Abstract
The TLR2 agonist, dipalmitoyl lipopeptide (Pam2LP), has been used as an immune adjuvant without much success. Pam2LP is recognised by TLR2/6 receptors in humans and in mice. This study examined the proliferative activity of cytotoxic T lymphocytes (CTL) using mouse Ag-presenting dendritic cells (DCs) and OT-I assay system, where a library of synthetic Pam2LP was utilised from the Staphylococcus aureus database. Ag-specific CTL expansion and IFN-γ levels largely depended on the Pam2LP peptide sequence. The first Aa is cysteine (Cys), which has an active SH residue to bridge fatty acids, and the second and third Aa are hydrophilic or non-polar. The sequence structurally adapted to the residual constitution of the reported TLR2/6 pocket. The inactive sequence contained proline or leucine/isoleucine after the first Cys. Notably, no direct activation of OT-I cells was detected without DCs by stimulation with the active Pam2LP having the Cys-Ser sequence. MyD88, but not TICAM-1 or IFN pathways, in DCs participates in DC maturation characterised by upregulation of CD40, CD80 and CD86. Hence, the active Pam2LPs appear suitable for dimeric TLR2/6 on DCs, resulting in induction of DC maturation.
Keywords: CTL proliferation, MyD88-mediated dendritic cell priming, Pam2 lipopeptide, TLR2 agonist
Introduction
Dendritic cells (DCs) evolve from myeloid progenitors and professionally participate in activation of the immune system.1 DCs recognise microbial patterns and mature to facilitate Ag presentation and appropriate immune activation.1 TLRs are a family of PRRs and are preferentially expressed by DCs.2,3 Mice and humans share most of the orthologues of the TLR family.4
Human Ag-presenting DCs (characterised by CD141) predominantly express TLR3, as well as members of the TLR2 families, consisting of TLR1, 2 and 6.3 TLR2 recognises peptidoglycan (PGN) by homodimerisation.5 TLR2 also recognises the dipalmitoyl lipopeptide (LP) Pam2 in combination with TLR6 and recognises tripalmitoyl Pam3 in combination with TLR1 in both humans and in mice.5−8 TLR2 agonists stimulate cytokines/IFN production and inflammation via MyD886–8 and, to a lesser extent, via the Toll-IL-1R homology domain-containing adaptor molecule-1 (TICAM-1; also called TRIF) adaptors, which appear to be bridged by TICAM-2 (also called TRAM).9 We have suggested that the TLR2 family consists of distinct members in humans and chickens. They have evolved to recognise bacterial LPs with a different set of TLR2 members.10 However, the principle of the bacterial pattern recognition by TLR2 family is conserved across the entire vertebrate species.11 The recognition of various LPs by TLR2/6 and/or TLR2/1 has been structurally defined,12 and the recognition of LPs and PGN by TLR2 family is evolutionally conserved in humans and mice.13
Previously, we have studied the role of the peptide portion of Pam2LP in TLR2/6 recognition using a DC–NK cell activation system.14,15 We chemically synthesised various Pam2LPs with modifications based on the predicted Aa sequences from the Staphylococcus aureus database.14 The results suggest that Pam2LP acts on both DCs (in this case, bone marrow–derived DC [BMDC]) and NK cells.15,16 In either case, the peptide sequence followed by Pam2 is crucial in NK cell activation. The levels of cytokines released in the co-culture (BMDC+NK cells) in response to Pam2LP correspond to the degrees of NK cell activation in vitro, measured by IFN-γ and cytotoxicity.15−17 However, in vivo administration of Pam2LP only marginally regresses NK-sensitive tumours implanted in mouse models.16,17 The tumour micro-environment, including prostanoids, reactive oxygen species (ROS), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), might be involved in this decreased activation of NK cells.18−21
We herein tested whether the peptide sequence of Pam2 is important for cytotoxic T lymphocytes (CTL) activation and proliferation using the OT-I assay. The results show that the second and third Aa in a TLR2 agonist Pam2LP play a critical role in DC maturation via the MyD88 but not TICAM-1-Type I IFN pathway. The MyD88 pathway plays an important role in sufficient proliferation of CTL.
Materials and methods
Mice
WT C57BL/6J mice were purchased from CLEA. Ticam1–/– mice were made in our laboratory.22 Ifnar–/–, Myd88−/− and OT-I mice were kindly provided by Dr Taniguchi (Tokyo University, Tokyo, Japan), Dr Akira (Osaka University, Osaka, Japan) and Dr Ishii (Tohoku University, Sendai, Japan), respectively. All mice were backcrossed more than eight times to C57BL/6 background and maintained under specific pathogen-free condition in the animal faculty of the Hokkaido University Graduate School of Medicine. Animal experiments were performed according to the guidelines set by the animal safety centre, Hokkaido University, Japan.
