Highlights
-
•
IdeSsuis vaccination of piglets significantly reduced survival of S. suis cps9 in blood.
-
•
IdeSsuis reactive T helper cells producing TNF-α, IL-17A or IFN-ɣ were detectable.
-
•
Vaccination resulted in protection against mortality induced by cps9 challenge.
Keywords: IgM protease, T helper cell, Antigen-reactive, Pigs, Bactericidal assay, Serotype 9
Abstract
Vaccination of weaning piglets with the recombinant IgM degrading enzyme of Streptococcus suis (S. suis), rIdeSsuis, elicits protection against disease caused by serotype (cps) 2 infection. In Europe, S. suis cps9 is at least as important as cps2 in causing severe herd problems associated with meningitis, septicemia and arthritis. The objective of this study was to determine humoral and cellular immunogenicities of rIdeSsuis suckling piglet vaccination and to investigate protection against a virulent cps9 strain. Vaccination in the 2nd and 4th week of life with rIdeSsuis and an oil-in-water adjuvant induced seroconversion against IdeSsuis in 13 of 20 vaccinated piglets. In the 5th week, survival of the S. suis cps9 strain was significantly reduced in the blood of prime-booster vaccinated piglets. After a 2nd booster vaccination IdeSsuis-reactive T helper (Th) cells partially producing TNF-α, IL-17A or IFN-ɣ were detectable in rIdeSsuis-vaccinated but not in placebo-treated piglets and frequencies of IdeSsuis-reactive Th cells correlated with α-IdeSsuis–IgG levels. An intravenous challenge, conducted with a cps9 strain of sequence type (ST) 94, led to 89% mortality in placebo-treated piglets due to septicemia and meningitis. In contrast, all rIdeSsuis prime-booster-booster vaccinated littermates survived the challenge despite signs of disease such as fever and lameness. In conclusion, the described rIdeSsuis vaccination induces humoral and detectable IdeSsuis-reactive Th cell responses and leads to protection against a highly virulent cps9 strain.
1. Introduction
S. suis cps9 has become the most important serotype in some European countries with a huge pig industry such as Spain and the Netherlands [1], [2]. In the field, prophylaxis against cps9 is very problematic, because vaccination with a bacterin protects against mortality, but not morbidity [3] and does not reduce colonization and transmission [4]. Furthermore, cps9 is a strong biofilm inducer and endocarditis is a main manifestation, which might also occur in vaccinated piglets [3], [5], [6].
A few S. suis proteins have been shown to provide protection against cps2 challenge in pigs, including a combination of muramidase-released protein (MRP) and extracellular factor (EF) [7], surface antigen one (SAO) [8], HP0197 [9], SsPepO [10] and IdeSsuis [11]. Interestingly, only SAO has been shown to protect against a further serotype, namely cps1 [12]. However, most cps1 strains are closely related to cps2. Strains of both serotypes often belong to clonal complex (CC) 1 [13]. Noteworthy, other independent studies have shown that piglets with high antibody titers against SAO are highly susceptible to S. suis challenge and that these antibodies are not opsonizing [14], [15]. Thus, it is currently unknown whether any of the identified protective antigens has the cross-protective potential needed for a universal S. suis vaccine.
Different S. suis serotypes express a highly specific immunoglobulin M-degrading enzyme, designated IdeSsuis [16]. The protein is homologous to the IgG protease IdeS of S. pyogenes [17], but cleaves solely class M antibodies of swine. Mutants expressing no or a point-mutated IdeSsuis variant deficient in IgM cleavage show enhanced deposition of C3b on the bacterial surface indicating that IgM cleavage by IdeSsuis is involved in complement evasion [18], [19]. Vaccination of weaning piglets with rIdeSsuis elicits antibodies neutralizing the IgM protease activity and protects against morbidity and mortality induced by cps2 challenge [11].
Read out parameters in vaccination trials with piglets focused on humoral immunity. However, cellular immunity is known to be crucial to restrict colonization of the respiratory tract by the related pathogen S. pneumoniae [20], [21]. Mice lacking CD4 were found to be more susceptible to systemic S. suis infection at a low infection dose [22], whereas in pigs the T cell immune response is not well characterized.
Here, we investigated humoral and cellular immunogenicities of IdeSsuis vaccinated suckling piglets, being the age class of choice for vaccination in the field. Furthermore, we asked if immunization with IdeSsuis protects against S. suis cps9, the most important and troublesome pathotype in Europe.
2. Materials and methods
2.1. Bacterial strains, growth conditions and profiling of virulence-associated factors
S. suis strain A3286/94 is a mrp* sly+ cps9 strain belonging to ST99 of CC16 originally isolated from a pig with meningitis [3], [15], [23], [24]. Strains 15-3/3, V5404/2 and 16085/3b were originally isolated from inner organs of diseased piglets with meningitis and/or septicemia and identified as mrp+ sly+ cps9+ in a described multiplex (MP) PCR [25]. S. suis strain 10 (mrp+ epf+ sly+) is a virulent serotype 2 strain [11], [15], [26]. The isogenic mutant 10ΔideSsuis deficient in IgM cleavage was included in the bactericidal assay to reveal effects mediated by antigen-specific immunity [11], [16]. S. suis was grown on Columbia agar plates supplemented with 6% sheep blood or in BactoTM Todd Hewitt broth (THB). Escherichia coli (E. coli) strains were cultured in Luria-Bertani (LB) medium including 100 µg/ml ampicillin, if appropriate.
2.2. Expression and purification of recombinant (r) proteins
The expression and the purification of His-tagged rIdeSsuis of serotype 2 strain 10 [16] and His-tagged fibronectin binding domain of streptococcal fibronectin-binding protein I of Streptococcus pyogenes (SfbI) [27] was performed as described previously [16]. SfbI was chosen as a control protein as Streptococcus pyogenes is not found in pigs.
2.3. Animal experiments
Piglets were infected experimentally and cared for in accordance with the principles outlined in the EU Directive 2010/63/EU (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm). All animal experiments or samplings were conducted by veterinarians and in accordance with the principles outlined in the European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes and the German Animal Protection Law (Tierschutzgesetz). The animal experiment of this study was approved by the Landesdirektion Sachsen (permit no. TVV28/16), which includes approval through the registered committee for animal experiments. The collection of blood samples was approved by the Landesdirektion Sachsen (permit no. N01/16 and N19/14).
