation rate was about 40%. The most commonly prescribed medications were analgesics, antitussives and antibiotics.
In this large study in Korea, the annual prevalence rate of SP was 39–66 per 100 000 individuals. These numbers were slightly higher than those previously reported: 1.2–7.4 per 100 000 individuals in Minnesota5 and 9.8–24 per 100 000 individuals in the UK.6 The reason for these differences may be because SP was defined as cases in which medical service was used, which could include follow-up appointments, rather than strictly number of incidents. In contrast, the prevalence rate of hospitalisation due to SP was 18–36 per 100 000 individuals overall. Hospitalisation rates have been previously reported as 14.3–22.7 per 100 000 individuals overall,7 8 and are similar to those found in our study. The similarity may be because hospitalisation was as a result of new SP incidents only. In addition, the prevalence rate of SP consistently increased from 2002 to 2011. This is consistent with a previous study that showed increased prevalence of SP.25 The cause of this problem is still controversial. Although deteriorating condition of the atmosphere is presumed to be the cause,26–28 increased underlying lung diseases such as COPD or lung cancer could be another cause. In this study, there was no significant change in the prevalence rate of SSP by year, which implies that increased SP was attributed to increased PSP, not increased underlying lung disease. To solve the problem of increasing SP prevalence, further study is needed to determine the exact cause.
The prevalence rate was much higher for men than in previous studies. The male to female ratio for all SP and for hospitalised SP was higher than those reported previously (2.4:1–3.3:1).6 8 9 In terms of age, the prevalence rate was highest for the 15–34 years old group, particularly for those aged 15–19 years old, followed by the ≥65 years old, 35–64 years old and <15 years old groups, respectively. Previous studies showed a peak age for SP at 20, followed by a later peak at 70.8 29 Overall, the incidence of SP was higher in Korea, particularly in men and at a slightly earlier age (15–19 years), than in other countries: for example, 20–25 years in France7 and Germany,8 and 20–24 years or 30–34 years in England.6 The prevalence of SP in men increased until 2012, while there was no clear trend in women. Since there has been no previous large-scale research in Eastern countries, we cannot conclude whether these results are due to ethnicity or environment. In addition, the choice of terms between ‘prevalence’ and ‘incidence’ was one of the challenges of this study. It was determined to use ‘prevalence’ because there were several previous studies that reported ‘prevalence’, and this study was based on occurrence within the period of a year.
SP has a high rate of hospitalisation30; 47%–57% of patients with SP in our study received hospital treatment. There were no marked differences due to sex, although the female hospitalisation rate was slightly lower. The hospitalisation rate was highest in the ≥65 years old group and lowest in the <15 years old group. The necessity of hospitalisation for SP is debated. Hallifax and Rahman10 stated in 2015 that outpatient treatment is sufficient for patients who are young, have no comorbidities and have stable vital signs; however, evidence on outpatient treatment stability is lacking.
SP has a high rate of relapse,24 25 31 with 1683 of 3003 first-time hospitalisation cases requiring rehospitalisation in our study. Although previous studies have shown that the relapse rate of SP is 15%–40%,7 11 12 25 32 these studies were based on small sample sizes (82–273 subjects).7 11 12 25 32 Although there was a large-scale study, only hospitalised patients were analysed.25 The risk factors for SP relapse are controversial; some studies indicate that elderly people and women are at higher risk,7 33 while other studies indicate that age and sex have no significant effect.34 Furthermore, low body mass index,12 35 non-surgical treatment and smoking habits12 34 have been implicated in SP, yet further study is required to delineate the risk factors.
SP can involve lung-related comorbidities.36 37 To distinguish between PSP and SSP, it is important to evaluate comorbidities. This distinction is important as these conditions have different characteristics and prognoses23 38; however, categorising these conditions based on administrative data remains challenging.19 This is because there are no codes to differentiate between PSP and SSP in the ICD categorisations. Nevertheless, our objective was to describe details related to SP; thus, we examined the current comorbidity status, rather than attempting to categorise patients with SP. Among comorbidities, COPD was the most common in all age groups. With increasing age, the proportion of patients with comorbidities increased; thus, with increasing age, SSP was more common than PSP. Therefore, the decrease in risk of SP with age, followed by an increase, may be due to an increase in SSP in particular.
