Abstract
Objective
To examine change in bone mineral density (BMD) and trabecular bone score (TBS) among older adult weight loss regainers (WR) and weight loss maintainers (WM).
Methods
Observational data come from 77 older adults (67±5 (SD) years, 69% women, 70% Caucasian) having obesity (BMI: 33.6±3.7 kg/m2) who lost weight during an 18 month weight loss intervention. Total body mass and composition, along with regional (total hip, femoral neck, lumbar spine) BMD and TBS, were measured at baseline, 18, and 30 months. WR (n=36) and WM (n=41) categories were defined as a ≥ or < 5% weight gain from 18 to 30 months, respectively.
Results
Among skeletal indices, only total hip BMD was significantly reduced during the 18 month intervention period in both WRs [−3.9 (95% CI: −5.8 to −2.0) %] and WMs [−2.4 (−4.3 to −0.5) %]; (p=0.07). After adjustment for relevant baseline covariates and weight change from 0-18 months, 30 month change in total hip BMD was −2.6 (−4.3 to −0.9) % and −3.9 (−5.7 to −2.1) % among WRs and WMs, respectively (p=0.07).
Conclusions
Loss of hip BMD persists in the year following a weight loss intervention among older adults having obesity, regardless of weight regain status.
Keywords: obesity, weight loss, weight regain, bone health
INTRODUCTION
Older adults are a rapidly growing segment of the world’s population (1, 2). The number of older adults classified as having obesity has also increased at an unprecedented rate over the past few decades, and currently 36.5% of U.S. older adults live with obesity (3). Historically obesity has been considered osteoprotective due to increased bone loading (4); however, this position has been recently challenged (5). Observational data show an inverse and non-linear relationship between body mass index (BMI) and bone mineral density (BMD), with lowest BMD observed in the BMI extremes (i.e., <15 kg/m2 and >30 kg/m2) (6, 7). Excess weight in the form of fat mass may be harmful to bone by exerting a detrimental effect on osteoblast activity (8). Additionally, data show that while underweight individuals are at higher risk for fracture, the majority of osteoporotic fractures occur in people having obesity due to the high prevalence of obesity, implying that the public health burden of osteoporotic fracture lies within this segment of the population (9).
Lifestyle-based weight loss is a first line therapy for obesity; however among older adults, weight loss recommendation is controversial (10). Although randomized controlled trial (RCT) data show weight loss interventions including diet and exercise can improve physical function and reduce fat mass, older adults often lose substantial bone mass (11–13) - roughly 1-4% BMD loss (depending on the skeletal site) concomitant with 10% weight loss (14). This is concerning because BMD is strong predictor of osteoporotic fracture risk (15), with every standard deviation decrease in BMD increasing fracture risk threefold (16). Indeed, recent data from the Look AHEAD study show intentional weight loss of 6-9% maintained throughout a decade increased hip, pelvis, and upper arm fractures by 39%, as compared to the education control condition (17).
Weight regain is also a common occurrence among those who lose weight, considering only l in 6 adults having obesity reported maintaining weight loss of at least 10% for 1 year at any point in their lives (18). Observational data indicate that weight variability and regain can contribute to increased risk of fracture (19–21), and emerging data suggest that BMD is not recovered by weight regain and that bone loss can continue to occur after weight loss ends (22–24). At present, the few studies conducted in this area primarily assessed post-menopausal women and only included BMD as a measure of bone health, lacking further measurement of bone integrity.
Therefore, the aim of this study was to assess the effect of weight regain or continued weight loss maintenance on BMD and trabecular bone score (TBS) among male and female older adults classified as having overweight/obesity and who lost weight during an 18 month, lifestyle based weight loss intervention. Secondary analysis were used to assess how the change in select independent variables (age, race/ethnicity, sex/gender, fat mass, lean body mass) over time influenced change in BMD at each site. We hypothesize that weight regain will partially restore bone loss associated with weight loss and that lean body mass will be a stronger positive predictor of bone health outcomes than fat mass.
METHODS
Study Design Overview
Data from the present analysis come from the Cooperative Lifestyle Extension Study II (CLIP II; ). The CLIP II study was a multisite, single-blinded, RCT examining the effect of exercise type during intentional weight loss on mobility and strength outcomes in older adults classified as having overweight/obesity with cardiovascular disease and/or metabolic dysfunction. Full inclusion/exclusion and intervention descriptions can be found in the study design paper (25). Briefly, 249 participants were allocated into three intervention arms: weight loss alone, weight loss with aerobic exercise training, and weight loss with resistance exercise training. The interventions lasted for 18 months and participants were asked to return for long term follow up assessments at 30 months, after a year (i.e., from 18 to 30 months post randomization) of free living conditions. Of the original 249 participants, 187 had regional (i.e., hip/spine) dual energy x-ray absorptiometry (DXA) data at baseline. Intervention effects on mobility and strength (26), body composition (27), and bone health (28) outcomes have been previously published. The present analysis involves only CLIP II participants who presented with complete baseline, 18, and 30 month DXA derived total body mass and regional BMD data, and lost any amount of weight during the 18 month intervention period (n=77).
