Background.
As our previous publications show, it is feasible to reverse type 1 diabetes (T1D) without insulin in multiple mouse models, through transplantation of embryonic brown adipose tissue (BAT) in the subcutaneous space. Embryonic BAT transplants result in rapid and long-lasting euglycemia accompanied by decreased inflammation and regenerated healthy white adipose tissue, with no detectable increase in insulin. To translate this approach to human patients, it is necessary to establish practical alternatives for embryonic tissue. Adult adipose tissue transplants or BAT-derived stem cell lines alone fail to reverse T1D. A likely reason is transplant failure resulting from lack of growth factors abundant in embryonic tissue. Adding growth factors may enable transplants to survive and vascularize as well as stimulate adipogenesis and decrease inflammation in the surrounding host tissue. Previous data points to insulin like growth factor 1 (IGF-1) as the most likely candidate. Embryonic BAT abundantly expresses IGF-1, and embryonic BAT transplant recipients exhibit increased plasma levels of IGF-1. Therefore, we tested the ability of temporary administration of exogenous IGF-1 to enable adult BAT transplants to correct T1D.
Methods.
Fresh BAT from healthy adult CB7BL/6 donors were transplanted in the subcutaneous space of hyperglycemic nonobese diabetic recipients. Exogenous IGF-1 was administered daily for a week following transplant, at 100 µg/kg SC.
Results.
Adult BAT transplants with IGF-1 supplementation produced rapid long-lasting euglycemia at a 57% success rate, in contrast with no recovery in the control groups who received adult BAT alone, IGF-1 alone, or no treatment.
Conclusions.
Temporary supplementation with IGF-1 enables adult BAT transplants to correct T1D phenotypes independent of insulin, providing a possible route to translate this treatment to human patients.
Type 1 diabetes (T1D) is a serious autoimmune disease requiring constant glucose management. T1D affects about 2 million Americans and an estimated 21 million people worldwide, with 40 000 new cases diagnosed each year. Traditional therapy involves insulin replacement, either directly or via islet/pancreas transplantation. Besides inconvenience, exogenous insulin administration carries the risk of hypoglycemia unawareness, a potentially fatal outcome. Pancreas transplantation involves the obvious limitations of invasive surgery and life-long immuno-suppression, while islet transplantation is also limited by availability of donor tissue, need for immuno-suppression, and return to insulin dependence over the long term.1-5 Despite continuous refinement through the past decades, effective solutions for these basic limitations have not been found. A comprehensive review of the safety and efficacy of commonly used type 2 diabetes drugs as add-on therapies to insulin in T1D concluded that their efficacy was minimal or modest, and such add-on therapy was not generally recommended for T1D.6 Thus, there is an ongoing need for better therapies for T1D.
The ultimate goal in treating T1D is to restore glucose homeostasis. Our work has shown that glucose homeostasis can be achieved without insulin, using subcutaneously placed embryonic brown adipose tissue (BAT) transplants.7,8 In both streptozotocin-diabetic and autoimmune-induced nonobese diabetic (NOD) mice, BAT transplants reestablish normoglycemia independent of insulin. T1D is associated with inflammation both generalized and in white adipose tissue (WAT), and loss of WAT.9-11 Embryonic BAT transplants lead to remarkable recovery of WAT, decreased inflammation, and reversal of diabetes.7,8 Euglycemia correlates with suppression of glucagon, and progressive increases in plasma adiponectin, insulin like growth factor 1 (IGF-1), and sometimes leptin. Thus, it appears that a combination alternate hormones arising from BAT transplant and/or regenerated WAT establishes a new physiological equilibrium, compensating for endocrine pancreas.
While this technique is proven effective in long-term reversal of T1D in mice, transplantation of embryonic tissue is not a practical solution for human patients. The goal of the current study was to implement appropriate modifications to reproduce the results with adult BAT transplants, a plausible alternative to embryonic BAT in clinical settings.
The presence of BAT in healthy human adults and its importance in combating metabolic disease is demonstrated in recent literature.12-14 While several studies show the ability of adult BAT and WAT transplants to alleviate diabetes and obesity, and to improve metabolism,13-19 complete reversal of insulin-dependent diabetes has not been achieved as it is with embryonic BAT transplants. According our own experience, transplantation of adult BAT or WAT alone fails to reverse T1D. Certain growth factors are abundant in embryonic tissue, and may enable embryonic BAT transplants to survive and vascularize in the recipients’ subcutaneous space as well as stimulate adipogenesis and decrease inflammation in the surrounding host tissue. Plausible candidate growth factors include IGF-1, fibroblast growth factor 21, and adiponectin, all of which are highly expressed in embryonic tissue, possess adipogenic and anti-inflammatory properties, and can mediate insulin-independent glucose transport and metabolism in peripheral tissues.20-40
Our data strongly point to donor-origin IGF-1 as a critical mediator in the early changes following BAT transplant.7,8 Reversal of diabetes occurs only with donor embryonic BAT from healthy C57BL/6 mice, and not with donor BAT from NOD mice who are deficient of IGF-1. IGF-1 levels in recipients’ plasma significantly increase within a week of BAT transplant placement, and progressively increase thereafter parallel to weight gain and WAT regeneration, in negative correlation with proinflammatory cytokines.7,8 IGF-1 is abundantly expressed in freshly isolated BAT from C57BL/6 embryos.7 Newly formed WAT in NOD transplant recipients continues to express IGF-1 for several months posttransplant, in contrast to little or no expression in control animals.7,8 It is noteworthy that only 50% of embryonic BAT transplants result in long-term euglycemia, and the ones that fail lack this increase of IGF-1 but instead show a progressive decrease of IGF-1.7 Thus, it seems that the presence of IGF-1 during the early stage of BAT transplant is critical for establishment of the new equilibrium of alternate hormones. Adipogenic and anti-inflammatory properties of IGF-1 might enable the regeneration of healthy WAT, which in turn could secrete other insulin-mimetic adipokines to compensate for the function of insulin. IGF-1 may also have direct actions on glucose lowering through insulin-independent glucose uptake via GLUT1 and GLUT3 transporters.34-36,40 Pharmacological inhibition of the insulin receptor in euglycemic BAT transplant recipients partially impairs their glucose tolerance,8 suggesting the occupation of insulin receptor by a noninsulin hormone. IGF-1 is a candidate here as well, due to the structural similarity of insulin and IGF-1 receptors.41,42
Based on the aforementioned data, we hypothesize that the failure of adult BAT transplants to reverse T1D is due to the lack of IGF-1, which may be corrected by supplementation with exogenous IGF-1 during the early stages. To test this hypothesis, we performed experiments to determine whether adult BAT transplants temporarily supplemented with IGF-1 can produce euglycemia in NOD mice with autoimmune-induced diabetes.