The approximate treatment period for SP was 11 days for outpatients and 17.7 days for hospitalised patients. The average number of follow-ups within 60 days was 1.73. Thus, many cases of SP were minor and a single outpatient visit is necessary to complete the treatment. For both hospitalisation and outpatient service users, 75% of the total follow-ups occurred within 1 month; hence, the follow-up duration for SP is short. Few studies have assessed the treatment or follow-up duration for SP, yet our results are similar to a previous study reporting a remission period of 7–15 days for SP.23 39 X-ray follow-up is recommended within 2–4 weeks of discharge,35 and the median value of length of stay is 6–7 days.7 33 No previous study has reported the socioeconomic costs due to SP; in our study, we found an average medical cost of $94.50 per person for outpatient services and of $2523.00 per person for hospitalisation. The maximum costs were $2673.60 per person for outpatient and $48 967.30 per person for hospitalisation.
For treatment, surgery and medication for patients with SP, we reported the frequency of prescriptions rather than the number of people. Oxygen inhalation among non-surgical treatments and thoracostomy among surgical treatments were the most common procedures. A previous study has also shown that the most common treatment for patients with SP is thoracostomy (92% of patients).32 The surgery most commonly performed after thoracostomy is lung wedge resection; this involves removing a portion of the lung, and is less invasive, preserves more lung function and has fewer side effects than lobar resection.40 41 The rate of surgery showed a mild tendency to increase until 2010 and then decreased slightly thereafter. It was not possible to confirm whether this was due to the severity of SP or the medical practice tendency in Korea. For medication, analgesics, antitussives and antibiotics were the most frequently prescribed, after excluding digestants. For convenience of analysis, we excluded outpatient prescriptions. Considering that the rate of hospitalisation exceeded 60% for patients with SP, prescription patterns are not likely to differ markedly between hospitalised and overall cases.
This study has several limitations. First, we could not distinguish between PSP and SSP due to data limitations. Accordingly, it was also impossible to present each treatment method. According to a study analysing the first-line treatment and management of PSP and SSP, there are differences with PSP and SSP in terms of management methods.15 Further research is needed on the epidemiology and medical service use of each SP category. Second, our results should be interpreted with caution as we could not ascertain whether medical service use was due to new incidents of SP or to follow-up. That is, rehospitalisations with the same episodes and recurrence 60 days before the last treatment were not detected. Although we attempted to select medical service use due to new incidents of SP by using an operational definition, this may not have been accurate. Third, our study may be limited by the lack of data on uncovered items and from patients who were not covered by health insurance. However, in Korea, because 98% of the total population is covered by health insurance, it seems to be the entire population data. Existence of uncovered items or indirect medical costs was a limitation in this study. In addition, as the diagnosis codes in the claims data that were the basis for identifying patients may not be completely accurate,42 it is not clear whether all patients with SP were listed under the appropriate corresponding diagnosis codes. Lastly, since analysis was performed using NHI data from 2002, there is a possibility of misclassification of new-onset SP due to patients with SP who ever suffered from SP before 2012.
However, despite these limitations, this study provided a detailed record of the epidemiology and treatment of SP. Our study is significant in that it provides novel information, contributing to an understanding of SP, a poorly understood condition with an increasing prevalence in Korea.
Conclusion
This study investigated the epidemiology and medical treatment of SP in Korea, which have not been studied before. The study showed the incidence of SP in Korea is increasing, and the incidence in men is higher than in women. It also showed a high rate of SP recurrence within a year and how treatment of pneumothorax in Korea is being done. However, this study did not identify the specific cause of the increase in SP. Therefore, future research is needed to identify the cause of increase of SP in Korea and to find ways to prevent this increase.
Supplementary Material
Acknowledgments
The authors thank Kyungwon Kang, who conducted data cleaning and initial analyses.
Footnotes
Contributors: I-HH, YJL and BJ contributed to the overall conception and design of the study protocol. BJ and DK contributed to the specific study design and data analysis. DK wrote the first draft of the manuscript. DK, BJ, SHC, B-HJ, YJL and I-HH contributed to interpretation of the analyses and revisions of the final manuscript. All authors gave final approval of the version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Institutional Review Board of Jaseng Hospital of Korean Medicine in Seoul, Korea (JASENG 2018-09-007).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: This study incorporated data from the National Health Insurance Service National Sample Cohort2002-2013, which were released by the Korean National Health Insurance Service in response to the researchers' request. Detailed cohort profile and the methods for obtaining data are explained in the following source: Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. International journal of epidemiology. 2016. doi: 10.1093/ije/dyv319. PubMed PMID:26822938.
