Abstract
Background
The Eastern Cape province of South Africa has one of the highest burdens of HIV in the world. Emergency Departments (EDs) can serve as optimal clinical sites for the identification of new HIV infections and entry into care. We sought to determine the current burden of HIV disease among ED patients in the Eastern Cape.
Methods
We conducted a prospective cross-sectional observational study in the EDs of three Hospitals in the Eastern Cape province of South Africa from June 2017 to July 2018. All adult, non-critical patients presenting to the ED were systematically approached and offered a Point-Of-Care (POC) HIV test in accordance with South African guidelines. All HIV-positive individuals had their blood tested for the presence of antiretroviral therapy (ART) and the presence of viral suppression (≤ 1000 copies/ml). HIV incidence was estimated using a multi-assay algorithm, validated for a subtype C epidemic.
Findings
Of the 2901 patients for whom HIV status was determined (either known HIV-positive or underwent POC HIV testing), 811 (28.0%) were HIV positive, of which 234 (28.9%) were newly diagnosed. HIV prevalence was higher in Mthatha [34% (388/1134) at Mthatha Regional Hospital and 28% (142/512) at Nelson Mandela Academic Hospital], compared to Port Elizabeth [22% (281/1255) at Livingstone Hospital]. HIV incidence was estimated at 4.5/100 person-years (95% CI: 2.4, 6.50) for women and 1.5 (CI 0.5, 2.5) for men. Of all HIV positive individuals tested for ART (585), 54% (316/585) tested positive for the presence of ARTs, and for all HIV positive participants with viral load data (609), 49% (299/609) were found to be virally suppressed.
Interpretation
Our study not only observed a high prevalence and incidence of HIV among ED patients but also highlights significant attrition along the HIV care cascade for HIV positive individuals. Furthermore, despite developing an optimal testing environment, we were only able to enrol a small sub-set of the ED population. Given the high HIV prevalence and high attrition in the ED population, HIV services in the ED should also develop strategies that can accommodate large testing volumes and ART initiation.
Keywords: HIV testing, HIV in men, South Africa, Emergency medicine, HIV and trauma
Research in context
Evidence before this study
Despite significant investments in HIV testing, nearly 25% of HIV-positive South Africans do not know their HIV status. Emergency Departments (EDs) provide care to large volumes of patients (in SA 150–300 patients per day). ED populations are typically younger and have a higher prevalence of vulnerable patients (i.e., persons with substance abuse, homelessness, mental health problems and victims of violent crime). These patients are 1.4–2 times more likely to seek care in the ED compared to other primary care settings. The ED population also has a higher prevalence of HIV infection compared to antenatal clinics and other primary care settings. Several studies have demonstrated high HIV prevalence in the ED, but little is known about the HIV care cascade in ED patients.
Added-value of this study
This study is the first to define not only the HIV prevalence and incidence in the ED population but also the HIV care cascade. Our study identified that ED-based HIV testing services could capture male patients who had a high burden of undiagnosed HIV infection. Furthermore, we identified that male patients were at significantly higher risk of attrition along the care cascade.
Implications of all the available evidence
Policymakers both in South Africa and beyond should critically evaluate the need to implement and support ED-based HIV care if we are to meet the UNAIDS 90-90-90 targets. However, ED-based HIV testing must be coupled with ART initiation and linkage to care strategies, which will require innovative solutions for long term-sustainability and implementation.
Alt-text: Unlabelled Box
1. Background
In recent years, significant strides have been made to develop and implement innovative strategies to improve the HIV care cascade, which refers to the percentage of people in a population that know their HIV status, are on antiretroviral therapy (ART), and are virally suppressed [1]. Compelling evidence from clinical and population studies supports HIV treatment as prevention [2]. Global increases in ART use have been shown to prevent viral transmission via the reduction in viral loads after the initiation of ART [3]. Furthermore, in the treatment as prevention paradigm, population-level viral suppression is strongly associated with decreases in HIV incidence [4].
Countries, such as South Africa, that shoulder the highest burden of HIV infection in the world, have implemented universal prevention, testing, and treatment policies to help curb the epidemic [5]. However, in Southern Africa, an estimated 86% of people living with HIV know their status, 61% are accessing ART, and only 47% are virally suppressed [6]. Despite sustained efforts for over two decades, critical coverage gaps remain. The Emergency Department (ED) in particular, is a key clinical venue where patients missed by current HIV testing and treatment programs can be accessed. It is well established that ED populations have a higher prevalence of HIV infection (both in the US and in low- and middle-income countries [LMICs]) than in the local community and other clinic-based facilities [7]. Furthermore, the ED may be a high-value testing venue that provides care to vulnerable populations (such as persons with substance abuse problems and victims of violent crime).
The Eastern Cape province in South Africa per the 2017 National HIV Prevalence Survey, has the third-highest burden of HIV (25.2%) after Kwazulu-Natal (27.0%) and Free State (25.5%) [8]. What is more concerning however is that the Eastern Cape has seen the highest rise in HIV prevalence in the country, over the preceding five years, in 2012 the HIV prevalence was reported as 19.9% (KwaZulu-Natal and Free State were 27.9% and 20.4% respectively) [9]. Compared to other provinces in the country, the Eastern Cape has a disproportionately high burden of HIV relative to the resources allocated to this region. HIV service delivery in the Eastern Cape, in particular, is hindered by limited resources, lack of standardised training, and competing clinical care priorities [10], [11]. Furthermore, even though universal provider-initiated testing and treatment is mandated in all health care facilities throughout South Africa, service provision is challenging in many clinic-based settings and virtually non-existent in the ED [12]. This study sought to characterise both the prevalence and incidence of HIV and to describe the current HIV care cascade in patients attending several EDs in the Eastern Cape.
2. Methods
2.1. Study Design and Setting
This prospective cross-sectional observational study was conducted in the ED in three hospitals in the Eastern Cape province between June 2017 and July 2018. Each hospital was sampled for a period of six weeks, during which all eligible patients, were approached study staff and offered point-of-care (POC) HIV testing. In addition, all previously known, and newly diagnosed HIV positive patients were requested to provide an additional blood sample for further laboratory testing.
2.2. Study Sites
The study was conducted in the Eastern Cape province of South Africa. Walter Sisulu University (WSU) is an academic health services complex that is spread throughout the Eastern Cape and hosts three academic centres located in East London, Mthatha and Port Elizabeth (PE), this study was conducted in the latter two. Nelson Mandela Academic Hospital (NMAH) (located in Mthatha) and Livingstone Hospital (LH) (located in Port Elizabeth), are tertiary care centres that provide 24-h emergency medical and trauma care and accept referrals from district hospitals up to 200 km away. Mthatha Regional Hospital (MRH) (located in Mthatha), is a district hospital with 24-h services and receives ambulances and walk-in patients, transferring all trauma patients to NMAH. All of the EDs had approximately 30–70 beds, were staffed by 1–2 doctors per shift, and used paper charts. Only Livingstone Hospital had an Infectious diseases specialist and Emergency Medicine specialist at the time of this study.