Weight Pattern Classification Exposure: Weight Regain versus Weight Loss Maintenance
Total body mass was obtained at baseline (n=187), 18 (n=138), and 30 (n=77) months using DXA (iDXA, GE Medical Systems, Madison, WI, USA) and used to classify participants into weight pattern categories. Participants were classified as weight regainers (WR) or weight maintainers (WM) based on: (1) a negative DXA-acquired total body mass change from baseline to 18 months, and (2) a 5% change in DXA-acquired total body mass from 18 to 30 month assessments (WR: ≥5% weight change; WM: <5% weight change; see Supplementary Figure 1).
Bone Outcome Measures: Bone Mineral Density and Trabecular Bone Score
Areal BMD of the total hip, the femoral neck, and the lumbar spine; and trabecular bone score (TBS) of the lumbar spine were also determined by DXA (iDXA, GE Medical Systems, Madison, WI, USA) at baseline, 18, and 30 months. All scans were performed and analyzed by a DXA technologist, certified by the International Society of Clinical Densitometry, who was blinded to the intervention assignment. Coefficients of variation (CVs) from repeated measurements (on the same individual by the same technician) at our institution are 1.21% for total hip BMD, 1.82% for femoral neck BMD, 1.38% for lumbar spine BMD, and 3.27% for TBS.
Relevant Covariate Measurements
Baseline demographic information, including age, sex/gender, race/ethnicity, education level, and medical history/comorbid status were captured via self-report at the baseline visit. Height was assessed without shoes to the nearest 0.25cm using a stadiometer (Health OMeter, Newell Brands Inc, Boca Raton, FL, USA) and body mass measured to the nearest 0.05 kg using a calibrated and certified digital scale (Health O Meter Professional 349KLX; Newell Brands Inc), with BMI calculated in kg/m2. Total body fat and lean masses were assessed using DXA at baseline, 18, and 30 months using previously described methodology. Lastly, the Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity questionnaire for older adults (29) was also administered at baseline, 18, and 30 months.
Statistical Analysis
Descriptive statistics were calculated overall and by weight pattern group at baseline. Means and 95% CIs were calculated for each measure of change in body mass and composition by weight pattern group from 0-18 months, 18-30 months, and 0-30 months, using a mixed-model repeated measures analysis of covariance (ANCOVA), with subject as a random effect to account for the fact that multiple measurement within a participant over time were not independent. Covariates used in the analysis included time, intervention group assignment, age, sex/gender, race/ethnicity, baseline value of the outcome, and recruitment wave (as a random effect). Sex/gender by weight pattern category interaction terms were tested in all models to determine whether stratified analyses should be pursued, and secondary models further adjusted for 0-18 month weight change. In exploratory analyses, simple linear regression lines and correlation coefficients were generated between hip BMD change and weight change during 0-18 months and 18-30 months among the subset of WRs who regained all lost weight (n=10). Lastly, to examine the effect of select demographic and modifiable determinants on bone change, a mixed-model ANCOVA was used (with subject as a random effect) and including the following covariates: time (18 or 30 months), intervention group assignment, age, sex/gender, race/ethnicity, baseline value of the outcome, wave (as a random effect) and total body fat mass (change from baseline), total body lean mass (change from baseline), and CHAMPS physical activity (change from baseline). Results are presented as beta-coefficients and associated p-values. All analysis were run using SAS version 9.4.
Results
Participant Characteristics
Descriptive information for the CLIP II participants with baseline, 18, and 30 month DXA-derived BMD/TBS data are presented in Table 1, overall and by weight pattern category, with no significant differences between groups (all p>0.05). Overall, participants were 67.0±4.9 (SD) years old with an average BMI of 33.6±3.7 kg/m2. Caucasian women made up the majority of the study sample, and nearly 60% of participants listed an associate degree or higher as their highest level of education received. Nearly 43% of participants presented with DXA-derived osteopenia at baseline. Lastly, responses to the modified CHAMPS physical activity questionnaire revealed participants engaged in an average 28.2 minutes of moderate to vigorous physical activity per week.
Table 1.