Cells
BMDCs were prepared, as described previously.22 CD8α+ DCs (Thy1.2− B220− DX5− CD8α+ population) were isolated from mouse spleen with a CD8α+ DC isolation kit (Miltenyi Biotec; cat. no. 130-091-169). Cells were cultured in RPMI 1640 (Gibco; 11875-093) supplemented with 10% heat-inactivated FBS (Thermo Fisher Scientific; SH30910.03), 10 mM of HEPES (Gibco; 15630-080) and 50 IU of penicillin/50 µg/ml of streptomycin (Gibco; 15070-063).
Reagents and Abs
Pam2LPs were synthesised, as described previously.14 Poly(I:C) was purchased from GE Healthcare Life Sciences (27-4732-01). EndoGrade® Ovalbumin was purchased from Hyglos (321001). Anti-TLR2 Ab (Clone: 6C2; cat. no. 12-19021-80) and a mouse IFN-γ ELISA KIT (88-7314) were purchased from eBioscience. ViaProbe was purchased from BD Biosciences (555816). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Molecular Probes (C1157). The following Abs were purchased from BioLegend: anti-CD3ɛ (145-2C11, 100314), anti-CD8α (53-6.7, 100712), anti-CD11c (N418, 117310), anti-CD16/32 (93, 101302), anti-CD28 (37.51, 102111), anti-CD40 (3/23, 124607), anti-CD80 (16-10A1, 104707), anti-CD86 (GL-1, 105005) and anti-TCR Vβ5.1,5.2 (MR9-4, 139504). An anti-TLR6 Ab (418601, MAB1533) was purchased from R&D Systems.
OT-I proliferation assay
OT-I T cells were isolated from spleens of OT-I mice by CD8-microbeads (Miltenyi Biotec; 130-049-401). OT-I T cells were labelled with 1 µM of CFSE for 10 min at 37℃. In the co-culture with OT-I cells and BMDCs, 5 × 105 BMDCs were seeded in a 24-well plate. PBS or 100 nM of Pam2LPs were added to a well. After 18 h, cells were added with 500 ng/ml of OVA and allowed to stand for 4 h. The cells were washed three times with saline. BMDCs (1 × 105) were reseeded in a 96-well plate and were co-cultured with 1 × 105 CFSE-labelled OT-I cells for 60 h. In the single culture of OT-I cells without DCs, 1 × 105 CFSE-labelled OT-I cells were seeded in a 96-well plate. PBS or Pam2LPs were added to the wells in the presence or absence of 0.25 µg/ml of anti-CD28 and 0.01 or 0.1 µg/ml of anti-CD3 Abs. The OT-I cells were cultured for 60 h. In the co-culture with OT-I cells and CD8α+ DCs, 3.5 × 104 CD8α+ DCs were seeded in a 96-well plate. PBS or Pam2LPs were added to the wells in the presence or absence of 2.5 µg/ml of OVA. After 3 h, CD8α+ DCs were co-cultured with 3.5 × 104 CFSE-labelled OT-I cells for 60 h. Then, the cells were stained with anti-CD8α and anti-TCR Vβ5.1,5.2 Abs. Dead cells were removed by 7-amino actinomycin D staining. The degree of OT-I proliferation was assessed by the attenuation of CFSE intensity by FACSCalibur™ (BD Biosciences). The concentration of IFN-γ in the culture medium was measured by ELISA.
Real-time PCR
For the Tlr2 gene expression analysis, OT-I cells and CD8α+ DCs were isolated from OT-I mouse spleen. The isolated cells were lysed with TRIzol reagent (Invitrogen; 15596018). Real-time PCR was performed, as described previously.23 Sequences of primers in this study are: Gapdh: 5′-GCCTGGAGAAACCTGCCA-3′ and 5′-CCCTCAGATGCCTGCTTCA-3′; Tlr2: 5′-AAGAGGAAGCCCAAGAAAGC-3′ and 5′-CGATGGAATCGATGATGTTG-3′.