MATERIALS AND METHODS
Experimental Design
All animal studies were approved by the Washington University Institutional Animal Care and Use Committee, under the Animal Welfare Assurance # A-3381-01, and Protocol # 20150121. BAT transplants were performed as described previously,7,8 on female NOD mice (Jackson labs, stock # 001976) 4–6 months old. Donor adipose tissue came from healthy young adult C57BL/6 females (Jackson labs, stock #000664) 1–2 months old. Experiments were performed on NOD mice determined diabetic by a repeated non-fasting blood glucose level over 200 mg/dL. The test group was provided with exogenous IGF-1 for the first 7 days following BAT transplant (100 µg/kg/day SC), in the lower end of the dosage range found in the literature on IGF-1 administration to mice and rats for various purposes. Control groups included diabetic mice who received adult BAT transplants alone, IGF-1 injections alone, or saline injections alone. Body weight was recorded, and non-fasting blood samples were collected before and at regular intervals after treatment, that is, every week for the first month following treatment, and every month thereafter. Metabolic parameters, such as blood glucose, insulin, glucagon, adiponectin, and IGF-1, were measured from transplant and control groups at regular intervals. Glucose tolerance tests were performed on representative euglycemic transplant recipients in comparison with normal and diabetic controls. Depending on the group and glycemic status, mice were euthanized at different time points after 1 month, and tissues were collected postmortem. The presence of insulin and glucagon in the pancreas, and the presence of insulin and IGF-1 in adipose tissue, were verified with immunofluorescence.
Isolation of Donor Tissue
Donor mice are anesthetized with ketamine/xylazine (200/40 mg/kg IP). Following aseptic preparation, interscapular adipose tissue is exposed through a dorsal midline skin incision. WAT is gently removed, and the BAT is dissected out. The mouse is immediately killed by cervical dislocation while under anesthesia. Freshly isolated BAT is placed in sterile ice-cold HBSS, and transplanted into recipients as quickly as possible.
Transplantation
Freshly isolated adult BAT is transplanted in the subcutaneous space of diabetic recipients. Following general anesthesia and aseptic preparation, a small (2–4 mm) incision is made in the skin of the dorsal body surface caudal to the endogenous BAT. A subcutaneous pocket is made by blunt dissection using a blunt-ended micro spatula. Donor tissue is introduced into the pocket with Dumont forceps and pushed in with blunt-ended micro spatula. The incision is closed by gentle pressure with hemostats alone, or with 1–2 simple interrupted sutures as necessary with 5–0 nonabsorbable sutures. One to 2 lobes of adult BAT are introduced into each recipient. Surgeries are performed under general anesthesia with ketamine/xylazine (100/20 mg/kg) IP, and postoperative analgesia is provided with ketoprofen 5 mg/kg/day SC as necessary. Sutures are removed in 7–10 days postop.
Metabolic Parameters
Blood samples were collected from a tail-nick under isoflurane/oxygen anesthesia, for measurement of glucose, insulin, and other hormones. Basal non-fasting blood samples were collected before treatment, at weekly intervals after treatment for the first month, and at monthly intervals thereafter. Plasma samples were analyzed for insulin, glucagon, adiponectin, and IGF-1. Intra-peritoneal glucose tolerance tests were performed on successful transplant recipients at 5 months posttransplant, in comparison with normal and diabetic controls. Intra-peritoneal glucose tolerance test involved tail-nick blood glucose measurements from 6-h fasted mice before (0 min) and 15, 30, 60, and 120 minutes after intraperitoneal injection of sterile glucose (Sigma) (1 g/kg body weight) under isoflurane/oxygen anesthesia.
Postmortem Tissue Collection
Mice were euthanized at different time points between 1 and 12 months after procedure, and adipose tissue and pancreas were retrieved for histology. The whole pancreas and the adipose tissue from the subcutaneous space of the dorsal body surface were collected, and the tissues were preserved in 70% ethanol for histological analysis.
Statistical Analysis
Groups were compared 2 at a time as denoted in each experiment, using Students homoscedastic t test. Values are expressed as mean ± SEM. Numbers (n) for each group and statistical probabilities (P) for each comparison are specified in the Results section, figures, or figure legends.
RESULTS
NOD female mice develop spontaneous diabetes after 12 weeks of age. Diabetic mice have basal non-fasting blood glucose over 200 mg/dL, which quickly rises to 600 mg/dL within weeks. If untreated, they die or require euthanasia soon after the onset of diabetes. Successful BAT transplant recipients are defined as those diabetic mice whose basal non-fasting blood glucose levels decreased below 180 mg/dL and stayed below 180 mg/dL for at least 2 months following transplant, with no other clinical signs of diabetes or metabolic abnormalities.