Five weeks prior to farrowing four pregnant sows of a German Landrace pig herd considered to be free of cps9+ S. suis strains were transported to the Faculty of Veterinary Medicine, Leipzig University. The classification as cps9 free was based on the genotyping results including a described MP-PCR [25] of S. suis isolates from the tonsils of more than 400 animals over the last 14 years. After birth the suckling piglets were treated as follows: Piglets of one litter were equally distributed to the vaccination and the placebo group with a final number of 20 piglets per group. At an age of 2 weeks a vaccine containing rIdeSsuis as antigen or a placebo was applied intramuscularly, both supplemented with 20% [vol/vol] Emulsigen as adjuvant. The piglets were boostered 14 days later. Nine piglets per group were boostered a second time another 14 days later. One dose of rIdeSsuis vaccination contained 0.4 mg rIdeSsuis. After the first booster vaccination at an age of 4 weeks, piglets were weaned and moved to the trial pen. All piglets (n = 18) used for experimental infection were placed in one pen. The person (CGB) who made the final decision of euthanasia did not know whether a specific piglet was vaccinated with rIdeSsuis or placebo-treated (partially blinded experiment). Piglets were challenged intravenously at an age of 8 to 9 weeks (17 days after the second booster) with 2 × 108 CFU of S. suis strain 16085/3b grown in Bacto™ Tryptic Soy Broth (TSB) without dextrose. Post infection, the health status of the animals was monitored every 8 h, including measurement of the inner body temperature, assessment of movements and feed intake (piglets were only fed at these time points). Based on predefined criteria (Table S1) clinical signs were scored. Piglets were classified as morbid if a body temperature of ≥40.2 °C or/and severe clinical signs of an acute disease were observed. In case of high fever (≥40.5 °C), apathy and anorexia persisting over 32 h as well as in all cases of central nervous system dysfunction or clinical signs of acute polyarthritis, animals were euthanized for animal welfare reasons. All surviving piglets were sacrificed 14 days post infection (dpi). After euthanasia every animal went through the same procedure of necropsy to collect the following samples for histological (h) and semi-quantitative bacteriological (b) investigations as described previously [3], [15]: cerebrospinal fluid (b); brain (b, h); tarsal and carpal joints (b, h), peritoneal, pleural and pericardial swabs (b), peritoneum, pleura and pericardium (h); cranial lobe of the left lung (b, h); liver (b, h); spleen (b, h); mitral valve (b, h) and tonsil (b, h). The histological screenings were scored as described [28] and briefly mentioned in the footnotes of Table 2. Isolation of the challenge strain was confirmed by MP-PCR detecting mrp, epf, sly, arcA, gdh, cps1, cps2, cps7 and cps9 [25].
Table 2.
Scoring of fibrinosuppurative lesions of piglets challenged with S. suis cps9 strain 16085/3b.
| Immunization antigen | Piglets without lesionsa | Piglets with lesions in two or more locationsa | Brain | Serosae | Joint | Spleen and liver | Lung | Heart | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| meningitis, chorioiditis |
pleuritis or peritonitis or pericarditis |
synovialitis |
splenitisb or hepatitis |
pneumonia |
endocarditis |
||||||||||||||||
| 5c | 3d | 1e | 4c | 2d | 1e | 4c | 2d | 1e | 4c | 2d | 1e | 4c | 2d | 1e | 4c | 2d | 1e | ωf | |||
| placebo | 0/9 | 6/9 | 3/9 | 1/9g | 0/9 | 0/9 | 3/9 | 1/9 | 1/9 | 2/9 | 0/9 | 0/9 | 6/9 | 1/9 | 0/9 | 0/9 | 1/9 | 0/9 | 3/9 | 1/9 | 3.0 h |
| rIdeSsuis | 3/9 | 2/9 | 0/9 | 0/9 | 2/9 | 0/9 | 1/9 | 0/9 | 1/9 | 3/9 | 0/9 | 0/9 | 0/9 | 6/9 | 0/9 | 0/9 | 0/9 | 1/9 | 2/9 | 2/9 | 2.0 |
Only fibrinosuppurative lesions are considered. Individual single perivascular neutrophils are not counted.
Neutrophilic accumulation of the splenic red pulp.
Scoring of 4 and 5 indicates moderate to severe diffuse or multifocal fibrinosuppurative inflammations.
Scoring of 2 and 3 indicates mild focal fibrinosuppurative inflammation.
Individual single perivascular neutrophils received a score of 1.
ω = Σscoremax/nanimals.
diffuse, mild plexus choroiditis.
Four of the nine placebo-treated piglets died within 8 h after experimental infection. These piglets reached comparable low histological scores of 2 (n = 3) and 3 (n = 1).
2.4. Bactericidal assay
Survival of S. suis in porcine blood ex vivo was determined as previously described [11]. Briefly, 500 µl of heparinized blood (16 I. U. heparin/ml) was mixed with 6 × 105 CFU of exponentially grown bacteria (OD600: 0.5–0.6). The samples were incubated for 2 h at 37°C on a rotator. Blood for bactericidal assays was drawn from all piglets of the vaccination trial 7 and 17 days after first and second booster vaccinations, respectively. The bactericidal assays were conducted within 4 h after blood collection. The specific bacterial contents in CFU/ml were determined by plating serial dilutions at t = 0 min and t = 120 min and the survival factor of S. suis for each sample was calculated by dividing the two values.
2.5. Detection of anti (α) -IdeSsuis IgG
The detection of α-IdeSsuis IgG was performed as previously described [11]. Sera of piglets immunized with rIdeSsuis and a truncated derivative (rIdeSsuis_homologue) in the previous study served as reference serum and positive control in the α-IdeSsuis ELISA, respectively.
2.6. Detection of IdeSsuis-reactive Th-cells
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples by density gradient centrifugation on Biocoll (Merck-Biochrom, Berlin, Germany) and cryo-conserved at −80 °C until usage for restimulation. PBMCs were cultivated in 96-well plates (1 × 106 cells/well) in complete Iscove's Modified Dulbecco's Medium (IMDM, Pan Biotech, Aidenbach, Germany) containing 10% FCS (Gibco) and penicillin/streptomycin (100 U/ml and 100 µg/ml, respectively; Merck-Biochrome, Berlin, Germany). Antigen-specific restimulation was conducted with 5 µg/ml rIdeSsuis or 5 µg/ml His-tagged rSfbI [27] as a non-relevant control antigen. Both recombinant antigens were expressed in E. coli and purified via Ni-affinity chromatography. rSfbI was included as a control to estimate background reactivity. PMA/Ionomycin (10 ng/ml and 500 ng/ml, respectively, both purchased from Merck-SIGMA-Aldrich, Taufkirchen, Germany) were used as a positive control added for the last 4 h of incubation time and medium alone was used as a negative control. PBMCs were stimulated for 18 h in presence of 2 µg/ml Brefeldin A (Enzo Life Science, Lörrach, Germany) for the last 14 h at 37°C, 5% CO2 in a humidified atmosphere. To label cells for flow cytometry analysis, the stimulated cells were harvested and stained as described in the section flow cytometry and as previously shown [29]. Measurement of samples was conducted at LSR-Fortessa™ (Beckton Dickinson, Germany, Heidelberg) recording 3–5 × 105 viable CD3+CD4+ cells. The frequency of antigen-reactive Th cells (CD154+) was determined from the antigen-experienced Th cell population, described as CD3+CD4+CD8α+ cells. Frequency of IdeSsuis-induced Th cells was calculated as the difference of the percentage of CD154+ cells after IdeSsuis re-stimulation minus the percentage of CD154+ cells of medium cultivated cells from the same sample.