Analysis of maturation markers on BMDCs
PBS and 100 nM of Pam2-13, Pam2-13s and Pam2CSK4 were added to BMDCs derived from various gene knockout mice. After 18 h, CD40, CD80 and CD86 expression levels on CD11+ cells were analysed by FACS AriaII (BD Biosciences). In Myd88–/– BMDCs, poly(I:C) was set as a positive control because upregulation of the co-stimulatory molecules by poly(I:C) is independent of MyD88.24
Results
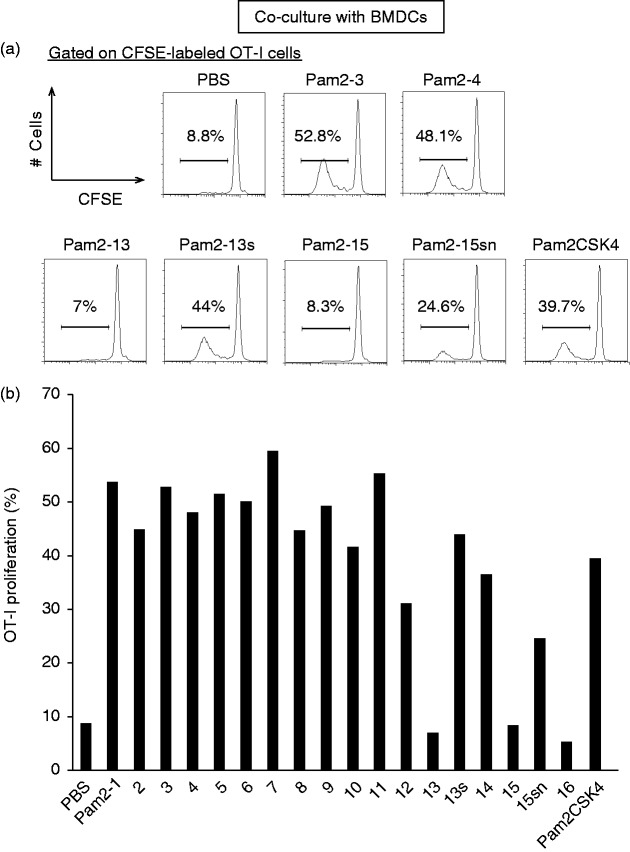
OT-I proliferation depends on the peptide sequence of Pam2LP
OT-I cells were prepared from OT-I mice, labelled with CFSE and co-cultured with BMDCs in the presence of various Pam2LP (Table 1) and OVA (Figure 1). The gating strategy and purity of DCs are in part shown in Supplementary Figure S1. The peptide sequences were obtained from the S. aureus database and artificially linked to Pam2.14 Pam2 OT-I proliferation was assessed by flow cytometry. PBS was the negative control, and Pam2CSK4, which is known as TLR2-agonistic Pam2LP, was the positive control. Pam2-1 to -12 and Pam2-14 induced potent OT-I proliferation comparable to Pam2CSK4 in the presence of DCs (Figure 1a and b). Under the same conditions, Pam2-13, -15 and -16 barely induced OT-I proliferation (Figure 1a and b). Based on the Aa sequences of Pam2LP in Table 1, when the second Aa was Pro or hydrophobic, OT-I proliferation was disrupted. When the second Aa was Gly/Ala/Thr/Ser, full DC maturation, in terms of CTL functional expansion, occurred. To confirm this finding, we used Aa substitutes for Pam2-13 (Pam2-13s: the -second Pro was replaced with Ser) and Pam2-15 (Pam2-15sn: the -second Leu and -third Ile were replaced with Ser and Asn). The results suggested that the second and third Aa positions are crucial for DC-mediated CTL induction by Pam2LP (Figure 1a and b). The first Aa must be Cys in Pam2LP. These results are consistent with those reported in the NK cell system.15
Table 1.
The sequences of Pam2LP used for the study.
| Name | Lipid+Aa sequence |
|---|---|
| Pam2-1 | Pam2+CANTRHSESDK |
| 2 | Pam2+CGTGGKQSSDK |
| 3 | Pam2+CGNGNKSGSDD |
| 4 | Pam2+CSNIEIFNAKG |
| 5 | Pam2+CTTDKKEIKAY |
| 6 | Pam2+CSFGGNHKLSS |
| 7 | Pam2+CGSQNLAPLEE |
| 8 | Pam2+CGQDSDQQKDG |
| 9 | Pam2+CGNDDGKDKDG |
| 10 | Pam2+CGNNSSKDKEA |
| 11 | Pam2+CSLPGLGSKST |
| 12 | Pam2+CSTSEVIGEKI |
| 13 | Pam2+CPFNCVGCYNK |
| 13s | Pam2+CSFNCVGCYNK |
| 14 | Pam2+CGSQNLAPLEEK |
| 15 | Pam2+CLILIIASETL |
| 15sn | Pam2+CSNLIIASETL |
| 16 | Pam2+CLILIIASETLFSFSHLTDVK |
| Pam2CSK 4 | Pam2+CSKKKK |
Figure 1.
The Aa following Pam2Cys determines the cross-priming activity in bone marrow–derived DC (BMDC). (a) and (b) BMDCs were stimulated with Pam2LPs in the presence of OVA. Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled OT-I cells were co-cultured with the BMDCs. After 60 h, the degree of OT-I proliferation was assessed by flow cytometry. (a) OT-I cells were gated as CD8α+ TCR Vβ5.1,5.2+ population. The histograms of representative samples are shown. (b) The result including all samples is shown. The results are representative of two independent experiments.