2.3. Recruitment and Enrolment
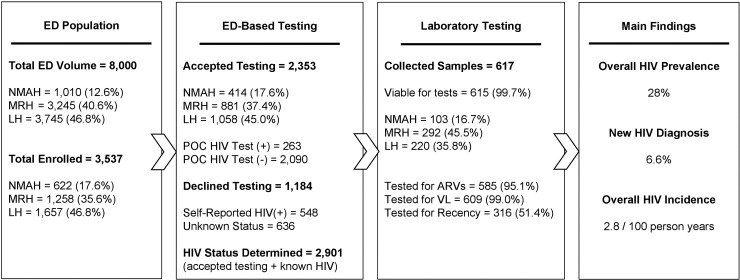
Staff were instructed to approach patients as soon as the triage process was completed. All patients presenting for care in the ED during the study period, and who were over the age of 18 years, fully conscious, and clinically stable were eligible to be enrolled by the study staff. Fig. 1 provides an overview of the baseline ED volume, number of patients enrolled, number of patients who consented for POC HIV testing and number of patients who provided a blood sample for additional laboratory testing across all three sites.
Fig. 1.
Overview of study population and enrollment in point-of-care testing and further laboratory analysis for HIV positive individuals across sites.
2.4. HIV Testing Intervention
During the study period, HIV testing was implemented and conducted in accordance with the 2015 National HCT Guidelines. The core recommendations of the national guidelines mandate an opt-in testing approach requiring informed consent with extensive pre- and post-test counselling in a confidential private setting. All study staff were trained in rapid POC HIV testing and counselling, were required to be fluent in English, Xhosa and/or Afrikaans and, completed Good Clinical Practice (GCP) and data collection training. All patients were first tested with the Advanced Quality™ Rapid Anti-HIV 1 & 2 test (InTec Products, Inc, Xiamen, China). Non-reactive specimens were considered HIV-negative. All reactive specimens were confirmed using an ABON™ HIV 1/2/O Tri-line HIV Rapid Test (ABON Biopharm, Hangzhou, China). As per the 2015 HCT Guidelines [13], patients with two reactive rapid tests were considered to be HIV positive, and no further confirmatory testing was done. Patients with discordant results were referred for re-testing using an ELISA at the local ARV clinic, but for the purpose of analysis, they were classified as HIV negative. Patients were informed of their HIV results immediately after testing completion. HIV negative patients were provided with standard post-test counselling on the HIV prevention strategies, the window period and importance of re-testing. HIV positive were provided with post-test counselling on linkage to care and a letter stating their results with directions to a local HIV clinic.
2.5. Further Laboratory Testing
All patients who self-reported as being HIV positive, or those who underwent POC testing and were identified as HIV positive, were further consented to provide a blood sample for laboratory testing. All tests were conducted based on sample availability. Serum was isolated and stored at − 80 °C. Presence of any ART drugs within serum samples was assessed using HPLC-High Resolution Accurate Mass (HRAM) spectrometry [13]. Samples with sufficient volume were tested for HIV viral load using the Abbott m2000 RealTime System with a detection limit of 320 copies/mL. (Abbott Park, IL), due to low volume testing algorithm, viral loads (VL) < 1000 copies/ml were considered virally suppressed. Samples were also tested with a limiting-antigen avidity enzyme immunoassay (LAg-Avidity). Using a multi-assay algorithm, which has been validated for a subtype C epidemic [14]. Subjects with a VL > 1000 copies/ml, a Limiting Antigen avidity assay normalised optical density of < 2.8 and a Johns Hopkins modified BioRad Avidity assay index result below 95% were considered recently infected. This testing algorithm has a window period of 248 days and has been objectively compared to provide near-identical incidence estimates observed in a longitudinal cohort [15].
2.6. Demographic Data Collection
We collected additional patient-level information such as age, sex, presenting complaint, the severity of illness, disposition, and previous diagnosis of HIV infection. The severity of illness was quantified using the South African Triage Scale (SATS) [16]. Data were prospectively collected on case report forms that were scanned and entered using intelligent character recognition (ICR) DataFax© software (DataFax, Clinical DataFax Systems Inc., Hamilton, Ontario, Canada) and centrally double-verified by independent data technicians.
2.7. Sample Size, Outcome Measures and Data Analysis
The 2012 South African National HIV prevalence study estimated the HIV prevalence in the Eastern Cape to be 19.9% [6], [9]. To detect a difference of greater than 5% from the baseline estimate of 19.9%, with a 95% confidence interval and power of 0.8, our study needed to recruit at least 534 patients per study site. Based on the ED volumes (approximately 100–150/day), we conservatively estimated the ability to enrol between 15 and 20 patients per day and thus planned to recruit patients for a six-week duration per study site.
Patients were defined as “HIV positive” if they self-reported a previous diagnosis of HIV infection (i.e., “Known HV”) or were “Newly Diagnosed with HIV” if they did not know their HIV status and then had two positive POC rapid HIV tests. Patients were defined as “HIV negative” if they had a single negative POC rapid HIV test. Patients were defined as “Unknown Status” if they were unaware of their status and refused a POC rapid HIV test.
The descriptive analysis explored the overall HIV prevalence, the proportion of newly diagnosed patients, and the proportion of patients on any ARTs and virally suppressed (VL < 1000 copies/ml). Comparisons of patient characteristics by HIV status were conducted using chi-squared tests. Log-binomial models were used to estimate to estimate crude (unadjPR) and adjusted (adjPR) prevalence ratios. In situations where the log-binomial model failed to converge, modified Poisson regression with robust variance were used. The following independent variables, age group, sex, presenting complaint, time of testing, symptomology, and severity of sickness were included in the multivariate model to examine the association between patient characteristics and attrition along the care cascade for those that are HIV positive. HIV incidence and 95% CI estimates were calculated using methods previously described using a window period of 248 days and a false recent ratio of 0% [14].
2.8. Ethical Considerations
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, the University of Cape Town Human Research Ethics Committee (HREC), and the Walter Sisulu University HREC. Written consent was obtained from all participants for the collection of demographic data, POC HIV testing, and sample collection if HIV positive.