2.7. Flow cytometry staining
To label antigen-reactive Th cells for flow cytometry analysis 6x106 PBMCs per stimuli were used after antigen-specific restimulation. Cells were washed two times with PBS before addition of fixable viability dye eFlour™ 506 (1:500, Thermo Fisher Scientific, San Diego, CA). For extracellular staining anti-porcine CD3-PE ~ Cy7 (clone: BB23-8E6-8C8), anti-porcine CD4-PE (clone: 74–12-4) and anti-porcine CD8α-FITC (clone: 76-2-11), all purchased from Beckton Dickinson (BD, Heidelberg, Germany), were used. Following fixation (2% paraformaldehyde) the cells were permeabilized in PBS buffer containing 3% FCS, 0.1% NaN3 and 0.5% saponin for intracellular staining with anti-human CD154-VioBlue (clone: 5c8, Miltenyi, Colone, Germany), anti-porcine IFN-γ-PerCP ~ Cy5.5 (clone: P2G10, BD), anti-human IL-17A-AlexaFlour®647 (clone: SCPL1362, BD) and anti-human TNF-α-BV421 (clone: MAb11, BioLegend, San Diego, CA).
2.8. Statistical analysis
The evaluation of more than two groups was carried out using one- or two- way analysis of variance (ANOVA) or Kruskall-Wallis with a subsequent Tukey’s or Dunn’s multiple comparisons test, respectively. Differences between two groups were analyzed with the Mann-Whitney U test. Correlation was calculated with the Pearson test. The Wilcoxon test was used for comparison of different time point values within the same group in case of no more than two repeated measures. The data presented in the Kaplan-Meier-diagrams were analyzed with the log rank test. Means and standard deviations of the results are shown. All statistical tests were conducted with GraphPad Prism 7.01 software. Probabilities lower than 0.05 were considered significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3. Results
3.1. S. suis 16085/3b exhibits increased bacterial survival in porcine blood in comparison to other cps9 strains
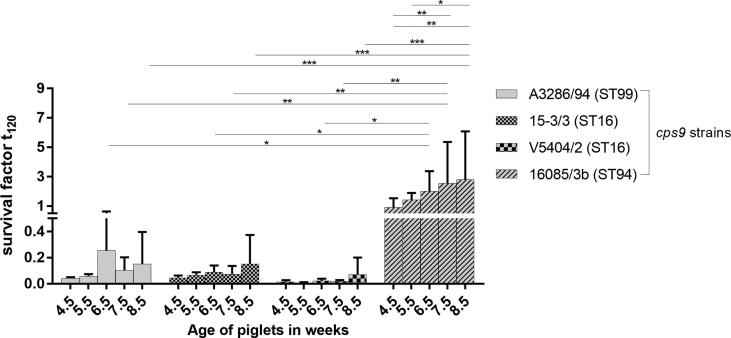
In Europe, S. suis cps9 is a major porcine pathogen causing herd problems associated mainly with meningitis, endocarditis and arthritis [2], [25]. Recently, a herd experienced an escalating S. suis cps9 problem in growing piglets due to meningitis and septicemia associated with cyanosis and sudden death. As the extent and severity of disease on this farm appeared extreme, the S. suis cps9 strain 16085/3b isolated from the spleen of a pig from this farm was compared to other S. suis cps9 strains by profiling of virulence-associated factors, multi locus sequence typing (MLST) and analysis of bacterial survival in porcine blood. Interestingly, strain 16085/3b is a mrp+ sly+ cps9+ strain of ST94 and thus genetically distinct to other invasive cps9 strains. As shown in Fig. 1 S. suis cps9 strain 16085/3b shows significantly higher bacterial survival factors than the other three tested cps9 strains of ST16 (V5402/2 and 15-3/3) and ST99 (A3286/94) in blood from cps9 free piglets at an age of 6.5, 7.5 and 8.5 weeks. In addition, for the 16085/3b strain we observed a significant increase of bacterial survival in blood of 8.5 week-old (mean = 2.8, SD = 3.3, n = 5) piglets in comparison to 4.5 week-old (mean = 0.9, SD = 0.6, n = 5) and 5.5 week-old piglets (mean = 1.4, SD = 0.5, n = 5) (Fig. 1). Due to this phenotype, we chose strain 16085/3b as challenge strain for this vaccination study, since previous experimental infection studies with other cps9 strains led to rather low rates of mortality making it difficult or even impossible to draw conclusions on protection against mortality [3], [4].
Fig. 1.
Strain 16085/3b shows increased survival in porcine blood compared to other cps9 strains. Bactericidal assays were conducted with S. suis cps9 strains A3286/94, 15-3/3, V5404/2 and 16085/3b belonging to the indicated sequence types (ST) with blood drawn from the same S. suis cps9 free piglets (n = 5) at the specified ages. Note, that data of strain 16085/3b was published recently [36]. The survival factor represents the ratio of CFU at 120 min to CFU at time zero. Bars and error bars represent mean values and standard deviations, respectively. Significant differences were determined using two-way ANOVA and a subsequent Tukey’s multiple comparisons test. Significances are indicated (*p < 0.05, **p < 0.01, ***p < 0.001).
3.2. Early prime-booster vaccination of piglets in the 2nd and 4th week with rIdeSsuis elicits specific IgG antibody titers and bactericidal immunity against S. suis cps9 strain 16085/3b
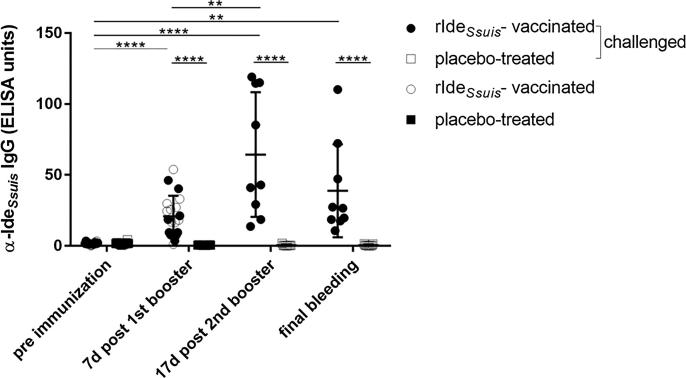
Induction of early protective immunity against S. suis by suckling piglet vaccination would be advantageous over weaning piglet vaccination, as diseases might occur after weaning. Thus, we investigated humoral immunogenicities of early prime-booster rIdeSsuis vaccination in the 2nd and 4th week of life, respectively. Prior to vaccination in the 2nd week of life, all suckling piglets had α-IdeSsuis IgG levels below 5 ELISA units (mean = 1.7; SD = 0.9; n = 20, Fig. 2). Seven days after the 1st booster vaccination, at an age of 5 weeks, a mean of 20.9 ELISA units (SD = 14.4, n = 20) α-IdeSsuis IgG was recorded in vaccinated piglets, which is significantly higher than the antibody level found in their placebo-treated littermates (mean = 0.5 ELISA units, SD = 0.2, n = 20; Fig. 2). However, 7 of 20 vaccinated piglets showed no or only a low increase in α-IdeSsuis IgGs with a difference of less than 10 ELISA units between the pre and post vaccination-serum.