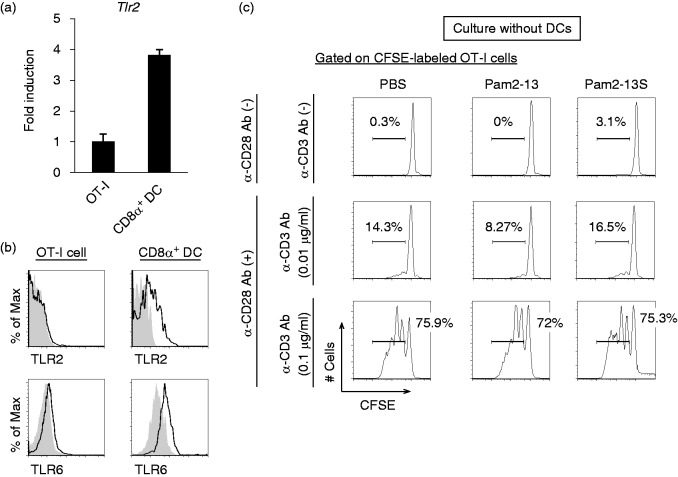
Pam2-13s does not act directly on OT-I cells
Pam2LP directly activates NK cells in the absence of DCs and/or Ag.16,21 In addition, our in vitro notion is that TLR2 on T cells plays a role in DC-independent T-cell activation.25,26 In fact, TLR2 activates the adaptor complex consisting of TRAM (TICAM-2)/TRIF (TICAM-1).9 In general, T cells express TLR2 and MyD88, which are associated with direct T-cell activation in vitro.25,26 Next, we assessed whether Pam2LP had a direct effect on OT-I cells in our experimental setting. The Tlr2 gene was expressed on OT-I cells, but the expression level was lower than that on CD8α+ DC which is a professional Ag-presenting DC subset (Figure 2a). In the analysis of protein expression, while TLR2 expressed in CD8α+ DCs, it barely expressed in OT-I cells (upper panels of Figure 2b). TLR6 was expressed on OT-I cells, but the expression level was lower than that on CD8α+ DCs (lower panels of Figure 2b). In the absence of the co-culture with DCs, Pam2-13s did not directly act on OT-I cells, even in the presence of CD28 and CD3 stimulation (Figure 2c). In the presence of the co-culture with either BMDCs or CD8α+ DCs, on the other hand, OT-I proliferation was initiated by TLR2 agonistic Pam2LPs.
Figure 2.
Pam2-13s is not directly recognized by OT-I cells. (a) Tlr2 gene expression level on OT-I cells and CD8α+ DCs isolated from OT-I mouse were analysed by real-time PCR. Error bars show ±SD. (b) Splenocytes were harvested from OT-I mouse and TLR2. TLR6 expression levels on OT-I cells (CD8α+ CD3+ population) and CD8α+ DCs (CD8α+ CD11chi population) were analysed by flow cytometry. Shaded and open histograms indicate isotype control and TLR2, TLR6 staining, respectively. (c) CFSE-labelled OT-I cells were cultured singularly in the presence or absence of anti-CD28 and anti-CD3 Abs. PBS, Pam2-13 and Pam2-13s were added to the culture media. After 60 h, the degree of OT-I proliferation was assessed. The histograms of each sample are shown.
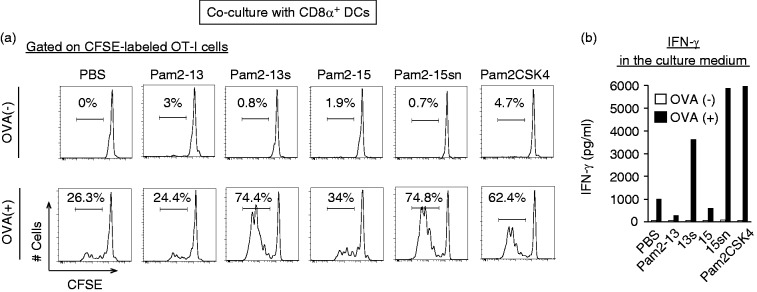
OVA Ag was necessary for Pam2LP-mediated OT-I proliferation by DCs, suggesting that OT-I cells proliferate in response to Ag (Figure 3a). The results were confirmed with the CD3/CD28 stimulation assay (Supplementary Figure S2). The conditions facilitated DC maturation-induced OT-I proliferation; Pam2-13s and Pam2-15sn induced DC-mediated CTL expansion comparable to Pam2CSK4, a representative positive control, whilst Pam2-13 and 15 did not (Figure 3a). IFN-γ levels reflected the degree of OT-I proliferation in this system (Figure 3b). In the absence of Ag, IFN-γ was not produced in the setting. These results reinforce the importance of the second and third Aa of the Pam2LP for the activation of DC-mediated acquired immunity.
Figure 3.
The conversion of an Aa following Pam2Cys to Serine gives rise to cross-priming in CD8α+ DC. (a) and (b) CD8α+ DCs (Thy1.2– B220– DX5– CD8α+ population) isolated from spleen were stimulated with Pam2LPs in the presence or absence of OVA. CFSE-labelled OT-I cells were co-cultured with the CD8α+ DCs. After 60 h, the degree of OT-I proliferation was assessed. (a) The histograms of each sample are shown. (b) The concentration of INF-γ in the culture medium was measured by ELISA. The results are representative of two independent experiments.
