Unidirectional cytoplasmic incompatibility (CI) results in a postfertilization incompatibility between Wolbachia-infected males and uninfected females. CI contributes to reproductive isolation between closely related species and is used in worldwide vector control programs to drastically lower arboviral vector population sizes or to replace populations that transmit arboviruses with those resistant to transmission. Despite decades of research on the factors that influence CI, penetrance is often variable under controlled laboratory conditions in various arthropods, suggesting that additional variables influence CI strength. Here, we demonstrate that paternal D. melanogaster grandmother age influences the strength of CI induced by their sons. Older D. melanogaster females have higher Wolbachia densities and produce offspring with higher Wolbachia densities that associate with stronger CI. This work reveals a multigenerational impact of age on CI and expands our understanding of host-Wolbachia interactions and the biology of CI induced by the Wolbachia strain infecting the most widely used arthropod model, D. melanogaster.
KEYWORDS: Wolbachia, Drosophila melanogaster, cytoplasmic incompatibility, maternal transmission
ABSTRACT
Wolbachia are obligate intracellular bacteria that are globally distributed in half of all arthropod species. As the most abundant maternally inherited microbe in animals, Wolbachia manipulate host reproduction via reproductive parasitism strategies, including cytoplasmic incompatibility (CI). CI manifests as embryonic death when Wolbachia-modified sperm fertilize uninfected eggs but not maternally infected eggs. Thus, CI can provide a relative fitness advantage to Wolbachia-infected females and drive the infection through a population. In the genetic model Drosophila melanogaster, the Wolbachia strain wMel induces variable CI, making mechanistic studies in D. melanogaster cumbersome. Here, we demonstrate that sons of older paternal D. melanogaster grandmothers induce stronger CI than sons of younger paternal grandmothers, and we term this relationship the “paternal grandmother age effect” (PGAE). Moreover, the embryos and adult sons of older D. melanogaster grandmothers have higher Wolbachia densities, correlating with their ability to induce stronger CI. In addition, we report that Wolbachia density positively correlates with female age and decreases after mating, suggesting that females transmit Wolbachia loads that are proportional to their own titers. These findings reveal a transgenerational impact of age on wMel-induced CI, elucidate Wolbachia density dynamics in D. melanogaster, and provide a methodological advance to studies aimed at understanding wMel-induced CI in the D. melanogaster model.
INTRODUCTION
Wolbachia are obligate intracellular bacteria that infect 40% to 65% of arthropod species (1–3) and 37% of the members of the Onchocercidae family of filarial nematodes (4). These bacteria are maternally transmitted from ova to offspring (5) and often cause cytoplasmic incompatibility (CI) to selfishly increase their transmission through the matriline (6–10). CI manifests as embryonic death when Wolbachia-modified sperm fertilize uninfected eggs but not when they fertilize infected eggs (11–13). Thus, infected transmitting females have a fitness advantage relative to their uninfected counterparts that leads to the spread of Wolbachia through host populations (6–10). Additionally, since CI reduces gene flow between Wolbachia-infected and uninfected populations or populations with different Wolbachia strains, it is associated with reproductive isolation and incipient speciation (14, 15).
Global vector control efforts have successfully leveraged CI to either suppress native populations (16–19) or promote the spread of disease-resistant Wolbachia strains (20–22) specifically through release of mosquitoes transinfected with the wMel Wolbachia strain of Drosophila melanogaster. wMel's success in these efforts is partially due to the strong CI that it induces in mosquito hosts (23, 24); however, in the native host D. melanogaster, wMel’s CI strength can range from an average of nearly 0% (no CI) to 100% (complete CI) (25–32). There are numerous factors reported to impact the penetrance of wMel-induced CI: Wolbachia density in the testes (25, 33), expression level of the CI genes cifA and cifB (29, 34), male age (30), male mating rate (30, 35), time of male emergence (32), fly rearing density (32), and temperature (30). However, these factors are not independent, and they have likely hampered the researcher's ability to use the vast resources of D. melanogaster for the study of reproductive parasitism and endosymbiosis. For example, CI strength rapidly decreases with male age (30), which also cocorrelates with cifA and cifB gene expression (29) and Wolbachia density in the testes (33).
Despite control of male age, time of emergence, rearing density, and temperature, we continued to see various levels of CI strength in our laboratory, suggesting that additional factors are involved. This variation in phenotype makes wMel in D. melanogaster difficult to study despite the fly’s extensive history as a powerful animal model. However, anecdotal observations in our laboratory suggested that stronger CI was induced in embryos when their infected paternal grandmothers were significantly aged before mating. Here, we used hatch rate analyses to formally test the hypothesis that paternal grandmother age influences the strength of CI induced by her sons. We also measured the effect of age and virginity on female Wolbachia titers and assessed whether females with higher Wolbachia titers deposited more Wolbachia into their progeny. Our results reveal a “paternal grandmother age effect” (PGAE) on CI strength, where older grandmothers produce males that induce stronger CI. We also characterize transgenerational Wolbachia density dynamics that correlate with CI penetrance. This work enhances our understanding of Wolbachia-host dynamics and provides methodological techniques of importance to studies of wMel-induced CI in D. melanogaster.
RESULTS
To test the hypothesis that D. melanogaster paternal grandmother age influences the strength of CI, we measured the percentage of surviving offspring produced by sons of differentially aged, infected y1w* grandmothers. CI strength increased with grandmother age when uninfected females were mated to infected sons of 2-, 5-, 11-, 14-, and 18-day-old grandmothers (Fig. 1). Sons of 2-day-old grandmothers produced statistically weaker CI than those of either 14-day-old (P = 0.0031) or 18-day-old (P = 0.0005) grandmothers, and the same was true for sons of 5-day-old grandmothers compared to those of either 14-day-old (P = 0.0095) or 18-day-old (P = 0.0018) grandmothers. Importantly, sons of 11-day-old uninfected grandmothers produced high hatch rates (Fig. 1), suggesting that the reduction in hatch rate in the remaining crosses was not associated with further aging of the flies. Together, these data suggest that CI is strongest in sons of older grandmothers (Fig. 1).
FIG 1.
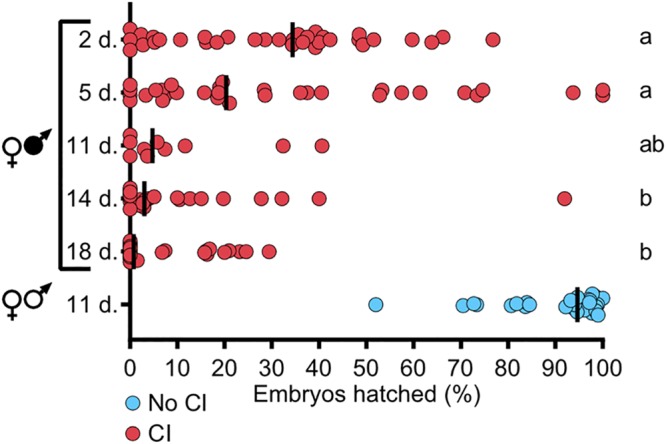
Paternal grandmother age effect impacts CI strength. Hatch rate assays were conducted with either uninfected y1w* males derived from uninfected females aged 11 days (d.) before mating or infected y1w* males derived from infected females aged 2, 5, 11, 14, or 18 days. Wolbachia infections are represented by filled sex symbols, and the age of the paternal grandmother is shown immediately to the left of the y axis. Each dot represents a replicate of offspring from single-pair matings. Vertical bars represent medians, and letters to the right indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all CI crosses. All statistical values are presented in Table S1 in the supplemental material.