Fig. 2.
rIdeSsuis vaccination induces specific IgG antibodies in serum. Time course of α-IdeSsuis IgG antibodies in rIdeSsuis-vaccinated and placebo-treated piglets during prime-booster-booster vaccination and after S. suis cps9 infection. IgG levels were determined in the 2nd week of life (pre immunization) and 7 days after the first booster in rIdeSsuis-vaccinated (○, ●) and placebo-treated (■, □) piglets (n = 20/group). In the 6th week of life, nine piglets per group were boostered (●) or placebo-treated (□), respectively, for a second time and challenged 17 days later. Blood samples taken from euthanized piglets are indicated as final bleeding and include the time period from 1 until 14 dpi. Mean values are indicated by horizontal lines, standard deviations by error bars. Statistical analyses were conducted with the Mann-Whitney U test (placebo-treated vs. rIdeSsuis-vaccinated), the Wilcoxon test (comparison of pre immunization and 7d post 1st booster immune sera, n = 20/group) or the one-way ANOVA and subsequently the Dunn’s multiple comparisons test (pre and post immune sera of the 18 piglets challenged with S. suis cps9). Significant differences are indicated (**p < 0.01, ****p < 0.0001).
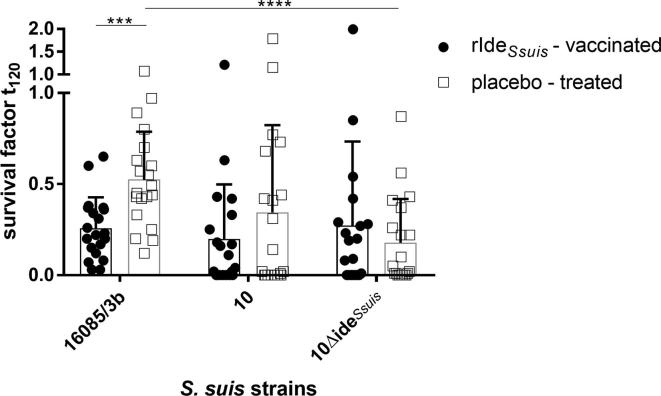
A bactericidal assay conducted 7 days after first booster vaccination revealed significantly lower survival factors of the cps9 strain 16085/3b in the blood of the vaccinated piglets (mean = 0.3, SD = 0.2, n = 20) compared to the blood of placebo-treated piglets (mean = 0.5, SD = 0.3, n = 20; Fig. 3). The cps2 wt strain 10 also had a lower survival factor in the blood of vaccinated piglets (mean = 0.2, SD = 0.3, n = 20) in comparison to placebo-treated piglets (mean = 0.3, SD = 0.5, n = 20), though differences were not significant (Fig. 3). In conclusion, an early prime-booster IdeSsuis vaccination (in the 2nd and 4th week of life) leads to an increase of α-IdeSsuis-IgG in serum at an age of 5 weeks which is associated with reduced survival of S. suis in blood.
Fig. 3.
Survival of S. suis cps9 strain 16085/3b is significantly reduced in blood of rIdeSsuis-vaccinated piglets (n = 20) in comparison to placebo-treated littermates 7 days after the first booster vaccination. Bacterial survival of cps2 strain 10 and its isogenic mutant 10ΔideSsuis was also determined in the blood of the same piglets. Bars and error bars represent mean values and standard deviations, respectively. The Mann-Whitney-U test was used for comparison of rIdeSsuis-vaccinated versus placebo-treated group. Two-way ANOVA and Tukey’s multiple comparisons post-hoc test were used for comparison of survival factors of the three S. suis strains per group. Significant differences are indicated (***p < 0.001, ****p < 0.0001).
3.3. Vaccination with rIdeSsuis protects growing piglets against mortality caused by S. suis cps9 infection
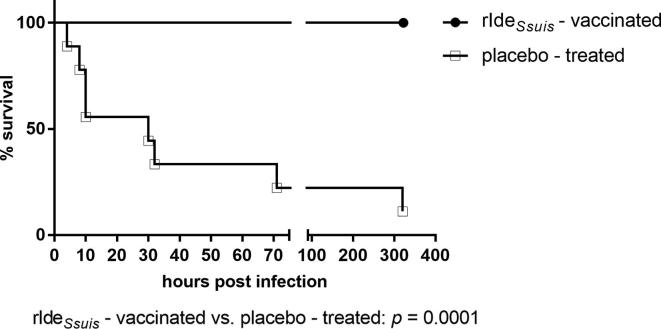
In contrast to the results of a previous vaccination trial with weaning piglets [11], early prime-booster vaccination with rIdeSsuis did not elicit a prominent systemic IgG response against rIdeSsuis in all vaccinated piglets. Piglets with a prominent IgG response against rIdeSsuis following prime-booster vaccination, which additionally showed neutralization of the IgM protease in Western blot analysis (as described previously by Seele et al., 2015), were chosen for another experiment not dealing with cps9 and thus not part of the study described here. A 2nd booster was applied to the remaining nine prime-booster vaccinated piglets which apart from two animals (>40 α-IdeSsuis IgG ELISA units) had responded poorly (<22 α-IdeSsuis IgG ELISA units) to the vaccine in terms of seroconversion against rIdeSsuis (Table S2). The 2nd booster led to a significant increase of the α-IdeSsuis IgG level to a mean of 64.4 ELISA units (SD = 43.9, n = 9) 17 days after the 2nd booster in the 8th week of life compared to a mean of 18.2 ELISA units (SD = 15.3, n = 9) 7 days after the 1st booster in these nine piglets (Fig. 2). These prime-booster-booster vaccinated piglets (n = 9) were used for the challenge experiment. A bactericidal assay conducted after the 2nd booster immunization suggested killing of the cps9 strain with a mean survival factor of 0.6 (SD = 0.6) in the rIdeSsuis-immunized group in contrast to substantially higher survival in placebo-treated littermates with a mean survival factor of 1.3 (SD = 1.15), respectively, although these differences were statistically not significant (Fig. S1). The intravenous challenge with the cps9 strain 16085/3b was conducted on the same day to test protection against this important serotype. A clinical scoring system was applied as shown in Table S1. Six of nine placebo-treated piglets died or were killed after reaching a clinical score of 25 within 32 h after experimental infection (Fig. 4). Four of them displayed a peracute course of disease with the inability to rise, pain vocalization, vomiting and apathy within less than 8 h after challenge. Further two showed signs of central nervous system dysfunction including convulsions, opisthotonus and tremor the day after challenge (Table 1). Another placebo-treated piglet showed signs of central nervous system dysfunction as late as 14 dpi. In contrast, none of the nine vaccinated piglets died or showed signs of acute septicemia, central nervous system dysfunction or the inability to rise in the observation period after the cps9 challenge. Only one vaccinated piglet received a clinical score above 10 after experimental infection. However, short time fever during the first three days following the challenge were recorded in seven of nine vaccinated piglets (Fig. S2) and lameness was observed in four of nine piglets. These piglets recovered within 24–32 h in all cases except for one. Thus, prime-booster-booster rIdeSsuis vaccination protects piglets against mortality but not morbidity in this intravenous cps9 challenge model.