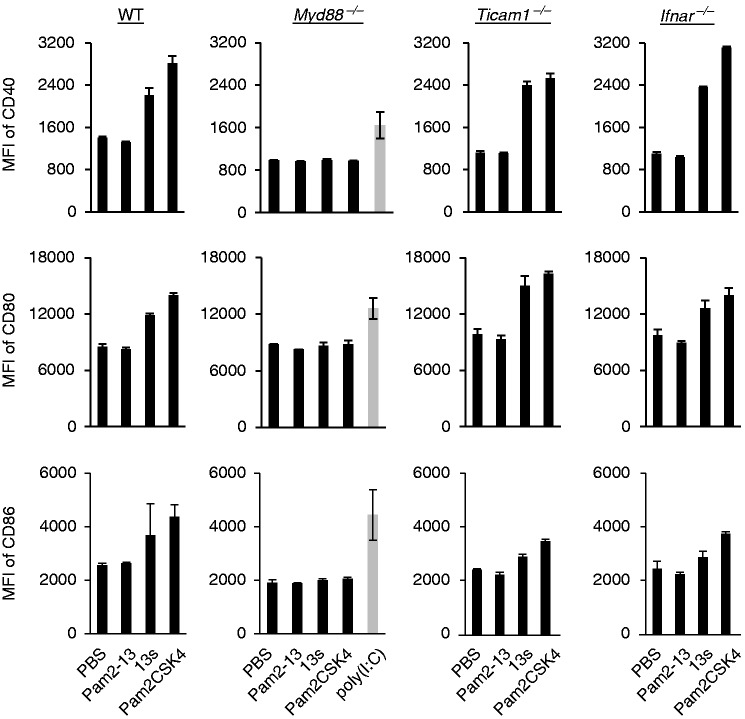
MyD88, but not type I IFN, is necessary for Pam2LP-mediated DC maturation
To identify the pathway responsible for Pam2LP-mediated BMDC maturation, BMDCs were isolated from a variety of gene-disrupted mice and stimulated with Pam2-13, -13s and Pam2CSK4 to test the upregulation of co-stimulatory molecules. Pam2-13 failed to elevate CD40, CD80 and CD86 levels, whereas Pam2-13s and Pam2CSK4 increased the levels of these molecules on wild type (WT) BMDCs (Figure 4 and Supplementary Figure S1). Notably, the Pam2-13s and Pam2CSK4 functions were abrogated in Myd88–/– BMDCs (Figure 4). Knockout of Ticam1 or Ifnar did not affect Pam2-13s and Pam2CSK4 functions in DCs. The co-stimulators appeared to be elevated on the surface of DCs, irrespective of the TICAM-1 or IFNAR pathway. The result is in part consistent with the IRF7 activation profile by Pam2LP.27 Therefore, the MyD88, but not the IFN-inducing axis, plays a key role in TLR2 agonistic Pam2LPs-mediated BMDC maturation and resultant CTL expansion.
Figure 4.
Pam2-13s induces DC maturation via MyD88 but not TICAM-1-Type I IFN pathway. PBS, Pam2-13, Pam2-13s and Pam2CSK4 were added to BMDCs derived from various gene knockout mice. In Myd88–/– BMDCs, poly(I:C) was also added as a positive control. After 18 h, CD40, CD80 and CD86 expression levels on CD11c+ populations were analysed by flow cytometry (gating strategy is shown in Supplementary Figure S1). Error bars show ±SD.
Discussion
Our motivation for publishing these data is to demonstrate that Pam2LP is a good candidate for immune-enhancing adjuvant for maturation of DCs. Co-stimulatory molecules and cross-presentation are tuned up by Pam2LP. Il-12p70 and IFN-induced gene Ifit1 levels are up-regulated in DCs by Pam2LP stimulation.16,28,29 The DC maturation profile was similar to that induced by another lipopeptide FSL-1 (Pam2CGDPKHSPKSF).30 The properties of the second and third Aa in the Pam2LP determine the DC maturation. CTL proliferation occurs exclusively through DCs in this context. The active Pam2LP containing serine after the first cysteine did not induce direct CTL proliferation in our experimental setting. However, we could not determine whether the given Aa are crucial for masking accessibility of the fatty acids in the peptide or changing affinity to the receptor. Since type I IFN-inducing pathways hardly participate in DC maturation by Pam2LP, the MyD88 axis of the TLR2 pathway actually works in DCs for T-cell priming. Thus, Pam2LP is a prime adjuvant for targeting DCs via MyD88.29,31
There is a long history in the study on the adjuvanticity of bacterial LPs. Earlier exploratory studies of the structural studies on bacterial (Escherichia coli) lipoproteins were summarised by Hantke and Braun.32,33 Jung and Bessler depicted the functional features of synthesised Pam3 bacterial lipoproteins.34,35 Their assays suggest that the synthetic tripalmitoyl (Pam3) pentapeptide has mitogenic activity for B-cell activation. The functional characteristics of the immune-enhancing LPs were summarised in their review, where T-cell activation by LP was mentioned.36 Pam3LP and Pam2LP share the receptor TLR2, although the pair co-receptors of TLR2 are differentially evolved for Pam3LP and Pam2LP, which are TLR1 and TLR6, respectively.7,8 These studies contributed to the development of biological response modifiers, which were later referred to as innate immune adjuvants. LPs are defined as TLR2 agonists. Mycoplasma harbours diacylated lipoproteins, whereas most Gram-negative and -positive bacteria possess triacylated lipoproteins, which reflects the difference in the repertoire of enzymes for lipoprotein synthesis.37 Tripalmitoyl Pam3CSK4 derived from E. coli and dipalmitoyl MALP-2 from Mycoplasma fermentans have been utilised as standard of lipoproteins. Their inflammatory properties are characterised by the receptor complex TLR1/2 and TLR6/2.