References
- 1. Tortora G, Anagnostakos N. Principles of anatomy and physiology. New York: Harper & Row, 1987. [Google Scholar]
- 2. Luh S-ping, Luh SP. Review: diagnosis and treatment of primary spontaneous pneumothorax. J Zhejiang Univ Sci B 2010;11:735–44. 10.1631/jzus.B1000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imran JB, Eastman AL. Pneumothorax. JAMA 2017;318 10.1001/jama.2017.10476 [DOI] [PubMed] [Google Scholar]
- 4. Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868–74. 10.1056/NEJM200003233421207 [DOI] [PubMed] [Google Scholar]
- 5. Melton LJ, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379–82. 10.1164/arrd.1979.120.6.1379 [DOI] [PubMed] [Google Scholar]
- 6. Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666–71. 10.1136/thorax.55.8.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bobbio A, Dechartres A, Bouam S, et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax 2015;70:653–8. 10.1136/thoraxjnl-2014-206577 [DOI] [PubMed] [Google Scholar]
- 8. Schnell J, Koryllos A, Lopez-Pastorini A, et al. Spontaneous pneumothorax: epidemiology and treatment in Germany between 2011 and 2015. Dtsch Arztebl Int 2017;114 10.3238/arztebl.2017.0739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987;92:1009–12. 10.1378/chest.92.6.1009 [DOI] [PubMed] [Google Scholar]
- 10. Hallifax RJ, Rahman NM. Epidemiology of pneumothorax--finally something solid out of thin air. Thorax 2015;70:921–2. 10.1136/thoraxjnl-2015-207438 [DOI] [PubMed] [Google Scholar]
- 11. Saito Y, Suzuki Y, Demura R, et al. The outcome and risk factors for recurrence and extended hospitalization of secondary spontaneous pneumothorax. Surg Today 2018;48:320–4. 10.1007/s00595-017-1585-8 [DOI] [PubMed] [Google Scholar]
- 12. Tan J, Yang Y, Zhong J, et al. Association between BMI and recurrence of primary spontaneous pneumothorax. World J Surg 2017;41:1274–80. 10.1007/s00268-016-3848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim D, Shin H-J, Kim S-W, et al. Psychological problems of pneumothorax according to resilience, stress, and post-traumatic stress. Psychiatry Investig 2017;14:795–800. 10.4306/pi.2017.14.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sousa I, Abrantes P, Francisco V, et al. Multicentric genome-wide association study for primary spontaneous pneumothorax. PLoS One 2016;11:e0156103 10.1371/journal.pone.0156103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kepka S, Dalphin JC, Pretalli JB, et al. How spontaneous pneumothorax is managed in emergency departments: a French multicentre descriptive study. BMC Emerg Med 2019;19:4 10.1186/s12873-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan 2009;24:63–71. 10.1093/heapol/czn037 [DOI] [PubMed] [Google Scholar]
- 17. National Health Insurance Service , 2011. Available: http://www.nhis.or.kr/retrieveHomeMain.xx
- 18. Lee J, Lee JS, Park S-H, et al. Cohort profile: the National health insurance Service-National sample cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46:e15 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 19. Frechette E, Guidolin K, Seyam A, et al. Identifying primary spontaneous pneumothorax from administrative databases: a validation study. Can Res J 2016;2016:1–6. 10.1155/2016/1690482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–94. 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 21. Bannay A, Chaignot C, Blotière P-O, et al. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care 2016;54:188–94. 10.1097/MLR.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 22. Charlson M, Wells MT, Ullman R, et al. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS One 2014;9:e112479 10.1371/journal.pone.0112479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British thoracic Society pleural disease guideline 2010. Thorax 2010;65 Suppl 2:ii18–31. 10.1136/thx.2010.136986 [DOI] [PubMed] [Google Scholar]
- 24. Skrbo A, Begović B, Skrbo S. [Classification of drugs using the ATC system (Anatomic, Therapeutic, Chemical Classification) and the latest changes]. Med Arh 2004;58:138–41. [PubMed] [Google Scholar]