Adult BAT transplants temporarily supplemented with IGF-1 produced rapid and long-lasting euglycemia, similar to the results previously observed with embryonic BAT transplants (Figure 1). Out of a total of 21 NOD mice that received adult BAT transplants with IGF-1 supplementation daily for the first week posttransplant, 12 recovered from diabetes (successful transplants) while 9 did not (failed transplants), providing a 57% success rate. The successful transplant recipients achieved euglycemia within a week of treatment (Figure 1A). Of the successful transplant group, 9 mice remained euglycemic until euthanasia at different endpoints ranging from 6 to 12 months, while 3 reverted to diabetes at months 3 and 7. Euglycemic transplant recipients also showed normal glucose tolerance comparable to normal nondiabetic controls (Figure 1B). Recovery from diabetes was not generally observed in the control groups that received adult BAT transplants alone, IGF-1 injections alone, or saline injections alone (Figure 1A). Due to increasing severity of clinical signs, these animals had to be euthanized at different time points within 3 months from development of hyperglycemia. One mouse from the group treated with IGF-1 injections alone showed euglycemia that lasted for 3 months post procedure. This mouse also showed abnormalities, such as excessive weight gain and organ enlargement, and therefore was removed from the study. One mouse from the control group receiving adult BAT transplants alone also showed some resistance to diabetes, where severe hyperglycemia did not develop immediately, and basal glucose levels stayed between 190 and 200 mg/dL for 5 weeks. While this caused an increase in the error bars in this group, the overall trend remained unchanged.
FIGURE 1.
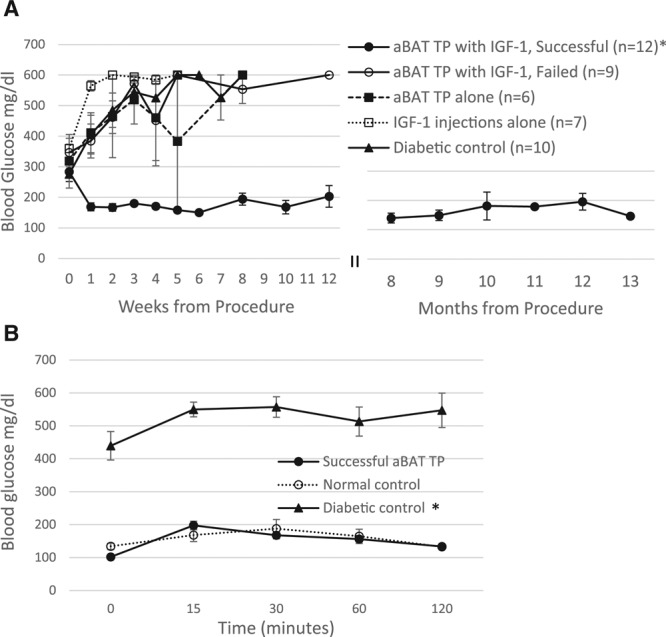
Glucose homeostasis in NOD mice following aBAT TP supplemented with IGF-1. A, Non-fasting blood glucose levels before and at weekly or monthly intervals after placement of aBAT TPs followed by supplementation with IGF-1 (100 µg/kg SC) for 1 wk, in comparison with control groups. Successful transplant recipients (closed circles) achieve and maintain euglycemia within a wk from transplant, while the control groups and failed transplants (open circles) become progressively hyperglycemic. Control groups included diabetic mice treated with aBAT TPs alone (closed squares), IGF-1 injections alone (open squares), or saline injections alone (closed triangles). *P < 0.004 when each posttransplant time point of the successful transplant group is compared with corresponding time points of other groups, or their own pretransplant values. Normal nondiabetic controls: 132.4 ± 11.2 mg/dL. B, Intra-peritoneal glucose tolerance test in 6-h-fasted mice. n = 3 for each group *P < 0.002 when the diabetic control group is compared with successful transplant group or normal control. aBAT TP, adult BAT transplant; BAT, brown adipose tissue; IGF-1, insulin like growth factor 1; NOD, non obese diabetic.
As previously reported with embryonic BAT transplants, the results are independent of insulin, as indicated by progressively decreasing levels of plasma insulin and the absence of insulin immunofluorescence in the pancreas postmortem (Figures 2A and 3). All groups show a progressive decline of plasma insulin as characteristic with NOD mice, compared with normal nondiabetic controls at 2457.4 ± 571.5 pg/mL. Pancreatic sections in diabetic control mice have little or no immuno-staining for insulin, while successful transplant recipients who remained euglycemic for 10 months show none at all (Figure 3, left panels). As diabetes progresses in NOD mice, there is a general decline in islet number and disruption of islet structure. Figure 3B and C (left panels) represent some of the remaining intact islets, to demonstrate the visible absence of insulin immunostaining. While acinar tissue and blood vessels can show some green autofluorescence, this is distinct from the actual immunofluorescence for insulin as seen in the control pancreas. As is characteristic in NOD mice, many islets in the diabetic control group show immune cell infiltration and disruption of islet structure upon H&E staining (Figure 3B, Right). Such cell infiltration was not evident in successful transplant recipients (Figure 3C, Right), presumably due to a widespread decrease of inflammation.
FIGURE 2.
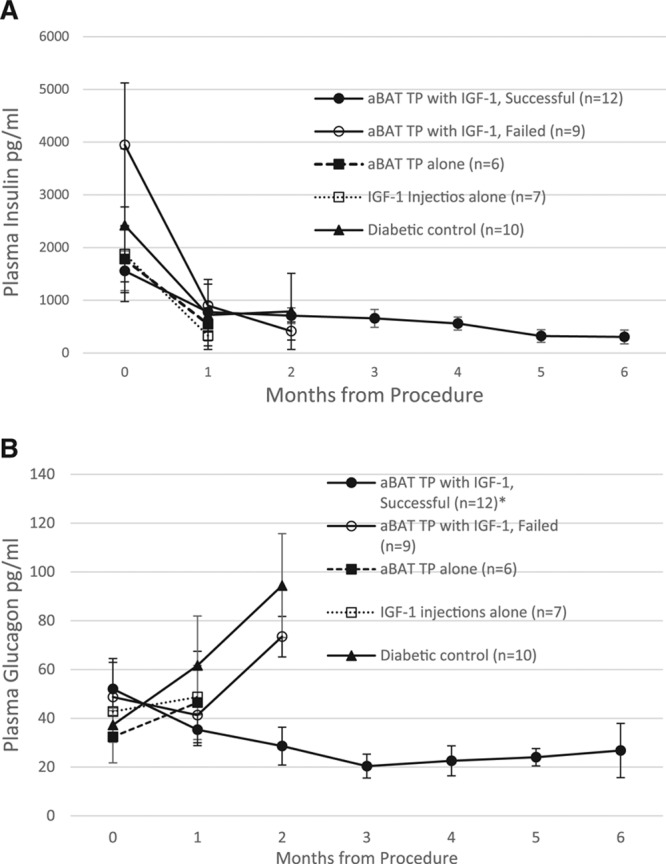
Changes to insulin and glucagon following aBAT TP. A, Plasma insulin levels at monthly intervals in the transplant group in comparison with control groups. Normal nondiabetic controls: 2457.4 ± 571.5 pg/mL. B, Plasma levels of glucagon before and at monthly intervals following transplant, in comparison with control groups. *P < 0.004 when the successful transplant group is compared with diabetic control group at the 2-mo time point. Normal nondiabetic controls: 33.6 ± 6.8 pg/mL. aBAT TP, adult BAT transplant; BAT, brown adipose tissue; IGF-1, insulin like growth factor 1.