Baseline descriptive characteristics of study sample by weight regain status and overall. Data are reported as mean (SD) or n (%).
| Descriptive Characteristic | Weight Regainers (N=36) | Weight Maintainers (N=41) | Overall (N=77) |
|---|---|---|---|
| Age (years) | 67.9 (5.5) | 66.2 (4.1) | 67.0 (4.9) |
| Female, n (%) | 27 (75.0) | 26 (63.4) | 53 (68.8) |
| Race/Ethnicity, n (%) | |||
| African American | 9 (25.0) | 11 (26.8) | 20 (26.0) |
| Hispanic | 1 (2.8) | 0 (0.0) | 1 (1.3) |
| White | 25 (69.4) | 29 (70.7) | 54 (70.1) |
| Other/Mixed/Missing | 1 (2.8) | 1 (2.4) | 2 (2.6) |
| Highest Level of Education, n (%) | |||
| Less than high school diploma | 2 (5.6) | 1 (2.4) | 3 (3.9) |
| High school/some college | 13 (36.1) | 15 (36.6) | 28 (36.4) |
| Associate’s degree or higher | 21 (58.3) | 25 (61.0) | 46 (59.7) |
| Comorbidities, n (%) | |||
| CVD History | 26 (72.2) | 28 (68.3) | 54 (70.1) |
| Diabetes | 8 (22.2) | 10 (24.4) | 18 (23.4) |
| Arthritis | 18 (51.4) | 24 (60.0) | 42 (56.0) |
| Hypertension | 25 (69.4) | 32 (78.0) | 57 (74.0) |
| Cancer | 8 (22.2) | 6 (14.6) | 14 (18.2) |
| Metabolic Syndrome | 22 (61.1) | 23 (56.1) | 45 (58.4) |
| BMI (kg/m2) | 33.5 (3.7) | 33.6 (3.8) | 33.6 (3.7) |
| DXA-derived Osteoporosis, n (%) | 0 (0.0) | 1 (2.4) | 1 (1.3) |
| DXA-derived Osteopenia, n (%) | 19 (52.8) | 14 (34.1) | 33 (42.9) |
| CHAMPS Physical Activity Questionnaire (min/week) | 16.8 (27.8) | 38.2 (63.6) | 28.2 (51.0) |
Continuous data are presented as means (standard deviations) and categorical data are presented as absolute number (percentage). Abbreviations: CVD: Cardiovascular Disease; BMI: Body Mass Index; kg: kilogram; m: meter; DXA: Dual-Energy X-ray Absorptiometry; CHAMPS: Community Healthy Activities Model Program for Seniors; min: minutes. There were no significant differences between groups regarding baseline characteristics (all p>0.05).
Intervention Effects on Fat, Lean, and Bone Mass
As these data come from a RCT of exercise and weight loss in older adults (25), treatment effects on mobility (26), body composition (27), and bone outcomes (28) have been previously reported. Briefly, with regard to body composition, significant reductions in total fat and lean mass were observed in all groups over the 18-month intervention period, with resistance exercise able to attenuate lean mass loss compared to aerobic exercise (−0.8 ± 0.3 kg, −1.5% vs −1.0 ± 0.3 kg; −2.0%), and weight loss combined with either exercise modality augmented fat mass loss compared to weight loss alone (WL+AT: −6.8 ± 0.6 kg, −16.4% and WL+RT: −7.8 ± 0.5 kg, −19.0% vs WL: −4.8 ± 0.6 kg, −10.9%) (27). Of bone outcomes, only total hip BMD was significantly reduced (by approximately 2% in all treatment groups) over the 18 month intervention, which persisted at the 30 month follow-up (28).
Associations between Weight Regain Status and Bone Outcome Measures
Based on the previously described 5% weight regain cut-point, 36 (47%) were classified as weight regainers (WR), and 41 (53%) participants were classified as weight maintainers (WM), with similar representation from each treatment group. Of the 36 WRs: 8 came from the weight loss alone, 13 from the weight loss with aerobic exercise, and 15 from the weight loss with resistance exercise groups. Of the 41 WMs: 15 came from the weight loss alone, 14 from the weight loss with aerobic exercise, and 12 from the weight loss with resistance exercise groups. Raw aggregate baseline and model-adjusted absolute change in total body composition and regional bone mass by weight pattern category are presented in Table 2, with percent changes discussed in the text. No sex/gender by weight pattern category interaction terms were significant, thus stratified analyses were not pursued.
Table 2.
Raw baseline and adjusted change in total body composition and regional bone mass by weight pattern category.
