Significance Statement
Because the presence of pretransplant donor-specific anti-HLA antibodies is associated with increased organ rejection risk, patients on transplant waiting lists are regularly monitored for changes in their alloimmune status. In this retrospective analysis, the authors investigated the dynamics of anti-HLA antibodies over time in patients on a kidney transplant waiting list. Their findings show that the kinetics of alloimmunity are highly individualized and do not appear to correlate with the interval between measurements. However, the magnitude of alloimmune status change increased significantly in patients with a previous transplant versus those without such a history. This suggests that an individualized strategy for alloimmune status monitoring of patients on organ transplant waiting lists on the basis of their alloimmunization history might be preferable to current recommendations for regular monitoring.
Keywords: anti-HLA antibodies, kidney transplantation, single antigen beads, donor specific antibodies, mean fluorescence intensity
Visual Abstract
Abstract
Background
Patients on organ transplant waiting lists are evaluated for preexisting alloimmunity to minimize episodes of acute and chronic rejection by regularly monitoring for changes in alloimmune status. There are few studies on how alloimmunity changes over time in patients on kidney allograft waiting lists, and an apparent lack of research-based evidence supporting currently used monitoring intervals.
Methods
To investigate the dynamics of alloimmune responses directed at HLA antigens, we retrospectively evaluated data on anti-HLA antibodies measured by the single-antigen bead assay from 627 waitlisted patients who subsequently received a kidney transplant at University Hospital Zurich, Switzerland, between 2008 and 2017. Our analysis focused on a filtered dataset comprising 467 patients who had at least two assay measurements.
Results
Within the filtered dataset, we analyzed potential changes in mean fluorescence intensity values (reflecting bound anti-HLA antibodies) between consecutive measurements for individual patients in relation to the time interval between measurements. Using multiple approaches, we found no correlation between these two factors. However, when we stratified the dataset on the basis of documented previous immunizing events (transplant, pregnancy, or transfusion), we found significant differences in the magnitude of change in alloimmune status, especially among patients with a previous transplant versus patients without such a history. Further efforts to cluster patients according to statistical properties related to alloimmune status kinetics were unsuccessful, indicating considerable complexity in individual variability.
Conclusions
Alloimmune kinetics in patients on a kidney transplant waiting list do not appear to be related to the interval between measurements, but are instead associated with alloimmunization history. This suggests that an individualized strategy for alloimmune status monitoring may be preferable to currently used intervals.
To minimize episodes of acute and chronic rejection of a transplanted organ, patients on the organ transplant waiting list are regularly monitored for signs of preexisting alloimmunity. The information on alloimmunity is then incorporated into the organ allocation algorithm used, so that organs will not be offered to potential recipients with preexisting alloimmunity to the specific donor organ. Optimally, this will lead to reduced incidences of acute and chronic graft rejection because evidence of preexisting donor specific alloimmunity has been associated with substantially increased incidence of both these rejection types in previously published studies.1,2 As the majority of alloimmune responses in human organ transplantation are directed against the polymorphic HLA proteins, the immunologic monitoring is focused on assessing preexisting immunity toward nonself HLAs. Assays to evaluate preexisting T cell alloimmunity have been difficult to develop, and thus the immunologic monitoring for alloimmunity has focused on evaluating antibodies directed against nonself HLA proteins.3 This is assessed, with high sensitivity, by use of single-antigen bead (SAB) technology, where different HLA protein variants are immobilized on fluorescent beads, so that one individual bead will only hold a single HLA antigen.4 Antibody reactivity to a specific SAB is assessed by evaluating the mean fluorescence intensity (MFI) of the bound anti-HLA antibodies. HLA typing of organ donors before transplantation, in combination with pretransplant SAB analysis in the recipient, facilitates the assessment of donor-specific antibodies (DSA), so that transplant pairs with presence of DSA can be avoided. Different clinical pretransplant SAB MFI cut-offs are used at different kidney transplant centers, and studies suggest that an optimal cut-off for identifying patients with increased risk of rejection could be somewhere between 1000 and 2000 MFI.5
Alloimmunity is a dynamic process and, as such, the alloimmune status of an individual patient may change over time. Recognized alloimmunization events include blood transfusions, pregnancies, and organ transplantations, but other immunologic events, such as vaccinations and changes in ongoing immunosuppressive therapies, may also have an influence on a patient’s alloimmune status.6–9 As the current alloimmune status of the transplant recipient is central to the pretransplant individualized immunologic risk stratification, patients on the organ transplant waiting list are usually monitored for changes in their alloimmune status at regular intervals, and after new alloimmunization events, using anti-HLA antibody detection assays. Before the introduction of recombinant erythropoietin, many patients with ESRD would regularly receive blood transfusions and this likely influenced the praxis of alloimmune status monitoring, with many centers testing serum from patients on the organ transplant waiting list every 3–4 months. A recommendation for alloimmune status monitoring every 3 months is also included in the current guidelines from the European Federation of Immunogenetics, whereas the American Society for Histocompatibility leaves it up to the regulations within different transplant networks to decide upon the appropriate interval of alloimmune status monitoring. There are few studies on how alloimmune responses change over time in patients on the transplant waiting list, and to our knowledge, there are no published studies supporting the 3-month recommendation. The paucity of knowledge within this field was also highlighted in a recent publication from the Sensitizing in Transplantation: Assessment of Risk (STAR) working group.10
In this study, we aim to investigate the dynamics of alloimmune responses, as assessed with the SAB assay, by retrospectively examining values collected from patients undergoing kidney transplantation at the University Hospital Zurich.