P values associated with all statistical comparisons corresponding to the main and extended bodies of data presented in the figures. Download Table S1, DOCX file, 0.01 MB (11.2KB, docx) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Next, we tested whether the increase in embryonic death with D. melanogaster grandmother age indeed represented CI and not some other transgenerational embryonic defect. In accordance with prior results (Fig. 1), there was an overall trend indicating that older grandmothers produced sons that induced stronger CI. Indeed, sons derived from 2-day-old infected grandmothers induced statistically weaker CI than sons of 11-day-old (P = 0.0008) and 14-day-old (P = 0.0110) grandmothers (see Fig. S1 in the supplemental material). Sons of 11-day-old grandmothers produced a lower median hatch rate than sons of 14-day-old grandmothers; however, the differences were not statistically significant (P > 0.9999). As expected for CI rescue, high rates of embryonic hatching were observed when infected females were mated to sons of infected 2-, 5-, 11-, and 14-day-old grandmothers and the rates did not differ significantly between groups (Fig. S1; P = 0.3705). Together, these results suggest that the PGAE is not attributable to other transgenerational, age-associated defects.
Paternal D. melanogaster grandmother age does not impact hatching in non-CI crosses. Hatch rate assays were conducted with uninfected or infected y1w* males derived from females aged 2, 5, 11, or 14 days (d.) followed by mating crossed to either infected or uninfected y1w* females. Each dot represents a replicate of offspring from single-pair matings. Wolbachia infections are represented by filled sex symbols, and the age of the paternal grandmother is shown immediately to the left of the y axis. Vertical bars represent medians, and letters to the right indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between crosses (in brackets). All statistical values are presented in Table S1. Download FIG S1, TIF file, 0.2 MB (198KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To test if the PGAE is specific to the y1w* strain, these experiments were repeated in a nos-GAL4-tubulin genetic background. The nos-GAL4-tubulin line was chosen because it was previously used to identify the cifA and cifB genes that underpin wMel-induced CI (29). The 2-, 5-, and 11-day time points were selected because they had demonstrated the greatest differences in hatch rate in the previous experiments. As predicted, CI strength correlated with the age of paternal grandmothers when uninfected nos-GAL4-tubulin females were mated to infected sons of 2-, 5-, and 11-day-old nos-GAL4-tubulin grandmothers (Fig. S2). Sons of 11-day-old grandmothers induced significantly stronger CI than sons of 2-day-old grandmothers (P = 0.0033; Fig. S2), suggesting that the PGAE is not specific to y1w* flies. When sons of uninfected grandmothers aged 2, 5, or 11 days were mated to uninfected females, there were no statistically significant differences in hatching rates across all three groups (P = 0.3907; Fig. S2), indicating that the PGAE is CI associated in nos-GAL4-tubulin flies as seen with y1w* flies.
The paternal D. melanogaster grandmother age effect is not specific to y1w* flies. Hatch rates assays were conducted with uninfected or infected nos-GAL4-tubulin males derived from females aged 2, 5, or 11 days (d.) followed by mating crossed to uninfected nos-GAL4-tubulin females. Each dot represents a replicate of offspring from single-pair matings. Wolbachia infections are represented by filled sex symbols, and the age of the paternal grandmother is shown immediately to the left of the y axis. Vertical bars represent medians, and letters to the right indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between crosses (in brackets). All statistical values are presented in Table S1. Download FIG S2, TIF file, 0.2 MB (188.4KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Since Wolbachia densities are positively associated with CI strength (25, 36–38), we then tested the hypothesis that infected sons derived from older D. melanogaster grandmothers have higher Wolbachia densities than infected sons from younger grandmothers. We did so by measuring the abundance of the single-copy Wolbachia groEL gene relative to that of the Drosophila rp49 housekeeping gene. Abdomen samples were taken from virgin male siblings of those used in the hatch rate experiment represented in Fig. 1. As predicted, Wolbachia densities in male abdomens positively correlated with paternal grandmother age, and sons of 18-day-old grandmothers had significantly higher Wolbachia densities than sons of 2-day-old grandmothers (P = 0.0450) (Fig. 2A). However, no significant differences were observed between sons of 5-, 11-, or 14-day-old grandmothers relative to any other group, presumably due to the variable penetrance of CI, low sample sizes, or biological reasons proposed in the Discussion. Taken together, these data suggest that older grandmothers produced sons with higher Wolbachia titers, which allowed the sons to induce stronger CI, though this density effect was weak relative to the effect that we see for CI.
FIG 2.
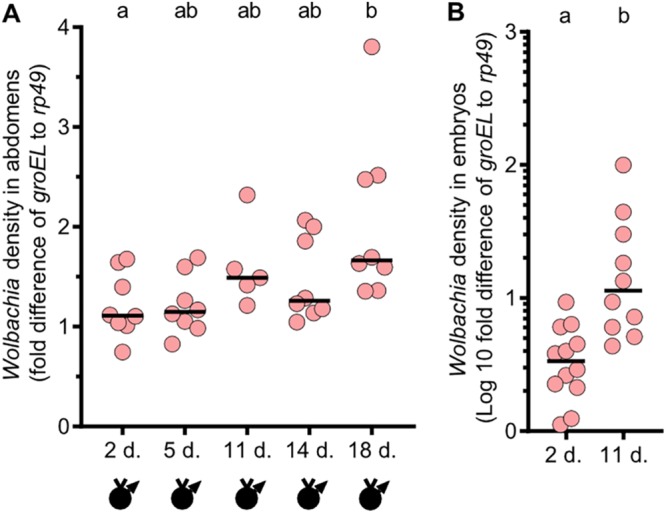
Wolbachia densities are highest in sons and embryos of older D. melanogaster grandmothers. (A) Wolbachia density assays were conducted with virgin females (indicated by a “v” above a sex symbol) and with infected y1w* males derived from grandmothers aged 2, 5, 11, 14, or 18 days (d.). Wolbachia infections are represented by filled sex symbols, and the age of the grandmother is shown immediately below the x axis. The samples analyzed were from abdomens of siblings of fathers corresponding to the hatch rate data in Fig. 1. (B) Wolbachia density assays were conducted with pools of 50 1-to-2-h-old embryos collected from 2-and-11-day-old grandmothers. The sex of the embryos was unknown since it cannot be determined visually. Wolbachia titers were lower in adults, requiring a standard linear scale (A), but higher in embryos, requiring a common logarithmic scale (B). Each dot represents the average of results from triplicate technical replicates for panel A and duplicates for panel B. Horizontal bars indicate medians, and the letters above the bars indicate significant differences based on α = 0.05 calculated by Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all groups (A) or by a Mann Whitney U test (B). All statistical values are presented in Table S1. Fold differences in Wolbachia densities (groEL) relative to D. melanogaster reference gene rp49 were determined with 2−ΔΔCT.
Next, we tested the hypothesis that embryos from older D. melanogaster grandmothers had higher Wolbachia titers than those from younger grandmothers. Wolbachia densities were measured in 0-to-1-h-old embryos produced by both 2-day-old and 11-day-old grandmothers (Fig. 2B). The 2-day and 11-day time points were chosen because they exhibited the greatest differences in CI strength over the shortest time interval. Here, embryos produced by 11-day-old grandmothers had significantly higher Wolbachia densities than embryos from 2-day-old grandmothers (P = 0.0006) (Fig. 2B). Thus, these data indicate that older females produce embryos with higher Wolbachia titers.