- 25. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of Inpatient-Treated spontaneous pneumothorax, 1968-2016. JAMA 2018;320:1471–80. 10.1001/jama.2018.14299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marx T, Bernard N, Parmentier A-L, et al. Does air pollution really impact the onset of spontaneous pneumothorax? A French case-crossover study. Environ Int 2019;127:317–23. 10.1016/j.envint.2019.01.056 [DOI] [PubMed] [Google Scholar]
- 27. Park JH, Lee SH, Yun SJ, et al. Air pollutants and atmospheric pressure increased risk of ED visit for spontaneous pneumothorax. Am J Emerg Med 2018;36:2249–53. 10.1016/j.ajem.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 28. Heyndrickx M, Le Rochais J-P, Icard P, et al. Do atmospheric conditions influence the first episode of primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2015;21:296–300. 10.1093/icvts/ivv145 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura H, Konishiike J, Sugamura A, et al. Epidemiology of spontaneous pneumothorax in women. Chest 1986;89:378–82. 10.1378/chest.89.3.378 [DOI] [PubMed] [Google Scholar]
- 30. Jheon SH, Lee EB, Choe JY, et al. Critical pathway for management of primary spontaneous pneumothorax. Korean J Thoracic and Cardiovascular Surg 2002;35:43–7. [Google Scholar]
- 31. Sadikot RT, Greene T, Meadows K, et al. Recurrence of primary spontaneous pneumothorax. Thorax 1997;52:805–9. 10.1136/thx.52.9.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aghajanzadeh M, Asgary MR, Delshad MSE, et al. Data on the epidemiology, diagnosis, and treatment of patients with pneumothorax. Data in Brief 2018;20:1053–6. 10.1016/j.dib.2018.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sjöström C, Stålenheim G, Janson C. [Spontaneous pneumothorax--a study of medical records. High age is the most significant risk factor of prolonged length of stay]. Lakartidningen 2002;99:1070–4. [PubMed] [Google Scholar]
- 34. Kepka S, Dalphin JC, Parmentier AL, et al. Primary spontaneous pneumothorax admitted in emergency unit: does first episode differ from recurrence? A cross-sectional study. Canadian Respiratory Journal 2017;2017:1–6. 10.1155/2017/2729548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang T-W, Lee S-C, Cheng Y-L, et al. Contralateral recurrence of primary spontaneous pneumothorax. Chest 2007;132:1146–50. 10.1378/chest.06-2772 [DOI] [PubMed] [Google Scholar]
- 36. Kwas H, Zendah I, Ghedira H. Spontaneous pneumothorax secondary to tuberculosis. Tunis Med 2017;95:276–9. [PubMed] [Google Scholar]
- 37. Iga N, Nishi H, Fujimoto N, et al. Clinical features of secondary spontaneous pneumothorax complicated with silicosis. Respir Investig 2018;56:144–9. 10.1016/j.resinv.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 38. Onuki T, Ueda S, Yamaoka M, et al. Primary and secondary spontaneous pneumothorax: prevalence, clinical features, and in-hospital mortality. Can Respir J 2017;2017:1–8. 10.1155/2017/6014967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chee CB, Abisheganaden J, Yeo JK, et al. Persistent air-leak in spontaneous pneumothorax--clinical course and outcome. Respir Med 1998;92:757–61. 10.1016/S0954-6111(98)90008-7 [DOI] [PubMed] [Google Scholar]
- 40. Cao J, Yuan P, Wang Y, et al. Survival rates after lobectomy, Segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg 2018;105:1483–91. 10.1016/j.athoracsur.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 41. Sihoe ADL, Van Schil P. Non-Small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115–20. 10.1016/j.lungcan.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 42. Kim JA, Yoon S, Kim LY, et al. Towards Actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017;32:718–28. 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028624supp005.pdf (118.3KB, pdf)
bmjopen-2018-028624supp001.pdf (52.9KB, pdf)
bmjopen-2018-028624supp002.pdf (1.1MB, pdf)
bmjopen-2018-028624supp003.pdf (269.7KB, pdf)
bmjopen-2018-028624supp004.pdf (262.3KB, pdf)