Methods
Patient Population
All patients transplanted with a kidney between 2008 and 2017 at the University Hospital Zurich for whom a SAB analysis had been performed were included in the study. Ethical approval of the study was given by the regional ethical review board. Clinical data were retrospectively collected and data on immunization events were available through the mandatory reporting system for patients listed for organ transplantation within the Swiss Organ Allocation System. The data on previous immunization included immunizing events occurring both before patients being listed and during the waiting time.
Laboratory Analysis
Presence of circulating anti–HLA-A, -B, -Cw, -DR, -DQ, and -DP antibodies was analyzed in native serum using SAB assays (One Lambda, Inc., Canoga Park, CA) on a Luminex platform. Only serum samples collected before transplantation, while the patients were actively listed on the kidney transplant waiting list, were analyzed. Upon being listed for kidney transplantation, the patients were initially screened using the Luminex Mix Assay (One Lambda, Inc.) or investigated with the SAB assay, depending on previous immunization history and time period. For SAB- and Mix-negative patients, subsequent monitoring was made using the Luminex Mix Assay. For SAB-positive patients, subsequent monitoring was performed with SAB. For patients that had a positive Luminex Mix Assay on subsequent screenings, a new SAB analysis was performed and if it was positive, all subsequent analyses were made with SAB. Depending on the patients’ accrued waiting time, the immunization status of the patient, and the presence of immunizing events, samples were analyzed with SAB at different time intervals in an individualized manner. Data on the occurrence of new immunizing events was obtained continuously from the mandatory reporting system for patients listed for organ transplantation within the Swiss Organ Allocation System. The analyses were also made in relation to the local transplant regulation in Switzerland that stipulates a 4-month interval for sampling of patients that are active on the kidney transplant waiting list. Serums were analyzed without the addition of EDTA during the whole study; if a prozone effect was suspected (highly immunized patient), the serum was heat inactivated and analyzed again. If a prozone effect was detected, all subsequent serums from the same patient were heat inactivated. HLA antibodies toward class 1 and class 2 HLA antigens were sometimes separately analyzed at different time points, depending on the immunization status of the patient. For each SAB analysis, the maximum MFI for an HLA antigen was defined as the highest-ranked bead carrying the designated HLA antigen. During the study period, the protocol for SAB measurements in our laboratory remained the same, and two different lot numbers from the SAB kits were used (One Lambda, Inc.). We were unable to find any statistically significant change between serums analyzed with the different lots. Our SAB analysis is subject to annual external and internal proficiency testing; for the external testing, single-bead MFIs from ten serums are compared between all six Swiss transplant laboratories. Assay agreement between laboratories on MFI cut-offs (1000 MFI) is typically between 90% and 99% and the coefficient of variation is typically around 15%. For internal evaluation, MFI values from single beads are compared as the same serum is analyzed by different laboratory staff at different time points, with intralaboratory coefficients of variation typically being around 7%.
Calculation of MFI Change
Changes in MFI over time were computed as the total absolute delta of the MFI values between two consecutive measurements, where all changes in MFI towards individual HLA antigens were compounded. All negative HLA antigens were given an arbitrary value of 499 MFI to allow for ΔMFI calculations. To capture all dynamical changes in MFI values contained within the dataset, only measurements of MFI change between consecutive measurements in individual patients were included. Mean absolute MFI change was calculated by dividing the total absolute MFI change on the number of positive HLA antigens detected within the dataset (80 for class 1 and 43 for class 2). For analysis of MFI changes that crossed common clinical MFI cut-offs, each anti-HLA antigen MFI change over a chosen cut-off (both in the positive and negative direction) between two consecutive measurements was awarded a point. The points were then added from consecutive measurements within the same time percentile and differences in points were analyzed between the different percentiles. Stratified analyses were performed by grouping MFI changes crossing clinical cut-offs by the clinical covariates of the patients at hand. For analyses on the effect of transfusions on MFI change, the mean absolute MFI change occurring between an SAB measurement performed before transfusion and an SAB measurement performed up to 200 days after the transfusion was calculated for class 1 and class 2 separately.
Data Exploration, Statistical Analyses, and Clustering Analysis
The data preprocessing and analysis was conducted using python and the following libraries: pandas, numpy, and scikit-learn. The figures were generated using the matplotlib and seaborn libraries. The Welch t test was used to determine significance for all tests to account both for differences in sample sizes and in variance of the distributions at hand. This choice was motivated by the large sample sizes of the group at hand. For the differences between time percentiles, a Bonferroni correction was applied to account for multiple hypotheses testing.
The scikit-learn library was used for the implementation of clustering (Kmeans, DBSCAN [R1], and hierarchical clustering) and dimensionality reduction algorithms (multidimensional scaling, t-SNE [R3], principal component analysis).
Code Availability
The code used to generate the figures is available at https://github.com/BorgwardtLab/alloimmunity-kinetics.