Fig. 4.
rIdeSsuis vaccination protects against mortality caused by S. suis cps9 challenge. The Kaplan-Meier diagram shows mortality of rIdeSsuis prime-booster-booster (n = 9) and placebo-treated (n = 9) growing piglets after infection. Piglets were challenged through intravenous application of 2 × 108 CFU of S. suis strain 16085/3b (mrp* sly+cps9) 17 days after 2nd booster immunization. In case of high fever (≥40.5 °C), apathy and anorexia persisting over 32 h as well as in all cases of central nervous system dysfunction or clinical signs of acute polyarthritis, animals were euthanized for animal welfare reasons. All surviving piglets were sacrificed 14 days post infection (dpi). Statistical analysis was conducted with the log-rank test (p value is shown below the diagram).
Table 1.
Assessment of the protection induced by the indicated treatments against morbidity and mortality after intravenous S. suis serotype 9 challenge.
| Immunization antigen | Morbidity | Mortality | Mean clinical scoree (SD) | Clinical signs |
Max. body temperature (°C) |
||||
|---|---|---|---|---|---|---|---|---|---|
| CNSa | Lameness | no feed intakee | <40 | ≥40 and ≤40.2 | >40.2 | ||||
| placebo | 8/9 | 8b/9 | 21 (8.1) | 4/9 | 3c/9 | 4(6)d/9 | 1d/9 | 0d/9 | 6d/9 |
| rIdeSsuis | 8/9 | 0/9 | 6 (3.9) | 0/9 | 4/9 | 1/9 | 1/9 | 1/9 | 7/9 |
Signs of central nervous system (CNS) dysfunction such as convulsions and opisthotonus.
One placebo-treated piglet showed central nervous system dysfunction and reached a score of 25 on the last day of the experiment.
Three additional piglets became recumbent.
Two piglets died a few hours after experimental infection prior to the first feeding and measurement of body temperature.
For the detailed clinical scoring system see Table S1.
The histological screening revealed that six of nine placebo-treated piglets had typical fibrinosuppurative lesions in at least two inner organs (Table 2). Accordingly, S. suis cps9 was detected in seven of nine placebo-treated piglets in two or more inner organs indicating severe bacteremia or infection of multiple organs after challenge (Table 3). The pathological score ω was substantially lower in rIdeSsuis-vaccinated piglets (2.0 versus 3.0) as moderate and severe fibrinosuppurative lesions were not detected in the brain of these animals (Table 2). Furthermore, the challenge strain was isolated from an inner organ of only one rIdeSsuis-vaccinated piglet (Table 3). In conclusion, recording of fibrinosuppurative inflammations and bacteriology indicated protection in prime-booster-booster rIdeSsuis-vaccinated piglets against meningitis and bacterial dissemination.
Table 3.
Reisolation of the challenge strain from piglets after intravenous challenge with S. suis cps9 strain 16085/3b.
| Immunization antigen | Number of piglets positive for the isolation of the challenge strain in an inner organb or in serosa or in joint fluid | Number of piglets positive for the isolation of the challenge strain in ≥ 3 inner organsb | Number of piglets in which the S.suis challenge straina was isolated from |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tonsils | Lungc | Serosad | Spleen | Liver | Brain, CSFe | Joint fluidf | Endocard | |||
| placebo | 8/9 | 7/9 | 0/9 | 5/9 | 4/9 | 7/9 | 5/9 | 7/9 | 6/9 | 6/9 |
| rIdeSsuis | 1/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1/9 | 0/9 | 0/9 | 1/9 |
The challenge strain was identified by PCR.
Inner organ refers to lung, spleen, liver, brain, CSF or endocard but not the tonsils.
One cranial lobe was investigated.
Pleural, peritoneal or pericardial cavity.
Cerebrospinal fluid.
Punctures of both tarsal and carpal joints were investigated in each animal. In case of lameness additional joint punctures of the respective limb were screened.
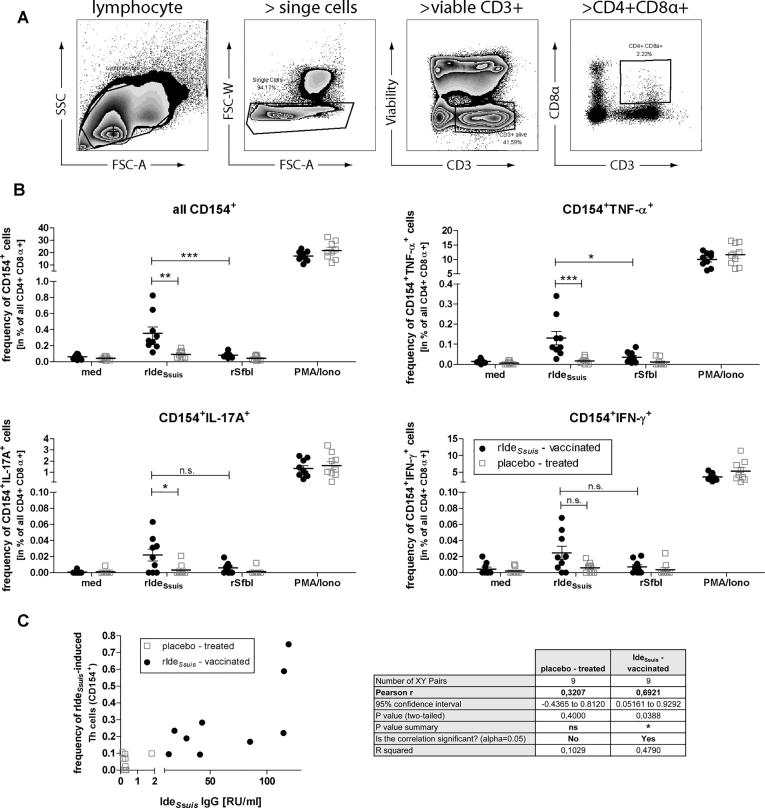
3.4. Prime-booster-booster rIdeSsuis vaccination elicits a detectable antigen-reactive Th cell response
We recently described a method to detect antigen-reactive Th cell responses in pigs using the transiently expressed Th cell activation marker CD154 [29]. Therefore we analysed antigen-experienced Th cells (pre-gated for lymphocytes > single cells > viable CD3+ > CD4+ CD8α+ ), described as CD3+CD4+CD8α+ [30], [31], shown in Fig. 5A. In comparison to piglets of the placebo-group the re-stimulation of PBMCs of rIdeSsuis-immunized piglets with rIdeSsuis induced a significantly increased number of CD154+ Th cells (Fig. 5B, upper left) within the antigen-experienced Th cell population. In contrast, stimulation with a non-relevant control antigen (recombinant fibronectin binding domain of SfbI) did not induce CD154+ Th cells in both groups that confirm the antigen-specific reactivity of antigen-experienced Th cells in the assay. Considering the cytokine profile of the rIdeSsuis-reactive CD154+ Th cells, we found a significantly increased number of TNF-α (Fig. 5B, upper right) and IL-17A producer (Fig. 5B, lower left) in the vaccination group in comparison to placebo-treated animals. The same trend was found for IFN-ɣ producing CD154+ Th cells, but not statistically significant (Fig. 5B, lower right).