Finally, this led to the hypothesis that older D. melanogaster grandmothers have higher Wolbachia densities than younger grandmothers and that they transfer more Wolbachia to their offspring. Supporting this hypothesis, Wolbachia densities were significantly higher in the ovaries of 11-day-old virgin females than in those of 2-day-old virgin females (P = 0.0045) (Fig. 3). Additionally, we predicted that Wolbachia densities would decrease in ovaries after egg-laying if grandmothers loaded Wolbachia into their offspring. As such, we measured Wolbachia densities in ovaries of mated grandmothers that laid eggs in the embryo density study described previously. We found that ovaries from mated 11-day-old females had significantly less Wolbachia than virgin 11-day-old females (P = 0.0240) (Fig. 3). Likewise, mated 2-day-old females had lower Wolbachia titers than virgin 2-day-old females, though the differences were not significant (P = 0.0882) (Fig. 3). Despite the overall decrease in relative Wolbachia densities after mating, ovaries from 11-day-old mated grandmothers had significantly higher densities than ovaries from 2-day-old mated grandmothers (P = 0.0087). Importantly, threshold cycle (CT) values remained consistent across age and virginity states for the Drosophila rp49 gene, suggesting that changes in the Wolbachia groEL gene, rather than in rp49 copy number, were responsible for the density dynamics that we report here (Fig. S3).
FIG 3.
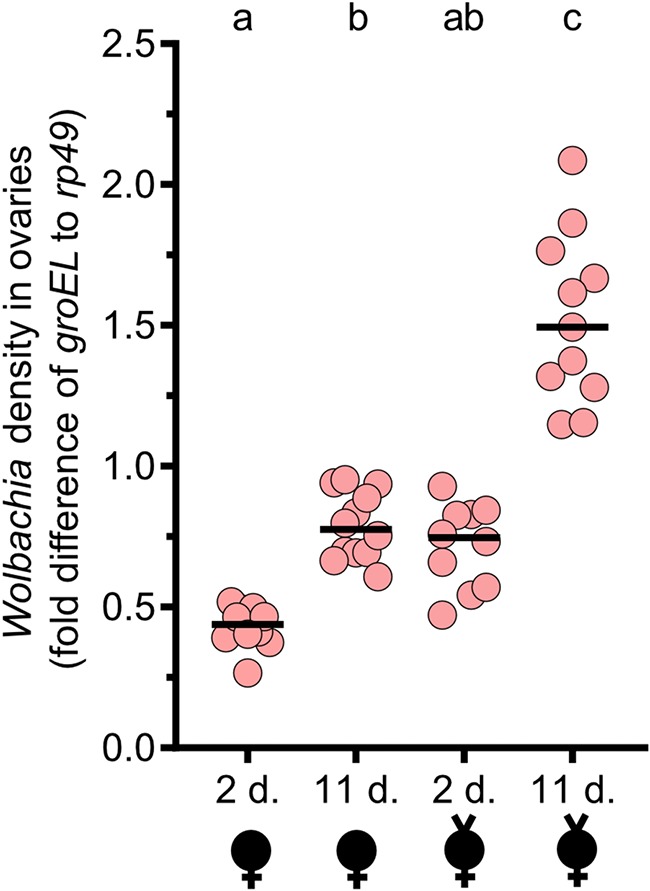
Wolbachia densities increase with female age in ovaries and decrease after mating. Wolbachia density assays were conducted with pools of 4 ovaries from virgin females (indicated by a “v” above a sex symbol) and nonvirgin females aged 2 or 11 days (d.). Wolbachia infections are represented by filled sex symbols, and the age of the sample is shown immediately below the x axis. Virgin and nonvirgin females were siblings. The nonvirgin females produced the embryos whose results are shown in Fig. 2B. Nonvirgin females were allowed to mate and lay for 48 h before ovary dissections. Nonvirgin and virgin females were incubated for that same period of time and dissected in parallel. Each dot represents the average of duplicate values. Horizontal bars indicate medians, and the letters above the bars indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all groups. All statistical values are presented in Table S1. Fold differences in Wolbachia density (groEL) relative to D. melanogaster reference gene rp49 were determined with 2−ΔΔCT.
rp49 CT values remain consistent across age and virginity states, whereas groEL fluctuates. CT values are from the Wolbachia density analysis described in the Fig. 3 legend. Wolbachia density assays were conducted with pools of 4 ovaries from virgin females (indicated by a “v” above a sex symbol) and nonvirgin females aged 2 or 11 days (d.). Wolbachia infections are represented by filled sex symbols, and the age of the sample is shown to the left of the sex symbol. Virgin and nonvirgin females were siblings. The nonvirgin females produced the embryos represented in Fig. 2B. Nonvirgin females were allowed to mate and lay for 48 h before ovary dissections were performed. Nonvirgin and virgin females were incubated for the same period of time and dissected in parallel. Each dot represents the average of duplicate values. Vertical bars indicate medians, and the letters above the bars indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all virgin females and mated females (separated by a horizontal dotted line). All statistical values are presented in Table S1. Download FIG S3, TIF file, 0.2 MB (176.3KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Similar results can be observed in measuring Wolbachia densities in abdomens instead of in ovaries (Fig. S4). Measuring abdominal titers, 11-day-old virgin females had statistically higher Wolbachia densities than 2-day-old virgin females (P < 0.0001). There was a detectable trend indicating that the mated females had less Wolbachia, though neither mated 11-day-old females (P = 0.2291) nor mated 2-day-old females (P > 0.9999) had titers significantly different from those of their virgin counterparts (Fig. S4). The titers in 11-day-old and 2-day-old mated females were not significantly different (P > 0.9999). Taken together, these data suggest that females accumulate Wolbachia as they age, that older females transfer more Wolbachia to their offspring, and that sons of older females induce stronger CI. Moreover, laying eggs appears to quickly reduce the amount of Wolbachia contained in the ovaries, suggesting that the PGAE is strongest soon after initial mating.
Wolbachia densities increase with female age in abdomens and decrease after mating. Wolbachia density assays were conducted with single abdomens from virgin females (indicated by a “v” above a sex symbol) and nonvirgin females aged 2 or 11 days (d.). Wolbachia infections are represented by filled sex symbols, and the age of the sample is shown immediately below the x axis. Virgin and nonvirgin females were siblings. The nonvirgin females produced the fathers corresponding to the hatch rate data in Fig. 1 and the males whose data are shown in Fig. 2A. Nonvirgin females were allowed to mate and lay for 48 h before abdomen dissections were performed. Nonvirgin and virgin females were incubated for that same period of time and dissected in parallel. Each dot represents the average of triplicate values. Horizontal bars indicate medians, and the letters above the bars indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all groups. All statistical values are presented in Table S1. Fold differences in Wolbachia density (groEL) relative to D. melanogaster reference gene rp49 were determined with 2−ΔΔCT. Download FIG S4, TIF file, 0.2 MB (249.6KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
D. melanogaster is a valued model system in studies of Wolbachia-host interactions due to its genetic tractability and the importance of its native Wolbachia strain, wMel, in vector control (39). However, the study of wMel-induced CI in D. melanogaster is inhibited by its variable penetrance, ranging from nearly complete embryonic death to none at all (26–32). Some phenotypic variation persists despite control of known variables of CI strength, leading to the hypothesis that as-yet-unknown factors contribute to CI variability. Anecdotal observations in our laboratory suggested that stronger CI may be induced by offspring of older virgin females, leading to the formal hypothesis that variation in CI penetrance is partly controlled by a paternal grandmother age effect (PGAE).
Here, we report evidence in support of the PGAE, namely, that sons of older D. melanogaster grandmothers induce stronger CI than sons of younger grandmothers. Paternal grandmother age did not influence the ability of CI to be rescued, suggesting that no other age-associated transgenerational deficiencies contributed to the increased embryonic death. Additionally, we found that embryos of older grandmothers had higher Wolbachia densities than the offspring of younger grandmothers. Likewise, older virgin females had more Wolbachia than younger virgin females. As such, the data support a model whereby PGAE is caused by an accumulation of Wolbachia in a virgin as she ages, leading to an increase in levels of Wolbachia passed on to her sons, who induce stronger CI in their offspring than sons of younger grandmothers.