Results
Dataset Structure
Anti-HLA antibody specificities and corresponding MFI values were consecutively collected for all patients who had undergone at least one SAB analysis and were transplanted with a kidney during the follow-up period, at the University Hospital Zurich, Switzerland between 2008 and 2017. In total 627 patients fulfilled these criteria and were included into the study. Additionally, because we focused on the temporal evolution of the MFI values over time, we excluded all patients where only a single SAB measurement had been performed before transplantation. The filtered dataset, comprising patients who had at least two SAB analyses, contains 1881 time points; SAB measurements were performed on a total of 467 patients, which equals an average of 4.03 measurement dates per patient. Additional clinical characteristics of included patients are presented in Table 1. Analyses on both class 1 and class 2 anti-HLA antibodies were conducted, but as these measurements were sometimes individually performed, there are slightly more class 1 analyses in the dataset according to the results of the anti-HLA antibody screening (Table 1). As repeated SAB-based monitoring was almost exclusively performed for patients with detectable anti-HLA antibodies (>1000 MFI), the filtered dataset consists of a group of patients where 92.3% had a peak anti-HLA antibody >1000 MFI (Table 1). This means that a large group of patients without evidence of preexisting alloimmunity (SAB negative) are not represented in the filtered dataset. In total, positive MFI values were found for SABs with 80 different HLA class 1 antigens and 43 different HLA class 2 antigens within the dataset.
Table 1.
Clinical characteristics and anti-HLA antibody status
| Characteristic | Patients in the Filtered Dataset | Age at Transplant | Mean Waiting Time | Previously Transplanted | Previous Pregnancy | Previous Transfusion | No Previous Immunizing Events |
|---|---|---|---|---|---|---|---|
| Men | 265 (56.7%) | 49.2 yr | 2.3 yr | 70 | — | 60 | 135 |
| Women | 202 (43.3%) | 50.0 yr | 2.3 yr | 45 | 98 | 21 | 38 |
| Total | 467 | 49.6 yr | 2.3 yr | 115 (24.6%) | 98 (21.0%) | 81 (17.3%) | 173 (37.0%) |
| HLA Class 1 | HLA Class 2 | Total | |||||
|---|---|---|---|---|---|---|---|
| A | B | Cw | DR | DQ | DP | ||
| Patients with SAB analysis | 455 | 440 | 467 | ||||
| Total dates | 1720 | 1651 | 3371 | ||||
| Total analyzed beads | 166,840 | 156,845 | 323,685 | ||||
| Proportion of patients with peak MFI>1000 | 48.6% | 56.1% | 30.4% | 45.8% | 40.9% | 33.4% | 92.3% |
| Proportion of patients with peak MFI>5000 | 19.3% | 23.3% | 7.7% | 12.2% | 15.2% | 4.9% | 38.1% |
| Proportion of patients with peak MFI>10,000 | 10.1% | 12.0% | 3.6% | 7.3% | 10.1% | 1.1% | 22.3% |
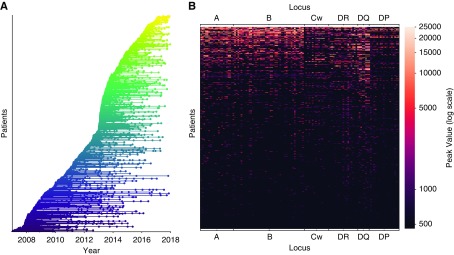
The interval between measurements (IBM) is a critically important component in understanding how anti-HLA antibodies evolve over time in individual patients, and is therefore a central part of this study. The dataset is very diverse in this regard because SAB analyses were performed at disparate time intervals, mostly depending on estimated additional waiting time for transplantation, but previous detection of anti-HLA antibodies and the occurrence of new immunizing events during the waiting time also influenced monitoring intervals. Figure 1A shows the distribution of the individual SAB measurements over time for all patients in the filtered dataset. We also analyzed peak MFI values for all detected anti-HLA antibodies from every single patient. We then plotted the peak MFI value signatures, according to locus, for every included patient, ordering them by their total peak MFI value (Figure 1B). Visual inspection allows one to distinguish clusters of patients with a stronger signal for each of the different loci. There are two major clusters of patients, one with a broader alloimmunization status showing positive MFI values across many of the assayed HLA antigens, and one cluster of patients with negative peak MFI values across most loci. Additionally, antibodies toward HLA-A, -B, -DR, and -DQ antigens are the most common within our dataset, as can also be seen in Table 1.
Figure 1.
Characteristics of the investigated dataset. (A) Sampling timeline of the 467 patients in the filtered dataset, every line represents an individual patient and every dot represents a SAB measurement. The lines are colored according to the date of the first SAB measurement. (B) The peak MFI values for anti-HLA antibodies directed at the different HLA antigens, for every patient, are displayed on a single line and colored according to MFI value. The measurements are grouped by locus and patients are sorted by the sum of their total peak MFI value.