Fig. 5.
Vaccination-induced IdeSsuis-reactive Th cell frequencies correlate with IdeSsuis-specific IgG levels. (A) Gating strategy to pre-gate antigen experienced Th cells (CD3+CD4+CD8α+). (B) Within this population frequency of IdeSsuis-reactive Th cells was determined 17 d post 2nd booster immunization (prior cps9 challenge). Therefore PBMCs from rIdeSsuis- vaccinated and placebo-treated pigs (n = 9 per group) were stimulated with 5 µg/ml rIdeSsuis or the recombinant fibronectin binding domain of SfbI as a non-relevant control antigen for 18 h and in presence of Brefeldin A (2 µg/ml) for the last 14 h to detect intracellular CD154 and cytokine expression (frequency of viable CD3+ cells; mean 41.8 ± SD 5.0%). As controls PMA/ionomycin (10 ng/ml and 500 ng/ml, respectively, positive control) and medium (med, negative control) were used. For statistical analysis non-parametric Kruskal-Wallis test and Dunn’s multiple comparisons post-hoc test was used (*p < 0.05, **p < 0.01, ***p < 0.001). (C) Pearson correlation between frequencies of IdeSsuis-induced CD154+ Th cells (calculation see materials/methods) and IdeSsuis IgG levels.
To analyze, whether the detected IdeSsuis-reactive Th cell frequencies are linked to the B cell-mediated IdeSsuis-specific IgG response described above (Fig. 2), correlation of the frequencies of rIdeSsuis-induced antigen-reactive CD154+ Th cell frequencies (representing the difference of IdeSsuis-stimulation minus medium control of the same sample) and IdeSsuis-specific IgG levels was performed using the Pearson test. In contrast to the placebo group (r2 = 0.10), within the rIdeSsuis-vaccinated group (n = 9) the number of IdeSsuis-reactive CD154+ Th cells correlated with the IdeSsuis-specific IgG level (r2 = 0.48) in this manner that animals with higher frequencies of rIdeSsuis-induced CD154+ Th cells showed higher levels of anti-IdeSsuis-IgGs (Fig. 5B). Taken together, rIdeSsuis-prime-booster-booster vaccination of piglets induces a Th cell response that is linked to IdeSsuis-specific IgG level.
In addition, we analyzed the frequency of IdeSsuis-reactive Th cells after challenge to prove whether vaccination-induced Th cells are re-activated by the challenge with cps9 strain 16085/3b. Therefore the two placebo-treated animals that survived until day 14 and five rIdeSsuis-immunized piglets, all with a maximal cumulative clinical score ≥ 4 were selected. We could not detect an increase of IdeSsuis-reactive Th cells after challenge, neither for the frequency of all rIdeSsuis-induced CD154+ cells nor for a cytokine producing subtype of them (Fig. S3A). Rather, by trend we observed a reduced number of IdeSsuis-reactive Th. We found a similar result for the α-IdeSsuis IgG levels, which did not increase after the challenge, but decreased in tendency (Fig. S3B).
In summary we observed an induction of IdeSsuis-reactive Th cells by immunization, but no recall upon challenge infection.
4. Discussion
S. suis cps2 and 9 have an enormous impact on animal health in Europe. A vaccine protecting against both serotypes is needed in the field to ensure return of investments in the pig industry and improve animal health and welfare. Though cps9 is a major porcine pathogen in the field, no recombinant S. suis vaccine has been tested in a challenge experiment with a cps9 strain [32]. This study revealed protection through prime-booster-booster vaccination with rIdeSsuis against mortality induced by intravenous application of a S. suis cps9 strain. rIdeSsuis vaccination has been shown to protect piglets also against S. suis cps2 challenge [11]. Thus, IdeSsuis is a cross-protective antigen covering at least related cps2 and cps9 strains. In contrast to the previous study including an intranasal cps2 challenge, we did not observe protection against morbidity following intravenous cps9 challenge, as vaccinated piglets showed temporarily elevated body temperature, reduced feed intake and lameness. However, sudden death, recumbency, convulsions and polyarthritis were observed in placebo-treated piglets only. As these signs occurred a few hours after challenge in contrast to the intranasal cps2 challenge, the intravenous application of the cps9 strain 16085/3b with 2 × 108 CFU conducted in this study is regarded as a very hard challenge. Noteworthy, sudden death is not a common sign in experimental infections with S. suis. Based on the rapid progression of disease and the high rate of mortality after challenge as well as the increased survival of this cps9 strain in porcine blood in comparison to the other investigated cps9 strains, strain 16085/3b is considered very virulent. MLST revealed that this strain is a ST94 strain which in contrast to other cps9 strains such as A3286/94 (ST99), V5402/2 and 15-3/3 (both ST16) does not belong to CC16. We cannot rule out differences in protective efficacies of rIdeSsuis vaccination against cps9 strains of different clonal complexes. However, experimental infections with cps9 strains of CC16 have resulted in low morbidity, making it very difficult if not impossible to draw conclusions on protection [4].
Pathohistological screenings revealed fibrinosuppurative meningitis only in placebo-treated piglets (4/9) and in accordance a substantially lower pathohistological score in rIdeSsuis-vaccinated piglets (ω = 2.0 versus ω = 3.0, respectively). Of note, meningitis is the most important pathology of S. suis infection in weaning piglets causing high numbers of losses [33]. Though fibrinous endocarditis was observed in three placebo-treated and also in three rIdeSsuis-vaccinated piglets, the challenge strain was detected on the respective mitral valve in six placebo-treated but only in one rIdeSsuis-vaccinated piglet. Whether rIdeSsuis vaccination might be less protective against endocarditis needs to be further clarified. As S. suis forms biofilms covered with fibrin on heart valves, killing of streptococci by humoral immunity is very limited once this vegetation is formed [6]. However, as intravenous application bypasses mucosal immunity, rIdeSsuis vaccination might still protect against endocarditis in mucosal infections. Compared to our previous S. suis cps2 study [11], the pathohistological score in placebo-treated piglets was notably lower (ω = 3.6 versus ω = 3.0, respectively). We suggest that this is due to the sudden death of four piglets on the day of infection. The peracute course of disease did most likely not leave enough time for severe lesions to develop. The piglets only reached pathohistological scores of 3 at the maximum.