3. Results
3.1. Overall Study Characteristics
Over the study period, a total of 3537 patients were approached across all sites, of which 2901 enrolled and had their HIV status determined (Fig. 1). The number of patients approached varied by site: LH (n = 1657); MRH (n = 1258); and NMAH (n = 622), however acceptance of HIV testing remained fairly even across all three sites at approximately 66.5% (range: 63.9%–70.0%) (Table 1). The age distribution remained consistent across all sites, and most ED patients were under the age of 35 [51.2% (1811/3537)] (Table 1). The two tertiary care facilities (NMAH and LH) provided care to a higher proportion of male patients (60.3% and 52.1% respectively) and to a higher proportion of trauma patients (42.1% and 54.4% respectively).
Table 1.
Characteristics of All Participants by Testing Site.
| Nelson Mandela Academic Hospital (NMAH) N = 622 |
Mthatha Regional Hospital (MRH) N = 1258 |
Livingstone Hospital (LH) N = 1657 |
Total N = 3537 |
|
|---|---|---|---|---|
| Age | ||||
| < 20 | 36 (5.79%) | 62 (4.93%) | 61 (3.68%) | 159 (4.50%) |
| 20–25 | 141 (22.67%) | 252 (20.03%) | 259 (15.63%) | 652 (18.43%) |
| 26–35 | 174 (27.97%) | 335 (26.63%) | 491 (29.63%) | 1000 (28.27%) |
| 36–45 | 79 (12.70%) | 192 (15.26%) | 337 (20.34%) | 608 (17.19%) |
| 46–55 | 55 (8.84%) | 116 (9.22%) | 260 (15.69%) | 431 (12.19%) |
| 56 + | 137 (22.03%) | 301 (23.93%) | 249 (15.03%) | 687 (19.42%) |
| Sex | ||||
| Female | 247 (39.71%) | 719 (57.15%) | 793 (47.86%) | 1759 (49.73%) |
| Male | 375 (60.29%) | 539 (42.85%) | 864 (52.14%) | 1778 (50.27%) |
| Presenting compliant | ||||
| Medical | 360 (57.88%) | 912 (72.50%) | 755 (45.56%) | 2027 (57.31%) |
| Trauma | 262 (42.12%) | 346 (27.50%) | 902 (54.44%) | 1510 (42.69%) |
| Time of presentation | ||||
| Routine hours | 249 (40.03%) | 540 (42.93%) | 625 (37.72%) | 1414 (39.98%) |
| Out of hours | 373 (59.97%) | 718 (57.07%) | 1032 (62.28%) | 2123 (60.02%) |
| SATSa | ||||
| Emergency | 0 (0.0%) | 0 (0.0%) | 31 (1.87%) | 31 (0.88%) |
| Very urgent | 186 (29.9%) | 358 (28.46%) | 114 (6.88%) | 658 (18.60%) |
| Urgent | 401 (64.47%) | 868 (69.0%) | 839 (50.63%) | 2108 (59.60%) |
| Routine | 35 (5.63%) | 32 (2.54%) | 673 (40.62%) | 740 (20.92%) |
| Deceased | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Disposition | ||||
| Death | 0 (0.0%) | 8 (0.64%) | 1 (0.06%) | 9 (0.25%) |
| Admission | 305 (49.04%) | 305 (24.24%) | 142 (8.57%) | 752 (21.26%) |
| Emergent surgery | 101 (16.24%) | 3 (0.24%) | 1 (0.06%) | 105 (2.97%) |
| ICU admission | 1 (0.16%) | 1 (0.08%) | 1 (0.06%) | 3 (0.08%) |
| Transfer | 54 (8.68%) | 58 (4.61%) | 278 (16.78%) | 390 (11.03%) |
| Discharge | 141 (22.67%) | 769 (61.13%) | 1120 (67.59%) | 2030 (57.39%) |
| Absconded | 3 (0.48%) | 1 (0.08%) | 103 (6.22%) | 107 (3.03%) |
| Unassigned | 17 (2.73%) | 113 (8.98%) | 11 (0.66%) | 141 (3.99%) |
| Consent to POC testing | ||||
| Declined (known positive)d | 98 (15.59%) | 253 (20.03%) | 197 (11.95%) | 548 (15.47%) |
| Declined (unknown status)e | 110 (17.86%) | 124 (9.86%) | 402 (24.20%) | 636 (17.95%) |
| Accepted test | 414 (66.72%) | 881 (70.11%) | 1058 (63.85%) | 2353 (66.58%) |
| HIV statusc | ||||
| Known HIV | 109 (17.52%) | 262 (20.83%) | 206 (12.43%) | 577 (16.31%) |
| HIV negative | 370 (59.49%) | 746 (59.30%) | 974 (58.78%) | 2090 (59.09%) |
| Newly diagnosed HIV | 33 (5.31%) | 126 (10.02%) | 75 (4.53%) | 234 (6.62%) |
| Unknownb | 110 (17.68%) | 124 (9.86%) | 402 (24.26%) | 636 (17.98%) |
South African Triage Score (SATS).
Unknown status is defined as those who were not known to be HIV positive, and refused to enrol in the study and undergo POC testing.
The number of patients with a documented test (N) was used as the denominator when calculating the proportion with a positive or negative result (n).
Declined - known positive defined as marking known positive but not consenting to test.
Declined – unknown defined as no known HIV diagnosis and did not consent to test.
3.2. HIV Prevalence
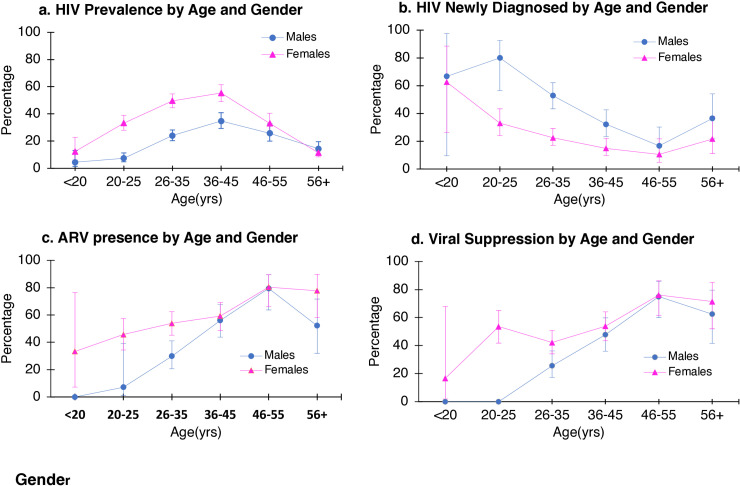
Of the 2901 patients for whom HIV status was determined (either known HIV-positive or underwent POC HIV testing), 811 (28.0%) were HIV positive, of which 234 (28.9%) had a new diagnosis. HIV prevalence was higher in Mthatha (34% [388/1134] at MRH, and 28% [142/512] at NMAH), compared to Port Elizabeth (22% [281/1255]) (Table 1). The prevalence of HIV was significantly higher in females (n = 509, 35.3%) compared to males (n = 302, 20.7%) (χ2 (df 1, N = 2901), p < 0.001). HIV prevalence was highest in females aged 36–45 years (55.4%; 95% CI: 49.0–61.5), and second highest in females aged 26–35 years (49.6%; 95% CI: 44.5–54.7) (Fig. 2a). The peak prevalence in males was highest at 34.8% (95% CI: 29.3, 40.9) also in the 36–45 years age category. Medical patients had a higher prevalence of HIV infection (34% [586/1723]) as did patients who were sicker (defined as SATS ‘emergency’/‘very urgent’) (27% [619/2314]), and those that were subsequently admitted (32% [332/1026]) (Table 2).