Time Dependency
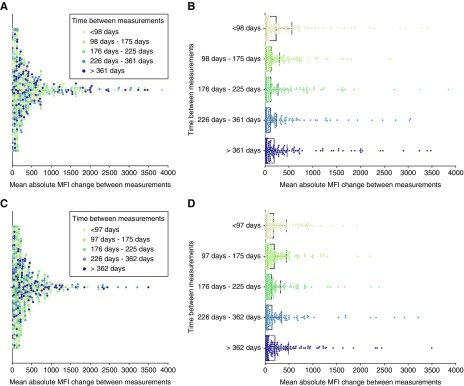
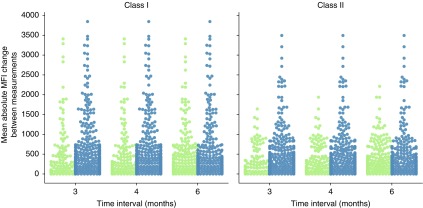
To investigate whether changes in alloimmunization status are influenced by the time between measurements, we investigated the relation between IBM and changes in anti-HLA antibody MFI in consecutive measurements, in individual patients. The IBM in days was compared with the mean of absolute ΔMFI values between measurements. The IBM categories were chosen so that they contain the same number of measurements (i.e., percentiles). In total, 1265 individual patient IBM were analyzed for class 1 measurements and 1210 IBM for class 2. No clear pattern of time dependency emerged in the visual presentation of the data for either class 1 or class 2 (Figure 2, A and C). From looking at the data, it is clear that changes in total mean MFI occur within all IBM categories in a subgroup of patients, whereas the majority of measurements show very little mean absolute MFI change regardless of the IBM (Figure 2, B and D). For statistical validation, we ran two-sample Welch tests on the five different percentiles to test the hypothesis that the means of the total absolute MFI change are not different from each other in the five temporal categories. No pair of time differences showed a significant total MFI change after a Bonferroni multiple hypothesis correction (α=0.005; P≥0.010 for class 1 and P≥0.06 for class 2; see Supplemental Tables 1 and 2 for detailed results). To test whether the nonsignificant total MFI change was a product of our division of the data into five percentiles, we tested if there were significant differences in total MFI change between measurements obtained before and after commonly used monitoring intervals. In line with our previous findings, we could not find any statistically significant differences in total MFI change when we used 3, 4, and 6 months as time intervals (Figure 3, Supplemental Table 3). To investigate if the lack of significant total MFI change between our investigated time points was influenced by individualized alloimmunity monitoring of immunized patients, we filtered our dataset by excluding all measurements from patients who encountered at least one documented immunizing event or who had measurements performed <30 days apart. This resulted in a reduced dataset containing 82 patients with multiple class 1 measurements and 75 patients with multiple class 2 measurements. Mean absolute MFI change between measurements were naturally much smaller in terms of absolute values in these patients without a documented immunization event (Supplemental Figure 1). Interestingly, here as well, no pair of time differences showed a significant total MFI change after multiple hypothesis correction (α=0.005; P≥0.08 for class 1 and P≥0.45 for class 2; see Supplemental Tables 4 and 5). In conclusion, across all patients and in a subgroup without a documented immunization event, we were unable to find any significant correlation between IBM and mean changes in anti-HLA antibody MFIs.
Figure 2.
MFI variability is, on average, not affected by the IBM. (A and C) Swarm plots depicting the mean of the absolute MFI change, between consecutive SAB measurements, for five different IBM categories (in days), for (A) class 1 and (C) class 2 anti-HLA antibodies. The IBM category is denoted by differently colored dots. (B and D) Mean and SD of the mean absolute MFI change for the five IBM categories. Each group contains the same number of measurements: (B) n=253 for class 1 and (D) n=242 for class 2. After correcting for multiple hypothesis testing, no pair of time differences showed a statistically significant mean absolute MFI change.
Figure 3.
MFI variability is not significantly different across three relevant clinical time intervals for class 1 and class 2 SAB measurements. The mean absolute MFI change between consecutive SAB measurements, in individual patients, performed at an interval shorter (in green) and longer (in blue) than the denoted time interval in months was compared. There were no statistically significant differences between measurements obtained with a shorter or longer time interval, for any of the investigated time intervals, either for class 1 (left) or class 2 (right) (P>0.06; see Supplemental Table 3 for details).
Time Dependency of Changes Over Clinical Cut-Offs
Decisions to accept or reject kidney offers for patients on the transplant waiting list are influenced by the presence of DSA in the recipient. This makes changes in MFI that cross commonly used clinical MFI cut-offs (in the positive or negative direction) important. To investigate whether the number of crossings of clinical cut-offs was associated with IBM, we investigated crossings over the clinical cut-offs 1000 and 5000 MFI. As can be seen in Figure 4, the pattern of crossings over the 1000 MFI cut-off is comparable with the previous analysis of total MFI change, with the number of crossings being similarly distributed over the different percentiles. In addition, no pair of time difference showed a statistically significant change in crossings after multiple hypothesis correction (α=0.005; P≥0.007 for class 1 and P≥0.009 for class 2; Supplemental Tables 6 and 7). An analog analysis of crossings over the 5000 MFI cut-off did not show any significant differences between the percentiles (P≥0.04; Figure 5, Supplemental Tables 8 and 9). Thus, changes in MFI over commonly used clinical MFI cut-offs are not associated with the IBM in our dataset.
Figure 4.
The IBM does not significantly affect the number of SAB specificities that cross the clinical cut-off of MFI 1000 between consecutive measurements. (A and C) Swarm plots of the number of SAB specificities, in individual patients, with MFI values that cross the 1000 MFI threshold between consecutive measurements for five different IBM categories (in days), for (A) class 1 and (C) class 2 HLA antigens. The IBM category is denoted by differently colored dots. (B and D) Individual swarm plots of the number of 1000 MFI threshold crossings for the five IBM categories. Each group contains the same number of measurements: (B) n=253 for class 1 and (D) n=242 for class 2. After correcting for multiple hypothesis testing, no pair of time differences showed a significantly different mean number of threshold crossings.
Figure 5.
The IBM does not significantly affect the number of SAB specificities that cross the clinical cut-off of MFI 5000 between consecutive measurements. (A and C) Swarm plots of the number of SAB specificities, in individual patients, with MFI values that cross the 5000 MFI threshold between consecutive measurements for five different IBM categories (in days), for (A) class 1 and (C) class 2 HLA antigens. The IBM category is denoted by differently colored dots. (B and D) Individual swarm plots of the number of 5000 MFI threshold crossings for the five IBM categories. Each group contains the same number of measurements: (B) n=253 for class 1 and (D) n=242 for class 2. After correcting for multiple hypothesis testing, no pair of time differences showed a significantly different mean number of threshold crossings.