A Gram-positive S. aureus express a number of LPs according to the database. Fujimoto’s group made a series of synthetic peptides based on the predicted proteins with N-terminal dipalmitoylation.14,38 Although the dipalmitoylation of these peptides appears to be artificial, they have good adjuvanticity in DC-mediated NK cell activation.15 We reason that the second and third Aa in the Pam2LP peptide facilitate the acquired immune response in this study. The first Aa, Cys, is connected to a diacylated palmitate in all LPs designed from the natural bacteria.39 The second and third Aa are categorised into non-polar/hydrophilic or hydrophobic groups. We found that only the former activates BMDCs and CD8α+ Ag-presenting DCs to expand CTL (represented by OT-I cell) in the presence of OVA Ag. Taken together, Pam2LP and Ag efficiently suppress tumour growth.20,29
In other reports, the TLR2 agonist Versican promotes progression and invasion of tumours.40 The TLR2-MyD88 pathway provokes monocytic-MDSCs, which induce iNOS up-regulation.19,20 An inducible ROS is a product which acts as an effector for the blocking of CTL reinvigoration in the tumour micro-environment.20 Additionally, TLR2 is involved in the expansion and activation of MDSCs,19,20 which in turn exacerbates the tumour micro-environment. TLR2 antagonists might relieve tumour-supportive functions of monocytic-MDSCs. Although DCs are classified into many subsets, BMDCs and splenic CD8α+ DCs are sufficiently activated by the TLR2 pathway.2,4,41 Designing the peptide portion of Pam2LP to target DCs but not MDSCs may lead to the development of specific DC-priming adjuvant. Segregation of Pam2-13 and 13s by DCs suggests that a TLR2 antagonist versus agonist would be an effective modulator of the tumour micro-environment for Pam2 adjuvant.
In comparison to the TLR3-TICAM-1 pathway, the TLR2-MyD88 pathway poorly activates NK cells in vivo, which permits tumour progression.16,17 Cox2 is a factor that suppresses NK activation.21 Treg induction may be a factor participating in this immune dysregulation.17 TLR2 induces a sequence of complex events in tumours, where DC-priming, Treg activation, MDSC expansion and Th2-skewing simultaneously occurs, and the balance of these effectors finally appears to determine the tumour fate. In fact, administration of TLR2 agonist plus Ag or tumour lysate (that includes tumour-associated Ag) yields a good prognosis in tumour-bearing mice, at least in some models.29,42
Nevertheless, Pam2LP sufficiently proliferates CTLs in a different fashion to poly(I:C) (activating MDA5 and TLR3) or the TLR3-specific adjuvant ARNAX.43,44 Pam2LP acts as an agonist for TLR2, but barely induces activation of TICAM-1- or type I IFN-inducing pathway in DCs. Mouse in vivo studies using Pam2LP and tumour Ag (OVA) suggest that the TLR2-MyD88 axis but not the TLR2-TICAM-1 axis takes part in DC maturation and anti-tumour CTL proliferation.29 This finding is in contrast to previous reports, which show that TICAM-1 is involved in TLR2 signalling in bone marrow–derived macrophages.9 Therefore, the TLR2-MyD88 pathway solely participates in DC maturation and successive CTL proliferation in the DC-OT-I model. It has been reported that the transcription factors of the Activator protein-1 (AP-1) family play an essential role in DC maturation.45 Thus, MyD88 signal links the AP-1 activation and induces the maturation of Ag-presenting DCs as well as TICAM-1 signal, independent of the type I IFN pathway. These two different signals result in the common features of DC maturation. This is not surprising, as a number of cell type-specific variations have been reported in TLR responses, particularly in DCs.1,46 These results support the previous advocation47,48 that TLR2 is a good adjuvant for CTL proliferation.29 Treg induction and MyD88-mediated cytokine production indeed remain problematic in terms of using TLR2 agonists as an adjuvant, in particular NK activation.17 However, these problems could be resolved by designing the peptide portion of Pam2LP to specifically target DCs.49
After completing this study, a paper describing a novel role of the long-chain N-acylated lipoprotein in the function of Pam3LP was reported.50 N-terminal hydrophobicity may further affect the TLR2-agonistic function of Pam2LP. However, the inter-association between the fatty acid and the peptide remains to be analysed in the context of these Pam2LP.