| Outcome Variable | Baseline (Raw) | Weight Regainers | Weight Maintainers | Group Comparison p-values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0-18 Month Change | 18-30 Month Change | 0-30 Month Change | 0-18 Month Change | 18-30 Month Change | 0-30 Month Change | 0-18 Months | 18-30 Months | 0-30 Months | ||
| Total Body Mass (kg) | 93.5±14.8 | −11.2 (−15.1, −7.3) | 7.2 (4.3, 10.2) | −4.0 (−7.9, −0.03) | −6.4 (−10.4, −2.4) | 0.8 (−1.9, 3.5) | −5.7 (−9.7, −1.7) | 0.002 | 0.002 | 0.26 |
| Total Body Fat Mass (kg) | 41.3±8.4 | −9.6 (−12.7, −6.4) | 6.5 (4.0, 8.9) | −3.1 (−6.3, 0.1) | −4.9 (−8.1, −1.7) | 1.3 (−1.0, 3.6) | −3.6 (−6.8, −0.4) | 0.0003 | 0.003 | 0.67 |
| Total Body Lean Mass (kg) | 49.5±10.3 | −1.6 (−2.7, −0.5) | 0.7 (−0.1, 1.6) | −0.9 (−2.0, 0.2) | −1.5 (−2.6, −0.3) | −0.5 (−1.3, 0.3) | −2.0 (−3.1, −0.8) | 0.78 | 0.04 | 0.01 |
| Total Hip BMD (g/cm2) | 1.016±0.138 | −0.038 (−0.057, −0.019) | 0.010 (−0.006, 0.026) | −0.028 (−0.047, −0.009) | −0.024 (−0.043, −0.004) | −0.004 (−0.019, 0.010) | −0.028 (−0.048, −0.009) | 0.09 | 0.19 | 0.95 |
| Femoral Neck BMD (g/cm2) | 0.950±0.132 | 0.002 (−0.024, 0.028) | −0.013 (−0.035, 0.008) | −0.011 (−0.037, 0.015) | 0.009 (−0.017, 0.036) | −0.012 (−0.033, 0.008) | −0.003 (−0.030, 0.023) | 0.53 | 0.96 | 0.48 |
| Lumbar Spine BMD (g/cm2) | 1.271±0.199 | −0.004 (−0.026, 0.018) | 0.002 (−0.017, 0.021) | −0.002 (−0.024, 0.020) | −0.005 (−0.028, 0.017) | 0.003 (−0.014, 0.021) | −0.002 (−0.025, 0.020) | 0.91 | 0.92 | 0.98 |
| Trabecular Bone Score | 1.421±0.100 | −0.018 (−0.056, 0.020) | −0.025 (−0.057, 0.008) | −0.043 (−0.080, −0.005) | −0.017 (−0.055, 0.020) | −0.005 (−0.035, 0.025) | −0.023 (−0.061, 0.015) | 0.97 | 0.38 | 0.22 |
Unadjusted baseline data are presented in aggregate and as means ± SD. Change data are presented as means (95% Confidence Interval), and estimated using a mixed-model repeated measures analysis of covariance (ANCOVA), with subject as a random. Covariates used in the analysis included time, intervention group assignment, age, sex/gender, race/ethnicity, baseline value of the outcome, and recruitment wave (as a random effect). Abbreviations: kg: kilogram; BMD: Bone Mineral Density; g: gram; cm: centimeter. Bolded p-values indicate statistical significance (p<0.05).
Briefly, WR lost more total body and fat mass from 0-18 months than WM (WR: −12.3% vs WM: −7.1%; p<0.01); however, by 30 months, weight change from baseline was similar between groups (WR: −4.6% vs WM: −6.2%; p=0.31). This observation was largely driven by fat mass, as fat mass loss from 0-18 months was nearly doubled in the WR group compared to the WM group (WR: −23.4% vs WM: −12.9%; p<0.01); yet, by 30 months change in fat mass from baseline was similar (WR: −8.1% vs WM: −9.6%; p=0.65). Lean mass was reduced by approximately 3% in both groups during the intervention period, yet by 30 months, change in lean mass from baseline was halved in the WR group compared to WM (WR: −2.0% vs WM: −4.0%; p=0.02). Of bone outcome measures, only total hip BMD was significantly reduced from 0-18 months, with marginally greater reductions observed in WR compared to WM (WR: −3.9% vs WM: −2.4%; p=0.07). Approximately 3% hip BMD loss persisted for both groups into the 30 month follow up period. By 30 months, TBS was modestly reduced from baseline in WRs, but not differently from WMs (WR: −2.9% vs WM: −1.6%; p=0.25).
Given the responsivity of bone to mechanical load (30), and the differential weight loss observed between groups during the active intervention period, a second set of models were run, further adjusting for weight change from 0-18 months. Regional BMD and TBS results are presented in Figure 1. Significantly reduced total hip BMD estimates were observed for both groups at 30 months, with marginally greater losses seen in WM (3.9 (−5.7, −2.1) %) compared to WR (2.6 (−4.3,−0.9) %); p=0.07, No other results were impacted by this additional adjustment.
Figure 1.