Stratified Analyses
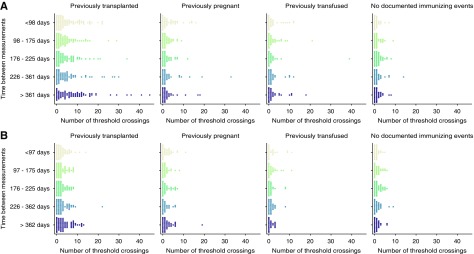
To investigate if we could find groups of patients with similar anti-HLA antibody kinetics we looked more closely at subgroups of patients according to clinical characteristics. We used our previous approach of quantifying the alloimmune status change over time, by analyzing the number of MFI changes over the 1000 MFI cut-off between measurements. Stratifying the dataset by sex or age did not show a difference in the pattern of alloimmune status change (Supplemental Figure 2). However, when the measurements were stratified on documented immunizing events, we found large differences in alloimmune status changes between these subgroups (Figure 6). As expected, patients with previous transplantations showed the largest extent of alloimmune status change, but the change interestingly appeared similar over the different investigated IBM (Figure 6). This was evident both in comparison with other documented immunizing events, such as previous pregnancies or blood transfusions, and with patients without previous documented immunization events. The amount of alloimmune status change was also significantly increased in the two groups of patients with previous pregnancies or transfusion, as compared with the group without a documented alloimmunization event (P<0.002; Figure 6). The quantity of alloimmune status change within the group of patients without a documented alloimmunization event was moderate for changes over the 1000 MFI cut-off, where a mean of 0.9 crossings per measurement were seen, compared with a mean of 5.0 crossings in the previously transplanted patients. Furthermore, 41.8% of the patients in the group without a documented immunizing event had at least one measured crossing over the 1000 MFI for class 1 (38.8% class 2) during their waiting time, as compared with 74.1% in the group of previously transplanted patients (63.4% class 2) (Supplemental Tables 10 and 11). As large, positive MFI movements over the different clinical cut-offs likely has more effect on organ allocation strategies than negative movements, we also investigated this in our subgroups. Previously transplanted patients show the largest percentage of patients with anti-HLA antibodies that increase from <1000 to >5000 MFI between measurements during the study period (9.5% for class 1 and 6.7% for class 2), as compared with the other groups (previously pregnant 1.9% and 1.1%, previously transfused 1.5% and 1.6%, and without a documented immunization event 0.3% and 0.3%, for class 1 and 2, respectively) (Supplemental Figure 3, Supplemental Table 12). To investigate how the alloimmune status changes after immunizing events in individual patients, we investigated the total MFI change associated with transfusions occurring during the waiting time. We identified 37 transfusion events in 24 patients within our dataset where MFI measurements had been performed before and after (1–200 days) the transfusion event. Total mean MFI change after transfusions for class 1 and class 2 antibodies for this subgroup is displayed in Supplemental Figure 4. The effect of transfusions was greater for antibodies against class 1 than for class 2, with 16% of the evaluated patients showing a mean total MFI increase >500 MFI after transfusion for class 1 as compared with 6% for class 2. In summary, our data show that the amount of alloimmunization status change over time is strongly related to alloimmunization history. This suggests that alloimmunity-monitoring schemes could be further individualized, and that immunization history is a relevant factor to include.
Figure 6.
The alloimmunization history affects pretransplant MFI variability. Individual swarm plots of the number of MFI crossings over the 1000 MFI threshold for five IBM groups and across four immunization categories: patients who had a previous transplant (n=115 overall), patients who did not have a previous transplant but who had been pregnant (n=98 overall), patients who did not have a previous transplant nor were pregnant but received blood transfusions (n=81 overall), and patients without a documented immunizing event (n=173 overall). The number of threshold crossing for (A) class 1 and (B) class 2 anti-HLA antibodies are reported. Patients without a documented immunizing event show significantly less threshold crossings as compared with the rest of the patients (P<3×10−11).