FIGURE 3.
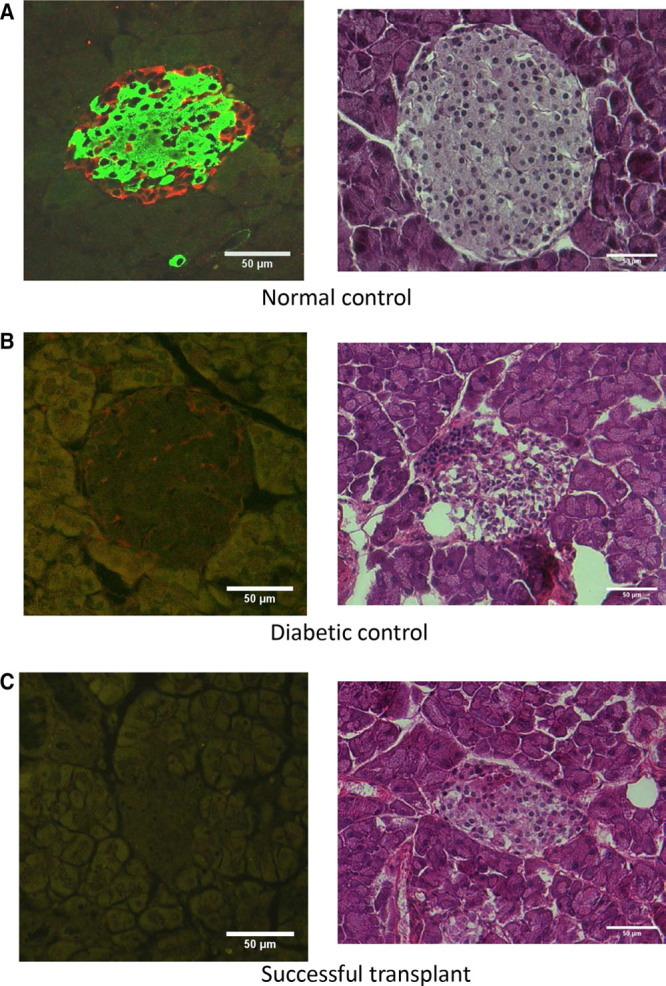
Pancreatic sections immuno-stained for insulin with Alexa-Fluor 488 (green), and for glucagon with Alexa-Fluor 594 (red), with the right column showing corresponding H&E stained sections. A, Normal nondiabetic control. B, Diabetic control treated with saline alone. C, Successful transplant recipient euglycemic at 10-mo.
Euglycemia is also accompanied by suppression of glucagon (Figure 2B) albeit to a lesser degree than previously observed with embryonic BAT transplants. Suppression of glucagon following transplants is further confirmed by the absence of glucagon immunostaining in the pancreas from successful transplant recipients as opposed to normal and diabetic controls (Figure 3, Left panels).
Euglycemia is also associated with increases in extra-pancreatic insulin-mimetic hormones, which may compensate for the lack of insulin. Successful transplant recipients show consistently higher levels of plasma adiponectin and IGF-1 compared with control groups (Figure 4). Euglycemia without insulin occurs only in those situations where suppression of glucagon is accompanied by a simultaneous increase of IGF-1 and adiponectin. Insulin-independence is further confirmed by the lack of insulin-immunostaining in the transplant or surrounding adipose tissue.
FIGURE 4.
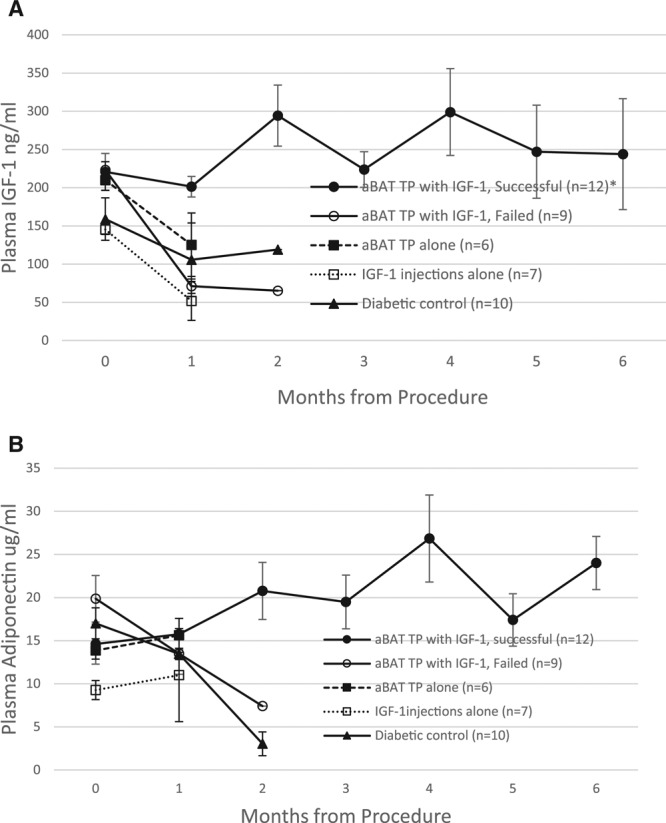
Euglycemia is associated with an increase of extra-pancreatic hormones: plasma levels of IGF-1 (A) and adiponectin (B) at monthly intervals following transplant, in comparison with control groups. Successful transplant recipients (closed circles) show a persistent increase in both hormones in contrast to a decrease observed in the other groups. *P < 0.05 when the successful transplant group is compared with other groups at the 1 mo time point. Normal nondiabetic controls: 16.6 ± 2.5 µg/mL adiponectin and 205.4 ± 61.8 ng/mL IGF-1. aBAT TP, adult BAT transplant; IGF-1, insulin like growth factor 1.