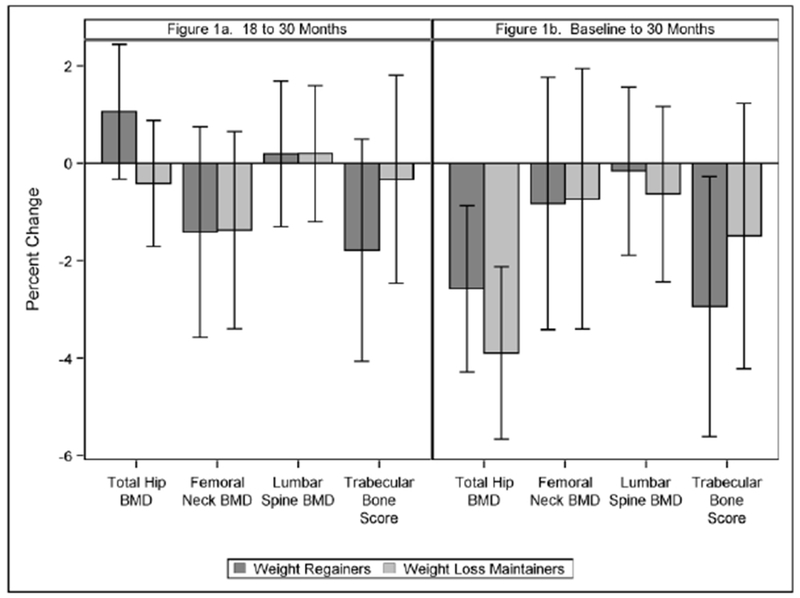
Percent change (95% CI) in regional bone mineral density and TBS by weight regain status from 18 to 30 months (a) and from baseline to 30 months (b) Model estimates were made using a mixed-model repeated measures analysis of covariance (ANCOVA), with subject as a random effect. Covariates used in the analysis included time, intervention group assignment, age, sex/gender, race/ethnicity, baseline value of the outcome, recruitment wave (as a random effect), and further adjusting for weight change from 0-18 months.
Lastly, to further elucidate the effect of weight loss and weight regain on change in total hip BMD, a simple regression line was fit between change in total body mass and change in hip BMD during weight loss (0-18 months) and weight regain (18-30 months) for WR individuals who regained all lost weight (n=10). Exploratory data are presented in Supplementary Figure 2. A stronger correlation between weight loss and hip bone loss was observed (r=0.23), as compared to weight regain and hip bone regain (r=0.06); yet, lines did not significantly differ from one another (p=0.48).
Select Demographic and Modifiable Determinants of Bone Change
Finally, results from analyses examining the effect of age, sex/gender, and race/ethnicity, as well as change in total body fat mass, total body lean mass, and CHAMPS score as predictors of change in regional BMD and TBS can be found in Table 3. Female sex/gender was associated with reduced lumbar spine (β= −0.027; p<0.01) and total hip (β= −0.015; p<0.01) BMD. Change in total hip BMD was directly associated with change in total body fat (β= 0.002; p<0.01) and lean (β= 0.004; p=0.01) masses. Although non-significant, marginal associations were observed for TBS, where change in total body lean mass was directly associated with change in TBS (β=0.005; p=0.09), while an inverse association was observed for change in total fat mass (β=−0.002; p=0.09).
Table 3.
Multiple regression model estimates of select demographic and modifiable determinants of bone change in all participants over the 30 month period.
| Outcome Variable | Predictor Variable | β | p-value |
|---|---|---|---|
| Δ in Total Hip BMD (g/cm2) | Age (years) | −0.0008 | 0.1383 |
| Female Sex/gender | −0.0146 | 0.0162 | |
| African American Race/Ethnicity | 0.0050 | 0.4489 | |
| Δ in Total Body Fat Mass (kg) | 0.0021 | <.0001 | |
| Δ in Total Body Lean Mass (kg) | 0.0035 | 0.0109 | |
| Δ in CHAMPS Physical Activity (min/week) | −3.4E-06 | 0.7623 | |
| Δ in Femoral Neck BMD (g/cm2) | Age (years) | −0.0014 | 0.1069 |
| Female Sex/gender | −0.0066 | 0.4489 | |
| African American Race/Ethnicity | 0.0148 | 0.1404 | |
| Δ in Total Body Fat Mass (kg) | 0.0009 | 0.2605 | |
| Δ in Total Body Lean Mass (kg) | 0.0001 | 0.9496 | |
| Δ in CHAMPS Physical Activity (min/week) | −9.4E-06 | 0.5858 | |
| Δ in Lumbar Spine BMD (g/cm2) | Age (years) | −0.0002 | 0.7487 |
| Female Sex/gender | −0.0271 | 0.0006 | |
| African American Race/Ethnicity | −0.0103 | 0.2251 | |
| Δ in Total Body Fat Mass (kg) | 0.0008 | 0.2214 | |
| Δ in Total Body Lean Mass (kg) | 0.0008 | 0.6488 | |
| Δ in CHAMPS Physical Activity (min/week) | 6.3E-06 | 0.6727 | |
| Δ in Trabecular Bone Score | Age (years) | 0.0016 | 0.2071 |
| Female Sex/gender | −0.0129 | 0.3021 | |
| African American Race/Ethnicity | 0.0079 | 0.5710 | |
| Δ in Total Body Fat Mass (kg) | −0.0019 | 0.0880 | |
| Δ in Total Body Lean Mass (kg) | 0.0052 | 0.0907 | |
| Δ in CHAMPS Physical Activity (min/week) | −1.8E-05 | 0.4920 |
Results are presented as beta-coefficients and associated p-values, and adjusted for baseline value of the outcome, intervention group, time, and random wave effect. Abbreviations: BMD: bone mineral density; g: grams; cm: centimeters; CHAMPS: Community Healthy Activities Model Program for Seniors, kg: kilograms, min: minutes. Bolded p-values indicate statistical significance (p<0.05).