Fig. 2.
HIV prevalence, newly diagnosed, ARV presence and viral suppression by age and gender.
Table 2.
Characteristics of participants with a determined HIV status across all sites.
| Total (n = 2901) (100.0%) |
HIV negative (n = 2090) (72.0%) |
Known HIV positive (n = 577) (19.9%) |
Newly diagnosed HIV positive (n = 234) (8.1%) |
p-Value | |
|---|---|---|---|---|---|
| Age | |||||
| < 20 | 132 | 121 (5.79%) | 4 (0.69%) | 7 (2.99%) | < 0.001 |
| 20–25 | 542 | 431 (20.62%) | 65 (11.27%) | 46 (19.66%) | |
| 26–35 | 816 | 526 (25.17%) | 192 (33.28%) | 98 (41.88%) | |
| 36–45 | 500 | 276 (13.21%) | 175 (30.33%) | 49 (20.94%) | |
| 46–55 | 358 | 253 (12.10%) | 91 (15.77%) | 14 (5.98%) | |
| 56 + | 553 | 483 (23.11%) | 50 (8.66%) | 20 (8.55%) | |
| Sex | |||||
| Female | 1443 | 934 (44.69%) | 399 (69.15%) | 110 (47.01%) | < 0.001 |
| Male | 1458 | 1156 (55.31%) | 178 (30.85%) | 124 (52.99%) | |
| Presenting compliant | |||||
| Medical | 1723 | 1137 (54.40%) | 430 (74.52%) | 156 (66.67%) | < 0.001 |
| Trauma | 1178 | 953 (45.60%) | 147 (25.48%) | 78 (33.33%) | |
| Time of presentation | |||||
| Routine hours | 1157 | 812 (38.85%) | 252 (43.67%) | 93 (39.74%) | 0.111 |
| Out of hours | 1744 | 1278 (61.15%) | 325 (56.33%) | 141 (60.26%) | |
| SATSa | |||||
| Emergency | 23 | 17 (0.81%) | 5 (0.87%) | 1 (0.43%) | < 0.001 |
| Very urgent | 564 | 378 (18.09%) | 130 (22.53%) | 56 (23.93%) | |
| Urgent | 1745 | 1235 (59.09%) | 363 (62.91%) | 147 (62.82%) | |
| Routine | 569 | 460 (22.01%) | 79 (13.69%) | 30 (12.82%) | |
| Disposition | |||||
| Death | 7 | 4 (0.19%) | 3 (0.52%) | 0 (0.0%) | |
| Admission | 639 | 419 (20.05%) | 165 (28.60%) | 55 (23.50%) | |
| Emergent surgery | 87 | 65 (3.11%) | 16 (2.77%) | 6 (2.56%) | < 0.001 |
| ICU admission | 3 | 2 (0.09%) | 0 (0.0%) | 1 (0.43%) | |
| Transfer | 297 | 208 (9.95%) | 68 (11.79%) | 21 (8.97%) | |
| Discharge | 1659 | 1259 (60.24%) | 272 (47.14%) | 128 (54.70%) | |
| Absconded | 80 | 66 (3.16%) | 9 (1.56%) | 5 (2.14%) | |
| Unassigned | 129 | 67 (3.21%) | 44 (7.62%) | 18 (7.69%) |
The number of patients with a documented test (N) was used as the denominator when calculating the proportion with a positive or negative result (n).
3.3. Burden of Undiagnosed HIV Infection
Younger ED patients were more likely to be unaware of their HIV positive status (Fig. 2). The peak prevalence of undiagnosed HIV infection was in patients younger than 25 years (males 80.0%; 95% CI: 56.4, 92.5 and, in females 33.0%; 95% CI: 24.0, 43.3). While the overall prevalence of HIV infection is higher in females, the burden of undiagnosed HIV infection remains similar across both genders [7.6% (110/1443) in females versus 8.5% (124/1458) in males]. Patients who were sicker (defined as SATS ‘emergency’/‘very urgent’) [9.7% (57/587)], and those who were admitted [8.6% (55/639)] also were more likely to be unaware of their status (Table 2).
3.4. Attrition Along the HIV Care Cascade
Of the 811 HIV positive patients, 234 (28.9%) were unaware of their HIV status. In an adjusted model, male patient were twice as likely to be unaware of their status (adj.PR 2.16; 95% CI: 1.66, 2.83) compared to their female counterparts (Table 3). Out of the 811 HIV positive patients, 617 patients (76.1%) consented to provide a sample for additional laboratory testing, of these 2 samples were discarded due to inadequate volumes (Fig. 1). Of the remaining 615 samples, there was sufficient serum volume to perform ART testing in 585 patients. Of all the patients tested, 54% (316/585) tested positive for the presence of ARTs. In our adjusted model, the only risk factor for the absence of ART was male sex (adj.PR 1.41; 95% CI 1.10, 1.81) (Table 3). Of the 615 samples 609 patients who had sufficient sample for viral load testing, of which 49% (299/609) were found to be virally suppressed; again, male sex was identified as an independent risk factor for not being virally suppressed (adj.PR 1.22; 95% CI: 1.04, 1.42) (Table 3).
Table 3.