We investigated cellular immunogenicities to find out if antigen-reactive Th cells are elicited by our vaccination protocol. Since Th cell-mediated B cell activation supports the production of highly affine specific IgGs [34], we investigated the correlation of rIdeSsuis-reactive Th cell frequencies with rIdeSsuis-specific IgG levels. For this Th cell support especially the activity of TfH cells (follicular T-helper cells) in draining lymph nodes is of importance, as recently also demonstrated in pigs [35]. However, lymph node tissue from immunized animals in a vaccination study is usually not accessible. Therefore, it is more beneficial to use readout-parameters for T cell reactivity from blood samples. The results of this study showed that prime-booster-booster vaccination with a vaccine containing rIdeSsuis and 20% Emulsigen results in induction of antigen-reactive Th cells detectable in porcine blood. The antigen-specific reactivity of this read out parameter is confirmed by using a S. pyogenes antigen (the fibronectin-binding domain of SfbI) as control that was also expressed as His-tagged protein in E. coli and purified via Ni-affinity chromatography [27]. Furthermore, the number of IdeSsuis-reactive CD154+ Th cells correlated with the IdeSsuis-specific IgG level within the immunization group. This is in accordance with our previous findings that IdeSsuis-reactive CD154+ Th cell frequencies correlated with IdeSsuis-specific IgG levels [29], although in contrast to our previous study, we did not observe a correlation with the frequency of IFN-γ producing CD154+ cells. In addition, we analyzed IdeSsuis-reactive Th cells in PBMCs after cps9 challenge (14 dpi.). Based on our data the adaptive immune response to IdeSsuis was not recalled by the S. suis cps9 challenge for both, IdeSsuis-specific-IgGs and IdeSsuis-reactive-Th cells. It is very unlikely that the time point of measurement (14 dpi) was too late after challenge as IdeSsuis-reative Th cells were detectable 17 days post 2nd booster and infection was not restricted to the day of challenge as indicated by the course of body temperature (Fig. S2) and the detection of the challenge strain in the mitral valve of piglet #1 (Table S2). In experiments designed to analyse antigen-specific recall of Th cells by using DO11.10/RagKO mice immunized and infected with Ova-expressing S. pneumoniae, Trzciński et al. demonstrated that vaccination-induced Th17 cells are only protective against S. pneumoniae by antigen-specific recall during challenge infection [21]. However, based on our results we suppose that IdeSsuis is not an immunodominant T cell antigen under invasive infection and therefore a direct involvement of IdeSsuis-reactive Th cells in protective mechanisms is improbable.
As we could previously demonstrate, the vaccination with rIdeSsuis elicits antibodies neutralizing the IgM protease activity of IdeSsuis [11]. Recently we demonstrated that the activity of complement is important for clearance of S. suis in porcine blood, whereas the complement deposition is reduced by the protease activity of IdeSsuis [19]. Thus, neutralization of the IgM protease by α-IdeSsuis-IgGs should improve the complement-mediated killing of S. suis in porcine blood.
Additionally, in our previous study we found a significantly lower bacterial survival factor of S. suis wt in blood of vaccinated piglets in comparison to the respective survival factor of the IdeSsuis mutant 10ΔideSsuis [11]. In agreement with the location of IdeSsuis on the bacterial surface [16], the most plausible explanation for this observation is that α-IdeSsuis antibodies mediate opsonophagocytosis. However, a significant difference was not observed in this study (Figs. 3 and S1).
As mentioned above, prime-booster-booster vaccination was necessary to elicit α-IdeSsuis-IgG levels comparable to those of our previous study. Still, in case of five piglets less than 43 ELISA units were measured at 17 d post 2nd booster (Table S2). α-IdeSsuis-IgG levels and the clinical scores obtained following experimental infection did not show a significant correlation (data not shown). Further studies are necessary to clarify if the data obtained in the described α-IdeSsuis-IgG ELISA allows setting up a threshold for protection against severe disease.
In summary, this study demonstrates protection against S. suis cps9 through prime-booster-booster vaccination of piglets with rIdeSsuis. This vaccination was associated with induction of α-IdeSsuis-IgG and IdeSsuis-reactive T cells. We suppose that IdeSsuis -reactive Th cells are important for the B cell immune response to IdeSsuis, but recall of IdeSsuis -reactive Th cells was not detectable after challenge and is thus not considered to be crucial for protection.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This study was financially supported by IDT Biologika GmbH. IDT Biologika GmbH applied for a European patent concerning an IdeSsuis-based S. suis vaccine (Nr. 14 170 637.4).