In this study, we measured Wolbachia densities by comparing the number of Wolbachia groEL gene copies to the number of Drosophila rp49 gene copies. Note that we cannot make direct claims about the density of Wolbachia per host cell based on these analyses, since doing so would assume that the number of host cells and host ploidy remain constant. Recent work has highlighted that a protein-enriched diet can influence relative estimates of Wolbachia density analysis in D. melanogaster by increasing ovary size and rp49 copy number (40). While age and mating state may be hypothesized to influence rp49 copy number, rp49 CT values remained constant across female age and mating states whereas groEL CT values changed (see Fig. S3 in the supplemental material). These data suggest that despite possible fluctuations in rp49 copy number across cell types within ovaries, the average rp49 copy number remains consistent across the extracted tissue samples. As such, we conclude that changes in Wolbachia groEL copy number, not rp49 copy number, underpin the results. However, future work will be necessary to describe how these density estimates explicitly relate to Wolbachia titers per host cell and across cell types in these tissues.
In addition to the PGAE, CI variation has previously been attributed to a “younger-brother effect” where the slowest-developing males, from a clutch of embryos within the 0-to-5-h age range, induced the weakest CI (32). If embryo deposition order correlates with maturation rate, then the younger-brother effect is at least in part explained by our findings that (i) Wolbachia densities in ovaries quickly decrease after mating and egg laying, (ii) the Wolbachia density in embryos correlates with ovary densities, and (iii) sons from eggs laid by mothers with lower Wolbachia densities induce weaker CI. As such, when a D. melanogaster female lays eggs, the amount of Wolbachia in her ovaries may be sequentially depleted after each embryo is produced. Thus, younger brothers that take longer to develop may receive fewer Wolbachia and then induce a weaker CI than their older counterparts that originally had received more Wolbachia. Therefore, the dynamics of the interaction that we observed between CI induction and Wolbachia densities across generations may explain the younger-brother effect, although this remains to be precisely established in future research.
Additionally, this paper adds to a growing body of literature reporting an influence of female insect age on Wolbachia densities. Indeed, older females harbor higher Wolbachia titers in wAlbA- and wAlbB-infected Aedes albopictus (41, 42), wVulC-infected Armadillidium vulgare (43), and wStri-infected Laodelphax striatellus (44). The relationship between paternal grandmother age and the strength of wMel-induced CI was explored once before; however, no relationship was found (32). Crucially, the virginity status of the grandmothers differs between the cited study and the one presented here and may in part explain the discrepancy. Our study maintained the virginity of all grandmothers as they aged, and grandmothers were allowed only 24 h of mating prior to egg deposition for hatch rate analysis. In contrast, the grandmothers in the prior study remained virgin until 3 days old and were then allowed to continuously mate until they were 11 days old, and the CI levels from sons produced at each of the two time points were compared (32). Our results suggest that mating has a detectable impact on Wolbachia densities and may explain why the PGAE was not observed in the earlier study. Additionally, we predict that the PGAE most strongly applies to aged virgins, since mating significantly reduced Wolbachia densities in our study.
The depletion of Wolbachia found in females following egg laying supports the hypothesis that the PGAE is caused by an effect of maternal loading of Wolbachia into her sons. However, the source of that loading is still unclear. In D. melanogaster, the following four sources of Wolbachia transfer to progeny are known: bacteriocyte-like cells (BLCs), germ line stem cells (GSCs), the somatic stem cell niche (SSCN), and late-stage oogenesis (5, 38, 45–48). BLCs found at the tip of the ovarioles are densely packed with Wolbachia and are predicted to transfer Wolbachia to GSCs (47). When a GSC asymmetrically undergoes mitosis (49, 50), its population of Wolbachia is divided between two daughter cells, one of which is an identical GSC that remains in the ovaries and the other a differentiating cytoblast that develops into the egg (5). Therefore, it is possible that the levels of Wolbachia allocated to the daughter cytoblast (and thus the offspring) are proportional to the densities in the parent GSC or the surrounding BLCs. Additionally, as the cytoblast develops into a germ line cyst, it comes into contact with the highly infected SSCN, acquiring additional Wolbachia (45, 46, 48). Finally, while Wolbachia replication in the oocyte occurs primarily at the beginning of oogenesis in wMel-infected D. melanogaster and halts at the onset of vitellogenesis, it can resume at a lower rate before egg laying in late-stage oogenesis (38). As such, prolonged retention of eggs in aged virgins may lead to an accumulation of Wolbachia in these developed oocytes. We hypothesize that Wolbachia replicate in the BLCs, GSCs, SSCNs, or late-stage oocytes as a mother ages, resulting in eggs with relatively high titers. Since eggs account for the greatest proportion of Wolbachia cells in the ovaries, this hypothesis could explain why titers are depleted after mating and egg laying.
Intriguingly, differences in CI strength more closely correlated with Wolbachia densities in embryos than with densities in adult males. CI is hypothesized to be caused by cif gene modifications of sperm-associated host products (51–58) or to be a consequence of loading of toxins into the sperm (52, 53, 59, 60); however, Wolbachia are stripped from the sperm during individualization (37, 61, 62). Therefore, Wolbachia titers are likely more important during a specific stage of spermatogenesis than at the time of CI induction. In D. melanogaster, spermatogenesis is a continuous process lasting approximately 11 days (63). As such, there may be a lag of several days between the time that sperm are subjected to the actions of cifA and cifB gene products and the time of CI induction. Spermatogenesis begins during larval development (63) and continues throughout the adult life span (64), though the first batches of mature sperm are produced soon after adult hatching (65). Since the males in our study were mated shortly after adult hatching, the majority of their sperm would have started spermatogenesis at a time closer to embryonic deposition than adult hatching, which may explain why CI strength correlates better with Wolbachia densities in embryos than in adult males. Additionally, spermatogenesis may incorporate and eliminate Wolbachia faster than they can multiply, resulting in the reduction and equalization of titers in adults (37). This may explain why some studies, including studies analyzing the younger-brother effect, found that CI strength did not always correlate with Wolbachia densities in adults (25, 32, 66). As such, we predict that the PGAE is the result of the presence of high Wolbachia densities during a critical time point in spermatogenesis when CI-defining changes occur, which may become the subject of future research.
It remains unclear if the association between female age and Wolbachia densities would be the case in wild populations. Since wild D. melanogaster females are estimated to mate, on average, every 27 h (67), it would seem unlikely that the Wolbachia accumulation reported here would occur in nature. However, infection status has been reported to influence mate choice behaviors in numerous animals, including D. subquinaria, D. paulistorum, Nasonia vitripennis, and Tetranychus urticae (68–71). For example, male mating rate affects CI strength (30), so wMel-infected males mate more frequently to reduce the impact of CI strength and therefore improve their lifetime reproductive success (35). Additionally, females infected with Wolbachia have a higher reproductive fitness when their daughters can sufficiently rescue CI and when their sons induce weak CI. Thus, it is plausible that the latency to copulation could be either lengthened in instances where a higher Wolbachia titer would be preferable (rescue efficiency) or, conversely, shortened in populations where a lower density is preferred (weakened CI). While it is unlikely that a fly in nature will remain virgin for as long as reported in this study, it is notable that CI strength increased substantially with every time point measured. As such, even small changes in mating latency may influence CI strength sufficiently to change the rate of spread through a population. Field studies measuring the latency toward copulation in sites with different infection rates would help determine if insects can modulate their mating latency, and thus Wolbachia titers, to increase their fitness and the fitness of their offspring.