Risk factors for attrition along the cascade of care.
| Prevalence of being unaware of HIV infection |
Prevalence of not being on ARVs |
Prevalence of not being virally suppressed |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 811 (100%) | Newly diagnosed | UnadPR (95% CI) |
adjPR (95% CI) |
N = 585 (100%) | Not on ART | UnadPR (95% CI) |
adjPR (95% CI) |
N = 609 (100%) | Not virally suppressed | UnadPR (95% CI) |
adjPR (95% CI) |
|
| Age | ||||||||||||
| < 20 | 11(1.36) | 66% (7/11) | 1.00 | 1.00 | 9(1.54) | 78%(7/9) | 1.00 | 1.00 | 9(1.48) | 89%(8/9) | 1.00 | 1.00 |
| 20–25 | 111(14) | 41% (46/111) | 0.65(0.40,1.07) | 0.66(0.30,1.48) | 84(14) | 61%(51/84) | 0.78(0.53,1.15) | 0.79(0.36,1.76) | 85(14) | 56%(48/85) | 0.64(0.30,1.34) | 0.61(0.29,1.31) |
| 25–35 | 290(36) | 34%(98/290) | 0.53(0.33,0.85) | 0.48(0.22,1.05) | 205(35) | 55%(113/205) | 0.71(0.49,1.03) | 0.66(0.31,1.44) | 217(36) | 64%(139/217) | 0.72(0.35,1.47) | 0.67(0.33,1.37) |
| 35–45 | 224(28) | 22%(49/224) | 0.34(0.21,0.57) | 0.32(0.14,0.71) | 152(26) | 42%(64/152) | 0.54(0.36,0.80) | 0.49(0.22,1.08) | 156(26) | 49%(76/156) | 0.55(0.26,1.14) | 0.49(0.24,1.03) |
| 45–55 | 105(13) | 13%(14/105) | 0.21(0.11,0.41) | 0.18(0.07,0.45) | 85(15) | 20%(17/85) | 0.23(0.15,0.45) | 0.23(0.09,0.56) | 90(15) | 24%(22/90) | 0.28(0.12,0.62) | 0.24(0.11,0.54) |
| > 55 | 70(9) | 29%(20/70) | 0.45(0.25,0.80) | 0.39(0.17,0.94) | 50(9) | 34%(17/50) | 0.44(0.26,0.74) | 0.39(0.16,0.95) | 52(9) | 33%(17/52) | 0.37(0.16,0.85) | 0.32(0.14,0.76) |
| Sex | ||||||||||||
| Female | 509(63)302(37) | 22% (110/509) | 1.00 | 1.00 | 363(62) | 42% (151/363) | 1.00 | 1.00 | 373(61) | 47% (175/373) | 1.00 | 1.00 |
| Male | 302(37)509(63) | 41% (124/302) | 2.0(1.53,2.35) | 2.16(1.66,2.83) | 222(38) | 53% (118/222) | 1.28(1.07,1.52) | 1.41(1.10,1.81) | 236(39) | 57% (135/236) | 1.22(1.04,1.42) | 1.33(1.05,1.67) |
| Presenting complaint | ||||||||||||
| Medical | 586(72) | 27%(156/586) | 1.00 | 1.00 | 423(72) | 44%(187/423) | 1.00 | 1.00 | 439(72) | 50%(219/439) | 1.00 | 1.00 |
| Trauma | 225(28) | 35%(78/225) | 1.30(1.04,1.63) | 1.22(0.91,1.64) | 162(28) | 51%(82/162) | 1.14(0.95,1.38) | 1.04(0.79,1.36) | 170(28) | 54%(91/170) | 1.07(0.91,1.27) | 0.99(0.77,1.28) |
| Time of presentation | ||||||||||||
| 9-5/M-F | 345(43) | 27%(93/345) | 1.00 | 1.00 | 256(44) | 46%(119/256) | 1.00 | 1.00 | 268(44) | 51%(138/268) | 1.00 | 1.00 |
| Out of hours | 466(57) | 30%(141/466) | 1.12(0.90,1.40) | 1.07(0.82,1.39) | 329(56) | 46%(150/329) | 0.98(0.82,1.17) | 0.92(0.72,1.17) | 341(56) | 50%(172/341) | 0.98(0.84,1.15) | 0.93(0.74,1.17) |
| SATS* | ||||||||||||
| Sick | 619(76) | 29%(177/619) | 1.00 | 1.00 | 449(77) | 46%(206/446) | 1.00 | 1.00 | 472(78) | 50%(237/472) | 1.00 | 1.00 |
| Very sick | 192(24) | 30%(57/192) | 1.04(0.81,1.33) | 1.07(0.78,1.45) | 136(23) | 46%(63/136) | 1.01(0.82,1.24) | 1.06(0.79,1.41) | 137(23) | 53%(73/137) | 1.06(0.89,1.27) | 1.10(0.84,1.44) |
| Site | ||||||||||||
| Livingstone | 281(35) | 27%(75/281) | 1.00 | 1.00 | 220(38) | 50%(109/220) | 1.00 | 1.00 | 220(36) | 55%(120/220) | 1.00 | 1.00 |
| MRH | 388(48) | 32%(126/388) | 1.22(0.96,1.55) | 1.26(0.94,1.71) | 268(46) | 46%(122/268) | 0.92(0.76,1.11) | 0.86(0.65,1.13) | 289(47) | 53%(152/289) | 0.96(0.82,1.13) | 0.90(0.70,1.16) |
| NMAH | 142(18) | 23%(33/142) | 0.87(0.61,1.24) | 0.84(0.56,1.27) | 97(17) | 39%(38/97) | 0.79(0.60,1.05) | 0.73(0.50,2.43) | 100(16) | 38%(38/100) | 0.70(0.53,0.92) | 0.63(0.44,0.91) |
3.5. Care Continuum
In a smaller proportion of patients, we were able to define the complete HIV care continuum. Of the 577 known positives we were able to test 386 for the presence of ARTs of which 76% (293/386) tested positive; of which 289 had enough serum to test for viral load, and 81% (233/289) were virally suppressed. Unfortunately, it is likely that these findings are biased by the fact that initial steps of the HIV care continuum relies on self-reporting of HIV status. In our study, of the patients who reported being unaware of their status at the time of enrolment, 10% (23/234) were actually taking ARVs.
3.6. HIV Incidence
Of the 811 HIV positive patients in our study, 29 patients were identified as having a recent infection giving an HIV incidence estimate of 2.8 per 100 person-years (95% CI: 1.7, 3.9). The incidence estimate was significantly higher (χ2 (df 1, N = 29), p = 0.01) in women at 4.5 per 100 person-years (95% CI: 2.4, 6.5) compared to males at 1.5 per 100 person-years (95% CI: 0.5, 2.5). In our study population, women were more likely to have recent infection [14.9% (15/101)] as well as patients younger than 20 [33.3% (2/6)] (Table 4).
Table 4.