Supplemental Material
Supplemental material for The second and third amino acids of Pam2 lipopeptides are key for the proliferation of cytotoxic T cells by Yohei Takeda, Masahiro Azuma, Ryoko Hatsugai, Yukari Fujimoto, Masahito Hashimoto, Koichi Fukase, Misako Matsumoto and Tsukasa Seya in Innate Immunity
Acknowledgments
This work was in part supported by the Uehara Memorial Foundation and Smoking Research Foundation. We are grateful to Drs K. Funami, T. Akazawa, H. Shime, J. Kasamatsu, M. Tatematsu and A. Maruyama for their thoughtful discussions. We are also grateful to Drs M. Kasahara (Hokkaido University), M. Fukushima and H. Nishimura (Translational Research Informatics Center, Kobe) for their support of our study. We had non-profit support from AID Co. Ltd (Kobe, Japan) through the university-company contract, which we acknowledge gratefully.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol 2006; 311: 17–58. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial Ags. J Exp Med 2001; 194: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell Ags. J Exp Med 2010; 207: 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004; 5: 987–995. [DOI] [PubMed] [Google Scholar]
- 5.Calcutt MJ, Kim MF, Karpas AB, et al. Differential posttranslational processing confers intraspecies variation of a major surface lipoprotein and a macrophage-activating lipopeptide of Mycoplasma fermentans. Infect Immun 1999; 67: 760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiguchi M, Matsumoto M, Takao T, et al. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J Immunol 2001; 166: 2610–2616. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Kawai T, Mühlradt PF, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 2001; 13: 933–940. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Sato S, Horiuchi T, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002; 169: 10–14. [DOI] [PubMed] [Google Scholar]
- 9.Nilsen NJ, Vladimer GI, Stenvik J, et al. A role for the adaptor proteins TRAM and TRIF in Toll-like receptor 2 signaling. J Biol Chem 2015; 290: 3209–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi M, Matsuo A, Shingai M, et al. Combinational recognition of bacterial lipoproteins and peptidoglycan by chicken Toll-like receptor 2 subfamily. Dev Comp Immunol 2008; 32: 147–155. [DOI] [PubMed] [Google Scholar]
- 11.Oshiumi H, Matsuo A, Matsumoto M, et al. Pan-vertebrate Toll-like receptors during evolution. Curr Genomics 2008; 9: 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang JY, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009; 31: 873–884. [DOI] [PubMed] [Google Scholar]
- 13.Ishii A, Kawasaki M, Matsumoto M, et al. Phylogenetic and expression analysis of amphibian Xenopus Toll-like receptors. Immunogenetics 2007; 59: 281–293. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto Y, Hashimoto M, Furuyashiki M, et al. Lipopeptides from Staphylococcus aureus as Tlr2 ligands: prediction with mRNA expression, chemical synthesis, and immunostimulatory activities. Chembiochem 2009; 10: 2311–2315. [DOI] [PubMed] [Google Scholar]
- 15.Azuma M, Sawahata R, Akao Y, et al. The peptide sequence of diacyl lipopeptides determines dendritic cell TLR2-mediated NK activation. PLos One 2010; 5: e12550–e12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawahata R, Shime H, Yamazaki S, et al. Failure of mycoplasma lipoprotein MALP-2 to induce NK cell activation through dendritic cell TLR2. Microbes Infect 2011; 13: 350–358. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki S, Okada K, Maruyama A, et al. TLR2-dependent induction of IL-10 and Foxp3+ CD25+ CD4+ regulatory T cells prevents effective anti-tumor immunity induced by Pam2 lipopeptides in vivo. PLoS One 2011; 6: e18833–e18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seya T, Kasamatsu J, Azuma M, et al. Natural killer cell activation secondary to innate pattern sensing. J Innate Immun 2011; 3: 264–273. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama A, Shime H, Takeda Y, et al. Pam2 lipopeptides systemically increase myeloid-derived suppressor cells through TLR2 signaling. Biochem Biophys Res Commun 2015; 457: 445–450. [DOI] [PubMed] [Google Scholar]
- 20.Shime H, Maruyama A, Yoshida S, et al. Toll-like receptor 2 ligand and interferon-γ suppress anti-tumor T cell responses by enhancing the immunosuppressive activity of monocytic myeloid-derived suppressor cells. Oncoimmunology 2017; 7: e1373231–e1373231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller C, Tufa DM, Chatterjee D, et al. The TLR-2/TLR-6 agonist macrophage-activating lipopeptide-2 augments human NK cell cytotoxicity when PGE2 production by monocytes is inhibited by a COX-2 blocker. Cancer Immunol Immunother 2015; 64: 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akazawa T, Ebihara T, Okuno M, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci U S A 2007; 104: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda Y, Azuma M, Matsumoto M, et al. Tumoricidal efficacy coincides with CD11c up-regulation in Ag-specific CD8 T cells during vaccine immunotherapy. J Exp Clin Cancer Res 2016; 35: 143–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoebe K, Janssen EM, Kim SO, et al. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol 2003; 4: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 25.Asprodites N, Zheng L, Geng D, et al. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J 2008; 22: 3628–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imanishi T, Hara H, Suzuki S, et al. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol 2007; 178: 6715–6719. [DOI] [PubMed] [Google Scholar]