Pattern of Individual Anti-HLA Antibody Kinetics
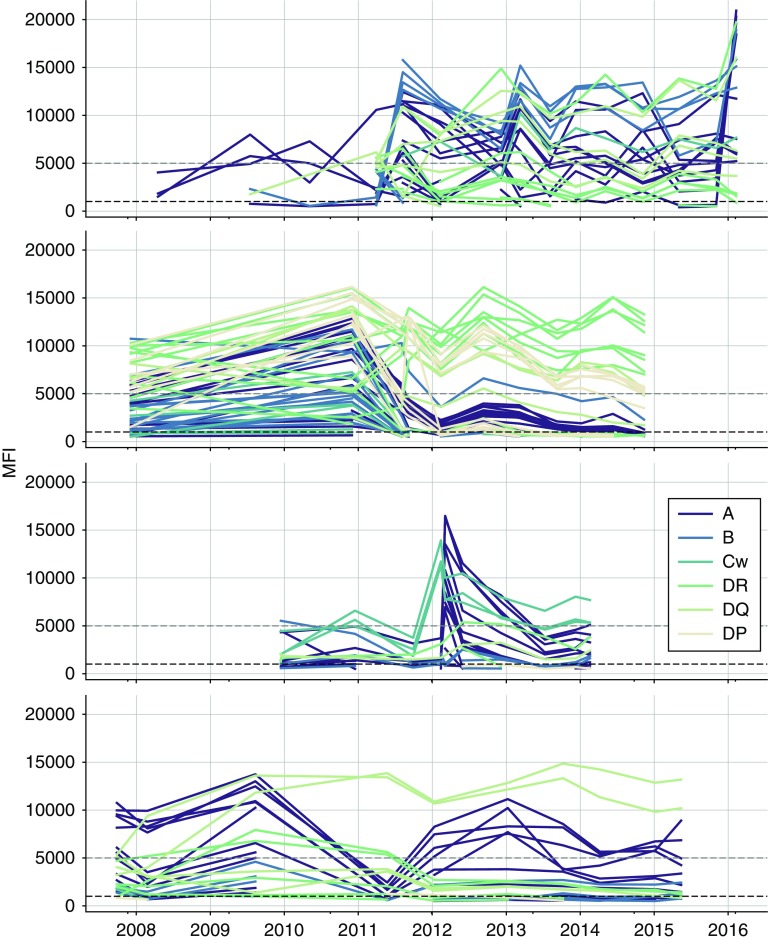
Although our stratified analysis using previous alloimmunization events was able to somewhat predict the likelihood of alloimmune status change over time, there was a lot of heterogeneity within the individual alloimmunized groups. Previously transplanted patients for instance showed the full spectrum of anti-HLA antibody status variability within all of our investigated temporal groups (Figure 6). This suggests that the kinetics of anti-HLA antibody change is individual and does not follow a typical pattern over time. We therefore studied anti-HLA antibody kinetics plots of individual patients with multiple measurements. As can be seen by the examples in Figure 7, the pattern of change over time is indeed highly individual. Not only are the individual patterns of alloimmune status change different ,but it also appears that the trajectories of antibodies directed at different HLA antigens vary considerably in individual patients (Figure 7). This suggests that other methods are needed to efficiently cluster patients on the basis of the kinetics of alloimmune status change.
Figure 7.
Individual variability in anti-HLA antibody kinetics likely explains the absence of clear global trends. Selected timelines for four individual patients with >10 SAB measurements; only values above the detection cut-off (MFI >500) are plotted. Trajectories for anti-HLA antibodies to individual HLA antigens are plotted and colored according to their respective HLA locus specificity. The gray line indicates 5000 MFI and the black line indicates 1000 MFI.
Clustering
We attempted to cluster patients according to the detailed temporal pattern of their MFI signal across time. We considered each patient as a multichannel, irregularly sampled time-series, meaning that we had N antibodies measurements at every time point and that time between measurements was not fixed. The main difficulty of comparing patients this way comes from two aspects: (1) the irregularly sampled nature of the data and (2) the fact that patients do not have the same number of time steps at which their alloimmune status was probed. As there are no standard methods to cluster multichannel, irregularly sampled time-series, we performed an analysis that considered summary statistics of the time-series of the patients, such as mean, SD, mean rate of change in MFI, and SD of the rate of change in MFI for each antibody. This resulted in a list of 320 features for class 1 and 174 features for class 2, for each patient. These extracted features were then used with three clustering algorithms: k-means, hierarchical clustering, and DBSCAN.11 Clustering success was measured using the silhouette coefficient12 and evaluated via visual inspection of t-SNE plots, a dimensionality reduction technique used to efficiently visualize high-dimensional data.13 No clear clusters could be obtained via these approaches, indicating that summary information on individual anti-HLA antibody kinetics are not detailed enough to provide valid ways of splitting the patients.
Discussion
Monitoring of alloimmune status by use of modern SAB technologies, in combination with improved HLA typing of donors, has vastly improved our ability to predict the immunologic risk associated with kidney transplantation for individual donor recipient combinations.2,14,15 SAB analyses are, however, both time consuming and costly, and it is therefore crucially important to optimize alloimmune status monitoring. Optimally, monitoring should be performed so that important changes in alloimmune status are not missed before transplantation, while at the same time limiting the analysis of patient samples were no alloimmune status changes are occurring. To design new strategies we first need to understand how allo-responses, as measured by the presence of anti-HLA antibodies, are changing over time in patients waiting for kidney transplantation. Our study aimed to investigate these kinetics and, interestingly, we were unable to find any significant differences in the alloimmune status change, as measured by anti-HLA antibody total MFI change, between measurements performed at different IBM. To investigate this further, we also looked at the difference using an alternative set of time intervals, in patients without a documented immunizing event and in MFI movement over clinically important MFI cut-offs. None of these approaches were able to identify an IBM with more change in alloimmune status, as compared with all other investigated IBM. A likely explanation for this is that alloimmune responses are highly individual and the pattern of change over time evolves in an individual fashion. The example plots of anti-HLA antibody kinetics in individual patients in our study are in line with this assessment.
Another explanation for our inability to find significant MFI differences between different IBM could be that our dataset includes short-interval measurements performed because of new immunizing events, with significant changes in MFI resulting from these events. These short interval measurements would then counteract a trend toward more changes in MFI over long IBM. However, comparison of measurements between later time points as well as analysis on other time intervals did not support a time dependency for MFI changes. Furthermore, when all patients with documented immunizing events or with measurements performed <30 days apart were excluded, there was still no significant effect of the IBM on MFI change. This somewhat argues against a general bias in our dataset that could counteract an underlying true difference in MFI change over time. However, we cannot exclude that our individualized SAB monitoring introduced confounding that might affect our analyses and results. Another important issue for longitudinal SAB analyses is assay variability, which could potentially both introduce additional MFI change between measurements as well as cloud our ability to detect real differences between different IBMs. Our measured internal coefficients of assay variability are, however, unlikely to substantially alter our results, even for highly immunized patients (where the effect on absolute ΔMFI measurements would be most significant), because measured ΔMFIs in our dataset markedly exceeded predicted effects of assay variability for a large proportion of the patients with detected MFI change. It is important to note that although the effect of variability likely had a limited effect on our results, SAB assay variability still affects our clinical ability to evaluate true alloimmune status change over time, especially over low MFI clinical cut-offs, in an individual patient. Our data does not include single-bead resolution MFI values, but instead uses values from the highest-ranked bead carrying the designated HLA antigen, and this could potentially influence our ability to evaluate MFI change over time. To improve upon these problems, additional studies using single-bead resolution data and a fixed short IBM, preferably in a large group of immunized patients, should be performed.