Characteristics of participants by time of infection as determined by MAA test.
| Newly diagnosed, not a recent infection (n = 186) (%) |
Newly diagnosed, recent HIV infection (n = 21) (%)a |
p-Value | |
|---|---|---|---|
| Age | |||
| < 20 | 4 (66.67%) | 2 (33.33%) | 0.332 |
| 20–25 | 38 (92.68%) | 3 (7.32%) | |
| 26–35 | 81 (92.05%) | 7 (7.95%) | |
| 36–45 | 36 (87.80%) | 5 (12.20%) | |
| 46–55 | 13 (92.86%) | 1 (7.14%) | |
| 56 + | 14 (82.35%) | 3 (17.65%) | |
| Sex | |||
| Female | 86 (85.15%) | 15 (14.85%) | 0.029 |
| Male | 100 (94.34%) | 6 (5.66%) | |
| Presenting compliant | |||
| Medical | 126 (89.36%) | 15 (10.64%) | 0.731 |
| Trauma | 60 (90.91%) | 6 (9.09%) | |
| Time of presentation | |||
| Routine hours | 77 (90.59%) | 8 (9.41%) | 0.771 |
| Out of hours | 109 (89.34%) | 13 (10.66%) | |
| SATSa | |||
| Emergency | 1 (100%) | 0 (0.00%) | 0.452 |
| Very urgent | 43 (89.58%) | 5 (10.42%) | |
| Urgent | 120 (91.60%) | 11 (8.40%) | |
| Routine | 22 (81.48%) | 5 (18.52%) | |
| Disposition | |||
| Death | 0 (0.00%) | 0 (0.00%) | 0.164 |
| Admission | 47 (92.16%) | 4 (7.84%) | |
| Emergent surgery | 6 (100%) | 0 (0.00%) | |
| ICU admission | 0 (0.00%) | 1(100%) | |
| Transfer | 16 (84.21%) | 3 (15.79%) | |
| Discharge | 102 (91.07%) | 10 (8.93%) | |
| Absconded | 2 (50.00%) | 2 (50.00%) | |
| Unassigned | 12 (85.71%) | 2 (14.29%) |
Note that 29 patients tested as recent using the MAA assay, however 8 of these patients also tested positive for ARVs and thus they have been excluded from the analysis above.
4. Discussion
Our study shows that EDs in the Eastern Cape treat a high HIV prevalence population with a high HIV incidence, and poorer ART use and viral suppression rates than National estimates. While the overall HIV prevalence of 28.0% in our study population is not substantially higher than the 2017 HSRC report of 25.2% (95% CI: 19.8, 31.5) for the Eastern Cape, there is a substantial difference among achievement of the 90–90-90 targets and incidence estimates [17]. Per the HSRC estimates, 85% of people aged 15–64 years in South Africa who are living with HIV know their HIV status, 71% are receiving ART, and 62.3% are virally suppressed [17]. In contrast, our study data revealed that 71.1% of ED patients were aware of their status, 54% were positive for ARTs, and 49% were virally suppressed. Given the attrition along the care cascade and the high number of young patients with undiagnosed infection, it is not surprising that the cross-sectional incidence estimate in our population was higher than the incidence nationally reported by the HSRC [17]. The estimated incidence yielded by our ED-based study was 2.8 per 100 person-years (4.5 among females and 1.5 among males) compared to the HSRC reported incidence of 0.79 per 100 person-years (0.93 among females and 0.69 among males) [17].
Numerous studies describe the need to target resources to areas of high HIV burden and transmission, referred to as “hotspots” [18], [19]. The ED has been identified in this study as an additional hotspot that is often missed during the implementation of universal test and treat policies where the implementation of targeted HIV care strategies could have a substantial impact on HIV transmission in areas of high HIV prevalence. Studies outside of South Africa have called for the implementation of routine testing and counselling in the ED after finding high rates of testing acceptance among ED patients, thereby catching significant numbers of undiagnosed HIV infections. A study in Uganda demonstrated that 76% of HIV-positive individuals had not received HIV/AIDS care (defined as prevention and testing messages, HAART, or co-trimoxazole prophylaxis) prior to enrolment in the ED-based HIV testing study [20]. For this reason, the ED is a prime location to identify undiagnosed HIV-positive patients and out-of-care HIV individuals to improve capture toward the 90-90-90 targets.
In addition to being hotspots, EDs capture the key and often missed demographic of young men. Evidence shows that men are significantly under-represented in HIV testing and treatment services–both in sub-Saharan Africa and globally [21]. Targeting men has the potential to significantly impact HIV-related mortality, incidence, and economic costs [22]. Due to men's vastly different health-seeking behaviours (women are more likely to seek healthcare), most facility-based programs will miss males [21]. Furthermore, most community-based interventions (such as community HIV-care providers), fail to capture young men, as evidenced by the poor recruitment of males in the HVTN071 (PopART) studies in South Africa and Zambia [23]. The ED provides a clinical venue where men, who would otherwise be missed, can be engaged for counselling, testing, and linkage to care.
Lastly, this study also exemplifies the well-known reality that high incidence and attrition along the care cascade go hand in hand. The ANRS 12249 TasP trial in South Africa evaluated the impact of Universal test and treat (UTT) on the HIV epidemic and identified an absence of a lowering of HIV incidence in UTT clusters [24]. They hypothesise that a lack of incidence reduction most likely resulted from poor linkage to care in the study population [24]. While the ED is a strategic venue in which to target HIV testing, it also is a complex clinical environment that provides care to vulnerable patients, most of whom do not routinely access healthcare services. Innovative solutions must be sought, not only provide HIV services, but also ART initiation and linkage to care. Understanding the clinical setting and the care cascade will help inform the design of tailored HIV services for ED patients.
The first challenge to implementing services in the ED is the high volume of patients and operational hours of the ED. Patients seeking care are generally in favour of making HIV testing a routine part of medical care [25]. Furthermore, by routinely offering HIV testing, the test will become normalised, decreasing stigma and removing highly personal prerequisite discussions about the risk of HIV infection. The challenge remains how best to implement routine testing in a complex clinical care environment, including ensuring confidentiality, dignity, and availability of care and treatment services after a new HIV diagnosis. Several studies have discussed opt-in versus opt-out testing approaches [26]. Another approach is to evaluate the utility of HIV self-testing and testing kiosks in the ED, which may combat the challenges around volumes and lack of HIV counsellors [27]. However, these approaches only decrease the burden of the initial time for consent; the time for completing the test, interpreting results and providing referral services still remains. Additionally, a study from the United States examined the effect of mandatory HIV screening in ED patients and found an increase in patient wait times, with the potential for an increase in patients absconding [28]. It is likely that hybrid models will need to be developed that combine both self-testing and provider-initiated testing approaches, with 24-hour access to counsellors and ART initiation teams.