While this work reports a PGAE for wMel in D. melanogaster, it is unknown if these dynamics occur for wMel in mosquito hosts. In wMel-infected Aedes aegypti mosquitoes, CI is consistently strong (23, 24). However, some factors such as Wolbachia densities and temperature were shown previously to correlate with CI penetrance (72). It is possible that other as-yet-unstudied factors in mosquitoes, such as the PGAE, can contribute to changes in CI strength. Since strong CI is crucial for rapid spread of wMel-infected mosquitoes through populations for successful vector control applications (73), understanding the factors that contribute to variation in CI strength would further inform the efficacy of population replacement and rearing strategies. Moreover, comparative studies exploring wMel-induced CI in D. melanogaster and A. aegypti could clarify the Wolbachia-host dynamics that govern the penetrance of CI.
Finally, there is a striking range of CI penetrance across Wolbachia and hosts, and more work is necessary to determine if the PGAE applies to other CI or reproductive parasite systems. For example, wRi in D. simulans consistently induces strong CI (7, 10, 74) and wYak and wTei in the D. yakuba clade cause weak and variable levels of CI similar to those seen with wMel (75). Intriguingly, wMel and wTei were initially thought not to cause CI until factors such as male age and host genotype were found to have a significant impact on CI strength (30, 75–79). Since it is clear that some Wolbachia cause CI only under strictly limited conditions, it remains possible that other weak-CI-inducing Wolbachia are mislabeled as non-CI strains because factors such as the PGAE had not been controlled for during initial testing. Indeed, while this work presents the first reported case of transgenerational Wolbachia titers influencing CI, it is not the first case of transgenerational Wolbachia titers influencing reproductive parasitism. In D. innubila, male-killing Wolbachia frequently kill all male offspring, but females with lower Wolbachia titers are known to produce some viable sons (80). The surviving female offspring inherit lower-than-average Wolbachia titers, leading to a greater-than-average chance that those infected females would also produce sons (80). Together, our results and those in D. innubila suggest that a transgenerational effect of titers may be common and consequential with respect to the expression of reproductive parasitism traits.
In conclusion, we characterize Wolbachia density dynamics in females in relation to age and mating, and we link a transgenerational influence of grandmother age to CI penetrance. This work highlights the importance of controlling grandparent age in future studies of wMel-induced CI in D. melanogaster and has implications for laboratory experiments where precise control over levels of CI would be valuable for dissecting the genetic and functional basis of CI. Additionally, it expands our understanding of Wolbachia-host interactions in relation to CI penetrance and titer dynamics and should motivate additional studies exploring these interactions in wMel-infected mosquitoes.
MATERIALS AND METHODS
Fly strains and maintenance.
The following D. melanogaster strains were used in this study: wMel-infected and uninfected variants of y1w* (BDSC 1495) and nos-GAL4-tubulin (BDSC 4442). Uninfected lines were generated through three generations of tetracycline treatment as previously described (29). All stocks were reared on 50 ml of a standard medium containing cornmeal, molasses, and yeast and were maintained at 25°C with a 12-h/12-h light:dark cycle and at 70% relative humidity (RH). All virgin flies were collected using CO2 anesthetization per standard procedures. Briefly, virgin flies were collected in the morning based on the presence of a meconium, bottles were subsequently cleared of adult flies, and flies collected in the evening were assumed virgin due to the standard time of latency until mating. All virgin flies were kept at room temperature prior to experimentation.
Hatch rate assays.
Hatch rate assays were used to assess the impact of D. melanogaster paternal grandmother age on the strength of CI induced by their sons. We conducted 3 variant hatch rate assays to test (i) whether paternal grandmother age influences CI hatch rates, (ii) whether this effect is specific to the y1w* genetic background, and (iii) whether the transgenerational impact of age on hatching is indeed caused by CI.
Source data for hatch rate and qPCR assays. Download Data Set S1, XLSX file, 0.7 MB (736.6KB, xlsx) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
First, we assessed if D. melanogaster paternal grandmother age influences CI hatch rates in the y1w* genetic background. Paternal y1w* grandmothers were collected as virgins and allowed to reach 2, 5, 11, 14, or 18 days of age before mating in parallel with paternal grandfathers aged 0 to 2 days. Paternal grandparents from each age cohort were crossed in single-pair matings in standard vials of media. Since rearing density influences CI strength (32), paternal grandparents were allowed 24 h to mate and to deposit eggs before the grandfathers were discarded and the grandmothers were flash frozen and stored at –80°C for Wolbachia titer analysis. To control for the younger-brother effect and the effect of male age on the strength of CI (30, 32), the earliest eclosing fathers were collected as virgins and left to age 1 day at room temperature before being used in hatch rate assays.
Maternal y1w* grandparents were crossed in standard medium bottles and allowed to mate for 4 days before flies were cleared, as described above for the paternal grandparents. Mothers were collected as virgins and allowed to reach 6 to 8 days of age at room temperature to maximize fertility (81).
Parental y1w* mating pairs were placed in 8-oz Drosophila stock bottles (Genesee Scientific) with a grape juice agar plate covered in yeast affixed to the top to collect embryos for hatch rate analysis as previously described (29, 34). Parents were allowed two back-to-back 24-h mating and laying periods, each with separate freshly yeasted grape juice agar plates. The plates from the first mating period were discarded due to the typically low levels of egg laying in the first 24 h. The embryos from the second mating period were immediately counted after 24 h of additional laying. Embryos were then incubated for 30 h at 25°C to allow time to hatch. The unhatched embryos were counted, and the percentage of embryonic hatching was determined by dividing the number of unhatched embryos by the total number of embryos laid during the second mating period.
To minimize the effect of female fecundity on embryo viability (81), any plate with fewer than 25 embryos was excluded. We measured the hatch rates of offspring produced by two sons of each paternal grandmother. If both sons from the same family produced 25 or more embryos, one was randomly selected and used in analysis.
Next, to assess if the PGAE was specific to the y1w* genetic background, a separate hatch rate assay was conducted using nos-GAL4-tubulin-infected and uninfected flies. This experiment was conducted similarly to the hatch rate experiment described above, with the following adjustments: age and virginity of paternal grandfathers were not controlled. Paternal nos-GAL4-tubulin-infected and uninfected grandmothers were collected as virgins and allowed to reach 2, 5, or 11 days of age before they were allowed to mate in standard medium bottles, and these bottles were cleared of flies after 4 days of laying to control rearing density (32).
Finally, to determine if the PGAE was in fact due to Wolbachia and not to other forms of inviability induced by a transgenerational impact on age, we conducted compatible rescue crosses with males derived from 2-, 5-, 11-, or 14-day-old females. This experiment was conducted similarly to the hatch rate experiment described above, with the following adjustments: both infected and uninfected males were produced from virgin females aged 2, 4, 11, or 14 days; the uninfected males were mated to uninfected females; and infected males were mated to infected females. Paternal grandparents were paired in 8-oz Drosophila stock bottles (Genesee Scientific) with a grape juice agar plate (29) covered in yeast affixed to the top for a 24-h mating and laying period, and then grandparents were collected from the bottles. The plates were maintained for 24 h, and then 20 of the largest larvae were transferred from each plate to a standard medium vial to control rearing density (32).
Wolbachia titer assays.