One way to optimize alloimmune status monitoring would be to divide patients on the waiting list into groups with different monitoring schedules on the basis of clinical parameters that could predict future alloimmune status change. Indeed, the investigated dataset already contains numerous data points that were the result of attempts at individualized monitoring, on the basis of waiting time, immunizing events, and the previous presence of anti-HLA antibodies. Despite this fact, many measurements were made that did not detect a significant change in alloimmune status, and it is highly likely that many relevant alloimmune status changes were also missed by the current approach.
To investigate potential individualized monitoring strategies, we tried several ways of stratifying the patients on the basis of different clinical parameters. A history of previous organ transplantation was, as predicted, associated with an increased amount of detectable anti-HLA antibodies, but was also associated with a highly significant increase in alloimmune status change over time. This was evident both in comparison with other immunizing events and with patients without previous documented immunization events. The amount of difference between these categories of patients suggests that individualized alloimmune monitoring could, in part, be structured so that patients with previous transplantations are monitored more frequently than patients in the other categories. Factors underlying increased MFI change in previously transplanted patients could be changes in ongoing immunosuppression, transplantectomies, or other proinflammatory events.16 Because of the lack of detailed information on these events, we were unfortunately not able to analyze this further in the current cohort. We were, however, able to investigate the effect of transfusions on alloimmune status change in a subgroup of patients with a documented transfusion during the waiting time. Our data underlines the importance of accurate monitoring of immunization events, as 22% of the transfusions were associated with marked changes in alloimmune status post-transfusion. Our dataset primarily comprises patients with detectable anti-HLA antibodies (92.3% >1000 MFI peak) that were followed with multiple SAB measurements throughout their waiting time. This means that our findings cannot be extended to nonalloimmunized patients without detectable anti-HLA antibodies, where clinical experience suggests that very little change in alloimmunization status occur over time, in the absence of new immunizing events.
Despite the large difference in alloimmune status change between the different categories, there are also huge differences within the immunized subgroups in the pattern of MFI change over time. We explored several different clustering approaches, on the basis of summary statistics, to generate groups of patients with similar alloimmune status change kinetics, but were unable to efficiently cluster the patients in a meaningful way. This suggests that the individual nature of alloimmune kinetics is extremely complex and cannot be easily categorized. The differentially spaced time points for alloimmune monitoring in the current dataset that allowed for the analysis of interaction between IBM and MFI change, unfortunately also make it difficult to design clustering algorithms. Further studies to improve on these analyses are underway.
In conclusion, we were unable to find a significant correlation between the change in alloimmune status, as measured by the amount of MFI change, and the time interval of SAB measurements. We were, however, able to find large differences in the magnitude of alloimmune status change between subgroups, stratified according to alloimmunization history. Our study has implications for the standardized monitoring of patients on the transplant waiting list and suggests that a more individualized monitoring schedule, partially on the basis of alloimmunization history, is preferable to current recommendations.
Disclosures
Prof. Borgwardt reports personal fees from mentoring and consulting for Roche Pharmaceutical Research and Early Development (pRED), Basel, outside the submitted work. Dr. Nilsson reports personal fees from One Lambda Inc., outside the submitted work. All of the remaining authors have nothing to disclose.
Supplementary Material
Acknowledgments
Mr. Togninalli, Prof. Borgwardt, and Dr. Nilsson designed the study, Mr. Togninalli, Dr. Yoneoka, and Dr. Nilsson analyzed the data. Dr. Kolios provided critically important intellectual content. Mr. Togninalli, Dr. Nilsson, Dr. Kolios, and Prof. Borgwardt drafted the manuscript. All authors approved the final version of the submitted manuscript and are accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Tracking HLA Antibody Changes among Kidney Waitlist Candidates: One Protocol May Not Fit All,” on pages 2042–2044.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060594/-/DCSupplemental.
Supplemental Table 1. Detailed P-values for percentile pairwise tests, class 1.
Supplemental Table 2. Detailed P-values for percentile pairwise tests, class 2.
Supplemental Table 3. P-values for Welch t-test statistics between groups of measurements across important clinical time intervals (3, 4, and 6 months). The analysis was performed both on the entire filtered dataset (marked with immunized) and on a subgroup of patients without a documented immunizing event (marked without immunized) (as denoted in Supplemental Figure 1).
Supplemental Table 4. Detailed values for percentile pairwise tests without immunized patients and measurements made closer than 30 days apart, class 1.
Supplemental Table 5. Detailed values for percentile pairwise tests without immunized patients and measurements made closer than 30 days apart, class 2.
Supplemental Table 6. Detailed P-values for percentile pairwise tests over the number of crossings over the clinical cut-off of MFI=1000, class 1.