ART initiation and linkage to care is a second challenge. A recent modelling study, in South Africa, demonstrated that it takes on average 4.9 years for 50% of HIV seroconverters to be linked to care (95% CI 4.2–5.7) [29]. Furthermore, they identified that men and participants aged less than 30 years were found to have the lowest rates of linkage-to-care [29]. Similarly, our study identified that young male patients were independently at higher risk of not being on ARTs and not being virally suppressed. A potential solution to this, within a healthcare context, is same-day ART initiation. The CASCADE study in South Africa found that offering same-day home-based ART initiation to individuals who tested positive during home-based HIV testing, compared with usual care and standard clinic referral, significantly increased linkage to care at 3 months and HIV viral suppression at 12 months [30]. Same-day ART initiation would need to be supported by clinical algorithms such as the Simplified Algorithm for Treatment Eligibility (“SLATE”) and will require training and implementation support [31]. Given the ED population of young males and the challenges of referral patterns, same-day initiation, and point-of-care, CD4 and viral load testing should be explored in the ED [32]. Lastly, our study does not directly address retention in care. We did find, however, that known HIV positive male patients in the ED were significantly less likely to be on ARTs and/or virally suppressed. One of the main weaknesses of this approach is getting HIV positive patients to link to care. Several studies in high resource settings have successful integrated mental health screening, violence screening and substance abuse screening, with promising linkage to care outcomes [33], [34]. There are a limited number of such studies in South Africa; however, there have been promising reports for pioneering screening and brief interventions for substance use in South African emergency care settings [35], [36], [37]. ED-based HIV care would need to address the known challenges of linkage and retention, such a location and access to care, in order to maximise impact. Innovative solutions targeted to male patients, such as video-based programming, linkages to social networks and community-based support programs should be explored [22].
5. Conclusion
Emergency Departments provide care to large volumes of HIV positive individuals who are at high risk of attrition along the care cascade. Furthermore, they are a venue to capture young men, often missed by current avenues of HIV service delivery. Beyond their role in surveillance of the HIV epidemic, emergency departments should develop innovative HIV testing and linkage to care strategies that in particular meet the needs of young men who do not routinely access clinic-based health services.
5.1. Limitations
This was a cross-sectional study, and thus, there are a number of limitations that need to be addressed. The lack of electronic medical records and a uniform process by which patients enter the ED made it difficult to capture all patients that presented for care. Only patients that were approached by HIV counsellors were included in the analysis, thus a significant proportion of patients were not captured i.e., those that were too sick for HIV testing (i.e., SATS category ‘emergency’ or being resuscitated), unable to consent for HIV testing (i.e., under the age of 18 or presenting with altered mental status), or were not approached by HIV counsellors (i.e., unable to be enrolled due to limitations in staffing availability). Furthermore, due to the lack of a patient tracking system and/or electronic medical records, we have no data on the patients that were missed by study staff, and thus we were unable to perform sensitivity analyses to evaluate for potential biases in our sample.
Funding
Research reported in this publication was supported by the South African Medical Research Council and in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. Funding agencies had no role in study design, data collection, data analysis, interpretation, or the writing of the report.
Acknowledgments
Acknowledgments
The authors would like to acknowledge the staff and patients in the Emergency Departments at Nelson Mandela Academic Hospital, Mthatha Regional Hospital and Livingstone Hospital for making this research possible; the HIV Counseling and Testing team for their dedication and hard work during the study; the research and support staff at Walter Sisulu University for their role in training and research implementation; the NHLS staff in the Eastern Cape for their role in sample collection and processing; and lastly the study participants.
Declaration of Competing Interest
The authors have nothing to disclose.
Author Contributions
BH, TQ, S Reynolds, A Redd, DS, AP, LW made substantial contributions to the conceptualisation and design of the work; BH, EH, S Ryan, A Rao, VC, JI, PM, JB, RM, NM, YN, AE, JM, RF, WC made substantial contributions to the acquisition of Data; BH, GM, EH, A Rao, OL made substantial contributions to the analysis and interpretation of data for the work. All authors contributed to the drafting the work and/or revising it critically for important intellectual content; AND approved the final version to be published. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.08.007.
Contributor Information
Bhakti Hansoti, Email: bhansot1@jhmi.edu.
George Mwinnyaa, Email: gmwinny1@jhu.edu.
Elizabeth Hahn, Email: ehahn6@jhmi.edu.
Aditi Rao, Email: aditi.rao@jhmi.edu.
Kathryn Clark, Email: kclark54@jhmi.edu.
William Clarke, Email: wclarke@jhmi.edu.
Anna L. Eisenberg, Email: aeisen14@jhmi.edu.
Reinaldo Fernandez, Email: rferna25@jhmi.edu.
Oliver Laeyendecker, Email: olaeyen1@jhmi.edu.
Jernelle Miller, Email: jmill249@jhmi.edu.
Steven J. Reynolds, Email: sjr@jhmi.edu.
Andrew D. Redd, Email: aredd2@jhmi.edu.
Sofia Ryan, Email: sryan24@jhu.edu.
Lee A. Wallis, Email: lee.wallis@uct.ac.za.
Thomas C. Quinn, Email: tquinn2@jhmi.edu.