To assess the relationship between the PGAE and Wolbachia titers, the following tissues were collected: ovaries, female abdomens, embryos, and male abdomens. Since the low biomass of Drosophila testes requires them to be pooled, abdomens were used instead of testes so that samples could be taken directly from the males used in hatch rate assays. To test if virginity and age impact female Wolbachia titers, virgin and nonvirgin females 2 and 11 days of age were reared in parallel, ovaries were dissected in phosphate-buffered saline (PBS), and samples were frozen in liquid nitrogen followed by storage at –80°C. Samples consisted of 4 pairs of ovaries. Nonvirgin females were mated in cohorts of 60 females to 12 males, provided grape juice plates, and allowed 48 h to mate and lay eggs before dissection. Additionally, full bodies from 2-or-11-day-old paternal grandmothers from a hatch rate assay were collected alongside virgin paternal grandaunts (siblings to the paternal grandmothers), frozen in liquid nitrogen, and stored at –80°C. To determine if embryos derived from older females had higher Wolbachia titers, 0-to-1-h-old embryos were collected from grape plates in batches of 50, frozen in liquid nitrogen, and stored at –80°C. Finally, to assess whether the sons of aged paternal grandmothers had higher Wolbachia titers, full bodies from virgin uncles (siblings of fathers used in a hatch rate assay) derived from 2-, 5-, 11-, 14-, or 18-day-old grandmothers were collected and aged 48 h at room temperature in a standard medium vial. Wolbachia titers were measured in virgin uncles rather than the fathers used in the hatch rate assay because of the relationship between CI strength and male mating rate (35).
Upon removal from –80°C conditions, abdomens were immediately dissected from full-body tissues, homogenized in liquid nitrogen, and mixed with 40 μl ice-cold RNase-free PBS. Each sample was split, and 30% (12 μl) was flash frozen and stored at –80°C for DNA extractions. The DNA was extracted from all tissue types using a Gentra PureGene tissue kit (Qiagen). Forty cycles of quantitative PCR (qPCR) were performed using rp49 and groEL primers (Table S2) for all DNA samples as well as positive controls (infected DNA), negative controls (uninfected DNA), no-reverse-transcription controls (RNA), and no-tissue controls (water). Male and female abdomen samples were tested in triplicate and ovaries and embryos in duplicate under the following qPCR conditions: 50°C for 10 min; 95°C for 5 min; 40 cycles of 95°C for 10 s and 55°C for 30 s; and 95°C for 30 s. Samples were excluded from analysis if the standard deviation of results of comparisons between replicates was >0.3. Fold difference between Wolbachia (groEL) density and that of the D. melanogaster rp49 reference gene was determined with 2−ΔΔCT.
Primers used for qPCR. Download Table S2, DOCX file, 0.01 MB (8.2KB, docx) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Statistical analyses.
All statistical analyses were conducted using GraphPad Prism 7. Wolbachia titers of embryos were analyzed using a Mann-Whitney U test. All other data (including data from hatch rate assays and ovary Wolbachia titer comparisons) were analyzed using the Kruskal-Wallis test followed by a Dunn’s multiple-comparison test. Figures were created in GraphPad Prism 7 and 8. All data used in these analyses have been made publicly available (see Data Set S1 in the supplemental material).
ACKNOWLEDGMENTS
We thank Daniel LePage for the initial hypothesis, Jane Meyers for assistance with hatch rate, and Sarah R. Bordenstein and Brittany Leigh for helpful comments throughout the work.
This work was supported by National Institutes of Health (NIH) awards R01 AI132581, R21 HD086833, and R21A133522 to S.R.B.; National Science Foundation (NSF) award IOS 1456778 and Vanderbilt Microbiome Initiative support to S.R.B.; and NSF Graduate Research Fellowship DGE-1445197 to J.D.S. and NIH F31AI143152 to J.I.P. E.M.L. was supported by the SyBBURE Searle Undergraduate Research Program and the National Science Foundation Tennessee Louis Stokes Alliance for Minority Participation.
Opinions, findings, and conclusions or recommendations expressed in this material are ours and do not necessarily reflect the views of the National Institutes of Health or the National Science Foundation.
We declare that we have no competing interests.
All of us contributed to the design of the study. E.M.L. and J.O. conducted hatch rate assays with J.I.P.'s assistance. E.M.L. and J.D.S. conducted titer studies. E.M.L. and J.D.S. analyzed data. E.M.L., J.D.S., and S.R.B. wrote the paper. J.D.S. and J.I.P. provided technical assistance throughout the study. All of us contributed to the final manuscript edits and revision.
Footnotes
Citation Layton EM, On J, Perlmutter JI, Bordenstein SR, Shropshire JD. 2019. Paternal grandmother age affects the strength of Wolbachia-induced cytoplasmic incompatibility in Drosophila melanogaster. mBio 10:e01879-19. https://doi.org/10.1128/mBio.01879-19.
REFERENCES
- 1.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. 2008. How many species are infected with Wolbachia? – a statistical analysis of current data: Wolbachia infection rates. FEMS Microbiol Lett 281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. 2015. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc Biol Sci 282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferri E, Bain O, Barbuto M, Martin C, Lo N, Uni S, Landmann F, Baccei SG, Guerrero R, de Souza Lima S, Bandi C, Wanji S, Diagne M, Casiraghi M. 2011. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS One 6:e20843. doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. 2008. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet 42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 6.Hancock PA, Sinkins SP, Godfray HCJ. 2011. Population dynamic models of the spread of Wolbachia. Am Nat 177:323–333. doi: 10.1086/658121. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann AA, Turelli M, Harshman LG. 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126:933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen VAA, Turelli M, Godfray HCJ. 2008. Stochastic spread of Wolbachia. Proc Biol Sci 275:2769–2776. doi: 10.1098/rspb.2008.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turelli M. 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48:1500–1513. doi: 10.1111/j.1558-5646.1994.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 10.Turelli M, Cooper BS, Richardson KM, Ginsberg PS, Peckenpaugh B, Antelope CX, Kim KJ, May MR, Abrieux A, Wilson DA, Bronski MJ, Moore BR, Gao J-J, Eisen MB, Chiu JC, Conner WR, Hoffmann AA. 2018. Rapid global spread of wRi-like Wolbachia across multiple Drosophila. Curr Biol 28:963–971.e8. doi: 10.1016/j.cub.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LePage D, Bordenstein SR. 2013. Wolbachia: can we save lives with a great pandemic? Trends Parasitol 29:385–393. doi: 10.1016/j.pt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor MJ, Bordenstein SR, Slatko B. 2018. Microbe profile: Wolbachia: a sex selector, a viral protector and a target to treat filarial nematodes. Microbiology 164:1345–1347. doi: 10.1099/mic.0.000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen JH, Barr AR. 1973. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22:242–250. doi: 10.1016/0022-2011(73)90141-9. [DOI] [PubMed] [Google Scholar]
- 14.Bordenstein SR, O'Hara FP, Werren JH. 2001. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409:707. doi: 10.1038/35055543. [DOI] [PubMed] [Google Scholar]
- 15.Brucker RM, Bordenstein SR. 2012. Speciation by symbiosis. Trends Ecol Evol 27:443–451. doi: 10.1016/j.tree.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Dobson SL, Fox CW, Jiggins FM. 2002. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc Biol Sci 269:437–445. doi: 10.1098/rspb.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laven H. 1967. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216:383. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- 18.Lees RS, Gilles JR, Hendrichs J, Vreysen MJ, Bourtzis K. 2015. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci 10:156–162. doi: 10.1016/j.cois.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor L, Plichart C, Sang AC, Brelsfoard CL, Bossin HC, Dobson SL. 2012. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis 6:e1797. doi: 10.1371/journal.pntd.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill SL. 2018. The use of Wolbachia by the world mosquito program to interrupt transmission of Aedes aegypti transmitted viruses. Adv Exp Med Biol 1062:355–360. doi: 10.1007/978-981-10-8727-1_24. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, Dong Y, Kenny N, Paton CJ, Ritchie SA. 13 August 2018, posting date Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res doi: 10.12688/gatesopenres.12844.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blagrove MSC, Arias-Goeta C, Failloux A-B, Sinkins SP. 2012. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci U S A 109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutra HLC, Dos Santos LMB, Caragata EP, Silva JBL, Villela DAM, Maciel-de-Freitas R, Moreira LA. 2015. From lab to field: the influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLoS Negl Trop Dis 9:e0003689. doi: 10.1371/journal.pntd.0003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourtzis K, Nirgianaki A, Markakis G, Savakis C. 1996. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann AA, Clancy DJ, Merton E. 1994. Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann AA, Hercus M, Dagher H. 1998. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holden PR, Jones P, Brookfield JFY. 1993. Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet Res 62:23. doi: 10.1017/s0016672300031529. [DOI] [PubMed] [Google Scholar]