DISCUSSION
This analysis was designed to assess change in BMD and TBS with weight regain or weight maintenance following intentional weight loss among older adults. Here we show that loss of hip BMD persists in the year following an 18 month weight loss intervention, regardless of weight regain status in a group of older men and women having obesity. However, after adjusting for 0-18 month weight change, data suggest that hip BMD may be partially restored with weight regain. Exploratory subgroup analysis also suggests that the association between weight loss and hip BMD loss is stronger than the association between weight regain and hip BMD regain. Lastly, we report loss in total fat and lean masses contribute to reduced hip BMD; yet, loss in fat mass may signal improved TBS. Together, our findings emphasize the importance of identifying and implementing interventions that can target fat, while minimizing lean, mass loss to optimize bone health response during weight loss in older adults.
To our knowledge, several studies have assessed the effects of weight loss and weight regain on site specific BMD (23, 24, 31–35), with some studies showing weight regain partially restores bone loss at the trochanter and radius (24, 32), and other studies showing no restoration (23, 31, 33–35). Inconsistencies may be attributed to varying study characteristics; thus, we interpret our findings in consideration of the two studies by Villalon et al (23) and Von Thun (24) with designs that most closely resemble the present analysis. In the first published study by Villalon et al (23), authors examined change in bone density among 23 older women assigned to a six month weight loss period (resulting in a ~5% weight loss) and after the following 12 months (resulting in a regain of ~75% of the lost weight). Lumbar spine and hip BMD were significantly decreased with weight loss; however, weight regain was not associated with bone regain at either site, and elevations of C-terminal telopeptide of type I collagen (CTX, a biomarker of bone resorption) noted during weight loss remained elevated during the weight regain period. In contrast, Von Thun et al (24) found that among postmenopausal women who experienced ~10% weight reduction as a part of an intentional weight loss intervention, and maintained the loss during the following 18 months, there was more than double the BMD loss at the trochanter and radial BMD sites compared to those who regained ~70% of lost weight. In our study, participants who maintained their weight did not experience accelerated bone loss compared to those who regained weight as found in the Von Thun study, possibly attributed to all female population compared to a mixed population in the current paper. However, weight loss associated hip bone loss, which was similar in magnitude to what was reported by Von Thun, persisted into the follow up period for both groups. Collectively, these findings suggest that at least some of the BMD lost during weight reduction among older adults is lasting. TBS data presented here also extend the current knowledge base, signaling that modest declines in TBS observed during weight loss may continue with weight regain.
In alignment with the mechanostat theory (36), results from our analyses also affirm that reduction in total body mass, regardless of compartment, is associated with loss of BMD, particularly at the total hip. This observation is likely due to decreased skeletal loading; however, negative energy state associated with weight loss can create biochemical alterations triggering increased bone resorption. For example, reduction in the hormone leptin, through obesity and weight loss, suppresses key regulatory pathways for growth and reproduction resulting in impaired bone growth, maturation, and turnover (30). The hormone estrogen also plays a dominant role in regulating the sensitivity of the skeleton to loading (37), particularly in post-menopausal women, and is known to be affected by weight loss (38). Admittedly, our study is limited in its ability to draw mechanistic inference, and we implore future research to examine the impact of non-mechanical stimuli of bone homeostasis during weight loss.
While associations were non-significant, the observed signal suggesting an inverse association between fat mass and TBS is an intriguing finding, and fits into a larger narrative where excess weight, when carried as fat, may adversely affect bone health (5). For example, increases in bone marrow adipogenesis are associated with osteoporosis and age related bone loss suggesting stromal cell differentiation into adipocytes rather than osteoblasts (39). Further evidence links elevated BMI with lower rates of bone formation (40) and, while a negative relationship between TBS and BMI has been previously described (41), to our knowledge this is the first study associating change in fat mass to change in TBS. Because meaningful change thresholds for TBS have yet to be defined, clinical interpretability of our findings is challenging; yet, lower TBS scores are associated with increased fracture risk (42), and may imply that fat versus lean loss with weight loss carry different fracture risk profiles.
This analysis was the first to examine the effect of weight regain following intentional weight loss on change in BMD and TBS among both older men and women. Our sample size was relatively large with a total follow up period of 30 months, allowing adequate time for bone remodeling. That said, although DXA is a clinically relevant tool to assess BMD and TBS, in the context of weight loss among older adults having obesity, significant measurement error can occur (34). Additionally, although inclusion of TBS as a surrogate for bone microarchitecture is novel, there are more advanced measures of bone health, such as a CT-derived thickness and finite element strength estimates, which we encourage future studies to include. Lastly, even though our data utilize a RCT of intentional weight loss, analyses pertaining to the present investigation are observational and thus susceptible to bias and confounding.