Acknowledgements
We thank H. Smith (DLO-Lelystad, Netherlands) for S. suis strain 10 and P. Valentin-Weigand (Veterinary University Hannover, Germany) for strains A3286/94 and V5404/2. This study was financially supported by IDT Biologika GmbH. Furthermore, we acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2019.100046.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Goyette-Desjardins G., Auger J.-P., Xu J., Segura M., Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3 doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisselink H.J., Smith H.E., Stockhofe-Zurwieden N., Peperkamp K., Vecht U. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet Microbiol. 2000;74:237–248. doi: 10.1016/s0378-1135(00)00188-7. [DOI] [PubMed] [Google Scholar]
- 3.Büttner N., Beineke A., de Buhr N., Lilienthal S., Merkel J., Waldmann K.H. Streptococcus suis serotype 9 bacterin immunogenicity and protective efficacy. Vet Immunol Immunopathol. 2012;146:191–200. doi: 10.1016/j.vetimm.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Dekker C.N.T., Bouma A., Daemen A.J.J.M., Van Leengoed L.A.M.G., Jonker F.H., Wagenaar J.A. Homologous whole bacterin vaccination is not able to reduce Streptococcus suis serotype 9 strain 7997 transmission among pigs or colonization. Vaccine. 2011;30:1379–1387. doi: 10.1016/j.vaccine.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Dawei G., Liping W., Chengping L. In vitro biofilm forming potential of Streptococcus suis isolated from human and swine in China. Brazilian J Microbiol. 2012;43:993–1004. doi: 10.1590/S1517-838220120003000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieckmann K., Müller K., Moter A., Baums C.G., Seydel A. Streptococcus suis serotype 9 endocarditis and subsequent severe meningitis in a growing pig despite specific bactericidal humoral immunity. JMM Case Rep. 2017;4:e005093. doi: 10.1099/jmmcr.0.005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisselink H.J., Vecht U., Stockhofe-Zurwieden N., Smith H.E. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet Rec. 2001;148:473–477. doi: 10.1136/vr.148.15.473. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Gottschalk M., Esgleas M., Lacouture S., Dubreuil J.D., Willson P. Immunization with recombinant Sao protein confers protection against Streptococcus suis infection. Clin Vaccine Immunol. 2007;14:937–943. doi: 10.1128/CVI.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang A., Chen B., Li R., Mu X., Han L., Zhou H. Identification of a surface protective antigen, HP0197 of Streptococcus suis serotype 2. Vaccine. 2009;27:5209–5213. doi: 10.1016/j.vaccine.2009.06.074. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Xia J., Tan C., Zhou Y., Wang Y., Zheng C. Evaluation of the immunogenicity and the protective efficacy of a novel identified immunogenic protein, SsPepO, of Streptococcus suis serotype 2. Vaccine. 2011;29:6514–6519. doi: 10.1016/j.vaccine.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Seele J., Hillermann L.M., Beineke A., Seitz M., von Pawel-Rammingen U., Valentin-Weigand P. The immunoglobulin M-degrading enzyme of Streptococcus suis, IdeSsuis, is a highly protective antigen against serotype 2. Vaccine. 2015;33:2207–2212. doi: 10.1016/j.vaccine.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 12.Hsueh K.J., Lee J.W., Hou S.M., Chen H.S., Chang T.C., Chu C.Y. Evaluation on a Streptococcus suis vaccine using recombinant Sao-L protein manufactured by bioreactors as the antigen in pigs. Transbound Emerg Dis. 2014;61:e35–e43. doi: 10.1111/tbed.12067. [DOI] [PubMed] [Google Scholar]
- 13.King S.J., Leigh J.A., Heath P.J., Luque I., Tarradas C., Dowson C.G. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol. 2002;40:3671–3680. doi: 10.1128/JCM.40.10.3671-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Martinez G., Gottschalk M., Lacouture S., Willson P., Dubreuil J.D. Identification of a surface protein of Streptococcus suis and evaluation of its immunogenic and protective capacity in pigs. Infect Immun. 2006;74:305–312. doi: 10.1128/IAI.74.1.305-312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baums C.G., Kock C., Beineke A., Bennecke K., Goethe R., Schröder C. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin Vacc Immunol. 2009;16:200–208. doi: 10.1128/CVI.00371-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seele J., Singpiel A., Spoerry C., von Pawel-Rammingen U., Valentin-Weigand P., Baums C.G. Identification of a novel host-specific IgM protease in Streptococcus suis. J Bacteriol. 2013;195:930–940. doi: 10.1128/JB.01875-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Pawel-Rammingen U., Johansson B.P., Björck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seele J., Beineke A., Hillermann L.M., Jaschok-Kentner B., Von Pawel-Rammingen U., Valentin-Weigand P. The immunoglobulin M-degrading enzyme of Streptococcus suis, IdeSsuis, is involved in complement evasion. Vet Res. 2015;46:45. doi: 10.1186/s13567-015-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rungelrath V., Weiße C., Schütze N., Müller U., Meurer M., Rohde M. IgM cleavage by Streptococcus suis reduces IgM bound to the bacterial surface and is a novel complement evasion mechanism. Virulence. 2018;9:1314–1337. doi: 10.1080/21505594.2018.1496778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson R., Cohen J.M., Jose R.J., De Vogel C., Baxendale H., Brown J.S. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015;8:627–639. doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trzciński K., Thompson C.M., Srivastava A., Basset A., Malley R., Lipsitch M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+T cells. Infect Immun. 2008;76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecours M.-P., Letendre C., Clarke D., Lemire P., Galbas T., Benoit-Biancamano M.-O. Immune-responsiveness of CD4+ T cells during Streptococcus suis serotype 2 infection. Sci Rep. 2016;6:38061. doi: 10.1038/srep38061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beineke A., Bennecke K., Neis C., Schröder C., Waldmann K.-H., Baumgärtner W. Comparative evaluation of virulence and pathology of Streptococcus suis serotypes 2 and 9 in experimentally infected growers. Vet Microbiol. 2008;128:423–430. doi: 10.1016/j.vetmic.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Rehm T., Baums C.G., Strommenger B., Beyerbach M., Valentin-Weigand P., Goethe R. Amplified fragment length polymorphism of Streptococcus suis strains correlates with their profile of virulence-associated genes and clinical background. J Med Microbiol. 2007;56:102–109. doi: 10.1099/jmm.0.46616-0. [DOI] [PubMed] [Google Scholar]
- 25.Silva L.M.G., Baums C.G., Rehm T., Wisselink H.J., Goethe R., Valentin-Weigand P. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet Microbiol. 2006;115:117–127. doi: 10.1016/j.vetmic.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Smith H.E., Damman M., van der Velde J., Wagenaar F., Wisselink H.J., Stockhofe-Zurwieden N. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinari G., Talay S.R., Valentin-Weigand P., Rohde M., Chhatwal G.S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baums C.G., Kaim U., Fulde M., Ramachandran G., Goethe R., Valentin-Weigand P. Identification of a novel virulence determinant with serum opacification activity in Streptococcus suis. Infect Immun. 2006;74:6154–6162. doi: 10.1128/IAI.00359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebner F., Schwiertz P., Steinfelder S., Pieper R., Zentek J., Schütze N. Pathogen-reactive T helper cell analysis in the pig. Front Immunol. 2017;8:179. doi: 10.3389/fimmu.2017.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerner W., Talker S.C., Koinig H.C., Sedlak C., Maira K.H., Saalmüller A. Phenotypic and functional differentiation of porcine αβ T cells: current knowledge and available tools. Mol Immunol. 2015;66:3–13. doi: 10.1016/j.molimm.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Zuckermann F.A., Husmann R.J. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500–512. [PMC free article] [PubMed] [Google Scholar]
- 32.Segura M. Streptococcus suis vaccines: candidate antigens and progress. Expert Rev Vacc. 2015;0584:1–22. doi: 10.1586/14760584.2015.1101349. [DOI] [PubMed] [Google Scholar]
- 33.Gottschalk M., Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol. 2000;76:259–272. doi: 10.1016/s0378-1135(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 34.Martins K.A.O., Cooper C.L., Stronsky S.M., Norris S.L.W., Kwilas S.A., Steffens J.T. Adjuvant-enhanced CD4 T cell responses are critical to durable vaccine immunity. EBioMedicine. 2016;3:67–78. doi: 10.1016/j.ebiom.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ugolini M., Gerhard J., Burkert S., Jensen K.J., Georg P., Ebner F. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat Immunol. 2018;19:386–396. doi: 10.1038/s41590-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 36.Rieckmann K., Seydel A., Szewczyk K., Klimke K., Rungelrath V., Baums C.G. Streptococcus suis cps7: an emerging virulent sequence type (ST29) shows a distinct, IgM-determined pattern of bacterial survival in blood of piglets during the early adaptive immune response after weaning. Vet Res. 2018;49:48. doi: 10.1186/s13567-018-0544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.