- 27.Stack J, Doyle SL, Connolly DJ, et al. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J Immunol 2014; 193: 6090–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seya T, Matsumoto M, Tsuji S, et al. Structural-functional relationship of pathogen-associated molecular patterns: lessons from BCG cell wall skeleton and mycoplasma lipoprotein M161Ag. Microbes Infect 2002; 4: 955–961. [DOI] [PubMed] [Google Scholar]
- 29.Takeda Y, Azuma M, Funami K, et al. Type I interferon-independent dendritic cell priming and antitumor T cell activation induced by a Mycoplasma fermentans lipopeptide. Front Immunol 2018; 9: 496–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiura K, Kataoka H, Nakata T, et al. The synthetic analogue of mycoplasmal lipoprotein FSL-1 induces dendritic cell maturation through Toll-like receptor 2. FEMS Immunol Med Microbiol 2006; 46: 78–84. [DOI] [PubMed] [Google Scholar]
- 31.Seya T, Shime H, Takeda Y, et al. Adjuvant for vaccine immunotherapy of cancer—focusing on Toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity. Cancer Sci 2015; 106: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hantke K, Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem 1973; 34: 284–296. [DOI] [PubMed] [Google Scholar]
- 33.Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta 1975; 415: 335–377. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RB, Köhl S, Wiesmüller K, Jung G, et al. Synthetic analogues of the N-terminal lipid part of bacterial lipoprotein are B-lymphocyte mitogens in vitro and in vivo. Immunobiology 1983; 165: 27–35. [DOI] [PubMed] [Google Scholar]
- 35.Bessler WG, Cox M, Lex A, et al. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol 1985; 135: 1900–1905. [PubMed] [Google Scholar]
- 36.Bessler WG, Jung G. Synthetic lipopeptides as novel adjuvants. Res Immunol 1992; 143: 548–553. [DOI] [PubMed] [Google Scholar]
- 37.Kurokawa K, Lee H, Roh KB, et al. The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for Toll-like receptor 2. J Biol Chem 2009; 284: 8406–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawaratsumida K, Furuyashiki M, Katsumoto M, et al. Characterization of N-terminal structure of TLR2-activating lipoprotein in Staphylococcus aureus. J Biol Chem 2009; 284: 9147–9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babu MM, Priya ML, Selvan AT, et al. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J Bacteriol 2006; 188: 2761–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009; 457: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaki H, Oshiumi H, Matsumoto M, et al. Dendritic cell subsets involved in type I IFN induction in mouse measles virus infection models. Int J Biochem Cell Biol 2014; 53: 329–333. [DOI] [PubMed] [Google Scholar]
- 42.Akazawa T, Masuda H, Saeki Y, et al. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res 2004; 64: 757–764. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto M, Tatematsu M, Nishikawa F, et al. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat Commun 2015; 6: 6280–6280. [DOI] [PubMed] [Google Scholar]
- 44.Takeda Y, Kataoka K, Yamagishi J, et al. A TLR3-specific adjuvant relieves innate resistance to PD-L1 blockade without cytokine toxicity in tumor vaccine immunotherapy. Cell Rep 2017; 19: 1874–1887. [DOI] [PubMed] [Google Scholar]
- 45.Murphy KM. Transcriptional control of dendritic cell development. Adv Immunol 2013; 120: 239–267. [DOI] [PubMed] [Google Scholar]
- 46.Joffre OP, Segura E, Savina A, et al. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12: 557–569. [DOI] [PubMed] [Google Scholar]
- 47.Seya T, Matsumoto M. A lipoprotein family from Mycoplasma fermentans confers host immune activation through Toll-like receptor 2. Int J Biochem Cell Biol 2002; 34: 901–906. [DOI] [PubMed] [Google Scholar]
- 48.Mühlradt PF, Kiess M, Meyer H, et al. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med 1997; 185: 1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akazawa T, Ohashi T, Nakajima H, et al. Development of a dendritic cell-targeting lipopeptide as an immunoadjuvant that inhibits tumor growth without inducing local inflammation. Int J Cancer 2014; 135: 2847–2856. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen MT, Uebele J, Kumari N, et al. Lipid moieties on lipoproteins of commensal and non-commensal staphylococci induce differential immune responses. Nat Commun 2017; 8: 2246–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for The second and third amino acids of Pam2 lipopeptides are key for the proliferation of cytotoxic T cells by Yohei Takeda, Masahiro Azuma, Ryoko Hatsugai, Yukari Fujimoto, Masahito Hashimoto, Koichi Fukase, Misako Matsumoto and Tsukasa Seya in Innate Immunity