- 29.LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD, Layton EM, Funkhouser-Jones LJ, Beckmann JF, Bordenstein SR. 2017. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543:243–247. doi: 10.1038/nature21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds KT, Hoffmann AA. 2002. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res 80:79–87. doi: 10.1017/S0016672302005827. [DOI] [PubMed] [Google Scholar]
- 31.Solignac M, Vautrin D, Rousset F. 1994. Widespread occurrence of the proteobacteria Wolbachia and partial cytoplasmic incompatibility in Drosophila melanogaster. C R Acad Sci III 317:461–470. [Google Scholar]
- 32.Yamada R, Floate KD, Riegler M, O'Neill SL. 2007. Male development time influences the strength of Wolbachia-induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics 177:801–808. doi: 10.1534/genetics.106.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark ME, Veneti Z, Bourtzis K, Karr TL. 2003. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech Dev 120:185–198. doi: 10.1016/S0925-4773(02)00424-0. [DOI] [PubMed] [Google Scholar]
- 34.Shropshire JD, On J, Layton EM, Zhou H, Bordenstein SR. 2018. One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster. Proc Natl Acad Sci U S A 115:4987–4991. doi: 10.1073/pnas.1800650115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crespigny FECD, Pitt TD, Wedell N. 2006. Increased male mating rate in Drosophila is associated with Wolbachia infection. J Evol Biol 19:1964–1972. doi: 10.1111/j.1420-9101.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 36.Boyle L, O'Neill SL, Robertson HM, Karr TL. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 37.Clark ME, Veneti Z, Bourtzis K, Karr TL. 2002. The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila. Mech Dev 111:3–15. doi: 10.1016/S0925-4773(01)00594-9. [DOI] [PubMed] [Google Scholar]
- 38.Veneti Z, Clark ME, Karr TL, Savakis C, Bourtzis K. 2004. Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl Environ Microbiol 70:5366–5372. doi: 10.1128/AEM.70.9.5366-5372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores HA, O'Neill SL. 2018. Controlling vector-borne diseases by releasing modified mosquitoes. Nat Rev Microbiol 16:508–518. doi: 10.1038/s41579-018-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen S, Camacho M, Sharmin Z, Momtaz AJMZ, Perez L, Navarro G, Triana J, Samarah H, Turelli M, Serbus L. 2019. Quantitative methods for assessing local and bodywide contributions to Wolbachia titer in maternal germline cells of Drosophila. BMC Microbiol 19:206. doi: 10.1186/s12866-019-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvitti M, Marini F, Desiderio A, Puggioli A, Moretti R. 2015. Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One 10:e0121813. doi: 10.1371/journal.pone.0121813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tortosa P, Charlat S, Labbé P, Dehecq J-S, Barré H, Weill M. 2010. Wolbachia sge-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS One 5:e9700. doi: 10.1371/journal.pone.0009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genty L-M, Bouchon D, Raimond M, Bertaux J. 2014. Wolbachia infect ovaries in the course of their maturation: last minute passengers and priority travellers? PLoS One 9:e94577. doi: 10.1371/journal.pone.0094577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Y, Hoffmann AA, Xu X-Q, Mo P-W, Huang H-J, Gong J-T, Ju J-F, Hong X-Y. 28 August 2018, posting date Vertical transmission of Wolbachia is associated with host vitellogenin in Laodelphax striatellus. Front Microbiol doi: 10.3389/fmicb.2018.02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, Frydman HM. 2011. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science 334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frydman HM, Li JM, Robson DN, Wieschaus E. 2006. Somatic stem cell niche tropism in Wolbachia. Nature 441:509–512. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- 47.Sacchi L, Genchi M, Clementi E, Negri I, Alma A, Ohler S, Sassera D, Bourtzis K, Bandi C. 2010. Bacteriocyte-like cells harbour Wolbachia in the ovary of Drosophila melanogaster (Insecta, Diptera) and Zyginidia pullula (Insecta, Hemiptera). Tissue Cell 42:328–333. doi: 10.1016/j.tice.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Toomey ME, Panaram K, Fast EM, Beatty C, Frydman HM. 2013. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc Natl Acad Sci U S A 110:10788–10793. doi: 10.1073/pnas.1301524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng W, Lin H. 1997. Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev Biol 189:79–94. doi: 10.1006/dbio.1997.8669. [DOI] [PubMed] [Google Scholar]
- 50.Lin H, Schagat T. 1997. Neuroblasts: a model for the asymmetric division of stem cells. Trends Genet 13:33–39. doi: 10.1016/s0168-9525(96)10050-0. [DOI] [PubMed] [Google Scholar]
- 51.Presgraves DC. 2000. A genetic test of the mechanism of Wolbachia-induced cytoplasmic incompatibility in Drosophila. Genetics 154:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shropshire JD, Bordenstein SR. 2019. Two-by-one model of cytoplasmic incompatibility: synthetic recapitulation by transgenic expression of cifA and cifB in Drosophila. PLoS Genet 15:e1008221. doi: 10.1371/journal.pgen.1008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shropshire JD, Leigh B, Bordenstein SR, Duplouy A, Riegler M, Brownlie JC, Bordenstein SR. 2019. Models and nomenclature for cytoplasmic incompatibility: caution over premature conclusions – a response to Beckmann et al. Trends Genet 35:397–399. doi: 10.1016/j.tig.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Tram U, Sullivan W. 2002. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296:1124–1126. doi: 10.1126/science.1070536. [DOI] [PubMed] [Google Scholar]
- 55.Ferree PM, Sullivan W. 2006. A genetic test of the role of the maternal pronucleus in Wolbachia-induced cytoplasmic incompatibility in Drosophila melanogaster. Genetics 173:839–847. doi: 10.1534/genetics.105.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poinsot D, Charlat S, Mercot H. 2003. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: confronting the models with the facts. Bioessays 25:259–265. doi: 10.1002/bies.10234. [DOI] [PubMed] [Google Scholar]
- 57.Bossan B, Koehncke A, Hammerstein P. 2011. A new model and method for understanding Wolbachia-induced cytoplasmic incompatibility. PLoS One 6:e19757. doi: 10.1371/journal.pone.0019757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landmann F, Orsi GA, Loppin B, Sullivan W. 2009. Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathog 5:e1000343. doi: 10.1371/journal.ppat.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, Mercot H, Weill M, Sicard M, Charlat S. 2019. The toxin-antidote model of cytoplasmic incompatibility: genetics and evolutionary implications. Trends Genet 35:175–185. doi: 10.1016/j.tig.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beckmann JF, Ronau J, Hochstrasser M. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol 2:17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bressac C, Rousset F. 1993. The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J Invertebr Pathol 61:226–230. doi: 10.1006/jipa.1993.1044. [DOI] [PubMed] [Google Scholar]
- 62.Snook RR, Cleland SY, Wolfner MF, Karr TL. 2000. Offsetting effects of Wolbachia infection and heat shock on sperm production in Drosophila simulans: analyses of fecundity, fertility and accessory gland proteins. Genetics 155:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindsley DL, Tokuyasu KT. 1980. Spermatogenesis, p 226–287. In Ashburner M, Wright TRF (ed), Genetics and biology of Drosophila (2nd ed). Academic Press, San Diego, CA. [Google Scholar]
- 64.ScienceDaily. 1 May 2018. Mechanisms for continually producing sperm. https://www.sciencedaily.com/releases/2015/05/150501095957.htm. Accessed 12 July 2019.