In conclusion, results from these analyses suggest that weight regain may only partially recover weight loss induced total hip BMD loss in older adults. These data add to a growing body of literature suggesting that bone loss persists after weight loss ends. Thus, to preserve the integrity of the skeletal system, geriatricians and their patients should strive to minimize bone loss during active weight loss attempts and approach weight loss as a permanent lifestyle change. Better understanding of the biologic mechanisms driving weight change and bone change are needed to inform weight loss intervention strategies designed to optimize the musculoskeletal, response.
Supplementary Material
Supplementary Figure 1. Illustration of weight pattern categorization based on percent change in weight from 0 to 18 months (x-axis) and 18 to 30 months (y axis). Data points represent the 77 CLIP II individuals who lost weight during the intervention period (0 to 18 months), and regained at least 5% (denoted by dashed line) of lost weight during the 18 to 30 month period [weight regainers (WR) denoted with +], or did not regain at least 5% of lost weight during the 18 to 30 month period [weight maintainers (WM) denoted with O).”
Supplementary Figure 2. Exploratory associations between change in weight and change in BMD during weight loss and weight regain, among individuals who completely regained lost weight (n=10).
What is already known about the subject:
Weight loss in older adults is accompanied by loss in bone mineral density (BMD) and increased fracture risk.
Many adults are not successful at long term weight loss maintenance and for most, weight regain is an expected occurrence.
Observational studies link weight variability with increased fracture risk, with limited data from smaller studies in middle-aged women indicating that weight regain does not fully restore weight loss associated bone loss, and may even cause further bone loss.
What this study adds:
Among older adults classified as having overweight or obesity who participated in an 18 month intentional weight loss intervention, loss of hip BMD persists in the following year, regardless of weight regain status; however, after adjusting for 0-18 month weight loss, hip BMD is partially recovered with weight regain.
Loss in total fat and lean masses are associated with reduced hip BMD; however, loss in fat mass may signal improved trabecular bone score.
Acknowledgments
Funding: This project was supported by grants R18 HL076441 (MPIs: Rejeski and Marsh) and K01 AG047921 (PI: Beavers).
Footnotes
Clinical Trial Registration: Observational Data from Trial
Disclosure: The authors declared no relevant conflicts of interest.
References
- 1.United States Census Bureau. (2018). Sixty-Five Plus in the United States. [Web page]. URL https://www.census.gov/population/socdemo/statbriefs/agebrief.html
- 2.American Psychological Association. (2018). Older Adults’ Health and Age-Related Changes. [Web page]. URL http://www.apa.org/pi/aging/resources/guides/older.aspx
- 3.Center for Disease Control and Prevention. (2018). Adult Obesity Facts | Overweight & Obesity CDC. [Web page]. URL https://www.cdc.gov/obesity/data/adult.html
- 4.Sharma S, Tandon VR, Mahajan S, Mahajan V, Mahajan A. Obesity: Friend or foe for osteoporosis. J -Life Health 2014;5:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obesity Compston J. and fractures in postmenopausal women. Curr Opin Rheumatol 2015;27:414–419. [DOI] [PubMed] [Google Scholar]
- 6.Lurati A, Laria A. Relationship between Lumbar Bone Mineral Density (BMD) and Body Mass Index (BMI) in Pre-Menopausal Population. A Large Cohort Study. Rheumatol Curr Res 2015;5:1–4. [Google Scholar]
- 7.Palermo A, Tuccinardi D, Defeudis G, et al. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int J Environ Res Public Health 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapses SA, Pop LC, Wang Y. Obesity is a concern for bone health with aging. Nutr Res N Y N 2017;39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res Off J Am Soc Bone Miner Res 2012;27:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: Do benefits exceed potential risks? Exp Gerontol 2016;86:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensrud KE, Ewing SK, Stone KL, et al. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 2003;51:1740–1747. [DOI] [PubMed] [Google Scholar]
- 12.Ensrud KE, Fullman RL, Barrett-Connor E, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab 2005;90:1998–2004. [DOI] [PubMed] [Google Scholar]
- 13.Dennison E, Eastell R, Fall CH, Kellingray S, Wood PJ, Cooper C. Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 1999;10:384–391. [DOI] [PubMed] [Google Scholar]
- 14.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr 2012;32:287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet Lond Engl 1993;341:72–75. [DOI] [PubMed] [Google Scholar]
- 16.Zibellini J, Seimon RV, Lee CMY, et al. Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J Bone Miner Res Off J Am Soc Bone Miner Res 2015;30:2168–2178. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KC, Bray GA, Cheskin LJ, et al. The Effect of Intentional Weight Loss on Fracture Risk in Persons With Diabetes: Results From the Look AHEAD Randomized Clinical Trial. J Bone Miner Res Off J Am Soc Bone Miner Res 2017;32:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes 2005 2010;34:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French SA, Folsom AR, Jeffery RW, Zheng W, Mink PJ, Baxter JE. Weight variability and incident disease in older women: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord J Int Assoc Study Obes 1997;21:217–223. [DOI] [PubMed] [Google Scholar]