Supplemental Table 7. Detailed P-values for percentile pairwise tests over the number of crossings over the clinical cut-off of MFI=1000, class 2.
Supplemental Table 8. Detailed P-values for percentile pairwise tests over the number of crossings over the clinical cut-off of MFI=5000, class 1.
Supplemental Table 9. Detailed P-values for percentile pairwise tests over the number of crossings over the clinical cut-off of MFI=5000, class 2.
Supplemental Table 10. Percentage of patients with more than N crossings of the 1000, 5000, and 10,000 MFI threshold (class 1) pretransplant, stratified on documented previous immunizing events.
Supplemental Table 11. Percentage of patients with more than N crossings of the 1000, 5000, and 10,000 MFI threshold (class 2) pretransplant, stratified on documented previous immunizing events.
Supplemental Table 12. Percentage of patients with at least one SAB specificity showing increase in MFI from <1000 to >5000 MFI pretransplant, stratified on documented previous immunizing events.
Supplemental Figure 1. MFI variability is, on average, not affected by the interval between measurements (IBM), in patients without documented immunizing events. When removing measurements made less than 30 days apart and patients with a documented immunizing events, no significant correlation between MFI variability and time between measurements can be seen for class I (A,B) and class II (C,D). Each IBM contains the same number of measurements (n=37 for class I, 36 for class II).
Supplemental Figure 2. Gender or age does not affect pre-transplant MFI variability. Individual swarm plots of the number of MFI crossings over the 1000 MFI threshold for five IBM groups stratified on sex (above) and age (below). For the analyses on the impact of patient age on pre-transplant MFI variability, the data set was split into three age groups (below 30 years, 30–60 years, and above 60 years). There were no significant differences in the number of crossings across either of these stratifications. Only class I measurements are shown but similar observations were also made for class II measurements (data not shown).
Supplemental Figure 3. The alloimmunization history affects pre-transplant MFI variability. Individual swarm plots of the number of MFI crossings over the 5000 MFI threshold for five IBM groups and across four immunization categories. Patients who had a previous transplant (n=115 overall), patients who did not have a previous transplant but who had been pregnant (n=98 overall), patients who did not have a previous transplant nor were pregnant but received blood transfusions (n=81 overall) and patients without a documented immunizing event (n=173 overall). The number of threshold crossing for class I (A) and class II (B) anti HLA-antibodies are reported.
Supplemental Figure 4. Mean total MFI change after transfusions occurring during the waiting time. Individual swarm plots showing the mean total MFI change after transfusion for class I (blue), and class II (green) measurements. Only measurements performed > 1 to < 200 days from the transfusion were included in the analyses.
References
- 1.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al.: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al.: Differential effects of donor-specific HLA antibodies in living versus deceased donor transplant. Am J Transplant 18: 2274–2284, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young JS, McIntosh C, Alegre ML, Chong AS: Evolving approaches in the identification of allograft-reactive T and B cells in mice and humans. Transplantation 101: 2671–2681, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tait BD: Detection of HLA antibodies in organ transplant recipients - triumphs and challenges of the solid phase bead assay. Front Immunol 7: 570, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al.: Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant 13: 1859–1870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akgul SU, Ciftci HS, Temurhan S, Caliskan Y, Bayraktar A, Tefik T, et al.: Association between HLA antibodies and different sensitization events in renal transplant candidates. Transplant Proc 49: 425–429, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Resse M, Paolillo R, Pellegrino Minucci B, Costa D, Fiorito C, Santangelo M, et al. : Effect of single sensitization event on human leukocyte antigen alloimmunization in kidney transplant candidates: A single-center experience. Exp Clin Transplant 16: 44–49, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Katerinis I, Hadaya K, Duquesnoy R, Ferrari-Lacraz S, Meier S, van Delden C, et al.: De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am J Transplant 11: 1727–1733, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Hricik DE, Formica RN, Nickerson P, Rush D, Fairchild RL, Poggio ED, et al.: Clinical Trials in Organ Transplantation-09 Consortium : Adverse outcomes of tacrolimus withdrawal in immune-quiescent kidney transplant recipients. J Am Soc Nephrol 26: 3114–3122, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tambur AR, Campbell P, Claas FH, Feng S, Gebel HM, Jackson AM, et al.: Sensitization in transplantation: Assessment of risk (STAR) 2017 working group meeting report. Am J Transplant 18: 1604–1614, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Ester M, Kriegel HP, Sander J, Xu X: A density-based algorithm for discovering clusters in large spatial databases with noise. Presented at the 2nd International Conference on Knowledge Discovery and Data Mining (KDD '96), Portland, OR, 1996 [Google Scholar]
- 12.Rousseeuw PJ: Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20: 53–65, 1987 [Google Scholar]
- 13.Maaten Lvd, Hinton GE: Visualizing data using t-SNE. J Mach Learn Res 9: 2579–2605, 2008 [Google Scholar]
- 14.Wehmeier C, Hönger G, Cun H, Amico P, Hirt‐Minkowski P, Georgalis A, et al. : Donor specificity but not broadness of sensitization is associated with antibody-mediated rejection and graft loss in renal allograft recipients. Am J Transplant 17: 2092–2102, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Amico P, Hirt-Minkowski P, Hönger G, Gürke L, Mihatsch MJ, Steiger J, et al. : Risk stratification by the virtual crossmatch: A prospective study in 233 renal transplantations. 24: 560–569, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Locke JE, Zachary AA, Warren DS, Segev DL, Houp JA, Montgomery RA, et al.: Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant 9: 2136–2139, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.