Appendix A. Supplementary Data
Supplementary Data to support Figure 2
References
- 1.Nachega J.B., Uthman O.A., del Rio C., Mugavero M.J., Rees H., Mills E.J. Addressing the Achilles' heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis. 2014;59(Suppl. 1):S21–S27. doi: 10.1093/cid/ciu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen M.S., Chen Y.Q., McCauley M. Prevention of HIV-1 infection with early antiretroviral therapy. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowski M.K., Serwadda D.M., Gray R.H. HIV prevention efforts and incidence of HIV in Uganda. 2017;377(22):2154–2166. doi: 10.1056/NEJMoa1702150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon S.S., Mehta S.H., McFall A.M. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. 2016;3(4):e183–e190. doi: 10.1016/S2352-3018(16)00019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suthar A.B., Ford N., Bachanas P.J. Towards universal voluntary HIV testing and counseling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10(8) doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS UNAIDS Data 2018. 2019. http://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
- 7.Hansoti B., Kelen G.D., Quinn T.C. A systematic review of emergency department based HIV testing and linkage to care initiatives in low resource settings. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simbayi L.C.Z.K., Zungu N. HSRC Press; Cape Town: 2017. HIV impact assessment summary.http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_Summary_ZA_ADS_cleared_PDFA4.pdf Available from. [Google Scholar]
- 9.Simbayi L., Shisana O., Rehle T., Onoya D., Jooste S., Zungu N. Human Sciences Research Council; Pretoria: 2014. South African national HIV prevalence, incidence and behaviour survey, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Bassett I.V., Regan S., Luthuli P. Linkage to care following community-based mobile HIV testing compared with clinic-based testing in Umlazi Township, Durban, South Africa. 2014;15(6):367–372. doi: 10.1111/hiv.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltzer K., Davids A. Lay counsellors' experiences of delivering HIV counseling services in public health facilities in a Eastern Cape Province District of South Africa. J Psychol Afr. 2011;21(1):53–61. [Google Scholar]
- 12.Hansoti B., Stead D., Eisenberg A. 2018. A window into the HIV epidemic from a South African emergency department. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Africa DoHRoS . 2015. Guidelines: national HIV counseling and testing (HCT) policy guidelines 2015. [Google Scholar]
- 14.Marzinke M.A., Breaud A., Parsons T.L. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta. 2014;433:157–168. doi: 10.1016/j.cca.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laeyendecker O., Konikoff J., Morrison D.E. Identification and validation of a multi-assay algorithm for cross-sectional HIV incidence estimation in populations with subtype C infection. J Int AIDS Soc. 2018;21(2):e25082. doi: 10.1002/jia2.25082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twomey M., Wallis L.A., Thompson M.L., Myers J.E. The South African Triage Scale (adult version) provides reliable acuity ratings. Int Emerg Nurs. 2012;20:142–150. doi: 10.1016/j.ienj.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 17.HSRC HSRC: research methodology and data centre, SA. 2018. http://www.hsrc.ac.za/en/departments/rmdc
- 18.Karim Q.A., Karim S.S.A., Frohlich J.A. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delva W., Karim Q.A. The HIV epidemic in Southern Africa–is an AIDS-free generation possible? Current HIV/AIDS reports. 2014;11(2):99–108. doi: 10.1007/s11904-014-0205-0. [DOI] [PubMed] [Google Scholar]
- 20.Nakanjako D., Kyabayinze D.J., Mayanja-Kizza H., Katabira E., Kamya M.R. Eligibility for HIV/AIDS treatment among adults in a medical emergency setting at an urban hospital in Uganda. Afr Health Sci. 2007;7(3) doi: 10.5555/afhs.2007.7.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shand T., Thomson-de Boor H., van den Berg W., Peacock D., Pascoe L.J.I.B. The HIV blind spot: men and HIV testing, treatment and care in sub-Saharan Africa. 2014;45(1):53–60. [Google Scholar]
- 22.Mills E.J., Beyrer C., Birungi J., MRJPm Dybul. Engaging men in prevention and care for HIV/AIDS in Africa. 2012;9(2) doi: 10.1371/journal.pmed.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes R., Floyd S., Schaap A. A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. 2017;14(5) doi: 10.1371/journal.pmed.1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwuji C.C., Orne-Gliemann J., Larmarange J. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. 2018;5(3):e116–e125. doi: 10.1016/S2352-3018(17)30205-9. [DOI] [PubMed] [Google Scholar]
- 25.Hansoti B., Hill S.E., Whalen M. Patient and provider attitudes to emergency department-based HIV counseling and testing in South Africa. South. Afr. J. HIV Med. 2017;18(1):7. doi: 10.4102/sajhivmed.v18i1.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvestri D.M., Modjarrad K., Blevins M.L., Halale E., Vermund S.H., McKinzie J.P. A comparison of HIV detection rates using routine opt-out provider-initiated HIV testing and counseling versus a standard of care approach in a rural African setting. J Acquir Immune Defic Syndr. 2011;56(1):e9. doi: 10.1097/qai.0b013e3181fdb629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai N.P., Sharma J., Shivkumar S. Supervised and unsupervised self-testing for HIV in high-and low-risk populations: a systematic review. PLoS Med. 2013;10(4) doi: 10.1371/journal.pmed.1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu N., Stone P.W., Schnall R. Impact of mandatory HIV screening in the emergency department: a queuing study. Res Nurs Health. 2016;vol. 39(2):121–127. doi: 10.1002/nur.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maheu-Giroux M., Tanser F., Boily M.-C., Pillay D., Joseph S.A., Bärnighausen T.J.A. Determinants of time from HIV infection to linkage-to-care in rural KwaZulu-Natal, South Africa. 2017;31(7):1017. doi: 10.1097/QAD.0000000000001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labhardt N.D., Ringera I., Lejone T.I. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomised clinical trial. 2018;319(11):1103–1112. doi: 10.1001/jama.2018.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen S., Fox M.P., Larson B.A. Simplified clinical algorithm for identifying patients eligible for immediate initiation of antiretroviral therapy for HIV (SLATE): protocol for a randomised evaluation. 2017;7(5) doi: 10.1136/bmjopen-2017-016340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CF Rowley. Developments in CD4 and viral load monitoring in resource-limited settings. Clin Infect Dis. 2013;58(3):407–412. doi: 10.1093/cid/cit733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Onofrio G., Degutis L.C. Preventive care in the emergency department: screening and brief intervention for alcohol problems in the emergency department: a systematic review. Acad Emerg Med. 2002;9(6):627–638. doi: 10.1111/j.1553-2712.2002.tb02304.x. [DOI] [PubMed] [Google Scholar]
- 34.Feldhaus K.M., Koziol-McLain J., Amsbury H.L., Lowenstein S.R., Abbott J.T. Accuracy of 3 brief screening questions for detecting partner violence in the emergency department. JAMA. 1997;277(17):1357–1361. [PubMed] [Google Scholar]
- 35.Sorsdahl K., Stein D.J., Naledi T., Breuer E., Myers B. Problematic alcohol and other substance use among patients presenting to emergency services in South Africa: who is ready for change? S Afr Med J. 2017;107(4):352–353. doi: 10.7196/SAMJ.2017.v107i4.10791. [DOI] [PubMed] [Google Scholar]
- 36.Myers B., Stein D.J., Mtukushe B., Sorsdahl K. Feasibility and acceptability of screening and brief interventions to address alcohol and other drug use among patients presenting for emergency services in Cape Town, South Africa. Adv Prev Med. 2012;2012 doi: 10.1155/2012/569153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorsdahl K., Myers B., Ward C., Matzopoulos R., Mtukushe B., Nicol A. Screening and brief interventions for substance use in emergency departments in the Western Cape province of South Africa: views of health care professionals. Int J Inj Control Saf Promot. 2014;21(3):236–243. doi: 10.1080/17457300.2013.811267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data to support Figure 2