- 65.Ruhmann H, Wensing KU, Neuhalfen N, Specker JH, Fricke C. 2016. Early reproductive success in Drosophila males is dependent on maturity of the accessory gland. Behav Ecol 27:1859–1868. [Google Scholar]
- 66.Karr TL, Yang W, Feder ME. 1998. Overcoming cytoplasmic incompatibility in Drosophila. Proc Biol Sci 265:391–395. doi: 10.1098/rspb.1998.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giardina TJ, Clark AG, Fiumera AC. 2017. Estimating mating rates in wild Drosophila melanogaster females by decay rates of male reproductive proteins in their reproductive tracts. Mol Ecol Resour 17:1202–1209. doi: 10.1111/1755-0998.12661. [DOI] [PubMed] [Google Scholar]
- 68.Chafee ME, Zecher CN, Gourley ML, Schmidt VT, Chen JH, Bordenstein SR, Clark ME, Bordenstein SR. 2011. Decoupling of host-symbiont–phage coadaptations following transfer between insect species. Genetics 187:203–215. doi: 10.1534/genetics.110.120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller WJ, Ehrman L, Schneider D. 2010. Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathog 6:e1001214. doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vala F, Egas M, Breeuwer JAJ, Sabelis MW. 2004. Wolbachia affects oviposition and mating behaviour of its spider mite host: Wolbachia induces assortative mating. J Evol Biol 17:692–700. doi: 10.1046/j.1420-9101.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- 71.Jaenike J, Dyer KA, Cornish C, Minhas MS. 2006. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biol 4:e325. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross PA, Ritchie SA, Axford JK, Hoffmann AA. 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 13:e0007357. doi: 10.1371/journal.pntd.0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritchie SA, van den Hurk AF, Smout MJ, Staunton KM, Hoffmann AA. 2018. Mission accomplished? We need a guide to the ‘post release’ world of Wolbachia for Aedes-borne disease control. Trends Parasitol 34:217–226. doi: 10.1016/j.pt.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann AA, Turelli M, Simmons GM. 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 75.Cooper BS, Ginsberg PS, Turelli M, Matute DR. 2017. Wolbachia in the Drosophila yakuba complex: pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics 205:333–351. doi: 10.1534/genetics.116.196238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charlat S, Ballard JWO, Mercot H. 2004. What maintains noncytoplasmic incompatibility inducing Wolbachia in their hosts: a case study from a natural Drosophila yakuba population. J Evol Biol 17:322–330. doi: 10.1046/j.1420-9101.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 77.Zabalou S, Charlat S, Nirgianaki A, Lachaise D, Mercot H, Bourtzis K. 2004. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics 167:827–834. doi: 10.1534/genetics.103.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez J, Longdon B, Bauer S, Chan Y-S, Miller WK, Bourtzis K, Teixeira L, Jiggins FM. 2014. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poinsot D, Bourtzis K, Markakis G, Savakis C, Mercot H. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dyer KA, Minhas MS, Jaenike J. 2005. Expression and modulation of embryonic male-killing in Drosophila innubila: opportunities for multilevel selection. Evol Int J Org Evol 59:838–848. doi: 10.1111/j.0014-3820.2005.tb01757.x. [DOI] [PubMed] [Google Scholar]
- 81.Miller PB, Obrik-Uloho OT, Phan MH, Medrano CL, Renier JS, Thayer JL, Wiessner G, Bloch Qazi MC. 2014. The song of the old mother: reproductive senescence in female Drosophila. Fly (Austin) 8:127–139. doi: 10.4161/19336934.2014.969144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
P values associated with all statistical comparisons corresponding to the main and extended bodies of data presented in the figures. Download Table S1, DOCX file, 0.01 MB (11.2KB, docx) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Paternal D. melanogaster grandmother age does not impact hatching in non-CI crosses. Hatch rate assays were conducted with uninfected or infected y1w* males derived from females aged 2, 5, 11, or 14 days (d.) followed by mating crossed to either infected or uninfected y1w* females. Each dot represents a replicate of offspring from single-pair matings. Wolbachia infections are represented by filled sex symbols, and the age of the paternal grandmother is shown immediately to the left of the y axis. Vertical bars represent medians, and letters to the right indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between crosses (in brackets). All statistical values are presented in Table S1. Download FIG S1, TIF file, 0.2 MB (198KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The paternal D. melanogaster grandmother age effect is not specific to y1w* flies. Hatch rates assays were conducted with uninfected or infected nos-GAL4-tubulin males derived from females aged 2, 5, or 11 days (d.) followed by mating crossed to uninfected nos-GAL4-tubulin females. Each dot represents a replicate of offspring from single-pair matings. Wolbachia infections are represented by filled sex symbols, and the age of the paternal grandmother is shown immediately to the left of the y axis. Vertical bars represent medians, and letters to the right indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between crosses (in brackets). All statistical values are presented in Table S1. Download FIG S2, TIF file, 0.2 MB (188.4KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
rp49 CT values remain consistent across age and virginity states, whereas groEL fluctuates. CT values are from the Wolbachia density analysis described in the Fig. 3 legend. Wolbachia density assays were conducted with pools of 4 ovaries from virgin females (indicated by a “v” above a sex symbol) and nonvirgin females aged 2 or 11 days (d.). Wolbachia infections are represented by filled sex symbols, and the age of the sample is shown to the left of the sex symbol. Virgin and nonvirgin females were siblings. The nonvirgin females produced the embryos represented in Fig. 2B. Nonvirgin females were allowed to mate and lay for 48 h before ovary dissections were performed. Nonvirgin and virgin females were incubated for the same period of time and dissected in parallel. Each dot represents the average of duplicate values. Vertical bars indicate medians, and the letters above the bars indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all virgin females and mated females (separated by a horizontal dotted line). All statistical values are presented in Table S1. Download FIG S3, TIF file, 0.2 MB (176.3KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Wolbachia densities increase with female age in abdomens and decrease after mating. Wolbachia density assays were conducted with single abdomens from virgin females (indicated by a “v” above a sex symbol) and nonvirgin females aged 2 or 11 days (d.). Wolbachia infections are represented by filled sex symbols, and the age of the sample is shown immediately below the x axis. Virgin and nonvirgin females were siblings. The nonvirgin females produced the fathers corresponding to the hatch rate data in Fig. 1 and the males whose data are shown in Fig. 2A. Nonvirgin females were allowed to mate and lay for 48 h before abdomen dissections were performed. Nonvirgin and virgin females were incubated for that same period of time and dissected in parallel. Each dot represents the average of triplicate values. Horizontal bars indicate medians, and the letters above the bars indicate significant differences based on α = 0.05 calculated by a Kruskal-Wallis test followed by a Dunn’s multiple-comparison test performed between all groups. All statistical values are presented in Table S1. Fold differences in Wolbachia density (groEL) relative to D. melanogaster reference gene rp49 were determined with 2−ΔΔCT. Download FIG S4, TIF file, 0.2 MB (249.6KB, tif) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Source data for hatch rate and qPCR assays. Download Data Set S1, XLSX file, 0.7 MB (736.6KB, xlsx) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used for qPCR. Download Table S2, DOCX file, 0.01 MB (8.2KB, docx) .
Copyright © 2019 Layton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.