Although IGF-1 is not generally expressed in WAT, euglycemia following embryonic BAT transplants is associated with increasing expression of IGF-1 in newly formed WAT.7,8 Similarly, current data show expression of IGF-1 in the WAT of successful transplant recipients who became euglycemic, compared with little or no expression observed in failed transplants and controls (Figure 5A). Again, even though adipocytes normally show some green autofluoresence, this is distinguishable from the bright immunofluorescence in response to the IGF-1 expression observed in successful transplant recipients. IGF-1 expression in adipose tissue is observed only in successful transplant recipients, and some areas show greater expression than others. Figure 5A top left shows IGF-1 expression over a large area at low magnification, while the top right image shows a brighter area of expression at higher magnification. While adult BAT lacks the IGF-1 expression normally abundant in embryonic BAT (Figure 5B), temporary supplementation with exogenous IGF-1 seems to enable the survival and function of adult BAT transplants. This leads to subsequent expression of IGF-1 in adipose tissue, increased secretion of beneficial adipokines, and insulin-independent glucose homeostasis.
FIGURE 5.
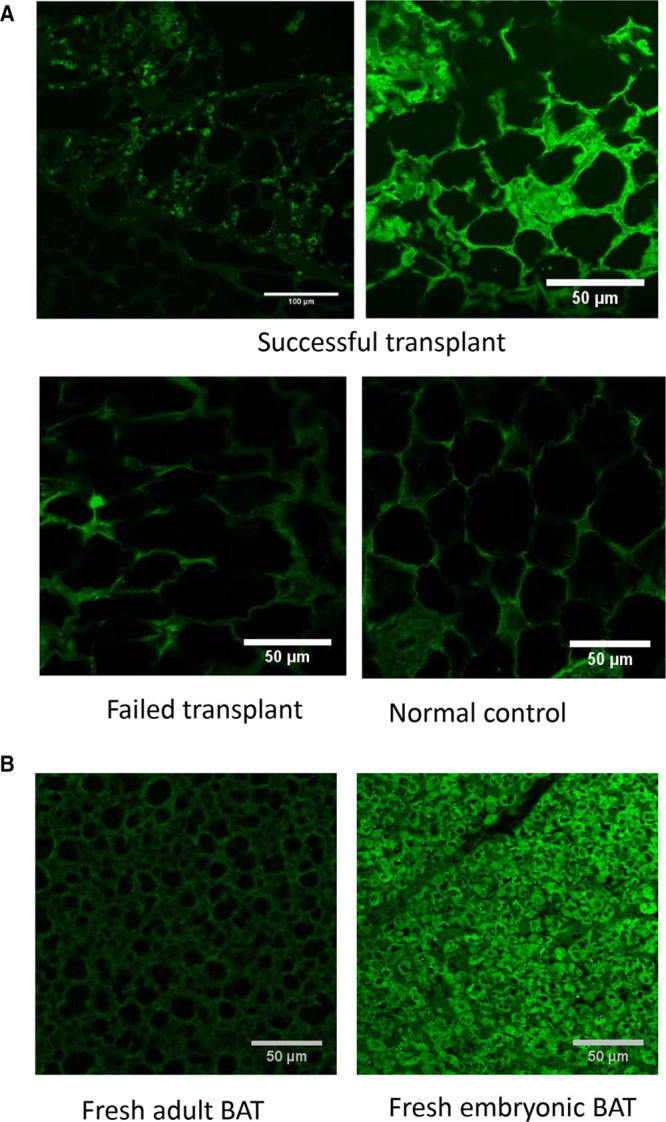
IGF-1 expression in adipose tissue: adipose tissue sections immuno-stained for IGF-1 with Alexa-Fluor 488 (green). A, NOD mice: IGF-1 is expressed in the adipose tissue of successful transplant recipients euglycemic at 10 mo, but not in failed transplants or controls. B, C57BL/6 mice: IGF-1 is expressed in freshly isolated embryonic BAT but not in adult BAT. BAT, brown adipose tissue; IGF-1, insulin like growth factor 1; NOD, non obese diabetic.
DISCUSSION
BAT is well known to improve metabolic regulation and alleviate obesity, effects generally attributed to its thermogenic properties.12-14 However, all of its beneficial effects cannot be explained by thermogenesis alone, particularly considering that reversal of T1D following BAT transplants is associated with a gain of WAT rather than loss. BAT is different from WAT in its developmental origin as well as in function and cellular composition. While WAT originates from mesodermal stem cells, BAT originates from dermatomyotomal precursor cells as does skeletal muscle, and is interchangeable with skeletal muscle during development. Unlike WAT which stores and accumulates fat, BAT is geared towards metabolism of fat and generation of heat, aided by large amounts of mitochondria and uncoupling protein 1. BAT shows greater vascularization and innervation compared with WAT, with small multilocular lipid droplets in brown adipocytes as opposed to the large unilocular droplets found in white adipocytes. WAT can be harmful under certain circumstances such as insulin-resistant obesity, where inflammation, hypertrophy, and necrosis of adipose tissue may cause/exacerbate the metabolic dysfunction. However, situations where BAT and WAT functionally complement each other can produce great benefits including correction of metabolic disease, as evidenced by the insulin-independent reversal of diabetes following BAT-transplants.