- 20.Søgaard AJ, Meyer HE, Tonstad S, Håheim LL, Holme I. Weight cycling and risk of forearm fractures: a 28-year follow-up of men in the Oslo Study. Am J Epidemiol 2008;167:1005–1013. [DOI] [PubMed] [Google Scholar]
- 21.Meyer HE, Tverdal A, Selmer R. Weight variability, weight change and the incidence of hip fracture: a prospective study of 39,000 middle-aged Norwegians. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 1998;8:373–378. [DOI] [PubMed] [Google Scholar]
- 22.Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging 2013;17:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villalon KL, Gozansky WS, Van Pelt RE, et al. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obes Silver Spring Md 2011;19:2345–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA. Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause N Y N 2014;21:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsh AP, Janssen JA, Ambrosius WT, et al. The Cooperative Lifestyle Intervention Program-II (CLIP-II): design and methods. Contemp Clin Trials 2013;36:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rejeski WJ, Ambrosius WT, Burdette JH, Walkup MP, Marsh AP. Community Weight Loss to Combat Obesity and Disability in At-Risk Older Adults. J Gerontol A Biol Sci Med Sci 2017;72:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beavers KM, Ambrosius WT, Rejeski WJ, et al. Effect of Exercise Type During Intentional Weight Loss on Body Composition in Older Adults with Obesity. Obes Silver Spring Md 2017;25:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beavers KM, Walkup MP, Weaver AA, et al. Effect of Exercise Modality During Weight Loss on Bone Health in Older Adults With Obesity and Cardiovascular Disease or Metabolic Syndrome: A Randomized Controlled Trial. J Bone Miner Res Off J Am Soc Bone Miner Res 2018;33:2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–1141. [DOI] [PubMed] [Google Scholar]
- 30.Iwaniec UT, Turner RT. Influence of body weight on bone mass, architecture and turnover. J Endocrinol 2016;230:R115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinton PS, Rector RS, Linden MA, et al. Weight-loss-associated changes in bone mineral density and bone turnover after partial weight regain with or without aerobic exercise in obese women. Eur J Clin Nutr 2012;66:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fogelholm GM, Sievänen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 2001;12:199–206. [DOI] [PubMed] [Google Scholar]
- 33.Fogelholm M, Sievänen H, Heinonen A, et al. Association between weight cycling history and bone mineral density in premenopausal women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 1997;7:354–358. [DOI] [PubMed] [Google Scholar]
- 34.Bosy-Westphal A, Later W, Schautz B, et al. Impact of intra- and extra-osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obes Silver Spring Md 2011;19:1503–1510. [DOI] [PubMed] [Google Scholar]
- 35.Avenell A, Richmond PR, Lean ME, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr 1994;48:561–566. [PubMed] [Google Scholar]
- 36.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec 1987;219:1–9. [DOI] [PubMed] [Google Scholar]
- 37.Westerlind KC, Wronski TJ, Ritman EL, et al. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci 1997;94:4199–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stolzenberg-Solomon RZ, Falk RT, Stanczyk F, et al. Sex hormone changes during weight loss and maintenance in overweight and obese postmenopausal African-American and non-African-American women. Breast Cancer Res BCR 2012;14:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop 1971;80:147–154. [DOI] [PubMed] [Google Scholar]
- 40.Papakitsou EF, Margioris AN, Dretakis KE, et al. Body mass index (BMI) and parameters of bone formation and resorption in postmenopausal women. Maturitas 2004;47:185–193. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y-S, Han J-J, Lee J, Choi HS, Kim JH, Lee T. The correlation between bone mineral density/trabecular bone score and body mass index, height, and weight. Osteoporos Sarcopenia 2017;3:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture Risk Prediction by Non-BMD DXA Measures: the 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J Clin Densitom Off J Int Soc Clin Densitom 2015;18:309–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Illustration of weight pattern categorization based on percent change in weight from 0 to 18 months (x-axis) and 18 to 30 months (y axis). Data points represent the 77 CLIP II individuals who lost weight during the intervention period (0 to 18 months), and regained at least 5% (denoted by dashed line) of lost weight during the 18 to 30 month period [weight regainers (WR) denoted with +], or did not regain at least 5% of lost weight during the 18 to 30 month period [weight maintainers (WM) denoted with O).”
Supplementary Figure 2. Exploratory associations between change in weight and change in BMD during weight loss and weight regain, among individuals who completely regained lost weight (n=10).