As demonstrated in our previous publications, euglycemia following embryonic BAT transplants is associated with generalized decrease of inflammation, regeneration of healthy WAT, suppression of glucagon, and progressive increases of nonpancreatic insulin-mimetic hormones primarily of adipose tissue origin.7,8 It is noteworthy that these results occur only with embryonic BAT. Adult BAT transplants or BAT-derived stem cell lines alone fail to produce insulin-independent euglycemia, as do other embryonic tissue types. Therefore properties of both BAT and embryonic tissue seem to be critical. T1D is associated with inflammation of WAT and loss of healthy functional WAT.11,15 The thermogenicity and increased metabolism characteristic of BAT, and the adipogenic and anti-inflammatory properties unique to embryonic tissue, may together improve the structure and function of WAT in diabetic animals, enabling it to secrete beneficial adipokines that compensate for the lack of insulin. The inability of adult BAT transplants to produce euglycemia is attributable to the lack of such adipogenic and anti-inflammatory growth factors, which are abundantly expressed in embryonic tissue. We hypothesized that exogenous administration of growth factors would correct this problem. Previous data suggest IGF-1 as a likely candidate growth factor.7
The current results confirm a critical role for IGF-1 in insulin-independent euglycemia brought about by BAT transplants. Insulin independence is confirmed by progressively decreasing plasma insulin levels comparable with the diabetic controls, and lack of insulin immunostaining either in pancreatic islets or in adipose tissue. It is noteworthy that IGF-1 alone or adult BAT transplants alone cannot achieve euglycemia, and the presence of both is required. As previously observed with embryonic BAT transplants, adult BAT transplants temporarily supplemented with IGF-1 result in rapid and long-lasting euglycemia in diabetic mice. Euglycemia is accompanied by suppression of glucagon, and increased levels of adiponectin and IGF-1 in plasma. The success rate in reversing diabetes is 50%–60% in both situations. Documenting the exact reasons for lack of a 100% success rate is beyond the scope of this study, considering the many variables and unknowns involved in a treatment that works through altering the whole body metabolism and establishing a new physiological equilibrium. Variables that may be tested and adjusted in a larger scale study include the age of donors, time from isolation of donor tissue to transplantation, site of transplantation, dosage of IGF-1, duration of IGF-1 administration, and possible combination with additional growth factors or anti-inflammatory agents. One consistent observation from all our experiments was that euglycemia occurs only when there is a simultaneous increase in plasma levels of IGF-1 and adiponectin together with suppression of glucagon. Control and failed transplant animals, either adult or embryonic, lack this particular hormonal profile, emphasizing the importance of alternate hormones compensating for the lack of insulin.
The importance of these extra-pancreatic hormones in glucose homeostasis is well documented. Both IGF-1 and adiponectin have beneficial effects on glucose regulation, including some insulin independent mechanisms.43-50 Metabolic disease states such as diabetes, obesity, and insulin resistance are associated with decreased plasma levels of both hormones.51-55 Monotherapy with IGF-1 or adiponectin are reported to alleviate diabetes and insulin resistance,43-50,56 even though such therapies are limited by side effects including hypoglycemia, tachycardia, edema, facial pain, or paralysis etc44,48,56 Side effects are unlikely with BAT transplants, since the increases in plasma adipokine levels are not supraphysiological, merely a return to normal from the decreased levels in T1D.
Recent literature shows the importance of glucagon in the pathogenesis of diabetes.57 The suppression of glucagon normally produced by glucose and insulin is lost in diabetes, and the resultant hyperglucagonemia exacerbates hyperglycemia. Persistent hyperglucagonemia, rather than insulin deficiency, has even been proposed to be the primary driver of hyperglycemia in diabetes. Inhibition of glucagon secretion or glucagon action has been shown to restore euglycemia in diabetic animals, even in the absence of insulin. Thus, suppression of glucagon by itself is a proposed therapeutic approach for diabetes.57 However, pharmacological agents for direct suppression glucagon secretion are not available, and glucagon receptor antagonists are limited by side effects and possible malignant transformation of α cells.58 The physiological equilibrium produced by BAT transplants involving a combination of endogenous insulin-mimetic hormones and suppression of glucagon may well be an alternative long-term solution.
In our previous studies, streptozotocin-treated C57BL/6 mice became euglycemic following BAT transplants, and showed a significant and progressive increase in leptin.8 In diabetic NOD recipients, we did not observe a significant increase of leptin, either with embryonic or adult BAT transplants. Leptin may not be a major player in BAT transplant-mediated glucose regulation, considering that BAT transplants are also associated with decreased inflammation, recovery of WAT, and a moderate weight gain.
Thus, the beneficial effects of embryonic BAT transplants could generally be reproduced by temporary supplementation of adult BAT transplants with exogenous IGF-1. While the tolerance of embryonic BAT allografts is attributable to the immune-privileged nature of embryonic tissue, we found that adult BAT transplants were also well tolerated. Literature shows that, unlike with other organs, rejection is not common with adipose tissue transplants.13-19 Transplanted adult BAT was identifiable by naked eye at 7 months posttransplant in some recipients, at considerably reduced size. In contrast, transplanted embryonic BAT soon becomes indistinguishable from surrounding host tissue and cannot be detected microscopically.7 Development of euglycemia does not seem to require the presence of intact transplanted tissue, but rather, the positive changes imparted to the host adipose tissue such as decrease of inflammation, proliferation of new healthy WAT cells, and secretion of insulin-mimetic adipokines.7
The current results demonstrate the feasibility of establishing long-term euglycemia in diabetic mice using adult BAT transplants, and are consistent with a model where beneficial insulin-mimetic hormones from adipose tissue maintain insulin-independent glucose homeostasis. This effect appears to occur partly through glucagon suppression and partly through direct effects on glucose uptake and metabolism. While the specific underlying mechanisms remain poorly understood, we can make some speculations based on what has been learned so far. Current data suggest that donor-origin IGF-1 is essential for the survival and function of BAT transplants in the early stages. This possibility can be tested by deleting IGF-1 from embryonic BAT transplants and verifying their ability to produce euglycemia. Successful BAT transplants establish a physiological equilibrium with increased adipokines and decreased glucagon, maintaining euglyemia in the absence of insulin. Preliminary data with BAT-conditioned media suggest that secreted peptides from BAT transplant may suppress glucagon secretion from pancreatic islets and stimulate IGF-1 secretion from liver and/or adipocytes. Studies are underway to identify and purify such messenger molecules.
Adult BAT transplants temporarily supplemented with IGF-1 is a plausible therapeutic approach transferable to human patients, that bypasses the complications associated with insulin replacement. Side effects are less likely since this treatment does not involve long-term administration of any exogenous compound, and glucose regulation is brought about by a physiological equilibrium of endogenous insulin-mimetic hormones. While concerns remain regarding the sources and availability of donor adult BAT, possible solutions include the implantation of BAT-derived stem cells or expansion of endogenous BAT with pharmacological agents such as beta-3 adrenergic agonists,59,60 together with temporary administration of IGF-1.
ACKNOWLEDGMENTS
Part of this work was performed by or with the help of the following core facilities: Metabolic Tissue Function Core, Histology Core, and Center for Cellular Imaging at Washington University; Immunomonitoring Laboratory at the Bursky Center for Human Immunology and Immunotherapy Programs at Washington University; Vanderbilt University Hormone Assay Core. Authors are grateful to Dr Peter Bayguinov (Washington University), Dr Zeno Lavagnino (IRCCS Ospedale San Raffaele), and Dr Alessandro Ustione (Washington University) for help with microscopy and image analysis.
Footnotes
Published online 8 October, 2019.
S.C.G. participated in the research design, performance of research, analysis and interpretation of data, and writing of the article. D.W.P. participated in the research design, interpretation of data, and writing of the article.
The authors declare no conflicts of interest.
This work was supported by grants from the Diabetes Research Connection (S.C.G.), Washington University Diabetes Center (DK-020579) (S.C.G.), Iacocca Family Foundation (D.W.P.), and Washington University School of Medicine (D.W.P.).
REFERENCES
- 1.Dholakia S, Oskrochi Y, Easton G, et al. Advances in pancreas transplantation. J R Soc Med 2016109141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caiazzo R, Vantyghem MC, Raverdi V, et al. Impact of procedure-related complications on long-term islet transplantation outcome. Transplantation 201599979–984 [DOI] [PubMed] [Google Scholar]
- 3.Niclauss N, Morel P, Berney T. Has the gap between pancreas and islet transplantation closed? Transplantation 201498593–599 [DOI] [PubMed] [Google Scholar]
- 4.Tekin Z, Garfinkel MR, Chon WJ, et al. Outcomes of pancreatic islet allotransplantation using the edmonton protocol at the University of Chicago. Transplant Direct. 2016;2:e105. doi: 10.1097/TXD.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Adra D, McGilvray I, Goldaracena N, et al. Preserving the pancreas graft: outcomes of surgical repair of duodenal leaks in enterically drained pancreas allografts. Transplant Direct. 2017;3:e179. doi: 10.1097/TXD.0000000000000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frandsen CS, Dejgaard TF, Madsbad S, et al. Non-insulin pharmacological therapies for treating type 1 diabetes. Expert Opin Pharmacother 201819947–960 [DOI] [PubMed] [Google Scholar]
- 7.Gunawardana SC, Piston DW. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab 2015308E1043–E1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 201261674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snell-Bergeon JK, West NA, Mayer-Davis EJ, et al. Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH case-control study. J Clin Endocrinol Metab 2010952868–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verrijn Stuart AA, Schipper HS, Tasdelen I, et al. Altered plasma adipokine levels and in vitro adipocyte differentiation in pediatric type 1 diabetes. J Clin Endocrinol Metab 201297463–472 [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro HQT, Coqueiro AY, Lima VBS, et al. Leucine and resistance training improve hyperglycemia, white adipose tissue loss, and inflammatory parameters in an experimental model of type 1 diabetes. Nutr Health 20182419–27 [DOI] [PubMed] [Google Scholar]
- 12.Sampath SC, Sampath SC, Bredella MA, et al. Imaging of brown adipose tissue: state of the art. Radiology 20162804–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013123215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarroya F, Giralt M. The beneficial effects of brown fat transplantation: further evidence of an endocrine role of brown adipose tissue. Endocrinology 20151562368–2370 [DOI] [PubMed] [Google Scholar]
- 15.Bahr J, Klöting N, Klöting I, et al. Transplantation of adipose tissue protects BB/OK rats from type 1 diabetes development. Transpl Immunol 201124238–240 [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014156304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster MT, Softic S, Caldwell J, et al. Subcutaneous adipose tissue transplantation in diet-induced obese mice attenuates metabolic dysregulation while removal exacerbates it. Physiol Rep. 2013;1:e00015. doi: 10.1002/phy2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klebanov S, Astle CM, DeSimone O, et al. Adipose tissue transplantation protects ob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. J Endocrinol 2005186203–211 [DOI] [PubMed] [Google Scholar]
- 19.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 20106195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mur C, Arribas M, Benito M, et al. Essential role of insulin-like growth factor I receptor in insulin-induced fetal brown adipocyte differentiation. Endocrinology 2003144581–593 [DOI] [PubMed] [Google Scholar]
- 21.Ohta H, Itoh N. Roles of FGFs as adipokines in adipose tissue development, remodeling, and metabolism. Front Endocrinol (Lausanne) 2014;5:18. doi: 10.3389/fendo.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh N. Hormone-like (endocrine) FGFs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res 20103421–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer S, Santos AN, Thieme R, et al. Adiponectin stimulates glucose uptake in rabbit blastocysts. Biol Reprod 201083859–865 [DOI] [PubMed] [Google Scholar]
- 24.Kim ST, Marquard K, Stephens S, et al. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum Reprod 20112682–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmerson E, Campbell L, Davies FC, et al. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: new insights into cutaneous IGF-1R/erα cross talk. J Invest Dermatol 20121322838–2848 [DOI] [PubMed] [Google Scholar]
- 26.Falcão-Pires I, Castro-Chaves P, Miranda-Silva D, et al. Physiological, pathological and potential therapeutic roles of adipokines. Drug Discov Today 201217880–889 [DOI] [PubMed] [Google Scholar]
- 27.Hansen-Pupp I, Hellström-Westas L, Cilio CM, et al. Inflammation at birth and the insulin-like growth factor system in very preterm infants. Acta Paediatr 200796830–836 [DOI] [PubMed] [Google Scholar]
- 28.Holly J, Sabin M, Perks C, et al. Adipogenesis and IGF-1. Metab Syndr Relat Disord 2006443–50 [DOI] [PubMed] [Google Scholar]
- 29.Iglesias P, Selgas R, Romero S, et al. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur J Endocrinol 2012167301–309 [DOI] [PubMed] [Google Scholar]
- 30.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 20111185–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanders D, Graff EC, White BD, et al. Niacin increases adiponectin and decreases adipose tissue inflammation in high fat diet-fed mice. Plos One. 2013;8:e71285. doi: 10.1371/journal.pone.0071285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, Xing C, Pan Y, et al. IGF-1 alleviates ox-LDL-induced inflammation via reducing HMGB1 release in haecs. Acta Biochim Biophys Sin (Shanghai) 201244746–751 [DOI] [PubMed] [Google Scholar]
- 33.Zhao P, Deng Y, Gu P, et al. Insulin-like growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroid-associated ophthalmopathy. Exp Eye Res 201310765–73 [DOI] [PubMed] [Google Scholar]
- 34.Baumann MU, Schneider H, Malek A, et al. Regulation of human trophoblast GLUT1 glucose transporter by insulin-like growth factor I (IGF-I). Plos One. 2014;9:e106037. doi: 10.1371/journal.pone.0106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copland JA, Pardini AW, Wood TG, et al. IGF-1 controls GLUT3 expression in muscle via the transcriptional factor Sp1. Biochim Biophys Acta 20071769631–640 [DOI] [PubMed] [Google Scholar]
- 36.Fladeby C, Skar R, Serck-Hanssen G. Distinct regulation of glucose transport and GLUT1/GLUT3 transporters by glucose deprivation and IGF-I in chromaffin cells. Biochim Biophys Acta 20031593201–208 [DOI] [PubMed] [Google Scholar]
- 37.Ge X, Chen C, Hui X, et al. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/ets-like protein-1 in adipocytes. J Biol Chem 201128634533–34541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mashili FL, Austin RL, Deshmukh AS, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 201127286–297 [DOI] [PubMed] [Google Scholar]
- 39.Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic β cells and adipocytes. Best Pract Res Clin Endocrinol Metab 20142843–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem 201134833–42 [DOI] [PubMed] [Google Scholar]
- 41.Hansen BF, Glendorf T, Hegelund AC, et al. Molecular characterisation of long-acting insulin analogues in comparison with human insulin, IGF-1 and insulin X10. Plos One. 2012;7:e34274. doi: 10.1371/journal.pone.0034274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab 20073302–310 [DOI] [PubMed] [Google Scholar]
- 43.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:E1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am 201241425–43, vii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunger D, Yuen K, Ong K. Insulin-like growth factor I and impaired glucose tolerance. Horm Res 200462Suppl 1101–107 [DOI] [PubMed] [Google Scholar]
- 46.Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. 2014;13:103. doi: 10.1186/1475-2840-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald A, Williams RM, Regan FM, et al. IGF-I treatment of insulin resistance. Eur J Endocrinol 2007157Suppl 1S51–S56 [DOI] [PubMed] [Google Scholar]
- 48.Quattrin T, Thrailkill K, Baker L, et al. ; rhIGF-I in IDDM Study Group Improvement of HbA1c without increased hypoglycemia in adolescents and young adults with type 1 diabetes mellitus treated with recombinant human insulin-like growth factor-I and insulin. rhIGF-I in IDDM study group. J Pediatr Endocrinol Metab 200114267–277 [DOI] [PubMed] [Google Scholar]
- 49.Quattrin T, Thrailkill K, Baker L, et al. Dual hormonal replacement with insulin and recombinant human insulin-like growth factor I in IDDM. Effects on glycemic control, IGF-I levels, and safety profile. Diabetes Care 199720374–380 [DOI] [PubMed] [Google Scholar]
- 50.Zenobi PD, Glatz Y, Keller A, et al. Beneficial metabolic effects of insulin-like growth factor I in patients with severe insulin-resistant diabetes type A. Eur J Endocrinol 1994131251–257 [DOI] [PubMed] [Google Scholar]
- 51.Chisalita SI, Ludvigsson J. Insulin-like growth factor-1 at diagnosis and during subsequent years in adolescents with type 1 diabetes. J Diabetes Res. 2018;2018:8623560. doi: 10.1155/2018/8623560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siddiqui MB, Patel S, Arshad T, et al. The relationship between hypoadiponectinemia and cardiovascular events in liver transplant recipients. Transplantation. 2019 doi: 10.1097/TP.0000000000002714. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics 200835321–326 [DOI] [PubMed] [Google Scholar]
- 54.Suda K, Matsumoto R, Fukuoka H, et al. The influence of type 2 diabetes on serum GH and IGF-I levels in hospitalized Japanese patients. Growth Horm IGF Res 2016294–10 [DOI] [PubMed] [Google Scholar]
- 55.Worda C, Leipold H, Gruber C, et al. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol 200419162120–2124 [DOI] [PubMed] [Google Scholar]
- 56.Thrailkill KM, Quattrin T, Baker L, et al. Cotherapy with recombinant human insulin-like growth factor I and insulin improves glycemic control in type 1 diabetes. RhIGF-I in IDDM study group. Diabetes Care 199922585–592 [DOI] [PubMed] [Google Scholar]
- 57.Hughes JW, Ustione A, Lavagnino Z, et al. Regulation of islet glucagon secretion: beyond calcium. Diabetes Obes Metab 201820Suppl 2127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearson MJ, Unger RH, Holland WL. Clinical trials, triumphs, and tribulations of glucagon receptor antagonists. Diabetes Care 2016391075–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broeders E, Bouvy ND, van Marken Lichtenbelt WD. Endogenous ways to stimulate brown adipose tissue in humans. Ann Med 201547123–132 [DOI] [PubMed] [Google Scholar]
- 60.Cypess AM, Weiner LS, Roberts-Toler C, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 20152133–38 [DOI] [PMC free article] [PubMed] [Google Scholar]


